Abstract
Hepatocellular carcinoma (HCC) is a common indication for liver transplantation (LT). Recent data suggest that body composition features strongly affect post-LT mortality. We examined the impact of body composition on post-LT mortality in patients with HCC. Methods: Data on adult LT recipients who received MELD exception for HCC between 2/29/02-12/31/13 and had CT scan anytime 6 months prior to LT were reviewed (N=118). All available CT scan DICOM (Digital Imaging and Communications in Medicine) files were analyzed using a semiautomated high throughput methodology with algorithms programmed in MATLAB®. Analytic morphomics measurements including dorsal muscle group (DMG) area, visceral and subcutaneous fat and bone mineral density (BMD) were taken at the bottom of the 11th thoracic vertebral level. Results: Thirty-two patients (28%) died during the median follow up of 4.4 years. Number of HCC lesions (HR=2.72; P<0.001), BMD (HR=0.90/HU; P=0.03), pre-LT loco-regional therapy (HR=0.34; P<0.001) and donor age (HR=1.05; P<0.001) were the independent predictors of post-LT mortality. DMG area did not affect post-LT survival. Conclusion: In addition to number of HCC lesions and pre-LT loco-regional therapy, low BMD, a surrogate for bone loss rather than DMG area, was independently associated with post-LT mortality in HCC patients. Bone loss may be an early marker of deconditioning that precedes sarcopenia and may affect transplant outcomes.
Introduction
Hepatocellular carcinoma (HCC) has become one of the leading indications for liver transplantation (LT) in the model for end stage liver disease (MELD) era.1, 2 Candidates with decompensated cirrhosis and HCC who meet Milan criteria (one lesion 2-5 cm or three lesions less than 3 cm in largest diameter) get listed with exception MELD score since the adoption of MELD-based allocation.3 Although MELD score is an excellent predictor of waitlist mortality, it does not perform well in predicting post-transplant survival.4
Emerging data suggest that frailty and sarcopenia affect survival after LT independent of recipient and donor factors. Using analytic morphomics, we and others have shown that body composition, especially; dorsal muscle group (DMG) area (psoas and paraspinal muscles) predicts post-operative complications 5-7 and survival after LT independent of MELD score.6, 8-12 Analytic morphomics is a novel approach that uses high throughput semi-automated image-processing techniques to assess body composition, such as body dimensions, visceral fat and muscle mass, and link it to clinical outcomes.13, 14 In one of our initial studies, Englesbe et al. showed that psoas muscle area was independently associated with post-transplant mortality.8 Psoas muscle area correlated with dorsal muscle group (DMG) area.10 Patients with larger DMG area have improved survival and fewer complications post-transplantation.10
Sarcopenia and bone loss are prevalent in the candidates awaiting LT. This is more pronounced among decompensated end-stage liver disease population with high MELD score awaiting LT because of debility and lower muscle mass. HCC candidates have lower calculated MELD score and well preserved muscle mass compared to non-HCC candidates with high lab MELD score because of exception MELD score policy for HCC.1, 15 Given that the HCC candidates have well-preserved muscle mass, we hypothesized that there might be other body composition components such as bone mineral density (BMD), visceral fat (VF) and subcutaneous fat (SF) rather than DMG area that affect post-transplant survival. Therefore, we examined the impact of body composition (DMG area, BMD, VF and SF) in addition to traditional recipient and donor factors on post-transplant survival among HCC LT recipients.
Methods
Medical records of all adult patients (age ≥ 18 years) who received deceased donor LT between February 28, 2002 and December 31, 2013 for HCC with exception MELD score and had a CT scan of chest or abdomen/pelvis any time prior to 6 months before LT at the University of Michigan were reviewed. The study was approved by University of Michigan Institutional Review Board. Candidates listed as status-1, received living donor LT, repeat LT, multi-organ transplant, found to have incidental HCC on explants or did not have the CT scan prior to 6 months before LT were excluded. Patients were followed till December 31st, 2014.
