Abstract
Sleep has a critical role in promoting health. Research over the past decade has documented that sleep disturbance has a powerful influence on the risk of infectious disease, the occurrence and progression of several major medical illnesses including cardiovascular disease and cancer, and the incidence of depression. Increasingly, the field has focused on identifying the biological mechanisms underlying these effects. This review highlights the impact of sleep on adaptive and innate immunity, with consideration of the dynamics of sleep disturbance, sleep restriction, and insomnia on antiviral immune responses with consequences for vaccine responses and infectious disease risk, and on proinflammatory immune responses with implications for cardiovascular disease, cancer, and depression. This review also discusses the neuroendocrine and autonomic neural underpinnings linking sleep disturbance and immunity, and the reciprocal links between sleep and inflammatory biology. Lastly interventions are discussed as effective strategies to improve sleep, and potential opportunities are identified to promote sleep health for therapeutic control of chronic infectious, inflammatory, and neuropsychiatric diseases.
Keywords: sleep, insomnia, sleep deprivation, sleep restriction, adaptive immunity, innate immunity, inflammation, infectious disease, vaccine, cardiovascular disease, cancer, depression, psychoneuroimmunology
INTRODUCTION
Over the past decade, there has been an explosion of research on the role of sleep in health, with compelling evidence that disturbances of sleep including insomnia complaints and extremes of sleep duration adversely influence risk of infectious and inflammatory disease and contribute to all-cause mortality (Dew et al. 2003, Kripke et al. 2002, Mallon et al. 2002, Vgontzas et al. 2013). These findings have substantial public health implications because about 25% of the population of the United States report insomnia complaints (LeBlanc et al. 2009), and nearly 10% fulfill diagnostic criteria for chronic insomnia (Morin et al. 2006b; Ohayon 1996, 2002), which is persistent for as long as three years in nearly 50% of patients (Morin et al. 2009). Importantly, sleep disturbance is a modifiable risk factor: Substantial evidence shows that behavioral treatments have robust efficacy, with remission of insomnia sustained in the long term (Irwin et al. 2006a, Morin et al. 2006a). Increasingly, research on sleep and health has focused on the biological mechanisms underlying these effects, which might identify those at greatest risk for adverse health consequences or might be targeted to attenuate, or even prevent, morbidity.
The purpose of the present review is to provide an integrated understanding of how “sleep health” (Buysse 2014) affects one salient biological mechanism, the immune system, with implications for infectious and inflammatory disease risk. This review considers (a) the restorative role of sleep on the immune system; (b) the influence of sleep disturbance on adaptive and innate immunity; and (c) the role of sleep disturbance in risk of infectious disease and three inflammation-related disorders: cardiovascular disease, cancer, and major depression. Furthermore, behavioral regulation of the immune system is embedded in a conceptual model in which inflammatory cytokines also have a reciprocal influence on sleep. Hence, the role of behavioral treatments that target either sleep complaints or inflammation is considered in the development of effective treatments that promote sleep and health.
SLEEP: ASSESSMENT OF SLEEP AND INSOMNIA
Sleep Characteristics
Sleep is first and foremost a behavior, which is characterized by changes in body posture and eye state; it is dimensionally evaluated by self-report, behavioral, physiological, circuit, cellular, and genetic levels of analysis (Buysse 2014). Whereas such analyses can be used to differentiate arousal states along a continuum from fully awake to deep sleep, it is widely recognized that the electroencephalogram (EEG) or polysomnography is the gold standard for the objective assessment of sleep.
Polysomnography records sleep continuity, sleep architecture, and rapid eye movement (REM) sleep. Sleep continuity measures include the duration of sleep (i.e., total sleep time), the length of time from turning off the lights until sleep onset (i.e., sleep onset latency), and the degree to which sleep is maintained continuously throughout the night (i.e., sleep efficiency or the ratio of wake time to time in bed; awake time after sleep onset). Sleep architecture measures categorically divide sleep into two major phases, non-rapid eye movement (NREM) and REM sleep, with further subdivision of NREM sleep into stages 1, 2, 3, and 4, although recent research has combined stages 3 and 4 into slow-wave sleep (SWS) because of the inherent difficulties in distinguishing these latter two stages of deep sleep. Stage 1 is the transition between wakefulness and sleep, whereas sleep onset is generally defined to occur when spindles and K-complexes characteristically appear during stage 2. SWS is identified by a preponderance of high-amplitude, low-frequency components as measured by EEG. Finally, REM sleep is defined by a rapid and low-voltage EEG, similar to the activity of the brain during waking hours, with rapid and random movement of the eyes and low muscle tone.
Despite the widespread use of criteria to define stages of sleep, sleep is not quantal but rather shows a continuous progression from wakefulness to NREM and REM sleep. Spectral analyses provide a continuous evaluation of shifts from mixed EEG frequencies to predominately lower EEG frequencies occurring during sleep.
In humans, the transition from wakefulness to sleep occurs by entry into NREM sleep and a later transition to REM. After a period of REM sleep, a brief arousal or awakening may occur before entry again into NREM sleep. Over the course of the night, four to six cycles of NREM to REM sleep typically occur, with each cycle lasting about 80 to 110 minutes. SWS predominates in the early part of the night, whereas more REM sleep occurs in the later part of the night. Hence, as discussed below, the effects of different stages of sleep on the regulation of immunity have often been inferred from sleep deprivation studies that have disrupted sleep in the early or late part of the night.
Assessment of Sleep Disturbance
Polysomnography provides a laboratory-based objective measure of sleep. Despite being the gold standard for sleep assessment, polysomnography may not adequately characterize sleep occurring at home or disturbances of sleep over time. To estimate sleep patterns in a natural sleep setting without the invasiveness of polysomnography, sleep actigraphy is used. The watch-shaped actigraph estimates sleep patterns and circadian rhythms and is coupled with a sleep diary, in which the patient reports sleep onset and morning awakening. As such, actigraphy provides reasonably accurate sleep continuity indices as compared to polysomnography (Ancoli-Israel et al. 2003).
In addition to the evaluation of sleep by polysomnography, actigraphy, and sleep diaries, the patient’s own sleep perceptions play a critical role in the assessment of sleep disturbance and diagnosis of insomnia. Indeed, in a study of insomniacs and controls, polysomnography testing provided little information to confirm or exclude insomnia, and a large multicenter field trial resulted in similar findings (for a review, see Vgontzas et al. 2013). The American Academy of Sleep Medicine does not recommend polysomnography for the routine assessment of insomnia, although it is helpful in the evaluation of other sleep disorders such as sleep apnea (Littner et al. 2003).
Consistent with these clinical perspectives, the diagnosis of insomnia disorder in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) is based solely on subjective complaints of difficulty initiating or maintaining sleep, early awakening, interrupted or nonrestorative sleep, and associated impairments in daytime functioning, which must be present at least three nights per week and last for three months or longer (Am. Psychiatr. Assoc. 2013) (Table 1). Unfortunately, no objective approaches are available to confirm insomnia diagnosis or its severity, although insomnia with short sleep duration (less than five hours) is conceptualized as being a potentially more severe phenotype (Vgontzas et al. 2013). Importantly, insomnia is common in other disorders such as depression, and DSM-5 classification acknowledges this comorbidity without assigning casual attributions inherent in primary and secondary labels.
Table 1.
Criteria for insomnia disorder*
| Insomnia disorder diagnostic criteria | |
|---|---|
| A. | A predominant complaint of dissatisfaction with sleep quantity or quality, associated with one (or more) of the following symptoms: |
| 1. Difficulty initiating sleep. (In children, this may manifest as difficulty initiating sleep without caregiver intervention.) | |
| 2. Difficulty maintaining sleep, characterized by frequent awakenings or problems returning to sleep after awakenings. (In children, this may manifest as difficulty returning to sleep without caregiver intervention.) | |
| 3. Early-morning awakening with inability to return to sleep. | |
| B. | The sleep disturbance causes clinically significant distress or impairment in social, occupational, educational, academic, behavioral, or other important areas of functioning. |
| C. | The sleep difficulty occurs at least three nights per week. |
| D. | The sleep difficulty is present for at least three months. |
| E. | The sleep difficulty occurs despite adequate opportunity for sleep. |
| F. | The insomnia is not better explained by and does not occur exclusively during the course of another sleep-wake disorder (e.g., narcolepsy, a breathing-related sleep disorder, a circadian rhythm sleep-wake disorder, a parasomnia). |
| G. | The insomnia is not attributable to the physiological effects of a substance (e.g., a drug of abuse, a medication). |
| H. | Coexisting mental disorders and medical conditions do not adequately explain the predominant complaint of insomnia. |
From Am. Psychiatr. Assoc. 2013. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: Am. Psychiatr. Publ. 5th ed.
In recognition that insomnia symptoms are often prodromal to the development of insomnia disorder, there has been much interest in brief and valid self-report instruments to screen for insomnia complaints and assess symptom severity. One example is the Insomnia Severity Index, which assesses sleep quality, fatigue, psychological symptoms, and quality of life, with a high sensitivity and specificity for the detection of insomnia cases (Morin et al. 2011). The Pittsburgh Sleep Quality Index, a 19-item self-report questionnaire that evaluates seven clinically derived domains of sleep difficulties (i.e., quality, latency, duration, habitual efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction), is used to identify clinically significant sleep impairment (Cole et al. 2006). Despite the validity and reliability of these simple questionnaires, assessment of sleep disturbance in many large-scale epidemiologic or naturalistic field studies is limited to evaluation of one or more questions about complaints, sleep quality, or sleep duration. Nevertheless, given that daytime dysfunction is a necessary criterion for the diagnosis of insomnia, it is important to note that certain single items predict daytime consequences, with the following hierarchical order: self-reported dissatisfaction, complaints of nonrestorative sleep, difficulty resuming or maintaining sleep, and difficulty initiating sleep (Ohayon et al. 2012).
IMMUNE SYSTEM
The two interconnected branches of the immune system, adaptive and innate immunity, serve to detect and eliminate molecules and cells that display foreign antigens, altered self-antigens, or evidence of cellular damage.
Adaptive Immunity
Adaptive immunity leads to the differentiation and proliferation of microbial-specific white blood cells (i.e., lymphocytes) that serve to eliminate microbes based on an immunological memory of having responded to a specific pathogen or antigen in the past. As such, the adaptive immune system coordinates a specific response to an infectious challenge by a sequence of coordinated steps (Murphy 2011). In response to an infectious challenge, antigen-presenting cells (APCs) such as macrophages or dendritic cells are attracted to a site of intrusion, take up invading antigen, and then migrate to local lymph nodes. Within the lymph node, the APCs present antigen to T helper (Th) cells followed by release of proinflammatory cytokines such as interleukin (IL)-6 from the APCs. In response to these inflammatory signals, Th cells activate, proliferate, and differentiate into one of two predominant cell types, which serve to help B cells become antibody-producing cells (i.e., plasma cells) or leave the lymph node to coordinate cytotoxic cell responses that, if successful, eliminate the pathogen. Once complete, a fraction of these antigen-specific Th cells as well as cytotoxic T cells and B cells survive to provide an immunological memory that achieves a more rapid response when that specific infectious challenge occurs again.
This multicell response is tightly regulated by both costimulatory signals (e.g., proinflammatory cytokines such as IL-6) and inhibitory signals (e.g., anti-inflammatory cytokines such as IL-10). Additionally, if exposure involves an intracellular pathogen such as a virus, then transcription factors such as interferon (IFN) regulatory factors are activated. These transcription factors induce antiviral immune response genes such as type I IFN genes, which through the translation of IFN can activate signal transducer and activator of transcription (STAT)-1, leading to the production of proinflammatory cytokines. A failure of these regulatory mechanisms can lead to an inadequate immune response (e.g., immune deficiency) or a response that is too robust and results in damage to the host (e.g., autoimmunity, septic shock). IL-1, IL-6, and tumor necrosis factor-α (TNFα) promote the differentiation of lymphocytes into cytotoxic T cells, which kill pathogens that are introduced into the body during physical wounding.
