Abstract
Introduction
A shorter delay time from chronic pain diagnosis to spinal cord stimulation (SCS) implantation may make it more likely to achieve lasting therapeutic efficacy with SCS. The objective of this analysis was to determine the impact of pain-to-SCS time on patients’ post-implant healthcare resource utilization.
Methods
A retrospective observational study was performed using a real-world patient cohort derived from MarketScan® Commercial and Medicare Supplemental claims databases from April 2008 through March 2013. The predictor variable was the time from the first diagnosis of chronic pain to permanent SCS implant. Using multivariable analysis, we studied the impact of pain-to-SCS time on healthcare resource utilization in the first year post-implant. For some regression tests, patients were grouped into terciles by healthcare resource utilization.
Results
A total of 762 patients met inclusion criteria, with a median pain-to-SCS time of 1.35 years (Q1: 0.8, Q3: 1.9). For every one-year increase in pain-to-SCS time, the odds increased by 33% for being in the high medical expenditures group (defined using the upper tercile: $4,133 over above) over the low group (first lower: $603 or less). The odds increased by 39% for being in the high opioid prescriptions group (10-58 prescriptions) over the low group (0-1). The odds increased by 44% and 55%, respectively, for being in the high office visits (8-77) or hospitalizations (3-28) group over the low office visits (0-2) or hospitalizations (0) group.
Conclusions
Healthcare resource utilization increased in the year following SCS implantation with longer pain-to-SCS time. These results suggest that considering SCS earlier in the care continuum for chronic pain may improve patient outcomes, with reductions in hospitalizations, clinic visits, and opioid usage.
Introduction
Chronic pain is a growing burden on the U.S. healthcare economy1-5. The Institute of Medicine Committee on Advancing Pain Research, Care, and Education estimates that 100 million American adults are suffering from chronic pain and the total costs of medical expenditures and lost productivity add up to an annual sum of between $560-635 billion5. Evidence continues to grow establishing spinal cord stimulation (SCS) as a safe, efficacious, and cost-effective treatment for chronic pain of the back and limbs. It has been used for an expanding number of chronic pain syndromes including failed back surgery syndrome (FBSS)6-9, chronic regional pain syndrome (CRPS)6,10, refractory angina pectoralis11, painful diabetic neuropathy12, neuropathic pain, and peripheral vascular disease13. Prior studies have shown that the efficacy of SCS for treatment of chronic pain, compared to conventional medical management (CMM) alone, is superior and more cost-effective over the lifetime of the patient6-14. Moreover, CMM commonly includes opioid usage, for which there is limited evidence of long-term therapeutic benefit and which has associated risks for serious harm15. Expert panels and professional societies suggest the use of SCS for chronic pain after CMM has been shown ineffective16,17. Specifically, the British Pain Society recommends, “SCS should be considered early in the patient's management when simple first-line therapies have failed.”17
Despite its proven efficacy, previous studies report that the majority of patients wait over 5 years after initial diagnosis of chronic pain to undergo SCS18,19. This extended wait time is suboptimal, as Kumar et al. showed that the efficacy of SCS in relieving chronic pain is inversely proportional to pain-to-SCS time13,16,18. In these studies, patients faced significant delays to SCS implantation at each level of the treatment continuum from self-management, to management by a family physician, and finally even at the level of pain specialists. It was found that non-implanting physicians took significantly longer to refer patients for SCS, thus highlighting the need to raise awareness of SCS's role in treating chronic pain18. To investigate the impact of pain-to-SCS times on outcomes, we studied a large, nationwide cohort using a database of medical and drug claims to analyze the effect of pain-to-SCS time on chronic pain-related healthcare resource utilization (HCRU).
Methods
The impact of chronic pain diagnosis to SCS implantation time on HCRU was evaluated using the Truven Health MarketScan Database. These databases contain de-identified, patient-level billing and payment data, with longitudinal tracking of over 50 million patients with private insurance or Medicare supplemental insurance in each calendar year. The data set includes integrated enrollment, inpatient, outpatient, and drug data. The patient cohort was derived from MarketScan data from April 1, 2008 through March 31, 2013.
