Abstract
Background
Naturally occurring polyphenols found in food sources provide huge health benefits. Several polyphenolic compounds are implicated in the prevention of disease states, such as cancer. One of the mechanisms by which polyphenols exert their biological actions is by interfering in the protein kinase C (PKC) signaling pathways. PKC belongs to a superfamily of serine-threonine kinase and are primarily involved in phosphorylation of target proteins controlling activation and inhibition of many cellular processes directly or indirectly.
Scope of review
Despite the availability of substantial literature data on polyphenols' regulation of PKC, no comprehensive review article is currently available on this subject. This article reviews PKC-polyphenol interactions and its relevance to various disease states. In particular, salient features of polyphenols, PKC, interactions of naturally occurring polyphenols with PKC, and future perspective of research on this subject are discussed.
Major conclusions
Some polyphenols exert their antioxidant properties by regulating the transcription of the antioxidant enzyme genes through PKC signaling. Regulation of PKC by polyphenols is isoform dependent. The activation or inhibition of PKC by polyphenols has been found to be dependent on the presence of membrane, Ca2+ ion, cofactors, cell and tissue types etc. Two polyphenols, curcumin and resveratrol are in clinical trials for the treatment of colon cancer.
General significance
The fact that 74% of the cancer drugs are derived from natural sources, naturally occurring polyphenols or its simple analogs with improved bioavailability may have the potential to be cancer drugs in the future.
Keywords: polyphenol, protein kinase C, signal transduction, cancer, antioxidant
1. Introduction
Understanding the action mechanisms of naturally occurring chemo-preventive agents is an important step in designing therapeutics for cancer and related diseases. These agents are known to exert a plethora of actions on multiple targets eliciting an either known or unknown hierarchy of biological responses. This review focuses on one such chemo-preventive class of agents, polyphenols, which are popularly known as antioxidants and are mostly found in dietary supplies. One of the mechanisms by which polyphenols exert their actions is by regulation of the protein kinase C (PKC), a central kinase in intracellular signal transduction. Discovered in the late 1970s, PKCs have been implicated in many disease states. This article focuses on the PKC-polyphenol interactions and its biological consequences. Despite the availability of substantial literature data on polyphenols' regulation of PKC and highlighting its therapeutic potential for disease states, no comprehensive review article is currently available on this important subject. Here we present important features of polyphenols, PKC, and interactions of naturally occurring polyphenols with PKC, and future perspective of research on this subject.
2. Polyphenols
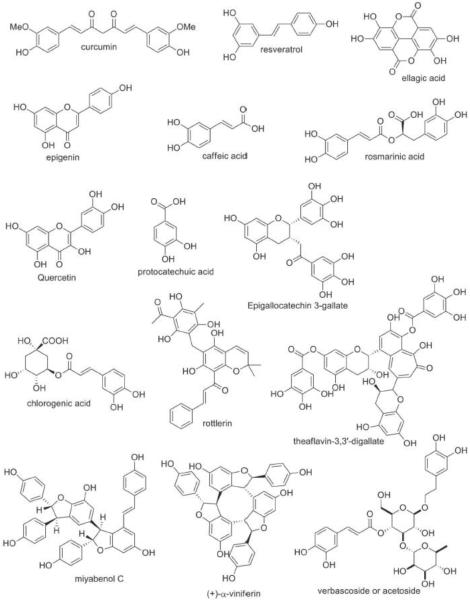
Polyphenols are a vast family of molecules characterized by more than one connected hydroxylated/multi-hydroxylated benzene rings-phenols. As a consequence of their enormous structural diversity in nature, they are often divided into four different classes- phenolic acid, stilbenes, lignans and flavonoids based on their structural properties alone [1]. These classes are consequently divided into an array of subclasses such as monohydroxybenzoic acids, dihydroxybenzoic acids, flavones, flavonols, flavanones, isoflavones, monomeric stilbene, oligomeric stilbene, diarylbutane derivatives, 2,3-dibenzylbutyrolactone derivatives, tetrahydrofuran derivatives, 4-aryltetrahydronaphthalene derivatives and a few others (Figure 1) [1–4]. Some natural sources are known to contain copious amounts of polyphenols including apples, cranberries, red grapes, blueberries black tea, pomegranate juice, red wine, and red onions, green tea, strawberry, bananas, corn, watermelon, plums, kiwi, potato, lemon juice, soy beans, cherries etc.[5].
Fig.1.
Chemical structure of the polyphenols.
Polyphenols have been implicated as potential preventive and curative therapeutic agents for a host of diseases like Alzheimer's [6, 7], Huntington[8], Parkinson's [9, 10], hypercholesterolemia [11], chronic fatigue syndrome [12, 13], diabetes[14], stroke [15, 16], various forms of cancer [17, 18], cardiovascular diseases [19, 20], autism [21, 22], vitiligo [23], etc. They have also been linked in curbing some of the phenotypes associated with senescence [24, 25]. In most of these instances, the antioxidant property of polyphenols is suggested as being responsible for their observed beneficial effects. Despite the predominant focus on the antioxidant activities, polyphenols have also been implicated in other roles independent of their antioxidant property like antibacterial [26, 27], anti-inflammatory [28] and modulation of ion transport [29].
2.1. Mechanisms of polyphenols' antioxidant properties
Currently, within the scientific community there is an ongoing debate on the primary mechanisms through which polyphenols exert their antioxidant property. Among the several possible mechanisms (Figure 2), the radical scavenging hypothesis postulates that polyphenols specifically `hunt' for free radicals and bind to them[30, 31]. Reactive Oxygen Species (ROS) is a class of molecules generated from metabolic reactions within aerobic organisms. ROS includes superoxide (O2−˙), hydroxyl radical (˙OH) and hydrogen peroxides (H2O2). Radical scavenging mechanism has been described as overly simplistic and also unsound in the case of radicals like ˙OH, which are highly reactive and react with almost all organic compounds within the cell [32]. The observation of direct scavenging of ROS by the higher concentrations of polyphenols in the in vitro cell culture seems unlikely to happen in the in vivo system where the concentrations of the consumed polyphenol concentrations are relatively low.
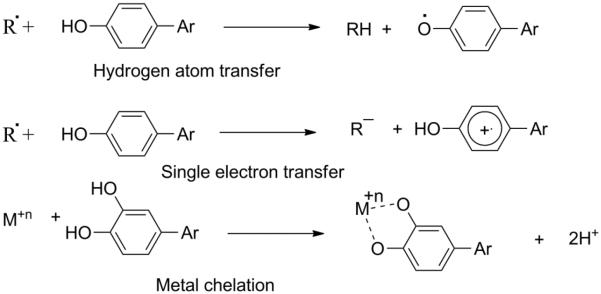
Fig.2.
Different mechanisms for polyphenols' antioxidant properties.
On the other hand, the transition metal chelating hypothesis contends that polyphenols bind free metals (especially iron) within the cells thus preventing the progression of the Fenton reaction (Figure 3)[5]. The Fenton reaction is a reaction pathway through which free iron (Fe2+) reacts with hydrogen peroxide to yield hydroxyl radical. This hypothesis has been questioned based on the fact that under normal human physiological condition iron concentration is nearly negligible due to its strict regulation by intracellular enzymes [33]. Nevertheless, in some disease states like Alzheimer and beta thalassemia, iron concentration is not negligible and is a primary contributor to oxidative stress [33]. Although, the radical scavenging hypothesis and the transition metal (primarily iron) chelation hypothesis are the most commonly cited and observed mechanisms of action in vitro and in vivo, evidence of stimulation of ROS-terminating enzymes and inhibition of ROS-producing enzymes by some polyphenols has also been reported. ROS are physiologically important as they are essential players in some cell signaling pathways [32, 34]. Nevertheless, beyond a certain threshold (not known in vivo or in vitro) they can have a particularly damaging physiological effect, for instance, both superoxide and hydroxyl radicals have been shown to cause damage to DNA [33]. Beyond this threshold, the cell is considered to be under oxidative stress. Oxidative stress caused by ROS has been correlated with the occurrence of some forms of cancers [35], cardiovascular diseases [36] and with neurodegenerative diseases like Alzheimer's [37] and Parkinson's [38]. Given the beneficial effects of ROS in the right amounts and the potentially harmful effects that may result from an excess, the formation and destruction processes of ROS is tightly controlled for maintaining proper balance. NADPH oxidase and xanthine oxidase are a few examples of enzymes that are known to regulate the production of some ROS. Conversely, superoxide dismutase, peroxidase and catalase are known to catalyze the destruction of ROS. Some polyphenols exert their antioxidant influence by inhibiting free radical generating enzymes like NADPH and xanthine oxidase [39] or via increasing expression levels/activities of radical terminating enzymes like superoxide dismutase and catalase [40, 41].
Fig.3.
Types of reaction mechanisms for polyphenols' antioxidant properties.
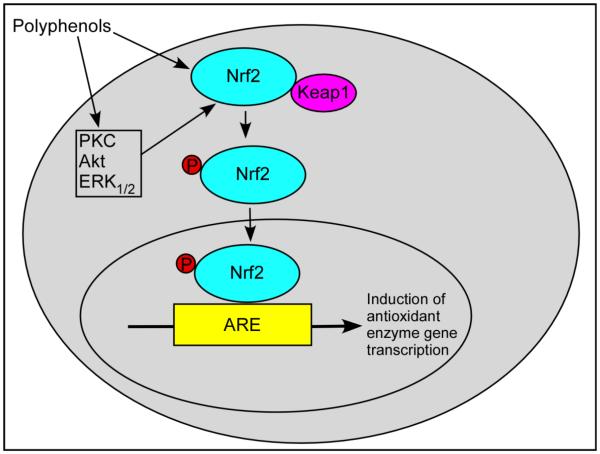
An elegant mechanism by which the polyphenols regulate the expression of antioxidant enzymes is through the activation of Keap1/Nrf2/ARE pathway (Figure 4). Kelch-like ECH-associated protein 1 (Keap 1) remains bound to transcription regulator nuclear factor E2-related factor 2 (Nrf2), and prevents its signaling. Polyphenols directly or indirectly cause dissociation of Keap1 from the Nrf2-Keap1 complex. Phosphorylation of Nrf2 and its dissociation from the complex allows it to translocate to the nucleus where it binds to the antioxidant response element (ARE) in the regulatory region of the target genes, and induce transcription of antioxidant/detoxification enzymes (Figure 4). Several upstream kinases such as, extracellular signal-regulated kinase (ERK), protein kinase B (Akt) and PKC regulate this translocation and transcriptional activation. Several polyphenols regulate the activity of these kinases thereby exerting their antioxidant properties.
Fig.4.
Mechanism of polyphenols' antioxidant properties. Polyphenols activate Keap1/Nrf2/ARE pathway and induce the expression of antioxidant/detoxification enzymes. Keap 1 protein always bound to Nrf2 transcription regulator and prevents its signaling. Polyphenols directly or indirectly cause dissociation of Keap1 from the Nrf2-Keap1 complex and subsequent translocation of Nrf2 to the nucleus where it binds to the ARE in the regulatory region of the target genes and induce transcription of antioxidant/detoxification enzymes. ARE, antioxidant response element; CAT, catalase; ERK, extracellular signal-regulated kinase; GCL, g-glutamylcysteine synthatase; GPx, glutathione peroxidase; GST, glutathione S-transferase; HO-1, heme oxygenase-1; Keap 1, Kelch-like ECH-associated protein 1; Nrf2, Nuclear factor E2-related factor 2; PRX, peroxiredoxin; SOD, superoxide dismutase; Trx, thioredoxin.
Despite existing evidence for the mechanisms mentioned above, most studies have not ruled out the possibility that polyphenols exert their antioxidant effects via a signal transduction cascade perhaps via interactions with cellular receptors. It has also been posited that polyphenols undergo further processing upon ingestion and lose their antioxidant property before reaching the cells hence their beneficial properties could be largely independent of their antioxidant properties [42]. Some studies have even proposed the antioxidant properties are exhibited in the gastrointestinal tract before absorption [43].
3. Protein Kinase C (PKC)
PKC belongs to a superfamily of serine-threonine kinase [44], primarily involved in phosphorylation of target proteins controlling activation and inhibition of many cellular processes directly or indirectly. Discovered by Yasutomi Nishizuka in the late seventies [45, 46], the PKC family plays a key role in many biological functions such as, apoptosis, cell proliferation [47], transcription regulation, immune responses, cell signaling [48], learning and memory [49], etc.
PKC family consists of 11 isozymes which are classified into three groups based on their N-terminal regulatory domain structure and activators bound to it (second messenger role) [50]. Conventional PKCs (α, βI, βII and γ) are activated by Ca2+, diacylglycerol (DAG)/phorbol ester (Figure 5), and phosphatidylserine (PS) whereas, novel PKCs (δ, ε, θ and η) are activated by DAG/phorbol ester but not by Ca2+. Atypical PKCs (ζ and ι / λ isoforms) are not activated by either DAG or Ca2+, but usually can be activated by phosphatidylinositol (3, 4,5)-trisphosphate (PIP3) and PDK1 [51]. A recent study showed that PKCζ_can be activated independent of PIP3 [52].
Fig.5.
DAG, sn-1, 2-dioleoylglycerol (top) and phorbol ester, phorbol 12-myristate 13- acetate (TPA) (bottom).
PKC isozymes are expressed ubiquitously in tissues. PKCα is expressed in the adult liver, heart, epithelium and brain. PKCθ is expressed in T lymphocytes and relatively high levels in platelets [53]. PKCγ, PKCδ and PKCε are present in the heart [54]. PKCδ is expressed in vascular smooth muscle cells. PKCε was detected in the adult brain, heart, and liver, but not the adult kidney and lung. PKCζ was more abundant in the fetal/neonatal than in the adult brain, lung, kidney, and heart. PKCβ immunoreactivity was prominent in brain tissue [55].
Abnormalities in PKC signaling play a major role in cancer. Because phorbol esters are tumor promoters and also high affinity PKC ligands, there exists a correlation between PKC and cancer. Most of the changes occur in expression and activation of PKC α, β, δ during cancer progression [56]. Role of different PKC isoforms in various types of cancer has been reviewed by Griner and Kazanietz [57]. PKCs are also implicated in cardiovascular diseases like ischemic heart disease [58, 59], acute and chronic heart disease [60], heart failure [61], stroke [62], lung [63] and kidney complications [64], diabetes [65], various dermatological conditions including psoriasis [66], neurological disease states like bipolar disorders [67], Parkinson's disease [68, 69] dementia [70], Alzheimer's disease [71, 72], and pain [73]. One of the major goals of studying PKC isozymes is to develop potent and selective ligands to regulate PKC signaling in these disease states and the significance of PKC as a therapeutic target has been elegantly reviewed by Mochley-Rosen et al [74].
3.1. PKC domains and structure
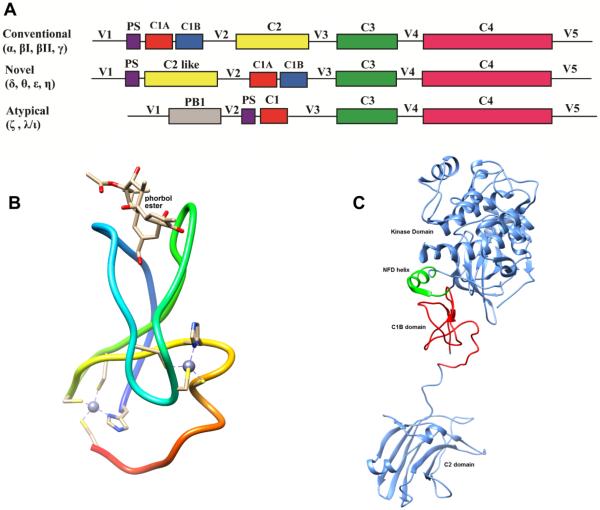
Structurally, PKC consists of four conserved (C1–C4) and five variable (V1–V5) regions (Figure 6A). The conserved region is divided into sub regions. Regulatory region is at the N–terminal, where several different second messengers bind and the highly conserved catalytic region is at the C-terminal. The regulatory region has C1 and C2 domains. The C1 domain of conventional and novel PKCs are composed of the C1A and C1B subdomains, each having approximately 50 amino acids [75]. These subdomains contain two zinc ions, each of which is coordinated by three cysteines and one histidine [76]. The C1A and C1B bind to DAG/phorbol ester. While DAG is an endogenous second messenger, phorbol ester is a tumor-promoter isolated from plant, and mimic the functions, but more potent than DAG [77–79]. The C2 domain binds to Ca2+ and phosphatidylserine in conventional PKCs, but in some cases novel PKCs bind to negatively charged phospholipids in a Ca2+-independent manner [76, 80]. PKC regulatory domain region contains a pseudosubstrate domain which helps in maintaining the enzyme in active or inactive conformation. The catalytic domain contains C3 and C4 domains having 12 highly conserved subdomains which fold into one catalytic core [81]. The C3 domain binds to ATP and C4 domain binds to substrate.
