Abstract
Fibrillin proteins are the major components of extracellular microfibrils found in many connective tissues. Fibrillin-1 and fibrillin-2 are well studied and mutations in these proteins cause a number of fibrillinopathies including Marfan syndrome and congenital contractural arachnodactyly, respectively. Fibrillin-3 was more recently discovered and is much less well characterized. Fibrillin-1 is expressed throughout life, whereas fibrillin-2 and -3 are thought to be primarily present during development. Here, we report detailed fibrillin-3 expression patterns in early human development.
A polyclonal antiserum against a C-terminal recombinant half of human fibrillin-3 was produced in rabbit. Anti-fibrillin-3 antibodies were affinity-purified and antibodies cross-reacting with the other fibrillins were removed by absorption resulting in specific anti-fibrillin-3 antibodies. Immunohistochemical analyses with these purified antibodies demonstrate that fibrillin-3 is temporally expressed in numerous tissues relatively evenly from the 6th to the 12th gestational week. Fibrillin-3 was found spatially expressed in perichondrium, perineurium, perimysium, skin, developing bronchi, glomeruli, pancreas, kidney, heart and testis and at the prospective basement membranes in developing epithelia and endothelia. Double immunohistochemical analyses showed that all fibrillins are globally expressed in the same organs, with a number of differences on the tissue level in cartilage, perichondrium and developing bronchi. These results suggest that fibrillin-3, compared to the other fibrillins, fulfills both overlapping and distinct functions in human development.
Keywords: Fibrillin, microfibrils, development, connective tissue, immunohistochemistry, basement membranes
INTRODUCTION
The extracellular matrix is a crucial player in development, not solely by acting as a scaffold for developing organs but also by providing cells with instructive cues (Rozario and DeSimone, 2010). One family of extracellular matrix proteins essential for development is represented by the fibrillins (Hubmacher et al., 2006). Fibrillins are large glycoproteins which assemble into higher order extended structures termed microfibrils (Kielty et al., 2005). Microfibrils are found associated with elastic fibers or they can remain devoid of elastin and are then frequently associated with basement membranes (Low, 1962; Ross and Bornstein, 1969). They fulfill a wide range of physiological functions from providing a scaffold for elastic fiber assembly (Mecham and Davis, 1994; Carta et al., 2006), to acting as stress-bearing entities for example in the ciliary zonules of the eye (Raviola, 1971), to serving as reservoirs and regulators for growth factors of the TGF-β superfamily (for reviews see Hubmacher et al., 2006; Ramirez and Rifkin, 2009; Nistala et al., 2010). These functional aspects of microfibrils are critical for proper development documented by the clinical symptoms found in microfibrillopathies and the phenotypes of several mouse models (Hubmacher et al., 2006; Robinson et al., 2006).
Fibrillin-1, -2 and -3 constitute the fibrillin family in higher vertebrates and fibrillin orthologs were identified in various invertebrates along the evolutionary tree. Expression of fibrillin-1 and -2 and their respective orthologs was studied in the development of lower and higher vertebrates including frog, zebrafish, chick, mouse and men (for reviews see Hubmacher et al., 2006; Ramirez and Dietz, 2009). Expression of the respective fibrillin genes is generally confined to mesenchymal derivatives and the onset of gene expression correlates with the beginning of gastrulation (Skoglund and Keller, 2007; Gansner et al., 2008). In mouse development, fibrillin-1 and -2 are present in most tissues in a diphasic expression pattern, with the onset of fibrillin-2 expression taking place earlier than fibrillin-1, except for the cardiovascular system (Zhang et al., 1995). In human, both fibrillins are expressed in most tissues as early as the 5th gestational week (GW)1, but it is not established whether fibrillin-2 expression also precedes fibrillin-1 expression (Zhang et al., 1994; Quondamatteo et al., 2002). However, it is now well established that mammalian fibrillin-2 expression phases out soon after birth, whereas fibrillin-1 expression remains active throughout adult life. In embryonic tissue, both fibrillins have a similar spatio-temporal expression patterns and are present in the same organ systems with some differences observed in kidney, liver, rib anlagen and notochord (Quondamatteo et al., 2002).
Fibrillin-1 and -2 play different as well as overlapping roles in vertebrate development. Mice lacking the fibrillin-2 gene, for example, are characterized by hindlimb syndactyly which is not observed in fibrillin-1 null mice despite similar expression patterns in developing autopods (Arteaga-Solis et al., 2001; Chaudhry et al., 2001; Miller et al., 2010). Ablation of fibrillin-1 in mice leads to impaired maturation and stability of the aortic wall resulting in dissecting aneurysm and neonatal death (Carta et al., 2006). Contrary, ablation of fibrillin-2 has no impact on the aortic wall architecture (Arteaga-Solis et al., 2001; Carta et al., 2006). However, mice doubly homozygous null for fibrillin-1 and -2 die in utero earlier than the single homozygous null mice with a poorly developed aortic media suggesting functional cooperation of both fibrillins in the development of the aortic matrix (Carta et al., 2006). The absence of fibrillin-1 in lung development leads to failure of distal alveolar septation mediated by elevated TGF-β signaling (Neptune et al., 2003). Treatment with fibrillin-2 antisense oligonucleotides induced dysmorphogenesis of rat lung explants (Yang et al., 1999). It remains to be established whether the roles of fibrillin-1 and -2 in lung development are overlapping or distinct. Further evidence for overlapping as well as distinct functions of fibrillin-1 and -2 in development stems from their involvement in genetic disorders. Mutations in fibrillin-1 lead to the Marfan syndrome (MFS) characterized by cardinal symptoms in the cardiovascular, skeletal and ocular systems including progressive aortic root enlargement, dolichostenomelia, scoliosis and ectopia lentis (Robinson et al., 2006). Mutations in fibrillin-2 on the other hand give rise to congenital contractural arachnodactyly (CCA) with some overlapping skeletal features with MFS (Frederic et al., 2009). In contrast to MFS, individuals with CCA are characterized by joint contractures and abnormally shaped ears, whereas cardiovascular and ocular manifestations are usually absent (Viljoen, 1994).
The third fibrillin family member, fibrillin-3, was relatively recently discovered and has not been extensively studied. The cDNA coding for fibrillin-3 has first been isolated from human fetal brain (Nagase et al., 2001). Corson and coworkers subsequently found that fibrillin-3, similar to fibrillin-2, is mainly expressed during embryonic development (Corson et al., 2004). Interestingly, the fibrillin-3 gene is not expressed in rodents, although it is expressed in many other organisms including primates, cow, sheep, dog, swine, chick, zebrafish and others (Corson et al., 2004). Based on a small set of analyses using indirect immunofluorescence labeling, the fibrillin-3 protein is expressed in some human, chick and bovine connective tissues including fetal lung, kidney, skin, muscle and perichondrium (Corson et al., 2004).
On the functional level, fibrillin-3 shares some similarities with the other fibrillin isoforms. The C-terminal half of fibrillin-3 multimerizes and strongly interacts with fibronectin, similar to the C-terminal halves of fibrillin-1 and -2 (Sabatier et al., 2009). Ultrastructural immunolocalization demonstrated association of fibrillin-3 with microfibrils in the perichondrium both in the presence and in the absence of elastic fibers demonstrating that the functional entity of fibrillin-3 is the 10–12 nm in diameter microfibril (Corson et al., 2004).