Inclusion and exclusion criteria were established to identify patients in the datasets who obtained SCS therapy. The index event was permanent SCS implantation, defined as a pulse generator implantation procedure and lead implantation procedure (Table S1), occurring on the same day, between April 1, 2010 and March 31, 2012. Performing a combined pulse generator with lead implant on a single day is the primary technique used to implant neurostimulators in the United States. The preoperative enrollment time for each patient was calculated as the difference between the start enrollment date and the first diagnosis of chronic pain; similarly, each patient's postoperative follow-up time was calculated as the difference from the index event date to the end enrollment date. Enrollment was truncated to the earliest (April 1, 2008) and latest (March 31, 2013) date of the data set, respectively. Patients were excluded from the study if they: (1) had less than 1 year of post-SCS follow-up time available, or (2) had a SCS procedure with a corresponding migraine diagnosis (Table S2), suggesting use of SCS for migraine treatment (e.g., occipital nerve stimulation), or (3) were less than 18 years of age at SCS implantation, or (4) did not have a chronic pain diagnosis within the data set that preceded the SCS procedure, or (5) did not have a pain-free period of at least 300 days prior to the initial chronic pain diagnosis during preoperative follow-up. The latter helped ensure that the true initial diagnosis was captured. The predictor variable was defined as the duration between a patient's initial chronic pain diagnosis and permanent SCS implantation.
Chronic pain diagnoses were classified into one of the following groupings: post-laminectomy syndrome, complex regional pain syndrome, neuritis / radiculitis, limb pain, degenerative spine disease, back pain, and chronic pain syndrome (Table S2). A patient could have multiple chronic pain diagnoses, and had to have least one of these diagnoses to be included in the analysis
The outcome variables evaluated chronic pain-related HCRU in the 1 year following SCS implantation. Outcomes included total (inpatient and outpatient) medical expenditures (in US dollars [USD]), number of office visits, number of hospitalizations, and number of ER visits, for which there was a chronic pain diagnosis. Additionally, total pharmaceutical payments were evaluated for the following drug classes commonly used for pain treatment: opioids, analgesics, anti-convulsants, NSAIDS, and muscle relaxants. Moreover, we evaluated opioid pharmaceutical payments and the number of opioid prescriptions filled. Finally, we investigated the number of delivered pain injections (epidural steroids, trigger point injections, and nerve blocks).
We summarized continuous patient descriptor variables using mean with standard deviation (SD). Alternatively, categorical variables, such as sex and chronic pain diagnoses were summarized with counts and percentages.
Multivariable regression was performed to establish the relationship between the pain-to-SCS time predictor variable and each HCRU outcome variable. Patients were divided into terciles based on HCRU: low utilization (lower tercile), medium utilization (middle tercile), and high utilization (upper tercile). This regression focused on the clinically meaningful categories by showing the change in the odds of being in the high utilization group, compared to the low utilization group, with each one-year increase in pain-to-SCS time. We believe the lower tercile reflects those who did clinically well and the upper tercile reflects those who did not respond well to SCS, which formed the basis for excluding the middle tercile from analysis since this group reflected a more typical patient who may or may not have responded well to SCS.
Chi-squared tests were used to compare the number of patients in the low, medium, and high utilization groups after binning patients by years of pain-to-SCS time (0-1 year, 1-2 years, 2-3 years, or >3 years). For this analysis, we included an additional group of patients in the >3 year pain-to-SCS time group for whom we could not verify that the first chronic pain diagnosis was the patients’ initial diagnosis (<300 days preceding pain-free clean period), but nevertheless could confirm that the pain-to-SCS time was at least 3 years.
Finally, Tobit regressions were performed for expenditure-based outcome variables and negative binomial regressions were performed for count-based variables. The Tobit model was used to estimate the linear relationships between HCRU and pain-to-SCS time with left-censoring, which in this case accounts for the minimum possible expenditure value of $0. All regressions used age, sex, prior back surgery, and Charlson comorbidity index (CCI) as covariates. All tests were two sided, and statistical significance was defined using α = 0.05. We used RStudio (R version 3.0.3) for data preparation and analysis.
