Abstract
Background
Dialysis-requiring acute kidney injury (AKI) is associated with substantial mortality and risk of end-stage renal disease (ESRD). Despite considerable growth in incidence of severe AKI, information pertaining to trends in outcomes remains limited. We evaluated time trends in one year risks of ESRD and death in patients with dialysis-requiring AKI over an eight year period in Denmark.
Methods
In a retrospective nationwide study based on national registers, all adults requiring acute renal replacement therapy between 2005 and 2012 were identified. Patients with preceding ESRD were excluded. Through individual-level cross-referencing of administrative registries, information pertaining to comorbidity, preceding surgical interventions, and concurrent other organ failure and sepsis was ascertained. Comparisons of period-specific one year odds ratios for ESRD and death were calculated in a multiple logistic regression model.
Results
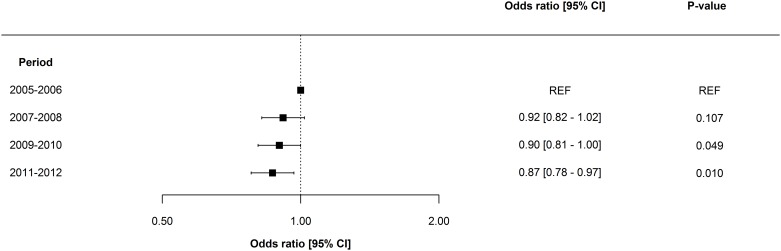
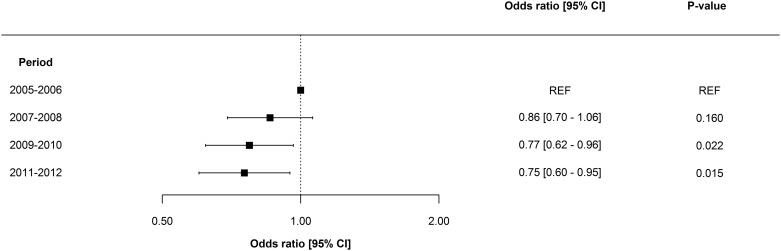
A total of 13,819 patients with dialysis-requiring AKI were included in the study. Within one year, 1,017 (7.4%) patients were registered with ESRD, and 7,908 (57.2%) patients died. The one-year rate of ESRD decreased from 9.0% between 2005 and 2006 to 6.1% between 2011 and 2012. Simultaneously, the one-year mortality rate decreased from 58.2% between 2005 and 2006 to 57.5% between 2011 and 2012. Consequently, the adjusted odds ratios for the period 2011–2012 (with the period 2005–2006 as reference) were 0.75 (0.60–0.95, p = 0.015) and 0.87 (95% CI 0.78–0.97, p = 0.010) for ESRD and death, respectively.
Conclusions
In a nationwide retrospective study on time trends in one year outcomes following dialysis-requiring AKI, risk of all-cause mortality and ESRD decreased over a period of 8 years.
Introduction
Acute kidney injury (AKI) continues to be a severe disease associated with poor prognosis [1, 2]. AKI is independently associated with diminished short- and long-term survival [3–5], and outcomes are proportional to AKI severity [6, 7]. Additionally, AKI is associated with substantially increased propensity for chronic kidney disease (CKD) including end-stage renal disease (ESRD) [8–11]. Crude incidence rates of dialysis-requiring AKI have increased substantially throughout the last two decades [12, 13]; however, Danish incidence rates have remained stable at approximately 350 per million since 2006 [14], and information pertaining to temporal change in outcomes following AKI continues to be limited [15, 16]. Development of AKI is predominantly characterized by concurrence of acute and chronic disease [17, 18]. Consequently, risk prediction in AKI is dependent on an accurate comprehension of the interplay between potential risk factors for adverse outcomes. The clinical implications associated with changing employment of acute renal replacement therapy (RRT) however remain undetermined. Accordingly, the aim of the present study was to investigate temporal change in outcomes following dialysis-requiring AKI. Consequently, we assessed one-year risk for ESRD and all-cause mortality following dialysis-requiring AKI between 2005 and 2012 in a nationwide population-based cohort.
Methods
Data sources
Danish residents are issued a civil registration number at birth or time of immigration. As health services are tax-funded; all Danish citizens receive comprehensive medical coverage through the public health care system. Treatment is registered according to the civil registration number, and consequently, cross-referencing of registers is possible. Information concerning public health care is recorded in multiple national administrative registers. All hospitalizations are recorded in the National Patient Registry, and reimbursement is contingent on accurate registration by departments. Diagnostic coding is registered in accordance with the 10th edition of the International Classification of Diseases (ICD), and procedural coding is registered in accordance with the Nordic Medico-Statistical Committee Classification of Surgical Procedures [19, 20]. The predicative value of diagnostic codes included in the Charlson Index Score has recently been validated with excellent results [21]. Additionally, chronic dialysis treatment and renal transplantations are recorded in the validated Danish National Registry on Regular Dialysis and Transplantation [22]. Dispensation of prescription medication by Danish pharmacies is recorded in the Danish Register of Medicinal Product Statistics[23]. The register records the Anatomical Therapeutic Chemical Classification System (ATC) code, quantity, strength and date of all prescriptions dispensed in Danish pharmacies. Prescription medication is partially reimbursed by the public healthcare system, and the register has been validated with excellent results [23, 24]. Limited biochemistry was available for a fraction of the study population; results were accessible from multiple laboratories across Denmark. Finally, information relating mortality and causes of death was determined via the National Registry of Causes of Death [25].
