Abstract
Purpose
To determine whether a hypocaloric diet alone (WL) or with exercise training (AEX+WL) is effective in improving body composition, fitness, glucose utilization and CVD risk factors in sedentary women with a history of gestational diabetes (GDM) and with type 2 diabetes (T2DM).
Materials and Methods
Longitudinal clinical investigation of 25 overweight/obese (BMI:32±1 kg/m2) women (59±1 yrs) with a GDM history (n=20) or T2DM (n=5). Women completed 6 months WL (n=10) or AEX+WL (n=15) with VO2max, body composition, and glucose tolerance testing. Insulin sensitivity was measured during the last 30 min of 2-hour hyperinsulinemic-euglycemic clamps (40 mU·m−2·min−1) before and after interventions.
Results
Body weight decreased ~7% after WL and AEX+WL (P<0.001), with an 11–12% decrease in fat mass (P<0.0001). Visceral fat and subcutaneous abdominal fat decreased 27 and 10% after WL (P<0.01) and 14 and 11% after AEX+WL (P<0.05). VO2max increased 16% after AEX+WL (P<0.001) and did not change after WL. Glucose AUC decreased 14 and 13% after WL (P<0.05) and AEX+WL (P<0.01) with a 42% decrease in insulin AUC after AEX+WL (P<0.01). Glucose utilization increased 25% (P=0.05) with AEX+WL and 7% with WL.
Conclusions
A six-month aerobic exercise program combined with moderate weight loss reduces body weight, visceral and subcutaneous abdominal fat, and improves insulin sensitivity in older women who had previously been diagnosed with GDM and those with T2DM. These findings should encourage women with a history of GDM to engage in an active lifestyle and reduce caloric intake to lower the risk for the development of T2DM.
Keywords: Insulin sensitivity, aging, exercise, nutrition, obesity
Introduction
Gestational diabetes mellitus (GDM), affecting 2–10% of pregnancies in the United States (1), is associated with long-term health risks including a higher risk of type 2 diabetes and obesity (2). Reports indicate that between 3–70% of women with a history of GDM both develop type 2 diabetes mellitus sometime after delivery (3–5) and have a 7–12 fold lifetime risk for subsequent development of type 2 diabetes (6). Defects in glucose tolerance, insulin secretion and insulin action are observed in women with a prior history of GDM (7–11). Since women with prior GDM are at a heightened risk for subsequent cardiovascular disease (CVD) complications, lifestyle modification may be an option to alter this course.
Current recommendations for exercise during uncomplicated pregnancies are supported in a systematic review (12). Physical activity during pregnancy results in a 28% reduced risk of GDM and thus, provides some protective effect against the development of GDM (13). Yet, there is a paucity of data on the association between post-partum physical activity and weight change and glucose metabolism in women with GDM. Modest weight loss within the first year post-partum is associated with improved glucose metabolism (14). Women in the Nurses Health Study II who had a history of GDM who increased their total physical activity levels by 150 minutes/week of moderate intense physical activity had almost a 50% lower risk of developing type 2 diabetes mellitus (15) suggesting that higher physical activity levels may lower the risk of diabetes. Up till now, there is a lack of literature targeting the effects of exercise and weight loss in older women with a history of GDM.
We hypothesized that the combination of diet and exercise would improve insulin sensitivity in middle-aged and older women who had a history of GDM and those with T2DM. In addition, we hypothesized that changes in central adiposity and intramuscular fat would be related to changes in glucose utilization. Thus, the purpose of this study was to determine whether a hypocaloric diet with and without exercise training is effective in improving body composition, fitness, glucose utilization and CVD risk factors in sedentary women with a history of GDM and women with type 2 diabetes. Insight into the benefits of these treatments could help promote preventive prescriptions to decrease the risk for type 2 diabetes and CVD in older women with a history of GDM.
Materials and Methods
Subjects
Overweight and obese women between the ages of 44–68 years with body mass index (BMI) range 29–40 kg/m2 with a history of GDM with confirmed diagnosis made by a physician or health care provider or who had type 2 diabetes (T2DM) were recruited from the Baltimore metropolitan area for participation in this study. Only women who were weight stable (<2.0 kg weight change in past year) and sedentary (<20 min of aerobic exercise 2x/wk) were recruited. The Institutional Review Board of the University of Maryland approved all methods and procedures for the study. Each participant provided written informed consent. Subjects were screened by medical history questionnaire, physical examination, fasting blood profile, and 12-lead resting electrocardiogram. On a second screening visit, subjects underwent a graded exercise treadmill test in an attempt to exclude those with CVD. All subjects were nonsmokers, showed no evidence of cancer, liver, renal or hematological disease, or other medical disorders and were not on hormone replacement therapy. Women who were premenopausal had regular menstrual cycles and had not used hormonal contraception during the previous year. Thirty women were eligible for the study and were enrolled randomly in groups to either a hypocaloric diet only (WL) or aerobic exercise + weight loss (AEX+WL) intervention for a period of 6 months described below. Five women dropped out due to time constraints or health reasons unrelated to the study and 25 women completed the interventions (n=10 for WL and n=15 for AEX+WL).
