Abstract
The AlloRep database (www.AlloRep.org) (Sousa et al., 2016) [1] compiles extensive sequence, mutagenesis, and structural information for the LacI/GalR family of transcription regulators. Sequence alignments are presented for >3000 proteins in 45 paralog subfamilies and as a subsampled alignment of the whole family. Phenotypic and biochemical data on almost 6000 mutants have been compiled from an exhaustive search of the literature; citations for these data are included herein. These data include information about oligomerization state, stability, DNA binding and allosteric regulation. Protein structural data for 65 proteins are presented as easily-accessible, residue-contact networks. Finally, this article includes example queries to enable the use of the AlloRep database. See the related article, “AlloRep: a repository of sequence, structural and mutagenesis data for the LacI/GalR transcription regulators” (Sousa et al., 2016) [1].
Specifications Table
| Subject area | Biology |
|---|---|
| More specific subject area | Protein biochemistry |
| Type of data | Text, figure |
| How data was acquired | Literature survey and computational calculations for LacI/GalR protein variants |
| Data format | Normalized; analyzed |
| Experimental factors | Mutational data were normalized to wild-type protein activity |
| Experimental features | For structural data, intra- and intermolecular non-covalent contacts were calculated at a 5A threshold. |
| Data source location | The University of Kansas Medical Center, Kansas City, KS |
| Data accessibility | Data is within this article and available atwww.AlloRep.org |
Value of the data
-
•
The AlloRep database (www.AlloRep.org) compiles extensive sequence, mutagenesis, and structural information for the LacI/GalR family of transcription regulators.
-
•
The AlloRep database simplifies the consolidation of non-covalent structural information with mutagenesis and sequence conservation data.
-
•
The AlloRep database can be used to benchmark computational predictions and to design synthetic transcription repressors for biotechnology.
-
•
The example queries contained in this article can be used to improve searches of the AlloRep database.
1. Data
The AlloRep database (www.AlloRep.org) [1] compiles extensive sequence, mutagenesis, and structural information for the LacI/GalR family of transcription regulators. Phenotypic and biochemical data on almost 6000 mutants have been compiled from an exhaustive search of the literature; citations for these data are listed in this publication [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82]. The data can be exported to build a local copy on the user׳s computer, but the insert and import features are disabled. New data are welcome and can be submitted to the corresponding author at lswint-kruse@kumc.edu. Here, we detail the organization of the 5 database modules and their components tables, and provide full descriptions for the contents of table columns. Fig. 1 overviews the structure of the database.
Fig. 1.
AlloRep database scheme. The five sections of the AlloRep database are contained within the dashed boxes. Each section contains one or more tables (smaller boxes with blue headings). Lines between tables indicate connections that may be accessed with SQL queries.
We also present a protein structural comparison that was facilitated by compiling the information in the structural module. Fig. 2 shows a comparison of intra- and inter-molecular contacts from a comparative study of 65 structures available for the LacI/GalR homologs.
Fig. 2.
Comparisons of inter- and intra-molecular contacts among 65 structures of LacI/GalR homologs. All available structures were collected for each equivalent state of a given protein (from the same species and bound the same ligands), including the occurrences of multiple structures present in a unit cell. Inter- and intra-monomeric contacts were determined as defined in the text, and the frequency of each contact was calculated for the set of structures. If only one structure was available, the frequency was set to 100% by default. As an example, panel (a) shows an excerpt from a matrix containing information about the frequency of various contacts for all structures of E.coli apo-PurR. Each contact matrix was then linearized in numerical order (b) to make one column of panel (c). As a second example, the dashed box contains the composite information for all structures of LacI bound to DNA and the small molecule NPF. In panel (c), the contacts were ordered on the Y axis so that those involving the N-terminal DNA binding domain are at the top, those of the linker come next (positions 45–62 in E. coli LacI), followed by contacts in the regulatory domain. Each column along the X axis corresponds to the named group of equivalent structures. Bound ligands are in parentheses and ligand abbreviations can be found in the table “struct2_ligand_description”. Different colors indicate the frequency that a particular contact occurs. Inter-monomeric contacts are collected on the left of panel (c). Some structures contained monomers that could not be dimerized by symmetry operations; thus their inter-monomer contacts could not be determined. Intra-monomeric contacts are shown on the right. Once contact frequency was calculated, agnostic, hierarchical clustering was used to order the inter- and intra-monomeric contacts in panel (c). These plots show that the inter-monomer contacts (left panel) cluster according to their ligand binding state. For example, the DNA bound structures for different homologs are more similar to each other than to their respective inducer bound structures. In contrast, the intra-monomeric contacts (right panel) cluster so that the structures for each LacI/GalR subfamily are most closely related, regardless of their binding state.
Finally, the database can be searched by selecting a table from one of the modules and using the built in search fields (search tab; Fig. 3). In addition, command line queries can be executed using the SQL tab. Example command line queries are listed in supplement to this manuscript.
Fig. 3.
