Abstract
Background
Alteramide B (ATB), isolated from Lysobacter enzymogenes C3, was a new polycyclic tetramate macrolactam (PTM). ATB exhibited potent inhibitory activity against several yeasts, particularly Candida albicans SC5314, but its antifungal mechanism is unknown.
Methods
The structure of ATB was established by extensive spectroscopic analyses, including high-resolution mass spectrometry, 1D- and 2D-NMR, and CD spectra. Flow cytometry, fluorescence microscope, transmission electron microscope, molecular modeling, overexpression and site-directed mutation studies were employed to delineate the anti-candida molecular mechanism of ATB.
Results
ATB induced apoptosis in C. albicans through inducing reactive oxygen species (ROS) production by disrupting microtubules. Molecular dynamics studies revealed the binding patterns of ATB to the β-tubulin subunit. Overexpression of the wild type and site-directed mutants of the β-tubulin gene (TUBB) changed the sensitivity of C. albicans to ATB, confirming the binding of ATB to β-tubulin, and indicating that the binding sites are L215, L217, L273, L274 and R282. In vivo, ATB significantly improved the survival of the candidiasis mice and reduced fungal burden.
Conclusion
The molecular mechanism underlying the ATB-induced apoptosis in C. albicans is through inhibiting tubulin polymerization that leads to cell cycle arrest at the G2/M phase. The identification of ATB and the study of its activity provide novel mechanistic insights into the mode of action of PTMs against the human pathogen.
General significance
This study shows that ATB is a new microtubule inhibitor and a promising anti-Candida lead compound. The results also support β-tubulin as a potential target for anti-Candida drug discovery.
Keywords: Alteramide B, Candida albicans SC5314, reactive oxygen species, apoptosis, β-tubulin
Graphical abstract
ATB, a new polycyclic tetramate macrolactam (PTM) compound, exhibited potent activity against Candida albicans SC5314 in vitro and in vivo. ATB induced apoptosis of C. albicans through inducing the production of reactive oxygen species (ROS) by disrupting microtubules. The binding model of ATB to β-tubulin was simulated by Amber12 and shown by PyMoL.
1. Introduction
The constant emergence of drug resistant pathogens is a serious threat to human health and demands continual discovery of new anti-infectives with new structures and novel modes of action. Bioactive natural products, especially those isolated from the Gram-positive soil bacteria Streptomyces, have traditionally served as the major source of anti-infectives. Relatively little effort has been given to exploitation of bioactive natural products from Gram-negative bacteria. The genus Lysobacter is a group of Gram-negative bacteria widely distributed in soil, freshwater and plants. This genus was first described in 1978 by Christensen and Cook, and Lysobacter species are known to produce abundant lytic enzymes [1]. We have been searching for new bioactive natural products from this largely untapped natural resource [2].
Among the Lysobacter natural products, Heat-Stable Antifungal Factor (HSAF) isolated from Lysobacter enzymogenes (L. enzymogenes) represents an antifungal compound with a structure distinct from any existing antifungal drug [3]. HSAF belongs to a recently described group of natural products called polycyclic tetramate macrolactams (PTMs) [4]. PTMs are emerging as a new class of natural products with distinct structural features, comprising of a macrocyclic lactam with an embedded tetramic acid ring. The group of compounds exhibit diverse biological activities, including antifungal activity of HSAF [5] and geodin [6], antiprotozoal activity of ikarugamycin [7], and antitumor activity of cylindramide [8] and discodermide [9]. Particularly, HSAF exhibited potent inhibitory activities against a broad spectrum of filamentous fungi, including Fusarium graminearum [10], Uromyces appendiculatus [11], and Bipolaris sorokiniana [12]. Another notable feature of HSAF is its novel mode of antifungal action, which is through disrupting the biosynthesis of sphingolipids in filamentous fungi [3].
As part of our efforts to identify new antifungal derivatives of HSAF, we have further investigated the metabolites of L. enzymogenes C3. In this study, we report the isolation and structural elucidation of a new PTM derivative, alteramide B (ATB). Surprisingly, ATB exhibits strong inhibitory activity against several yeasts including Candida albicans (C. albicans), and its mode of anti-Candida action is through inhibiting microtubule polymerization, arresting cells at G2/M phase and triggering apoptosis via a ROS-dependent pathway.
2. Material and methods
2.1 Strains and growth conditions
C. albicans SC5314 [13], Saccharomyces cerevisiae (S. cerevisiae) ATCC 24860, Kluyveromyces lactis (K. lactis) ATCC 64161 and azole-resistant C. albicans 23C, 23L, 24F, 28A and 28I were grown in yeast extract-peptone-dextrose (YPD) medium (1% yeast extract, 1% Bacto peptone, 2% -D-glucose) at 28 °C for 24 h and stored in medium supplemented with 20% (vol/vol) glycerol at −80 °C. C. albicans CAI4 (ura3::λimm 434/ ura3:: λimm 434) was grown in synthetic complete medium (SC). Staphylococcus aureus (S. aureus) ATCC 25923 and Bacillus subtilis (B. subtilis) ATCC 6051 were cultured in LB medium (1% tryptone, 0.5% yeast extract and 1%) at 37 °C for 24 h.
2.2 Fermentation, Extraction and Isolation of ATB
L. enzymogenes C3 (10 liters) was allowed to grow on the Fermentation Medium at 30 °C for 4 days. The whole solid cultures were diced and extracted three times with AcOEt/MeOH/AcOH (80 : 15 : 5, v/v/v) at room temperature, and the crude extract solution was concentrated under reduced pressure at 40 °C, and the concentrated extract was sequentially solvent partitioned into petroleum ether-soluble extract and MeOH-soluble extract. The MeOH extract was loaded to medium-pressure liquid chromatography (MPLC; 80 g RP-18 silica gel; acetonitrile/H2O 30%, 50%, 70%, 80%, and 100%, 200 mL each) to afford five fractions for separation. Fraction of 80% from MPLC (60 mg) was subjected to preparative HPLC (Agilent 1200, ZORBAX Eclipse XDB-C18, 9.4 × 250 mm, 5 µ), using an isocratic solvent of 55% acetonitrile, at flow rate of 4 ml/min, detected at UV 318 nm.
2.3 In vitro assay for antimicrobial activities
C. albicans SC5314, Saccharomyces cerevisiae ATCC 24860, Kluyveromyces lactis ATCC 64161, Staphylococcus aureus ATCC 25923, and Bacillus subtilis ATCC 6051, drug-resistant strains including 23C, 23L, 24F, 28A and 28I were grown to an optical density at 600 nm of 0.1 to 1.0 and diluted, coated plates, and counted to determine the relationship between OD600 and Log CFU/mL. The Minimum Inhibitory Concentrations (MICs) were determined by the broth microdilution procedure following the Clinical and Laboratory Standards Institute (CLSI) [14].
2.4 Assays for germ tube formation
A colony of C. albicans SC5314 was used to inoculate 5 mL of YPD medium. The cells were incubated at 25 °C for at least 48 h without shaking and then centrifuged for 5 min at 2000 g to pellet the cells. The pellet was then resuspended with RPMI 1640. The cells of C. albicans were diluted to OD600 0.2 and dispensed into a 96-well flat-bottom polystyrene plate. Samples were treated with 0.78, 1.56 and 3.12 µM ATB with dimethyl sulfoxide at a final concentration of 1%. The plate was placed in a 37 °C incubator for 4 h without shaking [15]. The medium in the plate was discarded and washed twice by 70% ethanol, and then added SDS to each well. The SDS was discarded, and the samples were washed three times with distilled water. Germ tubes attached to the wells were stained with 100 µL of 0.02% crystal violet dissolved in phosphate-buffered saline (PBS) for 15 min and then analyzed by ZEISS fluorescence microscope (ZEISS, Axio Observer A1, Germany) using a 40 × objective.
2.5 Measurement of ROS
Intracellular ROS production was determined by incubating the cells (2 × 106/mL) with 1.56, 3.12 and 6.25 µM ATB at 28 °C for 1, 2, 3 h, followed by staining with 5 µg/mL DHR-123. After incubation for 30 min, cells were washed with PBS twice [16]. Samples were quantitatively analyzed with a FACScan flow cytometer (Becton Dickinson, San Jose, CA, USA).
