Abstract
Didecyldimethylammonium chloride (DDAC) is a dialkyl-quaternary ammonium compound that is used in numerous products for its bactericidal, virucidal and fungicidal properties. There have been clinical reports of immediate and delayed hypersensitivity reactions in exposed individuals; however, the sensitization potential of DDAC has not been thoroughly investigated. The purpose of these studies was to evaluate the irritancy and sensitization potential of DDAC following dermal exposure in a murine model. DDAC induced significant irritancy (0.5 and 1%), evaluated by ear swelling in female Balb/c mice. Initial evaluation of the sensitization potential was conducted using the local lymph node assay (LLNA) at concentrations ranging from 0.0625–1%. A concentration-dependent increase in lymphocyte proliferation was observed with a calculated EC3 value of 0.17%. Dermal exposure to DDAC did not induce increased production of IgE as evaluated by phenotypic analysis of draining lymph node B-cells (IgE+B220+) and measurement of total serum IgE levels. Additional phenotypic analyses revealed significant and dose-responsive increases in the absolute number of B-cells, CD4+ T-cells, CD8+ T-cells and dendritic cells in the draining lymph nodes, along with significant increases in the percentage of B-cells (0.25% and 1% DDAC) at Day 10 following 4 days of dermal exposure. There was also a significant and dose-responsive increase in the number of activated CD44 + CD4 + and CD8+ T-cells and CD86+ B-cells and dendritic cells following exposure to all concentrations of DDAC. These results demonstrate the potential for development of irritation and hypersensitivity responses to DDAC following dermal exposure and raise concerns about the use of this chemical and other quaternary ammonium compounds that may elicit similar effects.
Keywords: DDAC, hypersensitivity, allergic disease, quaternary ammonium compound
Introduction
Quaternary ammonium compounds (QAC) are commonly used as water-based surface disinfectants due to their low volatility and they are increasingly being used in hospitals, hotels and in consumer products (Zhang et al. 2015) due to their broad antimicrobial capabilities. In healthcare settings they are frequently used in clinical settings for the decontamination of surgical instruments, endoscopes and other medical instruments. The use of QAC as antiseptics, disinfectants, detergents and preservatives has increased their incorporation into consumer products that are utilized orally (mouthwash) or applied to the skin or eyes for the purpose of decreasing microbial contamination and reducing the incidence of pathogen-induced illness.
All QAC are permanently charged ions with four alkyl side chains. Their structures contain at least one hydrophobic hydrocarbon chain linked to a positively charged nitrogen atom and other alkyl groups that are mostly short-chain substitutes such as methyl or benzyl groups. The biocidal activity is conferred through alkyl chain length (McBain et al. 2004). The ability to adapt and optimize QAC structure to target specific microbial species has recently increased the utilization of these compounds for use in consumer products (Carson et al. 2008). Dialkyl QAC represent the newest generation of QAC and exhibit a wide spectrum of activity. These new synthetic polymeric QAC contain multiple positively-charged amine centers that confer anti-microbial, antistatic, and surfactant properties in solution. One of the newer QAC in common use is didecyldimethylammonium chloride (DDAC). DDAC is a broad-spectrum bactericidal and fungicidal biocide that exhibits antimicrobial activity against several pathogens such as Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa (Walsh et al. 2003), Legionella pneumophilia (Skaliy et al. 1980), Stachybotrys chartarum and enveloped and non-enveloped viruses (Argy et al. 1999). It is used in several types of applications including: industrial processes, swimming pools and aquatic areas, wood treatment, healthcare and food handling and storage (Ohnuma et al. 2011).
QAC have been used for over 50 years and have generally been regarded as safe; however, there is very limited published research describing the toxicity of these compounds, especially regarding the newer formulations such as DDAC. A study conducted by Ohnuma et al. (2011) examined the pulmonary defense system following a single intratracheal instillation of DDAC (60 and 150 μg/kg) in C57BL/6J mice. Those authors found that exposure to the high dose induced lung injury as early as 1-d post-exposure, as evidenced by increased lactate dehydrogenase (LDH) activity and protein concentrations in the bronchoalveolar (BAL) fluid. There was also an increase in total cells in the BAL (specifically macrophages, neutrophils and lymphocytes), along with increases in interleukin (IL)-6 production by 7-days post-exposure. The authors also suggested that DDAC exposure altered oxidative stress and antimicrobial markers (evaluated by gene expression) in the lungs and systemic co-exposure with lipopolysaccharide (LPS) generated a further enhancement in pulmonary inflammation suggesting a potential increase in susceptibility to bacterial agents.
A more recent study investigated the effects of DDAC following a 2-week inhalation exposure (Lim & Chung 2014) in Sprague-Dawley rats. Aside from decreases in body weight at the highest exposure concentration (3.6 mg/m3), no changes in hematological and blood biochemistry parameters were observed and mild changes were observed in BAL cell counts and cell damage parameters. Interestingly, a recent study observed that, following the introduction of DDAC in an animal facility there was decreased reproductive performance of the laboratory mice. Additional examination identified decreases in fertility and fecundity including increased time to first litter, longer pregnancy intervals, fewer pups per litter and fewer pregnancies in mice following DDAC exposure (Melin et al. 2014).