Inflammatory cytokines promote vascular permeability and cellular adhesion, which makes it possible for immune cells to leave the blood vessels and migrate to tissues where they can eliminate pathogens. For example, IL-1 activates the endothelial adhesion molecule intercellular adhesion molecule-1 that facilitates adherence of immune cells to the endothelium and migration into tissues. Proinflammatory cytokines also signal sites of injury or infection by activating the release of chemokines that recruit immune cells to the site of inflammatory activity (Murphy 2011).
Innate Immunity
Innate immunity is evolutionally older than adaptive immunity[AU: Than adaptive immunity?] and serves as the body’s first line of defense against tissue damage and microbial infection (Medzhitov 2008). Composed of immune cells such as monocytes, macrophages, and dendritic cells that constantly circulate in the body, the innate immune system uses invariant receptors to detect a wide variety of pathogens. Activation of these cells leads to a rapid (i.e., minutes to hours) cascade of inflammatory processes that help contain an infection and ultimately promote healing and recovery (Medzhitov 2008).
When receptors of innate immune cells recognize “hard-wired” and highly conserved features of microbes or pathogen-associated molecular patterns, the acute-phase response is triggered, which leads to an increase in inflammatory activity that can occur both locally (i.e., at the site of tissue injury or infection) and systemically. This recognition strategy, which uses a relatively small number of immune cell types to detect and generate a response to a wide range of microbial diversity, is termed pattern recognition, and innate immune receptors that use this strategy are called pattern recognition receptors (Medzhitov 2008).
Among pattern recognition receptors, Toll-like receptors (TLRs) are found on macrophages, neutrophils, and dendritic cells that drive inflammation (Medzhitov 2008). Whereas TLRs recognize conserved components of microbes including bacteria, viruses, and fungi, there is some specificity of ligand recognition within this family of TLRs. For example, lipopolysaccharide, an endotoxin that is the major component of the outer membrane of Gram-negative bacteria, binds to TLR4 (Medzhitov 2008) Following binding of one of the TLR4, a conserved signaling cascade is initiated that is characterized by activation of key intracellular transcription factors such as nuclear factor-κB (NF-κB) and activator protein 1 (AP-1) (Karin 2006). Activation of NF-κB, for example, leads to the transcription of proinflammatory immune response genes such as TNF-α and IL-1 and the translation and production of proinflammatory cytokines that serve to coordinate the inflammatory response (Karin 2006). Hence, intracellular processes that occur at the level of the genome most proximally regulate the inflammatory response.
As described below, sleep plays an important role in the regulation of the adaptive and innate immune responses; evidence indicates that sleep disturbance induces a downregulation of adaptive immunity, as indexed by an impaired response to infectious challenge, along with an upregulation of innate immune responses, as indexed by increases in cellular and genomic markers of inflammation.
Psychoneuroimmunologic Pathways of Immune Regulation
Sleep influences the two primary effector systems, the hypothalamus-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS), which in turn regulate adaptive and innate immune responses. During sleep, blood levels of cortisol, epinephrine, and norepinephrine drop (Besedovsky et al. 2012), whereas mediators that subserve cell growth such as growth hormone, prolactin, and the pineal hormone melatonin show a steep increase in their blood levels. (For characterization of these latter neuroendocrine responses, which is beyond the scope of this review, see Besedovsky et al. 2012.) In contrast, chronic sleep disturbance leads to activation of the HPA and SNS pathways (Vgontzas et al. 2013), which together contribute to an increased proinflammatory and reduced antiviral skewing of the basal gene expression profile, termed the basal transcriptome. Such a conserved transcriptional response to adversity, of which sleep disturbance is one type, has been extensively discussed in prior reviews (Irwin & Cole 2011, Slavich & Irwin 2014).
Briefly, activation of the HPA axis releases cortisol that suppresses proinflammatory and antiviral immune responses by three mechanisms. First, glucocorticoids bind to the glucocorticoid receptor of gene promoter sequences and interrupt transcription of proinfammatory and antiviral genes. Second, activation of the glucocorticoid receptor induces transcription of certain anti-inflammatory genes, which serves to interfere with activation of the transcription factor NF-κB and leads to a blockade of the inflammatory cascade. Finally, protein-protein interactions interfere with proinflammatory transcription factors such as NF-κB and AP-1 and antagonize inflammatory gene transcription (Irwin & Cole 2011).
The second effector pathway is the SNS. In contrast to HPA activation, the SNS “steers” immune responses between antiviral and proinflammatory immune responses (Irwin & Cole 2011). Immune response gene transcription is regulated via stimulation of β-adrenergic receptors by the release of neurotransmitter norepinephrine into peripheral tissues, primary and secondary lymphoid organs, and all other major organ systems (including the vasculature and perivascular tissues) (Irwin & Cole 2011). This adrenergic signaling cascade suppresses transcription of antiviral type I IFN genes (Cole et al. 1998) and upregulates transcription of the proinflammatory immune response genes IL1B, TNF, and IL6, leading to increases in systemic inflammatory activity (Cole et al. 2010).
IMMUNITY DURING NOCTURNAL SLEEP: CIRCADIAN VERSUS SLEEP PROCESSES
Nocturnal sleep has a homeostatic role in the regulation of immunity, which is separate from the influence of circadian processes. In a series of elegant studies, the profile of immune measures during a regular 24-hour sleep-wake cycle has been compared to a 24-hour cycle of continuous nocturnal wakefulness to evaluate the influence of sleep versus circadian oscillators on nocturnal, as well as daytime, levels of immunity (Born et al. 1997, Lange et al. 2010). The studies reveal that certain aspects of immunity are mainly influenced by circadian processes, whereas other immune measures are primarily steered by sleep.
Nocturnal Profile of Adaptive Immunity
Circadian factors play a predominant role in regulating the distribution of immune cells. Regardless of nocturnal sleep, the numbers of leukocytes, granulocytes, and monocytes as well as the major lymphocyte subsets, including T-helper cells (CD4+), cytotoxic T cells (CD8+), activated T cells (HLA-DR+), and B cells (CD19+), reach a maximum in the evening or early night and then decline throughout the remaining night to reach a minimum in the morning hours (Born et al. 1997). In humans, this circadian rhythm of T cells in blood is independent of sleep and is coupled to the rhythm of cortisol such that the peak in cortisol in the beginning of the wake period precedes a decrease in blood T cell number by about 3 hours.
In contrast to circadian factors, nocturnal sleep plays a predominant role in the regulation of adaptive immune responses. During nocturnal sleep, but not during a period of nocturnal wakefulness, T cell production of IL-2 (Lissoni et al. 1998), as well as IFN-γ (Petrovsky et al. 1998), is enhanced. Even a modest amount of sleep can increase IL-2 production, as levels of IL-2 are similar during a night of uninterrupted sleep or partial sleep loss (Irwin et al. 1999). Nocturnal sleep also enhances the production of IL-12 by precursor dendritic cells as well as by monocytes (Lange et al. 2006b, 2010); IL-12 is a key cytokine for the induction of Th1- type adaptive immune responses. Finally, nocturnal sleep favors a shift toward Th1 (predominance of IFN-γ), with a peak around 03:00 h (Petrovsky & Harrison 1997) or even at 06:30 h (Redwine et al. 2003), along with a reduction in production of the anti-inflammatory cytokine IL-10 by monocytes (Lange et al. 2006b). The ratio of IFN-γ to IL-10 production is thought to be a determinant for the selection of the effector mechanisms of immune defense, in which Th1 cell release of mainly IFN-γ enhances response to intracellular viral and bacterial challenges and supports various cellular responses (i.e., type 1), including macrophage activation and antigen presentation. In sum, nocturnal sleep supports adaptive immunity, especially during the early portion of the night when SWS is dominant, whereas it is thought that counterregulatory processes develop during the late portion of the night, when REM sleep prevails.
Nocturnal Profile of Innate Immunity
Nocturnal sleep, as opposed to circadian processes, regulates aspects of innate immunity. For example, the nocturnal increase of natural killer (NK) cell activity, a type of cytotoxic lymphocyte critical to the innate immune system, is dependent in part on sleep. Counts of NK cells and NK activity are at a minimum during the early part of the night and reach a maximum in the late morning hours (Kronfol et al. 1997); this nocturnal increase is attenuated in persons who evidence sleep disturbance (Irwin et al. 1996, Redwine et al. 2003).
In contrast, both circadian- and sleep-dependent processes contribute to a nocturnal increase in inflammatory measures. Circulating concentrations of IL-6 show two peaks, at 19:00 and again at 05:00 (Vgontzas et al. 1999). Yet, early night sleep deprivation delays the nocturnal increase of IL-6 (Redwine et al. 2000), and total night sleep deprivation diminishes the increase in IL-6 by about half (Vgontzas et al. 1999). Nevertheless, circadian factors also contribute, as a transient peak in IL-6 occurs at 01:00 even in the absence of sleep (Redwine et al. 2000), with evidence that monocyte production of IL-6 also peaks at 02:00 even when the subject is awake (Dimitrov et al. 2006). Nocturnal sleep also plays a critical role in the regulation of IL-6 signaling of neural functions. An integrated action of IL-6 on brain and nonimmune tissues requires the release of IL-6R because binding of IL-6 with IL-6R is required to activate cells that lack membrane-bound IL-6R. Nocturnal sleep is associated with a robust (70%) increase in levels of soluble IL-6R (Dimitrov et al. 2006), which is most pronounced during the late part of the night, consistent with relative increases in REM sleep amounts and increases in IL-6 (Redwine et al. 2000). However, nocturnal increases in another proinflammatory cytokine, TNF, and its soluble receptor appear to be driven primarily by circadian factors (Born et al. 1997).
The signals that drive nocturnal increases in proinflammatory cytokines are not known, although it is speculated that danger signals such as reactive oxygen species, nucleotides (e.g., adenosine triphosphate), and heat shock proteins accumulate during the wake period and act like classical immunological stimulants for the production of proinflammatory cytokines, which then support the initiation of adaptive immune responses (Besedovsky et al. 2012). Alternatively, sleep induces the release of growth hormone (GH) and prolactin during the early night, and GH and prolactin are well known to enhance the proliferation and differentiation of T cells as well as to promote type 1 cytokine activity (Lange et al. 2006a). Interestingly, peaks of proinflammatory cytokines during the rest period have been observed, often during the early SWS-dominated portion of sleep, in humans as well as in animals, on the mRNA and protein levels in different tissues including the brain, adipose tissue, and lymph nodes (for a review, see Besedovsky et al. 2012).
