Abstract
Background
Depression is a risk factor for morbidity and mortality in patients with coronary heart disease. Finding effective methods for identifying and treating depression in these patients is a high priority. The purpose of this study was to determine whether collaborative care (CC) for patients who screen positive for depression during an outpatient cardiology visit results in greater improvement in depression symptoms and better medical outcomes than seen in patients who screen positive for depression but receive only usual care (UC).
Methods
Two hundred-one patients seen in an outpatient cardiology clinic who screened positive for depression during an outpatient visit were randomized to receive either CC or UC. Recommendations for depression treatment and ongoing support and monitoring of depression symptoms were provided to CC patients and their primary care physicians (PCPs) for up to 6 months.
Results
There were no differences between the arms in mean Beck Depression Inventory-II scores(CC, 15.9; UC, 17.4; p=.45) or in depression remission rates(CC, 32.5%; UC, 26.2%; p=0.34) after 6 months, or in the number of hospitalizations after 12 months(p=0.73). There were fewer deaths among the CC (1/100) than UC patients (8/101)(p=0.03).
Conclusions
This trial did not show that CC produces better depression outcomes than UC. Screening led to a higher rate of depression treatment than was expected in the UC group, and delays in obtaining depression treatment from PCPs may have reduced treatment effectiveness for the CC patients. A different strategy for depression treatment following screening in outpatient cardiology services is needed.
Keywords: Outpatient cardiology, depression, collaborative care
Depression and cardiovascular diseases are highly comorbid, and depression is a significant risk factor for psychosocial and medical morbidity and mortality in patients with coronary heart disease (CHD) [1, 2]. A 2008 American Heart Association Science Advisory recommended that cardiologists routinely screen their patients for depression [3], and this statement was endorsed by the American Psychiatric Association. However, critics argued that this recommendation was premature due to insufficient evidence that depression screening improves either depression or cardiac outcomes in patients with CHD [4, 5].
Both critics and supporters have generally agreed that in order for depression screening to improve depression, procedures must be in place to ensure that clinically appropriate actions are initiated when patients screen positive [6]. This concern is supported by a recent study of patients who were screened for depression following cardiac surgery without an organized response to a positive screen. The study found significant depression in many of these patients six months after the initial screening [7]. Collaborative care (CC), in which treatment is managed by a primary care physician (PCP) in consultation with a psychiatrist, is one of the best studied and most cost-effective models for depression management following routine depression screening in primary care and in some medical specialty settings [8–10]. At least 3 randomized controlled trials of CC for depression in cardiac care settings have been conducted since the publication of the AHA depression screening statement.
Rollman and his colleagues completed a study of depression screening and CC in patients recovering from coronary artery bypass surgery [11]. They compared UC provided by PCPs to an 8-month, nurse-delivered, telephone-based CC intervention for patients who screened positive for depression. The intervention included initiation or adjustment of antidepressants, watchful waiting, or referral to a mental health professional. CC participants received a median of 10 contacts of unreported duration. The CC patients had greater improvements in quality of life and depression symptoms than did those who received only UC. Forty-one percent of the patients were receiving antidepressants at baseline, and roughly half were receiving antidepressants by the end of the trial. The authors did not report the proportion of patients in each group who received antidepressants, but did indicate that the proportion was higher in the CC arm than in the UC arm.
In two separate trials [12, 13], Huffman and colleagues enrolled patients who screened positive for depression and subsequently found to be clinically depressed during hospitalization for a cardiac event. In the first trial, patients were assigned to receive either UC or a CC intervention that included education about depression and its impact on heart disease, encouragement to plan pleasurable activities after discharge, and specific recommendations for treatment when appropriate (pharmacotherapy or referral for psychotherapy). The second trial (MOSIACS) enrolled patients with depression, an anxiety disorder, or both. Using a protocol otherwise similar to the one employed in the first trial, patients preferring psychotherapy over antidepressants were offered 50-minute sessions of cognitive behavior therapy (CBT) inhospital and by telephone following hospital discharge for a minimum of 6 sessions. Most of the patients in the CC arm for whom antidepressants were recommended (approximately 80% in both studies) were prescribed the drug by their PCP or other physicians involved in their care, before hospital discharge. In the first trial, a study nurse acted as the care manager for patients randomized to the CC arm, whereas a social worker performed this function in the second trial. In both trials, patients in the CC arm received an average of 3 follow-up telephone calls after hospital discharge. Patients who received the intervention had significantly greater improvement in depressive symptoms and mental health quality-of-life at 6 and 12 weeks in the first trial, and after 24 weeks in the second trial.
These findings are very encouraging. However, there have not been any studies of routine screening and CC for depression in outpatient cardiology settings. Inpatient and outpatient settings pose different challenges for implementation of collaborative depression care. The purpose of this study was to determine the effectiveness of CC vs. UC in reducing depression symptoms in an outpatient cardiology setting.
METHODS
Screening/Enrollment
Participants were recruited from the outpatient cardiology services at the Washington University Center for Advanced Medicine in St. Louis, Missouri and its suburban satellite facility. Both facilities are administered and staffed by the Division of Cardiovascular Medicine at the Washington University School of Medicine. Patients seen at these facilities represent a cross-section of residents of the City of St. Louis and the surrounding suburbs, as well as from rural areas and small towns in eastern Missouri and western Illinois.
In accordance with the AHA recommendation [3], routine screening for depression with the Patient Health Questionnaire (PHQ-9) [14] was instituted by the outpatient cardiology service just prior to the start-up of this study. Patients with CHD who were being seen for their first outpatient cardiology appointment were asked by the receptionist to complete the PHQ-9 upon arrival at the clinic. Patients who received ongoing care at the clinic were rescreened annually. The cardiologists were notified if their patient screened positive for depression (PHQ-9>10), and this information was also entered in the patient’s electronic medical record.