Fig.6.
A. Schematic representation of the PKC domains. For conventional and novel PKCs, four distinct domains are C1, C2, C3 and C4 and five variable regions are V1, V2, V3, V4 and V5. PS is the pseudosubstrate domain. B, Structure of the phorbol ester-bound PKCδC1B (PDB ID: 1PTR). C, Ribbon representation of the structure of the full-length PKCβII (PDB ID: 3PFQ), comprising the C1B domain (red), C2 domain (cornflower blue), kinase domain (cornflower blue) and NFD helix (green).
The three-dimensional structure of each truncated domains of PKC has been determined. The structure of PKCδC1B complexed with phorbol 13-O-acetate[82] (Figure 6B) revealed that there are two sets of three cysteines and one histidine that form two Zn2+ ion coordination sites at the end of the β sheets. In order to allow for ligand binding, the two β sheets are pulled apart and an “unzipped” groove is created. PKCδC1B possesses two long beta sheets (20% β-sheet) forming a V-shaped activator (DAG/phorbol ester) binding groove and a short alpha helix (4% α-helix) at the C-terminal end. Phorbol-13-O-acetate forms five hydrogen bonds in the activator binding groove.
The C2 domain of PKCα is composed of 8 β-sheets and calcium binds to CBR1 and CBR3 formed by the N- and C-terminal loops of the C2-key motif [83]. The crystal structure of the kinase domain of PKCβII revealed that this domain is composed of a classical bilobal fold. The N-terminal lobe formed of a five stranded β-sheets and two α-helices whereas the C-terminal lobe consists of eight α-helices. The N- and C-terminal lobes are connected by a linker region and the binding site for ATP lies between these lobes [84].
Crystal structure of full-length PKCβII was determined at 4Å resolution by Leonard et al in 2011(Figure 6C) [85]. The C1B domain is located close to the kinase domain rather than the C2 domain and interacts with the residues 619–633 of the C-terminal tail. The kinase active site has an open conformation for substrate. Still, the conformation of the active site has a low-activity state, because the ATP-binding side chain of Phe-629 of the conserved NFD motif is displaced. The C1B domain forms a clamped conformation and the NFD helix is kept in a low-activity conformation, which is reversed upon membrane binding [85]. The C1A domain is undefined in this structure.
3.2.PKC activation
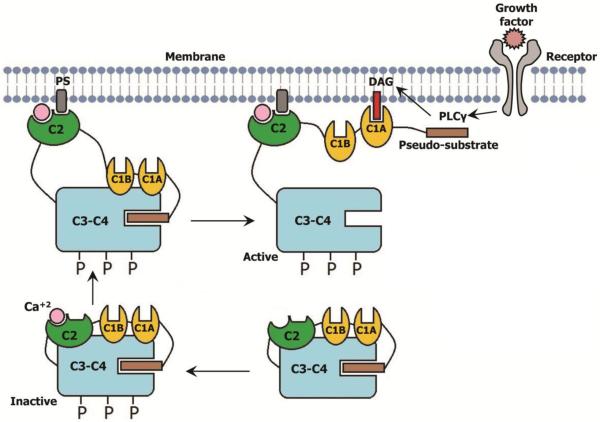
PKC activation, extensively studied for conventional isotypes, occurs in multiple steps [86, 87]. In the inactive state of the enzyme the pseudosubstrate domain at the N-terminus occupies the substrate binding site at the catalytic domain. This shielding the substrate binding domain prevents phosphorylation of the substrate. After assuming an active conformation by phosphorylation of the catalytic domain and calcium ion binding to the C2 domain, PKC then binds to the membrane anchoring through the C2 domain and phosphatidylserine (PS). In the next step endogenous DAG binds to the C1 domain. Membrane penetration and DAG binding pull the pseudosubstrate region from the active site in the kinase domain, resulting in enzyme activation and subsequent phosphorylation of the target proteins, thereby regulating apoptotic or anti-apoptotic pathways [88–90] (Figure 7). Phosphorylation of Nrf2 at its Ser-40 by PKC and subsequent regulation of the antioxidant enzymes is one of the mechanisms by which PKC participate in the antioxidant defense mechanism in the cell [91–93].
Fig.7.
Simplified scheme showing the activation process of Ca+2-sensitive PKCs. Embedded C2 domain in C3–C4 domain of inactive conventional PKC first binds to cytosolic calcium. In the next step the calcium bound C2 domain anchored to the PS of the membrane, which pulls out the C1 domains away from the catalytic domain. Then the C1A domain first binds to the DAG which eventually pulls out the pseudosubstrate motif from the substrate binding pocket and thereby activating the PKC.
3.3. PKC and small molecules
Several classes of natural and synthetic compounds are known to modulate PKC activity [87]. These include phorbol esters, prostratin, bryostatins, indo- and benzolactams, ingenol esters, aplysiatoxin, mezerein, resiniferatoxin, daphnoretin, iridals, anthracyclin derivatives, retinoids etc. The prominent synthetic class of molecule that modulate PKC activity are diacylglycerol lactone, isophthalic acid derivatives etc. While these molecules bind to the C1 domain with nanomolar affinity and activate different PKC isoforms, natural compounds such as, staurosporin are PKC inhibitors that compete with the ATP-binding site. In 2012, FDA and EMA approved PKC-targeting ingenol-3-angelate (Picato or PEP005) for treating actinic keratosis and several other PKC-based drugs are under clinical trials [74].
4. PKC modulation by polyphenols
PKCs are regulators of many intracellular signaling pathways. Their balanced activity is essential for a healthy cell [57, 94]. Hyperactivity and hypo-activity of different isoforms of PKCs have been linked with pathogenesis (e.g. carcinogenesis), hence, there is an immense focus and body of work on how they can be of therapeutic value. Since they contain redox susceptive residues, it is speculated that their activity could be controlled in a redox pathway [95, 96]. Polyphenols demonstrate for the most part antioxidant properties in vitro and influence cellular redox processes and could influence PKC activity.
Below is a list of polyphenols, their sources, biological importance and what is known about their effects on different PKC isoforms.
4.1. Curcumin
Curcumin (Figure 1) is a β-diketone constituent of turmeric obtained from the powdered root of Curcuma L [97, 98]. Not only is it used as a spice to give a specific flavor and yellow color to curry, which is consumed in trace quantities daily by millions of people, curcumin has also been used as traditional medicine for liver disease (jaundice), indigestion, urinary tract diseases, rheumatoid arthritis, and insect bites [97–99]. Curcumin is a promising therapeutic agent for diseases such as cancer, diabetes, multiple sclerosis, Alzheimer's, HIV and cardiovascular disease [100–102].
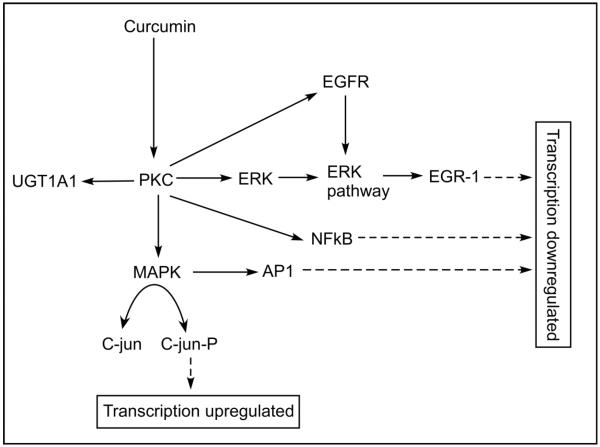
Modulation of PKC activity by curcumin can affect transcription up-regulation or down-regulation, thereby affecting many disease states [103, 104]. For example, inhibition of PKC can produce downstream effects in NFκB, activator protein-1 (AP-1), epidermal growth factor (EGFR), UGT1A1. In human colon cancer cells (HCT1) curcumin induces apoptosis indicating that curcumin could be a therapeutic intervention for colon cancer. In fact, curcumin is under clinical trial for colorectal cancer [104]. In colon cancer, several signaling molecules are affected by curcumin (Figure 8). It suppresses the activity of PKC, subsequently affecting MAPK and C-jun. The down-stream effects of PKC include inhibition of UGT, ERK pathway and EGFR affecting the transcription factors and regulators, AP-1, NF-kB and ERG-1. Studies indicate that curcumin also attenuates diabetic nephropathy [105]and diabetic cardiomyopathy [106] through PKC signaling pathways. In human monocyte, curcumin exerts its antioxidant property by activating the PKCδ/Nrf2/ARE system [107].
Fig.8.
Curcumin and PKC signaling pathway in colon cancer. Curcumin suppresses the activity of PKC subsequently affecting MAPK and C-jun. The down-stream effects of PKC include inhibition of UGT, ERK pathway and EGFR affecting the transcription factors and regulators AP-1, NF-kB and ERG-1. UGT, UDP-glucuronosyltransferase; ERK, Extracellular signal-regulated kinase; EGFR, Epidermal growth factor receptor; MAPK, Mitogen activated protein kinase; AP-1, Activated protein-1; EGR, Early growth response.
There are well documented studies on the modulation of PKC activity by curcumin [108–115]. However, the mechanism by which curcumin modulates PKC activity is poorly understood. In studies involving purified proteins, curcumin (<20 μM) was shown to activate PKCα in the presence of membrane [115]. At higher curcumin concentration (>20 μM), a decrease in activity was observed. In another study using purified protein, it was also shown that curcumin (6–48 μM) activated calcium sensitive PKC (e.g. PKCα) in the presence of membrane and inhibited it in the absence of membrane [114]. Similarly, another study also showed that in a membrane-free system, curcumin (100 μM) inhibited PKC [109]. All these results indicated that membrane, Ca2+ and curcumin concentration are important determinants for curcumin to behave as an activator or inhibitor. Studies using cultured NIH3T3 fibroblasts found that curcumin (15–20 μM) alone did not affect basal PKC activity, but it inhibited the TPA-induced PKC activity [108]. In a study involving mouse skin, curcumin (10 μmol) inhibited TPA-induced membrane translocation of both PKCα and PKCε [112]. However, in CHO-K1 cells curcumin and its derivatives show high selectivity for PKCα over PKCε. While curcumin (5–10 μM) inhibited TPA-induced translocation of PKCα, but not PKCε from cytosol to plasma membrane, the modification of its hydroxyl group with an unsaturated aliphatic chain completely abolished this PKCα inhibition[116].
These differences could be due to the differences in the membrane translocation machinery present in different cellular systems.
In studying the effect of curcumin on purified PKC, Mahmmoud proposed that curcumin may bind to the Ca2+ binding site in the regulatory domain and also at a site at the kinase domain [114]. However, the observed inhibition of membrane translocation of calcium insensitive PKCs (PKCε, PKCη) by curcumin in mouse skin [112] indicated that Ca2+ may not be the only factor that regulated this membrane translocation process by curcumin. From the recent binding and modeling studies [117, 118] it was proposed that curcumin could bind to the C1 domains of PKCs.
4.2.Resveratrol
Resveratrol (Figure 1) is a naturally occurring phytoalexin found in grapes, red wine, peanuts, olive oil, cranberries, and other food [119, 120]. Numerous studies highlighted the effects of resveratrol in a variety of human disease models, including cardio- and neuroprotection, immune regulation, and cancer chemoprevention [121–126].
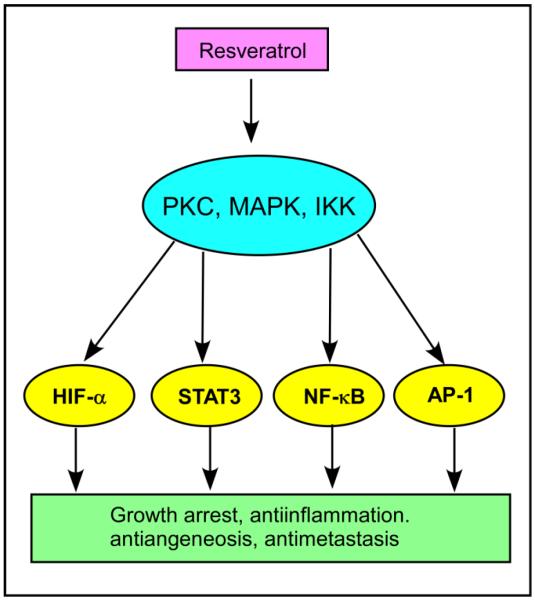
Resveratrol's direct and indirect effects on PKC and related signaling molecules have been implicated in several medical conditions [127]. Resveratrol (15 μM) inhibited TPA-induced PKC expression in human mammary and oral epithelial cells [128], inhibited TPA-induced expression of PKCδ and reduced the metastatic potential of human cervical cancer at 50 μM [129], and affects protein kinase C activity and promotes apoptosis in human colon carcinoma cells at a concentration as high as 200 μM [130]. Resveratrol suppresses the activity of kinases such as protein kinase C (PKC), mitogen-activated protein kinases (MAPK) and IκB kinase (IKK) and transcription factors such as hypoxia-inducible factor-1α (HIF-1α), signal transducer and activator of transcription 3 (STAT3), nuclear factor κB (NF-κB) and activator protein-1 (AP-1) leading to various conditions in response to oncogenic stimuli (Figure 9) [127]. Resveratrol is also currently in clinical phase II trials as an anti-cancer drug for treatment of human colon cancer [131, 132]. Recent studies show that resveratrol's neuroprotective effects are mediated by the activation of PKCγ at 3 μM [133] and anti-neutrophil activity by inhibiting PKC activity at 10 and 100 μM [134]. Resveratrol regulates cellular PKCα and PKCδ to inhibit growth and induce apoptosis in gastric cancer cells [135], antagonizes EGFR-dependent Erk1/2 activation in human androgen-independent prostate cancer cells associated with isozyme-selective PKCα inhibition [136], inhibits PKC-catalyzed phosphorylation of a cofactor-independent, arginine-rich protein substrate [137], and inhibits a PKCδ splice variant in 3T3L1 adipocytes indicating its role in controlling obesity [138].
Fig.9.
Effect of resveratrol on various PKC-mediated biological responses. Resveratrol suppresses the activity of kinases such as protein kinase C (PKC), mitogen-activated protein kinases (MAPK) and IκB kinase (IKK) and transcription factors such as hypoxia-inducible factor-1α (HIF-1α), signal transducer and activator of transcription 3 (STAT3), nuclear factor κB (NF-κB) and activator protein-1 (AP-1) leading to various conditions in response to oncogenic stimuli.
Effect of resveratrol on the activities of purified recombinant PKC isozymes in association with model lipid membranes was investigated using an in vitro assay system in which the cofactor and activator concentrations were systematically varied [139]. It inhibited PKCα with an IC50 of ~2 μM, PKCβ1 with an IC50 of ~105 μM, whereas PKCε and PKCζ remained unaffected. The authors proposed that resveratrol binds to the C1 domain of PKCα [139]. Recent studies showed that chemical modification of resveratrol moiety could attenuate activity of PKCα and PKCε [140, 141].
4.3.Epigallocatechin 3-gallate (EGCG)
Epigallocatechin 3-gallate (EGCG) (Figure 1) is present in green tea [142]. It has antiviral, antitumor, antihypertensive, antimalarial and hepatoprotective properties [143]. Some studies have further identified it as a potential therapeutic agent for diseases like Alzheimer's [144] and arthritis [145].