Results
Patient Cohort
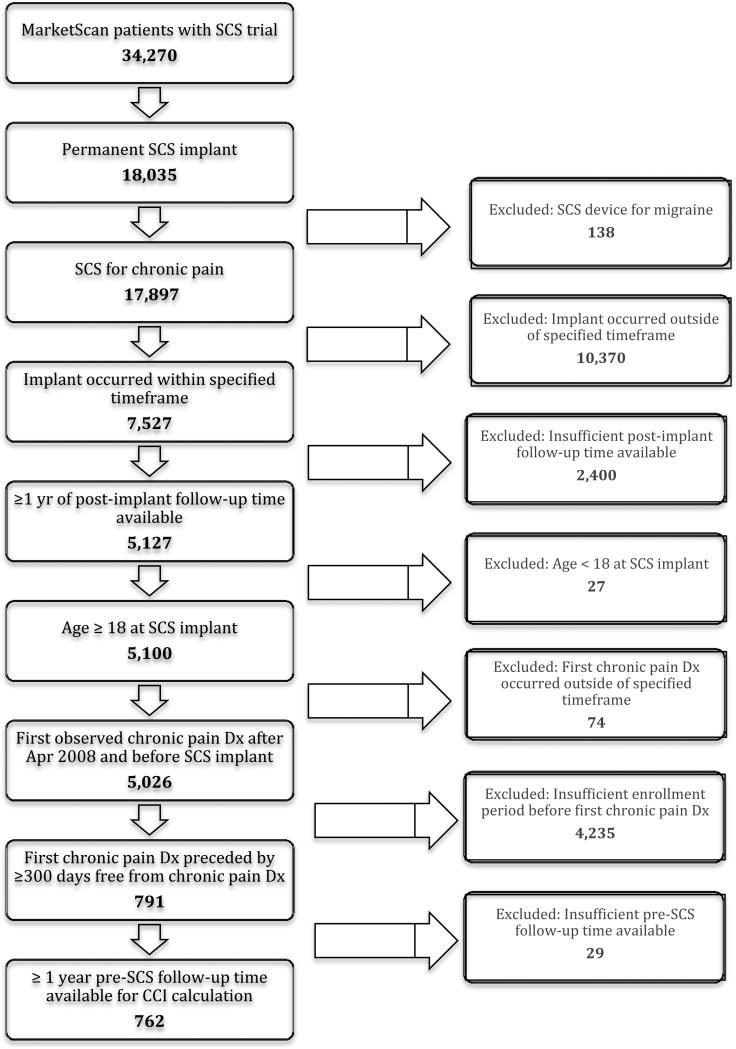
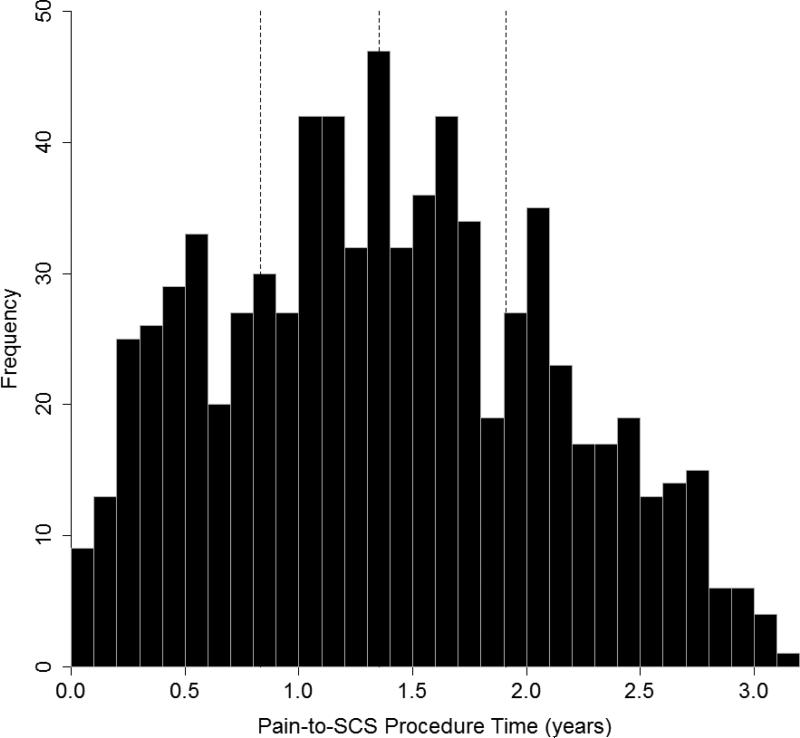
The overall demographics of the patient sample are outlined in Table 1. There were 762 patients that underwent permanent SCS implantation between April 1, 2010 and March 31, 2012 and met inclusion/exclusion criteria for the study (Figure 1). The mean patient age was 55.9 ± 13.9 years and 41.2% of patients were male. The average pain-to-SCS time was 1.39 ± 0.72 years (Figure 2). The most common initial chronic pain diagnoses in the cohort were back pain, degenerative spine disease, and neuritis/radiculitis. By the time of SCS implant, the most common pain diagnoses were chronic pain, neuritis/radiculitis, and post-laminectomy syndrome.
Table 1.
