Abstract
Background
Advanced high-grade serous ovarian carcinoma (HGSC) is commonly treated with surgery and chemotherapy. We investigated the survival of patients treated with primary or interval surgery at different times following neoadjuvant chemotherapy. Their survival was compared with that of patients treated with primary cytoreductive surgery and adjuvant chemotherapy.
Methods
Patients with stage III or IV HGSC were included in this retrospective cohort study. Clinical data were obtained from patient records. Patients were divided into 2 groups based on treatment with neoadjuvant chemotherapy and interval cytoreductive surgery (NAC) or with primary cytoreductive surgery and adjuvant chemotherapy (PCS). Study groups were stratified by several clinical variables.
Results
We included 334 patients in our study: 156 in the NAC and 178 in the PCS groups. Survival of patients in the NAC group was independent of when they underwent interval cytoreductive surgery following initiation of neoadjuvant chemotherapy (p < 0.001). Optimal surgical cytoreduction had no impact on overall survival in the NAC group (p < 0.001). Optimal cytoreduction (p < 0.001) and platinum sensitivity (p < 0.001) were independent predictors of improved survival in the PCS but not in the NAC group. Patients in the NAC group had significantly worse overall survival than those in the PCS group (31.6 v. 61.3 mo, p < 0.001).
Conclusion
Women with advanced HGSC who underwent PCS had better survival than those who underwent interval NAC, regardless of the number of cycles of neoadjuvant therapy. Optimal cytoreduction did not provide a survival advantage in the NAC group.
Abstract
Contexte
La chirurgie et la chimiothérapie sont habituellement le traitement recommandé pour les carcinomes ovariens séreux bien différenciés de haut grade. Nous avons étudié le taux de survie de patientes ayant subi une chirurgie initiale ou d’intervalle à divers moments après une chimiothérapie néoadjuvante et l’avons comparé avec celui de patientes ayant subi une chirurgie de réduction tumorale initiale et une chimiothérapie adjuvante.
Méthodes
Cette étude de cohorte rétrospective a été menée auprès de patientes présentant un carcinome de stade III ou IV. Les données cliniques ont été tirées de leur dossier médical. Les patientes ont été séparées en 2 groupes : le premier était formé des patientes ayant subi une chimiothérapie néoadjuvante et une chirurgie de réduction tumorale d’intervalle (groupe NAC), et le deuxième de celles ayant subi une chirurgie de réduction tumorale initiale et une chimiothérapie adjuvante (groupe PCS). On a stratifié les 2 groupes à l’aide de plusieurs variables cliniques.
Résultats
L’étude portait sur 334 patientes, soit 156 dans le groupe NAC et 178 dans le groupe PCS. Dans le groupe NAC, aucune corrélation n’a été observée entre le taux de survie des patientes et le temps écoulé entre la chimiothérapie néoadjuvante et la chirurgie de réduction tumorale d’intervalle (p < 0,001). La réduction tumorale optimale n’a eu aucune incidence sur le taux de survie global des patientes du groupe NAC (p < 0,001). La réduction tumorale optimale (p < 0,001) et la sensibilité au platine (p < 0,001) ont été ciblés comme étant 2 prédicateurs indépendants d’un taux de survie accru chez les patientes du groupe PCS, mais pas chez celles du groupe NAC. Le taux de survie des patientes du groupe NAC était beaucoup plus faible que celui des patientes du groupe PCS (31,6 mo contre 61,3 mo, p < 0,001).
Conclusion
Les femmes atteintes d’un carcinome ovarien séreux bien différencié de haut grade ayant subi une chirurgie de réduction tumorale initiale et une chimiothérapie adjuvante (PCS) ont affiché un taux de survie plus élevé que les patientes ayant subi une chimiothérapie néoadjuvante et une chirurgie de réduction tumorale d’intervalle (NAC), peu importe le nombre de cycles de chimiothérapie néoadjuvante. La réduction tumorale optimale n’a pas été associée à un taux de survie plus élevé chez ces dernières.
Epithelial ovarian cancer is the most common cause of death from gynecologic malignancy.1 High-grade serous ovarian cancer (HGSC) is the most common type of epithelial ovarian cancer, representing 70% of all diagnosed tumours.2 Most ovarian cancers (70%) are diagnosed at an advanced stage of disease (Stage III/IV), and 80%–90% of these advanced-stage tumours are HGSC.3 As such, the 5-year overall survival rate for women with HGSC is approximately 44%.
