Abstract
BACKGROUND
The subgenual anterior cingulate cortex (sgACC) and its connected circuitry have been heavily implicated in emotional functioning in adolescent-onset major depressive disorder (MDD). While several recent studies have examined sgACC functional connectivity (FC) in depressed youth at rest, no studies to date have investigated sgACC FC in adolescent depression during negative emotional processing.
METHODS
Nineteen medication-naïve adolescents with MDD and 19 matched healthy controls (HCL) performed an implicit fear facial affect recognition task during functional magnetic resonance imaging (fMRI). We defined seeds in bilateral sgACC and assessed FC using the psychophysiological interaction method. We also applied cognitive behavioral modeling to estimate group differences in perceptual sensitivity in this task. Finally, we correlated connectivity strength with clinical data and perceptual sensitivity.
RESULTS
Depressed adolescents showed increased sgACC-amygdala FC and decreased sgACC-fusiform gyrus, sgACC-precuneus, sgACC-insula, and sgACC-middle frontal gyrus FC compared to HCL (p<0.05, corrected). Among the MDD, sgACC-precuneus FC negatively correlated with depression severity (p<0.05, corrected). Lastly, MDD adolescents exhibited poorer perceptual sensitivity in the task than HCL, and individual differences in perceptual sensitivity significantly correlated with sgACC FC and depression scores (p<0.05, corrected).
LIMITATIONS
Subjects were clinically homogenous, possibly limiting generalizability of the findings.
CONCLUSIONS
Adolescent depression is associated with biased processing of negative stimuli that may be driven by sgACC dysregulation and may possibly lead to an imbalance among intrinsic functional brain networks. This work also establishes the use of combining neuroimaging and cognitive behavioral modeling methods to investigate cognitive and neural differences between psychiatric and healthy populations.
Keywords: functional connectivity, adolescent depression, linear ballistic accumulator model, functional magnetic resonance imaging, subgenual anterior cingulate cortex, psychophysiological interaction
Introduction
Functional magnetic resonance imaging (fMRI) studies have contributed greatly to the understanding of the neural networks in major depressive disorder (MDD). Recent evidence suggests that MDD is partially characterized by dramatic alterations in the functional connectivity (FC) of brain regions involved in emotion processing (Greicius 2008; Stuhrmann et al., 2011). Since MDD typically begins during adolescence (Avenevoli et al., 2008; Kessler et al., 2001; 2007) and confers a high risk of recurrence into adulthood (Lewinsohn et al., 1999), examining the FC of brain regions during adolescent depression could elucidate the etiology of this disorder in the context of brain changes that occur during this sensitive period of development (Somerville et al., 2010; Pine, 2007).
The subgenual anterior cingulate cortex (sgACC) and its connected circuitry have been heavily implicated in emotion function and in adult depression (Hamani et al., 2011; Mayberg, 1997; Mayberg et al., 1997; 2005; Drevets et al., 2008; Greicius et al., 2007; Johansen-Berg et al., 2008). Given its anatomical connections to subcortical and cortical structures, the sgACC is thought to lie at the interface of affective and cognitive processing, such that aberrant functioning in this region leads to impaired emotional regulation. In adolescents, altered resting-state FC of the sgACC has recently been documented in depressed adolescents and young adults relative to healthy controls (Cullen et al., 2009; Davey et al., 2012; Connolly et al., 2013, Gabbay et al., 2013). Specifically, aberrant FC has been observed between the sgACC and the amygdala (Connolly et al., 2013), insula (Cullen et al., 2009; Connolly et al., 2013), dorsal medial prefrontal cortex (Cullen et al., 2009; Davey et al., 2012), dorsolateral prefrontal cortex (Connolly et al., 2013), precuneus (Connolly et al., 2013), middle frontal gyrus (Connolly et al., 2013) and striatum (Gabbay et al., 2013). These results suggest an imbalance among salience (which include limbic, paralimbic, and striatal structures), cognitive executive (which include medial and lateral prefrontal and frontal cortices), and resting-state (which include posterior cingulate and precuneus) networks that may be mediated by the sgACC (Seeley et al., 2007; Dosenbach et al., 2008; Vincent et al., 2008; Fransson and Marrelec, 2008). However, these observed FC differences in adolescent and young adult depression have been inconsistent, in part because of the medication status, age range, and comorbidities of the participants recruited. It is therefore important to examine sgACC FC in non-medicated depressed adolescents with no comorbidities so that these factors do not confound interpretation of results.
Additionally, the aforementioned data were measured while subjects were at rest and are therefore unable to answer the question of how sgACC FC patterns among the salience, cognitive executive, and resting-state networks are affected during active emotional processing. Although there has yet to be any published work of sgACC-based functional connectivity during emotion processing in adolescents with MDD, recent neuroimaging work has examined FC differences in depressed adults during processing of negative material (Chen et al., 2008; Carballedo et al, 2011; Matthews et al., 2008; Almeida et al., 2011). These studies focused primarily on the amygdala and found disrupted functional connections with the sgACC and other nodes in the salience and cognitive executive networks. Given that adults (Foland-Ross and Gotlib, 2012; Foland-Ross et al., 2013; Gotlib et al., 2004; Joormann and Gotlib, 2006), adolescents with depression (Hankin et al., 2012), and even youth with a high familial risk for depression (Joormann et al., 2007; 2010; Kujawa et al., 2011; Romens and Pollack, 2012; Lopez-Duran et al., 2013) all exhibit behavioral biases towards affectively negative stimuli, we hypothesize that these sgACC-based FC disruptions among key brain networks may be reflective of the cognitive differences observed between MDD subjects and healthy controls (HCL) during the evaluation of negative material.