2.6 Measurement of mitochondrial electrical potentials
Mitochondrial electrical potential(ΔΨm) changes were assessed by using the membrane potential-dependent distributional probe DiOC6(3) as described previously [17]. Cells were harvested and incubated with 1.56, 3.12 and 6.25 µM ATB for 3 h at 28 °C. After that, cells were centrifuged and washed with PBS twice and incubated with 100 nM DiOC6(3) for 30 min in the dark. Samples were analyzed by a FACScan flow cytometer as previously described [18].
2.7 Flow cytometric analysis of cell cycles
The DNA content was quantified by the flow cytometry of the cells stained with propidium iodide (PI). The exponential phased cells of C. albicans were grown in YPD medium and then harvested and treated with 1.56, 3.12 and 6.25 µM of ATB, meanwhile adding 1 mM and 5 mM ascorbic acid (AA) for 30 min prior to the addition of 6.25 µM ATB. After incubation for 3 h, the cells were washed with PBS and fixed with 70% ethanol overnight at −20 °C. The cells were treated with 100 µg/ml RNase A for 2 h at 37 °C and added 50 mg/mL PI for 1 h at 4 °C in the dark. The samples were analyzed in a FACScan flow cytometer (Becton Dickinson, San Jose, CA, USA). The values represented the average of the measurements conducted in triplicate for three independent assays.
2.8 Analysis of apoptosis
Cells of C. albicans were digested with lywallzyme and lysis enzyme for 2 h at 28 °C as described previously [19]. Protoplasts were incubated with 6.25 µM ATB for 2 h at 28 °C, meanwhile adding 1 mM and 5 mM ascorbic acid (AA) for 30 min prior to the addition of ATB. Subsequently, the cells were washed with PBS three times and incubated with 5 µL FITC-Annexin V and 5 µL PI for 20 min in the dark and then analyzed by fluorescence microscope (OLYMPUS, IX71, Japan) using a 63 × objective.
DNA strand breaks in C. albicans cells were performed by the TUNEL method [20]. C. albicans cells grown to OD600 0.2 were collected by centrifugation and washed in PBS. Samples were treated with 6.25 µM ATB for 4 h, meanwhile adding 1 mM and 5 mM Vc for 30 min prior to the addition of ATB, and then washed with PBS for three times, permeabilized in a permeabilization buffer (0.1% sodium citrate and 0.1% Triton X-100) on ice for 10 min and washed again with PBS for three times. DNA ends were labeled with an in situ cell death detection kit at 37 °C for 1 h in the dark and then analyzed by fluorescence microscope (OLYMPUS, IX71, Japan) using a 63 × objective.
2.9 Polymerization assays
The ability of tubulin to polymerize into microtubules (MTs) can be followed by observing an increase in optical density of a tubulin solution at OD340. Tubulin protein from porcine brain was reconstituted to 5 mg/mL with cold tubulin buffer (80 mM PIPES, pH 6.9, 2 mM MgCl2, 0.5 mM EGTA). The tubulin solution (at 4°C), which was plus 5% glycerol and 1 mM GTP adding 10 µM ATB or 10 µM vincristine, was transferred into a microtiter plate, which had been pre-warmed to 37°C. Tubulin polymerization was measured by taking readings every 30 s for 30 min. Absorbance at 340 nm was monitored in the spectrophotometer.
2.10 Electron microscopy observations
Tubulin protein from porcine brain (2 µM) was incubated with 2 mM GTP, 5% glycerol at 37 °C for 15 min in the presence or absence of 10 µM ATB or 10 µM vincristine. The samples (5 µL) were placed on carbon-coated copper grids at the room temperature, fixed for 1 min and negatively stained with 2% uranyl acetate. Grids were air-dried and observed under a transmission electron microscope at 80 kV.
2.11 Molecular modeling
All the molecular modeling was performed on a Dell Precision T5500 workstation. The molecular docking was conducted using SYBYL-X 1.1 (Tripos, St. Louis, MO, USA). The wild type and mutant strains of C. albicans β-tubulin proteins were built by SWISS-MODEL, a fully automated protein structure homology-modelling server [21–24]. The compound ATB was optimized for 1000-generations until the maximum derivative of energy became 0.05 kcal/(mol*Å), using the Tripos force field. Charges were computed and added according to Gasteigere Hückel parameters. The optimized ATB was docked into the binding site of C. albicans β-tubulin proteins by means of surflex-dock, and the default parameters were used if it was not mentioned. Then a molecular dynamic (MD) study was performed to revise the docking result.
The Amber 12 and AmberTools 13 programs [25] were used for MD simulations of the selected docked pose. Compound ATB was first prepared by ACPYPE [26], a tool based on ANTECHAMBER for generating automatic topologies and parameters in different formats for different molecular mechanics programs, including calculation of partial charges. Then, the forcefield “leaprc.gaff” (generalized amber forcefield) was used to prepare the ligand, while “leaprc.ff12SB” was used for the receptor. The system was placed in a rectangular box (with a 10.0 Å boundry) of TIP3P water using the “SolvateOct” command with the minimum distance between any solute atoms. Equilibration of the solvated complex was done by carrying out a short minimization (1000 steps of each steepest descent and conjugate gradient method), 500 ps of heating, and 200 ps of density equilibration with weak restraints using the GPU (NVIDIA® Tesla K20c) accelerated PMEMD (Particle Mesh Ewald Molecular Dynamics) module. At last, 10 ns of MD simulations were carried out.
2.12 Construction of C. albicans mutants of β-tubulin overexpression and mutagenesis
The primers used for generating the vectors and strains used in this study are given in Table S1. The bacterial strain XL1-Blue used for cloning and purification of plasmids was grown in LB medium at 37 °C. Plasmid construction was based on the C. albicans integrating vector, CIp10 [27]. We first isolated the genomic DNA of C. albicans. The promotor of C. albicans actin-fragment (1121 bp) amplified by primers ProWT-F/ProWT-R was digested with PstI and NheI; the tubulin fragment (1728 bp) of C. albicans amplified by primers TubWT-F/TubWT-R was digested with NheI and SmaI (Figure S8). Plasmid pUC18 was digested by PstI and NheI, CaACT1 and CaTUB2 cloned between the PstI and NheI sites of pUC18. Then these three DNA fragments were purified by a gel extraction kit (Omega) and then ligated by a DNA ligation kit.C. albicans mutants were generated from the construct CaACT1-CaTUB2-pUC18, while the full length of C. albicans beta tubulin was used as positive control, and the empty vector (CIp10) as negative control. Amplified mutants WT+ and WT− were inserted in CIp10 vector between XhoI and MluI restriction sites respectively. The mutants were transformed into CIA4− using lithium acetate (LiAc) method [28]. The colonies were selected on SD Ura minimal medium.
2.13 Cell proliferation assays
The activity of 6.25 µM ATB on mutants was evaluated. The % inhibition for each well was calculated as follows: % Inhibition = (A570control cells −A570treated cells)/(A570control cells − A570blank cells)× 100. The percentages of inhibition were calculated using software Prism 6 (GraphPad Software, Inc., USA).
2.14 Statistical analysis
Means and standard deviations were calculated using GraphPad Prism 6 (GraphPad Software, Inc., USA), and the two-way analysis of variance (ANOVA) was used to determine the significance of differences among the groups method in this software. Results were presented as mean ± standard deviation (SD). The experiments were performed in triplicate and repeated at least three times independently.
2.15 In vivo antifungal test
Fifty male BALB/c mice (6–8 weeks old) were obtained from the Experimental Animal Center of Shandong University (Jinan, Shandong, China). The experiments were conducted according to the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). All efforts were made to minimize the number of animals used and their suffering.
Totally fifty mice were randomly divided into five groups of ten mice each. The mice were infected by injection of a 100 µL inoculum containing 1× 106 cells of C. albicans via the lateral tail vein. C. albicans was grown at 37 °C in YPD medium containing penicillin and streptomycin. Cells were centrifuged, washed in PBS, and diluted to OD600 = 0.2. ATB was intraperitoneal injection once daily at doses of 3 mg/kg of body weight. Fluconazole (FLU) and amphotericin B (AMB) were injected in the same way with ATB as the positive control or the same volume of solvent (5% glucose + 10% ethanol + 1% DMSO) as the vehicle control. After 3 d of therapy, three mice in each group were euthanized, kidneys were harvested, weighed and processed for histopathological analysis, while the other was homogenized in sterile PBS. Serial dilutions were prepared and plated on YPD, and following 24 h of incubation at 37 °C, fungal burden (CFU/g) was determined. In the survival arm, seven mice in each group were monitored off therapy until 26 d.