The standard of care treatment for HGSC has essentially remained the same for the past 2 decades and includes a combination of surgical cytoreduction and platinum-/taxane-based adjuvant chemotherapy.4,5 However, despite aggressive surgery and chemotherapy, cure is rare for the majority of women with HGSC. Survival in these patients largely depends on the tumour sensitivity to platinum-based chemotherapy6,7 and the degree of surgical cytoreduction.8,9 Even extensive surgeries leaving more than 1 cm of residual tumour have limited impact on survival.10–12 Since the original publication by Griffiths,9 which suggested an association between the amount of residual disease and survival, the definition of “optimal” cytoreduction has been shifting from an initial definition of no single residual lesion measuring less than 2 cm in diameter to a definition of less than 1 cm and, most recently, to a definition of no macroscopic disease.13–15
As optimal surgical cytoreduction is one of the strongest predictors of outcome for patients with HGSC, many studies have investigated the use of neoadjuvant chemotherapy as an alternative treatment strategy to reduce tumour burden before surgery.16 There are several putative advantages of the neoadjuvant treatment strategy, including less extensive surgery, reduced morbidity and increased optimal cytoreduction. Furthermore, it currently provides the only means to identify patients with platinum-resistant disease at presentation.17
Many studies suggest equivalent survival in patients receiving adjuvant versus neoadjuvant chemotherapy.18–28 Notably, Vergote and colleagues20 reported the only phase III randomized controlled trial in which patients with advanced-stage HGSC were treated with either primary surgery and adjuvant platinum-based chemotherapy (PCS group) or neoadjuvant platinum-based chemotherapy followed by interval cytoreductive surgery and additional adjuvant chemotherapy (NAC group). Although patients in the NAC group had higher rates of optimal cytoreduction and fewer perioperative complications, this did not translate into improved survival. This trial was criticized by many for poor progression-free and overall survival rates in both study arms.29–31
Importantly, several studies addressing the use of primary surgery versus neoadjuvant chemotherapy indicated that patients in the NAC group have inferior overall survival than patients in the PCS group.32–34 Bristow and Chi16 conducted a meta-analysis that suggested the number of neoadjuvant chemotherapy cycles before surgery was inversely proportional to the median overall survival. In addition, these authors demonstrated that although the difference in survival between the NAC and PCS groups did not reach statistical significance in previous studies, survival was often reduced by up to half in the NAC group.19
Hence, controversy remains about the use of neoadjuvant chemotherapy as a first-line treatment in patients with HGSC. Surveys of members of the Society of Gynecolologic Oncology35 and the European Society of Gynecologic Oncology36 suggest that 18% and 70% of gynecologic oncologists, respectively, routinely recommend neoadjuvant chemotherapy to their patients. Furthermore, the appropriate number of neoadjuvant chemotherapy cycles that should be administered before interval cytoreductive surgery is subject to debate. Hence, a deeper understanding of the effect of the treatment strategy, post-treatment tumour biology and survival outcomes are required.
In this study, we examine the progression-free and overall survival of patients in the NAC group as compared with patients in the PCS group. The objective of this work was to study surgical factors, including the timing of surgery, in relation to the number of preoperative neoadjuvant chemotherapy cycles and the rate of optimal cytoreduction in the NAC and PCS groups. We aimed to analyze the impact of these factors on survival in women with HGSC.
Methods
Patient selection and clinical data
We included patients with Stage III or IV HGSC diagnosed between 2003 and 2011 in this retrospective cohort study. Clinical data were extracted from the patients’ health records and from a prospectively maintained institutional database. Inclusion criteria consisted of a diagnosis of advanced-stage HGSC of mullerian origin. Patients were triaged to undergo primary cytoreductive surgery followed by adjuvant chemotherapy (PCS group) or to receive neoadjuvant chemotherapy with interval cytoreductive surgery (NAC group). Patients who underwent an “open and close” primary procedure were excluded from this analysis. In addition, patients who were identified as platinum-refractory following neoadjuvant chemotherapy treatment and those who did not undergo interval cytoreductive surgery were excluded from this study. This study was approved by the Research Ethics Board at Princess Margaret Cancer Centre, University Health Network, Toronto, Ont.
The decision regarding the treatment strategy depended solely on the treating gynecologic oncologist. The most common reasons for selecting the neoadjuvant treatment modality included substantial medical comorbidities, poor performance status, extent of disease on imaging, and prolonged wait time for surgery. In some instances, the specific reason for recommending neoadjuvant chemotherapy was clearly stated in the patient’s records; however, explicit treatment reasoning was not identified for all patients. The rationale for surgical timing depended on multiple variables, including results of interval imaging, such as the extent of upper abdominal disease, liver disease and thoracic disease.
Additional clinical data were collected to allow stratification of patients based on age at diagnosis, the number of chemotherapy cycles administered and surgical cytoreduction status. We appreciate that optimal cytoreduction to no residual disease is now recognized as superior to residual disease of less than 1 cm; however, this information was not consistently included in the operative reports during the study period. Optimal cytoreduction to less than 1 cm residual disease was used in this analysis as this was the “gold standard” at the time when these patients were treated. These parameters were used to further stratify the neoadjuvant and primary surgery groups and to control for the effects of confounding variables.
Statistical analyses
We used the Student t test to examine differences in the distributions of age and the number of cycles of primary platinum-based chemotherapy in the NAC and PCS groups. In addition, Fisher exact tests were used to compare rates of optimal cytoreduction and platinum sensitivity (defined as no disease recurrence or recurrence > 6 mo from last platinum-based treatment) between the 2 study groups. Log-rank (Mantel–Cox) tests were used to examine progression-free and overall survival, and Kaplan–Meier plots were generated. We performed all pairwise analyses using a Bonferroni multiple-comparisons correction. All analyses were performed using Graph Pad Prism 6 software. We performed a Cox proportional hazard regression analysis of overall survival. Treatment, age at diagnosis, debulking status, sensitivity to platinum-based chemotherapy and cycles of primary chemotherapy were included in univariate and multivariate analyses. We built the multivariable model using backward elimination, and only significant predictors were kept in the model. The selected multivariable model included treatment, debulking status, and sensitivity to platinum-based chemotherapy, which was included in the model as a stratification factor.