Baseline demographics of patients included in the study.
| Parameter | Units | N = 762 | |
|---|---|---|---|
| Age | Years | mean ± SD | 55.9 ± 13.9 |
| Sex | Male | count (%) | 314 (41.2%) |
| Charlson Comorbidity Index | -- | mean ± SD | 0.46 ± 1.21 |
| Wait Time | Years | mean ± SD | 1.39 ± 0.72 |
| Trial-to-Permanent Time (N = 658) | Days | mean ± SD | 43.2 ± 34.7 |
| Back Surgeries Before SCS | -- | mean ± SD | 0.17 ± 0.51 |
| ≥ 1 Back Surgery Before SCS | -- | count (%) | 102 (13.4%) |
| Initial Pain Diagnosis | CRPS | count (%) | 21 (2.8%) |
| Chronic Pain | 37 (4.9%) | ||
| Neuritis / Radiculitis | 186 (24.4%) | ||
| Degenerative Spine Disease | 213 (28.0%) | ||
| Post-Laminectomy Syndrome | 28 (3.7%) | ||
| Back Pain | 318 (41.7%) | ||
| Pain in Limb | 110 (14.4%) | ||
| Pain Diagnosis at Implant | CRPS | count (%) | 71 (9.3%) |
| Chronic Pain | 257 (33.7%) | ||
| Neuritis / Radiculitis | 235 (30.8%) | ||
| Degenerative Spine Disease | 146 (19.2%) | ||
| Post-Laminectomy Syndrome | 204 (26.8%) | ||
| Back Pain | 133 (17.5%) | ||
| Pain in Limb | 27 (3.5%) |
Figure 1.
Flow chart of numbers of patients that met inclusion/exclusion criteria.
Figure 2.
Histogram of distribution of pain-to-SCS procedure time for patients included in the study. The three vertical lines show the median, lower quartile, and upper quartile.
Healthcare Resource Utilization
It was found that increasing pain-to-SCS time was associated with significant increases in HCRU, after adjusting for covariates (age, sex, prior back surgery, and CCI). For every one year increase in pain-to-SCS time, the odds increased by 33% for being in the high total medical expenditures group (defined using the upper tercile: $4,133-359,084) over the low group (lower tercile: $0-603) (Table 2). However, with the Tobit regression, there was not a statistically significant increase in total medical expenditures for each year of increased pain-to-SCS time (Table 3).
Table 2.
Results of logistic regressions showing the odds of being in the high HCU group vs. the low HCU group with one additional year of pain-to-SCS procedure time.
| Variable | Low Utilization | High Utilization | Odds Ratio [95% CI] | P Value |
|---|---|---|---|---|
| Inpatient & outpatient medical expenditures | $0 - 603 | $4,133 - 359,084 | 1.33 [1.01, 1.77] | 0.047 |
| Pharmaceutical payments | $0 - 33 | $482 - 15,154 | 1.35 [1.04, 1.75] | 0.023 |
| Opioid payments | $0 - 5 | $186 - 15,154 | 1.43 [1.10, 1.85] | 0.0072 |
| Opioid prescriptions filled | 0 - 1 | 10 - 58 | 1.39 [1.09, 1.78] | 0.0091 |
| Office visits | 0 - 2 | 8 - 77 | 1.44 [1.12, 1.85] | 0.0049 |
| Hospitalizations | 0 | 3 - 28 | 1.55 [1.19, 2.03] | 0.0012 |
| ER visits | 0 | 1 - 6 | 1.07 [0.79, 1.44] | 0.66 |
| Pain injections | 0 | 1 - 9 | 1.10 [0.85, 1.43] | 0.48 |
Table 3.
Results of adjusted Tobit regression* demonstrating the increase in HCU with additional pain-to-SCS procedure time.
| Variable (Log10 Transformed) | Regression Estimate [95% CI] | % Increase in HCU with 1 Year Increase in Pain-to-SCS Time | P Value |
|---|---|---|---|
| Inpatient & outpatient medical expenditures | 0.09 [−0.045, 0.225] | -- | 0.193 |
| Pharmaceutical payments | 0.187 [0.013, 0.36] | 53.8% | 0.035 |
| Opioid payments | 0.246 [0.063, 0.429] | 76.2% | 0.0086 |
Covariates include age, sex, prior back surgery, and CCI.