SLEEP DISTURBANCE, ADAPTIVE IMMUNITY, AND INFECTIOUS DISEASE RISK
Given that nocturnal sleep is a prominent regulator of adaptive immune responses, it is logical to consider the effects of sleep disturbance on this branch of the immune system during the day and after a period of sleep disturbance. However, in some instances when sleep loss is prolonged or severe, such as total night sleep deprivation for several nights, the immune effects may not be specific to sleep disturbance, as there can be marked activation of stress response pathways such as the HPA axis during such profound sleep loss. Yet, when the sleep loss is limited to a single night or part of the night, or when sleep is fragmented as often occurs in naturalistic settings, activation of stress pathways appears limited to increases of SNS activity (Irwin et al. 1999, Irwin & Ziegler 2005). Adrenergic signaling then steers immune responses with the suppression of antiviral immune response genes and increases in inflammatory response genes as noted above (Irwin & Cole 2011).(See Figure 1)
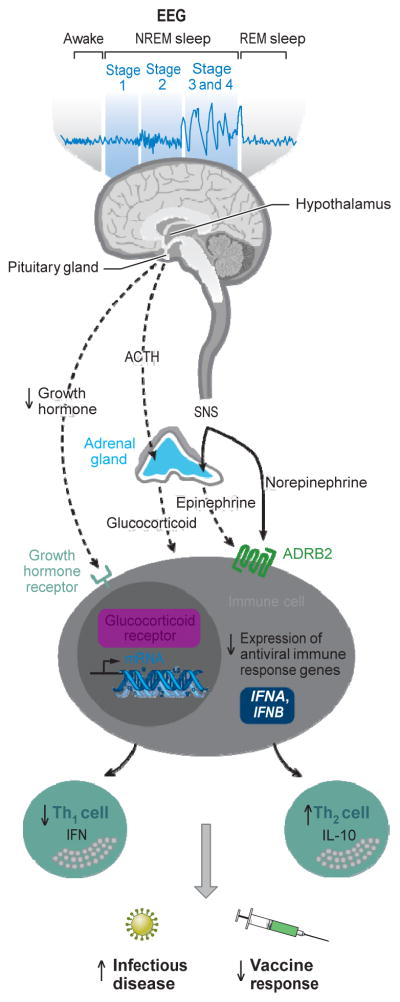
Figure 1.
Sleep disturbance and adaptive immunity. The hypothalamus-pituitary-adrenal axis distributes glucocorticoid hormones through the blood to regulate gene expression in virtually every cell of the body. Sleep disturbance results in hormone activation of the glucocorticoid receptor in leukocytes, which leads to a profound suppression antiviral gene programs [e.g., interferon regulatory factor (IRF)-mediated transcription of type I interferons (IFNs) such as the IFNA and IFNB genes]. Sleep disturbance also activates nerve fibers from the sympathetic nervous system (SNS) to release the neurotransmitter norepinephrine into primary and secondary lymphoid organs, all other major organ systems including the vasculature and perivascular tissues as well as many other peripheral tissues. SNS neural fibers can also stimulate the adrenal gland to release stored epinephrine into the systemic circulation. Both neuromediators stimulate leukocyte adrenergic receptors (e.g., ADRB2) to repress IRF-mediated antiviral IFN response gene (IRG) programs. Sleep also induces the release of growth hormone during the early part of the night, and this hormone acts to enhance the proliferation and differentiation of T cells as well as to promote type 1 cytokine activity; when sleep is disrupted, a reduced amount of growth hormone is released. Suppression of IRF-mediated IRG programs leads to a shift in Th1 to Th2 balance, with decreases in Th1 cell production of IFN and increases in Th2 cell production of interleukin-10 (IL-10). This suppression of adaptive immune response is thought to contribute to increased susceptibility to infectious disease and decreased response to vaccines. Abbreviations: ACTH, adrenocorticotropic hormone; EEG, electroencephalogram.
Experimental Sleep Loss and Adaptive Immunity
Experimental sleep loss, as defined by experimentally induced deprivation of sleep during all or part of the night, has minimal effects on immune cell numbers, in part due to the predominant circadian regulation of almost all white blood cell subpopulations. For example, the relative distribution of most major lymphocyte subsets does not change in response to sleep loss, with the exception of decreases in the numbers and relative percentage of NK cells (Born et al. 1997, Irwin et al. 1996). However, when sleep loss is extended beyond a single night of sleep deprivation, numbers of circulating granulocytes and monocytes increase (Dinges et al. 1994, Irwin et al. 1996).
In contrast, experimental sleep loss during all or part of the night has robust effects on the production of cytokines important in the regulation of adaptive immunity. Both partial- and total-night sleep deprivation reduce T cell production of IL-2, and this effect is independent of the total number of circulating T cells (Born et al. 1997; Irwin et al. 1996, 1999). In addition, a night of wakefulness is associated with a shift in the Th1 to Th2 cytokine balance toward increased Th2 cytokine activity, especially in the morning hours (Dimitrov et al. 2004, Lange et al. 2006a). Sleep deprivation also reduces monocyte production of IL-12, a cytokine that supports Th1 responses, whereas sleep loss increases the production of IL-10, a cytokine that promotes Th2 responses (Lange et al. 2006b). Yet, when sleep loss is prolonged for several nights, there are relatively minimal changes in T cell--derived cytokines such as IL-2, IL-4, and IFN-γ (Dinges et al. 1995).
Naturalistic Sleep Disturbance and Adaptive Immunity
Naturalistic sleep disturbance, in contrast to experimental sleep loss, is associated with alterations in the relative distribution of immune cells; marked decreases in the numbers of CD3+, CD4+, and CD8+ T cells have been reported in patients with chronic insomnia (Savard et al. 2003). Sleep disturbance also induces a relative shift toward a type 2 response as evidenced by a lower ratio of stimulated production of IFN-γ/IL-10 as compared to responses in those without a sleep disturbance (Redwine et al. 2003), which provides insight into the immune processes that might contribute to impaired vaccine responses or susceptibility to infections in persons who are experiencing acute or chronic sleep disturbances, as discussed below.
Sleep Disturbance and Vaccine Responses
Research on the effects of sleep loss on vaccine responses provides a translational perspective on the sleep-related changes in adaptive immunity by identifying clinically relevant endpoints related to infectious disease risk including immunologic response to vaccination. Indeed, some of the first observations linking sleep disturbance and infectious outcomes were generated in animal studies, which showed that the clearing of influenza was slowed in sleep-deprived mice as compared to animals that were not sleep deprived (Brown et al. 1989), although three subsequent studies have failed to replicate these initial findings (Renegar et al. 1998a, b, 2000).
In humans, partial sleep deprivation mimics the ubiquitous sleep disturbance found in persons exposed to social adversity or who are aged and most vulnerable to infectious diseases. Following a night of partial sleep loss, immunologic response to an influenza A vaccination was reported to be reduced by over 50% as compared to the response in those who maintained a regular sleep schedule (Spiegel et al. 2002). Similar results were found in response to immunization with the hepatitis A vaccine, in which total sleep deprivation also reduced virus-specific antibody titers (Lange et al. 2003). Furthermore, the effects of sleep loss were persistent for up to one year, with a reduced frequency of antigen-specific Th cells as well reduced levels of antigen Ag-specific immunoglobulin G1 (Lange et al. 2011).
Naturalistic sleep disturbance has also been found to be associated with a reduced magnitude of immune response to several types of vaccines. Initial studies focused on the link between psychological stress and vaccine response and revealed that sleep amounts partially explained the impaired vaccine responses. Miller et al. (2004) found an association between fewer hours of sleep, as indexed by ambulatory monitoring, and lower antibody response to the influenza vaccine, which accounted in part for the association between daily measures of psychological stress and the reduced response to the influenza vaccine. Likewise, Pressman et al. (2005) found that loneliness and poor sleep efficiency were associated with poorer antibody response to influenza vaccine.
Recent research has examined the association between sleep amounts and vaccine response. Prather et al. (2012) found that shorter sleep duration (i.e., less than six hours per night as confirmed by actigraphy) was strongly linked to a decreased likelihood of protection from a hepatitis B vaccination in 125 midlife adults. Importantly, this adverse effect of sleep disturbance persisted even when three doses of the vaccine were given, including a booster dose at six months. Together, these experimental and prospective observations demonstrate that variations in sleep duration decrease the immunologic protection offered by standard, clinical vaccines (i.e., influenza, hepatitis) and suggest the possibility that short sleep duration might increase infectious disease risk by increasing susceptibility to viral pathogens or expression of symptoms, or both.
Sleep Disturbance and Infectious Disease
Extremes of sleep duration are related to infectious disease risk, with epidemiologic evidence that both reduced (e.g., <5 h) and prolonged (e.g., >9 h) habitual sleep duration are correlated with an increased risk of pneumonia (Patel et al. 2012). However, Cohen et al. (2009) found that self-reported shorter sleep duration and sleep fragmentation were associated with susceptibility to the common cold. In experimental studies in which healthy adults are inoculated with a rhinovirus that produces symptoms of the common cold, poor sleep efficiency was associated with increased susceptibility to the common cold and greater symptom reporting (Cohen et al. 2009). Whether it is possible to modify infectious disease risk profiles in persons with sleep problems is not known, although Irwin et al. (2013) recently reported that treatment of depressed older adults with a selective serotonin reuptake inhibitor normalizes attenuated vaccine responses to the zoster vaccine. The age and depression status of older adults contribute to shorter sleep duration along with blunted viral-specific immune response to vaccines and an increased risk of herpes zoster as well as influenza. Additionally, it is important to note that sleep disturbance also occurs in the context of an acute infectious challenge (Toth 1995) as well as during chronic infections such as HIV (Reid & Dwyer 2005), although the impact of insomnia on infectious outcomes such as HIV is not known.
Psychoneuroimmunologic Mechanisms Linking Sleep Disturbance and Adaptive Immunity
The reasons that sleep contributes to dynamic variations in the adaptive immune system and response to vaccine are not known, although it is thought that the quiescent period of sleep serves to reallocate energy resources from functions related to wakefulness to processes that, for example, facilitate and promote immune responses to infectious challenge (Besedovsky et al. 2012, Motivala & Irwin 2007). As immune cells move out of the circulatory system during the nocturnal period, naive T and B cells relocate to the lymph nodes and are exposed to foreign antigens, which initiates an adaptive immune response. The subsequent immune cell division and differentiation require metabolic resources, which appear to be supported during sleep, especially SWS. For example, Lange et al. (2003) found that EEG slow-wave activity on the night after vaccination against hepatitis A correlated with the percentage of antigen-specific Th cells measured one year after vaccination, with evidence that increased release of growth hormone and prolactin but decreased cortisol mediated these effects. The release of growth hormone, as well as prolactin, primarily occurs early in the night, at the time that SWS is most prominent. Hence, the link between SWS and the formation of immunological memory, as well as neurobehavioral memories, provides insight into the role of sleep in the cross-links between the brain and the immune system and the function of sleep in supporting the organism’s strategic adaptation to different kinds of environmental stressors (i.e., behavioral, immunological). Another potential mechanism involves activation of adrenergic signaling that suppresses adaptive immunity. Whereas sleep disturbance induces increases in SNS outflow (Irwin et al. 1999, Irwin & Ziegler 2005), further research is needed to test explicitly whether antagonism of β-adrenergic signaling abrogates the effects of sleep on adaptive immunity, similar to the effects of β-adrenergic antagonism of stress-induced reduction of adaptive immune responses (Irwin & Cole 2011, Murray et al. 1992).
SLEEP DISTURBANCE, INNATE IMMUNITY, AND INFLAMMATORY DISORDERS
Nocturnal sleep, as described above, serves to prime the immune system to infectious challenge and to induce nocturnal activation of inflammatory signaling. Hence, in the absence of sleep, nocturnal levels of inflammatory cytokines are lower (Besedovsky et al. 2012, Redwine et al. 2000). However, loss of sleep also induces an activation of sympathetic outflow, and in the aftermath of sleep disruption, such adrenergic signaling is thought to steer inflammatory gene expression and lead to increases in daytime levels of markers of innate immunity and inflammation. Below, the effects of sleep loss and sustained sleep disturbance on daytime activity of the innate immune system are described.(See Figure 2)
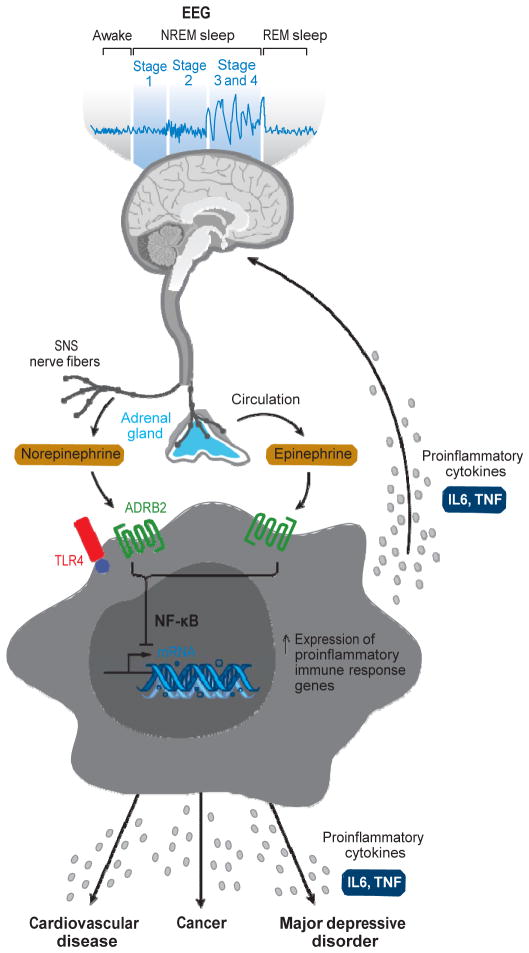
Figure 2.