Results
Characteristics of patients with HGSC typically treated with neoadjuvant chemotherapy
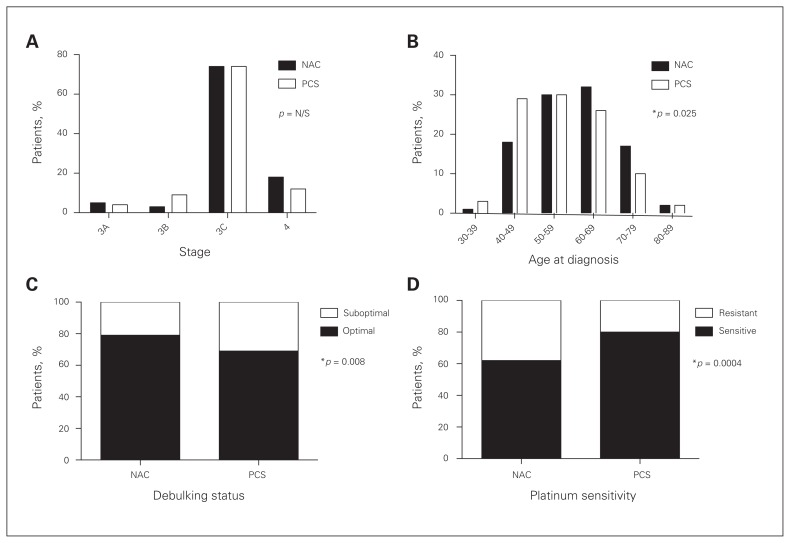
We included 398 patients with Stage III or IV HGSC in this study: 156 in the NAC group and 178 in the PCS group. Patients in the NAC group were treated with neoadjuvant chemotherapy and interval surgery, and those in the PSC group were treated with primary surgery followed by adjuvant chemotherapy. Comparison of patient characteristics between these groups revealed no difference in tumour stage (Fig. 1A). Patients in the NAC group were older than patients in the PCS group (median age 60 v. 56, p = 0.025; Fig. 1B). All patient characteristics are described in Table 1.
Fig. 1.
Comparison of clinical characteristics of patients with high-grade serous ovarian carcinoma (HGSC) treated with neoadjuvant chemotherapy and interval cytoreductive surgery (NAC) or primary cytoreductive surgery and adjuvant chemotherapy (PCS). (A) No significant difference in tumour stage was observed between the adjuvant and neoadjuvant groups. (B) Patients treated with neoadjuvant chemotherapy were significantly older (Student t test, p = 0.004). (C) Significantly more patients in the neoadjuvant group were optimally debulked (< 1 cm residual disease) than in the adjuvant group (Fisher exact test, p = 0.025). (D) Patients treated with neoadjuvant chemotherapy are more likely to demonstrate resistance to first-line platinum-based chemotherapy (i.e., progress on therapy or recurrence within 6 mo of completion of first-line therapy, Fisher exact test, p = 0.001). N/S = nonsignificant.
Table 1.
Demographic and clinical characteristics of patients
| Characteristic | Group; no. (%) | p value | |
|---|---|---|---|
| NAC, n = 156 | PCS, n = 178 | ||
| Stage | 0.23 | ||
| IIIa | 8 (5) | 8 (4) | |
| IIIb | 5 (3) | 16 (9) | |
| IIIc | 115 (74) | 132 (74) | |
| IV | 28 (18) | 22 (12) | |
| Age at diagnosis, yr | 0.004 | ||
| < 49 | 29 (19) | 58 (32) | |
| 50–59 | 47 (30) | 53 (30) | |
| 60–69 | 50 (32) | 46 (26) | |
| > 70 | 30 (19) | 21 (12) | |
| Debulking status | 0.025 | ||
| Optimal | 124 (79) | 122 (69) | |
| Suboptimal | 32 (21) | 56 (31) | |
| Sensitivity to platinum-based chemotherapy | 0.001 | ||
| Sensitive | 97 (62) | 141 (80) | |
| Resistant | 59 (38) | 36 (20) | |
| Unknown | — | 1 (0) | |
| Cycles of neoadjuvant chemotherapy | |||
| 0 | — | 178 (100) | |
| 3 | 48 (31) | — | |
| 4 | 76 (49) | — | |
| ≥ 5 | 32 (21) | — | |
| Cycles of primary chemotherapy | < 0.001 | ||
| < 5 | 10 (6) | 14 (8) | |
| 6 | 53 (34) | 128 (72) | |
| 7 | 38 (24) | 9 (5) | |
| 8 | 33 (21) | 14 (8) | |
| > 9 | 19 (12) | 9 (5) | |
| Unknown | 3 (2) | 4 (2) | |
As the volume of residual disease after surgery and the sensitivity to first-line platinum-based chemotherapy are the strongest known predictors of outcome in HGSC, these variables were compared in the 2 study groups. Patients treated with neoadjuvant chemotherapy were significantly more likely to achieve optimal debulking to less than 1 cm of residual disease (80% in the NAC group v. 68% in the PCS group, p = 0.008; Fig. 1C). Notably, patients in the NAC group were significantly more likely to demonstrate resistance to first-line platinum-based chemotherapy (38% in the NAC group v. 20% in the PCS group, p < 0.001; Fig. 1D).