Demographic and clinical data
Data collected included demographics (age, sex, race/ethnicity), date of listing, date of transplant, date of HCC recurrence, date of last follow-up and death, diagnosis, history of smoking, alcohol, pre-LT history of hypertension, diabetes, laboratory MELD score at the time of listing and transplant, alpha fetoprotein (AFP) within 6 months of listing and LT, pre-LT radiology data including number of lesions, size of each lesion (cm), size of largest lesion (cm), portal venous thrombosis, meeting Milan-criteria pre-LT, history of loco-regional therapy. We also collected data on donor factors such as donor age, sex and cold ischemia time, post-LT recipient factors such as immunosuppression, history of rejection, date of recurrence of HCC, site of recurrence, status at the end of follow-up, and history of graft failure.
Morphomics and body composition
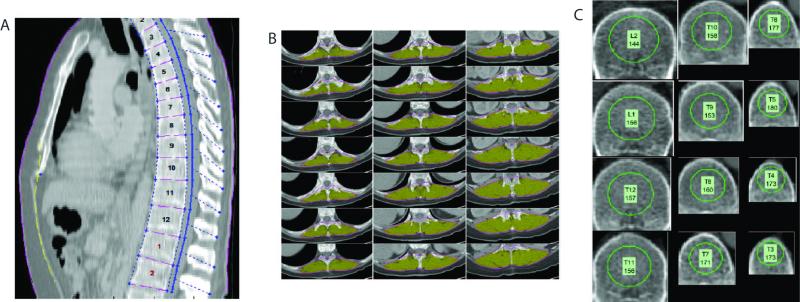
All available CT scan DICOM (Digital Imaging and Communications in Medicine) files were analyzed using a semi-automated high throughput methodology with algorithms programmed in MATLAB® (MathWorks Inc., Natick, MA) as described previously13, 16-19. Our methodology allowed for accurate anatomic indexing of every individual based on the spine. All algorithms involved a combination of user-defined points, automated image processing, and user editing and verification. All measurements were taken at the bottom of the 11th thoracic vertebral level. This anatomic landmark was chosen because this was felt to have the highest likelihood of being available on all abdominal and chest CT scans. The data included the DMG area, BMD, VF, and SF. Furthermore, the age related decline in trabecular bone strength at thoracic vertebral level is similar to lumbar vertebra.20 BMD was measured only on trabecular bone with calculation of the average pixel density within a circle defined as the mid vertebral core sample. A ratio of VF to subcutaneous fat was also calculated. An illustration of the measurements taken from one patient is shown in figure 1.
Figure 1.
Computer generated graphical user interface (GUI) of the (A) anatomic indexing showing the spinal vertebral levels which serve as anatomical reference system for each patient. B: Example of the dorsal muscle group measured (shaded in yellow) defined automatically after delineation of paraspinous lateral seams at specified vertebra points that is processed in each patient. C: Example of the trabecular bone density areas measured within the circle within the mid vertebral core.
Statistical Analysis
Continuous variables were expressed as median and interquartile range and the categorical variables were expressed as counts and percentage, respectively. The primary outcome was post-transplant mortality. For reference purposes, the DMG area and BMD of HCC LT recipients were compared with age and sex matched non-HCC LT recipients who were transplanted during the study duration at our institution using Mann-Whitney test. Cox regression analysis was used to examine the predictors of post-transplant mortality. DMG area, VF area and SF area and BMD were included as the variables of interest in addition to various donor and recipients factors including age at LT, sex, alpha fetoprotein (AFP), diabetes, etiology of liver disease, serum bilirubin, creatinine and INR at LT, pre-transplant number of lesions, largest diameter , donor age and cold ischemia time. Since explant information is not known prior to the time of LT, number of lesions and largest diameter on the explant were not included as predictive variables. Variable with p-value <0.1 on univariate analysis were included to perform the multivariable analysis. Since we were interested in the effect of DMG area on post-transplant mortality, DMG area was forced in the multivariable model.
All analyses were performed using SPSS 22.0 statistical package.
Results
Patient characteristics at LT
There were a total of 199 patients transplanted for HCC during the study period. One hundred and eighteen HCC patients met the inclusion criteria (availability of CT scan any time prior to 6 months before LT). The baseline characteristics of the cohort (N=118) at the time of LT were shown in Table 1. The median age was 56 years, 78% were males, 75% were Caucasians and 64% had hepatitis C as the etiology of liver disease. The median donor age was 41 years.
Table 1.