Clinic patients who had documented CHD (angiographic findings of >50% stenosis in one or more major coronary artery, or a history of either coronary revascularization or hospitalization for ACS), and who screened positive for depression (PHQ-9>10) between November, 2011 to January, 2015 were evaluated for study eligibility. Patients who were receiving an antidepressant at baseline were included if they had been taking a standard recommended dose of the prescribed agent for at least 4 weeks and met all other study criteria.
Patients were excluded from study participation for any of the following: 1) significant suicidal ideation or behavior; 2) significant cognitive impairment or inability to read or speak English; 3) schizophrenia, bipolar disorder, active substance abuse or alcoholism, or other severe Axis I comorbidities; 4) medical conditions including a recent (within the past 3 months) acute coronary syndome (ACS) or coronary artery bypass graft (CABG) surgery, severe valvular heart disease according to standard echocardiographic criteria, severe heart failure (NYHA class IV), malignancy, or physical limitations that would interfere with participation in the study protocol; 5) exemption by the patient’s cardiologist or primary care physician; 6) participation in a competing research protocol; or 7) refusal to participate or to sign an informed consent form.
With the permission of their cardiologist, eligible patients were contacted by telephone after their clinic visit by a research nurse to discuss study participation. Those who wished to participate were asked to provide written informed consent as approved by the Human Research Protection Office at Washington University School of Medicine conforming to the guidelines of the 1975 Declaration of Helsinki. An appointment for the patient to visit the Washington University Behavioral Medicine Center was scheduled to complete the baseline assessments. If an appointment could not be scheduled within one week, the informed consent and baseline questionnaires were mailed to the patient and the recruiting nurse again contacted the patient to review the consent form and answer questions. The first 201 patients meeting all study criteria who provided informed consent and completed baseline forms were randomly assigned to receive either UC or up to 6 months of a CC intervention in a 1:1 allocation ratio. Participants were randomized in permuted blocks, and randomization was stratified by antidepressant use at baseline. Treatment assignments were concealed in sequentially numbered, opaque envelopes (one set per stratum) and opened by the study coordinator after the baseline evaluation.
Treatment Groups
Collaborative Care (CC)
The CC intervention was designed to be both feasible and cost-effective for a nurse or social worker trained as a case manager (CM) to implement in cardiology outpatient practice settings. The CM was responsible for assessing the patient’s depression, treatment needs, and treatment preferences; meeting with a study psychiatrist (EHR) and psychologist (RMC) to establish a treatment plan; and encouraging the patient’s PCP (or cardiologist if the patient did not have a PCP) to prescribe an antidepressant or facilitate a referral for other appropriate treatments. A nurse assumed the role of CM for half of the CC participants, and a social worker functioned in that role for the other half.
A clinical interview was conducted by the CM to determine whether a provisional diagnosis of major depressive disorder could be made. The CM assessed depression treatment history, current medications, comorbid medical illnesses, and the patient’s treatment preferences, and these data were provided to the consulting psychiatrist and psychologist for review. Potential treatment recommendations included specific antidepressants or psychotherapy. For patients with mild depression, the options included exercise (if medically appropriate), support groups, pleasant activity scheduling, or watchful waiting with ongoing support and monitoring from the CM for patients who declined treatment. The patient was asked to rank order his/her treatment preferences during the initial contact with the CM. Selective serotonin reuptake inhibitors (SSRIs) such as sertraline or citalopram, which have generally been found to be safe in cardiac patients, were usually recommended unless contraindicated by treatment history, potential drug-drug interactions, or possible adverse effects on medical comorbidities. If the patient agreed with the recommendation and gave permission, his or her physician was given these recommendations. If antidepressants were chosen, the PCP was asked to write the prescription and encouraged to contact the CM should any questions or concerns arise. If psychotherapy was recommended, the CM worked with the patient and PCP to identify potential providers, taking into account location and insurance coverage. Because exercise training is contraindicated for some patients, it was recommended only if approved by the patient’s cardiologist and PCP. In these cases, the patient was referred to a cardiac rehabilitation facility, a community facility (e.g. health club or community center), or a home-based exercise program. Support groups such as those provided by cardiac rehabilitation facilities were also considered for those patients who were mildly depressed.
If a patient had been recently seen by a mental health professional, he or she was encouraged to contact this provider for re-evaluation. If the patient was already taking an antidepressant, the CM contacted the prescriber (usually the PCP), informed the prescriber that the patient was still depressed, and recommended a dosage adjustment, a switch to a different antidepressant, or another treatment option.
The participants were followed for up to 6 months. Initially, they received brief telephone calls as needed until the treatment was implemented and progress was made, as indicated by improvement in depression symptoms on the PHQ-9 administered at each contact. Efforts were made during this time to help the patient overcome any obstacles to implementing and following the recommended treatment regimen, including identifying cardiac rehabilitation programs, contacting social services for financial or transportation assistance, and recommending techniques to improve adherence to the depression treatment regimen. If the patient was doing well, he or she was encouraged to continue the intervention as directed, and the telephone contacts decreased to one or fewer each month until the end of 6 months. If the patient’s depression symptoms were not improving or were worsening, the CM informed the study psychiatrist or psychologist and a new or modified treatment plan was developed, including referrals as needed to mental health professionals.
A treatment form was completed for each patient during the first contact, and updated during the course of the intervention. It included information concerning personal and family depression history, psychiatric comorbidity, depression treatment history, and the duration and course of the present episode. The PHQ-9 forms that were administered during the intervention were also maintained in this file.