Survival of patients with HGSC treated with neoadjuvant chemotherapy
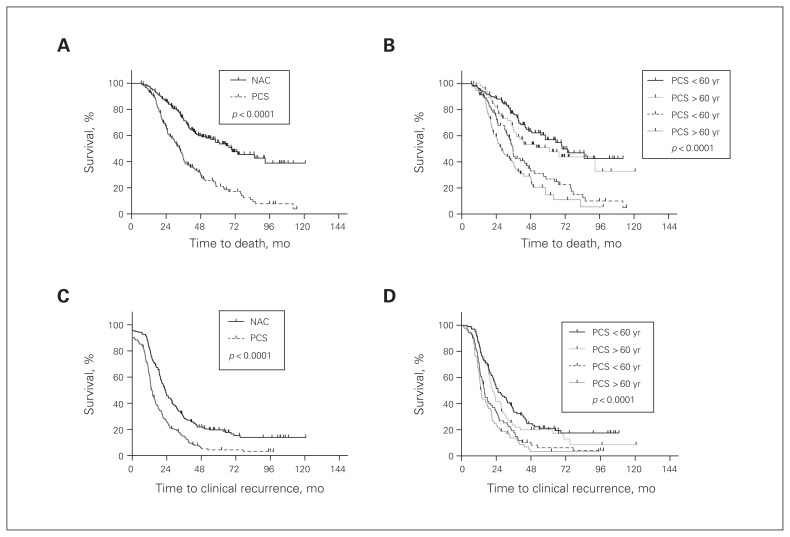
Overall survival of patients with HGSC in the NAC group was compared with that of patients in the PCS group. Patients in the NAC group had a significantly worse overall survival than patients in the PCS group (Fig. 2A), with a median overall survival of 33.4 and 69.5 months, respectively. This difference in survival was independent of the patients’ age (p < 0.001; Fig. 2B). Similarly, analysis of progression-free survival showed worse outcomes in the NAC group than the PCS group, independent of age (Fig. 2C and D). The survival difference between the treatment groups remained significant after adjusting for confounding factors, including age, debulking status and sensitivity to platinum-based chemotherapy (Table 2 and Table 3).
Fig. 2.
Overall and progression-free survival of patients treated with neoadjuvant chemotherapy and interval cytoreductive surgery (NAC) compared with primary cytoreductive surgery and adjuvant chemotherapy (PCS). (A) Patients treated with neoadjuvant chemotherapy followed by surgery have a significantly worse overall survival than patients treated with primary surgery and adjuvant chemotherapy (p < 0.001). (B) The difference in overall survival was independent of the patients’ age at diagnosis (p < 0.001). (C) Patients treated with neoadjuvant chemotherapy had a significantly worse progression-free survival measured by radiologic imaging (i.e., clinical recurrence) (p < 0.001). (D) The significant difference in progression-free survival is independent of the age of the patient at diagnosis. All statistics were calculated with a log-rank (Mantel–Cox) test.
Table 2.
Univariable and multivariable analysis of overall survival using Cox proportional hazards model
| Variable | Reference | Univariate | Multivariate | ||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95%CI) | p value | HR (95%CI) | p value | ||
| Treatment: NAC | PCS | 2.56 (1.91–3.42) | < 0.001 | 2.31 (1.70–3.13) | < 0.001 |
|
| |||||
| Age at diagnosis > 50 yr | ≤ 50 yr | 1.52 (1.09–2.11) | 0.014 | — | — |
|
| |||||
| Suboptimal debulking status | Optimal | 1.77 (1.30–2.40) | < 0.001 | 1.58 (1.15–2.17) | 0.005 |
|
| |||||
| Resistant to platinum-based chemotherapy | Sensitive | — | < 0.001* | — | — |
|
| |||||
| Cycles of primary chemotherapy > 6 | ≤ 6 | 1.92 (1.43–2.56) | < 0.001 | — | — |
CI = confidence interval, HR = hazard ratio; NAC = neoadjuvant chemotherapy and interval cytoreductive surgery; PCS = primary cytoreductive surgery and adjuvant chemotherapy.
Log-rank test. Sensitivity to platinum-based chemotherapy has nonproportional hazard. It was included in the multivariate analysis as a stratification factor.
Table 3.
Multivariable analysis of overall survival using Cox proportional hazards model by adjusting all covariates
| Variable | Reference | Multivariate analysis | |
|---|---|---|---|
| HR (95%CI) | p value | ||
| Treatment: NAC | PCS | 2.33 (1.66–3.28) | < 0.001 |
| Age at diagnosis > 50 yr | ≤ 50 yr | 1.12 (0.79–1.57) | 0.53 |
| Suboptimal debulking status | Optimal | 1.61 (1.14–2.26) | 0.007 |
| Resistant to platinum-based chemotherapy* | Sensitive | — | — |
| Cycles of primary chemotherapy > 6 | ≤ 6 | 0.96 (0.69–1.34) | 0.82 |
CI = confidence interval, HR = hazard ratio; NAC = neoadjuvant chemotherapy and interval cytoreductive surgery; PCS = primary cytoreductive surgery and adjuvant chemotherapy.
Sensitivity to platinum-based chemotherapy has nonproportional hazard. It was included in the multivariate analysis as a stratification factor.
Timing of surgery
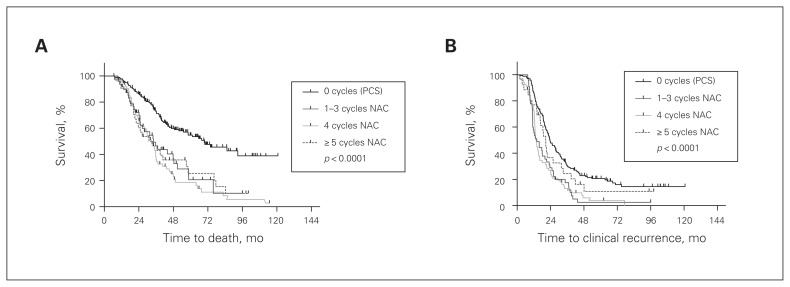
In the NAC group, no significant difference in overall or progression-free survival was noted based on the number of neoadjuvant chemotherapy cycles given before interval cytoreductive surgery. Patients who received up to 3 cycles, 4 cycles, or 5 or more cycles had a median overall survival of 34.1, 32.7 and 34.2 months, respectively, as compared with the adjuvant group (p < 0.001, Fig. 3A). The same trend was observed in the analysis of progression-free survival, with median progression-free survival of 14.1 (≤ 3 cycles), 13.7 (4 cycles) and 20.5 months (≥ 5 cycles; Fig. 3B).