Recipient and donor characteristics at LT
| Variables | Median (interquartile Range)/proportion N=118 |
|---|---|
| Age at LT (years) | 56(23-71) |
| % Males | 78% |
| Caucasians | 75% |
| African-American | 15% |
| Others | 10% |
| Hepatitis C | 64% |
| Hepatitis B | 4.2% |
| Alcohol | 15.3% |
| Cryptogenic | 9.3% |
| Other | 6.8% |
| Lab MELD | 14 (8-18) |
| Log AFP | 1.44 (0.08-4.30) |
| Bilirubin (mg/dl) | 2.5(1.4-3.6) |
| INR | 1.2 (0.9-1.4) |
| Creatinine (mg/dl) | 1.2 (0.9-1.4) |
| Donor age (years) | 41 (24-53) |
Pre-LT radiological and morphomics characteristics
Table 2 shows the pre-LT radiological and morphomics characteristics. The median number of HCC lesions was 1 with the median largest diameter was 2.5 cm. None of the patients had portal vein thrombosis. At presentation, 87% met Milan criteria, 59% received some type of loco-regional therapy and 12% were downsized. All the patients were within Milan criteria at the time of LT based upon the most recent imaging prior to LT.
Table 2.
Pre-LT radiological tumor characteristics and analytic morphomics characteristics
| Variables | Median (interquartile Range) or proportion |
|---|---|
| Number of lesions | 1 (1-2) |
| Largest Diameter (cm) | 2.5 (1.9-3.2) |
| Loco-regional therapy | 59% |
| Meeting Milan Criteria | 87% |
| Dorsal Muscle group (DMG) Area (mm2) | 3636.2 (2770-4903) |
| Dorsal Muscle group (DMG) Volume (mm3) | 77408.4 (49415-92153.8) |
| Visceral Fat (VF) area (mm2) | 8014.5(4203.8-13153.5) |
| Subcutaneous Fat (SF) area (mm2) | 9731.6 (6057.8-15398.8) |
| Ratio VF/SF | 0.87 (0.50-1.26) |
| Bone Mineral Density (Hounsfield units) | 184.9 (162-216.8) |
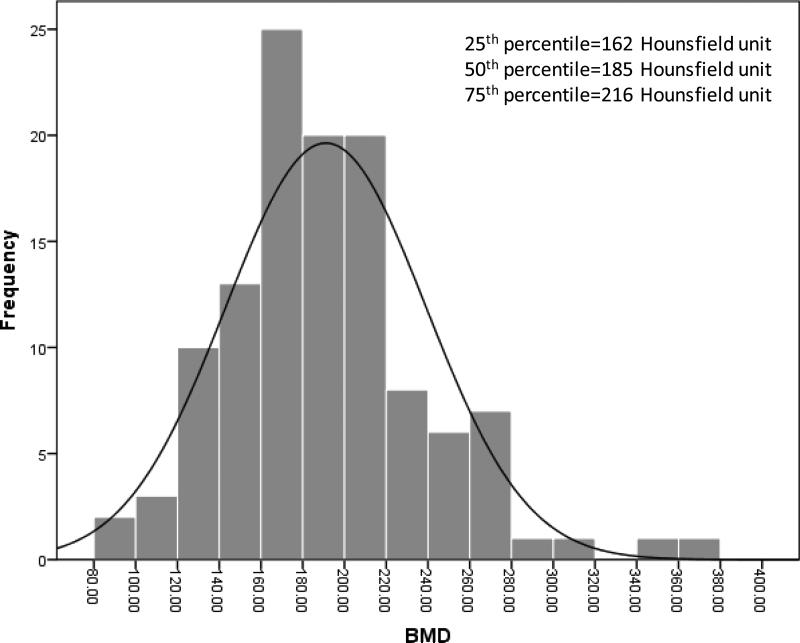
The median DMG area was 3636.2 mm2, VF area was 8014.5 mm2, SF was 9731.6 mm2 ratio of VF to SF was 0.87. Figure 2 shows the distribution of BMD in the cohort. The median BMD was 185 Hounsfield units. The 25th and 75th percentile was 162 Hounsfield units and 216 Hounsfield units, respectively. Only 2.5% of the patients had BMD≤100 Hounsfield units, suggestive of osteoporosis.