3. Results
3.1 Isolation and structure elucidation for Alteramide B
Alteramide B (ATB) was isolated from the fermentation products of L. enzymogenes C3 cultivated on TSB agar media. Its molecular formula was determined to be C29H38N2O6 by the high-resolution ESIMS m/z 511.2784 [M + H]+ (calcd. for 510.2730, C29H38N2O6). The 1H, 13C NMR and HSQC spectroscopic data were almost identical to that of alteramide A [29], establishing the planar structure of ATB (Table S2 and Fig. S1–S8). The structure was confirmed by the analysis of the HMBC and 1H-1H COSY correlations. The relative configurations of ATB were established by thorough analysis of NMR spectroscopic data. The large coupling constants (J > 15.0 Hz) of H-8 and H-9, H-21 and H-22, H-23 and H-24 led to assignment of the 8(9)E-, 21(22)E- and 23(24)E- configurations for ATB, respectively. The Z-configuration of the C-10/C-11 double bond was deduced from the coupling constant J = 11.5 Hz between H-10 and H-11. Additionally, strong NOE correlations between H-14 ↔ H-18 ↔ H-20, and H-16 ↔ H-29 ↔ H-18 ↔ H-31 suggested that H-14, H-16, H-18, H-20, H-29, and H-31 were in the syn orientation in ATB. The NOE correlations between H-17 ↔ H-19 ↔ H-12 further indicated that H-12, H-17 and H-19 was in the syn orientation in ATB. Computational analyses of two conformers, (12S,19R)- and (12R,19S)-isomers of ATB, generated ECD spectra, and the ECD spectrum of (12S,19R)-isomer matched to the experimental CD spectrum of ATB (Table S3 and Fig. S1). Thus, the structure of ATB was established (Fig. 1A).
Fig. 1.
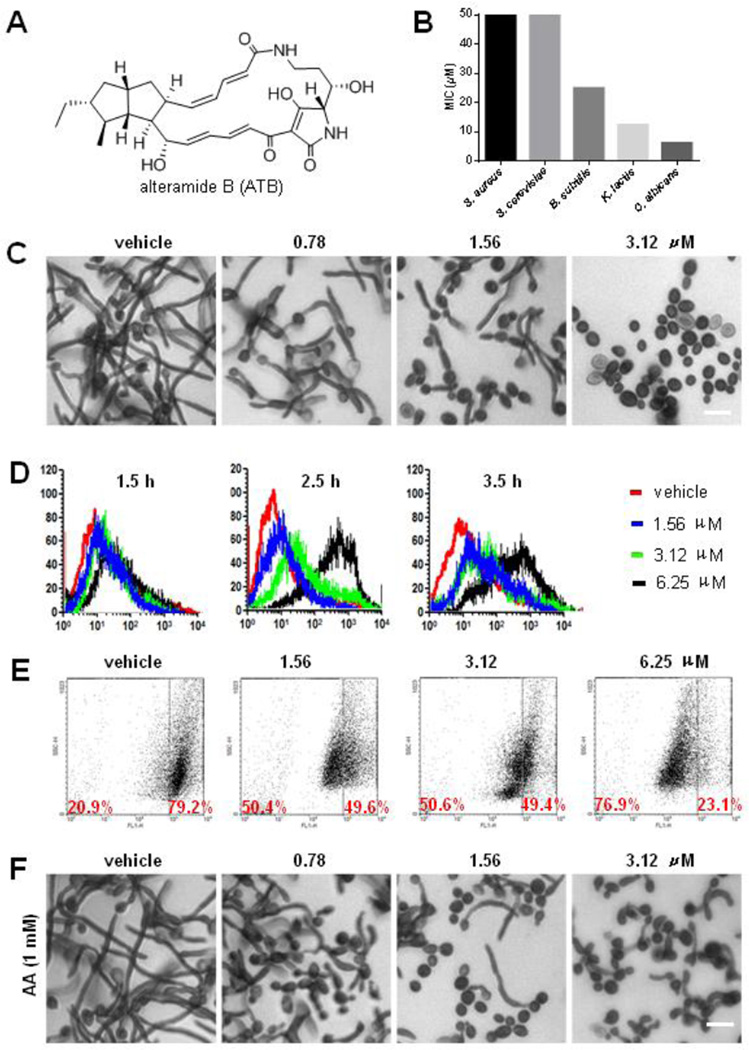
ATB inhibits the growth of C. albicans through inducing the production of ROS. Structure of alteramide B (A); The MIC values of ATB on S. aureus, B. subtllis, S. cerevisiae, K. lactis and C. albicans (B); ATB inhibits the tube formation of C. albicans. Stained by crystal violet and observed under a fluorescent microscope. The cultures were incubated for 48 h in RPMI 1640 medium at 25 °C without shaking, the cells were treated with DMSO, 0.78, 1.56 and 3.12 µM ATB at 37 °C for 4 h, respectively. Bar, 10 µm (C); Effect of ATB on the generation of ROS in C. albicans determined by flow cytometry using DHR-123. The cells were treated with 1.56, 3.12 and 6.25 µM ATB for 1, 2, 3 h at 28 °C, followed by staining with 5 µg/mL DHR-123, respectively. After incubation for 30 min, the cells were washed with PBS twice. Samples were quantitatively analyzed with a FACScan flow cytometer (D); ATB changes the mitochondrial potentials of C. albicans. The cells were treated with DMSO, 1.56, 3.12 and 6.25 µM ATB for 3.5 h, respectively. Stained with DiOC6(3) and analyzed by flow cytometry with excitation at 488 nm and emission at 535 nm (E); Ascorbic acid (AA) reduces the inhibitory effect of ATB on the tube formation of C. albicans. Stained by crystal violet and observed under a fluorescent microscope using a 40× objective. Bar, 10 µm (F).
3.2 ATB inhibits the growth of C. albicans through inducing the production of reactive oxygen species
The antimicrobial activities of ATB against bacteria and fungi were evaluated using the method described by CLSI. ATB exhibited modest antibacterial activities against gram-positive bacteria (Staphylococcus aureus ATCC 25923 and Bacillus subtilis ATCC 6051) (Fig. 1B), but evident activity against three test fungal strains. The MIC values of ATB against Saccharomyces cerevisiae ATCC 24860, Kluyveromyces lactis ATCC 64161and C. albicans SC5314 were 50, 12.5 and 6.25 µM, respectively (Fig. 1B), showing a selectivity on C. albicans. ATB was also effective on the azole-resistant C. albicans (Fig. S10) C. albicans is a dimorphic fungus, capable of growth in both yeast and filamentous forms. The yeast-to-hyphal transition is suggested to be a potential virulence factor in its pathogenesis [30]. ATB displayed evident inhibitory effect on the germ tube formation of C. albicans at the concentration of 3.12 µM in comparison to vehicle controls (Fig. 1C).
The formation of reactive oxygen species (ROS) has been suggested to be one of the fungicidal mechanisms of a large number of fungicidal antibiotics [31]. In order to determine whether ROS mediated the antifungal activity of ATB, we examined the ROS levels of C. albicans cells treated with or without ATB by fluorimetric assay. The generation of ROS in Candida cells was induced by ATB treatment for 1.5, 2.5, and 3.5 h at 1.56, 3.12 and 6.25 µM, respectively, which showed a dose- and time-dependent manner (Fig. 1D). We further investigated the effect of ATB on mitochondrial membrane potential (MMP) in C. albicans by DiOC6(3) assay [32]. Cells incubated with ATB at 1.56 µM showed intensive staining of the mitochondria, indicating that mitochondrial membrane was collapsed. The proportion of the stained cells after exposure for 3.5 h to 3.12 µM and 6.25 µM ATB was approximately 50.6 % and 76.9% (Fig. 1E), respectively, indicating that ATB damaged mitochondrial permeability by breakdown of membrane potential to induce the production of ROS in a dose-dependent manner.