Epidemiological data and case studies indicate that healthcare workers have an elevated risk for development of sensitization and allergic asthma from either dermal or inhalation exposure to chemicals compared to non-healthcare workers (Warshaw et al. 2008). Biocides such as QAC have been identified to be among the most common allergens in the healthcare profession (Bernstein et al. 1994; Purohit et al. 2000; Shaffer & Belsito 2000; Suneja & Belsito 2008; Gonzalez et al. 2014). A study evaluating 142 patients with suspected allergies to the most commonly used QAC, i.e. benzalkonium chloride (BAC) and benzethonium chloride (BEC), confirmed sensitization by patch test to these compounds in 20% of the patients and identified potential co-reactions between the two QAC in 85% of the subjects who tested positive (Dao et al. 2012).
While there have been fewer overall reported cases of sensitization to the newer formulations of QAC, allergic contact dermatitis and immediate-type allergic reactions caused by DDAC exposure have been recently reported. Four cases, which were confirmed by patch testing or open epicutaneous tests, describe contact dermatitis presenting in hospital and laboratory workers on the hands/wrists and face following exposure to DDAC present in a disinfectant (Dejobert et al. 1997; Dibo & Brasch 2001; Ruiz Oropeza et al. 2011). In addition, there is also a confirmed case of allergic contact dermatitis of the foot resulting from exposure to DDAC that was present in a shoe refresher spray (Mowitz & Ponten 2015). There has also been one case of immediate allergy reported in an individual in catering school who was exposed to DDAC in a cleaning product. This individual suffered from urticaria, facial angioedema and dyspnea within 10 min of exposure. A 20-min open epicutaneous test with the diluted cleaning product and DDAC confirmed immediate-type hypersensitivity (Houtappel et al. 2008). All symptoms in the individuals resolved when exposure to DDAC was eliminated.
While these studies suggest a role for QACs and DDAC in allergic disease, the exact mechanism of action for sensitization to these compounds remains to be investigated and explained. Due to the high potential for human exposure, epidemiological studies suggesting an association with allergic disease and the lack of dermal toxicological data, this study was performed to evaluate the irritancy and skin sensitization potential of DDAC using a murine model in an effort to assess its role in the development of allergic disease.
Materials and methods
Animals
Female BALB/c mice were used for the murine models. This mouse strain has a T-helper (TH)-2 bias and is commonly used to evaluate IgE-mediated sensitization (Woolhiser et al. 2000; Klink & Meade 2003). The mice were purchased from Taconic (Germantown, NY) at 6–8 weeks-of-age. Upon arrival, the animals were allowed to acclimate for a minimum of 5 days. Each shipment of animals was randomly assigned to a treatment group, weighed and individually identified (via tail marking) using a permanent marker or tattoo. A preliminary analysis of variance on body weights was performed to ensure a homogeneous distribution of animals across treatment groups. The animals were housed at a maximum of 5/cage in ventilated plastic shoebox cages with hardwood chip bedding. NIH-31 modified 6% irradiated rodent diet (Harlan Teklad) and tap water were provided from water bottles, ad libitum. The animal facility temperature was maintained at 68–72°F and the relative humidity between 36–57%. A light/dark cycle was maintained on 12-h intervals. All animal experiments were performed in the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited NIOSH animal facility in accordance with an animal protocol approved by the Institutional Animal Care and Use Committee.
Chemical exposure
Dimethyldidecylammonium chloride (DDAC; CAS#7173-51-5; Figure 1), acetone (CAS#67-64-1), α-Hexylcinnamaldehyde (HCA; CAS#101-86-0) and 2,4-toluene diisocyanate (TDI; CAS#584-84-9) were purchased from Sigma-Aldrich (Milwaukee, WI).
Figure 1.
Chemical structure of DDAC.
Concentration range-finding studies
Concentration range finding studies were performed to select the concentrations of DDAC to be used for dermal exposures. Mice (n = 3/group) were exposed topically to acetone vehicle or increasing concentrations of DDAC (up to 40%) in acetone on the dorsal surface of each ear (25 μl per ear) for three consecutive days. Acetone was selected as the appropriate vehicle based on solubility, historical control data and accepted use in skin sensitization studies (NIEHS 1999). Animals were allowed to rest for 2 days following the final exposure and then weighed and examined for signs of overt toxicity including loss of body weight, fatigue/lack of activity and ungroomed fur.
Combined irritancy and local lymph node assay (LLNA)
To determine the irritancy and sensitization potential of DDAC, a combined LLNA was conducted as previously described (Anderson et al. 2007) and according to the method described in the ICCVAM Peer Review Panel report (NIEHS 1999) with minor modifications. Briefly, mice (n = 5/group) were exposed topically to acetone vehicle, increasing concentrations of test agent or positive control (HCA) on the dorsal surface of each ear (25 μl per ear) for three consecutive days. HCA (30%) is an accepted and well-characterized positive control for the LLNA (NIEHS 1999). For irritancy evaluation, the thickness of the right and left ear pinnae of each mouse was measured using a modified engineer micrometer (Mitutoyo Co., Tokyo, Japan) before the first chemical administration and 24 h following the final exposure. The mean percentage of ear swelling was calculated based on the following equation: [(mean post-challenge ear thickness — mean pre-challenge ear thick-ness)/mean pre-challenge thickness] × 100.
Animals were allowed to rest for 2 days following the final exposure. On Day 6, mice were injected intravenously via the lateral tail vein with 20 μCi [3H]-thymidine (Dupont NEN; specific activity = 2 Ci/mmol). Five hours after [3H]-thymidine injection, the animals were euthanized via CO2 inhalation and the left and right auricular draining lymph nodes (DLN; drain site of chemical application) located at the bifurcation of the jugular vein were excised and pooled for each animal. Single cell suspensions were made and incubated overnight in 5% trichloroacetic acid and samples were then counted in a Packard Tri-Carb 2500TR liquid scintillation analyzer (Perkin Elmer, Waltham, MA). Stimulation indices (SI) were calculated by dividing the mean disintegrations per minute (DPM) per test group by the mean DPM for the vehicle control group. EC3 values (concentration of chemical required to induce a 3-fold increase over the vehicle control) were calculated based on the equation from Basketter et al. (1999). Dosing concentrations (0.0625–1.000%) were selected based on the results from the concentration range-finding studies. The concentration of chemical required to induce a 3-fold increase over the vehicle control (EC3) was calculated based on the equations from Basketter et al. (1999).