Thus, in order to better elucidate the role of the sgACC in adolescent depression as it pertains to negative emotional processing, the aim of the present study was two-fold: (1) investigate possible cognitive differences between MDD and HCL adolescents, and (2) examine and compare sgACC FC between these two groups to determine if and how salience, cognitive executive, and resting-state networks are affected by the processing of negative stimuli. To date, there are no studies of sgACC FC in adolescent depression during processing of negative emotional material. Thus, we applied functional magnetic resonance imaging (fMRI) to investigate sgACC FC in 19 adolescents (13–17 years old) with a current diagnosis of MDD and 19 matched HCL while subjects performed a gender discrimination task of face images exhibiting varying degrees of fear. Importantly, our depressed group was naïve to antidepressants and without psychiatric comorbidities. We defined seeds in bilateral sgACC and assessed FC using a psychophysiological interaction analysis (Friston et al., 1997). Depression severity was measured with the Beck Depression Inventory (BDI-II; Beck et al., 1996). To measure aspects of information processing in addition to simply mean accuracy and response time on the behavioral task, we adopted a commonly used cognitive behavioral model, the Linear Ballistic Accumulator (LBA; Brown and Heathcote, 2008), that allowed us to compute and localize cognitive differences in emotional processing between MDD and HCL adolescents. Based on prior literature in both adult and adolescent depression, we predict finding cognitive processing differences during evaluation of negative emotional stimuli between MDD and HCL adolescents and that these differences would be reflected as alterations in functional coupling between the sgACC and structures in the salience, cognitive executive, and resting-state networks.
Methods
Subjects
Forty-two right-handed adolescents (ages 13–17 years) were recruited for the study. Four subjects were excluded from the final analysis due to excessive motion. We therefore report results for 19 adolescents with a current primary DSM-IV diagnosis of MDD (mean age ± SD: 15.8 ± 1.4 years; 8 males) and 19 HCL adolescents (16.1 ± 1.2 years; 8 males). Subject groups were equivalent on major demographic variables (see Table 1). This study was approved by the Institutional Review Boards at the University of California, San Diego, Rady Children’s Hospital, and the County of San Diego. Please see Recruitment and Assessment of Subjects in Supplementary Material for more details.
Table 1. Summary of the sociodemographic and clinical data for the MDD and HCL adolescents.
Entries are of the form: mean ± standard error of mean (SEM). Statistical analyses were conducted with chi-squared tests (χ2), Student t-tests (t), and Wilcoxon Rank Sum test (U).
| Characteristic | MDD | HCL | df | Statistic | p-value |
|---|---|---|---|---|---|
| Gender (M / F) | 8 / 11 | 8 / 11 | 1 | χ2=1 | 0 |
| Age (years) | 15.8 ± 1.4 | 16.1 ± 1.2 | 36 | t=0.81 | 0.42 |
| Ethnicity (African / Asian / Hispanic / Caucasian / Mixed) | 1 / 1 / 8 / 6 / 3 | 0 / 2 / 5 / 10 / 2 | N/A | U =206.5 | 0.41 |
| Beck Depression Inventory II | 23.05 ± 2.6 | 2.94 ± 1.0 | 35 | t=7.20 | 0.0001 |
| Age of first episode onset (years) | 12 ± 0.73 | ||||
| Duration of MDD (months) | 25.8 ± 6.1 | ||||
| # of MDD episodes | 3 ± 1.2 | ||||
MDD=major depressive disorder; HCL=healthy control; df=degrees of freedom; N/A=not applicable.
Exclusionary criteria for adolescents with MDD included any psychiatric comorbidities, left-handedness, being color blind or having less than 20/40 correctable vision, contraindication to MR imaging (e.g., pregnancy, claustrophobia, metallic implants), a serious medical or neurological illness, a learning disability, prior or present use of antidepressants, the use of medication with CNS effects within the past 2 weeks, evidence of illicit drug use or misuse of prescription drugs, and more than 2 alcoholic drinks per week or within the previous month at the time of scanning. Please see Table 1 for a summary of the clinical characteristics of our depressed subjects.
HCL adolescents were excluded from the study for any of the exclusionary criteria for the MDD group, as well as any current or lifetime Axis I psychiatric disorder, any family history of mood or psychotic disorders in first- or second-degree relatives.
Image acquisition
All scanning was carried out on a GE Signa Excite 3T scanner (General Electric, Milwaukee, WI) with Twin Speed gradients and a GE 8-channel head coil. For details on scan parameters, see Image Acquisition under Supplementary Material. During scanning, subjects lay supine in the bore of the magnet and were instructed to relax but remain awake and as still as possible. Visual stimuli were projected onto a screen and viewed through a small, angled mirror mounted above the subject’s head.
Behavioral task and stimulus
Our behavioral task was adopted from a previously published PET paradigm (Morris et al., 1998) and was created and presented using an in-house Tcl script (http://www.tcl.tk/software/tcltk/). Ten faces (5 female) from a standardized series of facial expressions of fear (Ekman and Friesen, 1976) were morphed using computer graphical manipulation (Morris et al., 1998; Perrett et al., 1994) to represent three graded intensities of fear: strong (100%), moderate (50%), and neutral (0%). Facial stimuli and baseline trials (crosshair fixation) were presented in pseudorandom order. The facial stimuli were presented twice at each level of the fear intensities (see Figure 1a for faces representative of each fear level), along with 12 baseline trials, for a total of 72 trials. Each trial was presented for 3000 ms, with the inter-trial interval (ITI) randomly varying according to a Poisson distribution (mean ITI = 2000 ms). The total duration of the experimental run was therefore 360 seconds. For each facial trial, subjects were asked to indicate the gender of the face (male or female) by pushing one of two buttons on an MR compatible button box (Current Designs, Philadelphia, PA). These choices were displayed in boxed text on the bottom left and right corners but disappeared once a response was made (see Figure 1b for an example). Response time (RT) and accuracy of gender decision during scanning were recorded for each trial. However, several behavioral files were lost due to technical difficulties during data transfer. We therefore report behavioral data from 16 MDD and 13 HCL for all analyses involving RT and accuracy.
Figure 1. Implicit fear facial affective recognition paradigm.
60 facial trials and 12 baseline trials (crosshair fixation) were presented in pseudorandom order. Facial stimuli displayed one of three fear levels: FearStrong (FS, 1 00%), FearModerate (FM; 50%), and FearNeutral (FN; 0%) and were presented twice at each level of the fear intensities (a). Each trial lasted 3000 ms, with an inter-trial interval (ITI) randomly varying according to a Poisson distribution (mean ITI=2000 ms). For each facial trial, subjects were asked to indicate the gender of the face (male or female) by pushing one of two buttons on a button box. These choices were displayed in boxed text on the bottom left and right corners but will disappear once a response is made (b). See Behavioral task and stimuli under Methods for more details.