To determine if the elevated ROS in Candida cells contributes to ATB-induced cell death, the anti-Candida activity of ATB was examined with or without the supplement of the ROS scavengers, ascorbic acid (AA), acetyl cysteine (NAC), glutathione (GSH) and thiourea (TU). AA supplement reduced the cytotoxicity of ATB against C. albicans, increasing the MIC values by ca. 8-fold, from 6.25 to 50µM. NAC and GSH increased the MIC values of ATB by ca. 4-fold, from 6.25 to 25 µM, but TU had no obvious effects on the MIC of ATB in C. albicans (Fig. S11). Moreover, the anti-Candida activity of ATB including the growth inhibition of budding yeast and germ tube was strongly reduced by the addition of 1 mM AA (Fig. 1F). These results indicated that the anti-Candida activity of ATB was mediated by ROS production.
3.3 ATB induces cell cycle arrest at the G2/M phase and apoptosis in C. albicans through inducing the production of reactive oxygen species
A consequence of exposure of cells to diverse cytotoxic agents is DNA damage, which can lead to cell cycle arrest for DNA repair and, ultimately, to cell death. Using flow cytometric analysis, we observed that ATB led to an accumulation of C. albicans cells at the G2/M phase (from 18.00% to 33.29%), with the concomitant decrease at the G0/G1 phase (from 61.28% to 46.01%), and no significant change at the S phase in a dose-dependent manner (Fig. 2A). The cell cycle arrest at the G2/M phase induced by ATB could be recovered by the supplement of AA (Fig. 2A). These results indicated that ATB treatment induced a significant cell cycle arrest at the G2/M phase via the production of ROS.
Fig. 2.
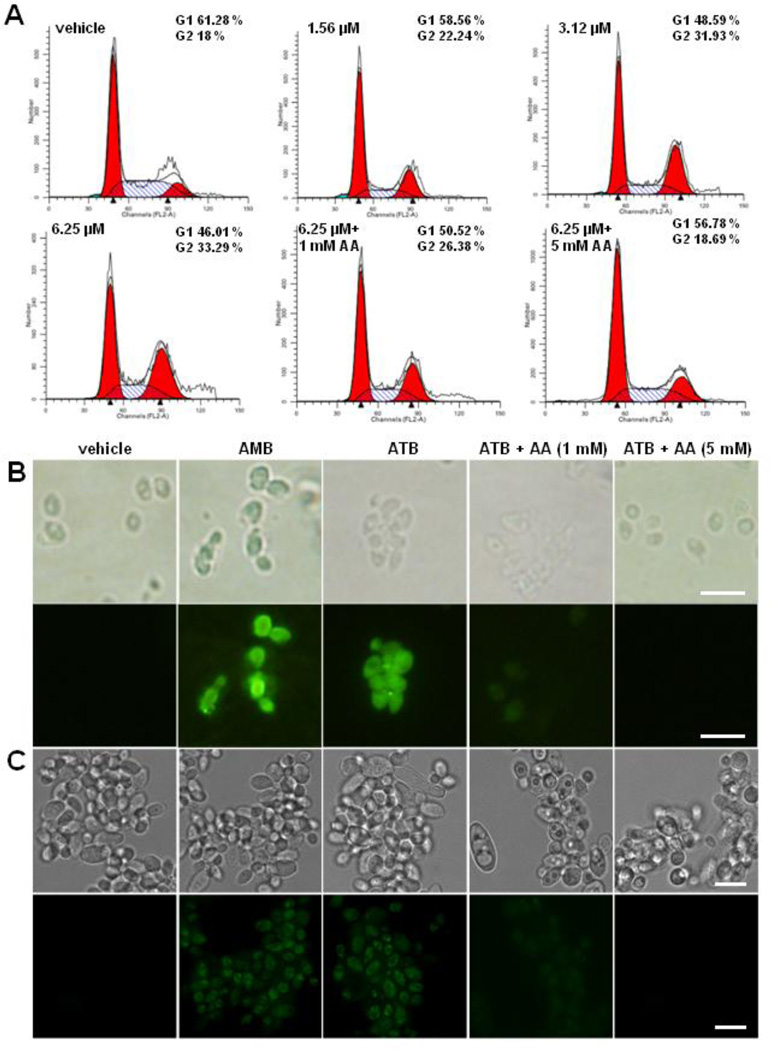
ATB induces G2/M phase cell cycle arrest and apoptosis of C. albicans through inducing the production of ROS. Cell cycles of C. albicans were assayed by flow cytometry. C. albicans protoplast cells were treated with 1.56, 3.12 and 6.25 µM of ATB, meanwhile 1 mM or 5 mM ascorbic acid (AA) was added for 30 min prior to the addition of 6.25 µM ATB. After incubation for 3 h, the cells were washed with PBS and fixed with 70% ethanol overnight at −20 °C. The cells were treated with 100 µg/mL RNase A for 2 h at 37 °C and added 50 mg/mL PI for 1 h at 4 °C in the dark. The samples were analyzed in a FACScan flow cytometer. Percentage values were calculated from the number of gated events in the G2/M or sub-G1 region out of the total events (20,000) (A). ATB induced the early and late apoptosis in C. albicans. Cells treated with DMSO, 10 µM AMB, 6.25µM ATB, 6.25µM ATB + 1 mM AA, 6.25µM ATB + 5 mM AA for 2 h at 28 °C shown by FITC-Annexin-V/PI staining (B), and by TUNEL staining (C) and observed under a fluorescent microscope. Bar, 10 µm.
Upstream cell cycle regulators are also involved in effector-triggered programmed cell death in Candida cells [33]. At the early stage of apoptosis of C. albicans cells, the phosphatidylserine distributed in the inner leaflet of the lipid bilayer of the plasma membrane will translocate to the outer leaflet of the cytoplasmic membrane. Annexin V-FITC and PI co-staining assay revealed a significant accumulation of green fluorescence in the protoplasts of C. albicans treated with ATB, similar to that of the positive control AMB, which was decreased by the supplement of 1 mM or 5 mM AA, whereas no fluorescence was found in the protoplasts treated with DMSO (Fig. 2B). These results showed that ATB could induce the early apoptosis of C. albicans.
DNA fragmentation was one of the late apoptotic phenotypes, which can be visualized using TUNEL assay. The cells treated for 4 h with ATB at 6.25 µM exhibited nuclear fragmentation, similar to that treated with AMB, which was reversed by the addition of the antioxidant AA (Fig. 2C). These results indicated that ATB induced apoptosis in C. albicans through a ROS-dependent pathway.
3.4 ATB inhibits the polymerization of β-tubulin of C. albicans
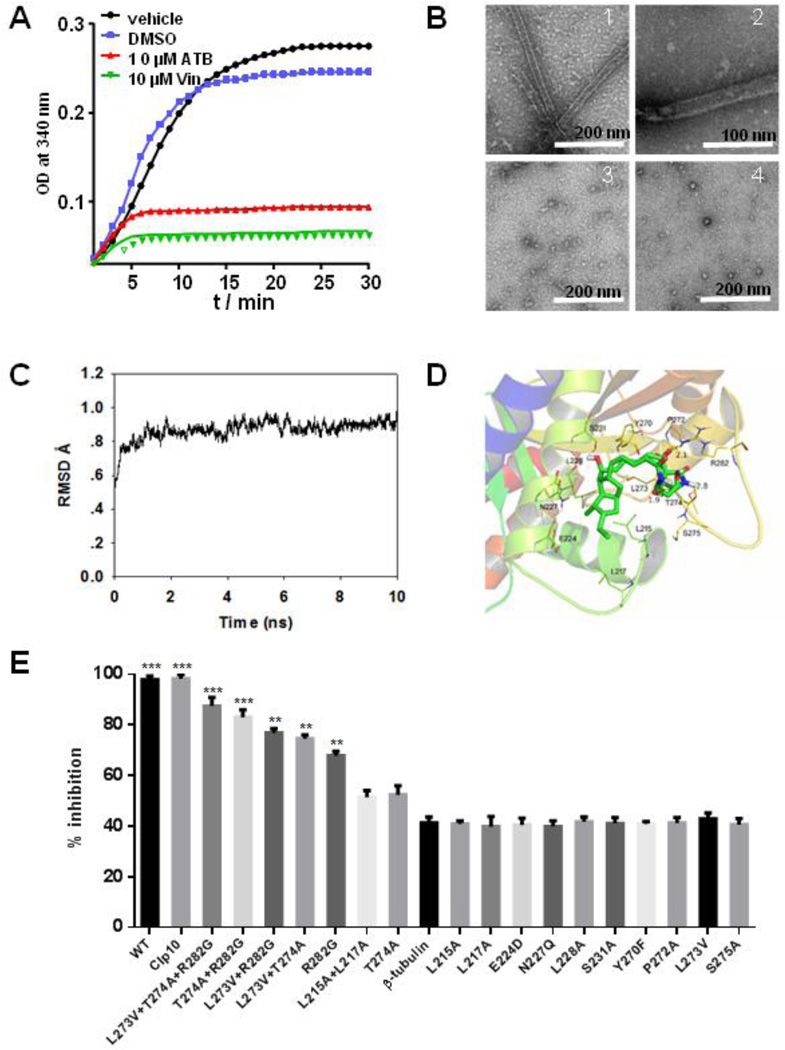
The cellular effects of ATB, disruption of mitochondrial membrane potential, cell cycle arrest at the G2/M phase and apoptosis, mirror the hallmarks of microtubule destabilizing agents [34]. To verify whether tubulins may be the potential target of ATB, ATB was assayed for the effect on the dynamics of tubulin polymerization in a biochemical assay. ATB strongly inhibited tubulin polymerization, similar to the activity exhibited by vincristine (Fig. 3A). To determine what types of microtubule dynamics was being affected, we used electron microscopy to analyze the fate of tubulin polymerization after exposure to ATB. Without ATB, tubulins observed remained in the polymerized state. In contrast, in the presence of ATB, nearly all tubulins had transited to the depolymerization state (Fig. 3B), similar to that of the treatment with vincristine (Fig. 3B). This result showed that ATB inhibited the polymerization of tubulin dimers to form microtubule.