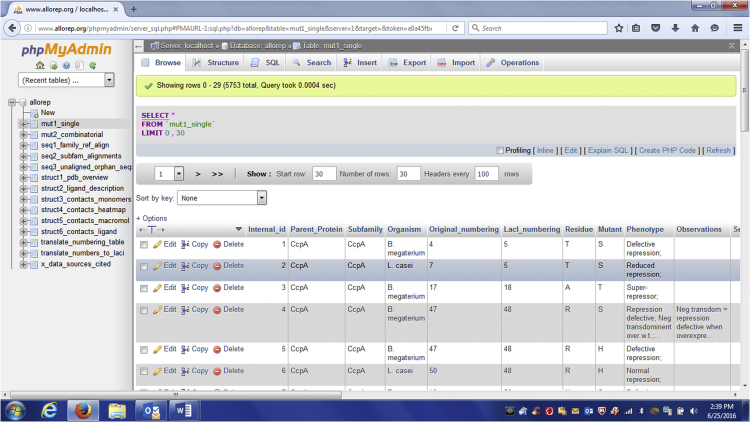
Screen shot of the AlloRep database. This screen shot was taken after entering the AlloRep database from the home webpage (www.AlloRep.org) and selecting the table “mut1_single” under the “allorep” tree that appears on the left-hand side of the window. When viewing this table under the “Browse” tab (the default option after choosing a table), individual entries can be browsed and specific features can be sorted by clicking on the column headings. For more advanced searches and filtering, the tabs near the top of the window can be used to reach the built-in search fields (“Search”) and the command-line tools (“SQL”). Example command-line queries are given in the supplement to this manuscript.
2. Experimental design, materials and methods
2.1. AlloRep database overview and description of modules
The AlloRep database, freely available at www.AlloRep.org, [1] is divided into five modules (Fig. 1). Below are explanations of relevant tables and abbreviation used in each section. The tables can be browsed within the website and sorted by clicking on various column headings. In addition, example command line SQL queries are given in the supplement that can be used to link the information between the various modules.
2.2. Module 1: mutagenesis data
This module contains information collected from an exhaustive literature search [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82]. The module entails two tables: “mut1_single” and “mut2_combinatorial”. For variants in “mut1_single”, all outcomes can be attributed to a single mutation, either by comparing the properties of a single mutation to those of the wild-type protein, or, for example, by comparing a double mutant to a variant that contains the relevant single mutation. Variants in the “mut2_combinatorial” table contain multiple mutations that have not yet been parsed into their component contributions.
Both tables contain fields for: a unique internal_id for each variant, the relevant LacI/GalR subfamily, species of origin, position number in the parent protein, position number translated to the LacI reference numbering system, one-letter codes for the original amino acid and the mutational variant, and PMIDs of the original publications. The mut1_single also contains the parent protein that provides the basis for comparison of experimental results.
In both tables, additional columns contain all available experimental information for the variant. Since experiments were carried out over several decades, in different laboratories, and sometimes under different experimental conditions (such as different buffers), the functional effects of each mutation are reported relative to the appropriate parent protein. Information regarding the effect on protein secondary structure and/or oligomerization state (where “D” stands for dimer, “T” for tetramer and “M” for monomer) are stored in columns with those names. Effects on urea stability, thermal denaturation, trypsin digestion assays, and temperature sensitivity are stored in other columns. The phenotypic and biochemical characterizations are provided in the “phenotype”, “allostery” and “reverse phenotype” columns. When possible, the relative differences are indicated with the symbols: [0] or [−−−] for total loss, [− −] for a significant decrease, [−] small decrease, [=] or ~ if comparable with wild type, [+] for small increase and [+ +] for a significant increase. Any additional information is provided in the “observation” column.
2.3. Module 2: sequence data
This module contains three tables with: (i) the manually-curated alignment of representative sequences for the entire LacI/GalR family (each homolog is contained in a separate row) [83]; (ii) the separate alignments for all subfamilies (each subfamily alignment is contained in one row); and (iii) a table containing unaligned “orphan” sequences (one per row) that do not match any of the current subfamilies. All data are stored in fasta format. After selecting a table of interest, it can be downloaded using the export button at the bottom of the page and selecting the desired format. Note that the output options can be customized for a better compatibility with the user׳s operating system. The subfamily alignments can be matched to the spacing of the manually-curated, whole-family alignment using the program MARS-Prot (https://github.com/djparente/MARS) [84].
2.4. Module 3: structural data
All available structures for LacI/GalR homologs [18], [21], [55], [57], [66], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102] were retrieved from the Protein Data Bank database [103]. This module contains all the information regarding the PDB description (struct1_pdb_overview table), available ligand information (struct2_ligand_description table), and four tables with different types of contacts.
For each LacI/GalR structure, non-covalent contacts were defined when any two residues had at least one non-hydrogen atom within 5 Å of the other. Angles and other geometries were not considered. For all structures, the full set of contacts is stored in the table “struct3_contacts_monomers” where contacts were grouped according to their protein subfamily, inter- or intra-monomeric nature, and ligand. Next, for the table “struct4_contacts_heatmap”, equivalent structures (those for the same protein and liganded state) were combined to calculate the frequency of each contact pair; these values are presented in a single column for each group of equivalent structures (Fig. 2). For example, apo LacI has two structures (1lbi and 3edc) each of which contains four monomers. In two of the 8 chains (25%), LacI residues E100 and C107 are within 5 Å of each other; thus the occupancy score for this contact is 25%. For states that have only one available structure, the default value is 100%.