Fig. 3.
Overall and progression-free survival stratified by the time to interval cytoreductive surgery. (A) Survival in the neoadjuvant group (NAC) was independent of the timing of surgery with respect to the number of cycles of neoadjuvant chemotherapy. Patients treated with neoadjuvant chemotherapy had a worse overall survival (p < 0.001), independent of whether they were treated with up to 3, 4, or 5 or more cycles before surgery (p = 0.92). (B) Patients treated with neoadjuvant chemotherapy had a significantly worse progression-free survival independent of the timing of interval cytoreductive surgery with respect to the number of cycles of neoadjuvant chemotherapy received (p < 0.001). All statistics were calculated with a log-rank (Mantel–Cox) test. PCS = primary cytoreductive surgery and adjuvant chemotherapy.
Effect of surgical cytoreduction
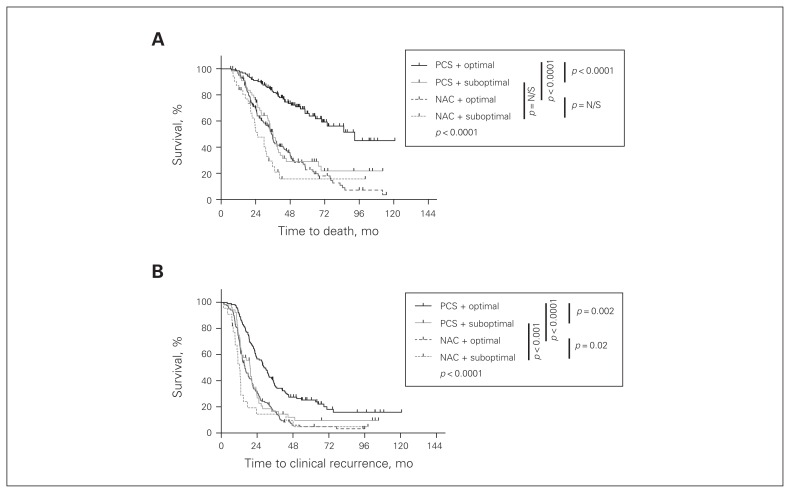
As optimal cytoreduction was more commonly achieved in the NAC group (Fig. 1C), we examined the impact of optimal cytoreduction on survival. Optimal cytoreduction was an independent predictor of improved overall survival in the PCS group (p < 0.001, Fig. 4A), with a median survival of 92.9 months versus 36 months in the PCS optimal and PCS suboptimal groups. In the NAC group, there was a trend toward improved survival if optimal cytoreduction was achieved; however, this didn’t reach statistical significance (p = 0.07). The median survival was 35.1 months in the NAC optimal group and 25.4 months in the NAC suboptimal group. When comparing all patients in the study who were optimally debulked, those who were pretreated with neoadjuvant chemotherapy had worse overall survival than patients who were chemotherapy-naive at the time of their surgery (35.1 mo v. 92.9 mo, p < 0.001). However, when comparing survival of study patients who underwent suboptimal cytoreduction, no significant difference was observed between the NAC and PCS groups (25.4 mo v. 36 mo, p = 0.24). The same trends were observed in the analysis of progression-free survival (Fig. 4B); however, all pairwise comparisons reached statistical significance.
Fig. 4.
Overall and progression-free survival of patients treated with neoadjuvant chemotherapy and interval cytoreductive surgery (NAC) or primary cytoreductive surgery and adjuvant chemotherapy (PCS) stratified by surgical cytoreduction status. (A) Optimal surgical cytoreduction to less than 1 cm residual tumour had no significant impact on overall survival in the neoadjuvant group. In addition, within the suboptimally debulked group, neoadjuvant chemotherapy had no significant effect. However, within the optimal subgroup, the neoadjuvant patients had a worse overall outcome (p < 0.001). Within the adjuvant group, patients who were optimally debulked had a significantly improved survival (p = 0.001). (B) Optimal surgical cytoredcution resulted in better progression-free survival in patients treated with neoadjuvant chemotherapy or primary surgical cytoreduction (p = 0.020 and p = 0.002, respectively). However, in the optimally and suboptimally surgically debulked groups, the patients who received neoadjuvant chemotherapy had a significantly worse progression-free survival (p < 0.001 for both comparisons). All statistics were calculated with a log-rank (Mantel–Cox) test. Bonferroni corrections were applied for all multiple comparisons. N/S = nonsignificant.