Phenotypic analysis of draining lymph node cells
For the phenotypic analysis, mice (n = 5/group) were exposed to 25 ml/ear of the acetone vehicle, increasing concentrations of test article (0.25%, 0.5% and 1% DDAC) or positive control (1.0% TDI) once daily for four consecutive days. Animals were euthanized by CO2 inhalation on Day 10, weighed and examined for gross pathology. The liver, spleen, kidneys and thymus were removed, cleaned of connective tissue and weighed. Serum was collected for total IgE analysis (see below). DLN cell suspensions (2 nodes/animal/3 ml PBS) were prepared by mechanical disruption of tissues between frosted microscope slides in phosphate buffered saline (PBS) and counted on a Cellometer (Nexcelom Bioscience, Lawrence, MA). Cells (1–2 × 106) were aliquoted into a 96-well U-bottom plates and washed in staining buffer (PBS+ 1% bovine serum albumin+0.1% sodium azide). Cells were re-suspended in staining buffer containing anti-mouse CD16/32 antibody (clone 2.4G2) for blocking of Fc receptors (BD Biosciences, San Jose, CA). Cells were next incubated with a cocktail of flurochrome-conjugated antibodies specific for cell surface antigens, including IgE-FITC (R35-72), B220-V500 (RA3-6B2), CD8a PE-CF594 (53-6.7) (BD Biosciences), CD11b-PerCP-Cyanine5.5 (M1/70), CD4 PE-Cyanine7 (GK1.5), CD86-APC (GL1), MHC II-Alexa Fluor F700 (M5/11.15.2) and CD44-eFluor 780 (IM7) (eBioscience, San Diego, CA). Cells were then washed in staining buffer and then fixed in Cytofix buffer according to manufacturer instructions (BD Biosciences). Within 24 h, the cells were re-suspended in staining buffer and 50 000 events collected and analyzed on an LSR II flow cytometer (BD Biosciences). Compensation controls were prepared with OneComp eBeads (eBioscience). The IgE+B220+ populations were analyzed as described by Manetz and Meade (1999). Data analysis was performed using FlowJo 7.6.5 software (TreeStar Inc., Ashland, OR).
Serum IgE antibody levels
Following euthanasia of animals included in the phenotyping study, blood samples were collected via cardiac puncture. Sera were separated by centrifugation and frozen at −20°C for subsequent analysis of total IgE by ELISA. A standard colorimetric sandwich ELISA was performed as previously described (Anderson et al. 2007).
Statistical analysis
For analysis, data was first tested for homogeneity using the Bartlett's Chi Square test. If homogeneous, a one-way analysis of variance (ANOVA) was conducted. If the ANOVA showed significance at p<0.05 or less, the Dunnett's Multiple Range t test was used to compare treatment groups with the control group. Linear trend analysis was performed to determine if the test articles had exposure concentration-related effects for the specified endpoints. Statistical analysis was performed using Prism software (v 5.0, Graph Pad, San Diego, CA). Statistical significance was designated by *p ≤ 0.05 and **p ≤ 0.01.
Results
Dermal exposure to high concentrations of DDAC resulted in toxicity
Exposure to 40% DDAC resulted in death of all animals by 24 h post-single dermal application; therefore, exposure concentrations were dramatically reduced for subsequent studies. Exposure to 1% DDAC for 3 days resulted in <10% loss in body weight and did not result in signs of overt toxicity or visual signs of excessive inflammation at the exposure sites. Therefore, this concentration was selected as the highest concentration used in subsequent studies. Significant decreases in percentage body weight (11% at 0.5% and 14% at 1%) were observed at Day 10 following a 4-day DDAC exposure regimen. Although no statistically significant changes in organ weight were observed following exposure to any tested concentrations of DDAC, a decreasing trend (Linear Trend Test, p<0.01 and p<0.05, respectively) in thymus and liver weight (but not percentage of body weight) was observed at Day 10 following a 4-day exposure (Table 1).
Table 1.
Body/organ weight of female Balb/c mice dermally exposed to DDAC.
| DDAC (w/v) | ||||
|---|---|---|---|---|
|
|
||||
| Parameter | 0% | 0.25% | 0.50% | 1% |
| Body Weight (g) | 19.73 ± 0.47 | 18.99 ± 0.57 | 17.46 ± 0.30* | 16.90 ± 0.32** |
| Kidney Weight | ||||
| mg | 263 ± 3 | 219 ± 11 | 225 ± 5 | 221 ± 9 |
| %bwa | 1.23 ± 0.03 | 1.20 ± 0.02 | 1.29 ± 0.05 | 1.30 ± 0.04 |
| Spleen Weight | ||||
| mg | 94 ± 2 | S3 ± 11 | 92 ± 13 | 97 ± 5 |
| %bw | 0.48 ± 0.01 | 0.43 ± 0.02 | 0.53 ± 0.04 | 0.57 ± 0.03 |
| Thymus Weight | ||||
| mg | 53 ± 6 | 59 ± 2 | 44 ± 2 | 39 ± 4# |
| %bw | 0.26 ± 0.02 | 0.31 ± 0.01 | 0.25 ± 0.01 | 0.23 ± 0.03 |
| Liver Weight | ||||
| mg | 917 ± 36 | 910 ± 46 | 838 ± IS | S00 ± 22## |
| %bw | 4.64 ± 0.08 | 4.79 ± 0.16 | 4.80 ± 0.10 | 4.73 ± 0.09 |
bw: body weight.