Fig. 3.
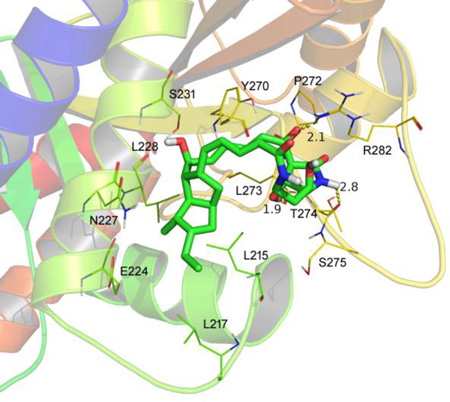
ATB inhibits the polymerization of β-tubulin. The tubulin from porcine brain was incubated at 37 °C in the presence of DMSO, 10 µM ATB and 10 µM vincristine, respectively, and tubulin polymerization was measured by taking readings every 30 s for 30 min. Absorbance at 340 mm was monitored in the spectrophotometer (A). Influence of ATB on tubulin polymerization in vitro observed by TEM. ATB was added to tubulin at a concentration of 2 µM. Tubulin incubated with 2 mM GTP, 5% glycerol (B1); 2 mM GTP, 5% glycerol and DMSO (B2); 2 mM GTP, 5% glycerol and 10 µM ATB (B3); 2 mM GTP, 5% glycerol and 10 µM vincristine (B4). The samples (5 µL) were placed on carbon-coated copper grids at the room temperature, fixed for 1 min and negatively stained with 2% uranyl acetate. Grids were air-dried and observed under a transmission electron microscope at 80 kV. Scale bar is 200 nm for (B1), (B3), (V4) and 100 nm for (B2) (B). The ATB was equilibrated after 10 ns MD simulation to produce the plot of RMSD (in ångstrom) of the ATB (C); The detailed interactions between ATB and β-tubulin. ATB selectively binds to the twelve residues of the β-tubulin, namely L215, L217, E224, N227, L228, S231, Y270, P272, L273, T274, S275 and R282 (D); The residues L215, L217, L273, L274 and R282 of β-tubulin may be the binding sites for ATB. The effects of 6.25 µM ATB on the mutants of C. albicans were measured and calculated as follows: % Inhibition = (A570control cells −A570treated cells)/(A570control cells − A570blank cells) × 100. The percentages of inhibition were calculated using software Prism 6. *** indicated P < 0.001 (E).
To gain further insights into the binding interaction of ATB with tubulin, we performed docking and molecular dynamics studies of ATB binding on tubulins. We found that ATB could physically interact with β-tubulin. The 3D structure of the C. albicans β-tubulin was generated by SWISS-MODEL, a fully automated protein structure homology-modelling server. A docking study of ATB was first performed using SYBYL-X 1.1 and then a molecular dynamics (MD) simulation was performed to refine the docking result by means of Amber 12 [25]. ATB was equilibrated after 10 ns MD simulation to afford a plot of RMSD (in ångstrom) for ATB (Fig. 3C). MD studies demonstrated that ATB selectively occupies twelve residues of the β-tubulin, namely L215, L217, E224, N227, L228, S231, Y270, P272, L273, T274, S275 and R282, respectively. The octahydropentalene scaffold of ATB fit into the hydrophobic domain of β-tubulin, surrounded by the residues L215, L217 and L228. Detailed analysis showed that 1-hydroxyl group (1.9 Å), 25-carbonyl group (2.1 Å) and 28-NH (2.8 Å) of ATB formed three hydrogen bond interactions with residues L273, R282 and T274, respectively, which was the main interactions between ATB and β-tubulin (Fig. 3D). Inspired by these results of MD study, more MD simulations were carried out for the complexes of ATB further docking into the C. albicans β-tubulin mutants (L215A+L217A, R282G, L273A+T274A, L273A+R282G, T274A+R282G and L273A+T274A+R282G), respectively. The β-tubulin mutants-ATB complexes were also equilibrated after 10 ns MD simulation to obtain the plots of RMSD (in ångstrom) these complexes (Fig. S13A–F), and the theoretical binding modes of ATB to the binding sites of β-tubulin mutants (Fig. S14G–L). The hydrophobic interactions were declined between the octahydropentalene scaffold of ATB and the β-tubulin mutant L215A+L217A compared with the wild type β-tubulin though two hydrogen bond interactions were observed between 27-carbonyl group of ATB and the residues R282 and M276 of L215A+L217A (Fig. S13G), Moreover, only one hydrogen bond interaction was observed between ATB and the β-tubulin mutants R282G (Fig. S13H), L273A+T274A (Fig. S13I), L273A+R282G (Fig. S13J), and T274A+R282G (Fig. S13K), respectively, while no hydrogen bond interaction was observed between ATB and the β-tubulin mutant L273A+T274A+R282G (Fig. S13L). The results of MD simulations with β-tubulin mutants further confirm that these twelve residues of β-tubulin are critical for the binding of ATB to β-tubulin.
Encouraged by these MD results, the wild type and the point mutants of the β-tubulin gene (TUBB) encoding the twelve amino acid residues were overexpressed in C. albicans under the actin promoter (Fig. S14), respectively. Meanwhile, the CIp10 plasmid was also transformed into C. albicans as control. The expression levels of all TUBBs were increased to more than two-fold as assessed by qPCR analysis (Fig. S15). The overexpression of wild type TUBB was sufficient to decrease the sensitivity of C. albicans cells to ATB, which showed 41.3% of growth rate inhibition compared with 97.9% of that in WT C. albicans cells treated with the same concentration of ATB (6.25 µM). Moreover, the TUBB point mutants L273A+T274A+R282G and L215A+L217A exhibited similar sensitivity to ATB as that of WT and CIp10 C. albicans (Fig. 3E), indicating that the β-tubulin was probably the target of ATB in C. albicans cells and the residues L215, L217, L273, L274 and R282 may be the binding sites for ATB.