Study Population
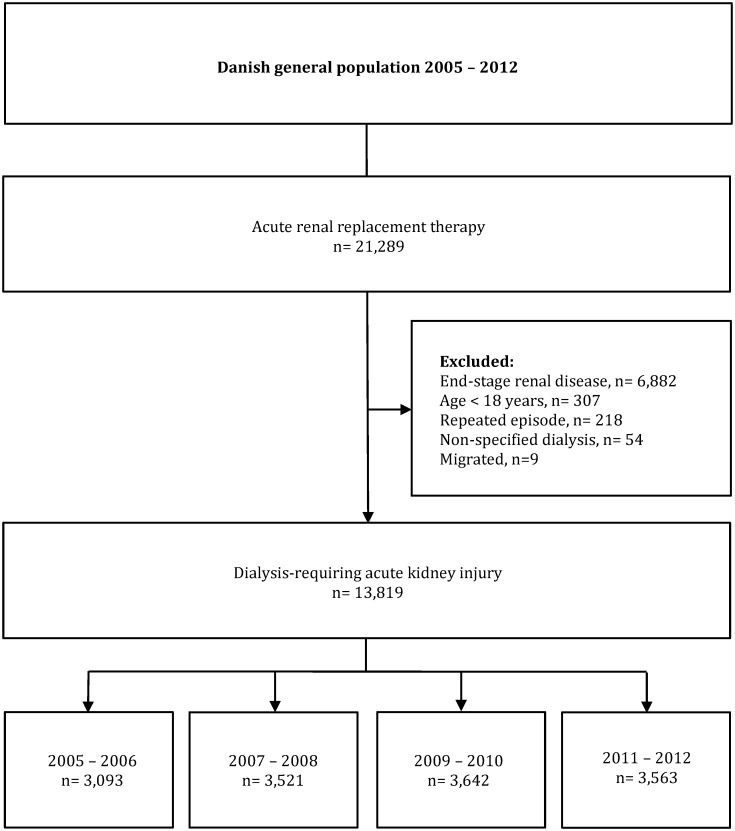
Dialysis-requiring AKI was defined as treatment with acute RRT in patients without previously registered chronic RRT. All patients requiring acute RRT between 1st January 2005 and 31st December 2012 were identified in the Danish National Patient Registry. Patients <18 years old, patients migrating within one year of treatment, patients previously treated with acute RRT, and patients previously registered with chronic RRT were excluded. All patients were followed until an endpoint or the end of the study. A flow chart depicting study design is shown in Fig 1.
Fig 1. Flow chart depicting study design.
Study Outcomes
Primary outcomes were defined as all-cause mortality and ESRD at one year. Secondary outcomes were defined as estimated glomerular filtration rate (eGFR) (plasma creatinine measurements recorded 52–104 weeks following index) in survivors with preserved renal function at one year, duration of dialysis-requirement, and duration of hospital admission.
Study Covariates
Comorbidities were identified based on diagnostic codes found in the National Patient Registry. Sensitivity for hypertension, and diabetes was augmented through the inclusion of information pertaining to outpatient medication. Preceding eGFRs were calculated as means all available plasma creatinine measurements recorded 52 to 2 weeks prior to index in accordance with the Chronic Kidney Disease Epidemiology Collaboration equation [26]. Azotemic parameters registered one day prior to index were included where available. Major cardiothoracic, gastric and orthopedic surgical interventions were identified based on procedural codes found in the National Patient Registry. Post-procedural dialysis-requirement was defined as acute RRT within 14 days of a major surgical intervention. Dialysis modalities were identified based on procedural codes found in the National Patient Registry as continuous RRT (CRRT), acute intermittent hemodialysis, or acute peritoneal dialysis. Similarly, requirements of mechanical ventilation and/or circulatory support were also identified based on procedural codes found in the National Patient Registry. Outpatient medications as baseline were identified in the prescription database. A comprehensive list of the medications, relevant codes, and algorithms are provided in S1 Appendix.
Statistical Analysis
Outcomes following dialysis-requiring AKI were compared between the periods; 2005–2006, 2007–2008, 2009–2010, and 2011–2012. Trends in patient characteristics were compared using the Cochrane-Armitage test. Durations of dialysis-requirement and hospital stay amongst patients surviving beyond hospital discharge were calculated in whole days in renal survivors. Statistical significance was defined as a two sided p-value <0.05. Analyses were performed using SAS software (versions 9.4, SAS Institute), and R version 2.15.2 (R Development Core Team). Summary results were reported with 95% confidence intervals (CI), means with standard deviations (SD), and medians with interquartile range (IQR). Odds ratios for one-year mortality and ESRD were calculated in multiple logistic regression analyses with adjustment for known risk factors associated with prognosis. As such, the models were adjusted for patient age, gender, dialysis modality, comorbidity, surgery, sepsis, mechanical ventilation, and circulatory support. Comorbidities included in the model were; non-insulin dependent and insulin-dependent diabetes, congestive heart failure, ischemic heart disease, cardiac arrhythmia, valvular heart disease, peripheral vascular disease, stroke, chronic obstructive pulmonary disease, solid and non-solid cancer, and chronic kidney disease. Preceding surgeries included in the model were any major cardiothoracic, gastric or orthopedic procedures.
Furthermore, odds ratios for one-year mortality and ESRD were also computed in various selected subpopulations. These included; gender-specific strata, age-specific strata, modality-specific strata, non-surgery-related and surgery-related strata, non-intensive care requiring and intensive care requiring strata, and Charlson Index Score stratified (<3, 3–6, and >6). In additional analyses, odds ratios were calculated for 90-day mortality and 90-day renal survival using multiple logistic regression analyses analogous to the principal analyses. As incidence of dialysis-requiring AKI in the general population was lowest in 2005, sensitivity analyses with exclusion of patients from the aforementioned year were performed to confirm the principal results; no differences were discernable. Finally, residual renal function was assessed in renal survivors by comparing the proportionate number of patients with significant (>20%) decrease in eGFR 90–365 days following index. Retrospective register based studies do not need ethical approval in Denmark; however, the Danish Data Protection Agency has approved use of data (ref. 2007-58-015 / I-suite nr 00916 GEH-2010-001).