The gene for fibrillin-3, located on chromosome 19, was originally suggested as a candidate gene for recessive Weill-Marchesani syndrome (Corson et al., 2004). However, subsequently mutations leading to recessive Weill-Marchesani syndrome were identified in ADAMTS10 (Dagoneau et al., 2004). Linkage and immunohistochemical analyses strongly suggests a role for fibrillin-3 in the pathogenesis of polycystic ovary syndrome (Urbanek et al., 2005; Stewart et al., 2006; Ewens et al., 2010; Jordan et al., 2010). Functional single nucleotide polymorphism analysis, however, argued against this possibility (Prodoehl et al., 2009).
It is clear that fibrillin-3, like fibrillin-2, is a developmentally expressed isoform of the fibrillin family, however, it remains to be established whether fibrillin-3 represents a redundant protein or whether it confers specific, yet-unknown functions in human development. In this study, we address the spatio-temporal expression pattern of fibrillin-3 in human embryos and fetuses between the 6–12th GW by single immunohistochemistry. We further correlate fibrillin-3 expression with the expression of fibrillin-1 and -2 using double immunohistochemistry staining. We observed that fibrillin-3 expression was widely distributed in connective tissue in a temporal manner similar to fibrillin-1 and -2. Fibrillin-3 is frequently associated with basement membranes in various tissues and its expression does not always colocalize with other fibrillin isoforms.
RESULTS
Antibody specificity
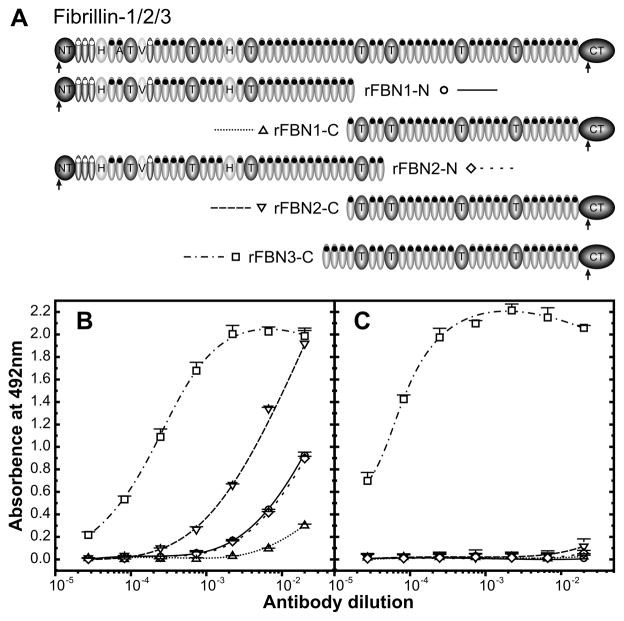
The C-terminal half of human fibrillin-3 was previously recombinantly expressed and purified (Sabatier et al., 2009) (Fig. 1A). A polyclonal antiserum against rFBN3-C was produced in rabbit and characterized by ELISA (Fig. 1B). The antiserum has a strong titer against rFBN3-C with half maximal binding observed at an antiserum dilution of ~1:5,000. The antiserum strongly cross-reacted with the C-terminal half of fibrillin-2 and to a much lesser extend the C-terminal half of fibrillin-1, indicating more shared antibody epitopes on the C-terminal half of fibrillin-3 and -2 compared to shared epitopes between fibrillin-3 and -1. Additional cross-reactivity of anti-rFBN3-C was observed with the N-terminal halves of fibrillin-1 and -2. To obtain antibodies monospecific for fibrillin-3, a two step procedure was employed. In the first step, cross-reacting antibodies were first removed from the antiserum by affinity absorption with the C- terminal half of fibrillin-2. In the second step, the anti-rFBN3-C antibodies were affinity purified with rFBN3-C protein (see Experimental Procedures; Fig. 1A). ELISA testing of the titers after this procedure demonstrated that the resulting antibodies only reacted with fibrillin-3 and not with fibrillin-1 or -2 (Fig. 1C). These specific antibodies were used to study the fibrillin-3 expression in human embryos and fetuses.
Figure 1. Antibody specificity.
A: Overview of previously produced recombinant halves of fibrillin-1, -2 and -3. B: Shown is an ELISA demonstrating the reactivity of untreated anti-rFBN3-C to the rFBN3-C fragment and its cross-reactivity with fibrillin-1 and fibrillin-2 N- and C-terminal halves. Serial dilutions of anti-rFBN3-C were added to immobilized rFBN1-N (circles), rFBN1-C (upward-pointing triangles), rFBN2-N (rhombuses), rFBN2-C (downward-pointing triangles) and rFBN3-C (squares). C: The ELISA as shown in panel B was performed after pre-clearing and affinity-purification of anti-rFBN3-C as detailed in Experimental Procedures. Note that antibodies cross-reacting with fibrillin-1 and -2 were completely removed, whereas the reactivity to rFBN3-C was fully maintained.
Single staining negative controls were performed using the pre-immune serum under the same conditions as anti-rFBN3-C. Only non-specific staining of collapsed chondrocytes was observed. No staining was detected in any other tissues examined (Supplemental Fig. 1).
Fibrillin-3 expression in human embryos
The global expression pattern of fibrillin-3 in embryonic and early fetal development is summarized in Table 1. On the whole organ level, fibrillin-3 is expressed continuously from the 6th to 12th GW in the connective tissue of every organ except in liver. The spatial expression patterns of fibrillin-3 and comparison to expression patterns for fibrillin-1 and -2 are described in the following paragraphs.
Table 1.
Summary of global fibrillin-3 expression in the human embryo and early fetus.
| GW (embryos analyzed)
|
6th (n = 3) | 8th (n = 6) | 9th (n = 5) | 10th (n = 10) | 11th (n = 3) | 12th (n = 6) |
|---|---|---|---|---|---|---|
| Organ anlage | ||||||
| Skin | + | + | + | + | + | + |
| Central nervous system | + | + | + | + | n/a | + |
| Peripheral nervous system | + | + | + | + | + | + |
| Heart | + | + | + | + | n/a | n/a |
| Blood vessels | + | + | + | + | n/a | + |
| Liver | n/a | − | − | − | − | − |
| Lung | + | + | + | + | + | + |
| Kidney | + | + | + | + | + | + |
| Pancreas | n/a | + | n/a | + | + | n/a |
| Axial skeleton | + | + | + | + | + | + |
| Ribs and perichondrium | + | + | + | + | + | + |
| Muscles | n/a | + | n/a | + | n/a | + |
| Pancreas | n/a | + | n/a | + | + | n/a |
+ = expressed; − = not expressed; n/a = tissues not available
Fibrillin-3 expression in basement membrane regions and connective tissues of various organs
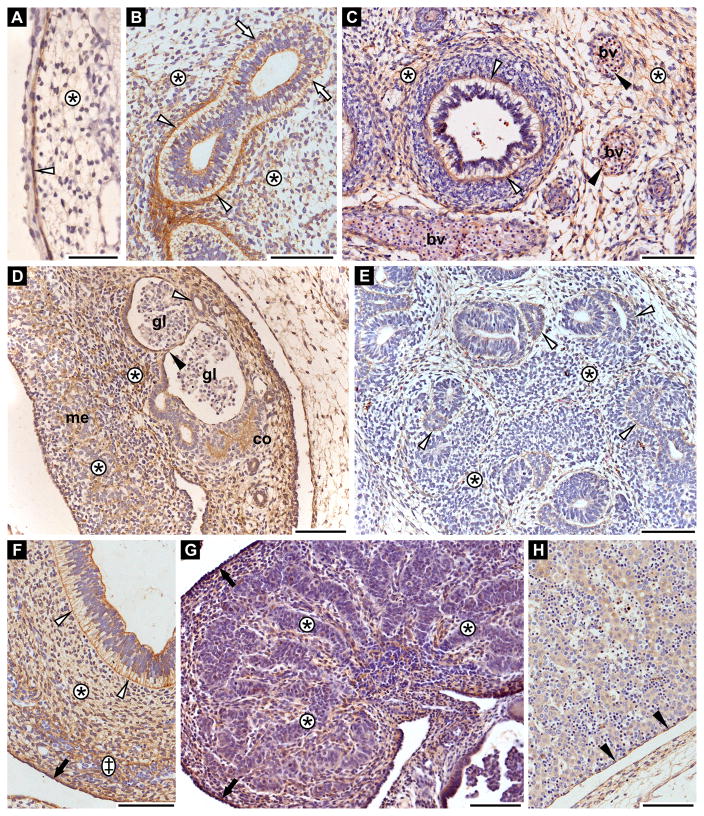
Fibrillin-3 is present during developing skin as early as 6th GW. The protein is strongly expressed in the basement membrane region of the periderm as well as in thin fibers throughout the dermis (Fig. 2A).