Usual Care (UC)
Because the PHQ-9 was routinely administered to all patients as part of their clinical care, the cardiologists were informed about the results regardless of whether their patient was participating in this study. In addition, the participants who were randomized to the UC arm were encouraged to discuss their depression symptoms with their cardiologist and PCP. Apart from follow-up assessments, the patients in this arm received no further contact from study personnel.
Baseline and Follow-up Assessments
The interviews, questionnaires, and medical chart reviews were performed by research staff who were blinded to the group status of the participants. Participants were paid $25.00 for completing each follow-up assessment.
Medical Assessments
Cardiovascular and Medical History
The participants’ medical records were reviewed to document history of ACS or other cardiac events, revascularizations, and comorbidities, as well as current medications and dosages. CHD risk factors including history of smoking, diabetes, hypertension, and hyperlipidemia were also recorded.
Depression and Depression Treatment Assessments
Beck Depression Inventory-II (BDI-II)
[15] The BDI-II, widely used as an index of depression severity and as an outcome measure in clinical trials, was the primary outcome measure of depression in this study. It was administered at baseline and by telephone 3, 6, and 12 months after enrollment. Depression remission was defined as a BDI-II score ≤9.
Longitudinal Course Chart: Depression Interview and Structured Hamilton (DISH)
[16] The Longitudinal Course section of the DISH interview was administered at the 3, 6, and 12-month follow-ups to determine the course of depression, including remission, relapses, and recurrences since the last assessment. The number of weeks spent depressed since the last assessment was estimated from these data.
Depression Treatment
The participant’s depression treatment was documented by interview at the 3, 6, and 12-month follow-ups. Patients were asked whether they had been prescribed an antidepressant, referred for a psychiatric evaluation, received individual or group psychotherapy, participated in a support group or an exercise program, or received other forms of depression treatment since the previous assessment.
Active treatment with an antidepressant medication was defined as receiving at least the minimum therapeutic dose for that antidepressant as recommended by current guidelines for a minimum of four weeks. Psychotherapy was defined as treatment by a licensed mental health professional with an evidenced-based psychotherapy for depression for a minimum of 6 sessions. Exercise was defined as regular participation in aerobic exercise program at least 3 times per week for at least 20 minutes per session [16].
Quality-of-life, Anxiety, and Physical Functioning
Patient Reported Outcomes Measurement Information System (PROMIS)
[17] The short forms of the PROMIS Anxiety, Physical Function, and Global Mental and Physical Quality-of-life scales were administered to assess anxiety, physical functioning, and mental and physical quality-of-life, respectively, at baseline, and at the 3, 6, and 12 month follow-ups.
Patient Satisfaction
Patients’ satisfaction with their depression treatment was assessed at the six month follow-up using a five-point Likert rating scale ranging from 1 (very dissatisfied) to 5 (very satisfied).
Hospitalizations and All-Cause Mortality
Cardiovascular and other hospitalizations and all-cause mortality were systematically documented at each follow-up. The electronic medical record system was monitored to document hospitalizations at facilities affiliated with the Washington University School of Medicine. Follow-up telephone interviews were used to identify hospitalizations at other facilities. Medical records were obtained to document hospitalizations and deaths at these facilities.
Statistical Analyses
The primary hypothesis was that patients who receive CC will have lower BDI-II scores 6 months after screening than patients who receive UC. Multiple imputation was used for data that were plausibly missing at random, consistent with the intention-to-treat analysis plan [18]. Separate imputers’ models were developed for each outcome; each one included the variables that were to be used in that analysis as well as variables that correlated with the presence or absence of the outcome data. Parameter estimates were aggregated over 100 imputed datasets for statistical inference [18].
Linear mixed regression models [19] were used to determine mean outcome scores within and between treatment groups over time. Each model included antidepressant use (the stratification factor), group, time, and the group by time interaction as fixed factors, and baseline intercept and patient as random factors. Because the assessment schedule was irregular, intra-subject variation was modeled with a spherical covariance structure which provided the lowest Bayesian information criterion. Pre-planned moderator analyses were conducted to determine whether the effects of treatment on the primary outcome depended on sex, race, or antidepressant use. A between-group difference of 3 points on the BDI-II scale was identified a priori as the smallest clinically meaningful difference for the primary outcome [20]. The target sample size (N=200) was adequate to detect a >3 point between-group difference with 80% power at α = 0.05. Between-group differences in imputed remission rates (BDI-II≤9) were tested at 3 and 6 months by comparing the difference in binomial proportions.
Chi-square tests and ANOVAs were used to compare the baseline demographic, medical, and depression characteristics of the UC and CC participants, treatments received during the intervention phase, and secondary outcomes at follow-up. All tests were two-tailed with a Type I error rate of 0.05. SAS 9.3 software (SAS Institute, Raleigh NC) was used to perform all statistical analyses.
