Abstract
Huanglongbing (HLB; citrus greening) is the most devastating disease of citrus worldwide. No cure is yet available for this disease and infected trees generally decline after several months. Disease management depends on early detection of symptoms and chemical control of insect vectors. In this work, different combinations of organic compounds were tested for the ability to modulate citrus molecular responses to HLB disease beneficially. Three small-molecule regulating compounds were tested: 1) L-arginine, 2) 6-benzyl-adenine combined with gibberellins, and 3) sucrose combined with atrazine. Each treatment contained K-phite mineral solution and was tested at two different concentrations. Two trials were conducted: one in the greenhouse and the other in the orchard. In the greenhouse study, responses of 42 key genes involved in sugar and starch metabolism, hormone-related pathways, biotic stress responses, and secondary metabolism in treated and untreated mature leaves were analyzed. TGA5 was significantly induced by arginine. Benzyladenine and gibberellins enhanced two important genes involved in biotic stress responses: WRKY54 and WRKY59. Sucrose combined with atrazine mainly upregulated key genes involved in carbohydrate metabolism such as sucrose-phosphate synthase, sucrose synthase, starch synthase, and α-amylase. Atrazine also affected expression of some key genes involved in systemic acquired resistance such as EDS1, TGA6, WRKY33, and MYC2. Several treatments upregulated HSP82, which might help protect protein folding and integrity. A subset of key genes was chosen as biomarkers for molecular responses to treatments under field conditions. GPT2 was downregulated by all small-molecule treatments. Arginine-induced genes involved in systemic acquired resistance included PR1, WRKY70, and EDS1. These molecular data encourage long-term application of treatments that combine these regulating molecules in field trials.
Introduction
Plant pests and diseases threaten agricultural systems. Huanglongbing (HLB) disease endangers the citrus industry and citrus cultivation worldwide [1]. Neither genetic resistance nor short- or long-term therapeutic strategies to mitigate HLB has been found. Huanglongbing disease is associated with three Candidatus liberibacter (C. Las) species: asiaticus, americanus, and africanus. C. Las is a member of the alpha subdivision of the proteobacteria based on ribosomal region sequence data [2]. Symptoms have been extensively described and all citrus species are susceptible to HLB to varying degrees [3,4].
Microarray analysis identified key genes and pathways affected by HLB at the transcriptomic level in mature, symptomatic leaves [5,6]. RNA-seq was applied to describe molecular responses in fruit with different degrees of HLB symptoms [7]. This allowed a comprehensive analysis of gene regulatory networks on source and sink tissues at different developmental stages [8,9]. Effects of C. Las infection on key genes involved in sugar and starch metabolism, disrupting source-sink relationships, was a key cause of the metabolic dysfunction. Fruits of infected plants remained immature and photosynthesizing while mature leaves became yellow and accumulated starch [9]. Protein expression has been linked to the nutritional condition of grapefruit plants before and after symptom appearance. C. Las-upregulated proteins were involved in redox reactions, cell wall modification, and biotic stress responses [10]. Isobaric tags for relative and absolute quantitation (iTRAQ) identified which pathways are affected post-transcriptionally by pathogen infections [11]. A predictive proteome analysis of C. Las has been conducted [12]. Because no toxins or other pathogenic substances were clearly identified in the genome [13], the pathogenetic mechanisms of HLB disease are still unclear, nor is it clear whether the HLB-associated changes in sugar and starch metabolism are a cause or an effect of the disease [9].
Current management procedures consist mainly of visual scouting for symptoms, PCR-based detection of the pathogen, and insecticides for vector control [14]. Although application of insecticides can reduce disease spread, the disease can spread with only a few infected psyllids in the orchard. Early disease detection and psyllid control are critical practices in areas where neither disease nor vector has yet been discovered [1]. At present, no chemical compounds have been tested to beneficially modulate Citrus host responses and eventually extend the life and production of HLB-infected trees, reducing high economic costs due to lost production. Research has focused only on testing compounds targeting the pathogen. A combination of two antibiotics (penicillin and streptomycin) applied by either root soaking or foliar spray decreased C. Las titer in infected plants [15]. Antimicrobial compounds have been delivered through graft-based chemotherapy [16]. Nanoemulsion formulations were evaluated for their ability to increase permeability of antimicrobial molecules with success dependent on the citrus cultivar and degree of HLB symptoms [17]. Small-molecule inhibitors designed by molecular docking were significantly effective in inhibiting SecA ATPase in vitro [18]. Extensive use of antibiotics in open fields is not desirable due to environmental and human health concerns. Host-based treatments that modulate key genes involved in metabolic HLB syndrome are highly desirable. Data on citrus molecular responses to HLB can now be exploited to design small-molecule combinations to ameliorate the devastating symptoms. The aim of this study was to determine if small molecules are effective in modulating expression of key HLB-regulated or innate response genes after three to six days of treatment.
Materials and Methods
Plant material
Greenhouse trial
In 2011, Valencia orange scions on Kuharske Carrizo rootstocks were grown in one-gallon plastic nursery containers and kept in the greenhouse under natural light at 17 to 25°C. Graft inoculations were performed using a standard inverted “T” budding technique with C. Las-infected budwood tested as described [19]. Starting three months after budding, each plant was tested monthly using quantitative RT-PCR for C. Las species as described [19]. Each control or treatment was represented by nine to 10 trees. The control consisted of trees sprayed with distilled water. The first treatment consisted of a Silwet (0.12%), DKP3XTRA (32.5 mL/20 L) and LK-phite spray (2 mL/20 L). The other six spray treatments were composed of three different small-molecule combinations at two different concentrations each: 1) L-arginine at 1 mM or 0.5 mM, 2) 120 μM 6-benzyl adenine in combination with 15 or 30 μM gibberellin, and 3) 80 mM sucrose combined with the herbicide atrazine (2 μM or 1 μM). All seven treatments contained the surfactant Silwet and LK-phite at the same concentration used for the first treatment. Phenotypes were evaluated to determine any phytotoxic effects of these sprays. All treatments were sprayed on the citrus foliage; the volume sprayed per tree was enough to wet both upper and lower leaf surfaces just to the point of runoff. Gene expression analyses were conducted on RNA extracted three days following treatment. Three biological replicates of nine mature leaves harvested from three trees (three leaves per tree) were analyzed for each treatment. Collected leaves were immediately frozen in liquid nitrogen and kept at -80°C until RNA was extracted. Forty-two host genes were analyzed in mature symptomatic leaves of treated and untreated trees.
Field trial
The same treatments were applied during the field experiments, which were conducted in a commercial citrus orchard in Indian Ricer County, FL, composed of Valencia orange scions on Swingle rootstocks. The study was conducted on private land and the owner of the land gave permission to conduct the study on the site of our experiments. We also confirm that the field studies did not involve endangered or protected species. Twenty-four trees of medium height, 12 trees per row, were selected. These trees were mildly HLB-symptomatic and confirmed infected by C. Las through the same qPCR assay used for the greenhouse trial. The experimental design was a completely randomized blocks. There were eight treatments with three single-tree replicates. Samples were collected at three and six days following treatment. Each replicate was a pool of 10 mature symptomatic leaves per tree.
RNA extraction and qRT-PCR analysis
Total RNA was extracted from mature, fully-expanded leaves of plants grown in the greenhouse or orchard using the Rneasy Plant RNA Isolation kit (Qiagen Inc., Germany). The RNA concentration and purity were assessed by Nanodrop (Thermo Fischer Scientific Inc., MA, USA). RNA was stored at −80°C until analyzed. For each target gene, PCR primers were designed using Primer Express software (Applied Biosystems, Foster City, CA; S1 Table). DNase treatment and cDNA synthesis were completed following a combined protocol based on the Quantitect Reverse Transcription Kit (Qiagen Inc., Germany). A standard curve determined the linearity of amplicon quantity vs. initial cDNA quantity for each gene. Five μL cDNA at five ng/μL was diluted to a 12-μL final volume using Sybr Green Master Mix (Bio Rad Laboratories, Hercules, CA, USA). Amplifications were performed using standard conditions: 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 s at 95°C, and 60 s at 60°C. Fluorescent signals were collected during the annealing temperature and ΔCT was calculated. Elongation factor 1 alpha (EF-1a, accession AY498567) was used as reference gene. ΔΔCT was determined by subtracting the average EF-1a CT from the average CT of the studied gene [9].