The table “struct5_contacts_macromol” contains information regarding the contacts between the LacI/GalR proteins and macromolecular ligands such as DNA or heteroproteins. Contacts between LacI/GalR proteins and small-molecule ligands are stored in the table “struct6_contacts_ligand table”, which also includes information on the total contact surface area and the number of contacts.
2.5. Module 4: translation tables
This section contains two tables – “translate_numbering_table” and “translate_numbers_to_laci” – that allow the conversion between the numbering system of Escherichia coli LacI and those of other LacI/GalR homologs. “Translate_numbers_to_laci” contains the necessary information for connecting both structural or mutagenesis data to the “translate_numbering_table”. The “translate_numbering_table” contains the structural alignment of all crystallographic structures as well as representative sequences for each protein subfamily that has available mutagenesis data.
Using either the PDB identifier and residue numbering as input (from tables in the structural module) or information regarding the LacI/GalR subfamily and residue numbering as input (from tables in the mutation module), the user can obtain the code to be used in the translation_numbers_to_laci and retrieve the original sequence numbering.
2.6. Module 5: citations
A final module comprises one table (“x_data_sources_cited”) that contains all bibliographic information and can be queried using the PMID or the citation code provided in the structural and mutagenesis tables.
Acknowledgments
This work was supported by Fundação para a Ciência e Tecnologia, SFRH/BPD/73058/2010 (FLS), NIH GM 079423 (LSK), and the University of Kansas Medical Center Biomedical Research Training Program (DJP). We thank Tina Perica for many stimulating discussions about this project.
Footnotes
Transparency data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.dib.2016.07.006..
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2016.07.006.
Transparency document. Supplementary material
Supplementary material
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Sousa F.L., Parente D.J., Shis D.L., Hessman J.A., Chazelle A., Bennett M.R. AlloRep: a repository of sequence, structural and mutagenesis data for the LacI/GalR transcription regulators. J. Mol. Biol. 2016;428:671–678. doi: 10.1016/j.jmb.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markiewicz P., Kleina L.G., Cruz C., Ehret S., Miller J.H. Genetic studies of the lac repressor. XIV. Analysis of 4000 altered E. coli lac repressors reveals essential and non-essential residues, as well as “spacers” which do not require a specific sequence. J. Mol. Biol. 1994;240:421–433. doi: 10.1006/jmbi.1994.1458. [DOI] [PubMed] [Google Scholar]
- 3.Miller J.H. Genetic studies of the lac repressor. XII. Amino acid replacements in the DNA binding domain of the E. coli lac repressor. J. Mol. Biol. 1984;180:205–212. doi: 10.1016/0022-2836(84)90438-8. [DOI] [PubMed] [Google Scholar]
- 4.Kleina L.G., Miller J.H. Genetic studies of the lac repressor. XIII. Extensive amino acid replacements generated by the use of natural and synthetic nonsense suppressors. J. Mol. Biol. 1990;212:295–318. doi: 10.1016/0022-2836(90)90126-7. [DOI] [PubMed] [Google Scholar]
- 5.Suckow J., Markiewicz P., Kleina L.G., Miller J., Kisters-Woike B., Müller-Hill B. Genetic studies of the lac repressor. XV: 4000 single amino acid substitutions and analysis of the resulting phenotypes on the basis of the protein structure. J. Mol. Biol. 1996;261:509–523. doi: 10.1006/jmbi.1996.0479. [DOI] [PubMed] [Google Scholar]
- 6.Chakerian A.E., Tesmer V.M., Manly S.P., Brackett J.K., Lynch M.J., Hoh J.T. Evidence for leucine zipper motif in lactose repressor protein. J. Biol. Chem. 1991;266:1371–1374. [PubMed] [Google Scholar]
- 7.Kraus A., Kuster E., Wagner A., Hoffmann K., Hillen W. Identification of a co-repressor binding site in catabolite control protein CcpA. Mol. Microbiol. 1998;30:955–963. doi: 10.1046/j.1365-2958.1998.01123.x. [DOI] [PubMed] [Google Scholar]
- 8.Kallipolitis B.H., Valentin-Hansen P. A role for the interdomain linker region of the E. coli CytR regulator in repression complex formation. J. Mol. Biol. 2004;342:1–7. doi: 10.1016/j.jmb.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 9.Miller J.H., Coulondre C., Hofer M., Schmeissner U., Sommer H., Schmitz A. Genetic studies of the lac repressor. IX. Generation of altered proteins by the suppression of nonsence mutations. J. Mol. Biol. 1979;131:191–222. doi: 10.1016/0022-2836(79)90073-1. [DOI] [PubMed] [Google Scholar]