RESULTS
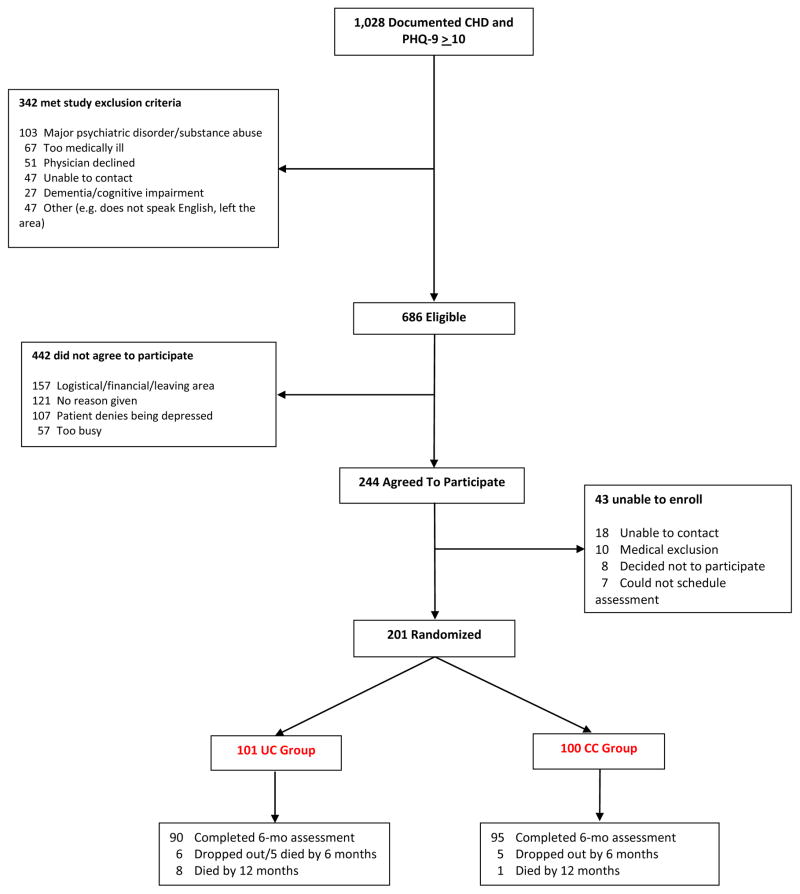
Two hundred-one patients who screened positive for depression and met all other study criteria were recruited, stratified by whether they were receiving an antidepressant at enrollment, and randomized to receive either UC (n=101) or CC (n=100). One hundred eighty-five (92%) of the participants completed the 6-month assessment (Figure 1). Table 1 presents the participants’ baseline demographic and medical characteristics. More patients assigned to the UC arm had a history of revascularization or myocardial infarction than those in the CC arm. There were no other differences between groups at baseline. Forty-eight percent of the participants in both arms were on an antidepressant at enrollment, and 80 (79.2%) of the UC and 83 (83%) of the CC participants reported prior episodes of major depression.
Figure 1.
Trial Flow Chart
Table 1.
Baseline demographic, medical, and depression characteristics by treatment arm* (N = 201)
| Baseline Characteristic | Usual Care (N=101) | Collaborative Care (N=100) | P |
|---|---|---|---|
| Demographics | |||
| Age (in years) | 63.1 SD=10.0 | 63.0 SD= 9.5 | 0.91 |
| Gender (female) | 37 (36.6) | 47 (47.0) | 0.14 |
| Race (Caucasian) | 71 (70.3) | 76 (76.0) | 0.36 |
| Education (12+ years) | 88 (87.1) | 89 (89.0) | 0.68 |
| Medical | |||
| Current Smoker | 20 (19.8) | 28 (28.0) | 0.17 |
| History of MI | 72 (71.3) | 49 (49.0) | 0.001 |
| Diabetes | 45 (44.6) | 42 (42.0) | 0.71 |
| Hypertension | 90 (89.1) | 87 (87.0) | 0.64 |
| History of Revascularization | 84 (83.2) | 67 (67.0) | 0.008 |
| Canadian Heart Class | 0.96 | ||
| Asymptomatic | 44 (62.0) | 37 (60.6) | |
| Class 1, 2, or 3 | 11 (15.5) | 9 (14.8) | |
| Class 4 | 16 (22.5) | 15 (24.6) | |
| Medications | |||
| Statin | 88 (87.1) | 86 (86.0) | 0.81 |
| Beta blocker | 82 (81.1) | 76 (76.0) | 0.37 |
| Aspirin | 93 (92.1) | 87 (87.0) | 0.24 |
| Calcium channel blocker | 31 (30.7) | 25 (25.0) | 0.37 |
| ACE Inhibitor | 60 (59.4) | 61 (61.0) | 0.82 |
| Diuretic | 58 (57.4) | 49 (49.0) | 0.23 |
| Anxiolytic | 17 (16.8) | 19 (19.0) | 0.69 |
| Depression History | |||
| Antidepressant at baseline | 48 (47.5) | 48 (48.0) | 0.95 |
| Prior episodes of major depression | 80 (79.2) | 83 (83.0) | 0.49 |
Continuous variables are reported as (mean, SD). Categorical variables represent number of patients (%).
Primary and Secondary Outcomes
The estimated mean BDI-II scores at baseline, 3, 6, and 12 months are presented in Table 2. There were no difference in BDI-II scores between groups over the course of follow-up (G × T interaction: F3,363=0.89;p=.45). There was significant improvement in depression during the six months of treatment in both the UC [mean = 7.5, SD=10.0, 95%CI: 5.5, 9.5; t181 = 7.55; p < .0001] and CC [mean = 9.2, SD=9.9, 95%CI: 7.2, 11.1; t187 = 9.31; p < .0001] groups, but no difference in BDI-II scores between the two arms at the primary outcome assessment at six months [mean difference= −1.6, CI:−4.0, 0.9; t185 = −1.26; p=.21].
Table 2.