Image preprocessing and analysis
All image processing and analyses were conducted with the Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). We employed standard steps for fMRI image preprocessing (see Image Processing under Supplementary Material for details). Briefly, regressors-of-interest modeled for each voxel’s time series included the three trial types: FearStrong, FearModerate, and FearNeutral. Six motion parameters and the time points flagged as outliers were considered nuisance regressors to account for motion artifacts. Linear trend was also modeled in the time series of each voxel to account for correlated drift. Finally, the data were converted to percent signal change by dividing the time series of each voxel by the mean global signal, smoothed with a Gaussian filter with a full-width half-maximum (FWHM) kernel of 4 mm, and transformed to stereotaxic coordinates (Talairach and Tournoux, 1988). Since the primary focus of this study was on negative emotion processing, we limited our voxel-based analyses to the linear contrast of FearStrong-FearNeutral to maximize fear-related activation.
Group and task effects
Regions of significant group differences (MDD versus HCL) were determined by running a voxel-based two-sample t-test on the beta weights estimated from the condition of interest (FearStrong-FearNeutral). An analysis of task effect was also conducted by running a one-sample t-test on the beta weights for this task condition from all subjects.
Controlling for multiple comparisons
For all fMRI analyses reported here, significant voxels were required to pass a voxel-wise statistical threshold of t36=2.029 (p=0.05, uncorrected). To control for multiple comparisons, we computed the minimum number of contiguous voxels passing the voxel-wise threshold that would result in a cluster-wise 5% probability of being due to chance using 10,000 iterations of Monte Carlo simulations based on an average skull-stripped whole brain mask created from all subjects (downsampled to 4×4×4 mm) and the applied FWHM values of the functional data. According to our simulations, this cluster threshold was 11 voxels (704 μL).
Region-of-interest (ROI) seed definitions
We defined anatomical bilateral sgACC seeds based on a prior study of cingulate connectivity (Marguiles et al., 2007) that were also recently used to examine sgACC connectivity in adolescent depression during resting-state (Connolly et al., 2013). The seeds were converted from MNI to Talairach space and resampled to 4×4×4mm, resulting in the following Talairach coordinates for right and left sgACC, respectively: x=6, y=−23, z=−8 and x=−2, y=−23, z=−8. Each seed comprised 7 voxels (448 μL).
Functional connectivity analysis
Functional connectivity methods were conducted according to previously published work (Simmons et al., 2008; Fonzo et al., 2010; Perlman et al., 2012) using the psychophysiological interaction method (PPI; Friston et al., 1997) adapted for AFNI (http://afni.nimh.nih.gov/sscc/gangc/CD-CorrAna.html). PPI analysis assesses whether connectivity between brain regions change under different psychological task conditions (Friston et al., 1997). Separate analyses were performed for each seed. The individual raw time series data underwent slice-time correction, Gaussian spatial smoothing with a 4.0 mm FWHM kernel, and bandpass filtering (0.009 < f < 0.08). Data points were despiked and censored if they differed by more than 2.5 standard deviations from the average EPI signal of the seed. For the first-level analysis, the deconvolved time series were extracted from each seed, multiplied with the condition regressor (FearStrong-FearNeutral), and then convolved with a modified gamma variate function to yield the interaction time series. Next, a multiple regression model was run separately for each seed to estimate the regression coefficient between all voxels and the interaction time series (along with task, movement, and linear drift as nuisance regressors). The strength of association between all voxels and the interaction time series was measured with R2 values. These coefficients of determination were square-rooted then multiplied by the sign of their respective estimated beta weights to obtain directionality of association. The correlation coefficients of the interaction time series were then converted to z-scores using Fisher’s transformation. The resulting statistical maps were then included in a second-level group analysis (MDD versus HCL) by running a voxel-based two-sample t-test on the z-scores of the interaction effect for each seed separately.
Linear ballistic accumulation (LBA) analysis
The LBA conceives of a two-choice decision as a race between two choice alternatives that begin at a start point (a) and accumulate evidence in favor of each respective choice (here, male or female; see Figure S1 for a schematic of this process). The first accumulator to gather the criterion amount of evidence (response threshold, b) determines the subject’s choice (e.g., male); the time taken to reach the response threshold (plus an extra constant time for sensory and motor processes, non-decision time, t0) determines the response latency. The average speed at which each accumulator (one representing the correct response and one representing the error response) approaches threshold is termed the accumulator’s drift rate. The difference between the drift rates of correct (vc) and error accumulators (ve) corresponds to perceptual sensitivity and can be thought of as a dynamic version of d’ in signal detection theory (Ratcliff and McKoon, 2008). One advantage of a measure like drift rate over d’, however, is that RT information is utilized in its calculation, instead of only hit rates and false alarms (Ratcliff and McKoon, 2008; White et al., 2010).
A hierarchical Bayesian method was employed to simultaneously uncover individual-participant parameters (which we used to correlate with individual differences in FC and clinical data) and group-level parameters (which we used to determine differences in cognitive processing between groups). Details of the estimation procedure can be found in LBA Parameter Estimation under Supplementary Material (see also Turner et al., 2012). Finally, we computed odds ratios (ORs) to provide a measure of statistical evidence for a difference between the group-level parameter distributions. For each group and each parameter we compared samples exhaustively drawn from the true distribution. A count was produced reflecting when the value drawn from the MDD distribution was larger than the value drawn from the HCL distribution. The mean count was then divided by 1 minus this count. All ORs were therefore calculated to be greater than 1, for ease of interpretation.
Sociodemographic and clinical scales analysis
Statistical analyses of all demographic and clinical scales were computed with R (R Development Core Team, 2012; http://www.r-project.org/) and Matlab (version 7.10; Natwick, MA). Within the MDD group only, correlations between extracted FC values (i.e., mean Fisher’s z-scores) in the significant clusters identified in the PPI analysis and depression severity (i.e., BDI-II scores) were examined using two-tailed tests of Spearman’s rank correlation coefficient (rs). Among all the subjects with behavioral data, participant-level parameter estimates from the LBA model were also correlated with extracted FC values in the significant clusters identified in the PPI analyses, as well as depression severity (two-tailed tests of rs).