- 10.Geanacopoulos M., Adhya S. Genetic analysis of GalR tetramerization in DNA looping during repressosome assembly. J. Biol. Chem. 2002;277:33148–33152. doi: 10.1074/jbc.M202445200. [DOI] [PubMed] [Google Scholar]
- 11.Kuster E., Hilbich T., Dahl M.K., Hillen W. Mutations in catabolite control protein CcpA separating growth effects from catabolite repression. J. Bacteriol. 1999;181:4125–4128. doi: 10.1128/jb.181.13.4125-4128.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatti-Lafranconi P., Dijkman W.P., Devenish S.R.A., Hollfelder F. A single mutation in the core domain of the lac repressor reduces leakiness. Microb. Cell Fact. 2013;12:67. doi: 10.1186/1475-2859-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoury A.M., Nick H.S., Lu P. in vivo interaction of E. coli lac repressor N-terminal fragments with the lac operator. J. Mol. Biol. 1991;219:623–634. doi: 10.1016/0022-2836(91)90659-t. [DOI] [PubMed] [Google Scholar]
- 14.Choi K.Y., Lu F., Zalkin H. Mutagenesis of amino acid residues required for binding of corepressors to the purine repressor. J. Biol. Chem. 1994;269:24066–24072. [PubMed] [Google Scholar]
- 15.Kuster-Schock E., Wagner A., Volker U., Hillen W. Mutations in catabolite control protein CcpA showing glucose-independent regulation in Bacillus megaterium. J. Bacteriol. 1999;181:7634–7638. doi: 10.1128/jb.181.24.7634-7638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esteban C.D., Mahr K., Monedero V., Hillen W., Perez-Martinez G., Titgemeyer F. Complementation of a Delta ccpA mutant of Lactobacillus casei with CcpA mutants affected in the DNA- and cofactor-binding domains. Microbiology. 2004;150:613–620. doi: 10.1099/mic.0.26658-0. [DOI] [PubMed] [Google Scholar]
- 17.Meyer S., Ramot R., Kishore Inampudi K., Luo B., Lin C., Amere S. Engineering alternate cooperative-communications in the lactose repressor protein scaffold. Protein Eng. Des. Sel. 2013;26:433–443. doi: 10.1093/protein/gzt013. [DOI] [PubMed] [Google Scholar]
- 18.Lu F., Schumacher M.A., Arvidson D.N., Haldimann A., Wanner B.L., Zalkin H. Structure-based redesign of corepressor specificity of the E. coli purine repressor by substitution of residue 190. Biochemistry. 1998;37:971–982. doi: 10.1021/bi971942s. [DOI] [PubMed] [Google Scholar]
- 19.Chou W.Y., Matthews K.S. Mutation in hinge region of lactose repressor protein alters physical and functional properties. J. Biol. Chem. 1989;264:6171–6176. [PubMed] [Google Scholar]
- 20.Geanacopoulos M., Vasmatzis G., Zhurkin V.B., Adhya S. Gal repressosome contains an antiparallel DNA loop. Nat. Struct. Biol. 2001;8:432–436. doi: 10.1038/87595. [DOI] [PubMed] [Google Scholar]
- 21.Huffman J.L., Lu F., Zalkin H., Brennan R.G. Role of residue 147 in the gene regulatory function of the E. coli purine repressor. Biochemistry. 2002;41:511–520. doi: 10.1021/bi0156660. [DOI] [PubMed] [Google Scholar]
- 22.Kraus A., Hillen W. Analysis of CcpA mutations defective in carbon catabolite repression in Bacillus megaterium. FEMS Microbiol. Lett. 1997;153:221–226. doi: 10.1111/j.1574-6968.1997.tb10485.x. [DOI] [PubMed] [Google Scholar]
- 23.Barbier C.S., Short S.A., Senear D.F. Allosteric mechanism of induction of CytR-regulated gene expression. Cytr repressor-cytidine interaction. J. Biol. Chem. 1997;272:16962–16971. doi: 10.1074/jbc.272.27.16962. [DOI] [PubMed] [Google Scholar]
- 24.Barbier C.S., Short S.A. Characterization of cytR mutations that influence oligomerization of mutant repressor subunits. J. Bacteriol. 1993;175:4625–4630. doi: 10.1128/jb.175.15.4625-4630.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swint-Kruse L., Zhan H., Fairbanks B.M., Maheshwari A., Matthews K.S. Perturbation from a distance: mutations that alter LacI function through long-range effects. Biochemistry. 2003;42:14004–14016. doi: 10.1021/bi035116x. [DOI] [PubMed] [Google Scholar]
- 26.Barry J.K., Matthews K.S. Substitutions at histidine 74 and aspartate 278 alter ligand binding and allostery in lactose repressor protein. Biochemistry. 1999;38:3579–3590. doi: 10.1021/bi982577n. [DOI] [PubMed] [Google Scholar]
- 27.Semsey S., Geanacopoulos M., Lewis D.E.A., Adhya S. Operator-bound GalR dimers close DNA loops by direct interaction: tetramerization and inducer binding. EMBO J. 2002;21:4349–4356. doi: 10.1093/emboj/cdf431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbier C.S., Short S.A. Amino acid substitutions in the CytR repressor which alter its capacity to regulate gene expression. J. Bacteriol. 1992;174:2881–2890. doi: 10.1128/jb.174.9.2881-2890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miura-Onai S., Yabuta M., Ohsuye K. Mutational study at Ser300 position of the E. coli lactose repressor. Biochem. Biophys. Res. Commun. 1995;209:126–130. doi: 10.1006/bbrc.1995.1479. [DOI] [PubMed] [Google Scholar]