Values are expressed as means (± SE) for each group.
Significantly different from acetone controls at
p<0.05 or
p<0.01
Linear Trend
p<0.001
p<0.05
In vivo studies identify DDAC to be an immune sensitizer and irritant
Dermal exposure to 0.5% and 1% DDAC induced significant ear swelling 24-h post-final chemical exposure (3 days) (Figure 2A). Exposure to 1% DDAC resulted in a 37% increase in ear swelling compared to the vehicle control (2%). Although there was an increasing trend (Linear Trend Test, p<0.05) in ear swelling following exposure to DDAC, this was not statistically significant at the lower concentrations (0.0625, 0.125 and 0.25%). DDAC tested positive in the LLNA and an EC3 value of 0.17% was calculated as previously described (Figure 2B). A dose-responsive increase (Linear Trend Test, p<0.05) in lymphocyte proliferation was observed following exposure to DDAC reaching statistical significance at 0.25%. HCA (30%) was used as a positive control for these experiments and resulted in a stimulation index (SI) value of 13 (data not shown).
Figure 2.
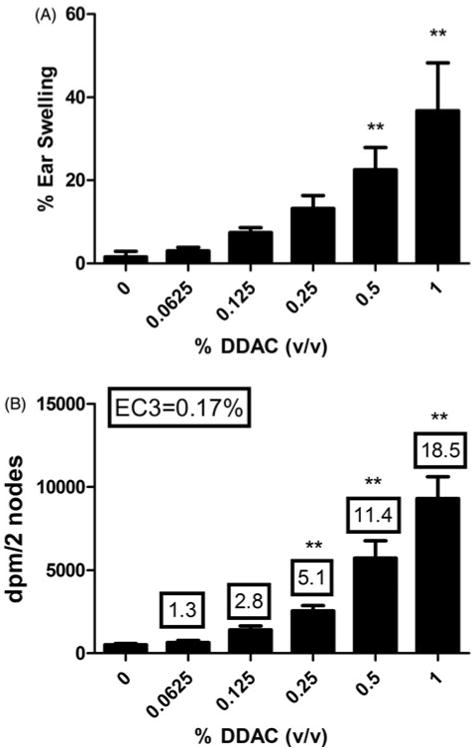
Irritancy and allergic sensitization potential after dermal exposure to DDAC. (A) Irritancy and (B) allergic sensitization potential of DDAC using the LLNA. Irritancy was determined using measurements collected at 24 h following the final DDAC exposure (3 days). DPM represent [3H]-thymidine incorporation into DLN cells from the BALB/c mice following exposure to vehicle or concentration of DDAC (0.0625–1%). SI value is the stimulation index (fold-change over vehicle control). Bars represent mean (±SE) of five mice per group.
Exposure to DDAC did not induce an increase in local or systemic IgE levels
The mechanisms of DDAC sensitization were further investigated using phenotypic analysis of IgE-expressing B-cells in the DLN. To further evaluate the mechanisms of the hypersensitivity response, the percentages of IgE+B220+ cells (IgE+ B-cells) in the DLN were evaluated using flow cytometry. Soluble IgE bound to the B-cell surface via the low affinity IgE receptor (CD23) is dependent on the level of soluble IgE present in the local DLN environment (representative of local IgE levels) and changes in this population following allergen exposure have been detected earlier than serum IgE levels. Manetz and Meade (1999) showed that select chemicals capable of inducing TH2-mediated allergic responses result in similar peak increases in the percentages of IgE+B220+ and total B-cell (B220+) populations and which become significantly elevated at equivalent concentrations of the test chemical. Consistent with the LLNA results, statistically significant and dose-responsive (Linear Trend Test, p<0.01) increases in the percentages of B-cells were observed (Figure 3A). However, there were no statistically significant increases in IgE+ B-cells in the DLN of mice treated with DDAC (Figure 3B). In contrast, the positive control TDI resulted in a statistically significant increase in the IgE+ B-cell (21%) and total B-cell (35%) populations compared to in the vehicle controls (0.44% and 14%, respectively). Serum IgE is commonly used as an indicator of Type I hypersensitivity to dermal sensitizers. Supporting the phenotyping results, exposure to DDAC did not produce a significant elevation in total serum IgE levels compared to levels in the vehicle control group (Figure 3C). However, exposure to TDI resulted in a significant elevation of total serum IgE (1697 ng/ml) compared to vehicle control levels (279 ng/ml).
Figure 3.