Sleep disturbance and innate immunity. Following a night of sleep loss, or during a period of sleep disturbance, nerve fibers from the sympathetic nervous system (SNS) release the neurotransmitter norepinephrine into primary and secondary lymphoid organs and stimulate the adrenal gland to release stored epinephrine into systemic circulation. Both neuromediators stimulate leukocyte adrenergic receptors (e.g., ADRB2) and activate nuclear factor (NF)-κB-mediated inflammatory programs. Intrinsic circuits detect microbes via pattern recognition receptors (PRRs) such as the Toll-like receptor-4 (TLR4) and stimulate inflammatory gene expression via transcription factors such as NF-κB. The production of proinflammatory cytokines interleukin (IL)-6 and tumor necrosis factor-α (TNFα) occurs. Bidirectional links between the brain and periphery allow the brain to regulate inflammatory activity, and inflammatory activity in turn can influence neural processes in the brain and alter sleep. When this dynamic is induced by sustained sleep disturbance, a feed-forward dysregulation of sleep can occur, which may also confer increased risk for inflammation-related disorders such as cardiovascular disease, cancer, and major depressive disorder.
Experimental Sleep Loss, Innate Immunity, and Inflammation
Over a decade ago, early data suggested that prolonged sleep deprivation induced an activation of innate immunity, although measures of circulating levels of cytokines relied on bioassays that lacked specificity and it was not known what cytokine contributed to reported increases in IL-1-like and IL-2-like activity (Moldofsky et al. 1989). However, the advent of cellular and molecular assays of inflammatory biology has exponentially increased interest in the role of sleep disturbance on inflammatory markers, with implications for understanding the association between sleep complaints and inflammatory disorders.
Partial-night sleep deprivation, when repeated for several nights (i.e., 10 nights), induces robust increases in C-reactive protein (CRP) (Meier-Ewert et al. 2004) and IL-6 (Haack et al. 2007). Even shorter periods of sleep restriction (i.e., seven nights) induce increases in plasma concentrations of IL-6 in men and women, increases of TNF in men only (Vgontzas et al. 2004a), and increases in inflammatory transcripts of IL-1β, IL-6, and IL-17 (van Leeuwen et al. 2009), with evidence that such increases persist even after a night of recovery sleep (van Leeuwen et al. 2009). However, when sleep restriction or sleep fragmentation is limited to only one or two nights (Abedelmalek et al. 2013, Schmid et al. 2011, Stamatakis & Punjabi 2010), or when sleep restriction occurs in the midst of intervening daytime naps (Faraut et al. 2011, Shearer et al. 2001), circulating levels of inflammatory markers do not appear to change. Yet, in a vulnerable population of individuals who report chronic sleep disturbance, a single night of sleep loss triggers an activation of inflammation with increases in IL-6 and TNF, which is not found in those who have no underlying sleep problems (Irwin et al. 2004).
Total-night sleep deprivation is also reported to induce increases in circulating markers of inflammation, with evidence of a dose response relationship in which progressive loss of sleep over four nights led to cumulative increases of CRP (Meier-Ewert et al. 2004). Increases of TNF emerge as early as one to two night (Chennaoui et al. 2011), although elevations in IL-6 do not appear to occur until after four nights (Shearer et al. 2001). Importantly, in healthy adults, an intervening nap appears to be protective, as increases of IL-6 following four nights of sleep restriction are reversed by a two-hour nap (Vgontzas et al. 2007). Activation of vascular endothelial markers (i.e., E-selectin, sICAM-1), which typically follows increases in inflammation, also occurs after a single night of sleep loss (Frey et al. 2007, Sauvet et al. 2010). Finally, consistent with the notion that nocturnal sleep primes inflammatory signaling but sleep loss leads to daytime increases of inflammation, Vgontzas et al. (1999) have found that sleep deprivation shifts the temporal pattern of circadian IL-6 secretion, with lower levels of IL-6 during the night and higher levels during the day.
Another component of the innate immunity that is altered by sleep loss is NK activity, although changes in NK activity parallel the suppressive effects of sleep loss on adaptive immunity, with robust reductions in NK activity (Irwin et al. 1994, Irwin et al. 1996) and in the ability of NK cells to become activated by IL-2 activation (Irwin et al. 1996). Additionally, sleep disturbance appears to be a critical pathway by which psychological stress and major depression lead to a reduction in NK activity (Cover & Irwin 1994, Irwin & Miller 2007).
Cellular and Molecular Mechanisms Linking Sleep Loss and Inflammation
Cellular production of IL-6 and TNF is thought to be due in part to aberrant increases of TLR activity, which in turn have been linked to inflammatory diseases such as rheumatoid arthritis (Andreakos et al. 2004) and heart failure (Satoh et al. 2005). Hence, to understand the functional basis for altered inflammatory response after sleep loss, research has examined the production of proinflammatory cytokines by monocytes following ligation of TLR-4 with lipopolysaccharide (Irwin et al. 2006b). Partial-night sleep deprivation induces an increase in TLR-4-stimulated production of IL-6 and TNF, which is especially prominent in women who show sustained elevations in production of proinflammatory cytokines throughout the day following sleep loss. Additionally, sleep loss induces an increase in transcription of IL-6 and TNF (Irwin et al. 2006b) due an activation of NF-κB, the key transcription control pathway in the inflammatory signaling cascade (Irwin et al. 2008b). Moreover, sleep has effects on transcriptome dynamics and induces an upregulation of a gene ensemble that includes the master circadian regulator, several immediate early genes marking cellular signal transduction, and multiple inflammatory response genes. Transcription factor binding motifs that were overrepresented in the sleep-deprivation condition included the promoters of genes involved in regulation by cyclic adenosine monophosphate/protein kinase A (cAMP/PKA)-induced transcription factors of the cAMP response element--binding protein/activating transcription factor (CREB/ATF) family, the protein kinase C-induced AP-1 family, the proinflammatory NF-κB/Rel family, and the mitogen-activated protein kinase-inducible E26 transformation-specific (ETS) transcription factor family typified by ELK1. In sum, the leukocyte transcriptional response to sleep deprivation involves multiple signal-transduction pathways including the NF-κB inflammatory signaling system.
Naturalistic Sleep Disturbance and Inflammation
Three prominent epidemiologic studies have specifically tested the relationship between insomnia complaints and elevated levels of CRP, a marker of systemic inflammation, and have found mixed results. The Nord-Trondelag Health Study (n = 8,547) found no significant relationship between insomnia symptoms and CRP after adjustment for cardiovascular risk factors (Laugsand et al. 2012), whereas the Northern Finland 1966 Birth Cohort study (n = 4,011) showed insomnia was associated with higher levels of CRP in men, but not in women, and that these relationships were apparent only if insomnia at least moderate to severe (Liukkonen et al. 2007). However, recent findings from the National Health and Nutrition Examination Survey (NHANES) (n = 10,908) showed that complaints of nonrestorative sleep, as opposed to nocturnal insomnia symptoms, were associated with increases in CRP in both sexes (Zhang et al. 2013a).
Naturalistic studies that have assessed sleep disturbance using validated sleep questionnaires such as the Pittsburgh Sleep Quality Index (PSQI) or that have diagnostically categorized insomnia have reported a more consistent effect of sleep disturbance on inflammation than have epidemiologic studies that have relied on one or two questions about sleep disturbance. Indeed, when sleep disturbance is defined by questionnaire threshold scores (i.e., PSQI >5), high levels of CRP have been found among poor sleepers in clinical (Chiu et al. 2009), adult (Okun et al. 2009), and older adult (Christian et al. 2011) populations. Elevated levels of proinflammatory cytokines (i.e., IL-6) are also reported in persons who report sleep impairment (Erten et al. 2005, Friedman et al. 2005, Okun et al. 2007), although not all studies have confirmed these findings (van Mark et al. 2010). Likewise, among patients with insomnia disorder, increases in daytime circulating levels of CRP (Razeghi et al. 2012) and IL-6 are found (Vgontzas et al. 2002), with elevated levels of IL-6, for example, extending into the mid-afternoon and early evening (Vgontzas et al. 2002) and even into the night (Burgos et al. 2006). Finally, given that women appear to be especially vulnerable to the effects of sleep loss on cellular inflammation (as noted above) (Irwin et al. 2008b, 2010), it is interesting to note that Suarez (2008) found that overall poor sleep quality was associated with increases inflammatory biomarkers, but only for women, similar to the findings of Friedman et al. (2005) in older adults.
Certain aspects of sleep disturbance, as well as sex differences, differentially impact inflammation, with evidence that poor sleep efficiency may be salient in the association with higher levels of CRP (Okun & Coussons-Read 2007) and IL-6 (Friedman et al. 2005), especially in women with poor social relationships. Further research should evaluate whether social ties might buffer the adverse effects of sleep disturbance on inflammation. Moreover, self-reported sleep duration may also impact inflammatory markers. Again, sex differences emerge with evidence that women, but not men, are more likely to show elevated levels of inflammation in association with short sleep duration. For example, in the Whitehall II epidemiologic study (n = 4,677) (Miller et al. 2009), women, but not men, who slept less than eight hours tended to have higher levels of IL-6 as well as higher levels of CRP. Other studies have focused solely on women and found that postpartum sleep duration of less than five hours was prospectively associated with increases of IL-6 and CRP one to three years later (Taveras et al. 2011); shorter sleep duration was also associated with increases of CRP in adolescent girls (Larkin et al. 2005).
Extreme sleep habits, i.e., short and long sleep duration, have been linked to adverse health outcomes (Youngstedt & Kripke 2004), with similar effects on inflammation. In the population-based InCHIANTI study (n = 751), levels of TNF, but not IL-6 or CRP, were increased at extremes of both short and long sleep as compared to those who slept seven to eight hours (Stenholm et al. 2011). However, in a five-year prospective observational study of an older adult Taiwanese population, only longer sleep (greater than eight hours) was associated with increases in markers of inflammation (Dowd et al. 2011). To further complicate these observations, the effects of sleep duration might depend on the proinflammatory cytokine; Patel et al. (2009) found that each additional hour of sleep duration resulted in an increase in CRP and an increase in IL-6, whereas a shorter sleep duration was associated with an increase in TNF. Given these mixed results, it is important to note that several high-quality studies have found no relationship between self-reported and objective (i.e., polysomnographic) sleep duration and markers of inflammation (Larkin et al. 2005, Lee et al. 2009, Marsland et al. 2008, Ramey et al. 2012, Rief et al. 2010, Taheri et al. 2007), even when measures of sleep quality and sleep efficiency were correlated with inflammation in the same population (Friedman 2005).