Results
Sociodemographic and clinical scales
The MDD and HCL groups did not significantly differ in age (t36=0.68, p=0.50), gender (χ21=0, p =1), and ethnicity (U=206.5, p=0.42). MDD adolescents endorsed significantly greater levels of depression as measured by the BDI-II (t35=7.20, p<0.0001). For more details, see Table 1.
Behavioral
A two-way ANOVA with group as a between-subject factor and fear level as a within-subject factor was run separately for accuracy and response time (RT) data. Accuracy data showed a main effect of group (F1,23=5.56, p<0.05), but not of fear level (F2,22=1.04, p>0.05) nor was there a significant interaction (F2,22=0.90, p>0.05). RT data showed no main effect of group (F1,23=1.47, p>0.05), fear level (F2,22=1.19, p>0.05) nor a significant interaction (F2,22=0.42, p>0.05). Overall accuracy (mean ± SEM) for MDD and HCL was 81.76% ± 1.9% and 86.96% ± 0.63%, respectively. Overall RT (mean ± SEM) for MDD and HCL was 1358.2ms ± 67.3ms and 1252.8ms ± 44.1ms, respectively. See Figure S2 for more details.
LBA parameter estimates
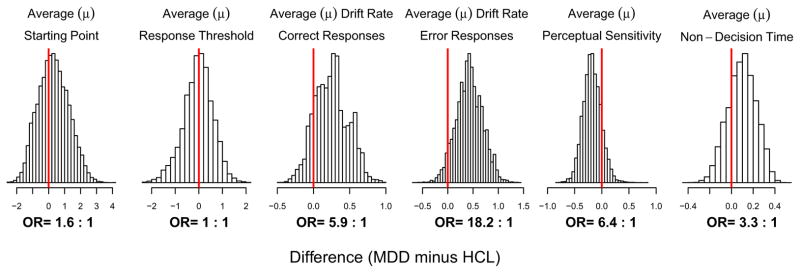
The LBA model yielded excellent fits to each subject’s RT data (see Figure S3 for more details). Table 2 summarizes the participant-level parameter estimates for each group. Figure 2 displays the difference between the MDD and HCL groups for each LBA model parameters (and corresponding latent cognitive process). At the group-level, MDD adolescents showed greater drift rates on trials where they responded correctly compared with HCL (OR=5.9:1), as well as on error trials (OR=18.2:1). As a result, perceptual sensitivity was lower in the MDD group (OR=6.4:1).
Table 2. Summary of participant-level LBA estimates.
Reported here is the mean ± standard error of the mean (SEM) of the median of posterior distributions of each participant-level parameter for each group. For more details on parameter estimation, see Methods and LBA Parameter Estimation in the Supplementary Material. See Figure S3 for model fits for each individual. For differences in group-level parameters, see Figure 2.
| LBA parameters | MDD | HCL |
|---|---|---|
| A (starting point) | 2.29 ± 0.41 | 1.31 ± 0.13 |
| b (response threshold) | 1.75 ± 0.12 | 1.68 ± 0.04 |
| vc (drift rate for correct responses) | 2.70 ± 0.02 | 2.45 ± 0.008 |
| ve (drift rate for error responses) | 1.23 ± 0.04 | 0.80 ± 0.007 |
| vc − ve (perceptual sensitivity) | 1.47 ± 0.02 | 1.64 ± 0.001 |
| t0 (non-decision time) | 0.22 ± 0.01 | 0.17 ± 0.03 |
Figure 2. Group differences in LBA parameters.
Each panel shows the posterior predictive distribution of the magnitude (μ) of the difference between the MDD and HCL groups for each parameter of the LBA model. Positive differences indicate larger parameter estimates for the MDD group, while negative differences indicate smaller parameter estimates for the MDD group. A distribution peaking at zero (denoted by the red line at x=0) indicates no difference between groups for that parameter. Odds ratios (ORs) indicating amount of evidence in favor of a difference are reported beneath each panel. See Figure S5 for posterior predictive distributions of the precision (σ) of the estimated group difference for each LBA hyper-parameter. To see posterior predictive distributions of each parameter for MDD and HCL separately, please see Figure S4.
Group and task effects
The MDD group showed reduced activation in the left precuneus, left anterior cingulate cortex, and right precentral gyrus relative to the HCL group in the contrast of interest (FearStrong-FearNeutral; see Figure S6 and Table S1 for more details). An examination of task effect showed greater activation in bilateral fusiform gyrus on FS compared to FN trials (see Figure S7 and Table S2 in for more details).
Functional connectivity
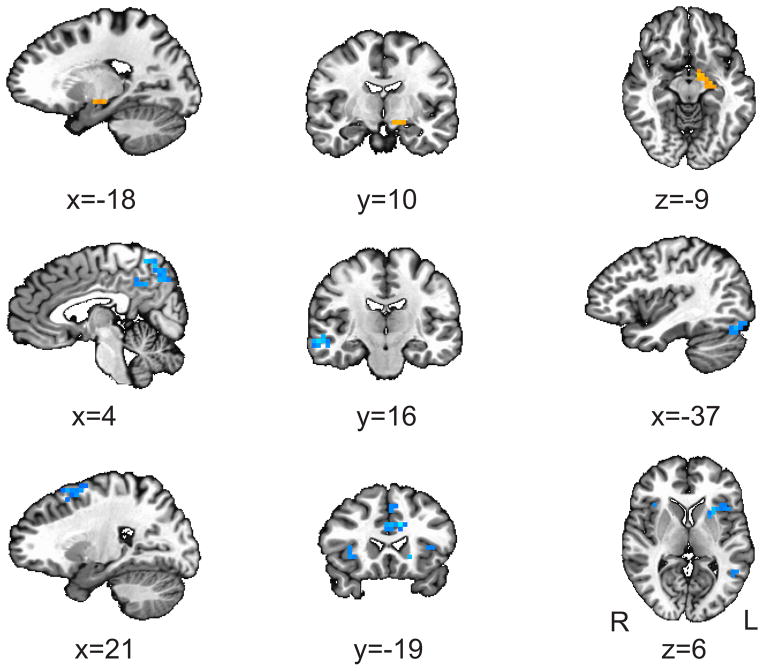
We observed greater mean FC (i.e., Fisher’s z-score) in the MDD relative to the HCL group between the right sgACC and a cluster in the left amygdala that extends into the striatum (see Figure 3 and Table 3). We also observed decreased FC in the MDD relative to the HCL group between the right sgACC and left fusiform, right precuneus (extending into posterior cingulate), right middle frontal gyrus, left cingulate, right superior temporal gyrus (extending into the insula), and right middle temporal gyrus, as well as between the left sgACC and the left insula (extending medially into the putamen), left cingulate, right insula, and left middle frontal gyrus, (see Figure 3, Table 3).