(−)-Epigallocatechin 3-gallate and its dehydroxy analog (−)-epicatechin gallate inhibit PKCα with IC50 values of 4.8 μM and 5.9 μM respectively [146]. EGCG competitively inhibited both ATP and phorbol ester binding to PKC [146, 147]. In human prostate cancer cells, EGCG down-regulated the expression of PKCα at 12 μM, while the expression of PKCβ, PKCδ, PKCε, PKCμ, PKCη and PKCζ were unaffected [148]. There are multiple studies showing EGCG's involvement in neuroprotection by attenuating PKC signaling pathways. Its neuroprotective effect was linked with the up-regulation of PKCα and PKCε [149]. Using rat hippocampal neuronal cells it was found that PKCγ plays a critical role in the neuro-protective action of EGCG and resveratrol [133]. Neuroprotective mechanism of EGCG can also involve a rapid PKC-mediated degradation of bcl-Associated Death Protein (Bad) by the proteasome [150]. The neuroprotective mechanism of EGCG against oxidative stress-induced cell death includes stimulation of PKC and modulation of cell survival/cell cycle genes [151]. Besides, EGCG (100 μM) down-regulated glucose-induced phosphorylation of PKCα and PKCβII in glomerular epithelial cells thereby providing nephroprotection [152]. EGCG's promotion of keratinocyte differentiation at 5–40 μM was found to be PKCδ-dependent, but neither PKCα nor PKCε-dependent [153]. It also exerts its inhibitory action of dopamine transporter internalization through PKC activation at the concentration range 1-100 μM [154]. EGCG effects a facilitation of glutamate release from glutamatergic terminals by positively modulating N- and P/Q-type Ca2+ channel activation through a signaling cascade involving PKC at 0.5 μM [155]. It attenuates high glucose-induced endothelial cell inflammation by suppression of PKC and NF-kappaB signaling in human umbilical vein endothelial cells at 10–50 μM, suggesting its therapeutic value in the treatment of diabetic vascular complications [156, 157].
Some actions of EGCG are mediated through its binding to cellular receptors. High affinity EGCG-binding proteins were identified by using affinity chromatography [158]. Tachibana et al. have shown that EGCG can bind with high affinity to 67-kDa laminin receptor and through which it can induce some anticancer effects [159]. Others have shown the importance of this receptor in neuroprotection occurring with submicromolar concentrations of EGCG [160]. Nevertheless, PKC may still play an important role in mediating the actions of EGCG as the downstream signaling of 67LR involves generation of reactive oxygen species and subsequent oxidative regulation of PKC isoenzymes [160, 161].
4.4. Quercetin
Quercetin (Figure 1) is a flavonol that can be obtained from curly kale, Ginkgo biloba, broccoli, apples, red wine, red onion, and lettuce [1, 162–164]. Quercetin has been shown to be radioprotective (protects cells/tissues against damage caused by radiation), antidiabetic, hepatoprotective, antitumor, and antihypertensive [165].
Quercetin inhibits PKC [166, 167]. In a study with rat brain and pig thyroid PKC, quercetin exhibited biphasic effect on PKC activity. At lower concentrations (10−7 M) it stimulated, whereas, at higher concentrations it inhibited calcium and phospholipid dependent PKCs [168]. Another study using breast cancer cell line (MCF-7) linked the observed inhibition of PKCδ translocation to the membrane fraction with its antitumor property [169]. Studies using human leukemia (HL 60) [170], mouse melanoma (B16-BL6) [171], and HepG2 [172, 173] cell lines linked the anti-metastatic properties of quercetin to its PKC regulation. Furthermore, a docking study showed quercetin as being able to directly bind to the catalytic domain of PKC [174]. Quercetin was also found to initiate apoptotic sensitivity in the human lymphocyte (HPB-all) cell line via activation of PKCα at a concentration of 50 μM [175]. It inhibits PKCθ phosphorylation indirectly in human mast cells at 1-100 μM, suggesting its therapeutic uses in inflammatory conditions [176]. Quercetin and its structural analog, catechin synergistically act in reducing platelet recruitment via inhibition of PKC-dependent NADPH oxidase activation, indicating their roles in cardiovascular diseases [177].
4.5. Ellagic acid
Ellagic acid (Figure 1) can be found in raspberry, strawberry, pomegranate, and red wine [162]. For the most part, in nature, they exist as complexes called ellagitannins, which usually undergo hydrolysis in the gastrointestinal tract to form ellagic acid [178]. In vitro and in vivo studies have shown ellagic acid to exert antidiarrheal [179], anti-diabetic [180], anti-carcinogenic [181, 182], anti-inflammatory [183] properties.
Ellagic acid (50 or 80 μM) has been shown to inhibit overall PKC activity in a pathway that prevents platelet aggregation [184]. It was suggested that ellagic acid initially inhibited the PLCγ2-PKC cascade and/or hydroxyl radical formation, followed by decreased phosphorylation of MAPKs and Akt, ultimately leading to the inhibition of platelet aggregation. This study also pointed out that ellagic acid did not play a direct role in PKC activation. Using Dalton lymphoma (DL) mice, however, another group observed that ellagic acid, at 40–80 mg/Kg doses, altered activity and expression of several PKC isoforms [95, 185–187] and affected apoptosis and antioxidant defense system.
4.6. Caffeic acid
Caffeic acid (Figure 1) belongs to the hydrocinnamic class of polyphenols. It can be found in coffee, blueberry, pear, apple and spinach [162]. It has been shown to have anti-inflammatory [188], antitumor, analgesic [189] properties in in vivo studies.
Caffeic acid is reported to be a noncompetitive inhibitor of overall PKC activity in partially purified human monocytes (U937 cell line) and in purified PKC containing PKCα, PKCβ and PKCγ at 100 μM [190].
4.7. Protocatechuic acid
Protocatechuic acid (Figure 1) can be found in raspberry, Cardiospermum halicacabum, and Harrisonia perforate [1, 191, 192]. It is one of the major metabolites of dietary anthocyanins [193] and has been implicated as having lifespan lengthening [194] cognitive improving [195] diabetic ameliorating and anti-inflammatory [196] functions.
Its extensive interactions with PKCs were identified in a study of Swiss mouse epidermis in which 16 μmol protocatechuic acid affected translocation of PKCα and reduced its activity by 59% [197]. In this study, PKCζ, PKCγ and PKCα translocation to membrane fractions was inhibited, however, PKCβI and βII were not affected. These effects were identified as a possible link to its antitumor properties. It has been observed that expression of PKCα and PKCβ was inhibited by protocatechuic acid (2 and 4%) which was associated with amelioration diabetic symptoms [198].
4.8.Tannic acid
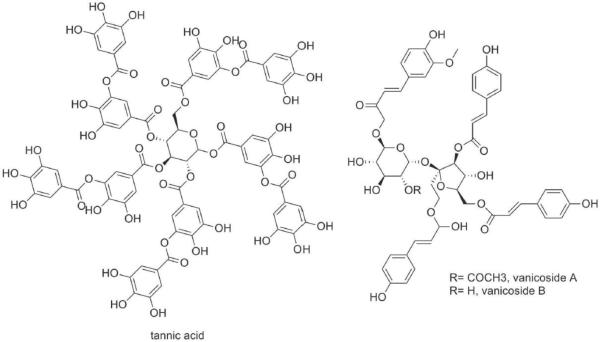
Tannin can be isolated from black tea, A. japonica and Pongamia pinnata [199–201]. Historically, tannic acid (Figure 1) has been used primarily to treat burns. More recently, it has been identified as suitable for treatment of abrasions. It has been linked as having antitumor, antihypertensive, antibacterial, antiviral, antibiofilm, and neuroprotective properties [202]. However, tannic acid has also been shown to be hepatotoxic [203] and cytotoxic to mouse fibroblasts at a concentration higher than 100 μM.
Tannic acid (16 μmol) decreases PKCα, PKCβI and PKCβII activities in mouse epidermal cell line, which is linked with its antitumor properties [197]. It also increased the levels of PKCα, PKCβI, PKCβII in the cytosolic fraction of mouse epidermis between 127% to 492% as compared with phorbol ester-treated group and decreased their activity by 94% in the membrane fraction [197]. Tannic acid inhibits PKC autophosphorylation without interfering with PKC translocation to membrane and consequently inhibits DNA synthesis in mouse fibroblast at 12–100 μM [204]. In human carcinoma cell line, tannic acid inhibited PKC activity at very high concentrations (IC50=350μM) [205]. It was also found that tannin inhibited chloride secretion in airway epithelial cells, in part, by inhibiting PKC [206]. Testing 56 different tannins on the inhibition of PKC, Kashiwada et al showed that some of them competed with phorbol ester with IC50 values ranging 2–20 μM, indicating the binding of tannins in the regulatory domain of PKC [207].
4.9.Chlorogenic acid
Chlorogenic acid (Figure 1) is an ester of caffeic acid and quinic acid [208]. It is present in large amounts in green coffee extract and also can be found in bamboo, peach and Chinese hawthorn [209]. Chlorogenic acid is considered to inhibit fat accumulation, helps lose weight [210] and has anti-hypertensive properties [211].
It affects TPA-stimulated activities of conventional PKCs, PKCζ and their distribution in mouse epidermis, and alters the tumor promotion. At 16 μmol, chlorogenic acid affected translocation of PKCα and reduced its activity by 43% [197].
4.10. Apigenin
Apigenin (Figure 1) belongs to the flavone polyphenol subclass [162]. It can be found in celery, hot pepper, oregano, thyme and olives [162]. In addition to its anti-proliferative effects, it has been implicated as a potential therapeutic agent for many forms of allergies, such as, cedar pollinosis and asthma [212].
In leukemia cells, apigenin (50 μM) increased PKCδ activity causing apoptosis [213]. A study using mouse embryonic fibroblasts also observed apigenin to be a competitive inhibitor of ATP binding to PKC [214]. In human epidermal keratinocytes, however, apigenin (20 μM) inhibited PKCδ activation and apoptosis of keratinocytes was not observed [215].
4.11. Theaflavin-3, 3'-digallate
Theaflavin-3, 3'-digallate (TF-3) (Figure 1) is found in black tea [216]. TF-3 is considered to have many health benefits, such as, it reduces risk of coronary heart disease, viral infection and prevention of cancer [217, 218].
TF-3 is known to repress the activities of xanthine oxidase, cyclooxygenase, EGF-receptor tyrosine kinase along with PKC. Inhibition of these proteins is thought to be by blocking the mitogenic and differentiating signals through modulating EGFR function, MAPK cascades, NF-κB activation, as well as c-myc, c-jun and c-fos expression, which leads to the suppression of tumor promotion. At 20 μM, TF-3 blocks PKCα translocation from cytosol to membrane in TPA-treated NIH3T3 cells [219].
4.12. Verbascoside
Verbascoside (Figure 1) is obtained from plant species such as, Ansbidaea pulchra, Buddleja brasiliensis, Verbasom phlomoides, Penstemon barbatus and Retzia capensis [220–224]. It is recognized for its anti-inflammatory [225], anti-tumor [226], analgesic [227], neuroprotective [228] and antihypertensive [229] properties as demonstrated in several in vitro and in vivo studies. It is a hetero-trimer made up of caffeic acid, glucose and a catechol unit.
Verbascoside competitively and non-competitively inhibits rat brain PKC for ATP (Ki= 22 μM) and histone IIIs respectively [230]. The authors further suggested that the antitumor activity of verbascoside could be in part through its binding to PKC.
4.13. Viniferin
Viniferin (Figure 1) is present in plant species such as, Paeonia suffruricosa, Paconia lactifora, Rheum lhasaense, Smilax scolanicaulis, Vitis amurensis, Vitis vinifera and Hopea chinensis [231–236]. It has been identified as a potential antifungal [237], antitumor [238], antibacterial [239], antidiabetic [240], neuroprotective [241] and cardioprotective [242] agent. Viniferin exists in nature in different isoforms such as α-viniferin, β-viniferin, γ-viniferin, δ-viniferin and ε-viniferin [243].
α-Viniferin inhibits PKCα, PKCβII, PKCγ, PKCδ and PKCε in a non-competitive manner at lower micromolar concentrations[244].The assay was performed with partially purified PKC isoforms expressed and purified from Sf9 cells.
4.14. Rosmarinic acid
Rosmarinic acid (Figure 1) is an ester of caffeic acid which is present in copious amounts in Salvia fruticosa tea, Prunella vulgaris, Sweet basil, Perrilla frutescens, and Melissia offinalis [245–248]. It shows anti-diabetic, melanogenic, tumor degenerative, antidepressive, and analgesic properties [248]. At 15 μM rosmarinic acid showed less than 20% inhibition rat brain PKC [146].
4.15. Miyabenol C
Miyabenol C (Figure 1) is present in plant species such as, Vitis vinifera, Vitis amurensis, Murabilis rotondifolia, Caragana sinica, and Sophora davidii [235, 249–251]. It also shows potent antifungal [252] and tumor inhibitory [253] properties in in vitro studies. Miyabenol C inhibits activity of partially purified rat brain PKC non-competitively with an IC50 of 27.5 μM [254]. Another study reported that inhibition of PKCα, PKCβII, PKCγ, PKCδ and PKCε by miyabenol C can occur at low micromolar concentrations[244].
4.16. Rottlerin
Rottlerin (Figure 1) is isolated from Mallotus philippenis (Kamala) found in Southeast Asia. It is used as laxatives. Rottlerin has many other functions, such as it acts on potassium (BK Ca2+) channel and improves cardiac performance after cardioplegia arrest through improved cardiomyocyte contraction and coronary perfusion [255]. This phenolic compound has antioxidant and anti-carcinogenic properties, and increases cardioplegia-induced phosphorylation of protein kinase B (Akt). Initially, rottlerin was known to inhibit calcium-unresponsive PKCδ but recent studies report that rottlerin also inhibits CAM kinase III and other protein kinases [256]. Liao et al. showed that rottlerin inhibited cell proliferation and activated cell apoptosis in different cell lines, which is possibly through the PKCδ pathway [257]. For many years rottlerin has been marketed as PKCδ inhibitor, but a recent study by Stephen P. Soltoff disapproves and reports that it inhibits other kinases and non-kinases, but not PKCδ [258].
4.17. Miscellaneous
Polyphenolic extracts from several plants and fruits were tested for their PKC activity. Apple polyphenol extract inhibited PKC in a cell-free system [259]. Other polyphenols such as vanicoside A1 and B2 (Figure 1) isolated from Polygonum pensylranicum are also reported to inhibit PKC [260]. A study involving the screening of 43 natural compounds from different sources for their inhibition against PKCα activity and competitive binding identified several flavonoid compounds such as isorhametin, kaempferol, luteolin, myricetin, rhamnetin, gallate such as, dodecyl gallate, and suggested that these compounds may bind to the catalytic domain of PKCα [146]. Ginseng is a Chinese herb that contains a polyphenol mixture. Its lipophilic fraction induced neurite extension and promoted survival of rat cortical neurons. These effects were blocked by PKC inhibitors, suggesting that the lipophilic fraction of ginseng exerts its neurotrophic effects via PKC-dependent pathways [261]. Flavonoids such as fisetin, luteolin, morin and rutin have been reported to inhibit PKC [166, 167]. Hydroxytyrosol, the major polyphenol in olive oil reduces metalloproteinase (MMP)-9 and COX-2 induction in activated human monocyte via PKCα and PKCβI inhibition, explaining its vascular protective (anti-inflammatory) effects [262]. Polymeric black tea polyphenols inhibit TPA-induced PKC phosphorylation to exert their anti-tumor promoting activity [263]. It has been also suggested that green tea polyphenols may act as potential neuroprotective agents against blood brain barrier damage through regulation of PKCα signaling pathway [264].
The effect of different polyphenols on PKC is summarized in Table 1.
Table 1.