Phenotypic measurements
Four phenotypic parameters for all treated and untreated trees were measured in the field: trunk diameter (mm), trunk height (m), width drill (m), and width row (m). These measurements were taken on 5/21/2014 and 6/28/2014 during the vegetative season.
Statistical analysis
All statistical analyses of gene expression and phenotypic data were performed using SAS II (2008) SAS/STAT software (SAS Institute). Gene expression and phenotypic data were analyzed using ANOVA and a post-hoc test to identify significant differences among treatments. Principal component analysis (PCA) was used to reduce the dimensionality of the gene expression data. Data analysis was performed to alleviate possible bias caused by the collected material for each class or by other confounding factors. Principal component analysis was applied to the ratio matrix of gene expression data to examine the contribution of each target parameter to the separation of the sample classes. A biplot was constructed based on the first two principal components.
Results
Gene expression analyses were conducted in the greenhouse and orchard. First, in greenhouse-grown plants, we quantified transcript abundance of 42 genes selected for their link with for being strongly up- or down-regulated by HLB syndrome [7,9] or for having a well-known role in plant responses to pathogen attacks [20]. Second, we selected a subset of five particularly representative genes to be analyzed in field-grown trees, to which we added an additional two genes previously linked with HLB syndrome in published data [5,9]. The presence of leaf drop or discoloration and other morphological features of trees were measured to check whether these treatments had deleterious effects on important vegetative parameters.
At advanced stages, HLB blocks sugar transport out of leaves, leading to starch accumulation in leaves, reduced photosynthesis and disrupted source-sink relationships [9]. Anatomical analysis showed that HLB caused phloem disruption, increased sucrose, and plugged sieve pores [6]. The disease also negatively modifies JA-SA crosstalk, leading to an ineffective innate immune response [7,9]. The three small-molecule treatments were selected for the potential to beneficially modulate these negative HLB-regulated responses. The combination of atrazine and sucrose upregulates genes associated with reactive-oxygen-species (ROS) defense mechanisms and sucrose metabolism [21,22]. We postulated that this treatment might upregulate genes that reduce sucrose and starch accumulation. Because L-arginine is the precursor of nitric oxide, which is involved in the SAR response and upregulates genes involved in secondary metabolism [23], we designed a second treatment consisting of two concentrations of L-arginine to induce upregulation of genes for secondary metabolic pathways such as phenols and terpenoids. Gibberellins boost systemic acquired resistance [20], favoring the resistance response against biotrophs such as C. Las [20]. Benzyladenine downregulates hexose transport in leaves, based on data deposited in the Genevestigator database. Indeed, we postulated that the combination of gybberellins and L-benzyladenine should have two synergistic effecs: 1) it should beneficially induce genes involved in the innate response against C. Las such as WRKYs, MYC2 and salycilic acid methyl transferase, and 2) it should repress the expression of GPT2 in symptomatic leaves, mitigating the deleterious HLB-driven upregulation [9].
Greenhouse trial
Atrazine combined with sucrose treatment
The transcript abundance of genes related to carbohydrate metabolism varied significantly in response to atrazine combined with sucrose (Table 1).
Table 1. Relative transcript abundance of genes involved in carbohydrate metabolism.
| Genes | Untreated | Control K-phite | 1 μM Atrazine + sucrose | 2 μM Atrazine + sucrose | 120 μM BA + 30 μM GA | 120 μM BA + 15 μM GA | 1 mM Arginine | 0.5 mM Arginine |
|---|---|---|---|---|---|---|---|---|
| Starch metabolism | ||||||||
| Alpha- amylase | 0.470 b | 4.950 ab | 3.320 ab | 4.860 ab | 87.140 a | 5.210 ab | 8.520 ab | 3.880 ab |
| Water dikinase starch degrad. | 0.317 d | 0.087 d | 0.063 d | 1.096 a | 0.185 d | 0.732 b | 0.635 bc | 0.364 cd |
| GPT2 | 0.569 a | 0.012 b | 0.219 b | 0.171 b | 0.025 b | 0.107 b | 0.243 b | 0.119 b |
| Starch synthase | 0.672 b | 2.429 ab | 4.256 a | 1.886 ab | 0.792 b | 2.870 ab | 2.016 ab | 3.029 ab |
| Sucrose metabolism | ||||||||
| Invertase | 0.839 a | 0.766 a | 0.351 a | 0.274 a | 0.296 a | 0.851 a | 0.758 a | 0.611 a |
| Sugar signaling | 0.546 a | 2.116 a | 0.937 a | 2.769 a | 7.359 a | 1.763 a | 1.750 a | 3.209 a |
| Susy | 2.310 b | 4.218 ab | 5.573 a | 2.131 b | 2.795 b | 3.412 ab | 4.296 ab | 2.022 b |
| Sps | 0.729 b | 2.023 b | 5.700 b | 13.486 a | 1.031 b | 3.393 b | 2.637 b | 5.966 b |
Means of three replicates were indicated. Letters means significant differences using ANOVA (P < = 0.05) and post-hoc test.
The sucrose synthase (Susy) and starch synthase transcripts were more abundant in 1 μM atrazine-treated plants. Sucrose-phosphate-synthase and water dikinase starch degradation (GWD) gene were upregulated by 2 μM atrazine + sucrose. Taken together, these findings highlight that activation of sucrose synthase should counter the accumulation of sucrose in symptomatic leaves, while the upregulation of GWD should promote degradation of accumulated starch.
Defense responses and hormone-related genes were affected by atrazine + sucrose treatments. WRKY33 was upregulated by 1 μM atrazine + sucrose (Table 2).
Table 2. Relative transcript abundance of genes involved in plant innate immune responses.
| Genes | Untreated | Control K-phite | 1 μM Atrazine + sucrose | 2 μM Atrazine + sucrose | 120 μM BA + 30 μM GA | 120 μM BA + 15 μM GA | 1 mM Arginine | 0.5 mM Arginine |
|---|---|---|---|---|---|---|---|---|
| RAD51 D | 0.197 b | 0.175 b | 0.690 ab | 1.878 a | 0.335 b | 0.350 b | 0.272 b | 1.362 ab |
| BZIP45 | 5.781 ab | 7.650 ab | 10.216 ab | 11.644 ab | 13.370 a | 5.197 a | 8.997 ab | 4.983 ab |
| TGA5 | 2.391 b | 3.107 ab | 4.370 ab | 3.366 ab | 2.203 b | 3.026 ab | 6.010 a | 2.201 b |
| RGA1 | 3.537 a | 4.092 a | 5.953 a | 5.301 a | 5.540 a | 4.968 a | 3.562 a | 13.320 a |
| WRKY33 | 1.752 b | 1.099 b | 6.372 a | 2.891 b | 2.425 b | 1.128 b | 1.510 b | 1.074 b |
| WRKY48 | 8.133 ab | 4.783 b | 6.387 ab | 13.873 a | 10.300 ab | 3.918 b | 3.405 b | 1.928 b |
| WRKY54 | 1.701 a | 4.708 a | 4.753 a | 6.452 a | 3.576 a | 6.850 a | 2.723 a | 1.808 a |
| EDS1 | 1.491 a | 1.905 a | 11.022 a | 9.360 a | 6.914 a | 8.289 a | 6.561 a | 4.319 a |
| ERF1 | 7.064 b | 6.616 b | 17.691 ab | 29.435 a | 18.566 ab | 9.641 b | 5.636 b | 11.654 b |
| MYC2 | 5.369 cd | 3.872 d | 10.282 abcd | 6.505 bcd | 17.029 ab | 18.585 a | 4.134 d | 6.320 bcd |
| PR1 | 0.053 b | 0.229 ab | 0.078 b | 0.970 ab | 0.454 ab | 0.119 ab | 0.832 ab | 2.27 ab |
| SR1 | 1.670 a | 2.690 a | 6.680 a | 4.720 a | 46.630 a | 5.590 a | 6.950 a | 5.210 a |
| WRKY59 | 1.376 b | 1.653 b | 1.709 b | 2.632 b | 8.332 a | 1.366b | 2.996 ab | 1.035 b |
Means of three replicates were indicated. Letters means significant differences using ANOVA (P < = 0.05) and post-hoc test.