Figure 3. Group differences in functional connectivity.
We employed a psychophysiological interaction (PPI) method of functional connectivity, with bilateral subgenual anterior cingulate cortex (sgACC) defined as seeds and FearStrong-FearNeutral as the condition of interest. This analysis revealed the following clusters with significantly differently functional connectivity with sgACC in MDD relative to HCL (orange=increased, blue=decreased): All coordinates are in Talairach space and results are overlaid over a standardized Talairach template. Significance of each cluster is p<0.05 (see Controlling for multiple comparisons under Methods for more details).
Table 3. Location and size of significant clusters from the functional connectivity analysis.
Results are based on a psychophysiological interaction (PPI) method of functional connectivity, with bilateral subgenual anterior cingulate cortex (sgACC) defined as seeds and FearStrong-FearNeutral as the condition of interest. Locations are reported according to center of mass of cluster in Talairach coordinates (radiological convention). See Figure 3 for more details.
| Seed | Direction | Cluster | x | y | z | # of voxels (volume) |
|---|---|---|---|---|---|---|
| R-sgACC | MDD > HCL | L Amygdala/Striatum | −16 | 10 | −8 | 11 (704 μL) |
| R-sgACC | HCL > MDD | L Fusiform Gyrus | −31, | 74 | −18 | 32 (2048 μL) |
| R-sgACC | HCL > MDD | R Precuneus/Posterior Cingulate | 23 | 64 | 49 | 30 (1920 μL) |
| R-sgACC | HCL > MDD | R Middle Frontal Gyrus | 21 | −10 | 54 | 26 (1664 μL) |
| R-sgACC | HCL > MDD | L Cingulate | −3 | 45 | 36 | 17 (1088 μL) |
| R-sgACC | HCL > MDD | R Superior Temporal Gyrus/Insula | 53 | 45 | 21 | 12 (768 μL) |
| R-sgACC | HCL > MDD | R Middle Temporal Gyrus | 57 | 16 | −9 | 11 (704 μL) |
| L-sgACC | HCL > MDD | L Putamen/Insula | −28 | −14 | 6 | 18 (1152 μL) |
| L-sgACC | HCL > MDD | L Cingulate | −4 | −20 | 26 | 14 (896 μL) |
| L-sgACC | HCL > MDD | R Insula/Putamen | 31 | −16 | 2 | 13 (832 μL) |
| L-sgACC | HCL > MDD | L Middle Frontal Gyrus/Cingulate | −5 | −13 | 41 | 13 (832 μL) |
| L-sgACC | HCL > MDD | L Middle Temporal Gyruss | −49 | 46 | 11 | 12 (768 μL) |
MDD=major depressive disorder; HCL=healthy controls; L=left; R=right.
Correlations
Within the MDD group only, depression severity correlated negatively with FC between the right sgACC and right precuneus (rs=−0.630, p=0.004).
Among all subjects, perceptual sensitivity (based on the participant-level estimates) correlated positively with FC between right sgACC and right middle frontal gyrus (rs=0.47, p=0.011) and also FC between right sgACC and left cingulate (rs=0.393, p=0.036).
Finally, among all subjects, higher estimates of perceptual sensitivity were significantly associated with lower BDI-II scores (rs=−0.46, p=0.014).
Discussion
To our knowledge, this study is the first to identify functional connectivity (FC) differences based in the subgenual anterior cingulate cortex (sgACC) during negative emotional processing in adolescents with major depressive disorder (MDD) compared to a sample of healthy controls (HCL). Importantly, all depressed subjects were antidepressant-naïve and with no diagnosed psychiatric comorbidities. We report three primary findings. First, adolescents with MDD showed altered FC in sgACC-based networks when evaluating negative emotional stimuli. Specifically, we found significantly greater FC between sgACC and amygdala and significantly decreased FC between sgACC and insula/putamen, fusiform gyrus, precuneus/posterior cingulate, and middle frontal gyrus in MDD relative to HCL (see Figure 3 and Table 3). Secondly, among the depressed adolescents only, sgACC-precuneus connectivity strength correlated significantly with depression severity. Lastly, MDD exhibited lower perceptual sensitivity of emotionally negative stimuli than HCL (see Figure 2 and Table 2). These individual differences in perceptual sensitivity were also significantly associated with sgACC-based functional connectivity, as well as with depression scores.
Based on prior resting-state studies in adolescent depression (Cullen et al., 2009; Davey et al., 2012; Gabbay et al., 2013; Connolly et al., 2013), we hypothesized finding differences in sgACC-based FC among salience, cognitive executive, and resting-state networks between adolescents with MDD and HCL subjects during the processing negative stimuli. However, the functional connectivity results in the aforementioned resting-state studies of adolescent depression are not entirely in agreement with one another, due to differences in medication status (only Connolly et al. and Gabbay et al. included medication-free subjects), age range (Davey et al. and Gabbay et al. included young adults), and comorbidities of their subjects, as well as differences in data acquisition (Cullen et al. allowed their participants to listen to music), data preprocessing, and analytical techniques.
Of these, the one by our group (Connolly et al.) is most directly comparable to the present study, due to similar sgACC seed definitions, age range of subjects, and inclusion of an antidepressant-naïve MDD cohort. Notably, our respective subject pools were completely independent, different MR scanners were used, and distinct preprocessing steps were employed on our respective fMRI datasets. Despite these differences, our results are strikingly similar. Specifically, we both find stronger sgACC-amygdala coupling, along with a decoupling between sgACC and the precuneus, superior temporal gyrus, and middle frontal gyrus in depressed adolescents. We also both report a significant negative correlation between sgACC-precuneus connectivity strength and depression severity. However, while Connolly et al. report greater sgACC-insula FC in their depressed group, we observe reduced sgACC-insula FC in ours. Additionally, we find decoupling between sgACC and fusiform gyrus and superior temporal gyrus in MDD relative to HCL, while no such group differences were seen in the study by Connolly et al.