Estimated mixed model means (SD) for BDI-II and secondary outcome measures at baseline, and 3, 6, and 12 month follow ups by treatment arm.
| Outcome Measure | Usual Care (N = 101) | Collaborative Care (N = 100) | Between-group Difference (95% CI) | P | Cohen’s D (95% CI) |
|---|---|---|---|---|---|
| Beck Depression-II Score (BDI-2) | |||||
| Baseline | 25.0 SD= 8.5 | 25.1 SD= 8.5 | 0.1 (−2.3, 2.5) | 0.93 | 0.01 (−0.26, 0.29) |
| 3 months | 19.1 SD= 8.9 | 17.5 SD= 8.8 | −1.5 (−4.0, 0.9) | 0.22 | 0.17 (−0.10, 0.45) |
| 6 months | 17.4 SD= 9.0 | 15.9 SD= 8.8 | −1.6 (−4.0, 0.9) | 0.21 | 0.18 (−0.10, 0.46) |
| 12 months | 16.1 SD= 9.5 | 14.7 SD= 8.9 | −1.5 (−4.0, 1.1) | 0.26 | 0.16 (−0.12, 0.44) |
| Anxiety-Distress (PROMIS) | |||||
| Baseline | 60.1 SD= 7.3 | 60.1 SD= 7.3 | 0.03 (−2.0, 2.1) | 0.97 | 0.01 (−0.27, 0.28) |
| 3 months | 56.3 SD= 7.6 | 55.5 SD= 7.6 | −0.7 (−2.8, 1.4) | 0.49 | 0.10 (−0.18, 0.38) |
| 6 months | 55.5 SD= 7.8 | 53.9 SD= 7.6 | −1.6 (−3.7, 0.5) | 0.14 | 0.21 (−0.07, 0.49) |
| 12 months | 56.0 SD= 8.2 | 53.1 SD= 7.9 | −2.8 (−5.1,−0.6) | 0.01 | 0.35 (0.08, 0.64) |
| Physical Functioning (PROMIS) | |||||
| Baseline | 38.4 SD= 3.9 | 38.4 SD= 3.9 | 0.04 (−1.0, 1.1) | 0.94 | 0.01 (−0.27, 0.29) |
| 3 months | 38.9 SD= 4.1 | 39.6 SD= 4.1 | 0.7 (−0.4, 1.9) | 0.20 | 0.18 (−0.08, 0.47) |
| 6 months | 39.2 SD= 4.2 | 39.8 SD= 4.1 | 0.7 (−0.5, 1.8) | 0.25 | 0.16 (−0.10, 0.45) |
| 12 months | 39.6 SD= 4.9 | 39.2 SD= 4.5 | −0.4 (−1.8, 0.9) | 0.53 | 0.09 (−0.18, 0.37) |
| Quality-of-life (PROMIS) | |||||
| Mental QOL Score | |||||
| Baseline | 37.1 SD= 6.9 | 37.2 SD= 6.9 | 0.13 (−1.8, 2.0) | 0.90 | 0.02 (−0.26, 0.30) |
| 3 months | 40.5 SD= 7.2 | 41.0 SD= 7.2 | 0.5 (−1.5, 2.5) | 0.65 | 0.06 (−0.21, 0.34) |
| 6 months | 41.5 SD= 7.3 | 43.0 SD= 7.3 | 1.5 (−0.6, 3.5) | 0.16 | 0.20 (−0.07, 0.48) |
| 12 months | 43.0 SD= 7.8 | 43.1 SD= 7.6 | 0.1 (−2.1, 2.3) | 0.94 | 0.01 (−0.27, 0.29) |
| Physical QOL Score | |||||
| Baseline | 35.7 SD= 5.7 | 35.7 SD= 5.7 | −0.01 (−1.6, 1.6) | 0.99 | 0.01 (−0.28, 0.28) |
| 3 months | 37.5 SD= 6.0 | 39.1 SD= 6.0 | 1.6 (−0.1, 3.3) | 0.06 | 0.27 (−0.01, 0.55) |
| 6 months | 38.1 SD= 6.1 | 39.2 SD= 6.1 | 1.1 (−0.6, 2.8) | 0.20 | 0.18 (−0.09, 0.47) |
| 12 months | 38.9 SD= 6.5 | 39.5 SD= 6.2 | 0.7 (−1.1, 2.4) | 0.44 | 0.11 (−0.17, 0.39) |
There was no difference in the remission rate between arms at the 3-month (20.7% UC vs. 25.5% CC) (difference in proportion 95%CI:−7.2%, 17.3%; p=0.42) or 6-month follow-up (26.2% UC vs. 32.5% CC; 95%CI:−6.7%, 19.5%; p=0.34), or in the mean number of weeks the UC (7.7, SD=8.3; 95%CI: 6.0, 9.4) or CC participants (9.1, SD=8.7; 95%CI: 7.4, 10.9)(p=0.24) reported being depressed during the 6 months following randomization. None of the moderator tests were statistically significant at 6 months, indicating that the relationship between depression outcome and treatment group did not differ by sex [F1,175 = 1.54; p=.22; partial eta2 = 0.3%, 95%CI:0.0%, 5.4%], race [F1,175 = 0.24; p=.63; partial eta2 = 0.1%, 95%CI:0.0%, 3.1%] or antidepressants at enrollment [F1,176 = 0.57; p=.45; partial eta2 = 0.3%, 95%CI:0.0%, 3.9%].
The estimated mean scores for the secondary outcomes at baseline, 3, 6 and 12 months are also presented in Table 2. The CC patients had a lower mean anxiety score than the UC patients at the 12 month follow-up. There were no other differences between the groups on any outcome assessment at the 3, 6, and 12 month follow-up.
Collaborative Care
Twenty-seven (27%) of the CC participants with relatively mild depression who declined treatment with either antidepressants or psychotherapy were initially advised to participate in support groups, exercise, pleasant activity scheduling, or watchful waiting. In addition, they received weekly calls from the CM for the first 8 weeks to offer support and determine whether their depression continued to improve. The remaining 73 participants in the CC arm were advised to obtain antidepressant treatment or psychotherapy based on patient preference, feasibility, and recommendations by the study psychiatrist and psychologist. Watchful waiting was initially recommended for 5 patients with mild depression who were later judged to require treatment. Thus, antidepressants or psychotherapy were recommended for a total of 78 CC patients during the trial. Six of these patients did not implement the initial recommendation but accepted a second recommendation. A median of 10 days elapsed between the time at which antidepressants were recommended to the PCP and when the patient began taking them. The CC participants received a mean of 13.4 (SD=4.3) contacts from the CM for an average of 13.0 (SD=3.8) minutes for each call, including the time required to administer the PHQ-9. Nearly one third of the CC participants required adjustments, changes, or additional depression treatments during the 6 months.