A zinc ion binding transcription factor, heat shock protein 82 (HSP82) and ERF1 were strongly induced by 2 μM atrazine (Tables 2 and 3).
Table 3. Relative transcript abundance of genes involved in stress responses and secondary metabolism.
| Genes | Untreated | Control K-phite | 1 μM Atrazine + sucrose | 2 μM Atrazine + sucrose | 120 μM BA + 30 μM GA | 120 μM BA + 15 μM GA | 1 mM Arginine | 0.5 mM Arginine |
|---|---|---|---|---|---|---|---|---|
| Stress-related and cell wall | ||||||||
| HSP21 | 23.350 c | 308.390 a | 24.110 c | 106.950 bc | 27.670 c | 83.290 bc | 97.860 bc | 288.346 a |
| HSP82 | 1.688 c | 5.208 ab | 0.729 c | 5.585 a | 3.364 abc | 5.448 ab | 4.332 ab | 0.920 c |
| ABC Transporter | 2.189 de | 1.110 de | 0.537 e | 15.798 b | 6.580 cd | 24.822 a | 9.245 c | 1.229 de |
| ATPtranslocase2 | 0.022 a | 0.052 a | 0.032 a | 11.499 a | 0.012 a | 0.148 a | 0.125 a | 0.029 a |
| Sulfotransfer. 1 | 0.298 c | 0.008 c | 0.6987 c | 0.6015 c | 2.678 b | 6.932 a | 0.483 c | 0.136 c |
| NNLTP | 0.022 a | 0.052 a | 0.032 a | 11.499 a | 0.012 a | 0.148 a | 0.125 a | 0.029 a |
| PSBW | 2.394 c | 3.178 c | 8.020 b | 6.470 bc | 2.287 c | 6.226 bc | 2.539 c | 12.839 a |
| Pectate lyase 5 | 0.427 b | 0.242 b | 0.169 b | 0.919 ab | 0.231 b | 4.233 a | 0.817 ab | 0.589 ab |
| Secondary metabolism | ||||||||
| Terpene synthase 14 | 0.198 b | 0.184 b | 0.402 b | 0.112 b | 0.101 b | 0.345 b | 0.051 b | 1.944 a |
| Terpene synthase 21 | 3.472 a | 1.309 a | 1.026 a | 5.870 a | 4.891 a | 1.849 a | 1.543 a | 6.564 a |
| Terpene synthase 3 | 0.76 a | 0.53 a | 4.92 a | 38.40 a | 0.64 a | 1.37 a | 0.32 a | 0.19 a |
| B-Amyrin | 1.457 b | 2.300 ab | 2.178 b | 3.504 ab | 22.614 a | 9.651 ab | 3.253 ab | 6.649 ab |
Means of three replicates were indicated. Letters means significant differences using ANOVA (P < = 0.05) and post-hoc test.
JIN1 was induced by 1 μM atrazine + sucrose (Table 4).
Table 4. Relative transcript abundance of genes involved in hormone-related pathways.
| Genes | Untreated | Control K-phite | 1 μM Atrazine + sucrose | 2 μM Atrazine + sucrose | 120 μM BA + 30 μM GA | 120 μM BA + 15 μM GA | 1 mM Arginine | 0.5 mM Arginine |
|---|---|---|---|---|---|---|---|---|
| Gibberellins | ||||||||
| GA2-oxidase | 0.395 a | 0.676 a | 0.556 a | 5.276 a | 2.718 a | 1.752 a | 0.199 a | 1.379 a |
| Gibberelin-2-oxygenase | 0.765 a | 0.357 a | 0.793 a | 1.367 a | 2.629 a | 3.965 a | 1.158 a | 0.487 a |
| Auxins and Benzyl-adenine | ||||||||
| GH3.1 | 0.077 b | 0.021 b | 0.135 b | 0.511 a | 0.048 b | 0.070 b | 0.118 b | 0.255 ab |
| Ka02 | 2.837 ab | 3.661 a | 2.532 ab | 3.608 a | 0.139 b | 4.164 a | 0.929 ab | 2.794 ab |
| Jasmonic acid | ||||||||
| 12-oxophytodi. reductase 1-like | 0.444 b | 0.290 b | 0.322 b | 1.986 a | 0.174 b | 1.063 ab | 1.964 a | 0.732 ab |
| Salicylic acid methyl transferase | ||||||||
| SA-methyl transferase | 0.033 b | 0.015 b | 0.052 b | 0.277 a | 0.314 a | 0.091 a | 0.360 a | 0.434 b |
| Ethylene | ||||||||
| ACS-1 | 3.960 a | 3.580 a | 5.039 a | 6.202 a | 2.025 a | 1.723 a | 4.595 a | 4.247 a |
Means of three replicates were indicated. Letters means significant differences using ANOVA (P < = 0.05) and post-hoc test.
Salicylic acid methyl transferase and 12-oxophytodienoate reductase 1-like were upregulated by 2 μM atrazine + sucrose.
Gibberellin and benzyl-adenine treatment
GA + BA treatments were tested to determine their effects on key genes involved in plant innate immune responses and carbohydrate metabolism. Among carbohydrate metabolism genes, alpha-amylase was significantly induced by 120 μM gibberellins combined with 30 μM benzyl-adenine (Table 1). Among innate immune response genes, WRKY54 was upregulated in response to 120 μM gibberellins + 15 μM benzyl-adenine while WRKY59 was enhanced by 120 μM gibberellins + 30 μM benzyl-adenine (Table 2). Sulfotransferase1 was enhanced by both gibberellin and benzyl-adenine treatments. MYC2 was upregulated by benzyladenine + gibberellin.
Among the secondary metabolism and stress response genes, 120 μM gibberellins + 15 μM benzyl-adenine enhanced expression of HSP82 and two genes encoding pectate lyases involved in cell wall metabolism (Table 3). β-amyrin was enhanced by 30 μM gibberellins combined with benzyl-adenine.
BA + GA treatments induced SA methyl transferase (Table 4). BA + GA partially induced HLB-related changes to SA-mediated defense response, but EDS1 was not altered by the treatment, so the plant probably still can’t downregulate jasmonic antagonistic signaling. Ka02 involved in cytokinin metabolism was higher in response to 15 μM than to 30 μM benzyl-adenine.
Arginine treatment and K-phite treatments
0.5 mM L-arginine upregulated HSP21 and terpene synthase3 (Table 3). Arginine treatments did not alter the expression of terpene synthase14 and terpene synthase21, so the treated plant is still unable produce important terpene compounds. TGA5 and HSP82 were enhanced by 1 mM L-arginine. K-phite treatment repressed glucose-phosphate-transporter2 (GPT2). This downregulation may have a beneficial effect since this gene allows glucose import into the chloroplast and starch accumulation. K-phite significantly induced HSP21, a chaperone involved in functional protein stability.