Figure 2. Fibrillin-3 expression in basement membrane regions of various epithelia.
Shown are immunohistochemical stainings for fibrillin-3. A: Skin anlage (6th GW). Dermis (asterisk); epidermal-dermal junction (white arrowhead). B: Lung anlage (8th GW). Lung stroma (asterisks) with strong staining (white arrowheads) and weaker staining (white arrows) in sub-epithelial region of bronchi anlagen. C: Lung anlage (11th GW). Lung stroma (asterisks); basement membrane region of terminal bronchi (white arrowheads); basement membrane of blood vessels (bv) (black arrowheads). D: Kidney anlage (8th GW). Stroma (asterisks); cortex (co); medulla (me); basement membrane region of the parietal layer of Bowman’s capsule (black arrowhead); glomeruli (gl); cortical tubules (white arrowhead). E: Pancreas anlage (8th GW). Stroma (asterisks); basement membrane of exocrine acini (white arrowheads). F: Gut anlage (8th GW). Basement membrane of gut epithelium (white arrowheads); mucosa (asterisk); submucosa (double cross); serosa (black arrow). G: Testis anlage (12th GW). Testis cords (asterisks); tunica albuginea (black arrows). H: Liver anlage (8th GW). Glisson’s capsule (black arrowheads). The diffuse, pale intracellular staining of hepatocytes is non-specific as it was not constantly observed. Scale bars = 100 μm.
Fibrillin-3 is also present in the lung stroma (Fig. 2B and C). At 8th GW, it is expressed in the sub-epithelial region of bronchi anlagen but with various intensities which could reflect different developmental stages (Fig. 2B). It remains present in the basement membrane region of airways even at 11th GW (i.e. in terminal bronchi) as well as in the lung parenchyma surrounding bronchi (Fig. 2C). Fibrillin-3 was also observed in endothelial basement membranes of pulmonary blood vessels (Fig. 2C).
In the kidney, fibrillin-3 is expressed in the stroma of the cortex and the medulla. Additionally, it is present in the basement membrane region of the parietal layer of the Bowman’s capsule and in the cortical tubules (i.e. 8th GW, Fig. 2D).
In pancreas, fibrillin-3 expression was detected in the stroma as well as in the basement membrane of anlagen of the exocrine acini (i.e. 8th GW, Fig. 2E). Furthermore, fibrillin-3 is expressed in the basement membrane region of the gastrointestinal epithelium, throughout the mucosa and submucosa, and in the serosa (Fig. 2F).
Fibrillin-3 is present in the interstitial tissue between the testis cords and in the tunica albuginea (i.e. 12th GW, Fig. 2G). However, it is not expressed in the liver excluding the Glisson’s capsule (Fig. 2H).
Fibrillin-3 expression in the cardiovascular system
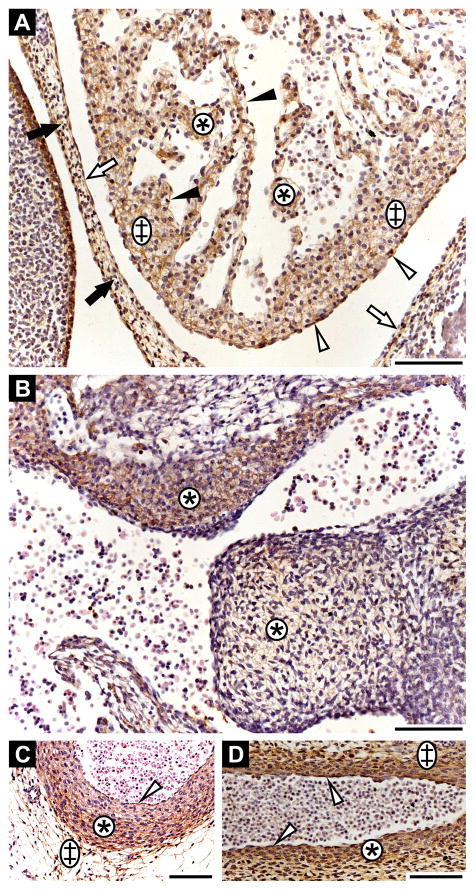
Fibrillin-3 staining was detected in the heart as early as 6th GW and up to 10th GW (heart was not available for 11–12th GW). It is expressed in fibrous pericardium and in serous pericardium, with especially strong staining in the visceral layer of the serous pericardium (Fig. 3A). It is also present in the spongy and the mantle layer of the myocardium, as well as in the endothelium. Fibrillin-3 is also strongly expressed in the endocardial cushion (Fig. 3B; 6th GW). Fibrillin-3 is expressed throughout blood vessel walls, as seen in cross-section at 8th GW (Fig. 3C) and in longitudinal section at 6th GW (Fig. 3D). It is present in the tunica intima, tunica media and tunica adventitia (Fig. 3C, D). However, differences in staining intensities are observed in the tunica media of the vessel wall (Fig. 3C). The inner tunica media demonstrates lighter fibrillin-3 staining compared to the outer tunica media. Using Hart’s staining for elastic fibers, we observed that the weaker fibrillin-3 staining in tunica media correlates with robust elastic fiber formation, whereas the most intense fibrillin-3 staining is seen where no elastic fibers are present (Supplemental Fig. 2).
Figure 3. Fibrillin-3 expression in the cardiovascular system.
A: Heart anlage (6th GW). Fibrous pericardium (black arrows); parietal layer of serous pericardium (white arrows); visceral layer of serous pericardium (white arrowheads); spongy layer (asterisks); mantle layer of myocardium (double crosses); endothelium (black arrowheads). Note that the strong staining on the left originates from periderm. B: Endocardial cushion (6th GW; asterisks). C: Descending aorta at the level of the tracheal bifurcation in cross-section (8th GW). Tunica intima (white arrowhead); tunica media (asterisk); tunica adventitia (double cross). D: Descending aorta in longitudinal section (6th GW). Tunica intima (white arrowheads); tunica media (asterisk); tunica adventitia (double cross). Scale bars = 100 μm.