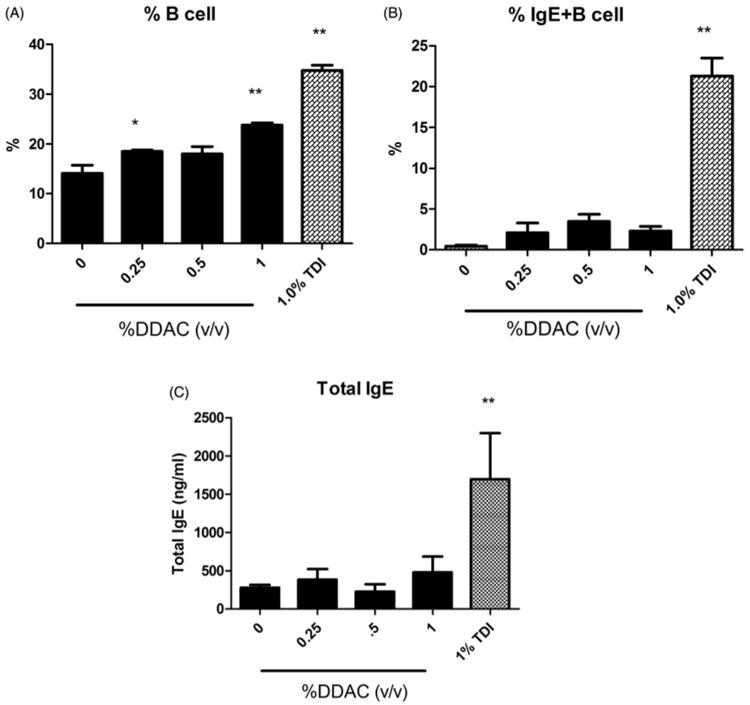
Local and systemic IgE levels following dermal DDAC exposure. Percentages of (A) B-cells and (B) IgE+ B-cells among total lymphocytes in the DLN and (C) total serum IgE (C) on Day 10 following 4 days of DDAC application. Bars represent mean (± SE) of five mice per group.
Exposure to DDAC induced increased DLN cellularity consisting of activated leukocytes
Dermal exposure to all concentrations of DDAC resulted in statistically significant increases in DLN cellularity (Table 2). There was almost a 6-fold increase in cellularity following exposure to 1% DDAC (1.70 × 10) compared to the vehicle control (3.65 × 10). Exposure to all concentrations of DDAC produced statistically significant increases in the absolute numbers of B-cells, CD4+ T-cells, CD8+ T-cells and dendritic cells (DC) (Table 2). Statistically significant increases in the percentages of the B-cell population were also observed (0.25% and 1% DDAC) as previously described, along with statistically significant decreases in the percentage of CD4+ T-cell (0.25%, 0.5% and 1%) and CD8+ T-cell (1%) populations. Exposure to DDAC did not alter the percentage of the DC population identified by high expression of CD11b and MHC II surface markers. The percentages of activated cells were also evaluated based on co-expression of surface markers CD44 (CD4+ and CD8+ T-cells) or CD86 (dendritic cells and B cells). Statistically significant increases in the percentage of activated CD4+, CD8+, B-cells and DC were observed after exposure to all concentrations of DDAC (Figure 4). Unlike DDAC, exposure to the TH2 typical asthmogen TDI did not induce significant changes in the percentage of activated CD8+ cells. However, the percentage of activated B-cells (61%) was much higher following TDI exposure compared to DDAC exposure (18–19%).
Table 2.
Effects of dermal exposure to DDAC on DLN cell number and lymphocyte sub-population in female Balb/c mice.
| DDAC | ||||
|---|---|---|---|---|
|
|
||||
| Parameter | 0% | 0.25% | 0.05% | 1% |
| DLN number (×106) | 3.65 ± 0.47 | 15.2 ± 0.83** | 15.5 ± 1.08** | 17.0 ± 1.42** |
| B-cell number (× 106) | 0.51 ± 0.09 | 2.8 ± 0.13** | 2.8 ± 0.21** | 4.0 ± 0.32** |
| % of DLN | 14.12 ± 1.5 | 18.52 ± 0.27* | 18.02 ± 1.45 | 23.80 ± 0.46** |
| CD4 + T-cell number (× 106) | 2.2 ± 0.29 | 8.4 ± 0.47** | 8.6 ± 0.68** | 8.7 ± 0.76** |
| % of DLN | 60.04 ± 0.96 | 55.28 ± 0.25** | 54.98 ± 0.25** | 51.02 ± 0.50** |
| CD8+-cells (× 106) | 0.92 ± 0.11 | 3.7 ± 0.21** | 3.5 ± 0.32** | 3.9 ± 0.29** |
| % of DLX | 25.26 ± 0.66 | 24.46 ± 0.22 | 2490 ± 0.46 | 22.98 ± 0.51* |
| Dendritic cells (× 105) | 0.4 ± 0.06 | 1.5 ± 0.08** | 2.0 ± 0.11** | 1.9 ± 0.19** |
| % of DLN | 1.18 ± 0.14 | 0.96 ± 0.05 | 1.33 ± 0.08 | 1.15 ± 0.09 |
Mice were dermally exposed to vechicle (acetone) or different concentrations of DDAC for 4 consecutive days. The mice were euthanized 6 days after the final exposure, DLN were removed, and total cells counted. Numbers and frequency of B- and T-cells, and subsets of T-cells (CD4+ and CD8+), and dendritic cells were enumerated. Values represent the means (± SE) for each group. Significantly different from acetone controls at
p<0.05 and
p<0.01
Figure 4.