- 30.Spott S., Dong F., Kisters-Woike B., Müller-Hill B. Dimerisation mutants of lac repressor. II. A single amino acid substitution, D278L, changes the specificity of dimerisation. J. Mol. Biol. 2000;296:673–684. doi: 10.1006/jmbi.1999.3469. [DOI] [PubMed] [Google Scholar]
- 31.Li L., Matthews K.S. Characterization of mutants affecting the KRK sequence in the carboxyl-terminal domain of lac repressor. J. Biol. Chem. 1995;270:10640–10649. doi: 10.1074/jbc.270.18.10640. [DOI] [PubMed] [Google Scholar]
- 32.Bandyopadhyay P.K., Wu C.W. Heterogeneity of the two tryptophanyl residues in the lac repressor of E. coli. Arch. Biochem. Biophys. 1979;195:558–564. doi: 10.1016/0003-9861(79)90382-5. [DOI] [PubMed] [Google Scholar]
- 33.Chang W.I., Barrera P., Matthews K.S. Identification and characterization of aspartate residues that play key roles in the allosteric regulation of a transcription factor: aspartate 274 is essential for inducer binding in lac repressor. Biochemistry. 1994;33:3607–3616. doi: 10.1021/bi00178a018. [DOI] [PubMed] [Google Scholar]
- 34.Xu J., Matthews K.S. Flexibility in the inducer binding region is crucial for allostery in the Escherichia coli lactose repressor. Biochemistry. 2009;48:4988–4998. doi: 10.1021/bi9002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falcon C.M., Matthews K.S. Engineered disulfide linking the hinge regions within lactose repressor dimer increases operator affinity, decreases sequence selectivity, and alters allostery. Biochemistry. 2001;40:15650–15659. doi: 10.1021/bi0114067. [DOI] [PubMed] [Google Scholar]
- 36.Thomsen L.E., Pedersen M., Norregaard-Madsen M., Valentin-Hansen P., Kallipolitis B.H. Protein-ligand interaction: grafting of the uridine-specific determinants from the CytR regulator of Salmonella typhimurium to E. coli CytR. J. Mol. Biol. 1999;288:165–175. doi: 10.1006/jmbi.1999.2668. [DOI] [PubMed] [Google Scholar]
- 37.Chakerian A.E., Pfahl M., Olson J.S., Matthews K.S. A mutant lactose repressor with altered inducer and operator binding parameters. J. Mol. Biol. 1985;183:43–51. doi: 10.1016/0022-2836(85)90279-7. [DOI] [PubMed] [Google Scholar]
- 38.Chakerian A.E., Matthews K.S. Characterization of mutations in oligomerization domain of lac repressor protein. J. Biol. Chem. 1991;266:22206–22214. [PubMed] [Google Scholar]
- 39.Zhan H., Sun Z., Matthews K.S. Functional impact of polar and acidic substitutions in the lactose repressor hydrophobic monomer.monomer interface with a buried lysine. Biochemistry. 2009;48:1305–1314. doi: 10.1021/bi801357f. [DOI] [PubMed] [Google Scholar]
- 40.Mikulskis A., Aristarkhov A., Lin E.C. Regulation of expression of the ethanol dehydrogenase gene (adhE) in E. coli by catabolite repressor activator protein Cra. J. Bacteriol. 1997;179:7129–7134. doi: 10.1128/jb.179.22.7129-7134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spotts R.O., Chakerian A.E., Matthews K.S. Arginine 197 of lac repressor contributes significant energy to inducer binding. Confirmation of homology to periplasmic sugar binding proteins. J. Biol. Chem. 1991;266:22998–23002. [PubMed] [Google Scholar]
- 42.Muller-Hartmann H., Müller-Hill B. The side-chain of the amino acid residue in position 110 of the lac repressor influences its allosteric equilibrium. J. Mol. Biol. 1996;257:473–478. doi: 10.1006/jmbi.1996.0176. [DOI] [PubMed] [Google Scholar]
- 43.Ebright R.H. Evidence for a contact between glutamine-18 of lac repressor and base pair 7 of lac operator. Proc. Natl. Acad. Sci. USA. 1986;83:303–307. doi: 10.1073/pnas.83.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerk L.P., Leven O., Müller-Hill B. Strengthening the dimerisation interface of lac repressor increases its thermostability by 40 deg. C. J. Mol. Biol. 2000;299:805–812. doi: 10.1006/jmbi.2000.3706. [DOI] [PubMed] [Google Scholar]
- 45.Zhan H., Swint-Kruse L., Matthews K.S. Extrinsic interactions dominate helical propensity in coupled binding and folding of the lactose repressor protein hinge helix. Biochemistry. 2006;45:5896–5906. doi: 10.1021/bi052619p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsieh M., Hensley P., Brenowitz M., Fetrow J.S. A molecular model of the inducer binding domain of the galactose repressor of E. coli. J. Biol. Chem. 1994;269:13825–13835. [PubMed] [Google Scholar]