Sympathetic Mechanisms Linking Sleep Disturbance and Inflammation
Activation of the sympathetic nervous system and β-adrenergic signaling activate the inflammatory cascade to induce increases in NF-κB, inflammatory gene expression, and production of proinflammatory cytokines, along with increases in markers of systemic inflammation such CRP (Irwin & Cole 2011). Given that normal nocturnal sleep is associated with a drop in sympathetic outflow (Irwin et al. 1999), one biologically plausible mechanism to explain the associations between sleep disturbance, short sleep duration, and increases in markers of inflammation is activation of sympathetic effector pathways.
Sleep and sleep depth regulate sympathetic outflow. In the transition from wakefulness to sleep, there is a shift from sympathetic to parasympathetic outflow, which is dependent on the stage of sleep as well as sleep depth (Boudreau et al. 2013). During NREM or SWS, sympathetic activity is decreased; during REM sleep, SNS outflow is higher, similar to daytime levels (Somers et al. 1993). Changes in plasma levels of the sympathetic neurotransmitters show a similar pattern (Irwin et al. 1999). In contrast, increases in norepinephrine, epinephrine, and other markers of sympathetic outflow (as measured by blood pressure, heart rate variability, and impedance cardiography) are found in patients with insomnia (Bonnet & Arand 1997, De Zambotti et al. 2011, Lanfranchi et al. 2009, Riemann 2010, Vgontzas et al. 2013) and following sleep deprivation (Irwin et al. 1999, Irwin & Ziegler 2005). Furthermore, patients with insomnia with short sleep duration are more likely to show increases in urinary catecholamines and levels of their metabolites than are insomniacs without objective sleep disturbance, similar to the greater activation of inflammatory biomarkers in this more severe phenotype (Vgontzas et al. 2013).
Sleep Disturbance, Sleep Duration, and Cardiovascular Disease
Inflammation has been found to have a fundamental role in mediating all stages of atherosclerosis, from its initiation through progression to cardiovascular disease, with evidence that the prospective risk for acute coronary syndromes as well as risk for other atherosclerotic complications is defined in part by high levels of CRP (Libby et al. 2002). In addition, sleep complaints---possibly through effects on inflammation---contribute to cardiovascular disease risk (Mullington et al. 2009). Finally, depression is well known to be a potent risk factor for cardiovascular disease (Joynt et al. 2003), with recent findings indicating that sleep disturbance has a critical role in mediating the association between depressive symptoms and hypertension incidence (Gangwisch et al. 2010) as well as all-cause and cardiovascular disease mortality (Azevedo Da Silva et al. 2014). However, no study has systematically evaluated whether elevated levels of inflammation mediate the association between sleep disturbance and cardiovascular disease, so the following section primarily describes the associations between sleep disturbance, hypertension, and cardiovascular outcomes.
Those who report sleep complaints and/or have short sleep duration appear to be at greatest risk of cardiovascular disease (Vgontzas et al. 2013). For example, a recent meta-analysis of 11 prospective studies (Meng et al. 2013) found that short sleep duration, as well as complaints of sleep maintenance and early-morning awakening, but not difficulty falling asleep, predicted an increased risk of hypertension; another systematic review (Palagini et al. 2013) as well as a large-scale epidemiologic study (n = 4,794) (Suka et al. 2003) had similar conclusions. The salient role of insomnia complaints combined with short sleep duration in predicting cardiovascular disease, and especially hypertension, has also been examined by polysomnography in a study of more than 1,700 adults (Vgontzas et al. 2009). Those who reported insomnia lasting more than one year and who also had short sleep duration (≤5 h) had a fivefold greater risk of having hypertension (Vgontzas et al. 2009), with prospective findings showing that this combination led to a fourfold increased risk of incident hypertension (Fernandez-Mendoza et al. 2012). In addition, in a nationwide study in Taiwan (n = 98,198), clinically significant insomnia disorder was associated with an increased risk of acute coronary syndrome, and this relationship was especially robust in young adult men (Chung et al. 2013). However, not all studies have demonstrated a relationship between insomnia complaints and objective measures of hypertension (Vozoris 2013), and some have found only modest or no associations when insomnia complaints are reported in the absence of short sleep duration (Phillips et al. 2009, Phillips & Mannino 2007).
Short sleep duration alone appears to have a robust effect on cardiovascular disease risk, similar to the effects of short sleep duration on inflammation (Ayas et al. 2003, Hoevenaar-Blom et al. 2011, Mallon et al. 2002). In a meta-analysis of 6 prospective (n = 9,959) and 17 cross-sectional (n = 105,432) studies, short sleep duration was associated with an increased risk of prevalent hypertension and, particularly in adults younger than 65 years old, and increased risk of incident hypertension (Wang et al. 2012). In addition to effects on hypertension (Grandner & Perlis 2013), another meta-analysis reported that short sleep duration is associated with morbidity and mortality from coronary heart disease and stroke but not with total cardiovascular disease (Cappuccio et al. 2011). Those sleeping less than five hours appear to show the greatest risk for cardiovascular events (Sabanayagam et al. 2011), although even sleeping less than seven hours per night entailed increased risk of cardiovascular mortality (Heslop et al. 2002), with a linear relationship between sleep duration and prospective risk of cardiovascular disease (Chien et al. 2010) that indicates an optimal sleep duration between seven and eight hours.
Extremes of sleep may contribute to cardiovascular disease, similar to the effects of sleep extremes on inflammation. Indeed, both meta-analyses noted above (Cappuccio et al. 2011, Wang et al. 2012) found that long duration of sleep was also associated with prevalent hypertension (Wang et al. 2012) as well as a greater risk of other cardiovascular disease (Kronholm et al. 2011, Wang et al. 2012), with a U-shaped risk profile in both men and women (Cappuccio et al. 2011, Ikehara et al. 2009, Wang et al. 2012). One study found that extremes of sleep duration are more robustly associated with cardiovascular morbidity and mortality in women but not in men (Kronholm et al. 2011), whereas other data suggest that only long sleep duration is associated with cardiovascular disease risk (Suzuki et al. 2009).
Sleep Disturbance and Cancer
Inflammation is increasingly thought to have a prominent role in cancer incidence and cancer recurrence. Elevated levels of CRP and other markers of inflammation are prospectively associated with increases in mortality and site-specific cancer mortality for lung, colorectal, liver, and prostate (Ko et al. 2012), especially in men (Baune et al. 2011), with other data showing that inflammation is linked to breast cancer incidence (Touvier et al. 2013) and interacts with other factors (e.g., smoking) to increase lung cancer risk (Shiels et al. 2013, Siemes et al. 2006). In addition, inflammation appears to be a prognostic factor predicting breast cancer recurrence (Cole 2009, Pierce et al. 2009). Inflammatory responses are thought to be linked to 15–20% of all deaths from cancer worldwide (Mantovani et al. 2008), in part due to cancer-related activation of NF-κB and multiple inflammatory cytokines (such as IL-1β, IL-6, and TNF-α) (Karin 2006). As with cardiovascular disease, no prospective study has examined whether sleep disruption elevates inflammation to mediate the relationships between sleep and cancer as described below.
Although beyond the scope of this review, circadian rhythm disturbances (i.e., shift work) and co-occurring sleep disturbance are implicated in cancer risk (for a review, see Haus & Smolensky 2013). Shift work and related circadian disruption contribute to a epigenetic modification of circadian genes, which serve as transcriptional regulators that affect the expression of many cancer-related genes and participate in regulating cell division and DNA repair. In longtime night workers and shift workers, slightly to moderately increased risk for breast, prostate, colon, and endometrial epithelial malignancies plus non-Hodgkin’s lymphoma is found (Conlon et al. 2007; Kubo et al. 2006, 2011; Parent et al. 2012), although other studies have reported no association (Schwartzbaum et al. 2007). The International Agency for Research on Cancer has concluded that there is sufficient experimental evidence in animals to support the carcinogenicity of circadian disruption, with adequate evidence in humans to denote long-term shift work as a probable carcinogen in humans (Haus & Smolensky 2013).
Given the associations between shift work and recurrent sleep disturbance, the association between sleep disturbance and cancer risk is being explicitly tested. In the Age, Gene/Environment Susceptibility (AGES)-Reykjavik cohort (n = 2,102), men who had problems falling and staying asleep had a significantly increased risk of prostate cancer compared to men without such sleep problems (Sigurdardottir et al. 2013), with an even more robust association among those with advanced prostate cancer. However, no prospective association between sleep complaints or sleep duration and risk of incident breast cancer was found in the Women’s Health Initiative (n = 110,011) (Vogtmann et al. 2013).
Short sleep duration alone may contribute to cancer risk (von Ruesten et al. 2012). In the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study (n = 23,620), those who reported a sleep duration of <6 h had a significantly increased risk of cancer as compared with those who reported 7 h of sleep, similar to the increased risk of stroke and overall chronic diseases (von Ruesten et al. 2012). Yet other data suggest that extremes of sleep duration, not simply short sleep duration, are associated with cancer risk. For example, compared with those with 7 h of sleep, both short sleep (<5 h) and long sleep (>9 h) were associated with an increased risk of colorectal cancer in the 11.3-year follow-up of the Women’s Health Initiative observational study (n = 75,828 postmenopausal women) (Jiao et al. 2013), although one other large-scale prospective report found that only long sleep duration (>9 h) was associated with the risk of colorectal cancer in the Health Professionals Follow-Up Study (n = 30,121) and the Nurses’ Health Study (n = 76,368), with the risk being relatively more robust in men than in women (Zhang et al. 2013b).
Sleep Disturbance and Depression: Role of Inflammation
Inflammation plays a prominent role in depression, which may be instigated in part by sleep disturbance. Multiple links exist between inflammation and depression (for a review, see Slavich & Irwin 2014). First, in patients with an inflammatory disorder, depression comorbidity is high. Second, markers of inflammation are elevated in depressed compared to nondepressed individuals, with prospective findings showing that increases of CRP and IL-6 predict the occurrence of depression (Gimeno et al. 2009). Third, experimental activation of inflammation induces increases of depressive symptoms along with activation of brain sites that regulate positive and negative affect (Eisenberger et al. 2009, 2010a, b). Finally, antagonism of endogenous inflammation shows promise in reducing depressive symptoms and possibly in the remission of depression in groups of depressed patients with high levels of inflammation (Raison et al. 2013, Tyring et al. 2006).
Symptoms of insomnia, including difficulties initiating and maintaining sleep, commonly co-occur with depression. However, insomnia is not simply a symptom of depression but rather may have a role in predicting depression incidence. Ford & Kamerow (1989) were among the first to note such a relationship in a longitudinal epidemiologic study, and to date more than 40 studies have been published that have tested the role of insomnia in the onset of depression (for a review, see Riemann 2010), with meta-analytic findings showing that sleep disturbance yields a twofold increased risk of depression (Baglioni et al. 2011) or is one of the first clinical signs of a depressive disorder (Dryman & Eaton 1991). Persistent insomnia is particularly potent in increasing the risk of depression, with a 14-fold greater risk of depression in the year following insomnia that had persisted for up to one year (Lee et al. 2013). In addition, this association appears to be specific to those with a history of depression (Cho et al. 2008), although persistent insomnia was found to predict risk for depressive symptoms over six years even in those without a depression history (Jaussent et al. 2011). Together, these findings have strong clinical implications for the development of studies to prevent the development of depression by targeting sleep disturbance, and some research has already found that adding cognitive behaviorial therapy for insomnia to standard antidepressant treatment generates a more rapid and durable remission of depression than does standard treatment alone (Manber et al. 2008). Again, the prospective role of sleep disturbance in driving increases in inflammation that mediate the risk of depression is not known.