Fibrillin-3 expression in bone, cartilage and muscles
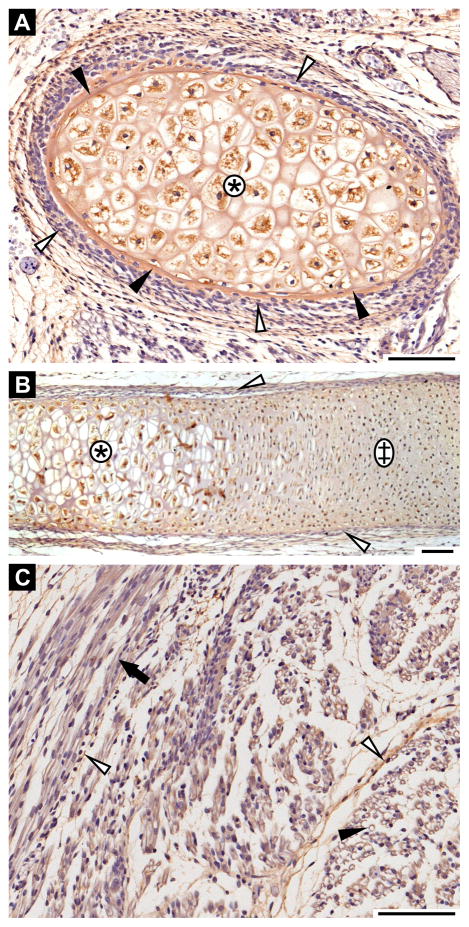
Fibrillin-3 is expressed in the interstitial matrix of hypertrophic cartilage of ribs, i.e. at 11th GW (Fig. 4A, B). It is also present in bony collar of the ribs (Fig. 4A) and in the perichondrium (Fig. 4A, B). Fibrillin-3 is present throughout endochondral ossification of the ribs, as it was also faintly detected in hyaline cartilaginous matrix of rib anlage (Fig. 4B).
Figure 4. Fibrillin-3 expression in bone, cartilage and muscle.
A and B: Rib anlagen in cross-section (A) and in longitudinal section (B) (11th GW). Hypertrophic cartilage (asterisks); hyaline cartilage (double cross); bony collar (black arrowheads); perichondrium (white arrowheads). C: Myotubules in cross-section (black arrowhead) and in longitudinal section (black arrow) (11th GW). Perimysium (white arrowheads). Scale bars = 100 μm.
Fibrillin-3 is not expressed in the myotubule anlagen (i.e. 11th GW). However, it is expressed in the surrounding perimysium (Fig. 4C).
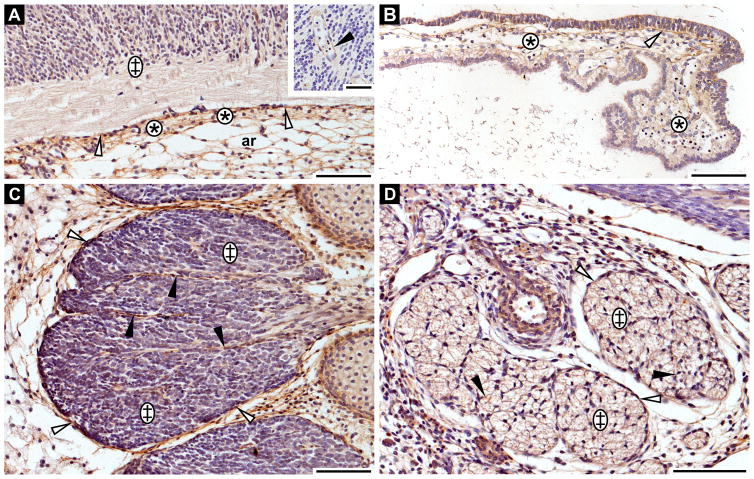
Fibrillin-3 expression in nervous tissues
Fibrillin-3 staining was not detected in the nervous parenchyma of the forebrain. However, it is expressed in the surrounding meningeal connective tissue. Here, staining was observed in the arachnoid layer and even stronger in the pia mater (Fig. 5A). Fibrillin-3 is also present in the capillary walls of the nervous parenchyma of the central nervous system (Fig. 5A, insert). The protein is expressed in the basement membrane region of the choroid ependymal cells as well as in the connective tissue of the choroid plexus at 8th GW (Fig. 5B).
Figure 5. Fibrillin-3 expression in the central and peripheral nervous system.
A: Forebrain anlage (8th GW). Nervous tissue (double cross); pia mater (asterisks); arachnoid layer (ar); external limiting membrane (white arrowhead). Insert: Capillary walls in nervous parenchyma (black arrowhead). B: Choroid plexus (8th GW). Basement membrane of ependymal cells (white arrowhead); connective tissue (asterisks). C: Spinal ganglions (8th GW). Epineurium (white arrowheads); perineurium (black arrowheads); nervous parenchyma (double crosses). D: Peripheral nerve (10th GW). Epineurium (white arrowheads); perineurium (black arrowheads); nervous parenchyma (double crosses). Scale bars = 100 μm.
Fibrillin-3 is not solely found in the connective tissue of the central nervous system, but also found in the connective tissue of the peripheral nervous system. For example, it was detected in the epineurium and the perineurium of spinal ganglions throughout the period studied (i.e. 8th GW, Fig. 5C) and of peripheral nerve (i.e. 10th GW, Fig. 5D). However, as for the central nervous system, it is not expressed in the nervous parenchyma of ganglions (Fig. 5C), nor in nerve fascicles (Fig. 5D).
Comparison of fibrillin expression patterns
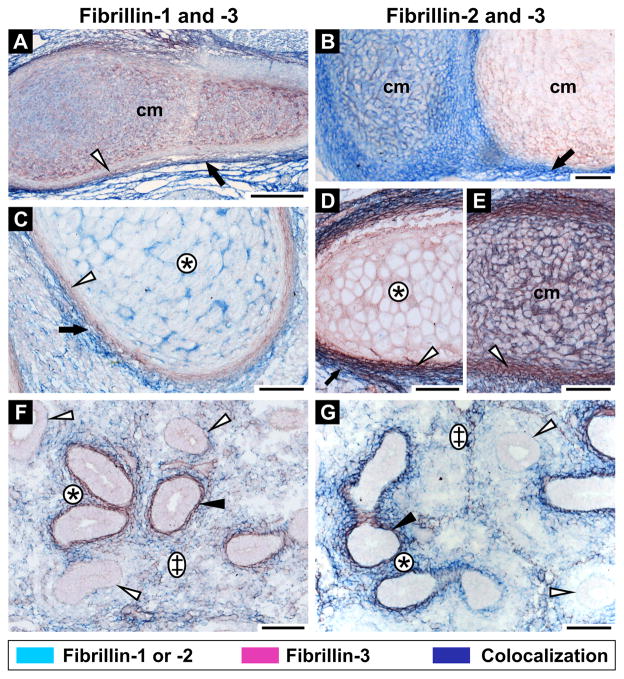
To study differences in fibrillin isoform expression patterns, double immunohistochemistry was performed. The fibrillins are generally expressed in the same organs, although we did observe differences in fibrillin expression patterns in some structures. For example, at 12th GW, all three fibrillins are expressed in cartilage but with striking differences in tissue distribution. Fibrillin-1 and -3 colocalize in the hyaline cartilage matrix (Fig. 6A). Both proteins are also present in hypertrophic cartilage matrix albeit not co-localized (compare Figs. 6C and D). On the other hand, fibrillin-2 is not expressed in hypertrophic cartilage (Fig. 6D). However, fibrillin-2 can be found in hyaline cartilaginous matrix in various co-expression patterns with fibrillin-3. It can be either present together with weakly expressed fibrillin-3 (Fig. 6B) or with strongly expressed fibrillin-3 (Fig. 6E). In other sections of hyaline cartilage, fibrillin-3 is expressed alone, whereas fibrillin-2 is absent (Fig. 6B). All fibrillins are expressed in perichondrium. Nonetheless, fibrillin-3 is primarily found in the inner chondrogenic layer (Fig. 6A–E), whereas fibrillin-1 (Fig. 6A, C) and fibrillin-2 (Fig. 6B, D, E) are mainly expressed in the outer fibrous layer. The proteins partially colocalize where both layers meet.