Principal component analysis
Two principal component analyses were conducted to independently assess two gene subsets: 1) genes involved in sucrose and starch metabolism (PCA-1; Figs 1 and 2) genes involved in hormone-related proteins and biotic stress responses (PCA-2; Fig 2).
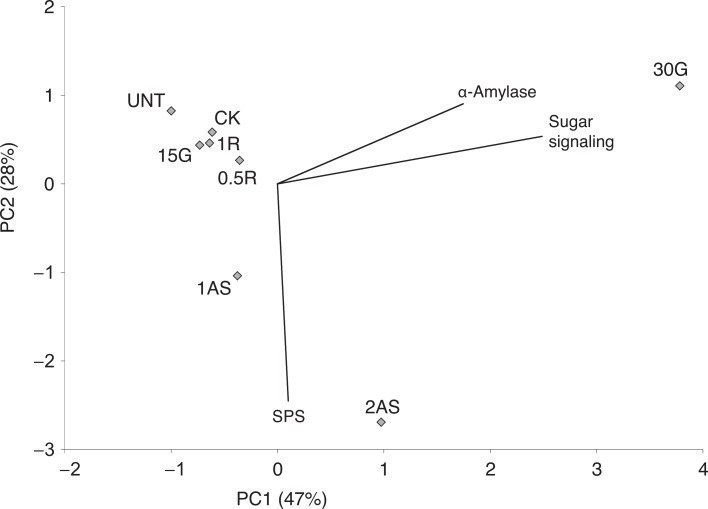
Fig 1. Overall analysis of HLB-regulated changes in carbohydrate metabolism.
Principal component analysis of treated and untreated Citrus categories in relation to genes involved in sucrose and starch pathways. UNT = Untreated with hormones (Control), CK = treated with K-phite, 30G = 30 μM gibberellin, 15G = 15 μM gibberellin, 1R = 1 mM L-arginine, 0.5R = 0.5 mM L-arginine, 2AS = sucrose combined with 2 μM atrazine, 1AS = sucrose combined with 2 μM atrazine. SPS = sucrose-phosphate-synthase.
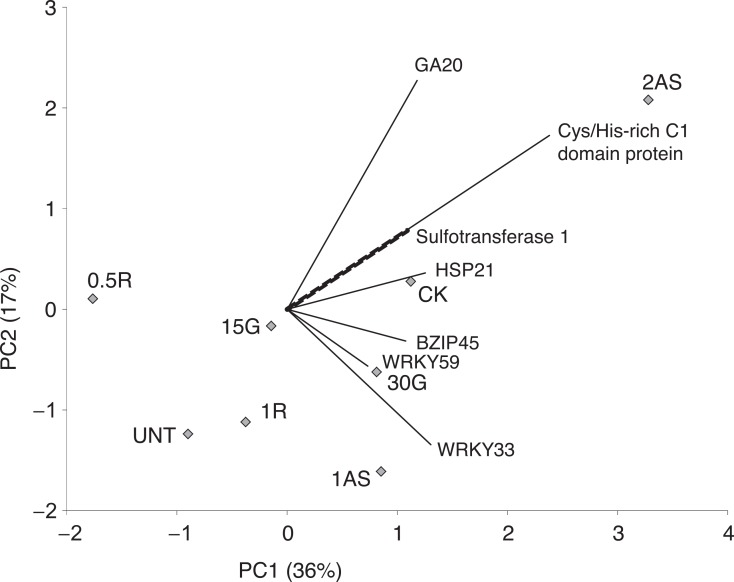
Fig 2. Overall analysis of HLB-regulated changes in biotic stress response.
Principal component analysis of treated and untreated Citrus categories in relation to genes involved in biotic stress responses. UNT = Untreated with hormones (Control), CK = treated with K-phite, 30G = 30 μM gibberellin, 15G = 15 μM gibberellin, 1R = 1 mM L-arginine, 0.5R = 0.5 mM L-arginine, 2AS = sucrose combined with 2 μM atrazine, 1AS = sucrose combined with 2 μM atrazine. GA20 = Ga2-oxidase, HSP21 = Heat shock protein 21.
In PCA-1, the first two principle components explained 47 and 28% of data variability, respectively. The 2 μM atrazine + sucrose treatment separated from the rest of the treatments. SPS greatly contributed to this separation. 120 μM BA + 30 μM GA was also highly discriminated from the rest of the treatments. The other treatments were not distinct from the untreated controls.
PC 1 and PC 2 of PCA-2 (Fig 2) explained 36 and 17% of data variability, respectively. The 1 mM L-arginine treatment was not distinct from untreated conditions, but the 2 μM atrazine + sucrose treatment was highly distant. GA2-oxidase, zinc ion binding, and cysteine-histine rich domain C1 gene contributed significantly to the separation of 2 μM atrazine + sucrose treatment. WRKY33 highly contributed to the separation of the 1 μM atrazine + sucrose treatment.
Field trial
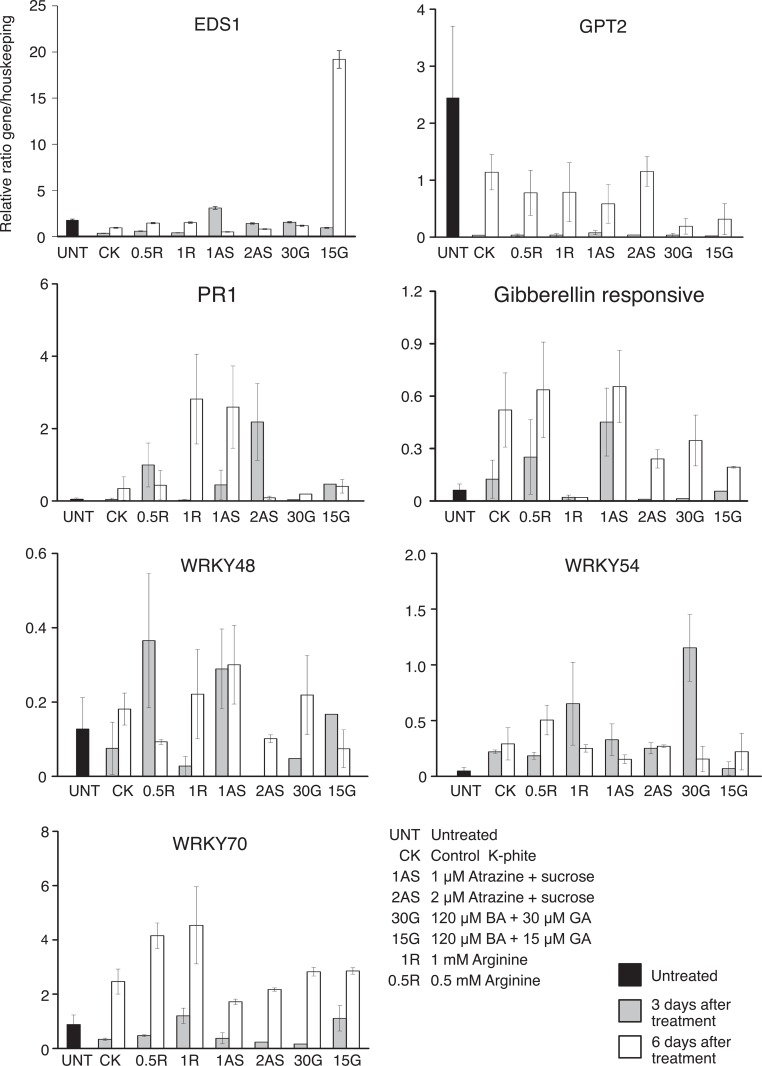
Small-molecule regulating treatments investigated in the greenhouse were also applied to HLB-symptomatic trees in a young orchard where disease symptoms were frequently present. The aim of this trial was to determine whether the same treatments used in the greenhouse could modulate the expression of key host genes at three and six days after their application. Seven key genes were selected to monitor the transcriptomic regulation of the treatments for two reasons: 1) previous data found them highly characteristic of an HLB-induced response [7,9] and 2) they play a key role in innate immune responses. A gibberellin responsive gene was used as a marker to determine the efficacy of GA + BA treatments to modulate gene expression under field conditions. GPT2 is a key HLB-regulated gene involved in glucose import into the chloroplast and is linked to the increased accumulation of starch in symptomatic leaves. The other genes were involved in plant defense and hormonal-mediated innate responses. WRKY70 and EDS1 are key points of regulation of JA-SA crosstalk. PR1 upregulation is a beneficial against C. Las since this gene is involved in the systemic acquired resistance response. WRKY48 and WRKY54 were induced by HLB in previous studies [7,9].