Study Design
During the 6-month interventions, all women attended weekly weight loss classes led by a registered dietitian for instruction in the principles of a hypocaloric diet that followed the American Heart Association (AHA) Step I (16) guidelines. Compliance was monitored by weekly review of 7-day food records and 24-hr dietary recalls during the six months. Women were instructed to restrict their caloric intake by 250–350 kcal/d. The program focused on eating behavior, stress management, control of portion sizes, and modification of binge eating. Women in the AEX+WL intervention exercised at the Baltimore VA Medical Center GRECC exercise facility three times a week for 6 months using treadmills, cycle ergometers, and a track. Each exercise session included a 5-10 minute stretching and warm-up phase and a 5-10 minute cool-down phase. Women exercised at ~50–60% heart rate reserve and gradually progressed in duration and intensity until able to exercise at >60% VO2max for 45 minutes. The average compliance to the weight loss and exercise classes was > 75%.
Maximal Oxygen Uptake (VO2max) and Blood Pressure
VO2max was measured using a continuous treadmill test protocol as previously described (17). Validation for attainment of VO2max included meeting two of the following three criteria: 1) a plateau in oxygen uptake with an increased work load as evidenced by a difference in oxygen uptake of < 2 ml·kg−1·min−1; 2) a respiratory exchange ratio >1.10; and 3) a maximal heart rate within 10 beats/min of the age-predicted maximal value. Seated blood pressures were determined twice prior to the exercise test and the average determination was used in the statistical analyses.
Body Composition
Anthropometry
Height (cm) and weight (kg) were measured to calculate body mass index (BMI) as weight (kg)/height (m2). Waist circumference was measured at the narrowest point superior to the hip, which was divided by the circumference of the hip, measured at its greatest gluteal protuberance, to calculate waist-to-hip ratio (WHR).
Dual-energy X-ray Absorptiometry (DXA)
Fat mass, lean tissue mass and bone mineral content (BMC) were determined by DXA (Model DPX-L LUNAR Radiation Corp., Madison, WI). Fat-free mass (FFM) is reported as lean tissue plus BMC. All DXA scans were analyzed using the LUNAR Corporation Version 1.3z DPX-L extended analysis program for body composition analyses.
Computed Tomography (CT)
A single 5-mm CT scan of the abdomen taken at the L4–L5 region was performed using a PQ 6000 Scanner (General Electric Hi-Light, Cleveland, Ohio) to quantitate the relative proportions of visceral (VAT) and subcutaneous abdominal adipose tissue (SAT) areas and sagittal diameter (17). A second scan performed at the level of the mid-thigh was used to quantify muscle area, total fat area, and low density lean tissue (intramuscular fat) of the thigh as previously described (17). Three women in the AEX+WL group did not have CT scans.
Metabolic Testing
To control nutrient intake prior to the metabolic studies, all subjects were provided with a eucaloric diet for two days before testing by a registered dietitian. The composition of this diet was 50–55% carbohydrate, 15–20% protein, ≤ 30% fat and 300–400 mg of cholesterol per day and a polyunsaturated to saturated fat ratio of 0.6–0.8. The diet was composed of at least 150-g carbohydrate/day for these two days before testing (18). The number of calories given to each woman was estimated from the 7-day food records and estimates of energy expenditure were based on the Harris-Benedict equation (19). All testing was performed in the morning after a 12-hr overnight fast. All subjects were weight stabilized (< 1 kg) for at least two weeks prior to metabolic testing.
Analysis of Blood Samples
The average of 2 to 3 fasting blood draws drawn on separate days was used in the determination of lipoprotein lipids. Blood samples were transferred into chilled tubes containing 1 mg of EDTA per cc of blood. Plasma was separated by centrifugation at 4°C for 15 min at 2000 × g. Total cholesterol (TC), triglyceride (TG) concentrations, high-density-lipoprotein (HDL) cholesterol, and HDL2 cholesterol was measured as previously described (17) and low-density-lipoprotein (LDL-C) was calculated by the Friedewald equation (20). Plasma leptin levels were measured in duplicate by RIA (21).
Oral Glucose Tolerance Tests
Subjects underwent a 75-g, 2-h oral-glucose-tolerance test (22). Blood samples were drawn every 30 min during the 2-h period for measurement of plasma glucose and insulin concentrations. Blood samples were collected in heparinized syringes and placed in prechilled test tubes containing 1.5 mg EDTA/ml of blood in a total volume that was 4% of the sample volume. The blood samples were centrifuged at 4°C and a 1ml aliquot of plasma was rapidly frozen (80°C) for subsequent hormone analysis. All determinations were performed in duplicate. Plasma glucose was measured with the glucose oxidase method (2300 STAT Plus; YSI, Yellow Springs, OH). Immunoreactive insulin was determined by RIA (Millipore, St. Charles, MO).
Hyperinsulinemic-euglycemic Clamps
Peripheral tissue sensitivity to exogenous insulin was measured using the hyperinsulinemic-euglycemic clamp technique (23). Clamps were not performed in some women (n=2 WL and n=7 AEX+WL) due to poor venous access; however, sample sizes before and after the interventions were equal between groups (n=8) for clamp data. Briefly, an intravenous catheter was inserted by percutaneous venipuncture for the infusion of glucose and insulin. A second catheter was inserted in a retrograde fashion into a dorsal hand or wrist vein, and the hand was enclosed in a grounded, insulated chamber, warmed to 70°C to “arterialize” (24) the blood obtained for all samples. For the assessment of basal glucose and insulin levels, three arterialized blood samples were drawn at 10-min intervals. Blood samples were obtained every 5 and 10 min thereafter for the determination of plasma glucose and insulin levels as described above. A 10 min priming and continuous infusion of insulin for two hours (240 pmol · m−2 · min1, Humulin, Eli Lilly Co., Indianapolis, IN) was performed. This resulted in a square wave of hyperinsulinemia at a level of 507 ± 28 pmol/l in WL and 557 ± 44 pmol/l in AEX+WL. The mean plasma glucose level during 10–120 min of the euglycemic clamp was computed for each individual study and expressed as a percentage of the desired goal. The plasma glucose levels during each clamp period averaged 5.32 ± 0.09 mmol/l for WL and 5.67 ± 0.02 mmol/l for WL+AEX which was 97.1 ± 0.4% of the desired goal with a coefficient of variation (CV) of 5.0 ± 0.2% in all clamps (n = 32).