Mode of regulation of PKC by dietary polyphenols
| Polyphenol | Mode of PKC regulation | References |
|---|---|---|
|
| ||
| Curcumin | Activation of PKCα in the presence of lipid | [115] |
| Inhibition of PKCα in the absence of lipid | [115] | |
| Activation of Ca++-sensitive PKC in the presence of lipid | [114] | |
| Inhibition of Ca++-sensitive PKC in the absence of lipid | [114] | |
| Inhibition of PKC in NIH 3T3 fibroblasts | [108] | |
| Inhibition of PKCα, PKCε, PKCη in mouse skm | [112] | |
| Inhibition of PKCα in CHO-K1 cells | [116] | |
| Inhibition of purified PKC | [109] | |
|
| ||
| Resveratrol | Activation of PKCγ | [133] |
| Inhibition of PKCα in prostate cancer cells | [136] | |
| Inhibition of PKC in neutrophil | [134] | |
| Inhibition of PKCδ splice variant in 3T3L1 adipocytes | [138] | |
| Inhibition of purified PKCα and PKCβI | [139] | |
| Inhibition of PKCα in CHO-K1 cells | [141] | |
| Downregulation of PKC expression in mammary and epithelial cells | [128] | |
| Downregulation of PKCδ expression | [129] | |
|
| ||
| Epigallocatechin 3-gallate | Inhibition of PKCα | [146] |
| Activation of PKC | [154] | |
| Inhibition of PKC | [156], [157] | |
| Downregulation of PKCα expression | [148] | |
| Upregulation of PKCα and PKCε | [149] | |
| Downregulation glucose-induced phosphorylation of PKCα and PKCβII in glomerular epithelial cells | [152] | |
|
| ||
| Quercetin | Inhibition of PKC | [166], [167] |
| Activation of Ca++-sensitive PKC at low concentration | [168] | |
| Inhibition of Ca++-sensitive PKC at higher concentration | [168] | |
| Inhibition of PKCδ in MCF-7 cell | [169] | |
| Activation of PKCα in human lymphocyte cell | [175] | |
| Inhibition of PKCθ in mast cell | [176] | |
|
| ||
| Ellagic acid | Inhibition of PKC in platelet | [184] |
| Upregulation of PKCδ activity in in DL mice | [187] | |
| Upregulation of expression of PKCδ, PKCε, PKCη and PKCθ in DL mice | [187] | |
|
| ||
| Caffeic acid | Inhibition of PKCα, PKCβ and PKCγ in monocyte and in partially purified protein | [190] |
|
| ||
| Protocatechuic acid | Inhibition of PKCα, PKCγ and PKCζ in Swiss mouse epidermis | [197] |
|
| ||
| Tannic acid | Inhibition of PKCα, PKCβI, PKCβII in mouse epidermis | [197] |
| Inhibition of PKC autophosphorylation in mouse fibroblasts | [204] | |
| Inhibition of PKC activity in human carcinoma cells | [205] | |
| Inhibition of PKC activity in airway epithelial cells | [206] | |
| Inhibition of PKC activity in purified protein | [207] | |
|
| ||
| Chlorogenic acid | Inhibition of PKCα and PKCζ in mouse epidermis | [197] |
|
| ||
| Apigenin | Activation of PKCδ in leukemia cell | [213] |
| Inhibition of PKCδ in keratinocyte | [214] | |
| Inhibition of PKC in embryonic fibroblasts | [215] | |
|
| ||
| Theaflavin 3,3-digallate | Inhibition of PKCα in NIH 3T3 cells | [219] |
|
| ||
| Verbacoside | Inhibition of rat brain PKC | [230] |
|
| ||
| Viniferin | Inhibition of PKCα, PKCβII, PKCγ, PKCδ, PKCε in partially purified protein | [244] |
|
| ||
| Rosmarinic acid | Inhibition of rat brain PKC | [146] |
|
| ||
| Miyabenol C | Inhibition of rat brain PKC | [254] |
| Inhibition of PKCα, PKCβII, PKCγ, PKCδ, PKCε in partially purified protein | [244] | |
|
| ||
| Vanicoside A1 and B2 | Inhibition of PKC | [260] |
|
| ||
| Hydroxytyrosol | Inhibition of PKCα and PKCβI | [262] |
|
| ||
| Isorhametin, kaempferol, luteolin, myricetin, rhmnetin, dodecyl gallate | Inhibition of PKCα | [146] |
|
| ||
| Fisetin, luteolin, morin, rutin | Inhibition of PKC | [166], [167] |
5. Summary and future perspectives
PKC and polyphenols are involved in the regulation of numerous diseases states. Whereas PKC can be regulated by several classes of natural and synthetic compounds, polyphenols have multiple biological targets. From the extensive literature data compiled on PKC-polyphenol interactions in this article, the following highlights emerge: (1) Polyphenols attenuate PKC signaling pathways directly or indirectly regulating many disease states. (2) Some polyphenols exert their antioxidant properties by regulating the transcription of antioxidant enzymes through PKC. (3) Regulation of PKC by polyphenols is isoform-dependent. (4) For a particular polyphenol, the activation or inhibition of PKC depends on the presence of membrane, Ca2+ ion, cofactors, cell and tissue types etc. (5) Polyphenols are low affinity PKC ligands as compared to phorbol ester/DAG. (6) The site of action of polyphenols on PKC is not unequivocally determined. (7) Two polyphenols, curcumin and resveratrol are in clinical trials for the treatment of colon cancer.
The facts that both PKC and polyphenols play important roles an anticancer mechanisms and that 74% of cancer drugs are derived from natural sources [265], naturally occurring polyphenols such as curcumin, resveratrol or EGCG or its simple analogs may have the potential to be cancer drugs in future. Several critical issues however should be addressed toward this end. First, the contribution of PKC-polyphenol interactions in a particular disease state should be dissected. For example, the anticancer activities of curcumin [266], resveratrol [267, 268] and EGCG [269] are exerted through multiple mechanisms with multiple targets including PKC. It is important to understand how significant PKC-polyphenol interaction is in the pathophysiology of this disease state. Second, the bioavailability of polyphenols after oral intake should be improved. While several polyphenols show promising therapeutic effect in the in vitro system, the effects could reduce to none in the in vivo system or in the clinical trials, indicating bioavailability as a major determinant of the therapeutic potency. Bioavailability of a polyphenol depends on its first pass metabolism, lipophilicity, phase II metabolism, stability in plasma etc. For example, poor bioavailability of curcumin (1%) [270], resveratrol (1%) [271, 272] and EGCG (0.1%) [273] has been a major obstacle in the in drug development relating to these agents. This will also have a significant bearing on the concentration of these agents in the brain after transporting through the blood brain barrier (BBB) [274, 275]. Improvement bioavailability of polyphenols using different delivery systems is a major area of current research [276].
Future research on PKC-polyphenol interactions is expected to evolve around identifying new biological targets of polyphenols in the PKC signaling pathways, systematic study of isoform-selective PKC for each polyphenol, determining the sites of action of polyphenols on PKC so that new molecules could be developed based on these sites. Because most of the high affinity PKC ligands are complex in structure and show poor isoform selectivity, use of simple polyphenol templates, such as curcumin and resveratrol could be used for structure/activity studies for developing isoform-selective PKC modulators for therapeutic purposes. A recent study of curcumin derivative in CHO-K1 cells provides a proof of principle toward this strategy [116]. Furthermore, polyphenol analogs should be designed focusing on the improved bioavailability and PK/PD properties. All these research should be directed toward the goal of developing new anticancer agents and/or identifying a dietary food combination that would be beneficial for a particular type of cancer.
Highlights.
Polyphenols attenuate PKC signaling pathways regulating many disease states
PKC regulation is dependent on membrane, Ca2+ ion, cofactors, cell and tissue types
Polyphenols are low affinity PKC ligands
Bioavailability is a major concern for polyphenol based drug development
Curcumin and resveratrol are in clinical trials for the treatment of colon cancer
Acknowledgements
This research has been supported by finding from NIH grant 1R01AA022414-01A1 to Joydip Das.
Abbreviations
- AP-1
Activated protein-1
- ARE
antioxidant response element
- BBB
Blood brain barrier
- CAT
catalase
- CHO
Chinese Hamster Ovary
- DAG
Diacylglycerol
- EGFR
Epidermal growth factor receptor
- EGR
Early growth response
- ERK
Extracellular signal-regulated kinase
- GCL
γ-glutamylcysteine synthatase
- GPx
Glutathione peroxidase
- GST
glutathione S-transferase
- HIF-1α
Hypoxia-inducible factor-1α
- HO-1
Heme oxygenase-1
- IKK
IκB kinase
- Keap 1
Kelch-like ECH-associated protein 1
- MAPK
Mitogen activated protein kinase
- NF-κB
Nuclear factor κB
- NFD
Asparagine Phenylalanine Aspartic acid
- Nrf2
Nuclear factor E2-related factor 2
- PDB
Protein data Bank
- PKC
protein kinase C
- PK/PD
Pharmacokinetic/Pharmacodynamic
- PRX
peroxiredoxin
- ROS
Reactive oxygen species
- STAT3
Signal transducer and activator of transcription 3
- SOD
superoxide dismutase
- TPA
phorbol 12-myristate 13- acetate
- Trx
thioredoxin
- UGT
UDP-glucuronosyltransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- [2].Hearon WM, Macgregor WS. The Naturally Occurring Lignans. Chem. Rev. 1955;55:957–1068. [Google Scholar]
- [3].Robbins RJ. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003;51:2866–2887. doi: 10.1021/jf026182t. [DOI] [PubMed] [Google Scholar]
- [4].Shen T, Wang XN, Lou HX. Natural stilbenes: an overview. Nat. Prod. Rep. 2009;26:916–935. doi: 10.1039/b905960a. [DOI] [PubMed] [Google Scholar]
- [5].Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding, Cell. Biochem. Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- [6].Pasinetti GM, Ho L. Role of grape seed polyphenols in Alzheimer's disease neuropathology. Nutr. Diet Suppl. 2010;2010:97–103. doi: 10.2147/NDS.S6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rojanathammanee L, Puig KL, Combs CK. Pomegranate polyphenols and extract inhibit nuclear factor of activated T-cell activity and microglial activation in vitro and in a transgenic mouse model of Alzheimer disease. J. Nutr. 2013;143:597–605. doi: 10.3945/jn.112.169516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Maher P, Dargusch R, Bodai L, Gerard PE, Purcell JM, Marsh JL. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Hum. Mol. Genet. 2011;20:261–270. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Anandhan A, Tamilselvam K, Radhiga T, Rao S, Essa MM, Manivasagam T. Theaflavin, a black tea polyphenol, protects nigral dopaminergic neurons against chronic MPTP/probenecid induced Parkinson's disease. Brain Res. 2012;1433:104–113. doi: 10.1016/j.brainres.2011.11.021. [DOI] [PubMed] [Google Scholar]
- [10].Vauzour D, Ravaioli G, Vafeiadou K, Rodriguez-Mateos A, Angeloni C, Spencer JPE. Peroxynitrite induced formation of the neurotoxins 5-S-cysteinyl-dopamine and DHBT-1: Implications for Parkinson's disease and protection by polyphenols. Arch. Biochem. Biophys. 2008;476:145–151. doi: 10.1016/j.abb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- [11].Duchnowicz P, Bors M, Podsedek A, Koter-Michalak M, Broncel M. Effect of polyphenols extracts from Brassica vegetables on erythrocyte membranes (in vitro study) Environ. Toxicol. Pharmacol. 2012;34:783–790. doi: 10.1016/j.etap.2012.09.008. [DOI] [PubMed] [Google Scholar]
- [12].Sathyapalan T, Beckett S, Rigby AS, Mellor DD, Atkin SL. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr. J. 2010;9 doi: 10.1186/1475-2891-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gupta A, Vij G, Sharma S, Tirkey N, Rishi P, Chopra K. Curcumin, a polyphenolic antioxidant, attenuates chronic fatigue syndrome in murine water immersion stress model. Immunobiol. 2009;214:33–39. doi: 10.1016/j.imbio.2008.04.003. [DOI] [PubMed] [Google Scholar]
- [14].Sabu MC, Smitha K, Ramadasan K. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J. Ethnopharmacol. 2002;83:109–116. doi: 10.1016/s0378-8741(02)00217-9. [DOI] [PubMed] [Google Scholar]
- [15].Wang LM, Wang YJ, Cui M, Luo WJ, Wang XJ, Barber PA, Chen ZY. A dietary polyphenol resveratrol acts to provide neuroprotection in recurrent stroke models by regulating AMPK and SIRT1 signaling, thereby reducing energy requirements during ischemia. Eur. J. Neurosci. 2013;37:1669–1681. doi: 10.1111/ejn.12162. [DOI] [PubMed] [Google Scholar]
- [16].Negishi H, Xu JW, Ikeda K, Njelekela M, Nara Y, Yamori Y. Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J. Nutr. 2004;134:38–42. doi: 10.1093/jn/134.1.38. [DOI] [PubMed] [Google Scholar]
- [17].Sarkar FH, Li YW. Mechanisms of cancer chemoprevention by soy isoflavone genistein. Cancer Metast. Rev. 2002;21:265–280. doi: 10.1023/a:1021210910821. [DOI] [PubMed] [Google Scholar]
- [18].Knekt P, Jarvinen R, Seppanen R, Heliovaara M, Teppo L, Pukkala E, Aromaa A. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997;146:223–230. doi: 10.1093/oxfordjournals.aje.a009257. [DOI] [PubMed] [Google Scholar]
- [19].Aviram M, Dornfeld L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis. 2001;158:195–198. doi: 10.1016/s0021-9150(01)00412-9. [DOI] [PubMed] [Google Scholar]
- [20].Zern TL, Fernandez ML. Cardioprotective effects of dietary polyphenols. J. Nutr. 2005;135:2291–2294. doi: 10.1093/jn/135.10.2291. [DOI] [PubMed] [Google Scholar]
- [21].Banji D, Banji OJF, Abbagoni S, Hayath MS, Kambam S, Chiluka VL. Amelioration of behavioral aberrations and oxidative markers by green tea extract in valproate induced autism in animals. Brain Res. 2011;1410:141–151. doi: 10.1016/j.brainres.2011.06.063. [DOI] [PubMed] [Google Scholar]
- [22].Dvorakova M, Jezova D, Blazicek P, Trebaticka J, Skodacek I, Suba J, Waczulikova I, Rohdewald P, Durackova Z. Urinary catecholamines in children with attention deficit hyperactivity disorder (ADHD): Modulation by a polyphenolic extract from pine bark (Pycnogenol((R))) Nutr. Neurosci. 2007;10:151–157. doi: 10.1080/09513590701565443. [DOI] [PubMed] [Google Scholar]
- [23].Jalel A, Soumaya GS, Hamdaoui MH. Vitiligo treatment with vitamins, minerals and polyphenol supplementation. Indian J. Dermatol. 2009;54:357–360. doi: 10.4103/0019-5154.57613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Khodja NI, Chataigneau T, Auger C, Schini-Kerth VB. Grape-Derived Polyphenols Improve Aging-Related Endothelial Dysfunction in Rat Mesenteric Artery: Role of Oxidative Stress and the Angiotensin System. PloS one. 2012;7 doi: 10.1371/journal.pone.0032039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mandel SA, Amit T, Weinreb O, Youdim MBH. Understanding the Broad-Spectrum Neuroprotective Action Profile of Green Tea Polyphenols in Aging and Neurodegenerative Diseases. J. Alzheimers Dis. 2011;25:187–208. doi: 10.3233/JAD-2011-101803. [DOI] [PubMed] [Google Scholar]