At three days after treatment, 1 μM atrazine + sucrose induced the gibberellin-responsive protein and PR1 and repressed WRKY48 (Fig 3).
Fig 3. Expression of seven host genes in response to spray treatments in field conditions.
Relative expression of each gene and treatment was shown as average of three biological replicates. Standard deviations were indicated.
In addition, 30 μM GA + 120 μM BA induced WRKY48 and WRKY54. This finding may have positive effects on infected Citrus since the two genes are involved in salicylic acid-mediated responses against biotrophs. 0.5 mM L-Arginine upregulated WRKY48 and EDS1. WRKY70 was enhanced by 1 mM arginine and 15 μM GA combined with 120 μM BA.
At six days following treatment, some important changes in expression of the seven host biomarkers were observed. 1 μM atrazine upregulated PR1. 2 μM atrazine and 0.5 mM L-arginine repressed WRKY48. A general inhibition of GPT2 was observed in all treated trees at both three and six days after treatment. This repression was particularly evident in leaves sprayed with gibberellins + benzyladenine.
Phenotypic measurements and pathogen qPCR detection
No obvious symptoms of a harmful spray as leaf drop or discoloration was observed in treated trees. The tree trunk diameter, height, width drill, and width row of the treated trees grown under field conditions were measured in May and June 2014 (Table 5).
Table 5. Phenotypic measurements in response to the seven treatments and control (untreated).
| 1st Measurement (5.21.14) | Untreated | 120 μM BA + 30 μM GA | 120 GA + 15 μM BA | 1 μM Atrazine | 2 μM Atrazine | .12% Siluet K-Phite + | 0.5 mM L-Arginine | 1.0 mM L-Arginine |
|---|---|---|---|---|---|---|---|---|
| Trunk Diameter (mm) | 90.770 a | 91.537 a | 82.280 a | 96.097 a | 88.197 a | 83.827 a | 96.427 a | 98.747 a |
| Tree Height (m) | 2.9533 ab | 3.0000 ab | 2.5533 b | 3.1667 ab | 3.1900 ab | 2.8133 ab | 3.2567 ab | 3.3200 a |
| Width Drill (m) | 2.4167 a | 2.7400 a | 2.4733 a | 2.7000 a | 2.6767 a | 2.4433 a | 2.7467 a | 2.6233 a |
| Width Row (m) | 2.4767 a | 2.7667 a | 2.4400 a | 2.7400 a | 2.5267 a | 2.5533 a | 2.7400 a | 2.6800 a |
| 2nd Measurement (6.28.13) | Untreated | 120 μM BA + 30 μM GA | 120 GA + 15 μM BA | 1 μM Atrazine | 2 μM Atrazine | .12% Siluet K-Phite + | 0.5 mM L-Arginine | 1.0 mM L-Arginine |
| Trunk Diameter (mm) | 70.940 a | 77.427 a | 70.503 a | 80.813 a | 75.207 a | 72.513 a | 82.293 a | 83.617 a |
| Tree Height (m) | 2.5500 abc | 2.6833 ab | 2.3533 bc | 2.7067 ab | 2.6733 abc | 2.3100 c | 2.7267 a | 2.7700 a |
| Width Drill (m) | 2.1767 a | 2.5400 a | 2.3133 a | 2.4233 a | 2.4000 a | 2.4200 a | 2.6533 a | 2.4400 a |
| Width Row (m) | 2.0700 c | 2.6167 ab | 2.2733 bc | 2.4900 abc | 2.3033 abc | 2.2667 bc | 2.7267 a | 2.4267 abc |
Means of three replicates were indicated. Letters means significant differences using ANOVA (P < = 0.05) and post-hoc test.
The aim of this analysis was to determine whether treatments were detrimental to tree growth or had undesirable phenotypic effects. No significant phenotypic differences were observed among untreated and treated trees except width row, which was significantly lower in untreated trees than in trees treated with 30 μM GA + 120 μM BA. There were no visible discolorations or other signs of plant distress from the spraying. No set of trees had visibly different vegetative vigor.
The quantification of pathogen titer was performed after three months from treatments as previously indicated to check if it was not changed in response to treatments.
Discussion
Our objective was to test the ability of six combinations of small-molecule compounds to modulate expression of key genes involved in HLB syndrome and innate immune responses shortly after treatment. We did not pretend to reduce pathogen titers or cure the plants with only one treatment. Before performing a long-term study, we wanted to evaluate the ability of the treatments to modulate expression of a subset of key genes that are altered during HLB syndrome. As we expected pathogen titers did not significantly differ among treated and control trees. A long-term study will reveal if repetitive and continuous applications will reduce pathogen concentrations and symptom severity.
Treatments were designed based on previously proposed hypotheses. Sucrose-induced protection against atrazine effects was linked to upregulation of reactive oxygen species (ROS) defence and repair mechanisms [21].
Nitric oxide (NO) is produced by l-arginine. Treatment with arginine provoked resistance against Botrytis cinerea in tomato at three to six days after treatment. Endogenous NO concentrations correlated positively with induction of key enzymes involved in biotic stress responses such as phenylalanine ammonia-lyase, chitinase, β-1,3-glucanase and polyphenoloxidase [23].
Gibberellins regulate plant growth by modulating degradation of growth-repressing DELLA proteins that promote susceptibility to biotrophic pathogens and resistance to necrotrophic pathogens [24]. This is accomplished by modulating the relative strength of the SA and JA signaling pathways [24]. Through regulation of DELLA stability, gibberellins affect the SA-JA-ET network and plant immune response. Genevestigator showed that benzyl-adenine downregulated the glucose-phosphate transporter in Arabidopsis. Since this gene is induced by HLB metabolic syndrome [5,9], benzyl-adenine treatments might help mitigate the negative effects of HLB on leaf metabolism. In combination, the two hormones may beneficially modulate key HLB-regulated genes involved in carbohydrate metabolism [7,9].
K-phite mineral solution was also tested, alone or in combination with the three small molecule compounds. This treatment was considered because of contrasting published reports on the effects of nutrient solutions such as K-phite [25]. Mineral solutions increased the concentrations of important N, Mn, Zn and B ions in leaves and long-term application reduced pathogen titer, leaf size, and leaf weight [25]. Although enhanced nutritional solutions composed of essential micronutrients did not improve fruit production and quality of C. Las-infected trees [26], others results support the hypothesis that the pathogen severely affects nutrient patterns [27]. In addition, foliar nutrition and soil conditioners helped reduce economic and production losses due to HLB [28,29].
To determine how the treatments affected the metabolism of infected trees, 42 genes were selected from previously published Citrus transcriptome data [7,9]. These genes fell into three subsets involved in: 1) carbohydrate metabolism and signaling, 2) innate immune responses, including key players in JA-SA signaling, crosstalk and induced responses, or 3) other genes involved in biotic stress responses such as those involved in hormone-related pathways, secondary metabolism and stress-preventing factors. From these biomarkers, we chose seven representative biomarkers to be followed under field conditions in response to the same treatments. Treated plants were tested for the presence of C. Las using qPCR and showed clear HLB symptoms.