- 47.Meinhardt S., Manley M.W.J., Becker N.A., Hessman J.A., Maher L.J.3, Swint-Kruse L. Novel insights from hybrid LacI/GalR proteins: family-wide functional attributes and biologically significant variation in transcription repression. Nucleic Acids Res. 2012;40:11139–11154. doi: 10.1093/nar/gks806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Semsey S. Mutations in transcriptional regulators allow selective engineering of signal integration logic. MBio. 2014;5 doi: 10.1128/mBio.01171-14. e01171–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Falcon C.M., Matthews K.S. Glycine insertion in the hinge region of lactose repressor protein alters DNA binding. J. Biol. Chem. 1999;274:30849–30857. doi: 10.1074/jbc.274.43.30849. [DOI] [PubMed] [Google Scholar]
- 50.Lu F., Brennan R.G., Zalkin H. E. coli purine repressor: key residues for the allosteric transition between active and inactive conformations and for interdomain signaling. Biochemistry. 1998;37:15680–15690. doi: 10.1021/bi981617k. [DOI] [PubMed] [Google Scholar]
- 51.Chang W.I., Olson J.S., Matthews K.S. Lysine 84 is at the subunit interface of lac repressor protein. J. Biol. Chem. 1993;268:17613–17622. [PubMed] [Google Scholar]
- 52.Roy S., Semsey S., Liu M., Gussin G.N., Adhya S. GalR represses galP1 by inhibiting the rate-determining open complex formation through RNA polymerase contact: a GalR negative control mutant. J. Mol. Biol. 2004;344:609–618. doi: 10.1016/j.jmb.2004.09.070. [DOI] [PubMed] [Google Scholar]
- 53.Qian Z., Dimitriadis E.K., Edgar R., Eswaramoorthy P., Adhya S. Galactose repressor mediated intersegmental chromosomal connections in E. coli. Proc. Natl. Acad. Sci. USA. 2012;109:11336–11341. doi: 10.1073/pnas.1208595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y.N., Chatterjee S., Roy S., Adhya S. The non-inducible nature of super-repressors of the gal operon in E. coli. J. Mol. Biol. 1995;253:414–425. doi: 10.1006/jmbi.1995.0563. [DOI] [PubMed] [Google Scholar]
- 55.Glasfeld A., Koehler A.N., Schumacher M.A., Brennan R.G. The role of lysine 55 in determining the specificity of the purine repressor for its operators through minor groove interactions. J. Mol. Biol. 1999;291:347–361. doi: 10.1006/jmbi.1999.2946. [DOI] [PubMed] [Google Scholar]
- 56.Xu J., Liu S., Chen M., Ma J., Matthews K.S. Altering residues N125 and D149 impacts sugar effector binding and allosteric parameters in E. coli lactose repressor. Biochemistry. 2011;50:9002–9013. doi: 10.1021/bi200896t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arvidson D.N., Lu F., Faber C., Zalkin H., Brennan R.G. The structure of PurR mutant L54M shows an alternative route to DNA kinking. Nat. Struct. Biol. 1998;5:436–441. doi: 10.1038/nsb0698-436. [DOI] [PubMed] [Google Scholar]
- 58.Gardner J.A., Matthews K.S. Characterization of two mutant lactose repressor proteins containing single tryptophans. J. Biol. Chem. 1990;265:21061–21067. [PubMed] [Google Scholar]
- 59.Meinhardt S., Swint-Kruse L. Experimental identification of specificity determinants in the domain linker of a LacI/GalR protein: bioinformatics-based predictions generate true positives and false negatives. Proteins. 2008;73:941–957. doi: 10.1002/prot.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tungtur S., Meinhardt S., Swint-Kruse L. Comparing the functional roles of nonconserved sequence positions in homologous transcription repressors: implications for sequence/function analyses. J. Mol. Biol. 2010;395:785–802. doi: 10.1016/j.jmb.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tungtur S., Egan S.M., Swint-Kruse L. Functional consequences of exchanging domains between LacI and PurR are mediated by the intervening linker sequence. Proteins. 2007;68:375–388. doi: 10.1002/prot.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meinhardt S., Manley M.W., Parente D.J., Swint-Kruse L. Rheostats and toggle switches for modulating protein function. PLoS One. 2013;8:e83502. doi: 10.1371/journal.pone.0083502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swint-Kruse L., Elam C.R., Lin J.W., Wycuff D.R., Shive Matthews K. Plasticity of quaternary structure: twenty-two ways to form a LacI dimer. Protein Sci. 2001;10:262–276. doi: 10.1110/ps.35801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi K.Y., Zalkin H. Role of the purine repressor hinge sequence in repressor function. J. Bacteriol. 1994;176:1767–1772. doi: 10.1128/jb.176.6.1767-1772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhan H., Camargo M., Matthews K.S. Positions 94–98 of the lactose repressor N-subdomain monomer-monomer interface are critical for allosteric communication. Biochemistry. 