- [26].Du WX, Olsen CW, Avena-Bustillos RJ, Friedman M, McHugh TH. Physical and Antibacterial Properties of Edible Films Formulated with Apple Skin Polyphenols. J. Food. Sci. 2011;76:M149–M155. doi: 10.1111/j.1750-3841.2010.02012.x. [DOI] [PubMed] [Google Scholar]
- [27].Daglia M. Polyphenols as antimicrobial agents. Curr. Opin. Biotech. 2012;23:174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- [28].Francisco V, Figueirinha A, Costa G, Lopes MC, Garcia-Rodriguez C, Cruz MT, Batista MT. Anti-inflammatory properties of polyphenols from Cymbopogon citratus by inhibition of NF-kappa B pathway. Planta Med. 2011;77:1419–1420. [Google Scholar]
- [29].Scholz EP, Zitron E, Katus HA, Karle CA. Cardiovascular Ion Channels as a Molecular Target of Flavonoids. Cardiovasc. Ther. 2010;28:e46–e52. doi: 10.1111/j.1755-5922.2010.00212.x. [DOI] [PubMed] [Google Scholar]
- [30].Lu JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J. Cell. Mol. Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chun OK, Kim DO, Lee CY. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J. Agric. Food Chem. 2003;51:8067–8072. doi: 10.1021/jf034740d. [DOI] [PubMed] [Google Scholar]
- [32].Forman HJ, Torres M, Fukuto J. Redox signaling. Mol. Cell. Biochem. 2002;234:49–62. [PubMed] [Google Scholar]
- [33].Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [34].Ahmed KA, Sawa T, Ihara H, Kasamatsu S, Yoshitake J, Rahaman MM, Okamoto T, Fujii S, Akaike T. Regulation by mitochondrial superoxide and NADPH oxidase of cellular formation of nitrated cyclic GMP: potential implications for ROS signalling. Biochem. J. 2012;441:719–730. doi: 10.1042/BJ20111130. [DOI] [PubMed] [Google Scholar]
- [35].Frohlich DA, McCabe MT, Arnold RS, Day ML. The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene. 2008;27:4353–4362. doi: 10.1038/onc.2008.79. [DOI] [PubMed] [Google Scholar]
- [36].Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, de Haan JB, Koulis C, El-Osta A, Andrews KL, Chin-Dusting JPF, Touyz RM, Wingler K, Cooper ME, Schmidt HHHW, Jandeleit-Dahm KA. NADPH Oxidase 1 Plays a Key Role in Diabetes Mellitus-Accelerated Atherosclerosis. Circulation. 2013;127:1888. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- [37].Mohsenzadegan M, Mirshafiey A. The Immunopathogenic Role of Reactive Oxygen Species in Alzheimer Disease. Iran. J. Allergy Asthm. 2012;11:203–216. [PubMed] [Google Scholar]
- [38].Cassarino DS, Fall CP, Swerdlow RH, Smith TS, Halvorsen EM, Miller SW, Parks JP, Parker WD, Jr., Bennett JP., Jr. Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson's disease. Biochim. Biophys. Acta. 1997;1362:77–86. doi: 10.1016/s0925-4439(97)00070-7. [DOI] [PubMed] [Google Scholar]
- [39].Lin HC, Tsai SH, Chen CS, Chang YC, Lee CM, Lai ZY, Lin CM. Structure-activity relationship of coumarin derivatives on xanthine oxidase-inhibiting and free radical-scavenging activities. Biochem. Pharmacol. 2008;75:1416–1425. doi: 10.1016/j.bcp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- [40].Farines V, Monje MC, Telo JP, Hnawia E, Sauvain M, Nepveu F. Polyphenols as superoxide dismutase modulators and ligands for estrogen receptors. Anal. Chim. Acta. 2004;513:103–111. [Google Scholar]
- [41].Li YB, Cao ZX, Zhu H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol. Res. 2006;53:6–15. doi: 10.1016/j.phrs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- [42].Azzi A, Davies KJA, Kelly F. Free radical biology - terminology and critical thinking. FEBS Lett. 2004;558:3–6. doi: 10.1016/s0014-5793(03)01526-6. [DOI] [PubMed] [Google Scholar]
- [43].Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005;81:268S–276S. doi: 10.1093/ajcn/81.1.268S. [DOI] [PubMed] [Google Scholar]
- [44].Azzi A, Boscoboinik D, Hensey C. The protein kinase C family. Eur. J. Biochem. 1992;208:547–557. doi: 10.1111/j.1432-1033.1992.tb17219.x. [DOI] [PubMed] [Google Scholar]
- [45].Takai Y, Kishimoto A, Inoue M, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J. Biol. Chem. 1977;252:7603–7609. [PubMed] [Google Scholar]
- [46].Inoue M, Kishimoto A, Takai Y, Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J. Biol. Chem. 1977;252:7610–7616. [PubMed] [Google Scholar]
- [47].Musashi M, Ota S, Shiroshita N. The role of protein kinase C isoforms in cell proliferation and apoptosis. Int. J. Hematol. 2000;72:12–19. [PubMed] [Google Scholar]
- [48].Nishizuka Y. Intracellular Signaling by Hydrolysis of Phospholipids and Activation of Protein-Kinase-C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- [49].Nogues X. Protein kinase C, learning and memory: A circular determinism between physiology and behaviour. Prog. Neuro-Psychoph. 1997;21:507–529. doi: 10.1016/s0278-5846(97)00015-8. [DOI] [PubMed] [Google Scholar]
- [50].Wu-Zhang AX, Newton AC. Protein kinase C pharmacology: refining the toolbox. Biochem. J. 2013;452:195–209. doi: 10.1042/BJ20130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Newton AC. Regulation of the structure, function, and localization of protein kinase C. FASEB J. 1997;11:A1298–A1298. [Google Scholar]
- [52].Tobias IS, Kaulich M, Kim PK, Simon N, Jacinto E, Dowdy SF, King CC, Newton AC. Protein kinase Czeta exhibits constitutive phosphorylation and phosphatidylinositol-3,4,5-triphosphate-independent regulation. Biochem. J. 2016;473:509–523. doi: 10.1042/BJ20151013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cohen S, Braiman A, Shubinsky G, Isakov N. Protein kinase C-theta in platelet activation. FEBS Lett. 2011;585:3208–3215. doi: 10.1016/j.febslet.2011.09.014. [DOI] [PubMed] [Google Scholar]
- [54].Duquesnes N, Lezoualc'h F, Crozatier B. PKC-delta and PKC-epsilon: Foes of the same family or strangers? J. Mol. Cell. Cardiol. 2011;51:665–673. doi: 10.1016/j.yjmcc.2011.07.013. [DOI] [PubMed] [Google Scholar]
- [55].Goldberg M, Steinberg SF. Tissue-specific developmental regulation of protein kinase C isoforms. Biochem. Pharmacol. 1996;51:1089–1093. doi: 10.1016/0006-2952(96)00046-9. [DOI] [PubMed] [Google Scholar]
- [56].Koivunen J, Aaltonen V, Peltonen J. Protein kinase C (PKC) family in cancer progression. Cancer Lett. 2006;235:1–10. doi: 10.1016/j.canlet.2005.03.033. [DOI] [PubMed] [Google Scholar]
- [57].Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nature Rev. Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- [58].Simonis G, Braun MU, Kirrstetter M, Schon SP, Strasser RH. Mechanisms of myocardial remodeling: ramiprilat blocks the expressional upregulation of protein kinase C-epsilon in the surviving myocardium early after infarction. J. Cardiovasc. Pharmacol. 2003;41:780–787. doi: 10.1097/00005344-200305000-00016. [DOI] [PubMed] [Google Scholar]
- [59].Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- [60].Palaniyandi SS, Sun L, Ferreira JC, Mochly-Rosen D. Protein kinase C in heart failure: a therapeutic target? Cardiovasc. Res. 2009;82:229–239. doi: 10.1093/cvr/cvp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ferreira JC, Brum PC, Mochly-Rosen D. betaIIPKC and epsilonPKC isozymes as potential pharmacological targets in cardiac hypertrophy and heart failure. J. Mol. Cell. Cardiol. 2011;51:479–484. doi: 10.1016/j.yjmcc.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bright R, Mochly-Rosen D. The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke. 2005;36:2781–2790. doi: 10.1161/01.STR.0000189996.71237.f7. [DOI] [PubMed] [Google Scholar]
- [63].Dempsey EC, Cool CD, Littler CM. Lung disease and PKCs. Pharmacol. Res. 2007;55:545–559. doi: 10.1016/j.phrs.2007.04.010. [DOI] [PubMed] [Google Scholar]
- [64].Li J, Gobe G. Protein kinase C activation and its role in kidney disease. Nephrol. (Carlton) 2006;11:428–434. doi: 10.1111/j.1440-1797.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- [65].Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- [66].Maioli E, Valacchi G. Rottlerin: bases for a possible usage in psoriasis. Current Drug Metabol. 2010;11:425–430. doi: 10.2174/138920010791526097. [DOI] [PubMed] [Google Scholar]
- [67].DiazGranados N, Zarate CA., Jr. A review of the preclinical and clinical evidence for protein kinase C as a target for drug development for bipolar disorder. Curr. Psychiatry Rep. 2008;10:510–519. doi: 10.1007/s11920-008-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhang D, Anantharam V, Kanthasamy A, Kanthasamy AG. Neuroprotective effect of protein kinase C delta inhibitor rottlerin in cell culture and animal models of Parkinson's disease. J. Pharmacol. Exp. Ther. 2007;322:913–922. doi: 10.1124/jpet.107.124669. [DOI] [PubMed] [Google Scholar]
- [69].Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472:319–324. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- [70].Sun MK, Alkon DL. Pharmacology of protein kinase C activators: cognition-enhancing and antidementic therapeutics. Pharmacol. Ther. 2010;127:66–77. doi: 10.1016/j.pharmthera.2010.03.001. [DOI] [PubMed] [Google Scholar]
- [71].Alkon DL, Sun MK, Nelson TJ. PKC signaling deficits: a mechanistic hypothesis for the origins of Alzheimer's disease. Trends Pharmacol. Sci. 2007;28:51–60. doi: 10.1016/j.tips.2006.12.002. [DOI] [PubMed] [Google Scholar]
- [72].Garrido JL, Godoy JA, Alvarez A, Bronfman M, Inestrosa NC. Protein kinase C inhibits amyloid beta peptide neurotoxicity by acting on members of the Wnt pathway. FASEB J. 2002;16:1982–1984. doi: 10.1096/fj.02-0327fje. [DOI] [PubMed] [Google Scholar]
- [73].Sweitzer SM, Wong SM, Peters MC, Mochly-Rosen D, Yeomans DC, Kendig JJ. Protein kinase C epsilon and gamma: involvement in formalin-induced nociception in neonatal rats. J. Pharmacol. Exp. Ther. 2004;309:616–625. doi: 10.1124/jpet.103.060350. [DOI] [PubMed] [Google Scholar]
- [74].Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nat. Rev. Drug Discov. 2012;11:937–957. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Steinberg SF. Structural basis of protein kinase C isoform function. Physiol. Rev. 2008;88:1341–1378. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Colon-Gonzalez F, Kazanietz MG. C1 domains exposed: from diacylglycerol binding to protein-protein interactions. Biochim. Biophys. Acta. 2006;1761:827–837. doi: 10.1016/j.bbalip.2006.05.001. [DOI] [PubMed] [Google Scholar]
- [77].Yamasaki T, Takahashi A, Pan JZ, Yamaguchi N, Yokoyama KK. Phosphorylation of Activation Transcription Factor-2 at Serine 121 by Protein Kinase C Controls c-Jun-mediated Activation of Transcription. J. Biol. Chem. 2009;284:8567–8581. doi: 10.1074/jbc.M808719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kazanietz MG, Krausz KW, Blumberg PM. Differential irreversible insertion of protein kinase C into phospholipid vesicles by phorbol esters and related activators. J. Biol. Chem. 1992;267:20878–20886. [PubMed] [Google Scholar]
- [79].Sharkey NA, Leach KL, Blumberg PM. Competitive inhibition by diacylglycerol of specific phorbol ester binding. Proc. Natl. Acad. Sci. U S A. 1984;81:607–610. doi: 10.1073/pnas.81.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Farah CA, Sossin WS. The role of C2 domains in PKC signaling. Adv Eexp Med Biol. 2012;740:663–683. doi: 10.1007/978-94-007-2888-2_29. [DOI] [PubMed] [Google Scholar]
- [81].Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- [82].Zhang G, Kazanietz MG, Blumberg PM, Hurley JH. Crystal structure of the cys2 activator-binding domain of protein kinase C delta in complex with phorbol ester. Cell. 1995;81:917–924. doi: 10.1016/0092-8674(95)90011-x. [DOI] [PubMed] [Google Scholar]
- [83].Verdaguer N, Corbalan-Garcia S, Ochoa WF, Fita I, Gomez-Fernandez JC. Ca(2+) bridges the C2 membrane-binding domain of protein kinase Calpha directly to phosphatidylserine. EMBO J. 1999;18:6329–6338. doi: 10.1093/emboj/18.22.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Grodsky N, Li Y, Bouzida D, Love R, Jensen J, Nodes B, Nonomiya J, Grant S. Structure of the catalytic domain of human protein kinase C beta II complexed with a bisindolylmaleimide inhibitor. Biochemistry. 2006;45:13970–13981. doi: 10.1021/bi061128h. [DOI] [PubMed] [Google Scholar]
- [85].Leonard TA, Rozycki B, Saidi LF, Hummer G, Hurley JH. Crystal structure and allosteric activation of protein kinase C betaII. Cell. 2011;144:55–66. doi: 10.1016/j.cell.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ananthanarayanan B, Stahelin RV, Digman MA, Cho W. Activation mechanisms of conventional protein kinase C isoforms are determined by the ligand affinity and conformational flexibility of their C1 domains. J. Biol. Chem. 2003;278:46886–46894. doi: 10.1074/jbc.M307853200. [DOI] [PubMed] [Google Scholar]
- [87].Das J, Rahman GM. C1 domains: structure and ligand-binding properties. Chem. Rev. 2014;114:12108–12131. doi: 10.1021/cr300481j. [DOI] [PubMed] [Google Scholar]
- [88].House C, Kemp BE. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987;238:1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- [89].Orr JW, Newton AC. Intrapeptide regulation of protein kinase C. J. Biol. Chem. 1994;269:8383–8387. [PubMed] [Google Scholar]
- [90].Kirwan AF, Bibby AC, Mvilongo T, Riedel H, Burke T, Millis SZ, Parissenti AM. Inhibition of protein kinase C catalytic activity by additional regions within the human protein kinase Calpha-regulatory domain lying outside of the pseudosubstrate sequence. Biochem. J. 2003;373:571–581. doi: 10.1042/BJ20030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc. Natl. Acad. Sci. U S A. 2000;97:12475–12480. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- [93].Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- [94].Antal CE, Newton AC. Tuning the signalling output of protein kinase C. Biochem. Soc. Trans. 2014;42:1477–1483. doi: 10.1042/BST20140172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mishra S, Vinayak M. Anti-carcinogenic action of ellagic acid mediated via modulation of oxidative stress regulated genes in Dalton lymphoma bearing mice. Leuk. Lymphoma. 2011;52:2155–2161. doi: 10.3109/10428194.2011.591014. [DOI] [PubMed] [Google Scholar]
- [96].Varadkar P, Dubey P, Krishna M, Verma N. Modulation of radiation-induced protein kinase C activity by phenolics. J. Radiol. Prot. 2001;21:361–370. doi: 10.1088/0952-4746/21/4/304. [DOI] [PubMed] [Google Scholar]
- [97].Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr. Cancer. 2010;62:919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- [98].Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br. J. Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- [99].Singh S. From exotic spice to modern drug? Cell. 2007;130:765–768. doi: 10.1016/j.cell.2007.08.024. [DOI] [PubMed] [Google Scholar]