QRT-PCR analyses were conducted at three to six days after treatment. As expected, we observed no significant changes in pathogen titer. Repeated applications of treatments (at least weekly) should eventually affect the titer. Since we only treated infected trees once, an analysis of pathogen titer after treaments was outside the scope of this study. Long-term studies on the effects of repeated applications of these treatments should test pathogen titer using qPCR.
Atrazine combined with sucrose
The first small-molecule treatment tested was the combination of sucrose and the herbicide atrazine. Atrazine is a well-known photosystem II inhibitor that affects plant gene expression, seedling physiology, and potentiality impairs protein translation and the ROS defense mechanism [30]. However, in combination with sucrose, atrazine induces xenobiotic and ROS signaling. In addition, this treatment upregulated important classes of antioxidant enzymes [21,22]. These findings were consistent with our unpublished findings that glutathione-S-transferases are upregulated in more tolerant Citrus genotypes.
Here we observed that atrazine combined with sucrose drastically affected some key genes responsible for HLB-induced carbohydrate changes. Increased sucrose concentrations have been found in in C. Las-infected leaves [6,11]. 1 μM atrazine + sucrose enhanced sucrose synthase while 2 μM atrazine + sucrose upregulated the water dikinase starch degradation gene and sucrose-phosphate-synthase. Atrazine upregulated alpha-amylase, which was repressed in mature HLB-infected Citrus leaves [9] but upregulated in infected Citrus stems [31]. Taken together, these findings lead us to speculate that atrazine + sucrose might help sucrose degradation by activating sucrose synthase. In addition, upregulation of alpha-amylase may increase starch degradation in HLB-infected plants where its accumulation is advanced.
Atrazine (1 μM) combined with sucrose upregulated WRKY33. Brassica napus plants overexpressing BnWRKY33 had increased resistance to Sclerotinia sclerotiorum infection [32]. This effect was mediated by SA [32]. WRKY33 upregulation allowed resistance to the necrotroph Botrytis cinerea in Arabidopsis [33]. Loss of WRKY33 function induces salicylic acid (SA)-mediated responses, increases salicylic acid and represses jasmonic acid (JA)-mediated responses [34].
Overall, our results support the hypothesis that this treatment could beneficially modulate key HLB-regulated genes associated with the well-known HLB carbohydrate metabolic syndrome [9]. The changes to expression of some key genes involved in sugar and starch metabolism could beneficially modulate the metabolic responses of HLB disease in photosynthesizing Citrus leaves, restoring a more normal source-sink relationship and potentially inhibiting the characteristic and deleterious syndrome in the fruit.
Gibberelllins combined with benzyl adenine treatments
A mixture of gibberellin (GA3) and 6-benzyladenine (BA) was tested to modulate jasmonic acid-salicylic acid (JA-SA) crosstalk in favor of responses to biotrophs such as C. Las. Our hypothesis was that gibberellin treatments may activate SAR responses through positive regulation of hormone-mediated crosstalk regulating biotic stress responses [19]. Some key genes in hormone-related pathways and JA-SA crosstalk were chosen as indicators of treatment effects.
MYC2 was significantly induced by both 15 and 30 μM gibberellin treatments. MYC2 is a transcription factor composed of a basic helix-loop-helix (bHLH) domain that activates and represses specific JA-responsive gene expression in Arabidopsis [35]. MYC2 also induced responses to abiotic stress mediated by abscissic acid in Arabidopsis [36] and suppressed salicylic acid-mediated responses in Arabidopsis [37]. Upregulation of WRKY54 in gibberellin-treated field trees is also interesting because this gene is a positive regulator of resistance against Erwinia amylovora, the agent of fire blight in the Rosaceae family.
The plant immune regulator EDS1 (Enhanced Disease Susceptibility1) plays a fundamental role in resistance mechanisms to biotrophs and hemi-biotrophs [38,39]. This role is due to the formation of complexes with PAD4 and SAD101 in both cytoplasm and nucleus [40]. The 15 μM GA + BA treatment enhanced EDS1 at six days after treatment in the field. The increase in EDS1 transcripts after application of these small molecules could help activate SAR response against pathogen infections. Mutant screening showed that upregulation of EDS1 induces non-host resistance against E. amylovora in Arabidopsis by activating WRKY46 and WRKY54 genes [41].
Our hypothesis was partially confirmed. GA + BA may beneficially increase innate responses by inducing EDS1 and MYC2. Although long-term field trials are required, we speculate that continuous application of this small molecule mixture could stimulate improved SA-JA crosstalk
L-arginine treatments
The third small-molecule treatment was composed of L-arginine, used in two concentrations. L-arginine positively regulates key genes involved in innate immune responses [23]. L-arginine may act on nitric oxide and directly upregulate key genes in salicylic acid signaling. Increased endogenous NO concentrations after L-arginine treatment in pre-harvest tomatoes correlated positively with increased defensive enzyme activity and postharvest disease resistance [15]. PR1, WRKY70 and WRKY54 were upregulated by 1 mM L-arginine under field conditions. WRKY transcription factors are important regulators of responses to abiotic and biotic stresses. WRKY54 and WRKY70 play a key role in a regulatory network that affects leaf senescence by interacting with another WRKY factor [42].
In our field trial, arginine induced some key important gene regulation that should benefit SAR responses.
Common effects among treatments
Glucose accumulation induced by C. Las infection is transported to the plastid by hexose transporters [5,9,43]. GPT2 is a key player in HLB-mediated starch accumulation in leaves because this gene mediates glucose import into the chloroplast in infected leaves [5,9]. GPT2 was significantly repressed by all spray applications at both three and six days after treatment under field conditions. This inhibition may reduce the amount of glucose carried into the plastid and thus starch accumulation, with consequent improvement of disrupted source-sink relationships.
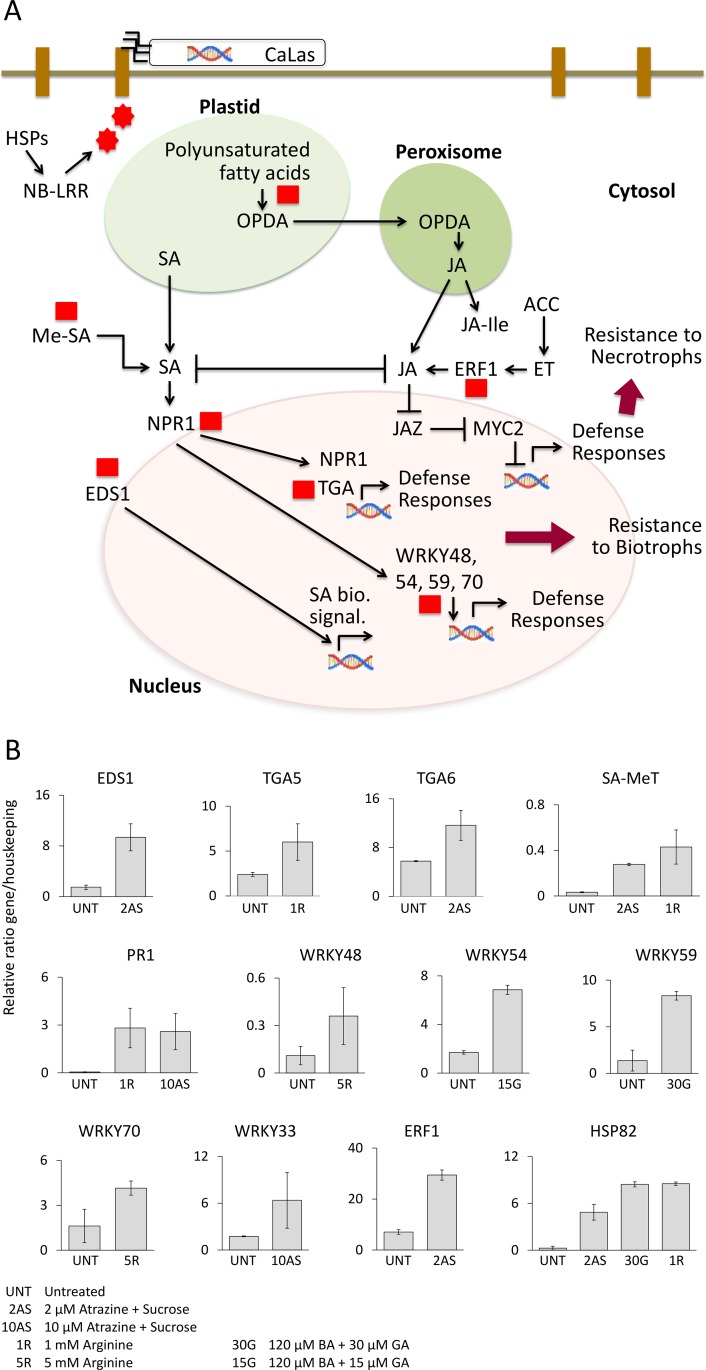
Gene expression changes observed in this work corroborated the hypothesis that these spray treatments may help stimulate systemic acquired resistance responses by activating key genes involved in innate responses (Fig 4).