Figure 2.
Distribution of BMD before LT among HCC LT recipients.
The age- and sex-matched median BMD at T11 of HCC LT-recipients was similar to the age- and sex- matched BMD of non-HCC LT recipients transplanted during the study period (HCC males: 176.6 Hounsfield units vs. non-HCC males: 173.6 Hounsfield units; HCC females: 209.8 Hounsfield units vs. non-HCC females: 182.9 Hounsfield units) were similar The median age- matched DMG area at T11 for female HCC LT recipients was also similar for HCC (2826.6 mm2) and female non-HCC LT recipients (3073.8 mm2). However, the age-matched median DMG of male HCC LT recipients was significantly higher than the age-matched male non- HCC LT recipients who were transplanted during the same study period (HCC: 4033 mm2 vs. non-HCC: 3167 mm2; P<0.001).
Post-LT deaths
There were a total of 32 post-LT deaths after a median follow up of 4.4 years. The unadjusted 1 year, 3 year and 5 year survival was 87%, 77% and 73%, respectively. The median time to death was 10.9 months from LT. The cause of death was primary non-function in five; hepatitis C related graft failure in four, sepsis and multi-organ failure in six, metastatic HCC in 11 and cause unknown in seven patients. The median BMD in 32 patients who died was 186.5 Hounsfield units (Q1=156.04; Q3=210.6) and only one (3%) patient had BMD<110 Hounsfield units. Of note fewer patients received loco-regional therapy among those who died compared to those who survived after LT (39% vs. 69%; P=0.006)
Independent predictors of post-LT deaths
Table 3 shows the independent predictors of post-LT mortality among HCC LT candidates, in a model adjusted for age and sex. Meeting pre-LT Milan criteria was not a predictor of post-LT mortality, although the number of patients transplanted outside of Milan criteria in this cohort was small. Evidence of loco-regional therapy before LT was associated with 86% lower risk of post-LT deaths. Factors independently associated with high post-LT mortality included higher number of HCC lesions, low BMD and older donor age (Table 3). Every 10 Hounsfield unit decrease in BMD was associated with 10% increase risk of post-LT mortality. In the multivariable model, DMG area was not associated with post-LT mortality.
Table 3.
Independent predictors of post-LT mortality among HCC LT recipients
| Covariates* | HR (95% CI) | P-Value |
|---|---|---|
| Dorsal Muscle area | 1.0 (1.00-1.00) | 0.7 |
| Number of lesion | 2.81 (1.74-4.53) | <0.001 |
| Loco-regional therapy (vs. not) | 0.14 (0.06-0.36) | <0.001 |
| BMD (per 10 Hounsfield Units) | 0.90 (0.83-0.99) | 0.03 |
| Donor Age (per year) | 1.05(1.02-1.07) | <0.001 |
Abbreviations: BMD=Bone mineral density.
Footnote:
Model was adjusted for age and sex.
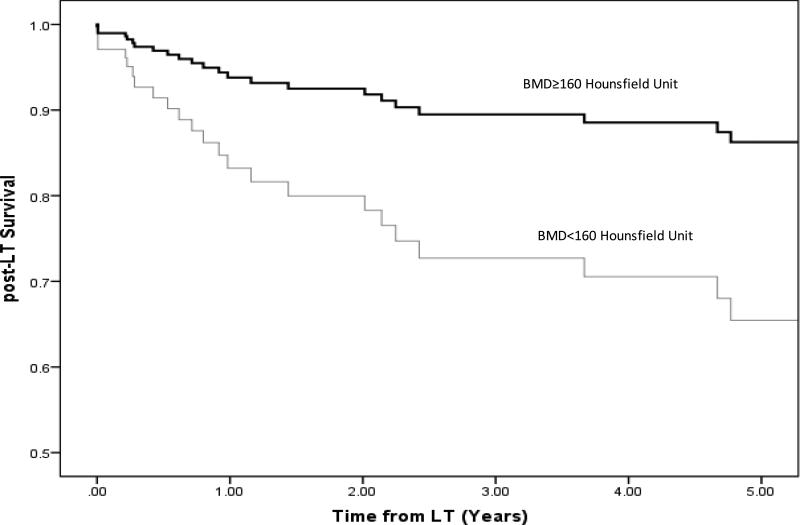
Based upon the published literature, BMD <160 Hounsfield unit is 90% sensitive for distinguishing osteoporosis.21 Therefore, we dichotomized BMD as <160 Hounsfield unit and ≥160 Hounsfield unit. After adjusting for sex, DMG muscle area, number of lesions, and donor age, BMD < 160 Hounsfield unit was associated with 2.8 fold higher hazard of post-LT mortality than those with BMD ≥ 160 Hounsfield unit (HR=2.87; P=0.018) (Figure 3).