Results
A total of 13,819 patients with dialysis-requiring AKI were identified between January 1st 2005 and December 31st 2012. Although patient characteristics principally remained stable, use of CRRT and mechanical ventilation increased over time. Period-specific baseline characteristics are shown in Table 1.
Table 1. Period-specific baseline characteristics of patients with dialysis-requiring AKI in Denmark between 2005 and 2012.
| Variables | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | |
|---|---|---|---|---|---|
| n = 3.093 | n = 3,521 | n = 3,642 | n = 3.563 | p-value | |
| Characteristics | |||||
| Gender (male), n (%) | 2022 (65.4) | 2164 (61.5) | 2264 (62.2) | 2227 (62.5) | 0.007 |
| Age (years) median [IQR] | 69.0 [59.2–76.3] | 68.1 [58.6–76.0] | 68.6 [59.3–76.3] | 69.0 [60.0–76.9] | 0.002 |
| Comorbidities | |||||
| Cardiac arrhythmia (%) | 691 (22.3) | 720 (20.4) | 813 (22.3) | 867 (24.3) | 0.002 |
| Chronic kidney disease, n (%) | 827 (26.7) | 900 (25.6) | 892 (24.5) | 876 (24.6) | 0.126 |
| Chronic obstructive pulmonary disease, n (%) | 339 (11.0) | 385 (10.9) | 375 (10.3) | 425 (11.9) | 0.174 |
| Diabetes, n (%) | 667 (21.6) | 835 (23.7) | 955 (26.2) | 977 (27.4) | <0.001 |
| Heart failure, n (%) | 496 (16.0) | 438 (12.4) | 481 (13.2) | 512 (14.4) | <0.001 |
| Ischemic heart disease, n (%) | 708 (22.9) | 653 (18.5) | 696 (19.1) | 681 (19.1) | <0.001 |
| Liver disease, n (%) | 247 (8.0) | 325 (9.2) | 350 (9.6) | 344 (9.7) | 0.069 |
| Non-solid cancer (%) | 166 (5.4) | 196 (5.6) | 225 (6.2) | 208 (5.8) | 0.506 |
| Peripheral vascular disease, n (%) | 369 (11.9) | 401 (11.4) | 365 (10.0) | 359 (10.1) | 0.022 |
| Solid cancer (%) | 461 (14.9) | 510 (14.5) | 584 (16.0) | 564 (15.8) | 0.217 |
| Stroke, n (%) | 456 (14.7) | 517 (14.7) | 517 (14.2) | 500 (14.0) | 0.792 |
| Valvular heart disease (%) | 331 (10.7) | 346 (9.8) | 405 (11.1) | 429 (12.0) | 0.027 |
| Charlson Index Score | |||||
| Charlson Score <3 (%) | 323 (10.4) | 434 (12.3) | 393 (10.8) | 372 (10.4) | 0.098 |
| Charlson Score 3–6 (%) | 1591 (51.4) | 1790 (50.8) | 1895 (52.0) | 1890 (53.0) | |
| Charlson Score >6 (%) | 1179 (38.1) | 1297 (36.8) | 1354 (37.2) | 1301 (36.5) | |
| Hospital admission | |||||
| Continuous renal replacement therapy, n (%) | 1499 (48.5) | 1858 (52.8) | 2057 (56.5) | 2094 (58.8) | <0.001 |
| Acute intermittent hemodialysis, n (%) | 1576 (51.0) | 1649 (46.8) | 1576 (43.3) | 1445 (40.6) | |
| Acute peritoneal dialysis, n (%) | 18 (0.6) | 14 (0.4) | 9 (0.2) | 24 (0.7) | |
| Admission to intensive care, n (%) | 2193 (70.9) | 2487 (70.6) | 2665 (73.2) | 2640 (74.1) | 0.002 |
| Mechanical ventilation, n (%) | 2010 (65.0) | 2279 (64.7) | 2434 (66.8) | 2395 (67.2) | 0.059 |
| Circulatory support, n (%) | 851 (27.5) | 964 (27.4) | 945 (25.9) | 868 (24.4) | 0.009 |
| Sepsis, n (%) | 760 (24.6) | 887 (25.2) | 930 (25.5) | 778 (21.8) | <0.001 |
| Cardiac surgery (%) | 447 (14.5) | 370 (10.5) | 434 (11.9) | 479 (13.4) | <0.001 |
| Gastric surgery (%) | 485 (15.7) | 532 (15.1) | 482 (13.2) | 503 (14.1) | 0.022 |
| Orthopedic surgery (%) | 160 (5.2) | 174 (4.9) | 191 (5.2) | 180 (5.1) | 0.942 |
| Outpatient medication | |||||
| Insulin (%) | 243 (7.9) | 313 (8.9) | 348 (9.6) | 351 (9.9) | 0.025 |
| Loop diuretics (%) | 929 (30.0) | 1063 (30.2) | 1062 (29.2) | 1113 (31.2) | 0.293 |
| Thiazides (%) | 409 (13.2) | 507 (14.4) | 551 (15.1) | 501 (14.1) | 0.162 |
| Aldosterone antagonist (%) | 267 (8.6) | 278 (7.9) | 287 (7.9) | 298 (8.4) | 0.611 |
| Renin-angiotensin system blocking treatment, n (%) | 869 (28.1) | 1067 (30.3) | 1199 (32.9) | 1191 (33.4) | <0.001 |
| Lipid-lowering treatment, n (%) | 651 (21.0) | 865 (24.6) | 1071 (29.4) | 1077 (30.2) | <0.001 |
| Non-steroidal anti-inflammatory drugs (%) | 342 (11.1) | 361 (10.3) | 340 (9.3) | 301 (8.4) | 0.002 |
| Proton-pump inhibitors (%) | 619 (20.0) | 780 (22.2) | 859 (23.6) | 911 (25.6) | <0.001 |
Preceding eGFRs were available in 16.5% (n = 2,286) of patients; period-specific eGFRs were; 55 ml/min/1.73m2 [IQR 33–76], 57 ml/min/1.73m2 [IQR 37–75], 60 ml/min/1.73m2 [IQR 41–81], and 66 ml/min/1.73m2 [IQR 42–86] for the periods 2005–2006, 2007–2008, 2009–2010, and 2011–2012, respectively (p<0.001). The median time from admission to initiation of dialysis decreased; the period-specific intervals were 6 [IQR 1–18], 5 [1–14], 5 [1–14], and 4 [1–13] for the periods 2005–2006, 2007–2008, 2009–2010, and 2011–2012, respectively (p<0.001). Additionally, blood samples pertaining to dialysis indications were available in a limited number of patients at index; results are shown in Table 2.