2010;49:8636–8645. doi: 10.1021/bi101106x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schumacher M.A., Allen G.S., Diel M., Seidel G., Hillen W., Brennan R.G. Structural basis for allosteric control of the transcription regulator CcpA by the phosphoprotein HPr-Ser46-P. Cell. 2004;118:731–741. doi: 10.1016/j.cell.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 67.Geanacopoulos M., Vasmatzis G., Lewis D.E., Roy S., Lee B., Adhya S. GalR mutants defective in repressosome formation. Genes Dev. 1999;13:1251–1262. doi: 10.1101/gad.13.10.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawabata T., Ota M., Nishikawa K. The Protein Mutant Database. Nucleic Acids Res. 1999;27:355–357. doi: 10.1093/nar/27.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swint-Kruse L., Zhan H., Matthews K.S. Integrated insights from simulation, experiment, and mutational analysis yield new details of LacI function. Biochemistry. 2005;44:11201–11213. doi: 10.1021/bi050404+. [DOI] [PubMed] [Google Scholar]
- 70.Alberti S., Oehler S., Wilcken-Bergmann von B., Krämer H., Müller-Hill B. Dimer-to-tetramer assembly of lac repressor involves a leucine heptad repeat. New Biol. 1991;3:57–62. [PubMed] [Google Scholar]
- 71.Nichols J.C., Matthews K.S. Combinatorial mutations of lac repressor. Stability of monomer-monomer interface is increased by apolar substitution at position 84. J. Biol. Chem. 1997;272:18550–18557. doi: 10.1074/jbc.272.30.18550. [DOI] [PubMed] [Google Scholar]
- 72.Royer C.A., Gardner J.A., Beechem J.M., Brochon J.C., Matthews K.S. Resolution of the fluorescence decay of the two tryptophan residues of lac repressor using single tryptophan mutants. Biophys. J. 1990;58:363–378. doi: 10.1016/S0006-3495(90)82383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barry J.K., Matthews K.S. Ligand-induced conformational changes in lactose repressor: a fluorescence study of single tryptophan mutants. Biochemistry. 1997;36:15632–15642. doi: 10.1021/bi971685r. [DOI] [PubMed] [Google Scholar]
- 74.Ozarowski A., Barry J.K., Matthews K.S., Maki A.H. Ligand-induced conformational changes in lactose repressor: a phosphorescence and ODMR study of single-tryptophan mutants. Biochemistry. 1999;38:6715–6722. doi: 10.1021/bi990242f. [DOI] [PubMed] [Google Scholar]
- 75.Dong F., Spott S., Zimmermann O., Kisters-Woike B., Müller-Hill B., Barker A. Dimerisation mutants of lac repressor. I. A monomeric mutant, L251A, that binds Lac operator DNA as a dimer. J. Mol. Biol. 1999;290:653–666. doi: 10.1006/jmbi.1999.2902. [DOI] [PubMed] [Google Scholar]
- 76.Schmitz A., Schmeissner U., Miller J.H. Mutations affecting the quaternary structure of the lac repressor. J. Biol. Chem. 1976;251:3359–3366. [PubMed] [Google Scholar]
- 77.Daly T.J., Matthews K.S. Characterization and modification of a monomeric mutant of the lactose repressor protein. Biochemistry. 1986;25:5474–5478. doi: 10.1021/bi00367a019. [DOI] [PubMed] [Google Scholar]
- 78.Falcon C.M., Swint-Kruse L., Matthews K.S. Designed disulfide between N-terminal domains of lactose repressor disrupts allosteric linkage. J. Biol. Chem. 1997;272:26818–26821. doi: 10.1074/jbc.272.43.26818. [DOI] [PubMed] [Google Scholar]
- 79.Semsey S., Tolstorukov M.Y., Virnik K., Zhurkin V.B., Adhya S. DNA trajectory in the Gal repressosome. Genes Dev. 2004;18:1898–1907. doi: 10.1101/gad.1209404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lehming N., Sartorius J., Kisters-Woike B., Wilcken-Bergmann von B., Müller-Hill B. Mutant lac repressors with new specificities hint at rules for protein–DNA recognition. EMBO J. 1990;9:615–621. doi: 10.1002/j.1460-2075.1990.tb08153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barker A., Fickert R., Oehler S., Müller-Hill B. Operator search by mutant lac repressor. J. Mol. Biol. 1998;278:549–558. doi: 10.1006/jmbi.1998.1729. [DOI] [PubMed] [Google Scholar]
- 82.Lehming N., Sartorius J., Niemöller M., Genenger G., Wilcken-Bergmann von B., Müller-Hill B. The interaction of the recognition helix of lac repressor with lac operator. EMBO J. 1987;6:3145–3153. doi: 10.1002/j.1460-2075.1987.tb02625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tungtur S., Parente D.J., Swint-Kruse L. Functionally important positions can comprise the majority of a protein׳s architecture. Proteins. 2011;79:1589–1608. doi: 10.1002/prot.22985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parente D.J., Ray J.C.J., Swint-Kruse L. Amino acids positions subject to multiple co-evolutionary constraints can be robustly identified by their eigenvector network centrality scores. Proteins. 