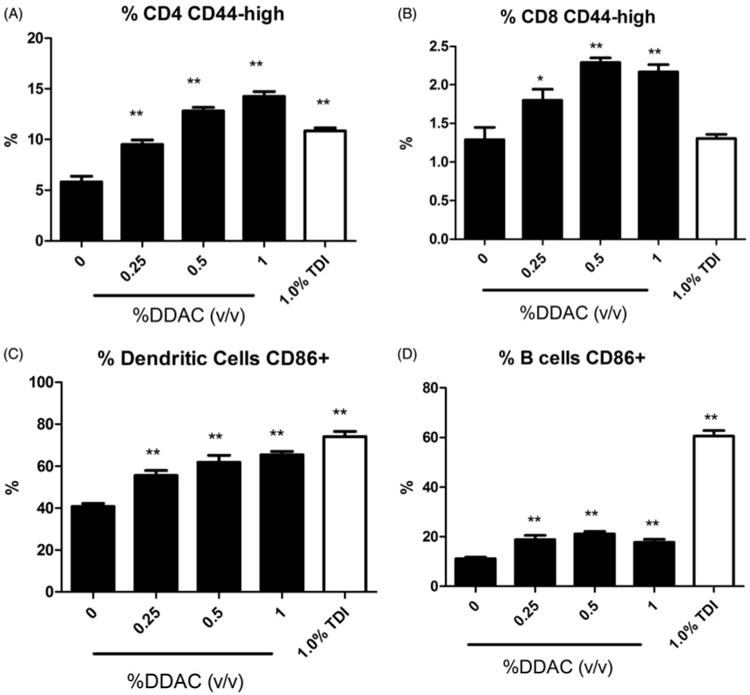
Increases in activated leukocytes following dermal exposure to DDAC. Percentages of (A) CD44highCD4+ cells, (B) CD44highCD8+ cells, (C) CD86+ dendritic cells and (D) CD86+ B-cells among total lymphocytes on Day 10 following 4 days of DDAC application. Bars represent mean (± SE) of five mice per group.
Discussion
The Centers for Disease Control and Prevention (CDC) estimates that more than 13 million workers in the US, spanning a variety of occupational industries and sectors, are at risk to be exposed to chemicals that can be absorbed through the skin. Approximately 82000 chemicals are currently in industrial use, with ≈700 additional chemicals being introduced annually, resulting in a high potential for dermal exposure (GAO 2005). Occupational skin exposures can result in numerous diseases which can adversely affect an individual's health and capacity to perform at work, resulting in significant economic losses including: decreased productivity, medical expenses, and loss of work due to illness, with associated costs estimated to exceed $1 billion annually in the US alone. Contact dermatitis is one of the most common types of occupational illnesses, accounting for ≈90–95% of all occupational skin disorders in the US, and results in a significant socioeconomic impact. Occupational skin diseases, including allergic contact dermatitis (ACD), occur commonly among healthcare workers (Warshaw et al. 2008). Epidemiological data and case studies indicate that healthcare workers have an elevated risk for development of sensitization and allergic asthma from either dermal or inhalation exposure to QAC (Bernstein et al. 1994; Shaffer & Belsito 2000; Suneja & Belsito 2008).
The studies described here use a standardized murine model to begin to evaluate the sensitization potential of DDAC. DDAC was identified as an irritant and strong sensitizing chemical. The lowest concentrations that induced a significant increase in lymphocyte proliferation (0.25%) were below concentrations that resulted in significant increases in ear swelling (0.5%). In addition, there was a significant increase in the percentage of B-cells in the absence of increasing IgE+ B-cells in the DLN and total IgE in the serum. TDI was included in these experiments as a chemical that induces a prototypical TH2 (IgE-mediated) hypersensitivity response. Although only a single concentration of TDI (1%) was included, it fell into the concentration range that was examined for DDAC (Figures 3 and 4), specifically activation percentages of the immune cell subsets examined. The percentage of activated CD8+ T-cells in the DLN was higher for DDAC compared to TDI for all concentrations tested. In contrast, the percentage of activated B-cells in the DLN was strikingly higher for TDI than for any concentration of DDAC. These findings demonstrate a lack of increase in both local and total IgE, along with an increased percentage of activated CD8+ T-cells in the DLN following exposure; this data suggests that DDAC may induce a T-cell or TH1-mediated hypersensitivity response.
There are several commercially-available QAC-containing products including Bardac 2280, Timbercote II and F-2 that contain DDAC at concentrations of 80%, 20% and 11.4%, respectively (Henderson 1992). Working concentrations of DDAC typically range from 0.01–1% and are similar to the concentrations tested in the present study. Since the concentrated solution typically requires dilution prior to use, there is the potential for skin exposure to high concentrations as a result of splashes and spills. Due to their low volatility it is presumed that the skin may be a significant route of exposure for DDAC and other QAC. It has been reported that QAC do not contaminate indoor air environments; however, once they are put into suspension, pulmonary exposure could potentially occur due to aerosolization. In addition, it has been determined that QAC may persist in the environment. A study found that QAC, including DDAC, were detectable for several months after the animal facility stopped using product that contained them (Melin et al. 2014). This suggests that continuous use may actually lead to accumulation of higher levels. While these companies typically regard these products as safe and non-sensitizing, specific toxicological information is lacking in the MSDS. Additionally, in the present study, a single dermal exposure to 40% DDAC resulted in deaths of all mice in the range-finding study within 24 h; this raised additional concern about the potential systemic toxicity of this chemical.
While epidemiological studies support a role for DDAC in allergic disease, these are the first animal studies to confirm that DDAC is a sensitizing chemical. QAC have been used as antimicrobial disinfectants in healthcare settings for over 40 years. Two of the most common QAC, i.e. BAC and BEC, have also been associated with allergic disease – based on epidemiologic studies. Interestingly, unlike DDAC and contradictory to the human data, animal data typically describes these compounds as irritants and/or very weak sensitizers (Manetz & Meade 1999; Gerberick et al. 2002). However, these animal models may lack the complexity associated with occupational exposures. Additionally, DDAC is considered to be in the next generation of QAC and, therefore, it is structurally different from BAC and BEC. In several of the epidemiological studies investigating DDAC, patients that patch tested positive for DDAC tested negative for BAC (Dibo & Brasch 2001; Geier et al. 2013). In addition, sensitization to DDAC may be under-reported since it is not included in the standard patch test series. Due to the emergence of a “new generation” of QAC that are structurally heterogeneous and potentially exhibit increased immunogenicity compared to their predecessors, it is imperative to analyze the immunotoxicological effects of these compounds. Typically in the healthcare setting there is a high potential for exposure to not only QAC, but other disinfectants and antimicrobials that have sensitizing and irritating properties. The immunological consequences of these types of mixed exposures has not thoroughly been studied, yet warrant further investigation.