3.5 ATB inhibits C. albicans in vivo
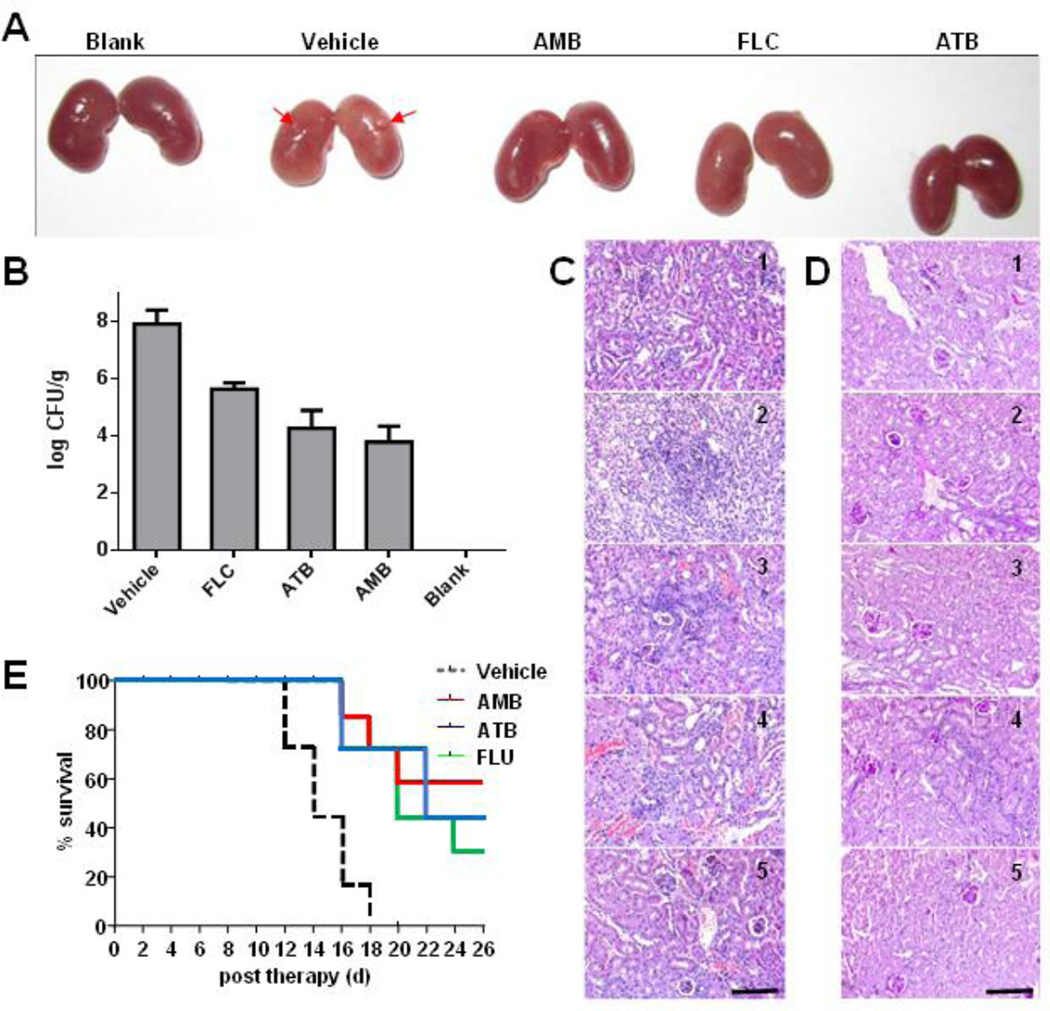
Encouraged by the potent in vitro anti-Candida activity of ATB, we went on to evaluate in vivo anti-Candida activity of ATB in a BALB/c mice model. The mouse model of systemic candidiasis was established by injecting 1 × 106 C. albicans cells into BALB/c mice via the lateral tail vein. After 24 h of infection, the mice were treated with ATB (3 mg/kg), FLC (3 mg/kg) or AMB (3 mg/kg), respectively. After 3 days’ treatment, three mice from each group were euthanized, and the kidneys were excised for analysis. Naked eye observations (Fig. 4A) and kidney fungal burden examinations (Fig. 4B) showed that ATB treatments reduced the pathogenesis of C. albicans. Histopathological analysis revealed that in contrast to the infected kidney of the vehicle control, the inflammatory areas decreased in the presence of ATB (Fig. 4C and 4D). The results of 26-day’s observations for seven mice in each group revealed that ATB, FLC and AMB promoted the longevity of mice as compared to the vehicle control (Fig. 4E).
Fig. 4.
ATB inhibits C. albicans in vivo. Fifty BALB/c mice were randomly divided into five groups of ten mice each. Four groups of mice were infected by injection of a 100 µL inoculum containing 1× 106 cells of C. albicans via the lateral tail vein. After 24 h of infection, mice were intraperitoneally injected once daily 3 mg/kg b w with ATB, and fluconazole (FLU) and amphotericin B (AMB) as the positive controls or the same volume of solvent (5% glucose + 10% ethanol + 1% DMSO) as the vehicle control, respectively. After 3 days’ treatment, three mice from each group were euthanized, and the kidneys were excised for naked eye observations (A), homogenized in sterile PBS for infectious burden analysis (B), and for histopathological analysis (C, D) from the group without treatment (1), and the groups treated with vehicle (2), AMB (3), FLU (4) and ATB (5) on the 3rd day of infection, respectively. The images of hematoxylin-eosin (C) or periodic-acid schiff (D) stained sections are presented at 200× magnification. Bar, 3 µm. Survival curve (E).
ATB considerably reduced the fungal burden in the kidneys of infected mice; it was slightly less active than AMB but exhibited a better effect than FLU against systemic candidiasis in mice. Moreover, the mode of the anti-Candida action of ATB is distinct from the antifungal drugs. ATB may be a new option to the treatment of invasive infections caused by C. albicans.
4. Discussion
C. albicans is a ubiquitous commensal fungus of the human microbiome, as well as the most common cause of disseminated fungal infections [35]. Candidiasis is now the third to fourth most common nosocomial infection in U.S. hospitals [36]. The burgeoning population of immunocompromised patients and the growing use of invasive medical devices and implants have dramatically increased the frequency of Candida infections. Current therapeutic approaches for Candida infections rely mainly on the azoles, the polyenes, and the echinocandins. The azoles are a class of five-membered nitrogen heterocyclic ring compounds, targeting the ergosterol biosynthesis for fungal cell membranes [37]. The polyenes are a class of poly-unsaturated organic compounds that contain one or more sequences of alternating double and single carbon-carbon bond, and their mode of action is through damaging the integrity of cell membranes [37]. The echinocandins are a class of cyclic hexapeptides with a linoleoyl side chain, targeting glucans of fungal cell walls [38]. The over-reliance on limited drug targets has led to the rapid emergence of anti-Candida drug resistance [39]. Thus, it is imperative to identify new anti-Candida drugs with distinct chemical structures and novel mode of actions.
Our previous works have shown that Lysobacter species are a new source of discovering bioactive natural products [40]. HSAF isolated from L. enzymogenes is a noteworthy antifungal compound due to its distinct chemical structure and new mode of action against filamentous fungi [41]. Here, we have isolated a new analog of HSAF. Although ATB belongs to the PTM family, its structure only contains a 5,5-bicyclic system, instead of a 5,5,6-tricyclic system in HSFA, that is fused to the characteristic tetramate-containing macrolactam common to the PTM family. In addition, ATB has an extended conjugation system, compared to the structure of HSAF [42]. These features may lead to an overall conformation enabling ATB to bind the tubulins of yeasts. Compounds in the PTM family exhibit diverse biological activities, but very little is known for the mechanism of their activities. Our results reveal that the molecular mechanism underlying the ATB-induced apoptotic cell death is through inhibiting tubulin polymerization that leads to cell cycle arrest at the G2/M phase. The identification of ATB and the study of its activity provide novel mechanistic insights into the mode of action of PTMs against the human pathogen.
Recently, several studies reported that ROS are both necessary and sufficient for inducing apoptosis in C. albicans, such as ROS induced by acetic acid [43], sugar-stress [44], salt-stress [45], plant antimicrobial peptide [46], hydrogen peroxide [47], farnesol [48], and plagiochin E [49]. These cases are commonly associated with the increase of ROS. ROS participates in the early and late stages of the regulation of apoptosis in C. albicans. These previous reports prompted us to determine whether the observed ROS accumulation induced by ATB was the causal effect of cell death or a side effect of other changes accompanying the killing process. In this study, we have shown that several inter-related events occurred when C. albicans cells were exposed to ATB, including phosphatidylserine exposure, nuclear chromatin condensation, DNA degradation and increase of intracellular ROS levels. We have also shown that microtubule dynamics were altered by ATB in a manner similar to that reported for vincristine, by inducing tubulin depolymerization in situ and in cell-free systems, causing cells to arrest in the G2-M phase of the cell cycle. Therefore, our findings suggest that ROS accumulation was the cause of ATB-induced cell death in C. albicans.