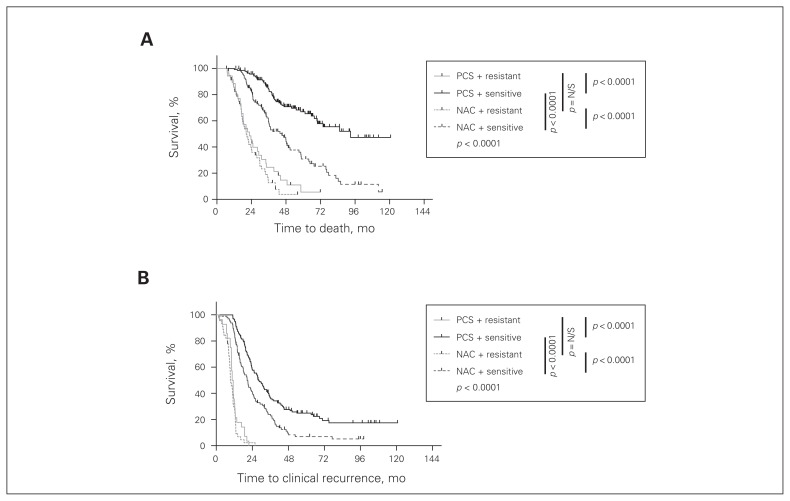
Sensitivity to platinum-based chemotherapy
Notably, patients who were resistant to first-line platinum-based chemotherapy demonstrated significantly worse overall survival than platinum-sensitive patients in both the PCS and NAC groups (p < 0.001, Fig. 5A). In addition, there was no difference in the survival of platinum-resistant patients in the PCS and NAC groups, with a median survival of 20.4 and 22.6 months, respectively. Among platinum-sensitive patients, those in the NAC group demonstrated a reduced overall survival compared with patients in the PCS group (p < 0.001), with a median survival of 45.2 versus 92.9 months, respectively. The same trends were observed when progression-free survival was assessed (Fig. 5B).
Fig. 5.
Overall and progression-free survival of patients treated with neoadjuvant chemotherapy and interval cytoreductive surgery (NAC) or primary cytoreductive surgery and adjuvant chemotherapy (PCS) stratified by sensitivity to platinum-based chemotherapy. All patients with first-line platinum-resistance had a worse (A) overall and (B) progression-free survival (p < 0.001). Treatment with neoadjuvant chemotherapy had no effect on the survival of the platinum-resistant patients. However, within the platinum-sensitive group, patients treated with neoadjuvant chemotherapy had worse overall and progression-free survival (p < 0.001). All statistics were calculated with a log-rank (Mantel–Cox) test. Bonferroni corrections were applied for all multiple comparisons. N/S = nonsignificant.
Discussion
The primary treatment of women with newly diagnosed ovarian HGSC commonly includes a combination of surgery and chemotherapy. The decision of whether to treat with upfront chemotherapy often rests with the treating physician. This study was conducted at the largest cancer centre in Canada. Given the open access health care system in Canada, all women with ovarian cancer had access to care at our academic centre. While there are inherent biases in a retrospective study design, this study included all women with HGSC treated at our centre between 2003 and 2011. All clinical charts were analyzed, and those who met the inclusion criteria were included in the analyses. We performed univariate and multivariate analyses to control for potential confounders of survival.
To our knowledge, this study represents the largest retrospective analysis worldwide examining survival of patients with HGSC, the most common and most lethal form of ovarian carcinoma. In this large patient cohort, women in the NAC group had a significantly worse progression-free and overall survival than women in the PCS group. This finding was independent of the patients’ age at diagnosis.
The use of neoadjuvant chemotherapy as a treatment strategy for patients with HGSC was proposed as a means to reduce tumour burden before surgical cytoreduction, thereby increasing the ability to achieve optimal surgical cytoreduction with less extensive surgery and reduced morbidity.16 The only phase III randomized controlled trial that compared patients treated with NAC or PCS showed equal survival between the 2 groups.20 Interestingly, despite the increased rate of optimal surgical cytoreduction reported in the neoadjuvant group, no concomitant increase in survival was observed. Other retrospective analyses have reported similar findings.18–28 By contrast, several studies have shown that patients treated with neoadjuvant chemotherapy have a worse survival than those treated with primary surgery.32–34 Overall, controversy remains about the use of neoadjuvant chemotherapy as a first-line treatment strategy for women with newly diagnosed HGSC.
One of the major concerns when giving neoadjuvant chemotherapy is that the administration of an increasing number of neoadjuvant chemotherapy cycles could increase the likelihood of selection for platinum-resistant clones.13 This viewpoint was largely driven by the meta-analysis by Bristow and Chi,16 which suggested a negative association between overall survival and the number of neoadjuvant chemotherapy cycles administered. By contrast, in this cohort, the decreased survival of patients with HGSC in the NAC group was independent of the number of cycles administered before surgery. This discrepancy might be due to all patients in our study receiving platinum-based neoadjuvant chemotherapy, whereas the study by Bristow and Chi16 included 16 different treatment combinations, which may account for the variable survival rates. Notably, our data suggest that the putative selective effects of neoadjuvant chemotherapy are incurred early, with as few as 3 cycles of treatment. Women receiving 3 or fewer cycles of neoadjuvant chemotherapy before interval surgery had equivalent survival to women receiving 4 cycles and to women receiving 5 or more cycles. The early clonal emergence hypothesis is supported by the observation that the most dramatic alteration in tumour bulk (measured by serum CA125 levels) is typically observed early in the course of treatment.37–39
Importantly, optimal surgical cytoreduction represents one of the strongest predictors of outcome in patients with HGSC treated with primary cytoreductive surgery.8,9 Although previous reports have suggested that neoadjuvant chemotherapy improves the rate of optimal surgical cytoreduction, none have demonstrated that this translates into improved survival.20–22,28,32 In our study, women in the NAC group had a significantly higher rate of optimal cytoreduction than chemothereapy-naive patients undergoing primary cytoreduction. Yet, this did not confer a survival advantage. The lack of survival difference between optimally and suboptimally cytoreduced patients in the NAC group calls into question the accuracy of estimating the cytoreduction status after neoadjuvant chemotherapy. Notably, chemotherapy can affect the gross morphologic appearance of tumour tissue and surgical planes, which could lead to anatomic variability and render the assessment of residual disease and optimal surgical excision challenging.40
Finally, when stratifying patients by platinum sensitivity, the negative impact of neoadjuvant chemotherapy on survival is noted. It is well known that patients with platinum-resistant disease have significantly worse survival.6,7 In this study, patients with platinum-sensitive disease had worse overall and progression-free survival when treated with neoadjuvant chemotherapy than with primary surgery. Importantly, these differences remained significant after adjusting for confounding factors, including age, debulking status and sensitivity to platinum-based chemotherapy.