Table 2. Period-specific baseline azotemia and lactate parameters.
| Variables | n | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | p-value |
|---|---|---|---|---|---|---|
| Serum creatinine (μmol/L), median [IQR] | 1,553 | 346 [188–559] | 338 [183–565] | 319 [156–562] | 378 [188–595] | 0.408 |
| Serum K+ (mEq/L), median [IQR] | 1,550 | 4.3 [3.7–4.9] | 4.2 [3.7–4.8] | 4.3 [3.8–4.9] | 4.2 [3.6–4.9] | 0.301 |
| Serum HCO3- (mEq/L), median [IQR] | 287 | 17.1 [15.3–20.4] | 18.3 [14.9–2.6] | 16.1 [12.9–19.5] | 16.5 [13.4–19.9] | 0.056 |
| Serum Lactate (mEq/L), median [IQR] | 165 | 2.0 L [1.1–2.0] | 1.6 [1.2–2.3] | 2.2 [1.3–6.1] | 2.5 [1.4–4.9] | 0.048 |
Within one year of dialysis-requiring AKI, 1,017 patients were registered with ESRD, and 7,908 patients died, corresponding to an absolute one-year rate of 7.4% and 57.2% for ESRD and death, respectively. Prevalence of ESRD in renal survivors only was 17.2%. Period-specific unadjusted one-year mortality rates were 58.2% (n = 1,799), 56.3% (n = 1,981), 57.1% (n = 2,081), and 57.5% (2,047) for the periods 2005–2006, 2007–2008, 2009–2010, and 2011–2012, respectively (p = 0.470). Period-specific unadjusted one year rates of ESRD were 11.1% (344), 9.2% (488), 14.9% (240), and 6.6% (354) for the periods 2005–2006, 2007–2008, 2009–2010, and 2011–2012, respectively (p<0.001).
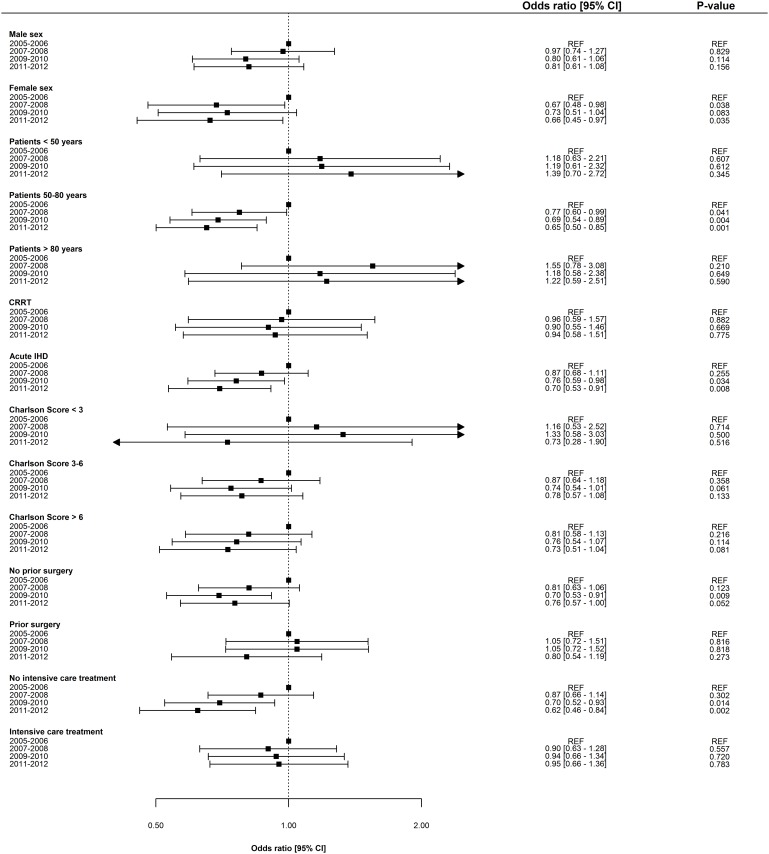
Overall, adjusted odds ratios for ESRD and death decreased incrementally over time. The adjusted one-year risk of death decreased by 3.8% [2.1–5.4] per year (p = 0.001), and the adjusted one-year risk of ESRD decreased by 6.6% [3.3–9.8] per year (p = 0.009). Odds ratios for one-year risk of death and ESRD are shown in Figs 2 and 3, respectively. Notably, mortality remained essentially unchanged in women, patients >80years, and in patients with Charlson Index score >6; however, ESRD prognosis for all subpopulations improved in all subpopulations. No significant interaction was determined between period and gender, period and patient age, and period and dialysis modality. Odds ratios for one-year risk of death and ESRD in subpopulations are shown in Figs 4 and 5. Improvement in short-term risk of death and ESRD was also observed. Odds ratios for 90-day mortality were 0.90 (0.81–1.00), 0.94 (0.84–1.04), and 0.86 (0.77–0.96), and odds ratios for 90-day risk of ESRD were 0.92 (0.75–1.12), 0.81 (0.66–1.00), and 0.76 (0.61–0.95) for the periods 2007–2008, 2009–2010, and 2011–2012, respectively (with 2005–2006 as reference).