In summary, based on calculated EC3 values from the LLNA, these studies demonstrate dermal sensitization potential (strong), based on the criteria set forth by ICCVAM (2011) for the disinfectant DDAC. While direct associations of dermal exposure to DDAC on human health have not been fully established, the above-mentioned studies raise concerns about exposure to this chemical.
Acknowledgments
The findings and conclusion in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. A portion of this project was funded by the NIOSH intramural National Occupational Research Agenda (NORA), under Project 939038G
Footnotes
This work was authored as part of the Contributor's official duties as an Employee of the United States Government and is therefore a work of the United States Government. In accordance with 17 U.S.C. 105, no copyright protection is available for such works under U.S. Law.
Disclosure statement: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Anderson SE, Wells JR, Fedorowicz A, Butterworth LF, Meade BJ, Munson AE. Evaluation of the contact and respiratory sensitization potential of volatile organic compounds generated by simulated indoor air chemistry. Toxicol Sci. 2007;97:355–363. doi: 10.1093/toxsci/kfm043. [DOI] [PubMed] [Google Scholar]
- Argy G, Bricout F, d'Hermies F, Cheymol A. Study of prophylaxis by didecyl dimethyl ammonium chloride against herpes simplex virus infection in nude mice. Comptes Rendus Acad Sci. 1999;322:863–870. doi: 10.1016/s0764-4469(00)86652-4. [DOI] [PubMed] [Google Scholar]
- Basketter DA, Lea LJ, Dickens A, Briggs D, Pate I, Dearman RJ, Kimber I. A comparison of statistical approaches to the derivation of EC3 values from local lymph node assay dose responses. J Appl Toxicol. 1999;19:261–266. doi: 10.1002/(sici)1099-1263(199907/08)19:4<261::aid-jat572>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Bernstein JA, Stauder T, Bernstein DI, Bernstein IL. A combined respiratory and cutaneous hypersensitivity syndrome induced by work exposure to quaternary amines. J Allergy Clinical Immunol. 1994;94:257–259. doi: 10.1016/0091-6749(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Carson RT, Larson E, Levy SB, Marshall BM, Aiello AE. Use of antibacterial consumer products containing quaternary ammonium compounds and drug resistance in the community. J Antimicrob Chemother. 2008;62:1160–1162. doi: 10.1093/jac/dkn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao H, Fricker C, Nedorost ST. Sensitization prevalence for benzalkonium chloride and benzethonium chloride. Dermatitis. 2012;23:162–166. doi: 10.1097/DER.0b013e318260d78d. [DOI] [PubMed] [Google Scholar]
- Dejobert Y, Martin P, Piette F, Thomas P, Bergoend H. Contact dermatitis from didecyldimethylammonium chloride and bis-(aminopropyl)-lauryl amine in a detergent-disinfectant used in hospital. Contact Dermatitis. 1997;37:95–96. doi: 10.1111/j.1600-0536.1997.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Dibo M, Brasch J. Occupational allergic contact dermatitis from N,N-bis(3-aminopropyl)dodecylamine and dimethyldidecylammonium chloride in 2 hospital staff. Contact Dermatitis. 2001;45:40–45. doi: 10.1034/j.1600-0536.2001.045001040.x. [DOI] [PubMed] [Google Scholar]
- GAO (General Accounting Office) Report to Congressional Requesters: CHEMICAL REGULATION options exist to improve EPA's ability to assess health risks and manage its chemical review program. Washington, DC: U.S.G.A. Office; 2005. GAO-05-458. [Google Scholar]
- Geier J, Lessmann H, Krautheim A, Fuchs T. Airborne allergic contact dermatitis caused by didecyldimethylammonium chloride in a geriatric nurse. Contact Dermatitis. 2013;68:123–125. doi: 10.1111/cod.12013. [DOI] [PubMed] [Google Scholar]
- Gerberick GF, Cruse LW, Ryan CA, Hulette BC, Chaney JG, Skinner RA, Dearman RJ, Kimber I. Use of a B-cell marker (B220) to discriminate between allergens and irritants in the local lymph node assay. Toxicol Sci. 2002;68:420–428. doi: 10.1093/toxsci/68.2.420. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Jegu J, Kopferschmitt MC, Donnay C, Hedelin G, Matzinger F, Velten M, Guilloux L, Cantineau A, de Blay F. Asthma among workers in healthcare settings: role of disinfection with quaternary ammonium compounds. Clin Exp Allergy. 2014;44:393–406. doi: 10.1111/cea.12215. [DOI] [PubMed] [Google Scholar]
- Henderson ND. Prepared for Environmental Protection Division, BC Environment. Victoria, British Columbia: Ministry of Environment, Lands and Parks; 1992. [cited 2015 Nov 2]. A review of the environmental impact and toxic effects of DDAC. Internet. Available from: http://infohousep2ricorg/ref/15/14383pdf. [Google Scholar]