Figure 6. Differential expression of fibrillins in human embryos.
Fibrillin-3 is stained in red together with either fibrillin-1 (left panel; A, C, F) or fibrillin-2 (right panel; B, D, E, G) in blue. Colocalization results in a dark purple staining. A: Hyaline cartilage from limb (12th GW), fibrillin-1 and -3 staining. Colocalization is observed in the cartilage matrix (cm). Fibrillin-1 is expressed in the outer fibrous layer of the perichondrium (black arrow), whereas fibrillin-3 is localized in the inner chondrogenic layer (white arrowhead). B: Hyaline rib cartilage (12th GW), fibrillin-2 and -3 staining. Fibrillin-3 is expressed alone in some cartilage matrices (cm) or with fibrillin-2 in others. Fibrillin-2 is expressed in the perichondrium (black arrow). C: Rib anlage (12th GW), fibrillin-1 and -3 staining. Fibrillin-1 is expressed alone in hypertrophic cartilage (asterisk) and is present in the fibrous layer of the perichondrium (black arrow). Fibrillin-3 is expressed in the chondrogenic layer of the perichondrium (white arrowhead). D, E: Rib anlagen (12th GW), fibrillin-2 and -3 staining. Fibrillin-3 is solely expressed in the hypertrophic matrix (asterisk). Fibrillin-2 and -3 are colocalized in the hyaline cartilage matrix (cm). Fibrillin-2 is present in the fibrous perichondrium (black arrow) and fibrillin-3 in the chondrogenic perichondrium (white arrowheads). Both proteins partially colocalize at the junction between both perichondrial layers. F: Lung anlage (12th GW), fibrillin-1 and -3 staining. Partial colocalization is found in lung stroma (double cross). Fibrillin-3 is found in basement membranes (black arrowhead) and fibrillin-1 is present in sub-epithelial regions (asterisk) of the bronchial anlagen. In some bronchi anlagen, no fibrillin staining is evident (white arrowheads). G: Lung anlage (12th GW), fibrillin-2 and -3 staining. Partial colocalization was observed in lung stroma (double cross). Fibrillin-2 is present alone in sub-epithelial regions of the bronchial anlagen (asterisk). Colocalization of fibrillin-2 and -3 was identified in the epithelial-mesenchymal interface (black arrowhead). Some bronchi anlagen did not stain for fibrillin-2 or -3 (white arrowheads). Scale bars = 100 μm.
At 12th GW, all three fibrillins partially colocalize in the stroma of the lung anlagen (Fig. 6F, G). Fibrillin-3 is strongly expressed in the basement membrane region of some developing bronchial anlagen (Fig. 6F, G), whereas fibrillin-1 is mainly found in the sub-epithelial region of the same anlagen (Fig. 6F). Interestingly in this tissue, the fibrillin-2 expression pattern is similar to both, fibrillin-1 and -3 (Fig. 6F and G). It colocalized partially with fibrillin-3 in the stroma as well as in the epithelial-mesenchymal interface and is expressed without fibrillin-3 in the sub-epithelial region of some developing bronchial anlagen where fibrillin-1 is expressed. Moreover, early developing bronchi do not show any fibrillin staining in their lamina propria, and all fibrillins are expressed around more differentiated bronchi (Fig. 6F, G).
DISCUSSION
It has been previously established that fibrillin-3, similar to fibrillin-2, is primarily expressed in developing fetal tissues with only low levels expressed in postnatal tissues (Corson et al., 2004). The goal of this study was to establish high resolution expression patterns of fibrillin-3 in human embryonic and early fetal tissues and to compare its expression with that of other fibrillins.
Fibrillin proteins are highly homologous to each other. On the primary amino acid sequence level, fibrillin-3 shares 61% homology with fibrillin-1 and 68% with fibrillin-2. A rabbit antiserum against the C-terminal half of fibrillin-3 generated for this study had a relatively high cross-reactivity with the C-terminal half of fibrillin-2 and a much lower cross-reactivity with the C-terminal half of fibrillin-1. The data indicate that the structural similarity, represented by surface epitope availability, is much higher between fibrillin-3 and -2 compared to the similarity between fibrillin-3 and -1. This differential epitope recognition was much more pronounced than expected from the similarities between the primary sequences. Antibodies reacting with fibrillin-1 and -2 were effectively removed using C-terminal fibrillin-2 and -3 affinity matrices, resulting in highly purified specific antibodies for fibrillin-3.
These antibodies were used for immunohistochemical labeling of fibrillin-3 either in single or in double immunohistochemical experiments together with other fibrillin isoforms in human embryos and fetuses between the 6th and 12th GW. We observed that fibrillin-3 expression was temporally and spatially expressed widely in connective tissues of many organs correlating frequently with the developmental expression of fibrillin-1 and -2. However, fibrillin-3 expression is frequently associated with developing basement membranes and differential expression patterns were observed on the tissue level in cartilage, perichondrium and developing bronchi.
A large sequencing project first identified the coding sequence for fibrillin-3 in a human brain cDNA library (Nagase et al., 2001). Subsequently, Corson et al. reported strong expression of fibrillin-3 in human fetal brain based on RT-PCR and Northern blotting (Corson et al., 2004). The immunohistochemical analysis of the human developing brain in the present study did not demonstrate fibrillin-3 expression in the nervous parenchyma of the forebrain, although the hindbrain was not present in our sections. However, the protein was strongly expressed in meningeal connective tissues as well as in blood vessels present in the nervous parenchyma. Both studies mentioned above used RNA from total brain extracts. Based on our observations, we conclude that the strong expression levels in brain observed in those studies originated from fibrillin-3 expression in meningeal connective tissue and supporting vasculature.
In previous indirect immunofluorescence analyses, fibrillin-3 expression was not evident in blood vessels, lung stroma, cartilage and basement membranes of kidney tubules (Corson et al., 2004). This is in contrast to our immunohistochemical analyses where we observed fibrillin-3 expression in each of those tissues. It has recently been demonstrated that fibrillin-2 epitopes are masked in postnatal microfibrils which can be revealed by enzymatic digestion (Charbonneau et al., 2010). In addition, that study showed that fibrillin-2 epitopes are accessible in early developmental stages. The following aspects may thus explain the discrepancies between the studies. We used an epitope retrieval protocol in our analyses (see Experimental Procedures), which may have allowed the detection of masked fibrillin-3 epitopes in the tissues. Masking of epitopes might be especially sensitive to detection by monoclonal antibodies which were used in the previous study (Corson et al., 2004). This possibility is less likely with specific polyclonal antibodies since they target more than one epitope. Corson and coworkers investigated fibrillin-3 expression in lung, blood vessels and kidney at later stages of development (20–21st GW) compared to the developmental stages in the present study (6–12th GW). Fibrillin-3 epitope masking might occur during development similar to the epitope masking in fibrillin-2 (Charbonneau et al., 2010). Alternatively, fibrillin-3 is not expressed at later developmental stages.