Fig 2. Period-specific one-year risk of death.
Multivariable logistic regression model adjusted for patient age, gender, dialysis modality, comorbidity, surgery, sepsis, mechanical ventilation, and circulatory support.
Fig 3. Period-specific one year risk of end-stage renal disease.
Multivariable logistic regression model adjusted for patient age, gender, dialysis modality, comorbidity, surgery, sepsis, mechanical ventilation, and circulatory support.
Fig 4. Period-specific one year risk of death in subgroups.
Multivariable logistic regression model adjusted for patient age, gender, dialysis modality, comorbidity, surgery, sepsis, mechanical ventilation, and circulatory support. CRRT = Continous renal replacement therapy. IHD = Intermitted hemodialysis.
Fig 5. Period-specific one year risk of end-stage renal disease in subgroups.
Multivariable logistic regression model adjusted for patient age, gender, dialysis modality, comorbidity, surgery, sepsis, mechanical ventilation, and circulatory support. CRRT = Continous renal replacement therapy. IHD = Intermitted hemodialysis.
Preceding and succeeding eGFRs were available in a total of 493 of 5,911 renal survivors. Overall, a ≥20% decreased in eGFR following dialysis-requiring AKI was observed in 43.2% (213) patients in the subsample; the period-specific proportions of patients with a ≥20% decrease in eGFR were 51.2%, 41.1%, 38.2%, and 43.6% for the periods 2005–2006, 2007–2008, 2009–2010, and 2011–2012, respectively (p = 0.125). Durations of dialysis-requirement decreased over time; the period-specific median requirements were 13 days [IQR 6–25], 12 days [IQR 5–24], 12 days [IQR 6–23], and 12 days [IQR 6–22], for the periods 2005–2006, 2007–2008, 2009–2010, and 2011–2012, respectively (p<0.001). Durations of hospital admission also decreased over time; the period-specific median requirements were 70 days [IQR 34–111], 63 days [IQR 24–104], 63 days [IQR 30–108], and 54 days [IQR 26–96], for the periods 2005–2006, 2007–2008, 2009–2010, and 2011–2012, respectively (p<0.001).
Discussion
In this nationwide retrospective study on outcomes following dialysis-requiring AKI, prognosis was observed to improve incrementally during an eight year study period. Specifically, the odds ratio of both all-cause mortality and ESRD was observed to decrease by ~20% between 2005 and 2012. Simultaneously, durations of dialysis-requirement and hospitalization decreased. Irrespectively, prognosis following dialysis-requiring AKI remains unfavorable, with only approximately one third of patients surviving without requiring chronic RRT. AKI is associated with significant mortality, with one-year mortality rates fluctuating from ~35% to ~70% dependent on population and AKI severity [4, 27–29]. However, mortality rates have been reported to be decreasing [13, 15, 16, 30, 31], although the attributable risk for death associated with AKI may be increasing [32]. Additionally, AKI survivors remain at increased risk of ESRD. Reported rates of ESRD range from 2% to 20% [3, 5, 8, 15, 33, 34]; however, risk of ESRD following dialysis-requiring AKI—as is the case with mortality—could be decreasing [15]. Regardless, our results indicate incremental improvement in prognosis following dialysis-requiring AKI from 2005 till 2012.
Although initiation of acute RRT in the context of imminent indications is associated with poorer outcomes [35], evidence supporting early initiation of acute RRT in AKI remains wholly insufficient [36]. Accordingly, initiation of acute RRT continues to be based on imminent indications with no apparent shift towards earlier initiation of therapy [37, 38]. Our results corroborate this observation, as we were unable to document any substantial change in pre-dialysis azotemia or serum lactate. Furthermore, incidence rates of non-dialysis-requiring AKI have increased throughout the last decade, while incidence rates of dialysis-requiring AKI have remained stable [14]. As such, no existing evidence clearly substantiates changing thresholds for initiation of acute RRT.
Although no novel interventions pertaining to dialysis-requiring AKI have demonstrated genuine benefit throughout the last decade [39–43], our results nonetheless provide further evidence of increments in survival and renal recovery following severe AKI. Use of CRRT increased throughout the study period. Dialysis-modality has previously been demonstrated to be associated with outcomes in observational data [38]; however, the association is plausibly driven by selection biases, and no evidence currently supports any benefit associated with a specific modality [44–48]. Improvements in prognoses could alternately represent the beneficial effects related to implementation of evidence-based goal-directed therapy in critical illness [49, 50]. Outcomes following sepsis and septic shock are known to be improving [51, 52], possibly due to the benefits associated with the surviving sepsis guidelines [53], and outcomes following acute respiratory failure may also be improving [54]; albeit, thresholds for use of mechanical ventilation could be changing [55]. As such, the observed improvement of outcomes following dialysis-requiring AKI remains plausible, although possibly driven by interventions targeting supportive and non-kidney related care.