Fig 4. Key differentially regulated genes in response to treatments involved in biotic stress responses.
PR gene induction is mediated through interaction with TGA transcription factors [15]. The observed upregulation of TGA5 and TGA6 by L-arginine and 2 μM atrazine + sucrose, respectively, might help stimulate defense responses against C. Las infection.
Arginine and atrazine sprays upregulated the PR1 gene under field conditions. PR1 protein is the hallmark of the defense response induction mediated by salicylic acid through systemic acquired resistance [44]. Molecular action of this protein against pathogens is still unclear, although antifungal properties have been attributed to it [45]. PR1 also interacted with fungal toxin activities, mediating necrosis in sensitive wheat [31]. This gene was not activated in response to C. Las infections in orchard trees [9].
Interestingly, all three treatments upregulated HSP82. Previous data on C. Las-infected citrus leaves and fruits showed that C. Las caused a significant repression of genes encoding chaperones [7–9]. Modified expression of these genes plays a key role in general stress conditions [46, 47]. A link between reduced HSP protein amount and HLB symptoms was also confirmed by analysis [10].
Conclusions
Present data confirmed our hypothesis that these small-molecule sprays may affect transcript abundance of key genes involved in HLB carbohydrate metabolic syndrome and innate responses. As expected, there were no phenotypic changes in response to treatments at one to two months after treatment. Treatment sprays did not cause negative effects such as leaf drop or discoloration. As expected, tree measurements showed almost no differences between treated and untreated trees in field conditions. We believe that beneficial effects are likely to be seen only if treatments are applied frequently before or at the onset of visible HLB symptoms. Here, our aim was to analyze the molecular effects of these treatments on gene expression several days after treatment. Future studies should examine long-term molecular and phenotypic improvements associated with ongoing applications to young trees infected with C. Las.
Supporting Information
(DOCX)
Acknowledgments
The authors wish to thank Mary Lou Mendum for her editorial review of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The research reported in this publication was supported by grant# CATAP09-305 received from the Citrus Research and Development Foundation and supported the work conducted by all of the authors except Veronica Fileccia who was supported by the University of Palermo. It should be noted that in either case the funders had no role in study design, data collection and analysis, decision to publish, or preparation of this manuscript.
References
- 1.Bove’ JM. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J Plant Pathol. 2006;88:7–37. [Google Scholar]
- 2.Jagoueix S, Bové JM, Garnier M. The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the Proteobacteria. Int J of Syst Bacteriol. 1994;44:397–86. [DOI] [PubMed] [Google Scholar]
- 3.Folimonova SY, Robertson CJ, Garnsey SM, Gowda S, Dawson WO. Examination of the responses of different genotypes of Citrus to Huanglongbing (Citrus greening) under different conditions. Phytopathology. 2009;99:1346–54. 10.1094/PHYTO-99-12-1346 [DOI] [PubMed] [Google Scholar]
- 4.Folimonova SY, Achor D. Early events of citrus greening (Huanglongbing) disease development at the ultrastructural level. Bacteriology. 2010;100:949–58. [DOI] [PubMed] [Google Scholar]
- 5.Albrecht U, Bowman KD. Gene expression in Citrus sinensis (L.) Osbeck following infection with the bacterial pathogen Candidatus Liberibacter asiaticus causing Huanglongbing in Florida. Plant Sci. 2008;175:291–306. [Google Scholar]
- 6.Kim J-S, Sagaram US, Burns JK, Li J-L, Wang N. Response of sweet orange (Citrus sinensis) to ‘Candidatus Liberibacter asiaticus’ infection: microscopy and microarray analyses. Phytopathology. 2009;99:50–57. 10.1094/PHYTO-99-1-0050 [DOI] [PubMed] [Google Scholar]
- 7.Martinelli F, Uratsu SL, Albrecht U, Reagan RL, Phu ML, Britton M, et al. Transcriptome profiling of Citrus fruit response to Huanglongbing disease. PLoS One. 2012;7:e38039 10.1371/journal.pone.0038039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dandekar AM, Martinelli F, Davis CE, Bhushan A, Zhao W, Fiehn O, et al. Analysis of Early Host Responses for Asymptomatic Disease Detection and Management of Specialty Crops. Crit Rev Immunol. 2010;30:277–89. [DOI] [PubMed] [Google Scholar]
- 9.Martinelli F, Reagan RL, Uratsu SL, Phu ML, Albrecht U, Zhao W, et al. Gene Regulatory Networks Elucidating Huanglongbing Disease Mechanisms. Plos One. 2013;8:e74256 10.1371/journal.pone.0074256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nwugo CC, Lin H, Duan Y, Civerolo EL. The effect of ‘Candidatus Liberibacter asiaticus’ infection on the proteomic profiles and nutritional status of pre-symptomatic and symptomatic grapefruit (Citrus paradisi) plants. BMC Plant Biology. 2013;13:59 10.1186/1471-2229-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan J, Chen C, Yu Q, Brlansky RH, Li Z-G, Gmitter FG. Comparative iTRAQ proteome and transcriptome analyses of sweet orange infected by “Candidatus Liberibacter asiaticus”. Physiol Plant. 2011;143:235–45. 10.1111/j.1399-3054.2011.01502.x [DOI] [PubMed] [Google Scholar]
- 12.Cong Q, Kinch LN, Kim B-H, Grishin NV. Predictive sequence analysis of the Candidatus liberibacter asiaticu proteome. Plos One. 2012;7(7):e41071 10.1371/journal.pone.0041071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyler HL, Roesch LFW, Gowda S, Dawson WO, Triplett EW. Confirmation of the sequence of ‘Candidatus Liberibacter asiaticus’ and assessment of microbial diversity in Huanglongbing-infected Citrus phloem using a metagenomic approach. Mol Plant Microbe Interact. 2009;22:1624–34. 10.1094/MPMI-22-12-1624 [DOI] [PubMed] [Google Scholar]
- 14.Khan AA, Afzal M, Qureshi JA. Botanicals, selective insecticides, and predators to control Diaphorina citri (Hemiptera: Liviidae) in citrus orchards. Insect Sci. 2014;21:717–26. 10.1111/1744-7917.12173 [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Powell CA, Zhou L, He Z, Stover E, Duan Y. Chemical compounds effective against the citrus Huanglongbing bacterium ‘Candidatus Liberibacter asiaticus’ in planta. Phytopathology. 2011;101:1097–1103. 10.1094/PHYTO-09-10-0262 [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Powell CA, Guo Y, Doud MS, Duan Y. A graft-based chemotherapy method for screening effective molecules and rescuing huanglongbing-affected citrus plants. Phytopathology. 2012;102:567–74. 10.1094/PHYTO-09-11-0265 [DOI] [PubMed] [Google Scholar]