- [100].Ataie A, Sabetkasaei M, Haghparast A, Moghaddam AH, Ataee R, Moghaddam SN. Curcumin exerts neuroprotective effects against homocysteine intracerebroventricular injection-induced cognitive impairment and oxidative stress in rat brain. J. Med. Food. 2010;13:821–826. doi: 10.1089/jmf.2009.1278. [DOI] [PubMed] [Google Scholar]
- [101].Cemil B, Topuz K, Demircan MN, Kurt G, Tun K, Kutlay M, Ipcioglu O, Kucukodaci Z. Curcumin improves early functional results after experimental spinal cord injury. Acta Neurochir. (Wien) 2010;152:1583–1590. doi: 10.1007/s00701-010-0702-x. [DOI] [PubMed] [Google Scholar]
- [102].Morimoto T, Sunagawa Y, Fujita M, Hasegawa K. Novel heart failure therapy targeting transcriptional pathway in cardiomyocytes by a natural compound, curcumin. Circ. J. 2010;74:1059–1066. doi: 10.1253/circj.cj-09-1012. [DOI] [PubMed] [Google Scholar]
- [103].Lin JK. Molecular targets of curcumin. Adv. Exp. Med. Biol. 2007;595:227–243. doi: 10.1007/978-0-387-46401-5_10. [DOI] [PubMed] [Google Scholar]
- [104].Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170–181. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- [105].Soetikno V, Watanabe K, Sari FR, Harima M, Thandavarayan RA, Veeraveedu PT, Arozal W, Sukumaran V, Lakshmanan AP, Arumugam S, Suzuki K. Curcumin attenuates diabetic nephropathy by inhibiting PKC-alpha and PKC-beta1 activity in streptozotocin-induced type I diabetic rats. Mol. Nutr. Food Res. 2011;55:1655–1665. doi: 10.1002/mnfr.201100080. [DOI] [PubMed] [Google Scholar]
- [106].Soetikno V, Sari FR, Sukumaran V, Lakshmanan AP, Mito S, Harima M, Thandavarayan RA, Suzuki K, Nagata M, Takagi R, Watanabe K. Curcumin prevents diabetic cardiomyopathy in streptozotocin-induced diabetic rats: possible involvement of PKC-MAPK signaling pathway. Eur. J. Pharmaceut. Sci. 2012;47:604–614. doi: 10.1016/j.ejps.2012.04.018. [DOI] [PubMed] [Google Scholar]
- [107].Rushworth SA, Ogborne RM, Charalambos CA, O'Connell MA. Role of protein kinase C delta in curcumin-induced antioxidant response element-mediated gene expression in human monocytes. Biochem. Biophys. Res. Commun. 2006;341:1007–1016. doi: 10.1016/j.bbrc.2006.01.065. [DOI] [PubMed] [Google Scholar]
- [108].Liu JY, Lin SJ, Lin JK. Inhibitory effects of curcumin on protein kinase C activity induced by 12-O-tetradecanoyl-phorbol-13-acetate in NIH 3T3 cells. Carcinogenesis. 1993;14:857–861. doi: 10.1093/carcin/14.5.857. [DOI] [PubMed] [Google Scholar]
- [109].Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Lett. 1994;341:19–22. doi: 10.1016/0014-5793(94)80232-7. [DOI] [PubMed] [Google Scholar]
- [110].Lin JK, Chen YC, Huang YT, Lin-Shiau SY. Suppression of protein kinase C and nuclear oncogene expression as possible molecular mechanisms of cancer chemoprevention by apigenin and curcumin. J. Cell. Biochem. Suppl. 1997;28–29:39–48. [PubMed] [Google Scholar]
- [111].Rungseesantivanon S, Thenchaisri N, Ruangvejvorachai P, Patumraj S. Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement Altern. Med. 2010;10:57. doi: 10.1186/1472-6882-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Garg R, Ramchandani AG, Maru GB. Curcumin decreases 12-O-tetradecanoylphorbol-13-acetate-induced protein kinase C translocation to modulate downstream targets in mouse skin. Carcinogenesis. 2008;29:1249–1257. doi: 10.1093/carcin/bgn114. [DOI] [PubMed] [Google Scholar]
- [113].Liao KK, Wu MJ, Chen PY, Huang SW, Chiu SJ, Ho CT, Yen JH. Curcuminoids promote neurite outgrowth in PC12 cells through MAPK/ERK- and PKC-dependent pathways. J. Agric. Food Chem. 2012;60:433–443. doi: 10.1021/jf203290r. [DOI] [PubMed] [Google Scholar]
- [114].Mahmmoud YA. Modulation of protein kinase C by curcumin; inhibition and activation switched by calcium ions. Br. J. Pharmacol. 2007;150:200–208. doi: 10.1038/sj.bjp.0706970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Perez-Lara A, Corbalan-Garcia S, Gomez-Fernandez JC. Curcumin modulates PKCalpha activity by a membrane-dependent effect. Arch. Biochem. Biophys. 2011;513:36–41. doi: 10.1016/j.abb.2011.06.010. [DOI] [PubMed] [Google Scholar]
- [116].Pany S, Majhi A, Das J. Selective Modulation of Protein Kinase C alpha over Protein Kinase C epsilon by Curcumin and Its Derivatives in CHO-K1 Cells. Biochemistry. 2016;55:2135–2143. doi: 10.1021/acs.biochem.6b00057. [DOI] [PubMed] [Google Scholar]
- [117].Majhi A, Rahman GM, Panchal S, Das J. Binding of curcumin and its long chain derivatives to the activator binding domain of novel protein kinase C. Bioorg. Med. Chem. 2010;18:1591–1598. doi: 10.1016/j.bmc.2009.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Das J, Pany S, Panchal S, Majhi A, Rahman GM. Binding of isoxazole and pyrazole derivatives of curcumin with the activator binding domain of novel protein kinase C. Bioorg. Med. Chem. 2011;19:6196–6202. doi: 10.1016/j.bmc.2011.09.011. [DOI] [PubMed] [Google Scholar]
- [119].Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- [120].Wang Y, Catana F, Yang Y, Roderick R, van Breemen RB. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J. Agric. Food Chem. 2002;50:431–435. doi: 10.1021/jf010812u. [DOI] [PubMed] [Google Scholar]
- [121].Wu JM, Hsieh TC. Resveratrol: a cardioprotective substance. Ann. N. Y. Acad. Sci. 2011;1215:16–21. doi: 10.1111/j.1749-6632.2010.05854.x. [DOI] [PubMed] [Google Scholar]
- [122].Petrovski G, Gurusamy N, Das DK. Resveratrol in cardiovascular health and disease. Ann. N. Y. Acad. Sci. 2011;1215:22–33. doi: 10.1111/j.1749-6632.2010.05843.x. [DOI] [PubMed] [Google Scholar]
- [123].Szekeres T, Saiko P, Fritzer-Szekeres M, Djavan B, Jager W. Chemopreventive effects of resveratrol and resveratrol derivatives. Ann. N. Y. Acad. Sci. 2011;1215:89–95. doi: 10.1111/j.1749-6632.2010.05864.x. [DOI] [PubMed] [Google Scholar]
- [124].Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann. N. Y. Acad. Sci. 2011;1215:150–160. doi: 10.1111/j.1749-6632.2010.05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann. N. Y. Acad. Sci. 2011;1215:1–8. doi: 10.1111/j.1749-6632.2010.05870.x. [DOI] [PubMed] [Google Scholar]
- [126].Richard T, Pawlus AD, Iglesias ML, Pedrot E, Waffo-Teguo P, Merillon JM, Monti JP. Neuroprotective properties of resveratrol and derivatives. Ann. N. Y. Acad. Sci. 2011;1215:103–108. doi: 10.1111/j.1749-6632.2010.05865.x. [DOI] [PubMed] [Google Scholar]
- [127].Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- [128].Subbaramaiah K, Chung WJ, Michaluart P, Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM, Dannenberg AJ. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J. Biol. Chem. 1998;273:21875–21882. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- [129].Woo JH, Lim JH, Kim YH, Suh SI, Min DS, Chang JS, Lee YH, Park JW, Kwon TK. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Oncogene. 2004;23:1845–1853. doi: 10.1038/sj.onc.1207307. [DOI] [PubMed] [Google Scholar]
- [130].Fang JY, Li ZH, Li Q, Huang WS, Kang L, Wang JP. Resveratrol affects protein kinase C activity and promotes apoptosis in human colon carcinoma cells. Asian Pac. J. Cancer Prev. 2012;13:6017–6022. doi: 10.7314/apjcp.2012.13.12.6017. [DOI] [PubMed] [Google Scholar]
- [131].Schwedhelm E, Maas R, Troost R, Boger RH. Clinical pharmacokinetics of antioxidants and their impact on systemic oxidative stress. Clin. Pharmacokinet. 2003;42:437–459. doi: 10.2165/00003088-200342050-00003. [DOI] [PubMed] [Google Scholar]
- [132].Patel KR, Scott E, Brown VA, Gescher AJ, Steward WP, Brown K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- [133].Menard C, Bastianetto S, Quirion R. Neuroprotective effects of resveratrol and epigallocatechin gallate polyphenols are mediated by the activation of protein kinase C gamma. Front. Cell. Neurosci. 2013;7:281. doi: 10.3389/fncel.2013.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Drabikova K, Perecko T, Nosal R, Harmatha J, Smidrkal J, Jancinova V. Polyphenol derivatives - potential regulators of neutrophil activity. Interdisciplinary Toxicol. 2012;5:65–70. doi: 10.2478/v10102-012-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Atten MJ, Godoy-Romero E, Attar BM, Milson T, Zopel M, Holian O. Resveratrol regulates cellular PKC alpha and delta to inhibit growth and induce apoptosis in gastric cancer cells. Invest. New Drugs. 2005;23:111–119. doi: 10.1007/s10637-005-5855-8. [DOI] [PubMed] [Google Scholar]
- [136].Stewart JR, O'Brian CA. Resveratrol antagonizes EGFR-dependent Erk1/2 activation in human androgen-independent prostate cancer cells with associated isozyme-selective PKC alpha inhibition. Invest. New Drugs. 2004;22:107–117. doi: 10.1023/B:DRUG.0000011787.75522.ec. [DOI] [PubMed] [Google Scholar]
- [137].Stewart JR, Ward NE, Ioannides CG, O'Brian CA. Resveratrol preferentially inhibits protein kinase C-catalyzed phosphorylation of a cofactor-independent, arginine-rich protein substrate by a novel mechanism. Biochemistry. 1999;38:13244–13251. doi: 10.1021/bi990875u. [DOI] [PubMed] [Google Scholar]
- [138].Patel R, Apostolatos A, Carter G, Ajmo J, Gali M, Cooper DR, You M, Bisht KS, Patel NA. Protein kinase C delta (PKCdelta) splice variants modulate apoptosis pathway in 3T3L1 cells during adipogenesis: identification of PKCdeltaII inhibitor. J. Biol. Chem. 2013;288:26834–26846. doi: 10.1074/jbc.M113.482638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Slater SJ, Seiz JL, Cook AC, Stagliano BA, Buzas CJ. Inhibition of protein kinase C by resveratrol. Biochim. Biophys. Acta. 2003;1637:59–69. doi: 10.1016/s0925-4439(02)00214-4. [DOI] [PubMed] [Google Scholar]
- [140].Das J, Pany S, Majhi A. Chemical modifications of resveratrol for improved protein kinase C alpha activity. Bioorg. Med. Chem. 2011;19:5321–5333. doi: 10.1016/j.bmc.2011.08.008. [DOI] [PubMed] [Google Scholar]
- [141].Pany S, Majhi A, Das J. PKC activation by resveratrol derivatives with unsaturated aliphatic chain. PloS one. 2012;7:e52888. doi: 10.1371/journal.pone.0052888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochemical Pharmacology. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Chowdhury A, Sarkar J, Chakraborti T, Pramanik PK, Chakraborti S. Protective role of epigallocatechin-3-gallate in health and disease: A perspective. Biomed. Pharmacother. 2016;78:50–59. doi: 10.1016/j.biopha.2015.12.013. [DOI] [PubMed] [Google Scholar]
- [144].Reznichenko L, Amit T, Zheng H, Avramovich-Tirosh Y, Youdim MBH, Weinreb O, Mandel S. Reduction of iron-regulated amyloid precursor protein and beta-amyloid peptide by (−)-epigallocatechin-3-gallate in cell cultures: implications for iron chelation in Alzheimer's disease. J. Neurochem. 2006;97:527–536. doi: 10.1111/j.1471-4159.2006.03770.x. [DOI] [PubMed] [Google Scholar]
- [145].Morinobu A, Biao W, Tanaka S, Horiuchi M, Jun L, Tsuji G, Sakai Y, Kurosaka M, Kumagai S. (−)-epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. 2008;58:2012–2018. doi: 10.1002/art.23594. [DOI] [PubMed] [Google Scholar]
- [146].Tammela P, Ekokoski E, Garcia-Horsman A, Talman V, Finel M, Tuominen R, Vuorela P. Screening of natural compounds and their derivatives as potential protein kinase C inhibitors. Drug Develop. Res. 2004;63:76–87. [Google Scholar]
- [147].Kitano K, Nam KY, Kimura S, Fujiki H, Imanishi Y. Sealing effects of (−)-epigallocatechin gallate on protein kinase C and protein phosphatase 2A. Biophys. Chem. 1997;65:157–164. doi: 10.1016/s0301-4622(96)02254-5. [DOI] [PubMed] [Google Scholar]
- [148].Wang SI, Mukhtar H. Gene expression profile in human prostate LNCaP cancer cells by (--) epigallocatechin-3-gallate. Cancer Lett. 2002;182:43–51. doi: 10.1016/s0304-3835(02)00065-4. [DOI] [PubMed] [Google Scholar]
- [149].Levites Y, Amit T, Mandel S, Youdim MB. Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (−)-epigallocatechin-3-gallate. FASEB J. 2003;17:952–954. doi: 10.1096/fj.02-0881fje. [DOI] [PubMed] [Google Scholar]
- [150].Kalfon L, Youdim MB, Mandel SA. Green tea polyphenol (−) -epigallocatechin-3-gallate promotes the rapid protein kinase C- and proteasome-mediated degradation of Bad: implications for neuroprotection. J. Neurochem. 2007;100:992–1002. doi: 10.1111/j.1471-4159.2006.04265.x. [DOI] [PubMed] [Google Scholar]
- [151].Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/ cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J. Biol. Chem. 2002;277:30574–30580. doi: 10.1074/jbc.M202832200. [DOI] [PubMed] [Google Scholar]
- [152].Park SJ, Jeong JM, Jeong HS, Park JS, Kim NH. Effects of Epigallocatechin-3-Gallate on the Expression of TGF-beta1, PKC alpha/betaII, and NF-kappaB in High-Glucose-Stimulated Glomerular Epithelial Cells. Chonnam. Med. J. 2011;47:116–121. doi: 10.4068/cmj.2011.47.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Adhikary G, Chew YC, Reece EA, Eckert RL. PKC-delta and -eta, MEKK-1, MEK-6, MEK-3, and p38-delta are essential mediators of the response of normal human epidermal keratinocytes to differentiating agents. J. Invest. Dermatol. 2010;130:2017–2030. doi: 10.1038/jid.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- [154].Li R, Peng N, Li XP, Le WD. (−)-Epigallocatechin gallate regulates dopamine transporter internalization via protein kinase C-dependent pathway. Brain Res. 2006;1097:85–89. doi: 10.1016/j.brainres.2006.04.071. [DOI] [PubMed] [Google Scholar]
- [155].Chou CW, Huang WJ, Tien LT, Wang SJ. (−)-Epigallocatechin gallate, the most active polyphenolic catechin in green tea, presynaptically facilitates Ca2+-dependent glutamate release via activation of protein kinase C in rat cerebral cortex. Synapse. 2007;61:889–902. doi: 10.1002/syn.20444. [DOI] [PubMed] [Google Scholar]
- [156].Yang J, Han Y, Chen C, Sun H, He D, Guo J, Jiang B, Zhou L, Zeng C. EGCG attenuates high glucose-induced endothelial cell inflammation by suppression of PKC and NF-kappaB signaling in human umbilical vein endothelial cells. Life Sci. 2013;92:589–597. doi: 10.1016/j.lfs.2013.01.025. [DOI] [PubMed] [Google Scholar]
- [157].Yang J, Han Y, Sun H, Chen C, He D, Guo J, Yu C, Jiang B, Zhou L, Zeng C. (−)-Epigallocatechin gallate suppresses proliferation of vascular smooth muscle cells induced by high glucose by inhibition of PKC and ERK1/2 signalings. J. Agric. Food Chem. 2011;59:11483–11490. doi: 10.1021/jf2024819. [DOI] [PubMed] [Google Scholar]