Our results confirm the detrimental consequences associated with severe AKI. Initial survivors of dialysis-requiring AKI remain at substantially increased risk of ESRD. Tentatively, our results indicate an improvement in residual eGFR in non-ESRD survivors; however, as preceding and succeeding eGFRs were only available in a small fraction of our population, the analysis was both underpowered and plausibly subject to certain biases. While outpatient care holds potential in identifying CKD in time to mitigate complications, only a fraction of patients with dialysis-requiring AKI are currently referred to a nephrologist following discharge [56, 57]. Notably, referral to a nephrologist following discharge is associated with interventions [58], and improved outcomes in retrospective cohorts [59].
Our study had numerous strengths. First, due to the structure of public health care in Denmark, national registries record comprehensive information pertaining to medical care of all Danish citizens. Follow-up is generous and essentially unflawed, and national registries are extensively validated. Second, the availability of reliable data from the Danish National Registry on Regular Dialysis and Transplantation ensures an accurate dissemination between dialysis-requiring AKI and acute RRT in ESRD. Additionally, the accuracy of CRRT in the intensive care setting has previously been validated with excellent results [60]. However, our study also had a number of limitations. First, correlation is not causation; due to the observational design, our results do not provide definite answers related to cause. Second, the absence of systematic and universal laboratory and clinical data is regrettable; consequently, a number of possible confounders remain unaddressed. Specifically, the limited data on biochemical and clinical indications for initiating acute RRT remains unfortunate; particularly as the availability of data pertaining to baseline azotemia was underpowered for definite conclusions regarding clinical implications. Furthermore our algorithm for identification of dialysis-requiring AKI does not rule out alternate indications for acute RRT to AKI. Additionally, prevalence of comorbidity may be underestimated due to a reliance on diagnostic and procedural coding. Finally, due to the particular demographics of Denmark, results cannot necessarily be extrapolated to other populations.
Conclusions
In a nationwide retrospective study on outcomes within the first year following dialysis-requiring AKI, we observed incremental decrease in odds ratios for all-cause mortality and ESRD between 2005 and 2012. Although mortality of patients with greatest vulnerability and women remained unchanged, an overall improvement in outcomes following dialysis-requiring AKI of all patients was observed. Although interpretation of our results is subject to limitations pertaining to residual confounding, dialysis-requiring AKI nonetheless continues to be associated with disheartening prognoses, with current one-year mortality and ESRD rates exceeding 50% and 10%, respectively.
Supporting Information
(DOCX)
Abbreviations
- AKI
Acute kidney injury
- ATC
Anatomical therapeutic chemical classification system
- CI
Confidence interval
- CKD
Chronic kidney disease
- CRRT
Continuous renal replacement therapy
- eGFR
Estimated glomerular filtration rate
- ESRD
End-stage renal disease
- ICD
International classification of diseases
- IQR
Interquartile range
- RRT
Renal replacement therapy
- SD
Standard deviation
Data Availability
Due to legal restrictions pertaining to use of Danish register-based data, deidentified data are available upon request from gg@heart.dk, provided relevant ethical and legal permissionshave been attained priorily, and researchers meet the criteria for access to confidential data.
Funding Statement
Dr. Carlson has received grants from The Danish Heart Foundation, The Danish Kidney Foundation, The Department of Cardiology at Gentofte Hospital, and the Department of Nephrology at Herlev Hospital, Helen and Ejnar Bjoernows Foundation, The Danish Society of Nephrology and the Health Foundation. Dr. Olesen has received speaker fees from Bristol-Myers Squibb and Boehringer Ingelheim, and funding for research from the Lundbeck Foundation, Bristol-Myers Squibb, and The Capital Region of Denmark, Foundation for Health Research. Dr. Kamper has received speaker fees from Eli Lilly, Boehringer Ingelheim and MSD. Dr. Vilsbøll has received lecture fees from Amgen, AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, Sanofi, and Zealand Pharma, and is a member of the Advisory Boards of AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Merck, Sharp & Dohme, Novo Nordisk and Sanofi. Dr. Torp-Pedersen has research contracts and speaking engagements with Sanofi, Bristol-Meyer, Merck, Biotronic and Bayer. Further, a research grant has been provided by Novo Nordisk Foundation. Dr. Gislason is supported by an unrestricted clinical research scholarship from the Novo Nordisk Foundation, and has received research grants from Pfizer/Bristol-Meyer Squibb, Bayer, Boehringer Ingelheim and AstraZeneca, and speaker fees from AstraZeneca, Bayer, Pfizer and Sanofi-Aventis. Funding from commercial sources, i.e., Bristol-Myers Squibb did not alter the authors' adherence to PLOS ONE policies on sharing data and materials. The opinions, results, and conclusions in this paper are solely representative of the authors, and are fully independent from funding sources, and no conflicts of interest exist. Additionally, the results presented in this paper have not been published previously in whole or part, except in abstract format. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript, and the opinions, results, and conclusions in this paper are solely representative of the authors, and are fully independent from funding sources.