Depression Treatments
Table 3 reports the proportion of patients in both groups who were receiving depression interventions at 3 and 6 months following enrollment. Patients who were enrolled on an antidepressant and subsequently prescribed a higher dose or a new antidepressant are included with patients who were not initially on an antidepressant but were prescribed one following enrollment. Participants in the CC arm were more likely to receive a new or dose-adjusted antidepressant or to begin psychotherapy in the first 3 months following enrollment than the participants in the UC group. However, the proportions of patients receiving any treatment for depression during the 6 months did not differ between arms. Patients in the UC arm rated their satisfaction as 3.3 (SD=1.3, 95% CI: 2.9, 3.8) on a 1 (very dissatisfied) to 5 (very satisfied) scale at 6 months, compared to 4.0 (SD=1.1, 95% CI: 3.7, 4.2) for the CC patients (p=0.01).
Table 3.
Depression Treatment during 6 months post-screening by treatment group
| Depression Treatment Received* | Usual Care** | Collaborative Care** | P |
|---|---|---|---|
| New or dose adjusted antidepressant*** | |||
| Baseline to 3 months | 14 (14.6%) | 33 (34.7%) | 0.001 |
| Baseline to 6 months | 24 (24.7%) | 36 (37.9%) | 0.049 |
| All patients receiving antidepressant | |||
| Baseline to 3 months | 56 (56%) | 66 (66.0%) | 0.08 |
| Baseline to 6 months | 59 (59%) | 66 (66.0%) | 0.19 |
| Psychotherapy | |||
| Baseline to 3 months | 11 (11.7%) | 23 (24.0%) | 0.028 |
| Baseline to 6 months | 14 (14.7%) | 25 (25.8%) | 0.057 |
| Support group | |||
| Baseline to 3 months | 8 (8.5%) | 5 (5.2%) | 0.37 |
| Baseline to 6 months | 10 (10.5%) | 11 (11.3%) | 0.86 |
| Exercise program | |||
| Baseline to 3 months | 9 (9.6%) | 7 (7.3%) | 0.57 |
| Baseline to 6 months | 10 (10.5%) | 8 (8.3%) | 0.59 |
| Depression treatment**** | |||
| Baseline to 6 months | 65 (66.3%) | 75 (76.5%) | 0.11 |
Some participants received more than one treatment during same time interval
Sample size for each analysis varies slightly due to missing data.
Newly prescribed, a change in dose or in antidepressant.
Includes treatments begun before and following enrollment.
Medical Outcomes
Thirty-one percent of the UC patients were hospitalized for any cause by the 12-month assessment, compared to 33% of the CC patients (p=0.73). Eight of the patients in the UC and one in the CC arm died during the 12-month follow-up (p=0.03).
DISCUSSION
The results of this study suggest that patients who screen positive for depression during an outpatient cardiology visit may not benefit more from a CC intervention than from usual care in a setting in which patients are routinely screened for depression. BDI-II scores significantly improved for both groups and the groups did not differ at any follow-up. There were no differences between the CC and UC arms in anxiety, mental or physical quality-of-life, or in the number of medical hospitalizations at 3 or 6 months after randomization. Patients in the CC arm did report significantly greater satisfaction with their depression treatment than did those in the UC arm. Fewer patients in the CC arm died during the 12 months following enrollment, although the number of deaths was small and more UC than CC patients had a history of MI and of revascularization.
By the end of the 6 month trial, 76.5% of the CC and 66.3% of the UC participants had received some form of depression treatment, with the majority in both groups receiving antidepressants. Although the patients in the CC arm were somewhat more likely (59%) than those in the UC arm (46%) (p=0.06) to receive new depression treatment, or a change in dosage or agent for patients already receiving an antidepressant during the course of the 6 months, the difference was smaller than expected.
Thus, depression treatment was often instituted or modified for patients in the UC group even though they and their cardiologists received nothing more than a notification about the patient’s depression screening results. The net result was that only 10% more of the patients in the CC arm received depression treatment during the 6 months than the patients in the UC arm, with a 7% greater improvement in BDI-II scores for the CC than the UC participants. The small difference between arms in the proportion of patients receiving depression treatment suggests that a positive depression screen alone may trigger the initiation or modification of depression treatment in a substantial number of cases. However, the trial did not include a comparison group of patients who were not screened for depression, so the effect of screening per se cannot be determined.
Despite our best efforts to help to implement appropriate treatments in the CC arm, after six months the mean BDI-II score for CC participants was 15.9 (SD=8.8), vs. 17.4 (SD=9.0) for the CC arm, indicating mild-to-moderate depression symptoms for both groups. Thus, although both groups showed improvement in BDI-II scores, both still scored in the “depressed” range on this measure.
Three earlier randomized controlled trials of CC following positive depression screening in cardiac care settings were summarized in the Introduction. In contrast to the present study, all reported greater improvement in patients who received the CC intervention than in those receiving only UC. Some important differences between them and the present study should be noted.
The patients in the earlier trials were identified, screened, and contacted by study personnel while still in the hospital following an acute cardiac event [12, 13] or CABG surgery [11]. Patients in the present study were medically stable, being seen for ongoing routine care, and were contacted at home by letter and/or telephone several days after an outpatient cardiology visit. Half of the enrolled patients were unable or unwilling to come to the medical center for the baseline assessment. These patients were enrolled by telephone and were never seen in person by the study staff. Transportation barriers, inflexible work schedules, and family responsibilities accounted for most of these cases.