A similar analysis was performed for the impact of pain-to-SCS time on the number of office visits, hospitalizations, and ER visits. The odds increased by 44% and 55%, respectively, for being in the high office visits (8-77) or hospitalizations (3-28) group over the low office visits (0-2) or hospitalizations (0) group for each year of delayed pain-to-SCS time. The incidence of annual office visits and hospitalizations increased by 13% and 20%, respectively, after one additional year (Table 4). Logistic regression and negative binomial regression failed to show any statistically significant difference in ER visits or pain injections associated with increased pain-to-SCS time.
Table 4.
Results of adjusted negative binomial regression* demonstrating an increase in HCU with a one year increase in pain-to-SCS procedure time.
| Variable | Regression Estimate [95% CI] | Increase in Incidence with 1 Year Increase in Wait Time | P Value |
|---|---|---|---|
| Opioid prescriptions filled | 0.18 [0.02, 0.33] | 19% | 0.025 |
| Office visits | 0.12 [0.01, 0.24] | 13% | 0.037 |
| Hospitalizations | 0.18 [0.03, 0.33] | 20% | 0.018 |
| ER visits | −0.02 [−0.34, 0.29] | -- | 0.89 |
| Pain Injections | 0.13 [−0.13, 0.41] | -- | 0.38 |
Covariates include age, sex, prior back surgery, and CCI.
The impact of pain-to-SCS time on pharmaceutical use for pain treatment was also investigated. The odds increased by 39% for being in the high opioid prescriptions filled group (10-58 prescriptions) over the low group (0-1), with a 19% increase in the incidence of annual opioid prescriptions for each additional year of pain-to-SCS time. Similarly, there was a 43% increase in odds of being in the high opioid payment group ($186-15,154) vs. the low group ($0-5), with a 76% increase in the expected value of opioid payments for a one year delay in pain-to-SCS time. For overall pharmaceutical payments, there was a 35% increase in the odds of being in the high group ($482-15,154) vs. the low group ($0-33) with a 54% increase in the expected value of pharmaceutical expenditures for each year increase in pain-to-SCS time.
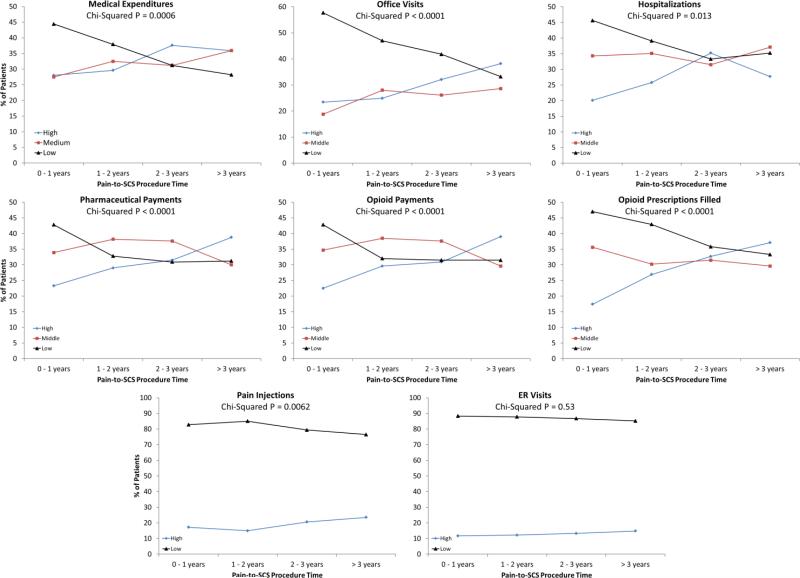
Finally, there were statistically significant changes in the number of patients that were categorized within the low, medium, and high HCRU terciles with each subsequent year increase in pain-to-SCS time (Figure 3). There was a greater percentage of patients in the 0-1 year pain-to-SCS time group that were categorized within the low medical expenditures tercile (44%) than in the medium (28%) or high (28%) terciles. However, with increases in pain-to-SCS time, the percentage of patients categorized into the low tercile decreased, and the percentage in the medium and high terciles increased. Similar trends were seen for office visits, hospitalizations, pharmaceutical payments, opioid payments, opioid prescriptions filled, and pain injections. No significant changes were found for ER visits.
Figure 3.
Percentage of patients in low, medium, and high utilization groups for all HCU variables. Patients are binned into one of four pain-to-SCS procedure time groups (0-1 years, 1-2 years, 2-3 years, >3 years).