- Houtappel M, Bruijnzeel-Koomen CA, Rockmann H. Immediate-type allergy by occupational exposure to didecyl dimethyl ammonium chloride. Contact Dermatitis. 2008;59:116–117. doi: 10.1111/j.1600-0536.2008.01336.x. [DOI] [PubMed] [Google Scholar]
- ICCVAM. Usefulness and limitations of the murine local lymph node assay for potency categorization of chemicals causing allergic contact dermatitis in humans. 2011 NIH Publication No 11-7709. [Google Scholar]
- Klink KJ, Meade BJ. Dermal exposure to 3-amino-5-mercapto-1,2,4-triazole (AMT) induces sensitization and airway hyper-reactivity in BALB/c mice. Toxicol Sci. 2003;75:89–98. doi: 10.1093/toxsci/kfg171. [DOI] [PubMed] [Google Scholar]
- Lim CH, Chung YH. Effects of didecyldimethylammonium chloride on sprague-dawley rats after two weeks of inhalation exposure. Toxicol Res. 2014;30:205–210. doi: 10.5487/TR.2014.30.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetz TS, Meade BJ. Development of a flow cytometry assay for the identification and differentiation of chemicals with the potential to elicit irritation, IgE-mediated, or T-cell-mediated hypersensitivity responses. Toxicol Sci. 1999;48:206–217. doi: 10.1093/toxsci/48.2.206. [DOI] [PubMed] [Google Scholar]
- McBain AJ, Ledder RG, Moore LE, Catrenich CE, Gilbert P. Effects of quaternary-ammonium-based formulations on bacterial community dynamics and antimicrobial susceptibility. Appl Environ Microbiol. 2004;70:3449–3456. doi: 10.1128/AEM.70.6.3449-3456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin VE, Potineni H, Hunt P, Griswold J, Siems B, Werre SR, Hrubec TC. Exposure to common quaternary ammonium disinfectants decreases fertility in mice. Reprod Toxicol. 2014;50:163–170. doi: 10.1016/j.reprotox.2014.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowitz M, Ponten A. Foot dermatitis caused by didecyldimethylammonium chloride in a shoe refresher spray. Contact Dermatitis. 2015;73:374–376. doi: 10.1111/cod.12456. [DOI] [PubMed] [Google Scholar]
- NIEHS. National Institute of Environmental Health Sciences; the Murine Local Lymph Node Assay: a test method for assessing the allergic contact dermatitis potential of chemicals/compounds. Fed Regist. 1999;64:14006–14007. [PubMed] [Google Scholar]
- Ohnuma A, Yoshida T, Horiuchi H, Fukumori J, Tomita M, Kojima S, Takahashi N, Fukuyama T, Hayashi K, Yamaguchi S, et al. Altered pulmonary defense system in lung injury induced by didecyldimethylammonium chloride in mice. Inhal Toxicol. 2011;23:476–485. doi: 10.3109/08958378.2011.584080. [DOI] [PubMed] [Google Scholar]
- Purohit A, Kopferschmitt-Kubler MC, Moreau C, Popin E, Blaumeiser M, Pauli G. Quaternary ammonium compounds and occupational asthma. Intl Arch Environ Health. 2000;73:423–427. doi: 10.1007/s004200000162. [DOI] [PubMed] [Google Scholar]
- Ruiz Oropeza A, Fischer Friis U, Duus Johansen J. Occupational contact urticaria caused by didecyl dimethyl ammonium chloride. Contact Dermatitis. 2011;64:297–298. doi: 10.1111/j.1600-0536.2011.01882.x. [DOI] [PubMed] [Google Scholar]
- Shaffer MP, Belsito DV. Allergic contact dermatitis from glutaraldehyde in healthcare workers. Contact Dermatitis. 2000;43:150–156. doi: 10.1034/j.1600-0536.2000.043003150.x. [DOI] [PubMed] [Google Scholar]
- Skaliy P, Thompson TA, Gorman GW, Morris GK, McEachern HV, Mackel DC. Laboratory studies of disinfectants against Legionella pneumophila. Appl Environ Microbiol. 1980;40:697–700. doi: 10.1128/aem.40.4.697-700.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suneja T, Belsito DV. Occupational dermatoses in health care workers evaluated for suspected allergic contact dermatitis. Contact Dermatitis. 2008;58:285–290. doi: 10.1111/j.1600-0536.2007.01315.x. [DOI] [PubMed] [Google Scholar]
- Walsh SE, Maillard JY, Russell AD, Catrenich CE, Charbonneau DL, Bartolo RG. Activity/mechanisms of action of selected biocidal agents on Gram-positive and -negative bacteria. J Appl Microbiol. 2003;94:240–247. doi: 10.1046/j.1365-2672.2003.01825.x. [DOI] [PubMed] [Google Scholar]
- Warshaw EM, Schram SE, Maibach HI, Belsito DV, Marks JG, Fowler JF, Rietschel RL, Taylor JS, Mathias CG, DeLeo VA, et al. Occupation-related contact dermatitis in North American health care workers referred for patch testing: cross-sectional data, 1998 to 2004. Dermatitis. 2008;19:261–274. [PubMed] [Google Scholar]
- Woolhiser MR, Munson AE, Meade BJ. Comparison of mouse strains using the local lymph node assay. Toxicology. 2000;146:221–227. doi: 10.1016/s0300-483x(00)00152-9. [DOI] [PubMed] [Google Scholar]
- Zhang C, Cui F, Zeng GM, Jiang M, Yang ZZ, Yu ZG, Zhu MY, Shen LQ. Quaternary ammonium compounds (QAC): A review on occurrence, fate and toxicity in the environment. Sci Total Environ. 2015;518:352–362. doi: 10.1016/j.scitotenv.2015.03.007. [DOI] [PubMed] [Google Scholar]