Figure 3.
Adjusted Post-LT Survival among HCC LT recipients stratified by BMD.
Discussion
This is the first study to identify the novel association between BMD and post-transplant survival among HCC LT recipients. Additionally tumor burden and advanced donor age were also associated with poor post-LT survival. Pre-LT loco-regional therapy of any kind was associated with lower risk of post-LT mortality. Our study did not find any association between DMG area and post-LT survival in HCC LT recipients.
BMD is the most important determinant of bone fragility. Dual x-ray absorptiometry (DXA) is currently the standard for assessing BMD and has been correlated with fracture risk and treatment efficacy. The use of Hounsfield units from the CT scans to assess regional BMD of the spine has recently been described, with several subsequent studies exploring its utility in assessing fracture risk and prognosticating fusion success.21-23 There is growing evidence that quantitative CT is excellent for predicting vertebral fractures and serial measuring for bone loss, with better sensitivity compared to DEXA as the trabecular bone can be selectively measured and overlying densities (such as aortic calcifications) can be excluded from the study.20, 21, 23 The added advantage is that BMD can be assessed accurately from the CT scans obtained for other indications without exposing patients to the additional risk for ionizing radiation.
We used the cut off of BMD < 160 Hounsfield units in order to further explore the association between BMD and post-LT mortality and found that the adjusted risk of death was close to 3 fold higher among those who have BMD<160 Hounsfield unit. Pickhardt et al. demonstrated that CT-attenuation threshold of 160 Hounsfield unit or less was 90% sensitive at first lumbar vertebrae and a threshold of 110 Hounsfield unit was more than 90% specific for distinguishing osteoporosis from osteopenia and normal BMD. Positive predictive values for osteoporosis were 68% or greater at first lumbar vertebrae CT-attenuation thresholds less than 200 Hounsfield unit; negative predictive values were 99% at thresholds greater than 200 Hounsfield unit. Although only 2.5% had BMD <110 Hounsfield unit suggestive of osteoporosis in our cohort but, 25% of the patients who died had BMD <160 suggestive of osteopenia. These results suggest that early intervention such as rehabilitation and medical therapy to improve low BMD (osteopenia) and prevent further bone loss prior to LT may improve post-LT outcomes. This becomes all the more relevant in the context of the revised HCC allocation policy where HCC candidates would have to wait for six months to receive exception MELD score of 28.
Sarcopenia and frailty are common among LT candidates and associated with poor post-transplant survival after solid organ transplant. 5, 8, 10, 24-26 Data suggest that the bone loss may begin before loss of muscle mass in patients with end stage liver disease and may become apparent before the loss of muscle mass.27 Our study appears to validate this observation. Decrease in bone strength, reflected by low BMD, may be the earliest hallmark of the ‘bone loss-sarcopenia-frailty’ spectrum among HCC candidates who have relatively preserved muscle mass compared to non-HCC candidates listed for LT of comparable allocation MELD. The definition of sarcopenia varies from study to study.5, 8-11, 13, 28-30 Englesbe et al. used total psoas area as a surrogate for sarcopenia in their pioneer study.8, 13 Some recent studies have used DMG area to define sarcopenia because it includes the core muscles which can be measured at T11.10 Our study used the DMG area as a surrogate of sarcopenia since psoas muscle is not generally present above L2 and thus would not be generally measureable in a thoracic CT. Moreover, there is an excellent correlation between total psoas muscle area and DMG area. 10
A recent study of 94 patients who underwent either resection or LT for primary liver tumor found that sarcopenia (defined as total psoas volume) was associated with a three-fold increased likelihood of postoperative complications.11 Almost all Clavien grade ≥3 complications occurred in the sarcopenic group.11 However, sarcopenia was not associated with long-term survival. 11 Similarly, our study did not find any relationship between sarcopenia (defined as DMG area) and post-transplant mortality among HCC LT recipients. It is plausible that while sarcopenia appears to be important for non-HCC patients with profound muscle mass loss, it does not really seem to impact post-LT survival in HCC patients due to MELD-exception policies.