2015;83:2293–2306. doi: 10.1002/prot.24948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee D., de Beer T.A.P., Laskowski R.A., Thornton J.M., Orengo C.A. 1,000 structures and more from the MCSG. BMC Struct. Biol. 2011;11:2. doi: 10.1186/1472-6807-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chavarria M., Santiago C., Platero R., Krell T., Casasnovas J.M., de Lorenzo V. Fructose 1-phosphate is the preferred effector of the metabolic regulator Cra of Pseudomonas putida. J. Biol. Chem. 2011;286:9351–9359. doi: 10.1074/jbc.M110.187583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schumacher M.A., Sprehe M., Bartholomae M., Hillen W., Brennan R.G. Structures of carbon catabolite protein A-(HPr-Ser46-P) bound to diverse catabolite response element sites reveal the basis for high-affinity binding to degenerate DNA operators. Nucleic Acids Res. 2011;39:2931–2942. doi: 10.1093/nar/gkq1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stenberg K.A.E., Vihinen M. Crystal structure of a 1.6-hexanediol bound tetrameric form of E. coli lac-repressor refined to 2.1 A resolution. Proteins. 2009;75:748–759. doi: 10.1002/prot.22284. [DOI] [PubMed] [Google Scholar]
- 89.Daber R., Stayrook S., Rosenberg A., Lewis M. Structural analysis of lac repressor bound to allosteric effectors. J. Mol. Biol. 2007;370:609–619. doi: 10.1016/j.jmb.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loll B., Saenger W., Biesiadka J. Structure of full-length transcription regulator CcpA in the apo form. Biochim. Biophys. Acta. 2007;1774:732–736. doi: 10.1016/j.bbapap.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 91.Schumacher M.A., Seidel G., Hillen W., Brennan R.G. Structural mechanism for the fine-tuning of CcpA function by the small molecule effectors glucose 6-phosphate and fructose 1,6-bisphosphate. J. Mol. Biol. 2007;368:1042–1050. doi: 10.1016/j.jmb.2007.02.054. [DOI] [PubMed] [Google Scholar]
- 92.Loll B., Kowalczyk M., Alings C., Chieduch A., Bardowski J., Saenger W. Structure of the transcription regulator CcpA from Lactococcus lactis. Acta Crystallogr. D Biol. Crystallogr. 2007;63:431–436. doi: 10.1107/S0907444907000546. [DOI] [PubMed] [Google Scholar]
- 93.Chaptal V., Gueguen-Chaignon V., Poncet S., Lecampion C., Meyer P., Deutscher J. Structural analysis of Bacillus subtilis CcpA effector binding site. Proteins. 2006;64:814–816. doi: 10.1002/prot.21001. [DOI] [PubMed] [Google Scholar]
- 94.Schumacher M.A., Seidel G., Hillen W., Brennan R.G. Phosphoprotein Crh-Ser46-P displays altered binding to CcpA to effect carbon catabolite regulation. J. Biol. Chem. 2006;281:6793–6800. doi: 10.1074/jbc.M509977200. [DOI] [PubMed] [Google Scholar]
- 95.Bell C.E., Lewis M. Crystallographic analysis of lac repressor bound to natural operator O1. J. Mol. Biol. 2001;312:921–926. doi: 10.1006/jmbi.2001.5024. [DOI] [PubMed] [Google Scholar]
- 96.Bell C.E., Lewis M. A closer view of the conformation of the lac repressor bound to operator. Nat. Struct. Biol. 2000;7:209–214. doi: 10.1038/73317. [DOI] [PubMed] [Google Scholar]
- 97.Hars U., Horlacher R., Boos W., Welte W., Diederichs K. Crystal structure of the effector-binding domain of the trehalose-repressor of E. coli, a member of the LacI family, in its complexes with inducer trehalose-6-phosphate and noninducer trehalose. Protein Sci. 1998;7:2511–2521. doi: 10.1002/pro.5560071204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schumacher M.A., Glasfeld A., Zalkin H., Brennan R.G. The X-ray structure of the PurR-guanine-purF operator complex reveals the contributions of complementary electrostatic surfaces and a water-mediated hydrogen bond to corepressor specificity and binding affinity. J. Biol. Chem. 1997;272:22648–22653. doi: 10.1074/jbc.272.36.22648. [DOI] [PubMed] [Google Scholar]
- 99.Lewis M., Chang G., Horton N.C., Kercher M.A., Pace H.C., Schumacher M.A. Crystal structure of the lactose operon repressor and its complexes with DNA and inducer. Science. 1996;271:1247–1254. doi: 10.1126/science.271.5253.1247. [DOI] [PubMed] [Google Scholar]
- 100.Schumacher M.A., Choi K.Y., Lu F., Zalkin H., Brennan R.G. Mechanism of corepressor-mediated specific DNA binding by the purine repressor. Cell. 1995;83:147–155. doi: 10.1016/0092-8674(95)90243-0. [DOI] [PubMed] [Google Scholar]
- 101.Friedman A.M., Fischmann T.O., Steitz T.A. Crystal structure of lac repressor core tetramer and its implications for DNA looping. Science. 1995;268:1721–1727. doi: 10.1126/science.7792597. [DOI] [PubMed] [Google Scholar]
- 102.Schumacher M.A., Choi K.Y., Zalkin H., Brennan R.G. Crystal structure of LacI member, PurR, bound to DNA: minor groove binding by alpha helices. Science. 1994;266:763–770. doi: 10.1126/science.7973627. [DOI] [PubMed] [Google Scholar]
- 103.Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material