Treatment was not initiated in the study by Rollman and colleagues until a week or more after hospital discharge, but treatment began in hospital in both studies by Huffman and colleagues. In the second study, 70% of patients were receiving “adequate” treatment for depression or anxiety before leaving the hospital, compared to 7% in the UC group. In the present study, due to difficulty in contacting eligible patients and their PCPs, and in scheduling baseline visits or telephone assessments, a median of 3 weeks elapsed before the patient was seen for baseline evaluation and randomization, a median of ten days after the PCP was contacted before the patient actually began receiving antidepressants, and even longer for those who chose psychotherapy. The high proportion of patients who were taking an antidepressant (48%) when they were enrolled in this study may have contributed to the relatively high BDI-2 scores at 6 months, since poor response to an initial antidepressant predicts a lower likelihood of response to subsequent treatments [21, 22]. However, a similar proportion of patients enrolled in the 3 earlier trials were also receiving antidepressants at baseline (40–44%). It has been shown that patients with CHD who do not respond to depression treatment are at high risk for cardiac mortality and morbidity [23]. Thus, it is important to identify these patients and to modify their treatment regimens.
As with the 3 earlier trials, the present study relied primarily on PCPs to prescribe the recommended antidepressants, and to approve and support other depression treatments as needed. PCPs provide a large proportion of depression treatment services in the United States and in many other Western countries, and may be better suited to manage routine cases of depression than cardiologists. However, the difficulty of providing prompt depression treatment for patients seen in outpatient cardiology services through collaboration with PCPs working in different locations and even hospital affiliations suggests that a different intervention may be needed.
One alternative to having PCPs prescribe and monitor recommended antidepressants would be to assign a dedicated nurse practitioner to the cardiology outpatient service to evaluate patients when they screen positive for depression. The nurse could then provide a prescription for an antidepressant or make a referral to a psychiatrist for further evaluation if needed, similar to the protocols used in successful post-ACS depression treatment trials [24, 25]. Perhaps screening for other cardiac risk factors including smoking and sedentary lifestyle, and offering appropriate interventions [26, 27] for those with these cardiac risk factors, could also be performed in this context. Smoking [28, 29] and a sedentary life style [30] are more prevalent among depressed than nondepressed patients, so there could be considerable overlap in these cases.
Conclusions
The study failed to find a difference in depression outcome between a CC intervention and usual care for patients who screen positive for depression during a routine outpatient cardiology visit. As there was little difference between arms in the proportion of participants who receive depression treatment, and both arms showed significant improvement in depression by six months, depression screening alone may result in the initiation or modification of depression treatment for many patients who screen positive for depression. This possibility deserves further study. However, even in outpatient primary care settings with PCPs working in the same facilities, effect sizes for CC trials [31] are often are at the lower end of what is reported in non-selected antidepressant and psychotherapy trials [4]. In outpatient cardiology practices, however, providing prompt depression treatment through patients’ PCPs who are spread out over a large geographic area may present an even bigger challenge. Alternatives to relying solely on PCPs to implement depression treatments should be considered.
Acknowledgments
Source of Funding: Supported by Grant Number RO1HS018335, Agency for Healthcare Research and Quality (AHRQ), Bethesda, Maryland.
Abbreviations
- BDI-II
Beck Depression Inventory-II
- CM
Case manager
- CBT
Cognitive behavior therapy
- CC
Collaborative care
- DISH
Depression Interview and Structured Hamilton
- PHQ-9
Patient Health Questionnaire
- PCP
Primary care physician
- UC
Usual care
Footnotes
This author takes responsibility for all aspects of the reliability and freedom of bias of the data presented and their discussed interpretation.3
Author’s Disclosure
Contributors:
Robert M. Carney, PhD Principal Investigator: participated in all phases of the study design and implementation, the analysis and interpretation of the data, and in drafting the manuscript.
Kenneth E. Freedland, PhD Co-investigator: participated in all phases of study design and implementation, in the analysis and interpretation of the data, and in drafting and revising the manuscript.
Brian C. Steinmeyer, MS Statistician/co-investigator: participated in study design, the analysis and interpretation of the data, and in drafting and revising the manuscript.
Eugene H. Rubin, MD, PhD Psychiatrist/co-investigator: participated in the study design and implementation of the intervention, the interpretation of the data, and in revising the manuscript.
Gregory Ewald, MD: Cardiologist/co-investigator, participated in all phases of study design and implementation, in the analysis and interpretation of the data, and in the revising the manuscript.
All of the authors had access to data from the study, made a substantial contribution to the study, and participated in the writing of the manuscript. All of the authors approve submission of the manuscript, and agree to convey all copyright ownership if the manuscript is accepted for publication.
All authors contributed to both the research study and to the preparation of the manuscript.
Conflicts of Interest: Dr. Carney or a member of his family owns stock in Pfizer, Inc. The other authors report no relevant conflicts of interest.