References
- 1.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193–207. 10.1038/nrneph.2013.282 [DOI] [PubMed] [Google Scholar]
- 2.Li PK, Burdmann EA, Mehta RL, World Kidney Day Steering C. Acute kidney injury: global health alert. Kidney Int. 2013;83(3):372–6. 10.1038/ki.2012.427 [DOI] [PubMed] [Google Scholar]
- 3.Gallagher M, Cass A, Bellomo R, Finfer S, Gattas D, Lee J, et al. Long-term survival and dialysis dependency following acute kidney injury in intensive care: extended follow-up of a randomized controlled trial. PLoS Med. 2014;11(2):e1001601 10.1371/journal.pmed.1001601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gammelager H, Christiansen CF, Johansen MB, Tonnesen E, Jespersen B, Sorensen HT. One-year mortality among Danish intensive care patients with acute kidney injury: a cohort study. Crit Care. 2012;16(4):R124 10.1186/cc11420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. Jama. 2009;302(11):1179–85. 10.1001/jama.2009.1322 [DOI] [PubMed] [Google Scholar]
- 6.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005;294(7):813–8. [DOI] [PubMed] [Google Scholar]
- 7.Grams ME, Sang Y, Coresh J, Ballew S, Matsushita K, Molnar MZ, et al. Acute Kidney Injury After Major Surgery: A Retrospective Analysis of Veterans Health Administration Data. Am J Kidney Dis. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gammelager H, Christiansen CF, Johansen MB, Tonnesen E, Jespersen B, Sorensen HT. Five-year risk of end-stage renal disease among intensive care patients surviving dialysis-requiring acute kidney injury: a nationwide cohort study. Crit Care. 2013;17(4):R145 10.1186/cc12824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung KC, Tonelli M, James MT. Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol. 2013;9(2):77–85. 10.1038/nrneph.2012.280 [DOI] [PubMed] [Google Scholar]
- 10.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–8. 10.1038/ki.2011.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58–66. 10.1056/NEJMra1214243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolhe NV, Muirhead AW, Wilkes SR, Fluck RJ, Taal MW. National trends in acute kidney injury requiring dialysis in England between 1998 and 2013. Kidney Int. 2015. [DOI] [PubMed] [Google Scholar]
- 13.Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37–42. 10.1681/ASN.2012080800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson N, Hommel K, Olesen JB, Soja AM, Vilsboll T, Kamper AL, et al. Dialysis-Requiring Acute Kidney Injury in Denmark 2000–2012: Time Trends of Incidence and Prevalence of Risk Factors-A Nationwide Study. PLoS One. 2016;11(2):e0148809 10.1371/journal.pone.0148809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wald R, McArthur E, Adhikari NK, Bagshaw SM, Burns KE, Garg AX, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis. 2015;65(6):870–7. 10.1053/j.ajkd.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 16.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17(4):1135–42. [DOI] [PubMed] [Google Scholar]
- 17.Kane-Gill SL, Sileanu FE, Murugan R, Trietley GS, Handler SM, Kellum JA. Risk factors for acute kidney injury in older adults with critical illness: a retrospective cohort study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2015;65(6):860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewington AJ, Cerda J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84(3):457–67. 10.1038/ki.2013.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 Suppl):30–3. 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 20.Jurgensen HJ, Frolund C, Gustafsen J, Mosbech H, Guldhammer B, Mosbech J. Registration of diagnoses in the Danish National Registry of Patients. Methods Inf Med. 1986;25(3):158–64. [PubMed] [Google Scholar]
- 21.Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sorensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83 10.1186/1471-2288-11-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hommel K, Rasmussen S, Madsen M, Kamper AL. The Danish Registry on Regular Dialysis and Transplantation: completeness and validity of incident patient registration. Nephrol Dial Transplant. 2010;25(3):947–51. 10.1093/ndt/gfp571 [DOI] [PubMed] [Google Scholar]
- 23.Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38–41. 10.1177/1403494810394717 [DOI] [PubMed] [Google Scholar]
- 24.Johannesdottir SA, Horvath-Puho E, Ehrenstein V, Schmidt M, Pedersen L, Sorensen HT. Existing data sources for clinical epidemiology: The Danish National Database of Reimbursed Prescriptions. Clin Epidemiol. 2012;4:303–13. 10.2147/CLEP.S37587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health. 2011;39(7 Suppl):26–9. 10.1177/1403494811399958 [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson FP, Yang W, Feldman HI. Predictors of death and dialysis in severe AKI: the UPHS-AKI cohort. Clin J Am Soc Nephrol. 2013;8(4):527–37. 10.2215/CJN.06450612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rimes-Stigare C, Frumento P, Bottai M, Martensson J, Martling CR, Walther SM, et al. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Critical care. 2015;19:221 10.1186/s13054-015-0920-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuchs L, Lee J, Novack V, Baumfeld Y, Scott D, Celi L, et al. Severity of acute kidney injury and two-year outcomes in critically ill patients. Chest. 2013;144(3):866–75. 10.1378/chest.12-2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponce D, Buffarah MB, Goes C, Balbi A. Peritoneal Dialysis in Acute Kidney Injury: Trends in the Outcome across Time Periods. PLoS One. 2015;10(5):e0126436 10.1371/journal.pone.0126436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khera S, Kolte D, Aronow WS, Palaniswamy C, Mujib M, Ahmed A, et al. Trends in acute kidney injury and outcomes after early percutaneous coronary intervention in patients >/ = 75 years of age with acute myocardial infarction. Am J Cardiol. 2013;112(9):1279–86. 10.1016/j.amjcard.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 32.Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20–8. 10.1016/j.athoracsur.2012.05.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harel Z, Bell CM, Dixon SN, McArthur E, James MT, Garg AX, et al. Predictors of progression to chronic dialysis in survivors of severe acute kidney injury: a competing risk study. BMC Nephrol. 