INFLAMMATORY REGULATION OF SLEEP
Whereas this review has primarily focused on the role of sleep disturbance in the regulation of immunity, the reciprocal links between the immune system and the brain (Irwin & Cole 2011), and their effects on sleep, also require consideration, although a detailed discussion of these mechanisms is beyond the scope of this review.
Extensive basic animal research has found that Th1 (i.e., IFN) and inflammatory cytokines are involved in the regulation of sleep; inflammatory cytokines increase amounts of NREM sleep, and specific antagonists to these cytokines decrease amounts of NREM sleep (Imeri & Opp 2009). In contrast, anti-inflammatory cytokines such as IL-4 and IL-10 act in the opposite direction and reduce the amount of NREM sleep (Imeri & Opp 2009).
However, findings in humans contrast with those in animal research, as some proinflammatory cytokines that promote NREM sleep in animals appear to suppress NREM sleep or sleep depth in humans. Acute administration of either IL-6 or IFN-α suppressed SWS during the early part of the night and reduced amounts of REM sleep (Spath-Schwalbe et al. 1998, 2000), whereas an acute dose of the T cell, IL-2, showed no effect on sleep amounts or measures of sleep architecture (Lange et al. 2002). When exposure to an inflammatory cytokine is chronic (i.e., IFN-alpha treatment), a similar suppression of SWS along with disturbances in sleep maintenance are found (Raison et al. 2010).
However, large and rapid increases in proinflammatory cytokine activity (i.e., single cytokine or endotoxin challenge) (Bauer et al. 1995, Mullington et al. 2000, Pollmacher et al. 2000) may not be a physiologically relevant signal for sleep regulation. To address this issue, cytokine antagonists that block physiologic levels of cytokines have been tested, and blockade of TNF has been found to induce a short-term normalization of REM sleep in alcohol-dependent patients with high levels of REM sleep (Irwin et al. 2009); the decrease in REM sleep correlated with pharmacologic neutralization of biologically active TNF. In addition, Zamarron et al. (2004) found that TNF blockade improved sleep continuity and increased sleep depth in patients with rheumatoid arthritis. Finally, TNF antagonism has been found to reduce daytime sleepiness in patients with sleep apnea (Vgontzas et al. 2004b) and possibly improves other depressive symptoms among those with high levels of inflammation (Raison et al. 2013).
SLEEP DISTURBANCE AND BEHAVIORAL CONTROL OF IMMUNITY
Several treatment options are available for patients with sleep disturbance and insomnia, including psychological and behavioral approaches (e.g., cognitive behavioral therapy for insomnia) and mind-body or relaxation-based therapies (e.g., tai chi and yoga) as well as various classes of medications. Here we focus on behavioral and mind-body interventions because of their potential to modulate certain aspects of the immune system, including adaptive and innate immune-response elements.
Among the various treatments for insomnia, cognitive behavioral therapies have a robust efficacy profile as compared with other approaches, including therapies that primarily target hyperarousal mechanisms using relaxation-based treatments (Morin et al. 2006a). Nevertheless, a number of studies have now demonstrated that these relaxation-based approaches (e.g., tai chi, yoga), which target stress-response pathways that are activated in association with insomnia, influence adaptive and innate immune responses and also improve insomnia symptoms. For example, tai chi has been found to improve insomnia complaints in older adults (Irwin et al. 2008a), augment vaccine responses to the herpes zoster virus (Irwin et al. 2007), and reduce inflammation (i.e., levels of IL-6) (Irwin & Olmstead 2012). Similarly, delivery of cognitive behavioral therapy in patients with rheumatoid arthritis improved depressive symptoms including insomnia complaints and reduced the stimulated production of the proinflammatory cytokine IL-6 (Zautra et al. 2008). More recent findings suggest that mindfulness-based meditation can reduce proinflammatory-response gene profiles (Creswell et al. 2012), and additional evidence indicates that yogic meditation reverses increased NF-κB-related transcription of proinflammatory cytokines and decreased interferon regulatory factor 1-related transcription of innate antiviral response genes (Black et al. 2013). Together, behavioral approaches might provide a strategy for redirecting the leukocyte transcriptome via the induction of multiple trans-acting transcription factors via β-adrenergic receptor signaling that is activated in association with life adversity and sleep loss.
Recently, we have focused on patients with insomnia complaints and tested whether behavioral interventions that target sleep complaints might reverse the activation of inflammatory pathways associates with insomnia. Along with inducing a remission of insomnia disorder, cognitive behavioral therapy was associated with decreases in CRP: Levels of this marker of inflammatory risk were 50% lower one year after treatment among those who showed insomnia remission (Irwin et al. 2014). Decreases in CRP following insomnia treatment were comparable to the benefits reported with vigorous physical activity (Ford 2002) or weight loss (Esposito et al. 2003). Similar findings are reported in a study of the effects of exercise training on sleep quality and inflammation in men on hemodialysis who had sleep problems (Afshar et al. 2011). Finally, although sedative hypnotic medications are widely used for the treatment of insomnia, no systematic studies have evaluated the effects of such medications on markers of inflammation, and no randomized controlled trials have assessed whether inflammation is altered in association with pharmacotherapy of insomnia.
PERSPECTIVES ON THE FUTURE
Important directions for future research include investigation of the environmental and personalized genomic inputs that contribute to the varying influence of sleep on immunity and inflammatory biological mechanisms and how distinct aspects of sleep map onto immunological signatures. With increased understanding of these inputs may come a better knowledge of the unique profile of sleep disturbance on infectious and inflammatory disease risk, the role of antiviral or proinflammatory immune response in mediating these respective adverse health outcomes, and the development of interventions that target sleep disturbance as one strategy to augment therapeutic control of chronic infectious, inflammatory, and neuropsychiatric diseases such as depression.
SUMMARY POINTS
Insomnia complaints are highly prevalent, occurring in nearly 25% of the US population, and adversely influence morbidity and mortality risk.
Sleep can be assessed objectively by polysomnography; other evaluations of sleep are provided by actigraphy, sleep diaries, and self-report questionnaires as well as clinician-based diagnosis of insomnia disorder.
Sleep influences the two primary effector systems, the hypothalamus-pituitary-adrenal axis and the sympathetic nervous system, which in turn regulate adaptive and innate immune responses.
Nocturnal sleep is associated with increases in adaptive and innate system activity (i.e., inflammation), which occur against the background of the strong circadian rhythm of cortisol and its suppressive effects on these aspects of the immune system, and a nocturnal decline in sympathetic outflow.
Sleep deprivation and naturalistic disturbance of sleep (i.e., short sleep duration or reduced sleep efficiency) impair adaptive immunity; this impairment is associated with reduced response to vaccines and increased susceptibility to infectious disease. Decreases in growth hormone during slow wave sleep and increases of sympathetic activation are two mechanisms implicated in the link between sleep disturbance and reduced antiviral immune responses.
Sleep loss, short sleep duration, and complaints of sleep disturbance are associated with increases in inflammation, which are thought to be due to the effects of sleep disruption on adrenergic signaling that steers inflammatory gene expression. Women appear to be more vulnerable to the effects of sleep disturbance on inflammatory dynamics, whereas men appear to be more at risk for cardiovascular disease and cancer, but not depression, in association with sleep disturbance, especially short sleep duration. However, prospective evidence that inflammation mediates the association between sleep disruption and inflammatory disorders, including depression, is not yet available.
Reciprocal links exist between the innate immune system and the brain, and pharmacologic blockade of physiologic levels of proinflammatory cytokine activity appears to normalize sleep continuity and REM sleep in clinical populations with either sleep disturbance or inflammation, or both.
Behavioral treatments that target stress response pathways or improve sleep have the potential to reduce systemic, cellular, and genomic markers of inflammation, with implications for cardiovascular or other inflammatory disease risk.
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abedelmalek S, Chtourou H, Aloui A, Aouichaoui C, Souissi N, Tabka Z. Effect of time of day and partial sleep deprivation on plasma concentrations of IL-6 during a short-term maximal performance. Eur J Appl Physiol. 2013;113:241–48. doi: 10.1007/s00421-012-2432-7. [DOI] [PubMed] [Google Scholar]
- Afshar R, Emany A, Saremi A, Shavandi N, Sanavi S. Effects of intradialytic aerobic training on sleep quality in hemodialysis patients. Iran J Kidney Dis. 2011;5:119–23. [PubMed] [Google Scholar]
- Am. Psychiatr. Assoc. Diagnostic and Statistical Manual of Mental Disorders. 5 Washington, DC: Am. Psychiatr. Publ; 2013. [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Andreakos E, Foxwell B, Feldmann M. Is targeting Toll-like receptors and their signaling pathway a useful therapeutic approach to modulating cytokine-driven inflammation? Immunol Rev. 2004;202:250–65. doi: 10.1111/j.0105-2896.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- Azevedo Da Silva M, Singh-Manoux A, Shipley MJ, Vahtera J, Brunner EJ, et al. Sleep duration and sleep disturbances partly explain the association between depressive symptoms and cardiovascular mortality: the Whitehall II cohort study. J Sleep Res. 2014;23:94–97. doi: 10.1111/jsr.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Bauer J, Hohagen F, Gimmel E, Bruns F, Lis S, et al. Induction of cytokine synthesis and fever suppresses REM sleep and improves mood in patients with major depression. Biol Psychiatry. 1995;38:611–21. doi: 10.1016/0006-3223(95)00374-x. [DOI] [PubMed] [Google Scholar]
- Baune BT, Rothermundt M, Ladwig KH, Meisinger C, Berger K. Systemic inflammation (interleukin 6) predicts all-cause mortality in men: results from a 9-year follow-up of the MEMO study. Age (Dordr) 2011;33:209–17. doi: 10.1007/s11357-010-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–37. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DS, Cole SW, Irwin MR, Breen E, St Cyr NM, et al. Yogic meditation reverses NF-κB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. 2013;38:348–55. doi: 10.1016/j.psyneuen.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–64. [PubMed] [Google Scholar]
- Boudreau P, Yeh WH, Dumont GA, Boivin DB. Circadian variation of heart rate variability across sleep stages. Sleep. 2013;36:1919–28. doi: 10.5665/sleep.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R, Pang G, Husband AJ, King MG. Suppression of immunity to influenza virus infection in the respiratory tract following sleep disturbance. Reg Immunol. 1989;2:321–25. [PubMed] [Google Scholar]
- Burgos I, Richter L, Klein T, Fiebich B, Feige B, et al. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. 2006;20:246–53. doi: 10.1016/j.bbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Buysse DJ. Sleep health: Can we define it? Does it matter? Sleep. 2014;37:9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- Chennaoui M, Sauvet F, Drogou C, Van Beers P, Langrume C, et al. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-α) levels in healthy men. Cytokine. 2011;56:318–24. doi: 10.1016/j.cyto.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Chien KL, Chen PC, Hsu HC, Su TC, Sung FC, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177–84. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YL, Chuang YF, Fang KC, Liu SK, Chen HY, et al. Higher systemic inflammation is associated with poorer sleep quality in stable haemodialysis patients. Nephrol Dial Transpl. 2009;24:247–51. doi: 10.1093/ndt/gfn439. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Lavretsky H, Olmstead R, Levin MJ, Oxman MN, Irwin MR. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165:1543–50. doi: 10.1176/appi.ajp.2008.07121882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Glaser R, Porter K, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Poorer self-rated health is associated with elevated inflammatory markers among older adults. Psychoneuroendocrin. 2011;36:1495–504. doi: 10.1016/j.psyneuen.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Lin CL, Chen YF, Chiang JY, Sung FC, et al. Sleep disorders and increased risk of subsequent acute coronary syndrome in individuals without sleep apnea: a nationwide population-based cohort study. Sleep. 2013;36:1963–68. doi: 10.5665/sleep.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169:62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh Sleep Quality Index in older adults. Sleep. 2006;29:112–16. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- Cole SW. Chronic inflammation and breast cancer recurrence. J Clin Oncol. 2009;27:3418–19. doi: 10.1200/JCO.2009.21.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo J, Takahashi R, Sloan EK, Lutgendorf S, et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci USA. 2010;107:5681–86. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J Immunol. 1998;161:610–16. [PubMed] [Google Scholar]
- Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18:182–83. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- Cover H, Irwin M. Immunity and depression: insomnia, retardation, and reduction of natural killer cell activity. J Behav Med. 1994;17:217–23. doi: 10.1007/BF01858106. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JM, et al. Mindfulness-based stress reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun. 2012;26:1095–101. doi: 10.1016/j.bbi.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zambotti M, Covassin N, De Min Tona G, Sarlo M, Stegagno L. Sleep onset and cardiovascular activity in primary insomnia. J Sleep Res. 2011;20:318–25. doi: 10.1111/j.1365-2869.2010.00871.x. [DOI] [PubMed] [Google Scholar]
- Dew MA, Hoch CC, Buysse DJ, Monk TH, Begely AE, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Benedict C, Nowell MA, Jones SA, et al. Sleep enhances IL-6 trans-signaling in humans. FASEB J. 2006;20:2174–76. doi: 10.1096/fj.06-5754fje. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Tieken S, Fehm HL, Born J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav Immun. 2004;18:341–48. doi: 10.1016/j.bbi.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Douglas SD, Hamarman S, Zaugg L, Kapoor S. Sleep deprivation and human immune function. Adv Neuroimmunol. 1995;5:97–110. doi: 10.1016/0960-5428(95)00002-j. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–39. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol. 2011;21:799–806. doi: 10.1016/j.annepidem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryman A, Eaton WW. Affective symptoms associated with the onset of major depression in the community: findings from the US National Institute of Mental Health Epidemiologic Catchment Area Program. Acta Psychiatr Scand. 1991;84:1–5. doi: 10.1111/j.1600-0447.1991.tb01410.x. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010a;68:748–54. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010b;24:558–63. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–90. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erten Y, Kokturk O, Yuksel A, Elbeg S, Ciftci TU, et al. Relationship between sleep complaints and proinflammatory cytokines in haemodialysis patients. Nephrology (Carlton) 2005;10:330–35. doi: 10.1111/j.1440-1797.2005.00418.x. [DOI] [PubMed] [Google Scholar]
- Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- Faraut B, Boudjeltia KZ, Dyzma M, Rousseau A, David E, et al. Benefits of napping and an extended duration of recovery sleep on alertness and immune cells after acute sleep restriction. Brain Behav Immun. 2011;25:16–24. doi: 10.1016/j.bbi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60:929–35. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S adults. Epidemiology. 2002;13:561–68. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–57. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Friedman EM. Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci USA. 2005;102:18757–62. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Hayney MS, Love GD, Urry HL, Rosenkranz MA, et al. Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci USA. 2005;102:18757–62. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Malaspina D, Posner K, Babiss LA, Heymsfield SB, et al. Insomnia and sleep duration as mediators of the relationship between depression and hypertension incidence. Am J Hypertens. 2010;23:62–69. doi: 10.1038/ajh.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39:413–23. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandner MA, Perlis ML. Short sleep duration and insomnia associated with hypertension incidence. Hypertens Res. 2013;36:932–3. doi: 10.1038/hr.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus EL, Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013;17:273–84. doi: 10.1016/j.smrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Heslop P, Smith GD, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: the effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Med. 2002;3:305–14. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34:1487–92. doi: 10.5665/sleep.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S, Iso H, Date C, Kikuchi S, Watanabe Y, et al. Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep. 2009;32:295–301. doi: 10.1093/sleep/32.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M, Mascovich A, Gillin JC, Willoughby R, Pike J, Smith TL. Partial sleep deprivation reduces natural killer cell activity in humans. Psychosom Med. 1994;56:493–98. doi: 10.1097/00006842-199411000-00004. [DOI] [PubMed] [Google Scholar]
- Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10:643–53. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, Ehlers C. Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain Behav Immun. 2004;18:349–60. doi: 10.1016/j.bbi.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Irwin M, Thompson J, Miller C, Gillin JC, Ziegler M. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrin Metab. 1999;84:1979–85. doi: 10.1210/jcem.84.6.5788. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24:54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006a;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–32. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Levin MJ, Laudenslager ML, Olmstead R, Lucko A, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56:1085–93. doi: 10.1093/cid/cis1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–83. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults: a randomized controlled trial of tai chi chih. Am J Geriatr Psychiatry. 2012;20:764–72. doi: 10.1097/JGP.0b013e3182330fd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Breen EC, et al. Cognitive behavioral therapy versus tai chi for late life insomnia and inflammation: a randomized controlled comparative efficacy trial. Sleep. 2014 doi: 10.5665/sleep.4008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Motivala SJ. Improving sleep quality in older adults with moderate sleep complaints: a randomized controlled trial of tai chi chih. Sleep. 2008a;31:1001–8. [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: a randomized, controlled trial of tai chi. J Am Geriatr Soc. 2007;55:511–17. doi: 10.1111/j.1532-5415.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Valladares EM, Breen EC, Ehlers CL. Tumor necrosis factor antagonism normalizes rapid eye movement sleep in alcohol dependence. Biol Psychiatry. 2009;66:191–95. doi: 10.1016/j.biopsych.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006b;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008b;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Ziegler M. Sleep deprivation potentiates activation of cardiovascular and catecholamine responses in abstinent alcoholics. Hypertension. 2005;45:252–57. doi: 10.1161/01.HYP.0000153517.44295.07. [DOI] [PubMed] [Google Scholar]
- Jaussent I, Bouyer J, Ancelin ML, Akbaraly T, Peres K, et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. 2011;34:1103–10. doi: 10.5665/SLEEP.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao L, Duan Z, Sangi-Haghpeykar H, Hale L, White DL, El-Serag HB. Sleep duration and incidence of colorectal cancer in postmenopausal women. Br J Cancer. 2013;108:213–21. doi: 10.1038/bjc.2012.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joynt KE, Whellan DJ, O’Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry. 2003;54:248–61. doi: 10.1016/s0006-3223(03)00568-7. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–36. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Ko YJ, Kwon YM, Kim KH, Choi HC, Chun SH, et al. High-sensitivity C-reactive protein levels and cancer mortality. Cancer Epidemiol Biomarkers Prev. 2012;21:2076–86. doi: 10.1158/1055-9965.EPI-12-0611. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–36. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- Kronfol Z, Nair M, Zhang Q, Hill EE, Brown MB. Circadian immune measures in healthy volunteers: relationship to hypothalamic-pituitary-adrenal axis hormones and sympathetic neurotransmitters. Psychosom Med. 1997;59:42–50. doi: 10.1097/00006842-199701000-00006. [DOI] [PubMed] [Google Scholar]
- Kronholm E, Laatikainen T, Peltonen M, Sippola R, Partonen T. Self-reported sleep duration, all-cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. 2011;12:215–21. doi: 10.1016/j.sleep.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Kubo T, Oyama I, Nakamura T, Shirane K, Otsuka H, et al. Retrospective cohort study of the risk of obesity among shift workers: findings from the Industry-Based Shift Workers’ Health study, Japan. Occup Environ Med. 2011;68:327–31. doi: 10.1136/oem.2009.054445. [DOI] [PubMed] [Google Scholar]
- Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164:549–55. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- Lanfranchi PA, Pennestri MH, Fradette L, Dumont M, Morin CM, Montplaisir J. Nighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep. 2009;32:760–66. doi: 10.1093/sleep/32.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Bollinger T, Diekelmann S, Born J. Sleep after vaccination boosts immunological memory. J Immunol. 2011;187:283–90. doi: 10.4049/jimmunol.1100015. [DOI] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Fehm HL, Born J. Sleep-like concentrations of growth hormone and cortisol modulate type1 and type2 in-vitro cytokine production in human T cells. Int Immunopharmacol. 2006a;6:216–25. doi: 10.1016/j.intimp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Fehm HL, Westermann J, Born J. Shift of monocyte function toward cellular immunity during sleep. Arch Intern Med. 2006b;166:1695–700. doi: 10.1001/archinte.166.16.1695. [DOI] [PubMed] [Google Scholar]
- Lange T, Marshall L, Spath-Schwalbe E, Fehm HL, Born J. Systemic immune parameters and sleep after ultra-low dose administration of IL-2 in healthy men. Brain Behav Immun. 2002;16:663–74. doi: 10.1016/s0889-1591(02)00018-1. [DOI] [PubMed] [Google Scholar]
- Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65:831–35. doi: 10.1097/01.psy.0000091382.61178.f1. [DOI] [PubMed] [Google Scholar]
- Larkin EK, Rosen CL, Kirchner HL, Storfer-Isser A, Emancipator JL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–84. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- Laugsand LE, Vatten LJ, Bjorngaard JH, Hveem K, Janszky I. Insomnia and high-sensitivity C-reactive protein: the HUNT study, Norway. Psychosom Med. 2012;74:543–53. doi: 10.1097/PSY.0b013e31825904eb. [DOI] [PubMed] [Google Scholar]
- LeBlanc M, Merette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32:1027–37. doi: 10.1093/sleep/32.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Cho HJ, Olmstead R, Levin MJ, Oxman MN, Irwin MR. Persistent sleep disturbance: a risk factor for recurrent depression in community-dwelling older adults. Sleep. 2013;36:1685–91. doi: 10.5665/sleep.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim SJ, Jung HH. Nocturnal sleep related with metabolic markers in end-stage renal disease patients receiving hemodialysis. Psychiatry Invest. 2009;6:34–38. doi: 10.4306/pi.2009.6.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridket PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Rovelli F, Brivio F, Brivio O, Fumagalli L. Circadian secretions of IL-2, IL-12, IL-6 and IL-10 in relation to the light/dark rhythm of the pineal hormone melatonin in healthy humans. Nat Immun. 1998;16:1–5. doi: 10.1159/000069464. [DOI] [PubMed] [Google Scholar]
- Littner M, Hirshkowitz M, Kramer M, Kapen S, Anderson WM, et al. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26:754–60. doi: 10.1093/sleep/26.6.754. [DOI] [PubMed] [Google Scholar]
- Liukkonen T, Rasanen P, Ruokonen A, Laitinen J, Jokelainen J, et al. C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 Birth Cohort study. Psychosom Med. 2007;69:756–61. doi: 10.1097/PSY.0b013e318157cb96. [DOI] [PubMed] [Google Scholar]
- Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–16. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–90. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res. 2013;36:985–95. doi: 10.1038/hr.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Pressman S, Barkin A, Rabin BS, Treanor JJ. Psychological stress and antibody response to influenza vaccination: When is the critical period for stress, and how does it get inside the body? Psychosom Med. 2004;66:215–23. doi: 10.1097/01.psy.0000116718.54414.9e. [DOI] [PubMed] [Google Scholar]
- Miller MA, Kandala NB, Kivimaki M, Kumari M, Brunner EJ, et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009;32:857–64. [PMC free article] [PubMed] [Google Scholar]
- Moldofsky H, Lue FA, Davidson JR, Gorczynski R. Effects of sleep deprivation on human immune functions. FASEB J. 1989;3:1972–77. doi: 10.1096/fasebj.3.8.2785942. [DOI] [PubMed] [Google Scholar]