Indirect Calorimetry
RMR was measured by indirect calorimetry (SensorMedics DeltaTrac cart (Yorba Linda, CA) while subjects rested quietly in the supine position for 30 minutes after a 12-hour fast. Energy expenditure was calculated by the Weir equation (25) and expressed per 24 hours.
Statistical Analyses
The mean concentration of glucose and insulin was calculated for each sample time point during the clamp. The trapezoidal rule was used to calculate the integrated response over 30 min intervals for each subject. The integrated response was divided by its time interval to compute mean concentrations. Glucose utilization (M) for 30 min intervals was calculated from the amount of glucose infused after correction for glucose equivalent space (glucose space correction). Statistical significance among groups was determined by one-way analysis of variance (ANOVA). Relationships between variables were determined by linear regression analyses and calculation of Pearson correlation coefficients. All data were analyzed by SPSS statistical software (SPSS Inc., Chicago). Data are expressed as mean ± standard error of the mean (SEM) and significance was set at the P < 0.05 level.
Results
Baseline Characteristics
The baseline physical characteristics of the women in each group are presented in Table 1. Age, body weight, waist and hip circumferences, WHR and VO2max were similar among WL and AEX+WL groups at baseline. In addition, there were no differences at baseline in percent body fat, fat mass, FFM, VAT, SAT, sagittal diameter, mid-thigh muscle, mid-thigh subcutaneous fat, and mid-thigh low density lean tissue area between groups (Table 1). BP and lipid profiles were not different between WL and AEX+WL groups at baseline (Table 2). Fasting plasma glucose, insulin, leptin concentrations, and all other measures of glucose metabolism did not differ between groups at baseline (Table 3). Of the 20 women with a history of GDM, eight women had T2DM, five women had IGT, and seven women had NGT. Five women had type 2 diabetes (22) but not a history of GDM (n=3 in WL and n=2 in AEX+WL). Since the effects of the interventions in women with T2DM only were similar to those women without a history of GDM, their results are presented with the GDM group. Only two women were on oral agents for T2DM and the remaining three were untreated.
Table 1.
Physical characteristics of women before and after the interventions.
| Weight Loss (n=10) | Aerobic Exercise + Weight Loss (n=15) | |||
|---|---|---|---|---|
|
| ||||
| Before | After | Before | After | |
|
| ||||
| Age (years) | 52 ± 2 | _____ | 54 ± 2 | _____ |
| Weight (kg) | 88.3 ± 3.3 | 82.6 ± 3.2‡ | 83.8 ± 2.6 | 77.6 ± 2.8‡ |
| BMI (kg/m2) | 32.4 ± 1.2 | 30.8 ± 1.3* | 32.2 ± 0.8 | 29.8 ± 1.0‡ |
| Waist circumference (cm) | 95.6 ± 3.6 | 90.3 ± 2.9† | 93.4 ± 2.2 | 88.2 ± 2.2† |
| Hip circumference (cm) | 116.2 ± 2.5 | 108.8 ± 2.2† | 110.5 ± 1.2 | 106.0 ± 2.0† |
| WHR | 0.82 ± 0.02 | 0.81 ± 0.01 | 0.84 ± 0.02 | 0.83 ± 0.02 |
| Percent body fat | 45.8 ± 1.3 | 42.9 ± 1.9† | 45.5 ± 1.4 | 41.9 ± 1.9† |
| Fat mass (kg) | 40.4 ± 2.2 | 35.8 ± 2.7‡ | 37.6 ± 2.3 | 32.2 ± 2.6‡ |
| Fat-free mass (kg) | 47.3 ± 1.3 | 46.5 ± 1.0 | 46.1 ± 2.5 | 43.3 ± 1.3 |
| RMR (kcal) | 1573 ± 37 | 1433 ± 52* | 1602 ± 46 | 1479 ± 52† |
| VO2max (ml/kg/min) | 21.0 ± 1.3 | 22.7 ± 1.7 | 20.6 ± 1.1 | 24.9 ± 1.5‡ |
| VO2max (l/min) | 1.85 ± 0.06 | 1.84 ± 0.08 | 1.72 ± 0.09 | 1.92 ± 0.09‡ |
| Mid-thigh muscle area (cm2) | 89.5 ±6.3 | 89.9 ± 4.1 | 85.2 ± 3.1 | 83.7 ± 3.4 |
| Mid-thigh subcutaneous fat area (cm2) | 171.2 ± 16.3 | 146.6 ± 11.6 | 185.8 ± 16.1 | 152.2 ± 13.9† |
| Mid-thigh low density lean tissue (cm2) | 15.5 ± 1.6 | 16.1 ± 1.2 | 16.5 ± 2.0 | 16.6 ± 1.4 |
Values are means ± SEM. Significantly different before vs. after the intervention:
p<0.05;
p<0.01;
P<0.0001.