Conclusion
Our data indicate that neoadjuvant chemotherapy is associated with inferior survival in patients with HGSC, independent of other prognostic factors. Women with advanced ovarian cancer who undergo neoadjuvant chemotherapy often undergo interval surgery at various points during their treatment. The variation in the timing of surgery depends on multiple factors, including patients’ medical conditions and surgical access. Importantly, this study demonstrates that surgical factors, including the timing of surgery and the rate of optimal cytoreduction, do not appear to add a survival advantage to women treated with neoadjuvant chemotherapy.
Our study raises important questions: Does early exposure to neoadjuvant chemotherapy provoke biologic and genetic changes in tumour cells that ultimately result in hastened platinum resistance? Is emergence of platinum resistance accelerated by the lack of true surgical cytoreduction in the neoadjuvant group? Given the increased adaptation of neoadjuvant chemotherapy in the management of women with advanced ovarian carcinoma, it is essential to examine the factors that influence treatment response and survival in this patient population. Studying the molecular alterations associated with neoadjuvant chemotherapy is imperative and may lead to greater understanding of the impact of early chemotherapy exposure on survival in women with ovarian HGSC.
Footnotes
Competing interests: None declared.
Contributors: J. Stewart and T. May designed the study. J. Stewart, M. Bernardini, S. Ferguson, S. Laframboise, J. Murphy, B. Rosen and T. May acquired the data, which J. Stewart, A. Tone, H. Jiang and T. May analyzed. J. Stewart and T. May wrote the article, which all authors reviewed and approved for publication.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 3.Vaughan S, Coward JI, Bast RC, Jr, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–25. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 5.du Bois A, Luck HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–9. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 6.Spriggs D. Optimal sequencing in the treatment of recurrent ovarian cancer. Gynecol Oncol. 2003;90:S39–44. doi: 10.1016/s0090-8258(03)00471-2. [DOI] [PubMed] [Google Scholar]
- 7.Ushijima K. Treatment for recurrent ovarian cancer-at first relapse. J Oncol. 2010;2010 doi: 10.1155/2010/497429. 497429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi DS, Liao JB, Leon LF, et al. Identification of prognostic factors in advanced epithelial ovarian carcinoma. Gynecol Oncol. 2001;82:532–7. doi: 10.1006/gyno.2001.6328. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975;42:101–4. [PubMed] [Google Scholar]
- 10.Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974–9. doi: 10.1016/s0002-9378(94)70090-7. [DOI] [PubMed] [Google Scholar]
- 11.Aletti GD, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006;107:77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 12.Chang SJ, Bristow RE, Ryu HS. Impact of complete cytoreduction leaving no gross residual disease associated with radical cytoreductive surgical procedures on survival in advanced ovarian cancer. Ann Surg Oncol. 2012;19:4059–67. doi: 10.1245/s10434-012-2446-8. [DOI] [PubMed] [Google Scholar]
- 13.Morrison J, Haldar K, Kehoe S, et al. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2012;8:CD005343. doi: 10.1002/14651858.CD005343.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison J. Improving outcomes in gynaecological cancer: the benefits of subspecialisation. Cochrane Database Syst Rev. 2012;4:ED000040. doi: 10.1002/14651858.ED000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thigpen T, duBois A, McAlpine J, et al. First-line therapy in ovarian cancer trials. Int J Gynecol Cancer. 2011;21:756–62. doi: 10.1097/IGC.0b013e31821ce75d. [DOI] [PubMed] [Google Scholar]
- 16.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2006;103:1070–6. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Baekelandt M. The potential role of neoadjuvant chemotherapy in advanced ovarian cancer. Int J Gynecol Cancer. 2003;13(Suppl 2):163–8. doi: 10.1111/j.1525-1438.2003.13354.x. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz PE, Rutherford TJ, Chambers JT, et al. Neoadjuvant chemotherapy for advanced ovarian cancer: long-term survival. Gynecol Oncol. 1999;72:93–9. doi: 10.1006/gyno.1998.5236. [DOI] [PubMed] [Google Scholar]
- 19.Vergote I, De Wever I, Tjalma W, et al. Neoadjuvant chemotherapy or primary debulking surgery in advanced ovarian carcinoma: a retrospective analysis of 285 patients. Gynecol Oncol. 1998;71:431–6. doi: 10.1006/gyno.1998.5213. [DOI] [PubMed] [Google Scholar]