Since the present study requires active task engagement, our results collectively suggest that increased FC between sgACC and the amygdala (a component of the salience network) and reduced FC between the sgACC and middle frontal gyrus (a component of the cognitive executive network) and precuneus (a component of the resting-state network) are potential functional identifiers of depression in youth regardless of brain state. This notion is consistent with work from adult depression (Greicius et al., 2007; Sheline et al., 2010; Menon, 2011; Drevets et al., 2008; Mayberg, 1997; Mayberg et al., 2005) suggesting that MDD is associated with sgACC dysregulation of stimulus-driven limbic activation (e.g., amygdala), which in turn may perturb communication with other sites involved in the immediate integration of salient and affective information (e.g., insula) and more higher order cognitive processing relating emotion with the self (e.g., precuneus, middle frontal gyrus).
However, the decoupling between sgACC and insula that we observe in our depressed adolescents stands in contrast to the increased coupling between these areas observed by Connolly et al. in their resting-state study. It may be that sgACC-insula FC depends on or even indicates brain state. Indeed, neuroimaging evidence has demonstrated that portions of the insula are responsible for switching between task-negative (rest) and task-positive (non-rest) brain states in healthy controls (Craig, 2009; Sridharan et al., 2008). Nevertheless, the fact that there is a significant difference in sgACC-insula connectivity strength between MDD and HCL in both of our studies (albeit in opposite directions, as we each assessed opposite brain states) suggests that depressed adolescents may have difficulty transitioning between rest and non-rest. As the insula is a major node of the salience network (Menon, 2011), switching between rest and non-rest may be particularly difficult for depressed adolescents during the processing of affective information. Such difficulty could possibly underlie some of cognitive symptoms associated with MDD, including rumination (Hamilton et al., 2011; Berman et al., 2011a; 2011b; Joormann et al., 2011) and trouble disengaging from negative material (Gotlib et al., 2004; Siegle et al., 2002; Joormann et al., 2011).
Unlike Connolly et al., we also observed FC differences in sgACC-fusiform gyrus and sgACC-superior temporal sulcus between MDD and HCL adolescents. Given that the fusiform gyrus is highly implicated in face processing (Haxby et al., 1994; Kanwisher et al., 1997) and the superior temporal sulcus is sensitive to mouth and eye movements during emotive facial expressions (Puce et al., 1995; 1998; Harris et al., 2012), the sgACC-based FC group differences we see in these areas may be stimulus-dependent. The reduced coupling between sgACC and face-processing areas – in conjunction with greater sgACC-amygdala functional connectivity – may partially explain why our depressed group possessed lower perceptual sensitivity in our task, as the affectively negative value of our facial stimuli may have impacted processing and impaired judgment.
Lastly, the results of our cognitive behavioral model are in line with evidence that both depressed adults (Foland-Ross and Gotlib, 2012; Foland-Ross et al., 2013; Gotlib et al., 2004) and adolescents (Hankin et al., 2012; Hommer et al., 2013) show biased processing to affectively negative material compared to healthy controls. We observed greater drift rates in depressed individuals on both correct and error responses (see Figure 2 and Table 2). Given that the decision in the task was to determine the gender of facial stimuli, these results suggest that depressed subjects may be more sensitive to or distracted by the negative value of the face and possibly less capable of inhibiting incorrect responses, resulting in poorer behavioral performance overall (i.e., lower accuracy and slower RT; see Figure S2). Moreover, individual differences in perceptual sensitivity to negative stimuli not only predicted connectivity strength between the sgACC and cingulate, the latter of which is part of the salience network (Menon and Uddin, 2010), but perceptual sensitivity to negative material among all our subjects also correlated negatively with depression severity. These results suggest that depressed adolescents may fundamentally perceive salient, negative affective material differently compared to healthy controls and that differences in functional connectivity which support or reflect these information processing differences may be a potential indicator of illness severity.
One clinical implication of this work is that biased processing of negative material stems from sgACC dysregulation of stimulus-driven responses, which further provokes an imbalance among salience, cognitive executive, and resting-state networks often seen in early-onset depression at rest (Cullen et al., 2009; Davey et al., 2012; Connolly et al., s2012; Gabbay et al., 2013). While studies with remitted or high-risk samples are needed to determine whether this imbalance of functional networks is a trait- or state-marker of MDD, viewing these results within the theoretical framework that they are indeed trait-markers may partially explain why depressed individuals preferentially process negative stimuli, even during remission (Hankin et al., 2012; Joormann and Gotlib, 2007; LeMoult et al. 2009). The results we report here raise the possibility that cognitive therapies which aim to reverse biased processing of negative information (e.g., Lang et al., 2009; Hazen et al., 2009; Joormann et al., 2009; 2011) may help build resilience in those at-risk for developing this disorder by thwarting cognitive mechanisms, such as rumination, that exacerbate negative mood states and possibly maintain depression (Hamilton et al., 2011; Nolen-Hoeksema, 2000). In addition to identifying sgACC-based functional connectivity patterns as potential biomarkers of adolescent MDD, our study also demonstrates that combining cognitive behavioral models with brain measures provides a richer understanding of information processing differences associated with pathologies like depression. Such knowledge could potentially lead to better assessment and treatment of major depression and other affective disorders.
Nevertheless, this study must be interpreted in the context of its methodological limitations. Firstly, functional connectivity is a measure of correlated activity and should not be interpreted as proving the presence of causal connections (McIntosh, 2010). Future studies using effective connectivity (Friston et al., 1997, 2009; McIntosh, 2010), which test model-based assumptions about the effect of one neural system or region has over another, are needed to assess whether and how sgACC causally affects structures in the salient, cognitive executive, and resting-state networks. However, effective connectivity requires a more focused approach with explicit assumptions on subsections of networks that need to first be identified and validated in fMRI studies using simpler analytical methods, such as functional connectivity (McIntosh, 2010; Büchel and Friston, 2000). As our study is the first to report sgACC-based functional connectivity patterns in adolescent depression during negative emotional processing, our hope is that these results will inform future effective connectivity studies. A second potential limitation is that we adhered to strict exclusion criteria in our depressed subjects so as to avoid bias from comorbidity when interpreting our findings. Since our MDD sample presented no psychiatric comorbidities and possessed little variability in age of illness onset, our results may not necessarily be generalizable to depressed youth more commonly seen in clinical practice. Investigating sgACC-based FC patterns in other subpopulations of adolescent MDD patients (e.g., prepubertal status, comorbidities, varying age of onset and duration of illness, etc) is needed to assess the generalizability of our results.