Table 2.
Blood pressure and lipoprotein lipids before and after the interventions.
| Weight Loss (n=10) | Aerobic Exercise + Weight Loss (n=15) | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| Systolic blood pressure (mm Hg) | 123 ± 5 | 129 ± 4 | 128 ± 4 | 124 ± 6 |
| Diastolic blood pressure (mm Hg) | 80 ± 4 | 77 ± 2 | 81 ± 3 | 81 ± 2 |
| Total cholesterol (mmol/l) | 5.16 ± 0.44 | 5.02 ± 0.40 | 5.05 ± 0.07 | 4.83 ± 0.23 |
| LDL-cholesterol (mmol/l) | 3.47 ± 0.43 | 3.47 ± 0.43 | 3.01 ± 0.19 | 3.00 ± 0.19 |
| HDL-cholesterol (mmol/l) | 1.16 ± 0.14 | 1.24 ± 0.09* | 1.18 ± 0.08 | 1.19 ± 0.07 |
| HDL2-cholesterol (mmol/l) | 0.11 ± 0.04 | 0.20 ± 0.06* | 0.16 ± 0.04 | 0.16 ± 0.03 |
| Triglycerides (mmol/l) | 1.25 ± 0.15 | 1.02 ± 0.13* | 1.91 ± 0.32 | 1.46 ± 0.13 |
Values are means ± SEM. Significantly different before vs. after the intervention:
p<0.05
Table 3.
Metabolic characteristics before and after the interventions.
| Weight Loss (n=10) | Aerobic Exercise+Weight Loss (n=15) | |||
|---|---|---|---|---|
|
| ||||
| Before | After | Before | After | |
|
| ||||
| Fasting plasma glucose (mmol/l) | 6.6 ± 0.5 | 6.1 ± 0.4* | 5.8 ± 0.2 | 5.6 ± 0.2 |
| Fasting plasma insulin (pmol/l) | 63 ± 9 | 55 ± 5 | 93 ± 12 | 65 ± 10* |
| 120-min glucose (mmol/l) | 10.3 ± 1.4 | 8.6 ± 1.3* | 24.9 ± 3.0 | 18.2 ± 2.2* |
| 120-min insulin (pmol/l) | 361 ± 74 | 342 ± 116 | 665± 125 | 354 ± 52* |
| GlucoseAUC (mmol/l/120min) | 1199 ± 112 | 1029 ± 102* | 1116 ± 61 | 974 ± 48† |
| InsulinAUC (pmol/l/120min) | 45213 ± 10624 | 37038 ± 8414 | 60658 ± 7060 | 35012 ± 6407† |
| Plasma leptin (pmol/l) | 23.8 ± 3.2 | 20.5 ± 3.3 | 26.5 ± 2.8 | 18.7 ± 2.4* |
Values are means ± SEM. Significantly different before vs. after the intervention:
P<0.05;
P=0.05;
P<0.01
Effects of the Interventions on Body Composition and VO2max (Table 1)
Overall, body weight decreased ~7% in both groups (P<0.001) along with significant decreases in waist and hip circumferences but no change in WHR. The changes in body composition with WL were an absolute decrease in percent body fat of 3% (P<0.01), an 11% decrease in total fat mass (P<0.001) and no significant change in FFM. With WL, there was a 27% decrease in VAT (P<0.01), a 10% decrease in SAT (P<0.01), a 7% decrease in sagittal diameter (P<0.01) (Figure 1), but no change in mid-thigh muscle area, subcutaneous fat, or intramuscular fat area. Likewise with AEX+WL, there was an absolute decrease in percent fat of 3% (P<0.001), a 12% decrease in fat mass (P<0.001), and no change in FFM. VAT and SAT decreased by 14% and 11%, respectively (P<0.05), sagittal diameter decreased by 4% (P<0.05) (Figure 2), mid-thigh subcutaneous fat decreased by 17% (P<0.001) with no change in mid-thigh muscle area or intra-muscular fat after WL+AEX. There were no significant differences in the changes in body composition between the two groups. However, percent changes in VO2max tended to be different between the WL and WL+AEX groups (P=0.06). VO2max (l/min) increased 12% after AEX+WL (P<0.001) and as expected, did not significantly change after WL. RMR decreased 9% after WL and 6% after AEX+WL (P<0.05).
Figure 1.
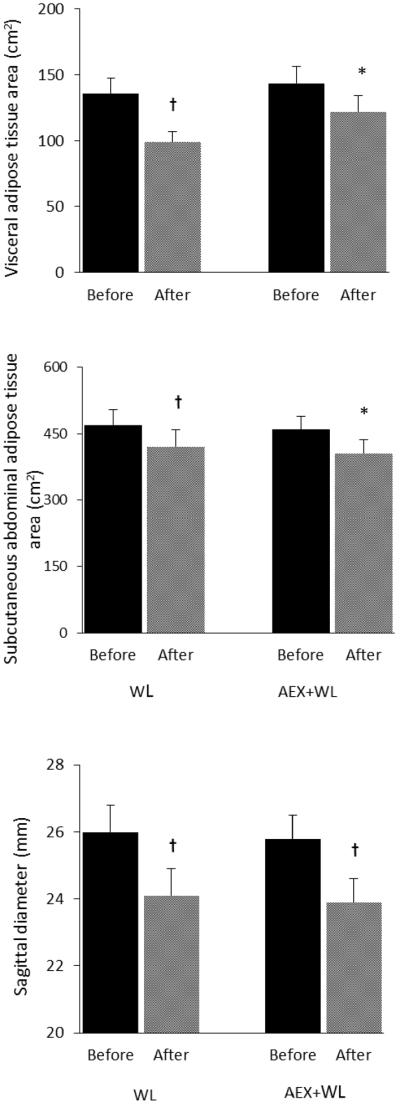
Abdominal adiposity before and after WL and AEX+WL. Values are means ±SEM. Significantly different before vs. after the intervention, *P<0.05; †P <0.01
Figure 2.