Globally, fibrillin-3 is expressed in the same organs as fibrillin-1 and -2 except for liver, which stained negative for fibrillin-3 in the present study and negative for fibrillin-2 but positive for fibrillin-1 in another study (Quondamatteo et al., 2002). However, we observed differential fibrillin expression patterns in some tissues including developing cartilage and lung airways. In perichondrium, fibrillin-3 is expressed in the chondrogenic layer, whereas fibrillin-1 and -2 are present in the fibrous layer (Fig. 6A–E). Fibrillin containing microfibrils in the perichondrium and periosteum may serve a mechanical function to restrain growth of long bones, which may be affected in genetic disorders resulting from mutations in fibrillins including Marfan syndrome and congenital contractural arachnodactyly. Long bone overgrowth is a common symptom seen in these disorders (Pyeritz and Dietz, 2002). Alternatively, dysregulated growth factor activities may be involved in pathological mechanisms resulting in long bone overgrowth. It is now well established that fibrillins and microfibrils are involved in signaling of TGF-β and potentially bone morphogenetic proteins (Ramirez and Rifkin, 2009; Nistala et al., 2010). Since fibrillin-3 is the only fibrillin found in the chondrogenic layer of the perichondrium, we propose that it may play a distinct role compared to fibrillin-1 and -2 in bone growth and development and that it may be involved in chondrocyte differentiation through modulation of growth factors.
We also observed different expression patterns between the three fibrillins in cartilage. The fact that fibrillin-3 is found expressed in the hyaline cartilaginous matrix, either together with fibrillin-2 (Fig. 6B, E) or without fibrillin-2 (Fig. 6B, D), likely represents differential developmental stages of this tissue. Further studies are required to distinguish between the two theoretical possibilities that either fibrillin-3 expression occurs earlier followed by fibrillin-2 expression, or that both fibrillins are expressed simultaneously in early cartilage before fibrillin-2 disappears. Quondamatteo et al. did not observe any fibrillin-2 staining in cartilage matrix in their study (Quondamatteo et al., 2002). It is again possible that the fibrillin-2 epitopes were selectively retrieved by our antigen unmasking technique. Fibrillin-3 expression also appears to be temporally regulated in rib hypertrophic cartilage. When the cells in the rib cartilaginous matrix appear to be in early stages of becoming hypertrophic, fibrillin-3 expression was not detected (Fig. 6C). However, when the chondrocytes are fully hypertrophic, fibrillin-3 was strongly expressed (Fig. 6D). Quondamatteo et al. never observed fibrillin-1 or -2 staining in hypertrophic cartilage matrices. We observed fibrillin-1 staining in early steps of cartilage hypertrophy (Fig. 6C) but only faint or no staining in fully hypertrophic cartilage (data not shown). Therefore, fibrillin-3 appears to be the main fibrillin present during cartilage hypertrophy, supporting the hypothesis that fibrillin-3 has a distinct function in cartilage development compared to other fibrillins.
In the lung, we observed expression of all three fibrillins around some airways, whereas other airways in the same section were negative for fibrillins (Fig. 6F, G). Our interpretation is that the latter are likely newly developed airways which do not yet express fibrillins. We observed the same characteristic differential staining in single immunohistochemical analyses where fibrillin-3 was strongly expressed around some airways but only weakly around others (Fig. 2B). We also observed differential spatial expression patterns in fibrillin-positive airways in lung. Fibrillin-3 was found strongly expressed at the basement membrane of the epithelial-mesenchymal interface, whereas fibrillin-1 was present in the sub-epithelial region further away from the basement membrane (Figs. 2B, 6G and F). The fibrillin-2 expression pattern overlaps with both, fibrillin-1 and -3. Based on this spatial distribution, we speculate distinct functions for fibrillin-1 and -3 and a potential redundant function for fibrillin-2 in human lung airway development. For fibrillin-1 and -2, morphogenetic roles emerged in lung development including branching morphogenesis and alveolar septation (Yang et al., 1999; Neptune et al., 2003). The precise role of fibrillin-3 in lung development remains to be established. Our immunohistochemical localization of fibrillin-3 expression in lung provides a solid basis for the development of new and testable hypotheses.
In summary, we provide a high resolution map for the distribution of fibrillin-3 in human embryonic and early fetal development. Fibrillin-3 is expressed abundantly together with other fibrillins in many tissues, and in some tissues it is differentially expressed. Provided the differential expression patterns in cartilage and lung, we speculate that potential mutations in human fibrillin-3 may lead to pathological manifestations in these tissues. Fibrillin-3 is frequently associated with developing basement membranes. These fibrillin-3 expression patterns together with its high conservation in human and other species suggest important functions in species where fibrillin-3 is expressed.
EXPERIMENTAL PROCEDURES
Proteins and Antibodies
N- and C-terminal halves of human fibrillin-1 (rFBN1-N, rFBN1-C) and fibrillin-2 (rFBN2-N, rFBN2-C) and the C-terminal half of human fibrillin-3 (rFBN3-C) were recombinantly expressed in human 293 cells and purified to homogeneity by metal ion chelating chromatography as described previously (Jensen et al., 2001; Lin et al., 2002; Sabatier et al., 2009). An overview of these fragments is presented in Fig. 1A.
A polyclonal antiserum (anti-rFBN3-C) was produced in rabbit against recombinant rFBN3-C using standard immunization procedures (McGill University Animal Resource Centre). To assess cross-reactivity of anti-rFBN3-C with fibrillin-1 and -2, the antiserum titers for the recombinant halves of fibrillin-1 and -2 were tested by standard enzyme-linked immunosorbent assays (ELISA) using 10 μg/ml coating concentrations. The antiserum was further purified by affinity chromatography using an Äkta Purifier system (GE Healthcare). Two affinity resins were generated: Recombinant rFBN2-C (25 mg) and rFBN3-C (14 mg) were individually coupled to 2.5–3.0 ml cyanogen bromide-activated Sepharose 4B (GE Healthcare) as instructed by the manufacturer and packed in 8 ml chromatography columns. Anti-rFBN3-C was loaded under low flow (0.1 ml/min) onto the rFBN2-C affinity column equilibrated in 50 mM Tris-HCl, 150 mM NaCl, pH 7.4 (TBS) including 2 mM CaCl2. The flow through was collected and loaded on the rFBN3-C column under identical conditions. Bound antibodies were eluted by 0.1 M glycine, pH 3.0 at 0.5 ml/min. The eluate was dialyzed against TBS and concentrated by ultrafiltration (Centriplus YM-30; Millipore) to reach an antibody concentration of 1.61 mg/ml determined by bicinchoninic acid protein assay (Thermo Scientific). The concentrated antibodies were retested by ELISA for reactivity with fibrillin-3 and for the removal efficiency of cross-reacting antibodies. The purified antibodies were used for immunohistochemical staining to detect fibrillin-3 in embryonic and fetal human tissues.
The mouse monoclonal anti-fibrillin-1 (mAb F2) and anti-fibrillin-2 (mAb 143) antibodies were generously provided by Dr. Lynn Sakai (Shriners Hospital for Children, Portland, OR). These antibodies have been characterized in detail elsewhere (Sakai et al., 1986; Charbonneau et al., 2003; Charbonneau et al., 2010).
Tissue preparation
Tissues from 33 human embryos and fetuses between the 6th and 12th GW were obtained from legal abortion procedures according to the guidelines of the Ethics Committee of the Faculty of Medicine of the University of Göttingen, Germany (approval #31/5/90/Sch). The number of specimen used for each gestational stage is indicated in Table 1. Clinical data on the gestational age are known and the crown-rump length was measured when possible. To further ensure reliable developmental age determinations of embryos and fetuses, the tissues were compared to developmental standards (O’Rahilly and Müller, 1987). No abnormalities or signs of autolytic processes were observed in the studied tissues. 33 embryos were used for single and 7 of them for double staining experiments. Tissues were fixed for 24h at 4°C in 4% phosphate-buffered paraformaldehyde. Fixation was followed by dehydration in an ascending ethanol series (60–100%), embedding in paraffin and cutting of 5 μm sections using a Leica 2035 microtome.