In our study, pre-LT tumor burden was independently associated with poor post-transplant survival. High tumor burden is one the most important causes of HCC recurrence and post-LT mortality among HCC LT recipients.31 Our study also showed that pre-LT loco-regional therapy improved patient survival after LT. This improved survival was likely related to pre-LT palliation of HCC tumors and change in programmatic practice of waiting for at least 3 months after loco-regional therapy. The waiting time eliminates HCC candidates with aggressive tumor biology (shorter doubling time) because of progression of HCC. The combination of these two practices has also attenuated early HCC recurrence and improved post-transplant survival among HCC LT recipients. Donor age is a known predictor of graft failure and post-LT mortality.32 Our study validated this finding in HCC LT recipients.
The limitations of our study include inherent biases associated with retrospective study design. Although analytic morphomics is versatile, at the present time we can only perform image analysis on CT data files and for patients who only had an MRI, we were unable to include them in the study. This resulted in attenuation of sample size and loss of power. Despite these limitations, our hypothesis generating study found a novel association between BMD and post-transplant survival among HCC LT recipients.
In conclusion, bone loss, high tumor burden and advanced donor age are the important determinants of post-LT mortality among HCC LT recipients. Modification of BMD, through pre-LT rehabilitation programs and medical therapy (e.g. calcium/Vitamin D, bisphosphonate therapy) and utilization of pre-LT loco-regional therapy may further improve post-LT survival among this subgroup of patients.
Acknowledgement
Dr. Sharma is supported by National Institutes of Health (NIH) grant KO8 DK-088946 and RO3 DK102480.
Abbreviations
- BMD
Bone Mineral Density
- DMG
Dorsal Muscle Group
- DICOM
Digital Imaging and Communications in Medicine
- HCC
Hepatocellular Carcinoma
- LT
Liver Transplantation
- MELD
Model for End Stage Liver Disease
- SF
Subcutaneous Fat
- VF
Visceral Fat
References
- 1.Sharma P, Balan V, Hernandez JL, et al. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004;10:36–41. doi: 10.1002/lt.20012. [DOI] [PubMed] [Google Scholar]
- 2.OPTN. HRSA, editor. Allocation of livers and intestines. http://optn.transplant.hrsa.gov/ContentDocuments/OPTN_Policies.pdf#nameddest=Policy_09.
- 3.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 4.Schaubel DE, Guidinger MK, Biggins SW, et al. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9:970–81. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Englesbe MJ. Quantifying the eyeball test: sarcopenia, analytic morphomics, and liver transplantation. Liver Transpl. 2012;18:1136–7. doi: 10.1002/lt.23510. [DOI] [PubMed] [Google Scholar]
- 6.Hamaguchi Y, Kaido T, Okumura S, et al. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl. 2014;20:1413–9. doi: 10.1002/lt.23970. [DOI] [PubMed] [Google Scholar]
- 7.Kirk PS, Friedman JF, Cron DC, et al. One-year postoperative resource utilization in sarcopenic patients. J Surg Res. 2015 doi: 10.1016/j.jss.2015.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–8. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krell RW, Kaul DR, Martin AR, et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013;19:1396–402. doi: 10.1002/lt.23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CS, Cron DC, Terjimanian MN, et al. Dorsal muscle group area and surgical outcomes in liver transplantation. Clin Transplant. 2014;28:1092–8. doi: 10.1111/ctr.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valero V, 3rd, Amini N, Spolverato G, et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg. 2015;19:272–81. doi: 10.1007/s11605-014-2680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaido T, Ogawa K, Fujimoto Y, et al. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant. 2013;13:1549–56. doi: 10.1111/ajt.12221. [DOI] [PubMed] [Google Scholar]