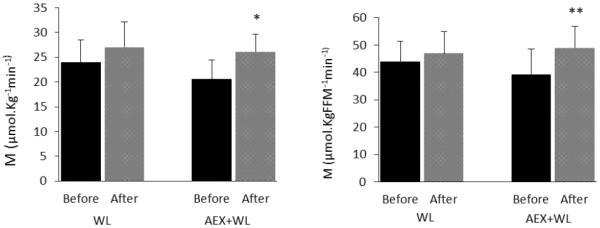
Glucose utilization (M) before and after WL and AEX+WL. Values are means ±SEM. Significantly different before vs. after the intervention, *P<0.05; **P=0.05
Effects of the Interventions on BP, Lipoprotein Lipids, and Glucose Metabolism (Tables 2 and 3)
Overall, there were no significant changes in either SBP or DBP in either group. In addition, total cholesterol and LDL-cholesterol did not change after WL or AEX+WL. There were no between group differences in changes in lipid profiles but HDL-cholesterol and HDL2-cholesterol increased and triglycerides decreased after WL (P<0.05).
Fasting glucose decreased with WL (P<0.05) but not with AEX+WL, and 120-min glucose decreased 17% after WL and 16% after AEX+WL (both P<0.05). Fasting and 120-min insulin concentrations decreased 30 and 47%, respectively after AEX+WL (both P<0.05) but did not significantly change after WL. Glucose AUC decreased 14 and 13% after WL (P<0.05) and AEX+WL (P<0.01) with a 42% decrease in insulin AUC after AEX+WL (P<0.01). Fasting leptin tended to decrease after WL (−14%, P=0.09) and decreased 29% after AEX+WL (P<0.05). Glucose utilization did not significantly change after WL and increased 25% (P<0.05) after AEX+WL. There were no significant differences in the changes in BP, lipids or glucose metabolism between groups.
Predictors of Glucose Utilization
At baseline in women with a history of GDM only, age was inversely related to M (r = −0.50, P<0.05); fasting insulin and insulin120min was inversely related to M (r = −0.58, and r=−0.54, both P<0.05). In prior GDM women, the relationships between glucose120min and M (r = −0.45) and as well as VAT with M (r = −0.46), approached significance (both P=0.08). At baseline in women with a history of GDM and those with T2DM and no history of GDM, relationships were similar. Namely, age was inversely related to M (r = −0.47, P<0.05) and fasting insulin was inversely related to M (r = −0.51, P<0.05). In all women, the relationships between fasting glucose and M (r = −0.38) and as well as VAT with M (r = −0.40), approached significance (both P=0.08). In both WL and AEX+WL groups combined, changes in M with the interventions were indirectly related to changes in body weight in prior GDM women (r=−0.56, P<0.05) and in all women (r=−0.60, P<0.05). Further, changes in M tended to be associated with changes in fat mass only in women with a history of GDM (r=−0.52, P=0.06). Changes in M were not related to changes in VO2max, VAT, or mid-thigh intra-muscular fat.
Discussion
We previously established that postmenopausal women with prior GDM are more insulin resistant than controls of similar age, adiposity, and fitness levels and have comparable glucose utilization rates as similarly characterized women with T2DM (26). This suggests that older overweight and sedentary women with a history of GDM are considerably insulin resistant and could potentially benefit from dietary induced weight loss or an exercise program. In the current study, we determined that a six-month supervised and progressive aerobic exercise program combined with moderate weight loss reduces body weight, visceral and subcutaneous abdominal fat, and improves insulin sensitivity in older women who had previously been diagnosed with GDM and those with T2DM.
Weight gain, overweight and obesity during mid-life contribute to cardiovascular disease, metabolic syndrome, and diabetes (27, 28). Further, women who have excess weight gain during pregnancy had an increased risk of developing type 2 diabetes 21 years after the index pregnancy (29). Our results are consistent with increased risk of developing impaired glucose metabolism after GDM as 65% of our women with a history of GDM had IGT or T2DM at the time of study enrollment. Furthermore, age was inversely associated with insulin sensitivity indicating that not only a history of GDM but aging itself, plays a role in the insulin resistance observed in this group of women. Abdominal obesity may also be important in the insulin resistance in our cohort of women with a history of GDM as there tended to be a relationship between visceral fat and glucose utilization which we have shown in postmenopausal women without a history of GDM (30).