Microtubules consist of α - and β-tubulin heterodimers and are responsible for a variety of functions including sustained shapes, the intracellular transport and the cell division [50]. Microtubule networks are highly dynamic and undergo dramatic reorganizations during the cell cycles [51]. Disruption of microtubule dynamics, results in cell cycle arrest and cell death. Microtubule inhibitors are classified into two main groups: microtubule stabilizing agents such as taxanes and epothilones, and microtubule destabilizing agents such as vinca alkaloids and colchicine [52]. These tubulin-binding compounds have been used in the treatment of many cancers. However, no compound in the PTM family has previously been demonstrated to affect microtubule mechanisms on C. albicans. ATB has the chemical structure of tetramic acid-containing macrolactam fused to a bicyclic system which is distinct from the classic microtubule inhibitors such as paclitaxel, vinca alkaloids and colchine. Importantly, unlike other traditional microtubule inhibitors, ATB induced apoptotic cell death through ROS dependent pathway. These findings indicated ATB may be a promising new tubulin-binding compound with potential for the infection management of C. albicans. The unique structure and novel mode of anti-Candida activity make ATB an interesting compound for further development.
In conclusion, ATB is a new anti-Candida natural product with microtubule-depolymerizing activity. The identification of ATB will enrich the limited number of known microtubule-stabilizing drugs available for antifungal pharmaceutical development, as ATB belongs to a growing group of natural products with structural features distinct from the existing microtubule-stabilizing compounds. Important properties that need to be considered in further development of ATB as an antifungal agent include the ease of producing synthetic analogues, its aqueous solubility and reducing toxicity to the body.
Supplementary Material
Highlights.
ATB is a novel PTM compound.
ATB shows potent in vitro and in vivo anti-Candida activity.
ATB induces apoptosis in C. albicans through increasing the production of ROS.
ATB is a new microtubule inhibitor and a promising anti-Candida lead compound.
Acknowledgments
We thank Dr. Xiaoyi Wei for assistance in calculation of low-energy conformers of ATB and obtaining the ECD spectra. This work was supported by National Key Basic Research Program of China (973 Program) (2012CB721005, 2013CB734002), Joint Research Fund for Overseas Chinese, Hong Kong and Macao Young Scholars to Y. S. & L. D. (31329005), the State Key Program of National Natural Science Foundation of China (81530091), the NIH (R01AI097260), Natural Science Foundation of Shandong Province, China (ZR2013HQ048), Program for Changjiang Scholars and Innovative Research Team in University (IRT13028) and National Natural Science Funds for Distinguished Young Scholars to Y. S. (30325044).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All the authors have declared no conflict of interest.
References
- 1.Christensen P, Cook FD. Lysobacter, a new genus of non-fruiting, gliding bacteria with a high base ratio. Int. J. Syst. Microbiol. 1978;28:367–393. [Google Scholar]
- 2.Xie YX, Wright S, Shen YM, Du LC. Bioactive natural products from Lysobacter. Nat. Prod. Rep. 2012;29:1277–1287. doi: 10.1039/c2np20064c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li SJ, Du LC, Yuen G, Harris SD. Distinct ceramide synthases regulate polarized growth in the filamentous fungus Aspergillus nidulans. Mol. Biol. Cell. 2006;17:1218–1227. doi: 10.1091/mbc.E05-06-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blodgett JAV, Oh DC, Cao SG, Currie CR, Kolter R, Clardy J. Common biosynthetic origins for polycyclic tetramate macrolactams from phylogenetically diverse bacteria. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11692–11697. doi: 10.1073/pnas.1001513107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Jochum C, Yu F, Zaleta-Rivera K, Du L, Harris S, Yuen G. An antibiotic complex from Lysobacter enzymogenes strain C3: antimicrobial activity and role in plant disease control. Phytopathology. 2008;98:695–701. doi: 10.1094/PHYTO-98-6-0695. [DOI] [PubMed] [Google Scholar]
- 6.Capon RJ, Skene C, Lacey E, Gill JH, Wadsworth D, Friedel T. Geodin A magnesium salt: a novel nematocide from a Southern Australian marine sponge, Geodia. J. Nat. Prod. 1999;62:1256–1259. doi: 10.1021/np990144v. [DOI] [PubMed] [Google Scholar]
- 7.Paquette LA, Macdonald D, Anderson LG, Wright J. A triply convergent enantioselective total synthesis of (+)-ikarugamycin. J. Am. Chem. Soc. 1989;111:8037–8039. [Google Scholar]
- 8.Hart AC, Phillips AJ. Total synthesis of (+)-cylindramide A. J. Am. Chem. Soc. 2006;128:1094–1095. doi: 10.1021/ja057899a. [DOI] [PubMed] [Google Scholar]
- 9.Gunasekera SP, Gunasekera M, McCarthy P. Discodermide: a new bioactive macrocyclic lactam from the marine sponge Discodermia dissoluta. The Journal of Organic Chemistry. 1991;56:4830–4833. [Google Scholar]
- 10.Jochum C, Osborne L, Yuen G. Fusarium head blight biological control with <i>Lysobacter enzymogenes </i> strain C3. Biol. Control. 2006;39:336–344. [Google Scholar]
- 11.Yuen G, Steadman J, Lindgren D, Schaff D, Jochum C. Bean rust biological control using bacterial agents. Crop Prot. 2001;20:395–402. [Google Scholar]
- 12.Li S, Jochum CC, Yu F, Zaleta-Rivera K, Du L, Harris SD, Yuen GY. An antibiotic complex from Lysobacter enzymogenes strain C3: Antimicrobial activity and role in plant disease control. Phytopathology. 2008;98:695–701. doi: 10.1094/PHYTO-98-6-0695. [DOI] [PubMed] [Google Scholar]
- 13.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5'-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 14.Fothergill AW. Antifungal Susceptibility Testing: Clinical Laboratory and Standards Institute (CLSI) Methods. 2012:65–74. [Google Scholar]
- 15.Brayman TG, Wilks JW. Sensitive assay for antifungal activity of glucan synthase inhibitors that uses germ tube formation in Candida albicans as an end point. Antimicrob. Agents Chemother. 2003;47:3305–3310. doi: 10.1128/AAC.47.10.3305-3310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawada T, Yoshino F, Kimoto K, Takahashi Y, Shibata T, Hamada N, Sawada T, Toyoda M, Lee MC. ESR detection of ROS generated by TiO2 coated with fluoridated apatite. J. Dent. Res. 2010;89:848–853. doi: 10.1177/0022034510370806. [DOI] [PubMed] [Google Scholar]
- 17.Hwang B, Hwang JS, Lee J, Lee DG. The antimicrobial peptide, psacotheasin induces reactive oxygen species and triggers apoptosis in Candida albicans. Biochem. Biophys. Res. Commun. 2011;405:267–271. doi: 10.1016/j.bbrc.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Andres MT, Viejo-Diaz M, Fierro JF. Human lactoferrin induces apoptosis-like cell death in Candida albicans: critical role of K+-channel-mediated K+ efflux. Antimicrob. Agents Chemother. 2008;52:4081–4088. doi: 10.1128/AAC.01597-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Lee DG. Novel Antifungal Mechanism of Resveratrol: Apoptosis Inducer in Candida albicans. Curr. Microbiol. 2014 doi: 10.1007/s00284-014-0734-1. [DOI] [PubMed] [Google Scholar]
- 20.Phillips AJ, Sudbery I, Ramsdale M. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14327–14332. doi: 10.1073/pnas.2332326100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 22.Guex N, Peitsch MC, Schwede T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis. 2009;30(Suppl 1):S162–S173. doi: 10.1002/elps.200900140. [DOI] [PubMed] [Google Scholar]