- [158].Bode AM, Dong Z. Epigallocatechin 3-gallate and green tea catechins: United they work, divided they fail. Cancer Prev. Res. (Phila.) 2009;2:514–517. doi: 10.1158/1940-6207.CAPR-09-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004;11:380–381. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- [160].Gundimeda U, McNeill TH, Elhiani AA, Schiffman JE, Hinton DR, Gopalakrishna R. Green tea polyphenols precondition against cell death induced by oxygen-glucose deprivation via stimulation of laminin receptor, generation of reactive oxygen species, and activation of protein kinase Cepsilon. J. Biol. Chem. 2012;287:34694–34708. doi: 10.1074/jbc.M112.356899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Tsukamoto S, Hirotsu K, Kumazoe M, Goto Y, Sugihara K, Suda T, Tsurudome Y, Suzuki T, Yamashita S, Kim Y, Huang Y, Yamada K, Tachibana H. Green tea polyphenol EGCG induces lipid-raft clustering and apoptotic cell death by activating protein kinase Cdelta and acid sphingomyelinase through a 67 kDa laminin receptor in multiple myeloma cells. Biochem. J. 2012;443:525–534. doi: 10.1042/BJ20111837. [DOI] [PubMed] [Google Scholar]
- [162].Han XZ, Shen T, Lou HX. Dietary polyphenols and their biological significance. Int J Mol Sci. 2007;8:950–988. [Google Scholar]
- [163].Bastianetto S, Zheng WH, Quirion R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br. J. Pharmacol. 2000;131:711–720. doi: 10.1038/sj.bjp.0703626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Crozier A, Lean MEJ, McDonald MS, Black C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997;45:590–595. [Google Scholar]
- [165].Kelly GS. Quercetin. Monograph. Altern. Med. Rev. 2011;16:172–194. [PubMed] [Google Scholar]
- [166].Ferriola PC, Cody V, Middleton E., Jr. Protein kinase C inhibition by plant flavonoids. Kinetic mechanisms and structure-activity relationships. Biochem. Pharmacol. 1989;38:1617–1624. doi: 10.1016/0006-2952(89)90309-2. [DOI] [PubMed] [Google Scholar]
- [167].End DW, Look RA, Shaffer NL, Balles EA, Persico FJ. Non-selective inhibition of mammalian protein kinases by flavinoids in vitro. Res. Commun. Chem. Pathol. Pharmacol. 1987;56:75–86. [PubMed] [Google Scholar]
- [168].Picq M, Dubois M, Munari-Silem Y, Prigent AF, Pacheco H. Flavonoid modulation of protein kinase C activation. Life Sci. 1989;44:1563–1571. doi: 10.1016/0024-3205(89)90450-5. [DOI] [PubMed] [Google Scholar]
- [169].Lin CW, Hou WC, Shen SC, Juan SH, Ko CH, Wang LM, Chen YC. Quercetin inhibition of tumor invasion via suppressing PKC delta/ERK/AP-1-dependent matrix metalloproteinase-9 activation in breast carcinoma cells. Carcinogenesis. 2008;29:1807–1815. doi: 10.1093/carcin/bgn162. [DOI] [PubMed] [Google Scholar]
- [170].Kang TB, Liang NC. Effect of quercetin on activities of protein kinase C and tyrosine protein kinase from HL-60 cells. Acta Pharmacologica Sinica. 1997;18:374–376. [PubMed] [Google Scholar]
- [171].Zhang XM, Huang SP, Xu Q. Quercetin inhibits the invasion of murine melanoma B16-BL6 cells by decreasing pro-MMP-9 via the PKC pathway. Cancer Chemother. Pharmacol. 2004;53:82–88. doi: 10.1007/s00280-003-0702-0. [DOI] [PubMed] [Google Scholar]
- [172].Maurya AK, Vinayak M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol. Biol. Rep. 2015;42:1419–1429. doi: 10.1007/s11033-015-3921-7. [DOI] [PubMed] [Google Scholar]
- [173].Maurya AK, Vinayak M. Modulation of PKC signaling and induction of apoptosis through suppression of reactive oxygen species and tumor necrosis factor receptor 1 (TNFR1): key role of quercetin in cancer prevention. Tumour Biol. 2015;36:8913–8924. doi: 10.1007/s13277-015-3634-5. [DOI] [PubMed] [Google Scholar]
- [174].Dok-Go H, Lee KH, Kim HJ, Lee EH, Lee J, Song YS, Lee YH, Jin C, Lee YS, Cho J. Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. saboten. Brain Res. 2003;965:130–136. doi: 10.1016/s0006-8993(02)04150-1. [DOI] [PubMed] [Google Scholar]
- [175].Russo M, Palumbo R, Mupo A, Tosto M, Iacomino G, Scognamiglio A, Tedesco I, Galano G, Russo GL. Flavonoid quercetin sensitizes a CD95-resistant cell line to apoptosis by activating protein kinase C alpha. Oncogene. 2003;22:3330–3342. doi: 10.1038/sj.onc.1206493. [DOI] [PubMed] [Google Scholar]
- [176].Kandere-Grzybowska K, Kempuraj D, Cao J, Cetrulo CL, Theoharides TC. Regulation of IL-1-induced selective IL-6 release from human mast cells and inhibition by quercetin. Br. J. Pharmacol. 2006;148:208–215. doi: 10.1038/sj.bjp.0706695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Pignatelli P, Di Santo S, Buchetti B, Sanguigni V, Brunelli A, Violi F. Polyphenols enhance platelet nitric oxide by inhibiting protein kinase C-dependent NADPH oxidase activation: effect on platelet recruitment. FASEB J. 2006;20:1082–1089. doi: 10.1096/fj.05-5269com. [DOI] [PubMed] [Google Scholar]
- [178].Larrosa M, Garcia-Conesa MT, Espin JC, Tomas-Barberan FA. Ellagitannins, ellagic acid and vascular health. Mol. Aspects Med. 2010;31:513–539. doi: 10.1016/j.mam.2010.09.005. [DOI] [PubMed] [Google Scholar]
- [179].Xiao HT, Tsang SW, Qin HY, Choi FF, Yang ZJ, Han QB, Chen HB, Xu HX, Sheng H, Lu AP, Bian ZX. A bioactivity-guided study on the anti-diarrheal activity of Polygonum chinense Linn. J. Ethnopharmacol. 2013 doi: 10.1016/j.jep.2013.07.007. [DOI] [PubMed] [Google Scholar]
- [180].Rani PU, Kesavan R, Ganugula R, T A, Kumar PU, Reddy GB, Dixit M. Ellagic acid inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and prevents atheroma formation in streptozotocin-induced diabetic rats. J. Nutr. Biochem. 2013 doi: 10.1016/j.jnutbio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- [181].Chung YC, Lu LC, Tsai MH, Chen YJ, Chen YY, Yao SP, Hsu CP. The inhibitory effect of ellagic Acid on cell growth of ovarian carcinoma cells. Evid Based Complement Alternat. Med. 2013;2013:306705. doi: 10.1155/2013/306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Qiu Z, Zhou B, Jin L, Yu H, Liu L, Liu Y, Qin C, Xie S, Zhu F. In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food Chem. Toxicol. 2013;59C:428–437. doi: 10.1016/j.fct.2013.06.025. [DOI] [PubMed] [Google Scholar]
- [183].Mo J, Panichayupakaranant P, Kaewnopparat N, Songkro S, Reanmongkol W. Topical Anti-inflammatory Potential of Standardized Pomegranate Rind Extract and Ellagic Acid in Contact Dermatitis. Phytother. Res. 2014;28:629–632. doi: 10.1002/ptr.5039. [DOI] [PubMed] [Google Scholar]
- [184].Chang Y, Chen WF, Lin KH, Hsieh CY, Chou DS, Lin LJ, Sheu JR, Chang CC. Novel bioactivity of ellagic Acid in inhibiting human platelet activation. Evid. Based Complement. Alternat. Med. 2013;2013:595128. doi: 10.1155/2013/595128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Mishra S, Vinayak M. Ellagic acid checks lymphoma promotion via regulation of PKC signaling pathway. Mol. Biol. Rep. 2013;40:1417–1428. doi: 10.1007/s11033-012-2185-8. [DOI] [PubMed] [Google Scholar]
- [186].Mishra S, Vinayak M. Ellagic acid inhibits PKC signaling by improving antioxidant defense system in murine T cell lymphoma. Mol. Biol. Rep. 2014;41:4187–4197. doi: 10.1007/s11033-014-3289-0. [DOI] [PubMed] [Google Scholar]
- [187].Mishra S, Vinayak M. Role of ellagic acid in regulation of apoptosis by modulating novel and atypical PKC in lymphoma bearing mice. BMC Complement Altern. Med. 2015;15:281. doi: 10.1186/s12906-015-0810-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Gamaro GD, Suyenaga E, Borsoi M, Lermen J, Pereira P, Ardenghi P. Effect of rosmarinic and caffeic acids on inflammatory and nociception process in rats. ISRN Pharmacol. 2011;2011:451682. doi: 10.5402/2011/451682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Takeda H, Tsuji M, Inazu M, Egashira T, Matsumiya T. Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur. J. Pharmacol. 2002;449:261–267. doi: 10.1016/s0014-2999(02)02037-x. [DOI] [PubMed] [Google Scholar]
- [190].Nardini M, Scaccini C, Packer L, Virgili F. In vitro inhibition of the activity of phosphorylase kinase, protein kinase C and protein kinase A by caffeic acid and a procyanidin-rich pine bark (Pinus marittima) extract. Biochim. Biophys. Acta. 2000;1474:219–225. doi: 10.1016/s0304-4165(00)00009-x. [DOI] [PubMed] [Google Scholar]
- [191].Chen J, Wei JH, Cai SF, Miao WS, Pan LW. Study on chemical constituents of Cardiospermum halicacabum. Zhong Yao Cai. 2013;36:228–230. [PubMed] [Google Scholar]
- [192].Yan XX, Liang ZF, Wang MY, Wang JR, Wang ZN. Study on the chemical constituents in the fruit of Harrisonia perforata. Zhong Yao Cai. 2013;36:223–225. [PubMed] [Google Scholar]
- [193].Vitaglione P, Donnarumma G, Napolitano A, Galvano F, Gallo A, Scalfi L, Fogliano V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. Journal of Nutrition. 2007;137:2043–2048. doi: 10.1093/jn/137.9.2043. [DOI] [PubMed] [Google Scholar]
- [194].Kim YS, Seo HW, Lee MH, Kim DK, Jeon H, Cha DS. Protocatechuic acid extends lifespan and increases stress resistance in Caenorhabditis elegans. Arch. Pharm. Res. 2014;37:245–52. doi: 10.1007/s12272-013-0183-6. [DOI] [PubMed] [Google Scholar]
- [195].Muley MM, Thakare VN, Patil RR, Bafna PA, Naik SR. Amelioration of cognitive, motor and endogenous defense functions with silymarin, piracetam and protocatechuic acid in the cerebral global ischemic rat model. Life Sci. 2013;93:51–57. doi: 10.1016/j.lfs.2013.05.020. [DOI] [PubMed] [Google Scholar]
- [196].Lin CY, Huang CS, Huang CY, Yin MC. Anticoagulatory, antiinflammatory, and antioxidative effects of protocatechuic acid in diabetic mice. J Agric. Food Chem. 2009;57:6661–6667. doi: 10.1021/jf9015202. [DOI] [PubMed] [Google Scholar]
- [197].Szaefer H, Kaczmarek J, Rybczynska M, Baer-Dubowska W. The effect of plant phenols on the expression and activity of phorbol ester-induced PKC in mouse epidermis. Toxicol. 2007;230:1–10. doi: 10.1016/j.tox.2006.10.001. [DOI] [PubMed] [Google Scholar]
- [198].Lin CY, Tsai SJ, Huang CS, Yin MC. Antiglycative effects of protocatechuic acid in the kidneys of diabetic mice. J. Agric. Food Chem. 2011;59:5117–5124. doi: 10.1021/jf200103f. [DOI] [PubMed] [Google Scholar]
- [199].Alasalvar C, Pelvan E, Ozdemir KS, Kocadagli T, Mogol BA, Pasli AA, Ozcan N, Ozcelik B, Gokmen V. Compositional, Nutritional, and Functional Characteristics of Instant Teas Produced from Low- and High-Quality Black Teas. J. Agric. Food Chem. 2013 doi: 10.1021/jf4015137. [DOI] [PubMed] [Google Scholar]
- [200].Lee JH, Park JH, Cho HS, Joo SW, Cho MH, Lee J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling. 2013;29:491–499. doi: 10.1080/08927014.2013.788692. [DOI] [PubMed] [Google Scholar]
- [201].Sajid ZI, Anwar F, Shabir G, Rasul G, Alkharfy KM, Gilani AH. Antioxidant, antimicrobial properties and phenolics of different solvent extracts from bark, leaves and seeds of Pongamia pinnata (L.) Pierre. Molecules. 2012;17:3917–3932. doi: 10.3390/molecules17043917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Sieniawska E. Activities of Tannins--From In Vitro Studies to Clinical Trials. Nat. Prod. Commun. 2015;10:1877–1884. [PubMed] [Google Scholar]
- [203].Chung KT, Wei CI, Johnson MG. Are tannins a double-edged sword in biology and health? Trends Food Sci. Tech. 1998;9:168–175. [Google Scholar]
- [204].Kuo ML, Wu WS, Lee KC, Lin JK. Effects of Tannic-Acid on 12-O-Tetradecanoylphorbol-13-Acetate-Induced Protein-Kinase-C Activation in Nih 3t3 Cells. Biochem. Pharmacol. 1993;46:1327–1332. doi: 10.1016/0006-2952(93)90095-e. [DOI] [PubMed] [Google Scholar]
- [205].Yang EB, Wei L, Zhang K, Chen YZ, Chen WN. Tannic acid, a potent inhibitor of epidermal growth factor receptor tyrosine kinase. J. Biochem. 2006;139:495–502. doi: 10.1093/jb/mvj050. [DOI] [PubMed] [Google Scholar]
- [206].Cloutier MM, Guernsey L. Tannin inhibition of protein kinase C in airway epithelium. Lung. 1995;173:307–319. doi: 10.1007/BF00176894. [DOI] [PubMed] [Google Scholar]
- [207].Kashiwada YN, G., Nishioka I, Ballas LM, Jiang JB, Janzen WP, Lee KH. Tannins as selective inhibitors of protein kinase C. Bioorg. Med. Chem. Lett. 1992;2:239–244. [Google Scholar]
- [208].Mubarak A, Hodgson JM, Considine MJ, Croft KD, Matthews VB. Supplementation of a High-Fat Diet with Chlorogenic Acid Is Associated with Insulin Resistance and Hepatic Lipid Accumulation in Mice. J Agric. Food Chem. 2013;61:4371–4378. doi: 10.1021/jf400920x. [DOI] [PubMed] [Google Scholar]
- [209].Jurikova T, Sochor J, Rop O, Mlcek J, Balla S, Szekeres L, Adam V, Kizek R. Polyphenolic profile and biological activity of Chinese hawthorn (Crataegus pinnatifida BUNGE) fruits. Molecules. 2012;17:14490–14509. doi: 10.3390/molecules171214490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Marcason W. What is green coffee extract? J. Acad. Nutr. Diet. 2013;113:364. doi: 10.1016/j.jand.2012.12.004. [DOI] [PubMed] [Google Scholar]
- [211].Zhao Y, Wang J, Ballevre O, Luo H, Zhang W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens Res. 2012;35:370–374. doi: 10.1038/hr.2011.195. [DOI] [PubMed] [Google Scholar]
- [212].Kawai M, Hirano T, Higa S, Arimitsu J, Maruta M, Kuwahara Y, Ohkawara T, Hagihara K, Yamadori T, Shima Y, Ogata A, Kawase I, Tanaka T. Flavonoids and related compounds as anti-allergic substances. Allergol. Int. 2007;56:113–123. doi: 10.2332/allergolint.R-06-135. [DOI] [PubMed] [Google Scholar]
- [213].Vargo MA, Voss OH, Poustka F, Cardounel AJ, Grotewold E, Doseff AI. Apigenin-induced-apoptosis is mediated by the activation of PKC delta and caspases in leukemia cells. Biochem. Pharmacol. 2006;72:681–692. doi: 10.1016/j.bcp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- [214].Huang YT, Kuo ML, Liu JY, Huang SY, Lin JK. Inhibitions of protein kinase C and proto-oncogene expressions in NIH 3T3 cells by apigenin. Eur. J. Cancer. 1996;32A:146–151. doi: 10.1016/0959-8049(95)00540-4. [DOI] [PubMed] [Google Scholar]
- [215].Balasubramanian S, Zhu L, Eckert RL. Apigenin inhibition of involucrin gene expression is associated with a specific reduction in phosphorylation of protein kinase Cdelta Tyr311. J. Biol. Chem. 2006;281:36162–36172. doi: 10.1074/jbc.M605368200. [DOI] [PubMed] [Google Scholar]
- [216].Lin JK, Chen PC, Ho CT, Lin-Shiau SY. Inhibition of xanthine oxidase and suppression of intracellular reactive oxygen species in HL-60 cells by theaflavin-3,3'-digallate, (−)-epigallocatechin-3-gallate, and propyl gallate. J. Agric. Food Chem. 2000;48:2736–2743. doi: 10.1021/jf000066d. [DOI] [PubMed] [Google Scholar]