Given the convincing evidence for the need for interventions to prevent weight gain, reduce body weight, or improve insulin sensitivity, it is remarkable that there are no interventional studies of older women with a history of GDM. In younger women with a history of GDM, as little as a 2 kg weight loss 12 months post-partum, resulted in significantly lower fasting glucose and insulin and HOMA values than women who maintained or gained weight post-partum (14). Our study is unique in that 1) we conducted an interventional weight loss study with nutrition and caloric instruction by a registered dietician; 2) the subject sample was significantly older and more than one-year post-partum; and 3) body weight loss was three-fold greater than previously published work. Moreover, our subjects lost between 10 and 27% visceral and subcutaneous abdominal fat with lower 120-min glucose values and glucose areas under the curve from the OGTT, illustrating a loss of abdominal obesity accompanied by improved glucose tolerance after weight loss in middle-aged and older women with a history of GDM. In addition, the small number of women with T2DM and no history of GDM who were included (N=5) had similar changes in body composition and glucose metabolism suggesting that these improvements are consistent with the women with a history of GDM.
Aerobic exercise training has been shown to be beneficial during pregnancy. In a meta-analysis of studies published between 1966 and 2014, women assigned to a physical activity intervention compared to control, had almost 30% lower risk of GDM during pregnancy (13). Less is known regarding physical activity in the post-partum period in women with a history of GDM. In a prospective study of over 4500 women with a history of GDM, women who increased their total physical activity levels by 150 min/week had a 47% lower risk of type 2 diabetes mellitus (15). In women with a history of GDM within the previous seven years, time constraints was a major barrier to participation in a lifestyle intervention program and finding a group or exercise buddy was seen as a facilitator to physical activity (31). Our results indicate that middle-aged and older women at least 5 years post-partum, an increase in physical activity three days a week for six months, improves fitness (VO2max), glucose tolerance, and glucose utilization, and reduces fasting insulin and insulin area under the curve. These findings suggest that changing sedentary behaviors is beneficial many years post-partum in women with a history of GDM.
Central distribution of adiposity and intramuscular fat are associated with impaired glucose tolerance, type 2 diabetes, and insulin resistance (17, 32, 33). We are unaware of any studies that have examined changes in body composition, including visceral or subcutaneous abdominal fat after interventions in women with a prior history of GDM. One study reported increased intramyocellular lipid (soleus and tibialis-anteiror muscles) in young women studied approximately 6 months after delivery with GDM (34). In our larger sample of women with a history of GDM (26), total body fat mass and subcutaneous abdominal fat are associated with insulin resistance demonstrating the importance of increased body fat and central obesity to increase the risk for the development of type 2 diabetes after pregnancy complicated by GDM. The relative loss of total body fat, abdominal fat, and leptin in the current study is similar to that which we have previously observed using the same WL and AEX+WL interventions in postmenopausal women without a history of GDM (35, 36) but the reduction in RMR observed here contrasts a lack of change in RMR after WL and AEX+WL(37).
Changes in CVD risk factors were also examined in our study in women with a history of GDM and those with T2DM. It has been reported that women with prior GDM have a worse lipid profile including an increased TC, LDL cholesterol and triglyceride levels than women without a history of GDM (38). Further, obesity by BMI, hypertension, and abnormal lipids are higher at the postpartum visit in women with prior GDM with impaired fasting glucose than prior GDM women with normal glucose status suggesting that a continuation of abnormal glucose metabolism following delivery is associated with other CVD risk factors (39). Although the literature (26, 38, 39) indicates worse CVD risk factors in prior GDM women, the women with normal BP in this study did not change BP with WL or AEX+WL but did have some minor improvements in lipoprotein lipid profiles.
Our study has several strengths including a long-term weight loss program of six months, supervised and well-controlled exercise intervention, detailed assessments of body composition and fitness, and sophisticated and comprehensive measures of glucose metabolism (OGTT's, hyperinsulinemic-euglycemic clamps). The study was limited in sample size for the glucose clamps and the inability to determine cellular changes in skeletal muscle or adipose tissue before and after the weight loss and exercise training. Future studies could be designed to examine the mechanistic underpinnings for the improvements in insulin action.
Conclusions
Our previous results(26) indicate that postmenopausal women with a history of GDM have comparable insulin stimulated glucose utilization rates as similar phenotypic women with T2DM and suggest that a prior history of GDM has detrimental effects on glucose homeostasis from 5 to over 30 years after the index pregnancy. Our results from this longitudinal study indicate that weight loss and aerobic exercise training reduce body fat, improve glucose tolerance, and increase insulin sensitivity in overweight and obese sedentary women with a history of GDM and those with T2DM. These findings should encourage middle-aged and older women with a history of GDM to engage in an active lifestyle and reduce their caloric intake to lower the risk for the development of type 2 diabetes mellitus.
Acknowledgments
Our appreciation is extended to those women who participated in this study. We are grateful to Andrew Goldberg, M.D., the nurses in the Geriatrics Services for technical assistance and to Melissa Gray, Agnes Kohler, and Carole St. Clair for laboratory assistance. This study was supported by funds from: Baltimore VA Medical Research Service, Research Career Scientist Award, NIH grants K01-AG00747, RO1-AG19310, P60-AG12853, and Baltimore Geriatric Research and Clinical Center.
Footnotes
Declaration of Interest The author has no conflict of interest. The author alone is responsible for the content and writing of the paper.