- 17.Yang C, Powell CA, Duan Y, Shatters R, Zhang M. Antimicrobial nanoemulsion formulation with improved penetration of foliar spray through citrus leaf cuticles to control citrus huanglongbing. Plos One. 2015;10(7):e0133826 10.1371/journal.pone.0133826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akula N, Trivedi P, Han FQ, Wang N. Identification of small molecule inhibitors against SecA of Candidatus Liberibacter asiaticus by structure based design. Eur J Med Chem. 2012;54:919–24. 10.1016/j.ejmech.2012.05.035 [DOI] [PubMed] [Google Scholar]
- 19.Li W, Hartung JS, Levy L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J Microbiol Methods. 2006;66:104–15. [DOI] [PubMed] [Google Scholar]
- 20.Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 2009;5:308–16. 10.1038/nchembio.164 [DOI] [PubMed] [Google Scholar]
- 21.Ramel F, Sulmon C, Cabello-Hurtado F, Tacconat L, Martin-Magniette M-L, Renou J-P, et al. Genome-wide interacting effects of sucrose and herbicide-mediated stress in Arabidopsis thaliana: Novel insights into atrazine toxicity and sucrose-induced tolerance. BMC Genomics. 2007;8:450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramel F, Sulmon C, Gouesbet G, Couee I. Regulatory effects of atrazine differentially override sucrose repression of amino acid catabolism. Acta Physiol Plant. 2013;35:2329–37. [Google Scholar]
- 23.Zheng Y, Sheng J, Zhao R, Zhang J, Lv S, Liu L, et al. Preharvest l -arginine treatment induced postharvest disease resistance to botrysis cinerea in tomato fruits. J Agric Food Chem. 2011;59:6543–49. 10.1021/jf2000053 [DOI] [PubMed] [Google Scholar]
- 24.Navarro L, Bari R, Achard P, Lison P, Nemri A, Harberd N.P, et al. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol. 2008;18:650–55. 10.1016/j.cub.2008.03.060 [DOI] [PubMed] [Google Scholar]
- 25.Shen W, Cevallos-Cevallos JM, Nunes da Rocha U, Arevalo HA, Stansly PA, Roberts PD, et al. Relation between plant nutrition, hormones, insecticide applications, bacterial endophytes, and Candidatus Liberibacter Ct values in citrus trees infected with Huanglongbing. Eur J Plant Pathol. 2013;137:727–42. [Google Scholar]
- 26.Gottwald TR, Graham JH, Irey M, McCollum G, Wood B. Inconsequential effect of nutritional treatments on huanglongbing control, fruit quality, bacterial titer and disease progress. Crop Protection. 2012;36: 72–82. [Google Scholar]
- 27.Cao J, Cheng C, Yang J, Wang Q. Pathogen infection drives patterns of nutrient resorption in citrus plants. Scientific Reports. 2015;5:e14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stansly PA, Arevalo HA, Qureshi JA, Jones MM, Hendricks K, Roberts PD, et al. Vector control and foliar nutrition to maintain economic sustainability of bearing citrus in Florida groves affected by huanglongbing. Pest Man Sci. 2014;70:415–26. [DOI] [PubMed] [Google Scholar]
- 29.Xu M, Liang M, Chen J, Xia Y, Zheng Z, Zhu Q, et al. Preliminary research on soil conditioner mediated citrus Huanglongbing mitigation in the field in Guangdong, China. Eur J of Plant Pathol. 2013;137:283–93. [Google Scholar]
- 30.Flores F, Collier CJ, Mercurio P, Negri AP. Phytotoxicity of Four Photosystem II Herbicides to Tropical Seagrasses. PLoS ONE. 2013;8:e75798 10.1371/journal.pone.0075798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aritua V, Achor D, Gmitter FG, Albrigo G, Wang N. Transcriptional and Microscopic Analyses of Citrus Stem and Root Responses to Candidatus Liberibacter asiaticus Infection. PLoS ONE. 2013;8, e73742 10.1371/journal.pone.0073742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Fang H, Chen Y, Chen K, Li G, Gu S, et al. Overexpression of BnWRKY33 in oilseed rape enhances resistance to Sclerotinia sclerotiorum. Mol Plant Pathol. 2014;15:677–89. 10.1111/mpp.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X-M, Gaudet DA, Liu W, Frick M, Puchalski B, Lu Z-X, et al. Defence responses including hypersensitive cell death, oxidative burst and defence gene expression in Moro wheat inoculated with Puccinia striiformis. Can J Plant Pathol. 2014;36:202–15. [Google Scholar]
- 34.Birkenbihl RP, Diezel C, Somssich IE. Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiol. 2012; 159:266–85. 10.1104/pp.111.192641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R. JASMONATE- INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell. 2004;16:1938–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 2003;15:63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurie-Berry N, Joardar V, Street IH, Kunkel BN. The Arabidopsis thaliana JASMONATE INSENSITIVE 1 gene is required for suppression of salicylic acid–dependent defenses during infection by Pseudomonas syringae. Mol Plant Microbe Interact. 2006;19:789–800. [DOI] [PubMed] [Google Scholar]
- 38.Falk A, Feys BJ, Frost LN, Jones JDG, Daniels MJ, Parker JE. EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc Natl Acad Sci USA. 1999;96:3292–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, et al. Pre- and Postinvasion Defenses Both Contribute to Nonhost Resistance in Arabidopsis. Science. 2005;310:1180–83. [DOI] [PubMed] [Google Scholar]
- 40.Feys BJ, Wiermer M, Bhat RA, Moisan LJ, Medina-Escobar N, Neu C, et al. Arabidopsis Senescence-associated gene101 stabilizes and signals within an enhanced disease susceptibility1 complex in plant innate immunity. Plant Cell. 2005;17:2601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreau M, Degrave A, Vedel R, Bitton F, Patrit O, Renou J-P, et al. EDS1 contributes to nonhost resistance of Arabidopsis thaliana against Erwinia amylovora. Mol Plant Microbe In. 2012;25:421–30. [DOI] [PubMed] [Google Scholar]
- 42.Besseau S, Li J, Palva ET. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J Exp Bot. 2012; 63:2667–679. 10.1093/jxb/err450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ibanez AM, Martinelli F, Reagan RL, Uratsu SL, Vo A, Tinoco MA, et al. Transcriptome and metabolome analysis of Citrus fruit to elucidate puffing disorder. Plant Science. 2014;217:87–98. 10.1016/j.plantsci.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 44.van Loon LC, Rep M, Pieterse CM. Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol. 2006;44:135–62. [DOI] [PubMed] [Google Scholar]
- 45.Kiba A, Maimbo M, Kanda A, Tomiyama H, Ohnishi K, Hikichi Y. Isolation and expression analysis of candidate genes related to Ralstonia solanacearum-tobacco interaction. Plant Biotechnol. 2007;24:409–16. [Google Scholar]
- 46.Martinelli F, Scalenghe R, Giovino A, Pasquale M, Aksenov AA, Pasamontes A, Peirano DJ, Davis CE, Dandekar AM. Proposal of a Citrus translational genomic approach for early and infield detection of Flavescence dorée in Vitis. Plant Biosystems 2016;150:43–53. [Google Scholar]
- 47.Martinelli F, Scalenghe R, Davino SW, Panno S, Suderi G, Ruisi P, Villa P, Stroppiana D, Boschetti M, Goulart LR, Davis CE, Dandekar AM. Advanced methods of plant disease detection. A Review. Agronomy for Sustainable Development 2015;35:1–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.