- [217].Isaacs CE, Xu WM. Theaflavin-3,3'-Digallate and Lactic Acid Combinations Reduce Herpes Simplex Virus Infectivity. Antimicrob. Agents Chemother. 2013;57:3806–3814. doi: 10.1128/AAC.00659-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Schuck AG, Ausubel MB, Zuckerbraun HL, Babich H. Theaflavin-3,3'-digallate, a component of black tea: an inducer of oxidative stress and apoptosis. Toxicol. In Vitro. 2008;22:598–609. doi: 10.1016/j.tiv.2007.11.021. [DOI] [PubMed] [Google Scholar]
- [219].Chen YC, Liang YC, Lin-Shiau SY, Ho CT, Lin JK. Inhibition of TPA-induced protein kinase C and transcription activator protein-1 binding activities by theaflavin-3,3'-digallate from black tea in NIH3T3 cells. J. Agric. Food Chem. 1999;47:1416–1421. doi: 10.1021/jf981099k. [DOI] [PubMed] [Google Scholar]
- [220].Filho AG, Morel AF, Adolpho L, Ilha V, Giralt E, Tarrago T, Dalcol II. Inhibitory effect of verbascoside isolated from Buddleja brasiliensis Jacq. ex Spreng on prolyl oligopeptidase activity. Phytother. Res. 2012;26:1472–1475. doi: 10.1002/ptr.4597. [DOI] [PubMed] [Google Scholar]
- [221].Gvazava LN, Kikoladze VS. Verbascoside from Verbascum phlomoides. Chem. Nat. Compd. 2007;43:710–711. [Google Scholar]
- [222].Xie J, Tan F, Zhu J, Yue C, Li Q. Separation, purification and quantification of verbascoside from Penstemon barbatus (Cav.) Roth. Food Chem. 2012;135:2536–2541. doi: 10.1016/j.foodchem.2012.07.021. [DOI] [PubMed] [Google Scholar]
- [223].Damtoft S, Franzyk H, Jensen SR, Nielsen BJ. Iridoids and Verbascosides in Retzia. Phytochemistry. 1993;34:239–243. [Google Scholar]
- [224].Brandao GC, Kroon EG, Souza DE, Filho JD, Oliveira AB. Chemistry and Antiviral Activity of Arrabidaea pulchra (Bignoniaceae) Molecules. 2013;18:9919–9932. doi: 10.3390/molecules18089919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Speranza L, Franceschelli S, Pesce M, Reale M, Menghini L, Vinciguerra I, De Lutiis MA, Felaco M, Grilli A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother. Res. 2010;24:1398–1404. doi: 10.1002/ptr.3173. [DOI] [PubMed] [Google Scholar]
- [226].Zhao XH, Yue HL, Li P, Zeng X, Zhang G. Evaluation of the Antitumor Activity by Cdte Qds with Verbascoside. Nano. 2013;8 [Google Scholar]
- [227].Hausmann M, Obermeier F, Paper DH, Balan K, Dunger N, Menzel K, Falk W, Schoelmerich J, Herfarth H, Rogler G. In vivo treatment with the herbal phenylethanoid acteoside ameliorates intestinal inflammation in dextran sulphate sodium-induced colitis. Clin. Exp. Immunol. 2007;148:373–381. doi: 10.1111/j.1365-2249.2007.03350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Esposito E, Dal Toso R, Pressi G, Bramanti P, Meli R, Cuzzocrea S. Protective effect of verbascoside in activated C6 glioma cells: possible molecular mechanisms. N-S Arch. Pharmacol. 2010;381:93–105. doi: 10.1007/s00210-009-0466-0. [DOI] [PubMed] [Google Scholar]
- [229].Ahmad M, Rizwani GH, Aftab K, Ahmad VU, Gilani AH, Ahmad SP. Acteoside - a New Antihypertensive Drug. Phytother. Res. 1995;9:525–527. [Google Scholar]
- [230].Herbert JM, Maffrand JP, Taoubi K, Augereau JM, Fouraste I, Gleye J. Verbascoside isolated from Lantana camara, an inhibitor of protein kinase C. J. Nat. Prod. 1991;54:1595–1600. doi: 10.1021/np50078a016. [DOI] [PubMed] [Google Scholar]
- [231].He CN, Peng Y, Xu LJ, Liu ZA, Gu J, Zhong AG, Xiao PG. Three new oligostilbenes from the seeds of Paeonia suffruticosa. Chem. Pharm. Bull. (Tokyo) 2010;58:843–847. doi: 10.1248/cpb.58.843. [DOI] [PubMed] [Google Scholar]
- [232].Yuk HJ, Ryu HW, Jeong SH, Curtis-Long MJ, Kim HJ, Wang Y, Song YH, Park KH. Profiling of neuraminidase inhibitory polyphenols from the seeds of Paeonia lactiflora. Food Chem. Toxicol. 2013;55:144–149. doi: 10.1016/j.fct.2012.12.053. [DOI] [PubMed] [Google Scholar]
- [233].Liu WB, Hu L, Hu Q, Chen NN, Yang QS, Wang FF. New resveratrol oligomer derivatives from the roots of Rheum lhasaense. Molecules. 2013;18:7093–7102. doi: 10.3390/molecules18067093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Wang P, Xu J, Wang Q, Feng SX, Chen T, Zhang CL. Phenylpropanoids and diphenylethene compounds from roots and rhizomes of Smilax scobinicaulis. Zhongguo Zhong Yao Za Zhi. 2013;38:1531–1535. [PubMed] [Google Scholar]
- [235].Pawlus AD, Sahli R, Bisson J, Riviere C, Delaunay JC, Richard T, Gomes E, Bordenave L, Waffo-Teguo P, Merillon JM. Stilbenoid profiles of canes from Vitis and Muscadinia species. J. Agric. Food Chem. 2013;61:501–511. doi: 10.1021/jf303843z. [DOI] [PubMed] [Google Scholar]
- [236].Yan T, Wang T, Wei W, Jiang N, Qin YH, Tan RX, Ge HM. Polyphenolic acetylcholinesterase inhibitors from Hopea chinensis. Planta Med. 2012;78:1015–1019. doi: 10.1055/s-0031-1298623. [DOI] [PubMed] [Google Scholar]
- [237].Schnee S, Queiroz EF, Voinesco F, Marcourt L, Dubuis PH, Wolfender JL, Gindro K. Vitis vinifera canes, a new source of antifungal compounds against Plasmopara viticola, Erysiphe necator, and Botrytis cinerea. J. Agric. Food Chem. 2013;61:5459–5467. doi: 10.1021/jf4010252. [DOI] [PubMed] [Google Scholar]
- [238].Gonzalez-Sarrias A, Gromek S, Niesen D, Seeram NP, Henry GE. Resveratrol oligomers isolated from Carex species inhibit growth of human colon tumorigenic cells mediated by cell cycle arrest. J. Agric. Food Chem. 2011;59:8632–8638. doi: 10.1021/jf201561e. [DOI] [PubMed] [Google Scholar]
- [239].Yim N, Ha do T, Trung TN, Kim JP, Lee S, Na M, Jung H, Kim HS, Kim YH, Bae K. The antimicrobial activity of compounds from the leaf and stem of Vitis amurensis against two oral pathogens. Bioorg. Med. Chem. Lett. 2010;20:1165–1168. doi: 10.1016/j.bmcl.2009.12.020. [DOI] [PubMed] [Google Scholar]
- [240].Morikawa T, Chaipech S, Matsuda H, Hamao M, Umeda Y, Sato H, Tamura H, Kon'i H, Ninomiya K, Yoshikawa M, Pongpiriyadacha Y, Hayakawa T, Muraoka O. Antidiabetogenic oligostilbenoids and 3-ethyl-4-phenyl-3,4-dihydroisocoumarins from the bark of Shorea roxburghii. Bioorg. Med. Chem. 2012;20:832–840. doi: 10.1016/j.bmc.2011.11.067. [DOI] [PubMed] [Google Scholar]
- [241].Kim JY, Jeong HY, Lee HK, Kim S, Hwang BY, Bae K, Seong YH. Neuroprotection of the leaf and stem of Vitis amurensis and their active compounds against ischemic brain damage in rats and excitotoxicity in cultured neurons. Phytomedicine. 2012;19:150–159. doi: 10.1016/j.phymed.2011.06.015. [DOI] [PubMed] [Google Scholar]
- [242].Zghonda N, Yoshida S, Ezaki S, Otake Y, Murakami C, Mliki A, Ghorbel A, Miyazaki H. epsilon-Viniferin Is More Effective Than Its Monomer Resveratrol in Improving the Functions of Vascular Endothelial Cells and the Heart. Biosci. Biotechnol. Biochem. 2012;76:954–960. doi: 10.1271/bbb.110975. [DOI] [PubMed] [Google Scholar]
- [243].Zhou J, Xie G, Yan X. SpringerLink (Online service), Encyclopedia of Traditional Chinese Medicines - Molecular Structures, Pharmacological Activities, Natural Sources and Applications Vol. 1: Isolated Compounds A-C. Springer-Verlag Berlin Heidelberg; Berlin, Heidelberg: 2011. [Google Scholar]
- [244].Kulanthaivel P, Janzen WP, Ballas LM, Jiang JB, Hu CQ, Darges JW, Seldin JC, Cofield DJ, Adams LM. Naturally-Occurring Protein-Kinase-C Inhibitors .2. Isolation of Oligomeric Stilbenes from Caragana-Sinica. Planta Med. 1995;61:41–44. doi: 10.1055/s-2006-957996. [DOI] [PubMed] [Google Scholar]
- [245].Aggarwal BB, Kunnumakkara AB, ebrary Inc . Molecular targets and therapeutic uses of spices modern uses for ancient medicine. World Scientific; Singapore; Hackensack, NJ: 2009. [Google Scholar]
- [246].Azevedo MF, Lima CF, Fernandes-Ferreira M, Almeida MJ, Wilson JM, Pereira-Wilson C. Rosmarinic acid, major phenolic constituent of Greek sage herbal tea, modulates rat intestinal SGLT1 levels with effects on blood glucose. Mol. Nutr. Food Res. 2011;55(Suppl 1):S15–25. doi: 10.1002/mnfr.201000472. [DOI] [PubMed] [Google Scholar]
- [247].Feng L, Jia X, Zhu MM, Chen Y, Shi F. Antioxidant activities of total phenols of Prunella vulgaris L. in vitro and in tumor-bearing mice. Molecules. 2010;15:9145–9156. doi: 10.3390/molecules15129145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Petersen M, Simmonds MS. Rosmarinic acid. Phytochemistry. 2003;62:121–125. doi: 10.1016/s0031-9422(02)00513-7. [DOI] [PubMed] [Google Scholar]
- [249].Chaher N, Arraki K, Dillinseger E, Temsamani H, Bernillon S, Pedrot E, Delaunay JC, Merillon JM, Monti JP, Izard JC, Atmani D, Richard T. Bioactive stilbenes from Vitis vinifera grapevine shoots extracts. J. Sci. Food Agric. 2014;94:951–954. doi: 10.1002/jsfa.6341. [DOI] [PubMed] [Google Scholar]
- [250].Jin Q, Han XH, Hong SS, Lee C, Choe S, Lee D, Kim Y, Hong JT, Lee MK, Hwang BY. Antioxidative oligostilbenes from Caragana sinica. Bioorg. Med. Chem. Lett. 2012;22:973–976. doi: 10.1016/j.bmcl.2011.12.012. [DOI] [PubMed] [Google Scholar]
- [251].Tanaka T, Ito T, Iinuma M, Ohyama M, Ichise M, Tateishi Y. Stilbene oligomers in roots of Sophora davidii. Phytochemistry. 2000;53:1009–1014. doi: 10.1016/s0031-9422(00)00016-9. [DOI] [PubMed] [Google Scholar]
- [252].Lambert C, Bisson J, Waffo-Teguo P, Papastamoulis Y, Richard T, Corio-Costet MF, Merillon JM, Cluzet S. Phenolics and Their Antifungal Role in Grapevine Wood Decay: Focus on the Botryosphaeriaceae Family. J. Agric. Food Chem. 2012;60:11859–11868. doi: 10.1021/jf303290g. [DOI] [PubMed] [Google Scholar]
- [253].Barjot C, Tournaire M, Castagnino C, Vigor C, Vercauteren J, Rossi JF. Evaluation of antitumor effects of two vine stalk oligomers of resveratrol on a panel of lymphoid and myeloid cell lines: Comparison with resveratrol. Life Sci. 2007;81:1565–1574. doi: 10.1016/j.lfs.2007.08.047. [DOI] [PubMed] [Google Scholar]
- [254].Xu G, Zhang LP, Chen LF, Hu CQ. Inhibition of protein kinase C by stilbenoids. Yao Xue Xue Bao. 1994;29:818–822. [PubMed] [Google Scholar]
- [255].Clements RT, Cordeiro B, Feng J, Bianchi C, Sellke FW. Rottlerin Increases Cardiac Contractile Performance and Coronary Perfusion Through BKCa++ Channel Activation After Cold Cardioplegic Arrest in Isolated Hearts. Circulation. 2011;124:S55–S61. doi: 10.1161/CIRCULATIONAHA.110.012112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Liao YF, Hung YC, Chang WH, Tsay GJ, Hour TC, Hung HC, Liu GY. The PKC delta inhibitor, rottlerin, induces apoptosis of haematopoietic cell lines through mitochondrial membrane depolarization and caspases' cascade. Life Sci. 2005;77:707–719. doi: 10.1016/j.lfs.2005.01.010. [DOI] [PubMed] [Google Scholar]
- [258].Soltoff SP. Rottlerin: an inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol. Sci. 2007;28:453–458. doi: 10.1016/j.tips.2007.07.003. [DOI] [PubMed] [Google Scholar]
- [259].Kern M, Pahlke G, Balavenkatraman KK, Bohmer FD, Marko D. Apple polyphenols affect protein kinase C activity and the onset of apoptosis in human colon carcinoma cells. J. Agric. Food Chem. 2007;55:4999–5006. doi: 10.1021/jf063158x. [DOI] [PubMed] [Google Scholar]
- [260].Zimmermann ML, Sneden AT. Vanicosides A and B, protein kinase C inhibitors from Polygonum pensylvanicum. J. Nat. Prod. 1994;57:236–242. doi: 10.1021/np50104a007. [DOI] [PubMed] [Google Scholar]
- [261].Mizumaki Y, Kurimoto M, Hirashima Y, Nishijima M, Kamiyama H, Nagai S, Takaku A, Sugihara K, Shimizu M, Endo S. Lipophilic fraction of Panax ginseng induces neuronal differentiation of PC12 cells and promotes neuronal survival of rat cortical neurons by protein kinase C dependent manner. Brain Res. 2002;950:254–260. doi: 10.1016/s0006-8993(02)03049-4. [DOI] [PubMed] [Google Scholar]
- [262].Scoditti E, Nestola A, Massaro M, Calabriso N, Storelli C, De Caterina R, Carluccio MA. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCalpha and PKCbeta1 inhibition. Atherosclerosis. 2014;232:17–24. doi: 10.1016/j.atherosclerosis.2013.10.017. [DOI] [PubMed] [Google Scholar]
- [263].Kumar G, Dange P, Kailaje V, Vaidya MM, Ramchandani AG, Maru GB. Polymeric black tea polyphenols modulate the localization and activity of 12-O-tetradecanoylphorbol-13-acetate-mediated kinases in mouse skin: mechanisms of their anti-tumor-promoting action. Free Rad. Biol. Med. 2012;53:1358–1370. doi: 10.1016/j.freeradbiomed.2012.07.017. [DOI] [PubMed] [Google Scholar]
- [264].Liu X, Wang Z, Wang P, Yu B, Liu Y, Xue Y. Green tea polyphenols alleviate early BBB damage during experimental focal cerebral ischemia through regulating tight junctions and PKCalpha signaling. BMC Complement Altern. Med. 2013;13:187. doi: 10.1186/1472-6882-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- [266].Tuorkey MJ. Curcumin a potent cancer preventive agent: Mechanisms of cancer cell killing. Interv. Med. Appl. Sci. 2014;6:139–146. doi: 10.1556/IMAS.6.2014.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Varoni EM, Lo Faro AF, Sharifi-Rad J, Iriti M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016;3:8. doi: 10.3389/fnut.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Kulkarni SS, Canto C. The molecular targets of resveratrol. Biochim. Biophys. Acta. 2015;1852:1114–1123. doi: 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- [269].Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res. Treat. 2014;46:2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Gambini J, Ingles M, Olaso G, Lopez-Grueso R, Bonet-Costa V, Gimeno-Mallench L, Mas-Bargues C, Abdelaziz KM, Gomez-Cabrera MC, Vina J, Borras C. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015;2015:837042. doi: 10.1155/2015/837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Walle T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- [273].Mereles D, Hunstein W. Epigallocatechin-3-gallate (EGCG) for clinical trials: more pitfalls than promises? Int. J. Mol. Sci. 2011;12:5592–5603. doi: 10.3390/ijms12095592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Wang J, Bi W, Cheng A, Freire D, Vempati P, Zhao W, Gong B, Janle EM, Chen TY, Ferruzzi MG, Schmeidler J, Ho L, Pasinetti GM. Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer's disease-experimental approach and therapeutic implications. Front. Aging Neurosci. 2014;6:42. doi: 10.3389/fnagi.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Pandareesh MD, Mythri RB, Srinivas Bharath MM. Bioavailability of dietary polyphenols: Factors contributing to their clinical application in CNS diseases. Neurochem. Int. 2015;89:198–208. doi: 10.1016/j.neuint.2015.07.003. [DOI] [PubMed] [Google Scholar]
- [276].Aras A, Khokhar AR, Qureshi MZ, Silva MF, Sobczak-Kupiec A, Pineda EA, Hechenleitner AA, Farooqi AA. Targeting cancer with nano-bullets: curcumin, EGCG, resveratrol and quercetin on flying carpets. Asian Pac. J. Cancer Prev. 2014;15:3865–3871. doi: 10.7314/apjcp.2014.15.9.3865. [DOI] [PubMed] [Google Scholar]