REFERENCES
- 1.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Preventing chronic disease. 2014;11:E104. doi: 10.5888/pcd11.130415. doi: 10.5888/pcd11.130415. PubMed PMID: 24945238; PubMed Central PMCID: PMC4068111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verier-Mine O. Outcomes in women with a history of gestational diabetes. Screening and prevention of type 2 diabetes. Literature review. Diabetes Metab. 2010;36(6 Pt 2):595–616. doi: 10.1016/j.diabet.2010.11.011. doi: 10.1016/j.diabet.2010.11.011. PubMed PMID: 21163424. [DOI] [PubMed] [Google Scholar]
- 3.Metzger BE, Cho NH, Roston SM, Radvany R. Prepregnancy weight and antepartum insulin secretion predict glucose tolerance five years after gestational diabetes mellitus. Diabetes Care. 1993;16(12):1598–605. doi: 10.2337/diacare.16.12.1598. Epub 1993/12/01. PubMed PMID: 8299456. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann RC, Schleyhahn FT, Huffman DG, Amankwah KS. Gestational diabetes diagnostic criteria: long-term maternal follow-up. Am J Obstet Gynecol. 1995;172(2 Pt 1):621–5. doi: 10.1016/0002-9378(95)90582-0. Epub 1995/02/01. doi: 0002-9378(95)90582-0 [pii]. PubMed PMID: 7856695. [DOI] [PubMed] [Google Scholar]
- 5.Kim C, Berger DK, Chamany S. Recurrence of gestational diabetes mellitus: a systematic review. Diabetes Care. 2007;30(5):1314–9. doi: 10.2337/dc06-2517. Epub 2007/02/10. doi: dc06-2517 [pii] 10.2337/dc06-2517. PubMed PMID: 17290037. [DOI] [PubMed] [Google Scholar]
- 6.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–9. doi: 10.1016/S0140-6736(09)60731-5. doi: 10.1016/S0140-6736(09)60731-5. PubMed PMID: 19465232. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan TA, Xiang A, Kjos SL, Lee WP, Trigo E, Nader I, et al. Gestational diabetes: antepartum characteristics that predict postpartum glucose intolerance and type 2 diabetes in Latino women. Diabetes. 1998;47(8):1302–10. doi: 10.2337/diab.47.8.1302. Epub 1998/08/14. PubMed PMID: 9703332. [DOI] [PubMed] [Google Scholar]
- 8.Catalano PM, Bernstein IM, Wolfe RR, Srikanta S, Tyzbir E, Sims EA. Subclinical abnormalities of glucose metabolism in subjects with previous gestational diabetes. Am J Obstet Gynecol. 1986;155(6):1255–62. doi: 10.1016/0002-9378(86)90155-9. Epub 1986/12/01. doi: 0002-9378(86)90155-9 [pii]. PubMed PMID: 3538877. [DOI] [PubMed] [Google Scholar]
- 9.Ryan EA, Imes S, Liu D, McManus R, Finegood DT, Polonsky KS, et al. Defects in insulin secretion and action in women with a history of gestational diabetes. Diabetes. 1995;44(5):506–12. doi: 10.2337/diab.44.5.506. Epub 1995/05/01. PubMed PMID: 7729607. [DOI] [PubMed] [Google Scholar]
- 10.Ward WK, Johnston CL, Beard JC, Benedetti TJ, Halter JB, Porte D., Jr Insulin resistance and impaired insulin secretion in subjects with histories of gestational diabetes mellitus. Diabetes. 1985;34(9):861–9. doi: 10.2337/diab.34.9.861. Epub 1985/09/01. PubMed PMID: 3896896. [DOI] [PubMed] [Google Scholar]
- 11.Ward WK, Johnston CL, Beard JC, Benedetti TJ, Porte D., Jr Abnormalities of islet B-cell function, insulin action, and fat distribution in women with histories of gestational diabetes: relationship to obesity. J Clin Endocrinol Metab. 1985;61(6):1039–45. doi: 10.1210/jcem-61-6-1039. Epub 1985/12/01. doi: 10.1210/jcem-61-6-1039. PubMed PMID: 3902865. [DOI] [PubMed] [Google Scholar]
- 12.Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Current opinion in obstetrics & gynecology. 2012;24(6):387–94. doi: 10.1097/GCO.0b013e328359f131. doi: 10.1097/GCO.0b013e328359f131. PubMed PMID: 23014142. [DOI] [PubMed] [Google Scholar]
- 13.Russo LM, Nobles C, Ertel KA, Chasan-Taber L, Whitcomb BW. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstetrics and gynecology. 2015;125(3):576–82. doi: 10.1097/AOG.0000000000000691. doi: 10.1097/AOG.0000000000000691. PubMed PMID: 25730218. [DOI] [PubMed] [Google Scholar]
- 14.Ehrlich SF, Hedderson MM, Quesenberry CP, Jr, Feng J, Brown SD, Crites Y, et al. Post-partum weight loss and glucose metabolism in women with gestational diabetes: the DEBI Study. Diabet Med. 2014;31(7):862–7. doi: 10.1111/dme.12425. doi: 10.1111/dme.12425. PubMed PMID: 24597974; PubMed Central PMCID: PMC4065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao W, Tobias DK, Bowers K, Chavarro J, Vaag A, Grunnet LG, et al. Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: a prospective cohort study. JAMA internal medicine. 2014;174(7):1047–55. doi: 10.1001/jamainternmed.2014.1795. doi: 10.1001/jamainternmed.2014.1795. PubMed PMID: 24841449; PubMed Central PMCID: PMC4209161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Heart Association: Heart and Stroke facts. AHA; Dallas,TX: 1997. [Google Scholar]
- 17.Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord. 1999;23(2):126–32. doi: 10.1038/sj.ijo.0800777. [DOI] [PubMed] [Google Scholar]
- 18.Meneilly GS, Elliott T. Metabolic alterations in middle-aged and elderly obese patients with type 2 diabetes. Diabetes Care. 1999;22(1):112–8. doi: 10.2337/diacare.22.1.112. Epub 1999/05/20. PubMed PMID: 10333911. [DOI] [PubMed] [Google Scholar]