2014;15:114 10.1186/1471-2369-15-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stads S, Fortrie G, van Bommel J, Zietse R, Betjes MG. Impaired kidney function at hospital discharge and long-term renal and overall survival in patients who received CRRT. Clin J Am Soc Nephrol. 2013;8(8):1284–91. 10.2215/CJN.06650712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaara ST, Reinikainen M, Wald R, Bagshaw SM, Pettila V, Group FS. Timing of RRT based on the presence of conventional indications. Clin J Am Soc Nephrol. 2014;9(9):1577–85. 10.2215/CJN.12691213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karvellas CJ, Farhat MR, Sajjad I, Mogensen SS, Leung AA, Wald R, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Critical care. 2011;15(1):R72 10.1186/cc10061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thakar CV, Rousseau J, Leonard AC. Timing of dialysis initiation in AKI in ICU: international survey. Crit Care. 2012;16(6):R237 10.1186/cc11906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46–61. 10.1038/ki.2014.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Investigators RRTS, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–38. 10.1056/NEJMoa0902413 [DOI] [PubMed] [Google Scholar]
- 40.Network VNARFT, Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. 10.1056/NEJMoa0802639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vesconi S, Cruz DN, Fumagalli R, Kindgen-Milles D, Monti G, Marinho A, et al. Delivered dose of renal replacement therapy and mortality in critically ill patients with acute kidney injury. Critical care. 2009;13(2):R57 10.1186/cc7784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wald R, Friedrich JO, Bagshaw SM, Burns KE, Garg AX, Hladunewich MA, et al. Optimal Mode of clearance in critically ill patients with Acute Kidney Injury (OMAKI)—a pilot randomized controlled trial of hemofiltration versus hemodialysis: a Canadian Critical Care Trials Group project. Critical care. 2012;16(5):R205 10.1186/cc11835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitchlu A, Adhikari N, Burns KE, Friedrich JO, Garg AX, Klein D, et al. Outcomes of sustained low efficiency dialysis versus continuous renal replacement therapy in critically ill adults with acute kidney injury: a cohort study. BMC Nephrol. 2015;16:127 10.1186/s12882-015-0123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wald R, Shariff SZ, Adhikari NK, Bagshaw SM, Burns KE, Friedrich JO, et al. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: a retrospective cohort study*. Crit Care Med. 2014;42(4):868–77. 10.1097/CCM.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 45.Vinsonneau C, Camus C, Combes A, Costa de Beauregard MA, Klouche K, Boulain T, et al. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;368(9533):379–85. [DOI] [PubMed] [Google Scholar]
- 46.Schefold JC, von Haehling S, Pschowski R, Bender T, Berkmann C, Briegel S, et al. The effect of continuous versus intermittent renal replacement therapy on the outcome of critically ill patients with acute renal failure (CONVINT): a prospective randomized controlled trial. Critical care. 2014;18(1):R11 10.1186/cc13188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedrich JO, Wald R, Bagshaw SM, Burns KE, Adhikari NK. Hemofiltration compared to hemodialysis for acute kidney injury: systematic review and meta-analysis. Critical care. 2012;16(4):R146 10.1186/cc11458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabindranath K, Adams J, Macleod AM, Muirhead N. Intermittent versus continuous renal replacement therapy for acute renal failure in adults. Cochrane Database Syst Rev. 2007;(3):CD003773 [DOI] [PubMed] [Google Scholar]
- 49.Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752–61. 10.1164/rccm.200912-1918OC [DOI] [PubMed] [Google Scholar]
- 50.Cecconi M, Corredor C, Arulkumaran N, Abuella G, Ball J, Grounds RM, et al. Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Critical care. 2013;17(2):209 10.1186/cc11823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA: the journal of the American Medical Association. 2014;311(13):1308–16. 10.1001/jama.2014.2637 [DOI] [PubMed] [Google Scholar]
- 52.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis*. Crit Care Med. 2014;42(3):625–31. 10.1097/CCM.0000000000000026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med. 2015;43(1):3–12. 10.1097/CCM.0000000000000723 [DOI] [PubMed] [Google Scholar]
- 54.Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Steingrub JS, Lagu T, et al. Epidemiology and outcomes of acute respiratory failure in the United States, 2001 to 2009: a national survey. J Hosp Med. 2013;8(2):76–82. 10.1002/jhm.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gacouin A, Jouneau S, Letheulle J, Kerjouan M, Bouju P, Fillatre P, et al. Trends in Prevalence and Prognosis in Subjects With Acute Chronic Respiratory Failure Treated With Noninvasive and/or Invasive Ventilation. Respir Care. 2015;60(2):210–8. 10.4187/respcare.03467 [DOI] [PubMed] [Google Scholar]
- 56.Silver SA, Goldstein SL, Harel Z, Harvey A, Rompies EJ, Adhikari NK, et al. Ambulatory care after acute kidney injury: an opportunity to improve patient outcomes. Can J Kidney Health Dis. 2015;2:36 10.1186/s40697-015-0071-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siew ED, Himmelfarb J. The inexorable rise of AKI: can we bend the growth curve? Journal of the American Society of Nephrology: JASN. 2013;24(1):3–5. 10.1681/ASN.2012111115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silver SA, Harel Z, Harvey A, Adhikari NK, Slack A, Acedillo R, et al. Improving Care after Acute Kidney Injury: A Prospective Time Series Study. Nephron. 2015;131(1):43–50. 10.1159/000438871 [DOI] [PubMed] [Google Scholar]
- 59.Harel Z, Wald R, Bargman JM, Mamdani M, Etchells E, Garg AX, et al. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney international. 2013;83(5):901–8. 10.1038/ki.2012.451 [DOI] [PubMed] [Google Scholar]
- 60.Blichert-Hansen L, Nielsson MS, Nielsen RB, Christiansen CF, Norgaard M. Validity of the coding for intensive care admission, mechanical ventilation, and acute dialysis in the Danish National Patient Registry: a short report. Clin Epidemiol. 2013;5:9–12. 10.2147/CLEP.S37763 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Due to legal restrictions pertaining to use of Danish register-based data, deidentified data are available upon request from gg@heart.dk, provided relevant ethical and legal permissionshave been attained priorily, and researchers meet the criteria for access to confidential data.