In summary, the present work is the first to examine functional connectivity of the subgenual anterior cingulate cortex (sgACC) in antidepressant-naïve adolescents with major depressive disorder compared to a group of matched healthy controls. Our results join a growing body of resting-state and task-based fMRI research that point to dysfunction in sgACC-based circuits as a potential hallmark of depression in adults (Mayberg, 1997; 2005; Drevets et al., 2008; Matthews et al., 2008; Almeida et al., 2011; Pezawas et al., 2005; Chen et al., 2008; Hamani et al., 2011; Greicius et al., 2008; Stuhrmann et al., 2011; Johansen-Berg et al., 2008), adolescents (Yang et al., 2009; Cullen et al., 2009; Davey et al., 2012; Connolly et al., 2013; Ho et al., 2013; Gabbay et al., 2013), and even children (Gaffrey et al., 2010; 2012; Luking et al., 2011). Our findings therefore support the idea that the sgACC acts as a mediator between emotional and cognitive processing regions. Under this theoretical framework, biased processing of negative information in adolescent MDD may engage sgACC circuitry and possibly result in a greater imbalance among the salience, cognitive executive, and resting-state functional brain networks. Lastly, our study is the first to establish the use of a cognitive behavioral model to examine information processing differences between depressed and healthy populations that can potentially be used to augment understanding of the relationship between cognition and brain activation patterns in affective disorders.
Supplementary Material
Acknowledgments
Role of funding source.
All funding sources played no part in the design, data collection and analysis, decision to publish, or preparation of this manuscript.
Funding: This work was supported by a NARSAD award and grants from NIMH (R01MH085734, R01MH085734-02S1) to T.T.Y. and ARC grant FT120100244 to S.D.B.
We would like to thank Mr. Kevin Hahn (affiliated with Stanford University), Dr. Greg Fonzo (affiliated with Stanford University), and Dr. Robert Hendren (affiliated with the University of California, San Francisco).
Footnotes
Contributors: M.P.P, A.N.S., and T.T.Y. designed the study and wrote the protocol. T.C.H., G.Y., J.W., P.C., S.D.B., N.H., M.C., and A.N.S. analyzed the data. T.C.H. wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflicts of interest:
Dr. Tiffany Ho reports no biomedical financial interests or potential conflicts of interest.
Mr. Guang Yang reports no biomedical financial or potential conflicts of interest.
Mr. Jing Wu reports no biomedical financial interests or potential conflicts of interest.
Mr. Pete Cassey reports no biomedical financial interests or potential conflicts of interest.
Dr. Scott Brown sits on the editorial board of the Journal of Mathematical Psychology.
Mr. Napoleon Hoang works as an assistant editor at Elsevier.
Ms. Melanie Chan reports no biomedical financial interests or potential conflicts of interest.
Dr. Colm Connolly reports no biomedical financial interests or potential conflicts of interest.
Dr. Eva Henje-Blom receives grant or research support from the Swedish Research Council, the Swedish Society of Medicine, and the Swedish American Association.
Dr. Larissa Duncan receives grant or research support from the National Institutes of Health.
Dr. Margaret Chesney receives grant or research support from the National Institutes of Health.
Dr. Martin Paulus receives grant or research support from the National Institutes of Health.
Dr. Jeffrey Max receives grant or research support from the National Institutes of Health. He also provides expert testimony in cases of traumatic brain injury on an ad hoc basis for plaintiffs and defendants on a more or less equal ratio. This activity constitutes approximately 5% of his professional activities.
Mr. Ronak Patel reports no biomedical financial interests or potential conflicts of interest.
Dr. Alan Simmons receives grant or research support from the Veterans Affairs and the Center of Excellence in Stress and Mental Health.
Dr. Tony Yang reports no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida JR, Kronhaus DM, Sibille EL, Langenecker SA, Versace A, Labarbara EJ, Phillips ML. Abnormal left-sided orbitomedial prefrontal cortical-amygdala connectivity during happy and fear face processing: a potential neural mechanism of female MDD. Front Psychiatry. 2011;2:69. doi: 10.3389/fpsyt.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico F, Carballedo A, Lisiecka D, Fagan AJ, Boyle G, Frodl T. Functional anomalies in healthy individuals with a first degree family history of major depressive disorder. Biol Mood Anxiety Disord. 2012;2(1):1. doi: 10.1186/2045-5380-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenevoli S, Knight E, Kessler RC, Merikangas KR. Epidemiology of depression in children and adolescents. Handbook of depression in children and adolescents. 2008:6–32. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-Second Edition Manual. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Berman MG, Nee DE, Casement M, Kim HS, Deldin P, Kross E, Jonides J. Neural and behavioral effects of interference resolution in depression and rumination. Cogn Affect Behav Neurosci. 2011;11(1):85–96. doi: 10.3758/s13415-010-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6(5):548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SD, Heathcote A. The simplest complete model of choice response time: linear ballistic accumulation. Cogn Psychol. 2008;57(3):153–178. doi: 10.1016/j.cogpsych.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Buchel C, Friston K. Assessing interactions among neuronal systems using functional neuroimaging. Neural Netw. 2000;13(8–9):871–882. doi: 10.1016/s0893-6080(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Carballedo A, Scheuerecker J, Meisenzahl E, Schoepf V, Bokde A, Moller HJ, Frodl T. Functional connectivity of emotional processing in depression. J Affect Disord. 2011;134(1–3):272–279. doi: 10.1016/j.jad.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Ooi C, Fu CH, Williams SC, Walsh ND, Bullmore E. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. 2008;33(8):1909–1918. doi: 10.1038/sj.npp.1301593. [DOI] [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, Paulus MP. Resting-State Functional Connectivity of Subgenual Anterior Cingulate Cortex in Depressed Adolescents. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, Milham MP. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009;460(3):227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Harrison BJ, Yucel M, Allen NB. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol Med. 2012;42(10):2071–2081. doi: 10.1017/s0033291712000323. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2):240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen W. Pictures of Facial Affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Foland-Ross LC, Gotlib IH. Cognitive and neural aspects of information processing in major depressive disorder: an integrative perspective. Front Psychol. 2012;3:489. doi: 10.3389/fpsyg.2012.