- 19.Harris JA. In: A biometric study of basal metabolism in man. Benedict FG, editor. Carnegie Institute of Washington; Washington: 1919. p. 1919. [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. ClinChem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 21.Ryan AS, Elahi D. The effects of acute hyperglycemia and hyperinsulinemia on plasma leptin levels: its relationships with body fat, visceral adiposity, and age in women. J Clin Endocrinol Metab. 1996;81(12):4433–8. doi: 10.1210/jcem.81.12.8954055. Epub 1996/12/01. doi: 10.1210/jcem.81.12.8954055. PubMed PMID: 8954055. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes A Diagnosis and Classification of Diabetes Mellitus. Diabetes. 2009;32(Supplement):S62–7. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 24.McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J ApplPhysiol. 1976;41(4):565–73. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- 25.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109(1–2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan AS, McLenithan JC, Zietowski GM. Accelerated metabolic susceptibility to type 2 diabetes in older women with a history of gestational diabetes. Endocrine connections. 2013;2(2):79–86. doi: 10.1530/EC-12-0072. doi: 10.1530/EC-12-0072. PubMed PMID: 23781323; PubMed Central PMCID: PMC3680953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willett WC, Manson JE, Stampfer MJ, Colditz GA, Rosner B, Speizer FE, et al. Weight, weight change, and coronary heart disease in women. Risk within the `normal' weight range. JAMA. 1995;273(6):461–5. doi: 10.1001/jama.1995.03520300035033. PubMed PMID: 7654270. [DOI] [PubMed] [Google Scholar]
- 28.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122(7):481–6. doi: 10.7326/0003-4819-122-7-199504010-00001. PubMed PMID: 7872581. [DOI] [PubMed] [Google Scholar]
- 29.Al Mamun A, Mannan M, O'Callaghan MJ, Williams GM, Najman JM, Callaway LK. Association between gestational weight gain and postpartum diabetes: evidence from a community based large cohort study. PLoS One. 2013;8(12):e75679. doi: 10.1371/journal.pone.0075679. doi: 10.1371/journal.pone.0075679. PubMed PMID: 24348988; PubMed Central PMCID: PMC3862846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan AS, Nicklas BJ, Berman DM. Aerobic exercise is necessary to improve glucose utilization with moderate weight loss in women. Obesity(SilverSpring) 2006;14(6):1064–72. doi: 10.1038/oby.2006.122. [DOI] [PubMed] [Google Scholar]
- 31.Nicklas JM, Zera CA, Seely EW, Abdul-Rahim ZS, Rudloff ND, Levkoff SE. Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a history of gestational diabetes. BMC pregnancy and childbirth. 2011;11:23. doi: 10.1186/1471-2393-11-23. doi: 10.1186/1471-2393-11-23. PubMed PMID: 21435246; PubMed Central PMCID: PMC3076295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan AS, Nicklas BJ, Berman DM. Racial differences in insulin resistance and mid-thigh fat deposition in postmenopausal women. Obes Res. 2002;10(5):336–44. doi: 10.1038/oby.2002.47. [DOI] [PubMed] [Google Scholar]
- 33.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46(10):1579–85. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 34.Kautzky-Willer A, Krssak M, Winzer C, Pacini G, Tura A, Farhan S, et al. Increased intramyocellular lipid concentration identifies impaired glucose metabolism in women with previous gestational diabetes. Diabetes. 2003;52(2):244–51. doi: 10.2337/diabetes.52.2.244. PubMed PMID: 12540593. [DOI] [PubMed] [Google Scholar]
- 35.Ryan AS, Ortmeyer HK, Sorkin JD. Exercise with calorie restriction improves insulin sensitivity and glycogen synthase activity in obese postmenopausal women with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2012;302(1):E145–52. doi: 10.1152/ajpendo.00618.2010. Epub 2011/10/20. doi: ajpendo.00618.2010 [pii] 10.1152/ajpendo.00618.2010. PubMed PMID: 22008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan AS, Nicklas BJ, Berman DM, Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int J Obes Relat Metab Disord. 2003;27(9):1066–71. doi: 10.1038/sj.ijo.0802387. [DOI] [PubMed] [Google Scholar]
- 37.Serra MC, Treuth MS, Ryan AS. Dietary prescription adherence and non-structured physical activity following weight loss with and without aerobic exercise. The journal of nutrition, health & aging. 2014;18(10):888–93. doi: 10.1007/s12603-014-0481-9. doi: 10.1007/s12603-014-0481-9. PubMed PMID: 25470804; PubMed Central PMCID: PMC4440863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyers-Seifer CH, Vohr BR. Lipid levels in former gestational diabetic mothers. Diabetes Care. 1996;19(12):1351–6. doi: 10.2337/diacare.19.12.1351. PubMed PMID: 8941463. [DOI] [PubMed] [Google Scholar]
- 39.Kjos SL, Buchanan TA, Montoro M, Coulson A, Mestman JH. Serum lipids within 36 mo of delivery in women with recent gestational diabetes. Diabetes. 1991;40(Suppl 2):142–6. doi: 10.2337/diab.40.2.s142. PubMed PMID: 1748245. [DOI] [PubMed] [Google Scholar]