- 20.Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–53. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 21.Kayikçioglu F, Kose MF, Boran N, et al. Neoadjuvant chemotherapy or primary surgery in advanced epithelial ovarian carcinoma. Int J Gynecol Cancer. 2001;11:466–70. doi: 10.1046/j.1525-1438.2001.01064.x. [DOI] [PubMed] [Google Scholar]
- 22.Vrscaj MU, Rakar S. Neoadjuvant chemotherapy for advanced epithelial ovarian carcinoma: a retrospective case-control study. Eur J Gynaecol Oncol. 2002;23:405–10. [PubMed] [Google Scholar]
- 23.Ushijima K, Ota S, Komai K, et al. Clinical assessment of neoadjuvant chemotherapy and interval cytoreductive surgery for unresectable advanced ovarian cancer. Int Surg. 2002;87:185–90. [PubMed] [Google Scholar]
- 24.Fanfani F, Ferrandina G, Corrado G, et al. Impact of interval debulking surgery on clinical outcome in primary unresectable FIGO stage IIIc ovarian cancer patients. Oncology. 2003;65:316–22. doi: 10.1159/000074644. [DOI] [PubMed] [Google Scholar]
- 25.Morice P, Brehier-Ollive D, Rey A, et al. Results of interval debulking surgery in advanced stage ovarian cancer: an exposed-non-exposed study. Ann Oncol. 2003;14:74–7. doi: 10.1093/annonc/mdg003. [DOI] [PubMed] [Google Scholar]
- 26.Morice P, Dubernard G, Rey A, et al. Results of interval debulking surgery compared with primary debulking surgery in advanced stage ovarian cancer. J Am Coll Surg. 2003;197:955–63. doi: 10.1016/j.jamcollsurg.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Loizzi V, Cormio G, Resta L, et al. Neoadjuvant chemotherapy in advanced ovarian cancer: a case-control study. Int J Gynecol Cancer. 2005;15:217–23. doi: 10.1111/j.1525-1438.2005.15206.x. [DOI] [PubMed] [Google Scholar]
- 28.Hegazy MA, Hegazi RA, Elshafei MA, et al. Neoadjuvant chemotherapy versus primary surgery in advanced ovarian carcinoma. World J Surg Oncol. 2005;3:57. doi: 10.1186/1477-7819-3-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi DS, Bristow RE, Armstrong DK, et al. Is the easier way ever the better way? J Clin Oncol. 2011;29:4073–5. doi: 10.1200/JCO.2011.35.9935. [DOI] [PubMed] [Google Scholar]
- 30.Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy is the better treatment option in some patients with stage IIIc to IV ovarian cancer. J Clin Oncol. 2011;29:4076–8. doi: 10.1200/JCO.2011.36.9785. [DOI] [PubMed] [Google Scholar]
- 31.Robinson WR. Neoadjuvant chemotherapy is rarely the easy way out. J Clin Oncol. 2012;30:1563. doi: 10.1200/JCO.2011.40.8401. [DOI] [PubMed] [Google Scholar]
- 32.Luyckx M, Leblanc E, Filleron T, et al. Maximal cytoreduction in patients with FIGO stage IIIC to stage IV ovarian, fallopian, and peritoneal cancer in day-to-day practice: a retrospective French multicentric study. Int J Gynecol Cancer. 2012;22:1337–43. doi: 10.1097/IGC.0b013e31826a3559. [DOI] [PubMed] [Google Scholar]
- 33.Chi DS, Musa F, Dao F, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT) Gynecol Oncol. 2012;124:10–4. doi: 10.1016/j.ygyno.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Shibata K, Kikkawa F, Mika M, et al. Neoadjuvant chemotherapy for FIGO stage III or IV ovarian cancer: survival benefit and prognostic factors. Int J Gynecol Cancer. 2003;13:587–92. doi: 10.1046/j.1525-1438.2003.13388.x. [DOI] [PubMed] [Google Scholar]
- 35.Dewdney SB, Rimel BJ, Reinhart AJ, et al. The role of neoadjuvant chemotherapy in the management of patients with advanced stage ovarian cancer: survey results from members of the Society of Gynecologic Oncologists. Gynecol Oncol. 2010;119:18–21. doi: 10.1016/j.ygyno.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Cornelis S, Van Calster B, Amant F, et al. Role of neoadjuvant chemotherapy in the management of stage IIIC-IV ovarian cancer: survey results from the members of the European Society of Gynecological Oncology. Int J Gynecol Cancer. 2012;22:407–16. doi: 10.1097/IGC.0b013e31823ea1d8. [DOI] [PubMed] [Google Scholar]
- 37.Makar AP, Kristensen GB, Bormer OP, et al. Serum CA 125 level allows early identification of nonresponders during induction chemotherapy. Gynecol Oncol. 1993;49:73–9. doi: 10.1006/gyno.1993.1089. [DOI] [PubMed] [Google Scholar]
- 38.Sevelda P, Schemper M, Spona J. CA 125 as an independent prognostic factor for survival in patients with epithelial ovarian cancer. Am J Obstet Gynecol. 1989;161:1213–6. doi: 10.1016/0002-9378(89)90668-6. [DOI] [PubMed] [Google Scholar]
- 39.Redman CW, Blackledge GR, Kelly K, et al. Early serum CA125 response and outcome in epithelial ovarian cancer. Eur J Cancer. 1990;26:593–6. doi: 10.1016/0277-5379(90)90085-8. [DOI] [PubMed] [Google Scholar]
- 40.McCluggage WG, Lyness RW, Atkinson RJ, et al. Morphological effects of chemotherapy on ovarian carcinoma. J Clin Pathol. 2002;55:27–31. doi: 10.1136/jcp.55.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]