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Hamilton JP, Joormann J, Berman MG, Jonides J, Gotlib IH. The neural basis of difficulties disengaging from negative irrelevant material in major depression. Psychol Sci. 2013;24(3):334–344. doi: 10.1177/0956797612457380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68(5):433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS biology. 2009;7(2):e1000033. doi: 10.1371/journal.pbio.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Milham MP. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013;52(6):628–641. e613. doi: 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Luby JL, Botteron K, Repovs G, Barch DM. Default mode network connectivity in children with a history of preschool onset depression. J Child Psychol Psychiatry. 2012;53(9):964–972. doi: 10.1111/j.1469-7610.2012.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Luby JL, Repovs G, Belden AC, Botteron KN, Luking KR, Barch DM. Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport. 2010;21(18):1182–1188. doi: 10.1097/WNR.0b013e32834127eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113(1):121–135. doi: 10.1037/0021-843x.113.1.121. [DOI] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69(4):301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70(4):327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Wetter EK, Flory K. Appetitive motivation and negative emotion reactivity among remitted depressed youth. J Clin Child Adolesc Psychol. 2012;41(5):611–620. doi: 10.1080/15374416.2012.710162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RJ, Young AW, Andrews TJ. Morphing between expressions dissociates continuous from categorical representations of facial expression in the human brain. Proc Natl Acad Sci U S A. 2012;109(51):21164–21169. doi: 10.1073/pnas.1212207110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994;14(11 Pt 1):6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Wu J, Shin DD, Liu TT, Tapert SF, Yang G, Wolkowitz O. Altered Cerebral Perfusion in Executive, Affective, and Motor Networks During Adolescent Depression. J Am Acad Child Adolesc Psychiatry. 2013 doi: 10.1016/j.jaac.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer RE, Meyer A, Stoddard J, Connolly ME, Mogg K, Bradley BP, Brotman MA. ATTENTION BIAS TO THREAT FACES IN SEVERE MOOD DYSREGULATION. Depress Anxiety. 2013 doi: 10.1002/da.22145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18(6):1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gilbert K, Gotlib IH. Emotion identification in girls at high risk for depression. J Child Psychol Psychiatry. 2010;51(5):575–582. doi: 10.1111/j.1469-7610.2009.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. J Abnorm Psychol. 2006;115(4):705–714. doi: 10.1037/0021-843x.115.4.705. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. 2007;116(1):80–85. doi: 10.1037/0021-843x.116.1.80. [DOI] [PubMed] [Google Scholar]
- Joormann J, Hertel PT, Brozovich F, Gotlib IH. Remembering the good, forgetting the bad: intentional forgetting of emotional material in depression. J Abnorm Psychol. 2005;114(4):640–648. doi: 10.1037/0021-843x.114.4.640. [DOI] [PubMed] [Google Scholar]
- Joormann J, Hertel PT, LeMoult J, Gotlib IH. Training forgetting of negative material in depression. J Abnorm Psychol. 2009;118(1):34–43. doi: 10.1037/a0013794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. J Abnorm Psychol. 2007;116(1):135–143. doi: 10.1037/0021-843x.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Angermeyer M, Anthony JC, RDEG, Demyttenaere K, Gasquet I, Ustun TB. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6(3):168–176. [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biol Psychiatry. 2001;49(12):1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, Klein DN. Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. J Child Psychol Psychiatry. 2012;53(2):207–215. doi: 10.1111/j.1469-7610.2011.02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Klein DN, Seeley JR. Natural course of adolescent major depressive disorder: I. Continuity into young adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38(1):56–63. doi: 10.1097/00004583-199901000-00020. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kuhlman KR, George C, Kovacs M. Facial emotion expression recognition by children at familial risk for depression: high-risk boys are oversensitive to sadness. J Child Psychol Psychiatry. 2013;54(5):565–574. doi: 10.1111/jcpp.12005]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Repovs G, Belden AC, Gaffrey MS, Botteron KN, Luby JL, Barch DM. Functional connectivity of the amygdala in early-childhood-onset depression. J Am Acad Child Adolesc Psychiatry. 2011;50(10):1027–1041. e1023. doi: 10.1016/j.jaac.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37(2):579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord. 2008;111(1):13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Maxwell M. Family Interview for Genetic Studies (FIGS): a manual for FIGS. Bethesda, Md: NIMH; 1992. [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8(4):1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Moving between functional and effective connectivity. Paper presented at the Society for Neuroscience; Washington, D.C. 2010. [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(Pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 2000;109(3):504–511. [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Perlman G, Simmons AN, Wu J, Hahn KS, Tapert SF, Max JE, Yang TT. Amygdala response and functional connectivity during emotion regulation: a study of 14 depressed adolescents. J Affect Disord. 2012;139(1):75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett DI, May KA, Yoshikawa S. Facial shape and judgements of female attractiveness. Nature. 1994;368(6468):239–242. doi: 10.1038/368239a0. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci. 1998;18(6):2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Gore JC, McCarthy G. Face-sensitive regions in human extrastriate cortex studied by functional MRI. J Neurophysiol. 1995;74(3):1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput. 2008;20(4):873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romens SE, Pollak SD. Emotion regulation predicts attention bias in maltreated children at-risk for depression. J Child Psychol Psychiatry. 2012;53(2):120–127. doi: 10.1111/j.1469-7610.2011.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/jneurosci.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol Psychiatry. 2008;64(8):681–690. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1(1):10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxis Atlas of the Human Brain. New York: Theime; 1998. Three-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CN, Ratcliff R, Vasey MW, McKoon G. Using diffusion models to understand clinical disorders. J Math Psychol. 2010;54(1):39–52. doi: 10.1016/j.jmp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Bischoff-Grethe A, Paulus MP. Depressed adolescents demonstrate greater subgenual anterior cingulate activity. Neuroreport. 2009;20(4):440–444. doi: 10.1097/WNR.0b013e3283262e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.