Immunohistochemical and histochemical staining procedures
To evaluate the tissue histology, sections were deparaffinized, hydrated and stained by a standard haematoxilin-eosin protocol. The sections were then dehydrated, cover-slipped and examined on an Axioskop 2 microscope using the Axiovision software version 4.6 (Zeiss).
Single immunostaining for fibrillin-3 was performed as described previously with minor modifications (Quondamatteo et al., 2002). In brief, tissue sections were deparaffinized in xylene, rehydrated in a descending ethanol series (100–60%) and washed for 10 min with TBS. To block the endogenous peroxidase activity, sections were incubated in 0.32% (v/v) hydrogen peroxide in methanol for 45 min in the dark at 22°C. Sections were rinsed with TBS before and between each subsequent treatment with ProTaqs I Antigen-Enhancer (Quartett, Berlin, Germany) for 20 min at 60 °C and with 10 μg/ml of protease XXIV (Sigma) for 5 min at 22°C. The sections were further incubated with primary anti-rFBN3-C antibody diluted 1:50 in 1% (w/v) bovine serum albumin (BSA; Fisher Scientific) in TBS for 1 h at 37°C in a humid chamber followed by a TBS wash. To detect bound antibodies, indirect immunoperoxidase staining was performed. Sections were incubated with swine-anti-rabbit immunoglobulins (Dako, Hamburg, Germany) 1:50 diluted in 1% BSA (w/v) in TBS followed by incubation with rabbit peroxidase anti-peroxidase complex (Dako, Hamburg, Germany) 1:150 diluted in 1% BSA (w/v) in TBS for 30 min at 22°C. Color reactions were developed with 0.69 mM 3,3′-Diaminobenzidine tetrahydrochloride hydrate (Sigma), 3.7 mM imidazole (Serva), 0.005% H2O2 (Merck) in 100 mM Tris-HCl, pH 7.4 for 5 min at 22°C in the dark. The sections were rinsed in TBS before cell nuclei were counter-stained with haematoxilin for 6 s at 22°C. Sections were washed with water, dehydrated, cover-slipped and microscopically examined as described above.
Colocalization of fibrillin-3 with either fibrillin-1 or fibrillin-2 was performed with the MultiVision polymer detection system including MultiVision anti-rabbit/horse radish peroxidase + anti-mouse/alkaline phosphatase polymers (Thermo Scientific; product TL-012-MARH) according to the manufacturer’s protocol with three modifications described in the following. In brief, the sections were deparaffinized in xylene, rehydrated in graded ethanol (100–30%) and washed twice for 5 min in TBST washing buffer (TBS including 0.05% Tween-20). An antigen retrieval step was performed in 10 mM sodium citrate, 0.05% Tween 20, pH 6.0 for 20 min at 98°C followed by cooling to 22°C for 20 min. The slides were then washed four times for 5 min with distilled water, incubated with the “Hydrogen Peroxidase Block” for 10 min and washed again four times for 5 min. Treatment with protease XXIV was performed as described above for single labeling, followed by washes with TBST. The “Ultra V block” was applied for 5 min and washed once. Sections were incubated overnight at 4°C with anti-rFBN3-C antibody diluted 1:50 in 1% BSA in TBS followed by washes with TBST. The sections were then incubated for 2 h at 37°C with either monoclonal anti-fibrillin-1 (mAb F2) or anti-fibrillin-2 (mAb 143) antibodies diluted 1:100 in 1% BSA in TBS. After washing, sections were developed with “LVBlue Solution” for 10 min, washed and developed with “LVRed Solution” for 10 min. The slides were washed four times with distilled water for 5 min, dried for 1 h at 22°C and cover-slipped. Light microscopic analysis and image recording were performed as described above.
To stain elastic fibers by Hart’s staining, sections were deparaffinized in CitriSolv (Fisher Scientific), rehydrated in a descending ethanol series (100–30%) and washed with water. Sections were immersed for 1.75 h in Hart’s resorcin-fuchsin solution, washed with water and then counter-stained with 6.7 mM Metanil Yellow dye for 1min 45s. The sections were again washed with water and dehydrated with 95% and 100% ethanol for 1 min each. After treatment with CitriSolv for 10 min, the sections were mounted with cover slips.
Supplementary Material
Acknowledgments
We thank Dr. Lynn Sakai (Shriners Hospital for Children, Portland, OR) for generously providing the F2 and the 143 monoclonal antibodies. This work was supported by the Canadian Institutes of Health Research grants MOP-68836, MOP-106494 and IMH-102821 and the Natural Sciences and Engineering Research Council of Canada grant RGPIN 375738-09.
Footnotes
ABBREVIATIONS: BSA, bovine serum albumin; CCA, congenital contractural arachnodactyly; ELISA, enzyme-linked immunosorbent assay; GW, gestational week; MFS, Marfan syndrome; TBS, Tris-buffered saline
References
- Arteaga-Solis E, Gayraud B, Lee SY, Shum L, Sakai LY, Ramirez F. Regulation of limb patterning by extracellular microfibrils. J Cell Biol. 2001;154:275–281. doi: 10.1083/jcb.200105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta L, Pereira L, Arteaga-Solis E, Lee-Arteaga SY, Lenart B, Starcher B, Merkel CA, Sukoyan M, Kerkis A, Hazeki N, Keene DR, Sakai LY, Ramirez F. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J Biol Chem. 2006;281:8016–8023. doi: 10.1074/jbc.M511599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau NL, Dzamba BJ, Ono RN, Keene DR, Corson GM, Reinhardt DP, Sakai LY. Fibrillins can co-assemble in fibrils, but fibrillin fibril composition displays cell-specific differences. J Biol Chem. 2003;278:2740–2749. doi: 10.1074/jbc.M209201200. [DOI] [PubMed] [Google Scholar]
- Charbonneau NL, Jordan CD, Keene DR, Lee-Arteaga S, Dietz HC, Rifkin DB, Ramirez F, Sakai LY. Microfibril structure masks fibrillin-2 in postnatal tissues. J Biol Chem. 2010;285:20242–20251. doi: 10.1074/jbc.M109.087031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry SS, Gazzard J, Baldock C, Dixon J, Rock MJ, Skinner GC, Steel KP, Kielty CM, Dixon MJ. Mutation of the gene encoding fibrillin-2 results in syndactyly in mice. Hum Mol Genet. 2001;10:835–843. doi: 10.1093/hmg/10.8.835. [DOI] [PubMed] [Google Scholar]
- Corson GM, Charbonneau NL, Keene DR, Sakai LY. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics. 2004;83:461–472. doi: 10.1016/j.ygeno.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Dagoneau N, Benoist-Lasselin C, Huber C, Faivre L, Megarbane A, Alswaid A, Dollfus H, Alembik Y, Munnich A, Legeai-Mallet L, Cormier-Daire V. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am J Hum Genet. 2004;75:801–806. doi: 10.1086/425231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewens KG, Stewart DR, Ankener W, Urbanek M, McAllister JM, Chen C, Baig KM, Parker SC, Margulies EH, Legro RS, Dunaif A, Strauss JFI, Spielman RS. Family-based analysis of candidate genes for polycystic ovary syndrome. J Clin Endocrinol Metab. 2010;95:2306–2315. doi: 10.1210/jc.2009-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederic MY, Monino C, Marschall C, Hamroun D, Faivre L, Jondeau G, Klein HG, Neumann L, Gautier E, Binquet C, Maslen C, Godfrey M, Gupta P, Milewicz D, Boileau C, Claustres M, Beroud C, Collod-Beroud G. The FBN2 gene: new mutations, locus-specific database (Universal Mutation Database FBN2), and genotype-phenotype correlations. Hum Mutat. 2009;30:181–190. doi: 10.1002/humu.20794. [DOI] [PubMed] [Google Scholar]