- 23.Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Case DA, Cheatham TE, 3rd, Darden T, Gohlke H, Luo R, Merz KM, Jr, Onufriev A, Simmerling C, Wang B, Woods RJ. The Amber biomolecular simulation programs. J. Comput. Chem. 2005;26:1668–1688. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sousa da Silva AW, Vranken WF. ACPYPE - AnteChamber PYthon Parser interfacE. BMC Res. Notes. 2012;5:367. doi: 10.1186/1756-0500-5-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murad AM, Lee PR, Broadbent ID, Barelle CJ, Brown AJ. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast. 2000;16:325–327. doi: 10.1002/1097-0061(20000315)16:4<325::AID-YEA538>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Yadav A, Singh SL, Yadav B, Komath SS. Saccharomyces cerevisiae Gpi2, an accessory subunit of the enzyme catalyzing the first step of glycosylphosphatidylinositol (GPI) anchor biosynthesis, selectively complements some of the functions of its homolog in Candida albicans. Glycoconj. J. 2014;31:497–507. doi: 10.1007/s10719-014-9536-8. [DOI] [PubMed] [Google Scholar]
- 29.Shigemori H, Bae MA, Yazawa K, Sasaki T, Kobayashi J. Alteramide A, a new tetracyclic alkaloid from a bacterium Alteromonas sp. associated with the marine sponge Halichondria okadai. The Journal of Organic Chemistry. 1992;57:4317–4320. [Google Scholar]
- 30.Pozzatti P, Loreto ES, Mario DAN, Rossato L, Santurio JM, Alves SH. Activities of essential oils in the inhibition of Candida albicans and Candida dubliniensis germ tube formation. J. Mycol. Med. 2010;20:185–189. [Google Scholar]
- 31.Hwang JH, Hwang IS, Liu QH, Woo ER, Lee DG. (+)-Medioresinol leads to intracellular ROS accumulation and mitochondria-mediated apoptotic cell death in Candida albicans. Biochimie. 2012;94:1784–1793. doi: 10.1016/j.biochi.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Petit PX, Lecoeur H, Zorn E, Dauguet C, Mignotte B, Gougeon ML. Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J. Cell Biol. 1995;130:157–167. doi: 10.1083/jcb.130.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zebell SG, Dong X. Cell-Cycle Regulators and Cell Death in Immunity. Cell host & microbe. 2015;18:402–407. doi: 10.1016/j.chom.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin KD, Yoon YJ, Kang YR, Son KH, Kim HM, Kwon BM, Han DC. KRIBB3, a novel microtubule inhibitor, induces mitotic arrest and apoptosis in human cancer cells. Biochem. Pharmacol. 2008;75:383–394. doi: 10.1016/j.bcp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Alby K, Schaefer D, Bennett RJ. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature. 2009;460:U890–U127. doi: 10.1038/nature08252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler G, Rasmussen MD, Lin MF, Santos MAS, Sakthikumar S, Munro CA, Rheinbay E, Grabherr M, Forche A, Reedy JL, Agrafioti I, Arnaud MB, Bates S, Brown AJP, Brunke S, Costanzo MC, Fitzpatrick DA, de Groot PWJ, Harris D, Hoyer LL, Hube B, Klis FM, Kodira C, Lennard N, Logue ME, Martin R, Neiman AM, Nikolaou E, Quail MA, Quinn J, Santos MC, Schmitzberger FF, Sherlock G, Shah P, Silverstein KAT, Skrzypek MS, Soll D, Staggs R, Stansfield I, Stumpf MPH, Sudbery PE, Srikantha T, Zeng QD, Berman J, Berriman M, Heitman J, Gow NAR, Lorenz MC, Birren BW, Kellis M, Cuomo CA. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–662. doi: 10.1038/nature08064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belenky P, Camacho D, Collins JJ. Fungicidal Drugs Induce a Common Oxidative-Damage Cellular Death Pathway. Cell Rep. 2013;3:350–358. doi: 10.1016/j.celrep.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denning DW. Echinocandins and pneumocandins--a new antifungal class with a novel mode of action. J. Antimicrob. Chemother. 1997;40:611–614. doi: 10.1093/jac/40.5.611. [DOI] [PubMed] [Google Scholar]
- 39.Brown JCS, Nelson J, VanderSluis B, Deshpande R, Butts A, Kagan S, Polacheck I, Krysan DJ, Myers CL, Madhani HD. Unraveling the Biology of a Fungal Meningitis Pathogen Using Chemical Genetics. Cell. 2014;159:1168–1187. doi: 10.1016/j.cell.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W, Li YY, Qian GL, Wang Y, Chen HT, Li YZ, Liu FQ, Shen YM, Du LC. Identification and Characterization of the Anti-Methicillin-Resistant Staphylococcus aureus WAP-8294A2 Biosynthetic Gene Cluster from Lysobacter enzymogenes OH11. Antimicrob. Agents Chemother. 2011;55:5581–5589. doi: 10.1128/AAC.05370-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lou L, Qian G, Xie Y, Hang J, Chen H, Zaleta-Rivera K, Li Y, Shen Y, Dussault PH, Liu F, Du L. Biosynthesis of HSAF, a tetramic acid-containing macrolactam from Lysobacter enzymogenes. J. Am. Chem. Soc. 2011;133:643–645. doi: 10.1021/ja105732c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu F, Zaleta-Rivera K, Zhu X, Huffman J, Millet JC, Harris SD, Yuen G, Li XC, Du L. Structure and biosynthesis of heat-stable antifungal factor (HSAF), a broad-spectrum antimycotic with a novel mode of action. Antimicrob. Agents Chemother. 2007;51:64–72. doi: 10.1128/AAC.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ludovico P, Sousa MJ, Silva MT, Leao C, Corte-Real M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiol-Sgm. 2001;147:2409–2415. doi: 10.1099/00221287-147-9-2409. [DOI] [PubMed] [Google Scholar]
- 44.Granot D, Levine A, Dor-Hefetz E. Sugar-induced apoptosis in yeast cells. FEMS Yeast Res. 2003;4:7–13. doi: 10.1016/S1567-1356(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 45.Wadskog I, Maldener C, Proksch A, Madeo F, Adler L. Yeast lacking the SRO7/SOP1-encoded tumor suppressor homologue show increased susceptibility to apoptosis-like cell death on exposure to NaCl stress. Mol. Biol. Cell. 2004;15:1436–1444. doi: 10.1091/mbc.E03-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narasimhan ML, Damsz B, Coca MA, Ibeas JI, Yun DJ, Pardo JM, Hasegawa PM, Bressan RA. A plant defense response effector induces microbial apoptosis. Mol. Cell. 2001;8:921–930. doi: 10.1016/s1097-2765(01)00365-3. [DOI] [PubMed] [Google Scholar]
- 47.Madeo F, Carmona-Gutierrez D, Ring J, Buttner S, Eisenberg T, Kroemer G. Caspase-dependent and caspase-independent cell death pathways in yeast. Biochem. Biophys. Res. Commun. 2009;382:227–231. doi: 10.1016/j.bbrc.2009.02.117. [DOI] [PubMed] [Google Scholar]
- 48.Shirtliff ME, Krom BP, Meijering RAM, Peters BM, Zhu J, Scheper MA, Harris ML, Jabra-Rizk MA, et al. Farnesol-Induced Apoptosis in Candida albicans. Antimicrob. Agents Chemother. 2009;53:2392–2401. doi: 10.1128/AAC.01551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu XZ, Cheng AX, Sun LM, Sun SJ, Lou HX. Plagiochin E, an antifungal bis(bibenzyl), exerts its antifungal activity through mitochondrial dysfunction-induced reactive oxygen species accumulation in Candida albicans. Bba-Gen Subjects. 2009;1790:770–777. doi: 10.1016/j.bbagen.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Zhang R, Alushin GM, Brown A, Nogales E. Mechanistic Origin of Microtubule Dynamic Instability and Its Modulation by EB Proteins. Cell. 2015;162:849–859. doi: 10.1016/j.cell.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinberg G, Wedlich-Soldner R, Brill M, Schulz I. Microtubules in the fungal pathogen Ustilago maydis are highly dynamic and determine cell polarity. J. Cell Sci. 2001;114:609–622. doi: 10.1242/jcs.114.3.609. [DOI] [PubMed] [Google Scholar]
- 52.Li CM, Wang Z, Lu Y, Ahn S, Narayanan R, Kearbey JD, Parke DN, Li W, Miller DD, Dalton JT. Biological Activity of 4-Substituted Methoxybenzoyl-Aryl-Thiazole: An Active Microtubule Inhibitor. Cancer Res. 2011;71:216–224. doi: 10.1158/0008-5472.CAN-10-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.