- 13.Englesbe MJ, Lee JS, He K, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256:255–61. doi: 10.1097/SLA.0b013e31826028b1. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamurthy V, Zhang P, Ethiraj S, et al. Use of analytic morphomics of liver, spleen, and body composition to identify patients at risk for cirrhosis. Clin Gastroenterol Hepatol. 2015;13:360–368. e5. doi: 10.1016/j.cgh.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P, Harper AM, Hernandez JL, et al. Reduced priority MELD score for hepatocellular carcinoma does not adversely impact candidate survival awaiting liver transplantation. Am J Transplant. 2006;6:1957–62. doi: 10.1111/j.1600-6143.2006.01411.x. [DOI] [PubMed] [Google Scholar]
- 16.Aaij R, Abellan Beteta C, Adametz A, et al. First evidence for the decay B(s)(0)-->mu+ mu. Phys Rev Lett. 2013;110:021801. doi: 10.1103/PhysRevLett.110.021801. [DOI] [PubMed] [Google Scholar]
- 17.Harbaugh CM, Terjimanian MN, Lee JS, et al. Abdominal aortic calcification and surgical outcomes in patients with no known cardiovascular risk factors. Ann Surg. 2013;257:774–81. doi: 10.1097/SLA.0b013e31826ddd5f. [DOI] [PubMed] [Google Scholar]
- 18.Huhdanpaa H, Douville C, Baum K, et al. Development of a quantitative method for the diagnosis of cirrhosis. Scand J Gastroenterol. 2011;46:1468–77. doi: 10.3109/00365521.2011.613946. [DOI] [PubMed] [Google Scholar]
- 19.Zhang P, Peterson M, Su GL, et al. Visceral adiposity is negatively associated with bone density and muscle attenuation. Am J Clin Nutr. 2015;101:337–43. doi: 10.3945/ajcn.113.081778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samelson EJ, Christiansen BA, Demissie S, et al. QCT measures of bone strength at the thoracic and lumbar spine: the Framingham Study. J Bone Miner Res. 2012;27:654–63. doi: 10.1002/jbmr.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickhardt PJ, Pooler BD, Lauder T, et al. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158:588–95. doi: 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber JJ, Anderson PA, Rosas HG, et al. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011;93:1057–63. doi: 10.2106/JBJS.J.00160. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Chung CK, Oh SH, et al. Correlation between Bone Mineral Density Measured by Dual-Energy X-Ray Absorptiometry and Hounsfield Units Measured by Diagnostic CT in Lumbar Spine. J Korean Neurosurg Soc. 2013;54:384–9. doi: 10.3340/jkns.2013.54.5.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dasarathy S. Posttransplant sarcopenia: an underrecognized early consequence of liver transplantation. Dig Dis Sci. 2013;58:3103–11. doi: 10.1007/s10620-013-2791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuda T, Shirabe K, Ikegami T, et al. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl. 2014;20:401–7. doi: 10.1002/lt.23811. [DOI] [PubMed] [Google Scholar]
- 26.Bari K, Sharma P. Impact of body mass index on posttransplant outcomes reexamined. Liver Transpl. 2015;21:1238–40. doi: 10.1002/lt.24227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira FB, Leite AF, Paula AP. Relationship between pre-sarcopenia, sarcopenia and bone mineral density in elderly men. Arch Endocrinol Metab. 2015;59:59–65. doi: 10.1590/2359-3997000000011. [DOI] [PubMed] [Google Scholar]
- 28.Pereira RA, Cordeiro AC, Avesani CM, et al. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv133. [DOI] [PubMed] [Google Scholar]
- 29.Meza-Junco J, Montano-Loza AJ, Baracos VE, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:861–70. doi: 10.1097/MCG.0b013e318293a825. [DOI] [PubMed] [Google Scholar]
- 30.Ilich JZ, Inglis JE, Kelly OJ, et al. Osteosarcopenic obesity is associated with reduced handgrip strength, walking abilities, and balance in postmenopausal women. Osteoporos Int. 2015 doi: 10.1007/s00198-015-3186-y. [DOI] [PubMed] [Google Scholar]
- 31.Sharma P, Welch K, Hussain H, et al. Incidence and risk factors of hepatocellular carcinoma recurrence after liver transplantation in the MELD era. Dig Dis Sci. 2012;57:806–12. doi: 10.1007/s10620-011-1910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–90. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]