- Gansner JM, Madsen EC, Mecham RP, Gitlin JD. Essential role for fibrillin-2 in zebrafish notochord and vascular morphogenesis. Dev Dyn. 2008;237:2844–2861. doi: 10.1002/dvdy.21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubmacher D, Tiedemann K, Reinhardt DP. Fibrillins: From biogenesis of microfibrils to signaling functions. Curr Top Dev Biol. 2006;75:93–123. doi: 10.1016/S0070-2153(06)75004-9. [DOI] [PubMed] [Google Scholar]
- Jensen SA, Reinhardt DP, Gibson MA, Weiss AS. MAGP-1, Protein interaction studies with tropoelastin and fibrillin-1. J Biol Chem. 2001;276:39661–39666. doi: 10.1074/jbc.M104533200. [DOI] [PubMed] [Google Scholar]
- Jordan CD, Bohling SD, Charbonneau NL, Sakai LY. Fibrillins in human adult ovary and polycystic ovary syndrome: Is fibrillin-3 affected in PCOS? J Histochem Cytochem. 2010 doi: 10.1369/jhc.2010.956615. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Marson A, Baldock C. Fibrillin microfibrils. Adv Protein Chem. 2005;70:405–436. doi: 10.1016/S0065-3233(05)70012-7. [DOI] [PubMed] [Google Scholar]
- Lin G, Tiedemann K, Vollbrandt T, Peters H, Bätge B, Brinckmann J, Reinhardt DP. Homo- and heterotypic fibrillin-1 and -2 interactions constitute the basis for the assembly of microfibrils. J Biol Chem. 2002;277:50795–50804. doi: 10.1074/jbc.M210611200. [DOI] [PubMed] [Google Scholar]
- Low FN. Microfibrils: fine filamentous components of the tissue space. Anat Rec. 1962;142:131–137. doi: 10.1002/ar.1091420205. [DOI] [PubMed] [Google Scholar]
- Mecham RP, Davis E. Elastic fiber structure and assembly. In: Yurchenco PD, Birk DE, Mecham RP, editors. Extracellular Matrix Assembly and Structure. Academic Press; New York: 1994. pp. 281–314. [Google Scholar]
- Miller G, Neilan M, Chia R, Gheryani N, Holt N, Charbit A, Wells S, Tucci V, Lalanne Z, Denny P, Fisher EMC, Cheeseman M, Askew GN, Dear TN. ENU mutagenesis reveals a novel phenotype of reduced limb strength in mice lacking fibrillin 2. PLoS One. 2010;5:e9137. doi: 10.1371/journal.pone.0009137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase T, Nakayama M, Nakajima D, Kikuno R, Ohara O. Prediction of the coding sequences of unidentified human genes. XXII The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 2001;8:85–95. doi: 10.1093/dnares/8.2.85. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Nistala H, Lee-Arteaga S, Siciliano G, Smaldone S, Ramirez F. Extracellular regulation of transforming growth factor beta and bone morphogenetic protein signaling in bone. Ann N Y Acad Sci. 2010;1192:253–256. doi: 10.1111/j.1749-6632.2009.05350.x. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, Müller F. Developmental stages in human embryos: including a revision of Streeter’s ‘Horizons’ and a survey of the Carnegie collection. Carnegie Institution of Washington; Washington, D.C: 1987. [Google Scholar]
- Prodoehl MJ, Hatzirodos N, Irving-Rodgers HF, Zhao ZZ, Painter JN, Hickey TE, Gibson MA, Rainey WE, Carr BR, Mason HD, Norman RJ, Montgomery GW, Rodgers RJ. Genetic and gene expression analyses of the polycystic ovary syndrome candidate gene fibrillin-3 and other fibrillin family members in human ovaries. Mol Hum Reprod. 2009;15:829–841. doi: 10.1093/molehr/gap072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyeritz R, Dietz H. Marfan syndrome and other microfibrillar disorders. In: Royce PM, Steinmann B, editors. Connective Tissue and its Heritable Disorders. 2. Wiley-Liss, Inc; New York: 2002. pp. 585–626. [Google Scholar]
- Quondamatteo F, Reinhardt DP, Charbonneau NL, Pophal G, Sakai LY, Herken R. Fibrillin-1 and fibrillin-2 in human embryonic and early fetal development. Matrix Biol. 2002;21:637–646. doi: 10.1016/s0945-053x(02)00100-2. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGF-beta and BMP signaling. Curr Opin Cell Biol. 2009;21:616–622. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Dietz HC. Extracellular microfibrils in vertebrate development and disease processes. J Biol Chem. 2009;284:14677–14681. doi: 10.1074/jbc.R900004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola G. The fine structure of the ciliary zonule and ciliary epithelium. Invest Ophthalmol. 1971;10:851–869. [PubMed] [Google Scholar]
- Robinson P, Arteaga-Solis E, Baldock C, Collod-Beroud G, Booms P, De Paepe A, Dietz HC, Guo G, Handford PA, Judge DP, Kielty CM, Loeys B, Milewicz DM, Ney A, Ramirez F, Reinhardt DP, Tiedemann K, Whiteman P, Godfrey M. The molecular genetics of Marfan syndrome and related disorders. J Med Genet. 2006;43:769–787. doi: 10.1136/jmg.2005.039669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R, Bornstein P. The elastic fiber: the separation and partial characterization of its macromolecular components. J Cell Biol. 1969;40:366–381. doi: 10.1083/jcb.40.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, Mosher DF, Reinhardt DP. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20:846–858. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986;103:2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund P, Keller R. Xenopus fibrillin regulates directed convergence and extension. Dev Biol. 2007;301:404–416. doi: 10.1016/j.ydbio.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DR, Dombroski BA, Urbanek M, Ankener W, Ewens KG, Wood JR, Legro RS, Strauss JFI, Dunaif A, Spielman RS. Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab. 2006;91:4112–4117. doi: 10.1210/jc.2006-0951. [DOI] [PubMed] [Google Scholar]
- Urbanek M, Woodroffe A, Ewens KG, Diamanti-Kandarakis E, Legro RS, Strauss JFI, Dunaif A, Spielman RS. Candidate gene region for polycystic ovary syndrome on chromosome 19p13.2. J Clin Endocrinol Metab. 2005;90:6623–6629. doi: 10.1210/jc.2005-0622. [DOI] [PubMed] [Google Scholar]
- Viljoen D. Congenital contractural arachnodactyly. J Med Genet. 1994;31:640–643. doi: 10.1136/jmg.31.8.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Ota K, Tian Y, Kumar A, Wada J, Kashihara N, Wallner E, Kanwar YS. Cloning of rat fibrillin-2 cDNA and its role in branching morphogenesis of embryonic lung. Dev Biol. 1999;212:229–242. doi: 10.1006/dbio.1999.9331. [DOI] [PubMed] [Google Scholar]
- Zhang H, Apfelroth SD, Hu W, Davis EC, Sanguineti C, Bonadio J, Mecham RP, Ramirez F. Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J Cell Biol. 1994;124:855–863. doi: 10.1083/jcb.124.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hu W, Ramirez F. Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J Cell Biol. 1995;129:1165–1176. doi: 10.1083/jcb.129.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.