Clinical Trial Registration: https://clinicaltrials.gov.NCT01552889
Role of Funding Source: This research study was supported by Grant Number RO1HS018335, Agency for Healthcare Research and Quality (AHRQ), Bethesda, Maryland. The funding agency was not directly involved in the study design, the collection, analysis, and interpretation of data, or the writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129:1350–69. doi: 10.1161/CIR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 2.Meijer A, Conradi HJ, Bos EH, Thombs BD, van Melle JP, de Jonge P. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33:203–16. doi: 10.1016/j.genhosppsych.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lesperance F, et al. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–75. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 4.Thombs BD, de Jonge P, Coyne JC, Whooley MA, Frasure-Smith N, Mitchell AJ, et al. Depression screening and patient outcomes in cardiovascular care. JAMA. 2008;300:2161–71. doi: 10.1001/jama.2008.667. [DOI] [PubMed] [Google Scholar]
- 5.Ziegelstein RC, Thombs BD, Coyne JC, de Jonge P. Routine screening for depression in patients with coronary heart disease never mind. J Am Coll Cardiol. 2009;54:886–90. doi: 10.1016/j.jacc.2009.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pignone MP, Gaynes BN, Rushton JL, Burchell CM, Orleans CT, Mulrow CD, et al. Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136:765–76. doi: 10.7326/0003-4819-136-10-200205210-00013. [DOI] [PubMed] [Google Scholar]
- 7.Tully PJ, Baumeister H, Bennetts JS, Rice GD, Baker RA. Depression screening after cardiac surgery: A six month longitudinal follow up for cardiac events, hospital readmissions, quality of life and mental health. Int J Cardiol. 2016;206:44–50. doi: 10.1016/j.ijcard.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Katon W, Robinson P, Von Korff M, Lin E, Bush T, Ludman E, et al. A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry. 1996;53:924–32. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- 9.Katon W, Russo J, Von Korff M, Lin E, Simon G, Bush T, et al. Long-term effects of a collaborative care intervention in persistently depressed primary care patients. J Gen Intern Med. 2002;17:741–8. doi: 10.1046/j.1525-1497.2002.11051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unutzer J, Katon W, Callahan CM, Williams JW, Jr, Hunkeler E, Harpole L, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 11.Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Houck PR, Counihan PJ, et al. Telephone-delivered collaborative care for treating post-CABG depression: a randomized controlled trial. JAMA. 2009;302:2095–103. doi: 10.1001/jama.2009.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huffman JC, Mastromauro CA, Sowden G, Fricchione GL, Healy BC, Januzzi JL. Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes. 2011;4:198–205. doi: 10.1161/CIRCOUTCOMES.110.959379. [DOI] [PubMed] [Google Scholar]
- 13.Huffman JC, Mastromauro CA, Beach SR, Celano CM, DuBois CM, Healy BC, et al. Collaborative care for depression and anxiety disorders in patients with recent cardiac events: the Management of Sadness and Anxiety in Cardiology (MOSAIC) randomized clinical trial. JAMA Intern Med. 2014;174:927–35. doi: 10.1001/jamainternmed.2014.739. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 15.Beck AT, Steer RA, Brown GK. BDI-II Manual. 2. San Antonio: Harcourt Brace & Company; 1996. [Google Scholar]
- 16.Freedland KE, Skala JA, Carney RM, Raczynski JM, Taylor CB, Mendes de Leon CF, et al. The Depression Interview and Structured Hamilton (DISH): rationale, development, characteristics, and clinical validity. Psychosom Med. 2002;64:897–905. doi: 10.1097/01.psy.0000028826.64279.29. [DOI] [PubMed] [Google Scholar]
- 17.Rose M, Bjorner JB, Becker J, Fries JF, Ware JE. Evaluation of a preliminary physical function item bank supported the expected advantages of the Patient-Reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2008;61:17–33. doi: 10.1016/j.jclinepi.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–76. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- 19.Littell RC. SAS system for mixed models. Cary, N.C: SAS Institute Inc; 1996. [Google Scholar]
- 20.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 21.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 22.Bennabi D, Aouizerate B, El-Hage W, Doumy O, Moliere F, Courtet P, et al. Risk factors for treatment resistance in unipolar depression: a systematic review. J Affect Disord. 2015;171:137–41. doi: 10.1016/j.jad.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Carney RM, Freedland KE. Treatment-resistant depression and mortality after acute coronary syndrome. Am J Psychiatry. 2009;166:410–7. doi: 10.1176/appi.ajp.2008.08081239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson KW, Rieckmann N, Clemow L, Schwartz JE, Shimbo D, Medina V, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med. 2010;170:600–8. doi: 10.1001/archinternmed.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson KW, Bigger JT, Burg MM, Carney RM, Chaplin WF, Czajkowski S, et al. Centralized, stepped, patient preference-based treatment for patients with post-acute coronary syndrome depression: CODIACS vanguard randomized controlled trial. JAMA Intern Med. 2013;173:997–1004. doi: 10.1001/jamainternmed.2013.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banham L, Gilbody S. Smoking cessation in severe mental illness: what works? Addiction. 2010;105:1176–89. doi: 10.1111/j.1360-0443.2010.02946.x. [DOI] [PubMed] [Google Scholar]
- 27.Berra K, Rippe J, Manson JE. Making physical activity counseling a priority in clinical practice: The time for action is now. JAMA On-line. 2015:E1–E2. doi: 10.1001/jama.2015.16244. [DOI] [PubMed] [Google Scholar]
- 28.Pratt LA, Brody DJ. Depression and smoking in the U.S. household population aged 20 and over, 2005–2008. NCHS Data Brief. 2010:1–8. [PubMed] [Google Scholar]
- 29.Thorndike AN, Regan S, McKool K, Pasternak RC, Swartz S, Torres-Finnerty N, et al. Depressive symptoms and smoking cessation after hospitalization for cardiovascular disease. Arch Intern Med. 2008;168:186–91. doi: 10.1001/archinternmed.2007.60. [DOI] [PubMed] [Google Scholar]
- 30.Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. 2009;31:306–15. doi: 10.1016/j.genhosppsych.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Gilbody S, Sheldon T, House A. Screening and case-finding instruments for depression: a meta-analysis. CMAJ. 2008;178:997–1003. doi: 10.1503/cmaj.070281. [DOI] [PMC free article] [PubMed] [Google Scholar]