- Morin CM, Belanger L, LeBlanc M, Ivers H, Savard J, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Belanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998–2004) Sleep. 2006a;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006b;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Motivala S, Irwin MR. Sleep and immunity: cytokine pathways linking sleep and health outcomes. Curr Dir Psychol Sci. 2007;16:21–25. [Google Scholar]
- Mullington J, Korth C, Hermann DM, Orth A, Galanos C, et al. Dose-dependent effects of endotoxin on human sleep. Am J Physiol Regul Integr Comp Physiol. 2000;278:R947–55. doi: 10.1152/ajpregu.2000.278.4.R947. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K. Janeway’s Immunobiology. New York: Garland Sci; 2011. [Google Scholar]
- Murray DR, Irwin M, Rearden CA, Ziegler M, Motulsky H, Maisel AS. Sympathetic and immune interactions during dynamic exercise: mediation via beta 2-adrenergic-dependent mechanism. Circulation. 1992;83:203–13. doi: 10.1161/01.cir.86.1.203. [DOI] [PubMed] [Google Scholar]
- Ohayon M. Epidemiological study on insomnia in the general population. Sleep. 1996;19:S7–15. doi: 10.1093/sleep/19.suppl_3.s7. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Riemann D, Morin C, Reynolds CF., 3rd Hierarchy of insomnia criteria based on daytime consequences. Sleep Med. 2012;13:52–57. doi: 10.1016/j.sleep.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain Behav Immun. 2009;23:351–54. doi: 10.1016/j.bbi.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Coussons-Read ME. Sleep disruption during pregnancy: How does it influence serum cytokines? J Reprod Immunol. 2007;73:158–65. doi: 10.1016/j.jri.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Okun ML, Hall M, Coussons-Read ME. Sleep disturbances increase interleukin-6 production during pregnancy: implications for pregnancy complications. Reprod Sci. 2007;14:560–67. doi: 10.1177/1933719107307647. [DOI] [PubMed] [Google Scholar]
- Palagini L, Bruno RM, Gemignani A, Baglioni C, Ghiadoni L, Riemann D. Sleep loss and hypertension: a systematic review. Curr Pharm Des. 2013;19:2409–19. doi: 10.2174/1381612811319130009. [DOI] [PubMed] [Google Scholar]
- Parent ME, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012;176:751–59. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- Patel SR, Malhotra A, Gao X, Hu FB, Neuman MI, Fawzi WW. A prospective study of sleep duration and pneumonia risk in women. Sleep. 2012;35:97–101. doi: 10.5665/sleep.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Zhu X, Storfer-Isser A, Mehra R, Jenny NS, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32:200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky N, Harrison LC. Diurnal rhythmicity of human cytokine production. J Immunol. 1997;158:5163–68. [PubMed] [Google Scholar]
- Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10:307–12. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- Phillips B, Bůzková P, Enright P Cardiovasc. Health Study Res. Group. Insomnia did not predict incident hypertension in older adults in the cardiovascular health study. Sleep. 2009;32:65–72. [PMC free article] [PubMed] [Google Scholar]
- Phillips B, Mannino DM. Do insomnia complaints cause hypertension or cardiovascular disease? J Clin Sleep Med. 2007;3:489–94. [PMC free article] [PubMed] [Google Scholar]
- Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–44. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmacher T, Schuld A, Kraus T, Haack M, Hinze-Selch D, Mullington J. Experimental immunomodulation, sleep, and sleepiness in humans. Ann N Y Acad Sci. 2000;917:488–99. doi: 10.1111/j.1749-6632.2000.tb05413.x. [DOI] [PubMed] [Google Scholar]
- Prather AA, Hall M, Fury JM, Ross DC, Muldoon MF, et al. Sleep and antibody response to hepatitis B vaccination. Sleep. 2012;35:1063–69. doi: 10.5665/sleep.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychol. 2005;24:297–306. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rye DB, Woolwine BJ, Vogt GJ, Bautista BM, et al. Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiatry. 2010;68:942–49. doi: 10.1016/j.biopsych.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramey SL, Perkhounkova Y, Moon M, Budde L, Tseng HC, Clark MK. The effect of work shift and sleep duration on various aspects of police officers’ health. Workplace Health Saf. 2012;60:215–22. doi: 10.1177/216507991206000505. [DOI] [PubMed] [Google Scholar]
- Razeghi E, Sahraian MA, Heidari R, Bagherzadeh M. Association of inflammatory biomarkers with sleep disorders in hemodialysis patients. Acta Neurol Belg. 2012;112:45–49. doi: 10.1007/s13760-012-0003-7. [DOI] [PubMed] [Google Scholar]
- Redwine L, Dang J, Hall M, Irwin M. Disordered sleep, nocturnal cytokines, and immunity in alcoholics. Psychosom Med. 2003;65:75–85. doi: 10.1097/01.psy.0000038943.33335.d2. [DOI] [PubMed] [Google Scholar]
- Redwine L, Hauger RL, Gillin JC, Irwin M. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85:3597–603. doi: 10.1210/jcem.85.10.6871. [DOI] [PubMed] [Google Scholar]
- Reid S, Dwyer J. Insomnia in HIV infection: a systematic review of prevalence, correlates, and management. Psychosom Med. 2005;67:260–69. doi: 10.1097/01.psy.0000151771.46127.df. [DOI] [PubMed] [Google Scholar]
- Renegar KB, Crouse D, Floyd RA, Krueger J. Progression of influenza viral infection through the murine respiratory tract: the protective role of sleep deprivation. Sleep. 2000;23:859–63. [PubMed] [Google Scholar]
- Renegar KB, Floyd R, Krueger JM. Effect of sleep deprivation on serum influenza-specific IgG. Sleep. 1998a;21:19–24. [PubMed] [Google Scholar]
- Renegar KB, Floyd RA, Krueger JM. Effects of short-term sleep deprivation on murine immunity to influenza virus in young adult and senescent mice. Sleep. 1998b;21:241–48. [PubMed] [Google Scholar]
- Rief W, Mills PJ, Ancoli-Israel S, Ziegler MG, Pung MA, Dimsdale JE. Overnight changes of immune parameters and catecholamines are associated with mood and stress. Psychosom Med. 2010;72:755–62. doi: 10.1097/PSY.0b013e3181f367e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann D. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:17. doi: 10.1016/j.smrv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Sabanayagam C, Shankar A, Buchwald D, Goins RT. Insomnia symptoms and cardiovascular disease among older American Indians: the Native Elder Care Study. J Environ Public Health. 2011;2011:964617. doi: 10.1155/2011/964617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M, Shimoda Y, Maesawa C, Akatsu T, Ishikawa Y, et al. Activated toll-like receptor 4 in monocytes is associated with heart failure after acute myocardial infarction. Int J Cardiol. 2005;109:226–34. doi: 10.1016/j.ijcard.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol. 2010;108:68–75. doi: 10.1152/japplphysiol.00851.2009. [DOI] [PubMed] [Google Scholar]
- Savard J, Laroche L, Simard S, Ivers H, Morin CM. Chronic insomnia and immune functioning. Psychosom Med. 2003;65:211–21. doi: 10.1097/01.psy.0000033126.22740.f3. [DOI] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Lehnert H, et al. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2011;34:371–77. doi: 10.1093/sleep/34.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbaum J, Ahlbom A, Feychting M. Cohort study of cancer risk among male and female shift workers. Scand J Work Environ Health. 2007;33:336–43. doi: 10.5271/sjweh.1150. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, et al. Soluble TNF-α receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–70. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst. 2013;105:1871–80. doi: 10.1093/jnci/djt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemes C, Visser LE, Coebergh JW, Splinter TA, Witteman JC, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24:5216–22. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- Sigurdardottir LG, Valdimarsdottir UA, Mucci LA, Fall K, Rider JR, et al. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:872–79. doi: 10.1158/1055-9965.EPI-12-1227-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014 doi: 10.1037/a0035302. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–7. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, et al. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998;83:1573–79. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E, Lange T, Perras B, Fehm HL, Born J. Interferon-α acutely impairs sleep in healthy humans. Cytokine. 2000;12:518–21. doi: 10.1006/cyto.1999.0587. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288:1471–72. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137:95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenholm S, Kronholm E, Bandinelli S, Guralnik JM, Ferrucci L. Self-reported sleep duration and time in bed as predictors of physical function decline: results from the InCHIANTI study. Sleep. 2011;34:1583–93. doi: 10.5665/sleep.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun. 2008;22:960–68. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suka M, Yoshida K, Sugimori H. Persistent insomnia is a predictor of hypertension in Japanese male workers. J Occup Health. 2003;45:344–50. doi: 10.1539/joh.45.344. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Yorifuji T, Ueshima K, Takao S, Sugiyama M, et al. Sleep duration, sleep quality and cardiovascular disease mortality among the elderly: a population-based cohort study. Prev Med. 2009;49:135–41. doi: 10.1016/j.ypmed.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of serum C-reactive protein (CRP)---no association with sleep duration or sleep disordered breathing. Sleep. 2007;30:991–96. doi: 10.1093/sleep/30.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveras EM, Rifas-Shiman SL, Rich-Edwards JW, Mantzoros CS. Maternal short sleep duration is associated with increased levels of inflammatory markers at 3 years postpartum. Metabolism. 2011;60:982–86. doi: 10.1016/j.metabol.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth LA. Sleep, sleep deprivation and infectious disease: studies in animals. Adv Neuroimmunol. 1995;5:79–92. doi: 10.1016/0960-5428(94)00045-p. [DOI] [PubMed] [Google Scholar]
- Touvier M, Fezeu L, Ahluwalia N, Julia C, Charnaux N, et al. Association between prediagnostic biomarkers of inflammation and endothelial function and cancer risk: a nested case-control study. Am J Epidemiol. 2013;177:3–13. doi: 10.1093/aje/kws359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- van Leeuwen WMA, Lehto M, Karisola P, Lindholm H, Luukkonen R, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS ONE. 2009;4:e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mark A, Weiler SW, Schröder M, Otto A, Jauch-Chara K, et al. The impact of shift work induced chronic circadian disruption on IL-6 and TNF-α immune responses. J Occup Med Toxicol. 2010;5:18. doi: 10.1186/1745-6673-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–54. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–97. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–7. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bixler EO, et al. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007;292:E253–61. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004a;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-α antagonist. J Clin Endocrinol Metab. 2004b;89:4409–13. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002;51:887–92. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- Vogtmann E, Levitan EB, Hale L, Shikany JM, Shah NA, et al. Association between sleep and breast cancer incidence among postmenopausal women in the women’s health initiative. Sleep. 2013;36:1437–44. doi: 10.5665/sleep.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ruesten A, Weikert C, Fietze I, Boeing H. Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. PLoS ONE. 2012;7:e30972. doi: 10.1371/journal.pone.0030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozoris NT. The relationship between insomnia symptoms and hypertension using United States population-level data. J Hypertens. 2013;31:663–71. doi: 10.1097/HJH.0b013e32835ed5d0. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta-analysis. Hypertens Res. 2012;35:1012–18. doi: 10.1038/hr.2012.91. [DOI] [PubMed] [Google Scholar]
- Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;8:159–74. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Zamarron F, Maceiras F, Mera A, Gomez-Reino JJ. Effects of the first infliximab infusion on sleep and alertness in patients with active rheumatoid arthritis. Ann Rheum Dis. 2004;63:88–90. doi: 10.1136/ard.2003.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra AJ, Davis MC, Reich JW, Nicassio P, Tennen H, et al. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J Consult Clin Psychol. 2008;76:408–21. doi: 10.1037/0022-006X.76.3.408. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lamers F, Hickie IB, He JP, Feig E, Merikangas KR. Differentiating nonrestorative sleep from nocturnal insomnia symptoms: demographic, clinical, inflammatory, and functional correlates. Sleep. 2013a;36:671–79. doi: 10.5665/sleep.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Giovannucci EL, Wu K, Gao X, Hu F, et al. Associations of self-reported sleep duration and snoring with colorectal cancer risk in men and women. Sleep. 2013b;36:681–88. doi: 10.5665/sleep.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]