Discussion
Despite the proven cost-effectiveness of SCS in treating chronic pain, patients still experience significant delays from time of pain diagnosis to SCS implantation. Kumar et al. have shown that the most significant delays occurred with physicians that are not familiar with the role of SCS in treating chronic pain, and that these delays led to decreased efficacy of SCS18. Our results are in line with these prior findings, as we found significant increases in HCRU with longer pain-to-SCS time. Our study found that longer pain-to-SCS time resulted in increases in total medical expenditures, number of opioid prescriptions filled, payments for opioid prescriptions, and number of hospital and office visits. Specifically, undergoing SCS even a year sooner in the care continuum has the potential to save thousands of dollars on pain related costs and a significant amount of time spent on visits or hospitalizations following SCS surgery. Given these results, raising awareness of the role of SCS in treating chronic pain could avoid unnecessary delays in treatment associated with increased HCRU.
The mean time from initial chronic pain diagnosis to permanent SCS implantation found in our study was lower than that previously reported. We found a mean pain-to-SCS time of 1.39 years compared to 5.45 years reported by Kumar et al.18 and 6.5 years reported by Veizi and Hayek19. It is important to note that pain-to-SCS time was defined differently in the Kumar et al. study than in our study. Kumar et al. defined pain-to-SCS time as time from initial onset of chronic pain symptoms to permanent SCS implantation, whereas our study defined this as time from initial chronic pain diagnosis to permanent SCS implantation. Our definition of chronic pain using a retrospective analysis required specific codes, while Kumar reported on initial onset of symptoms. Kumar et al. report that the average time from pain onset to initial physician consult is 3.4 months. Accounting for this additional time to pain diagnosis, the duration from initial physician consult to permanent SCS implantation in the Kumar et al. study was 5.17 years, which still demonstrates a longer time to SCS implantation compared to our study. More recent work by Kumar et al. also showed that pain-to-SCS time has decreased over the past decades. The average wait time in the 1980s was 154 months, with a decline to 71 months in the 1990s, and finally 62 months in the 2000s18. Our shorter pain-to-SCS time may suggest that this trend of decreasing wait times has continued more recently, as our study sampled patients from 2008 to 2013. Our shorter pain-to-SCS time may also be explained by the relatively short window of data used for analysis, which precluded analysis on patients having pain-to-SCS times of greater than approximately 3.2 years (after accounting for inclusion/exclusion criteria). Moreover, Kumar reported on his system in Canada, and our analysis utilized data from the United States. The differences in pain-to-procedure time may reflect inherent differences in health care systems.
Our study demonstrates that increasing time from initial chronic pain diagnosis to permanent SCS implantation is associated with increased HCRU after implantation. Logistic regression analysis separated patients into low and high HCRU groups, and showed that patients are significantly more likely to require higher HCRU with each additional year of pain-to-SCS time for total medical expenditures, pharmaceutical payments, opioid payments, prescriptions filled, office visits, and hospitalizations. Likewise, the chi-squared analysis showed that there was a significant change in the proportion of patients falling into low, medium, and high HCRU groups with increasing pain-to-SCS time. The trend was for more patients to fall into higher HCRU groups with longer pain-to-SCS times. This was statistically significant for total medical expenditures, pharmaceutical payments, opioid payments, prescriptions filled, office visits, hospitalizations, and number of pain injections.
We also quantified the increase in HCRU with delays in pain-to-SCS time using a Tobit regression for expenditure-based variables and a negative binomial regression for count-based variables. Our Tobit regression showed no significant change in total medical expenditures for increased pain-to-SCS time. There may have been other confounding factors beyond age, sex, prior back surgery, and CCI that impacted total medical expenditures, such as insurance, geographic location, and weight, but these were not considered in our model. In contrast, Tobit regression did show significant increases in the expected value of pharmaceutical payments and opioid payments. Negative binomial regression showed that with a one year increase in pain-to-SCS time, there were increases in the incidence of opioid prescriptions filled, office visits, and hospitalizations, whereas ER visits and pain injections showed no significant change.
In prior studies, Kumar et al. have shown that the efficacy of SCS is inversely proportional to pain-to-SCS time13,16,18, despite multiple other studies showing no association between pain duration and the success of SCS21-24. De Ridder et al. failed to find any predictive value of pain-to-SCS time on the overall reduction of pain on a numeric rating scale or the number of patients that responded to SCS21. Taylor et al. conducted a meta-analysis of the available literature in 2013 and found that longer pain duration was associated with decreased efficacy of SCS using univariate analysis; however, when these authors controlled for appropriate covariates using multivariate regressions, pain duration was no longer a statistically significant prognostic factor for SCS efficacy22. Although we were not able to directly measure the efficacy of SCS in our study, we do believe that the trends observed in post-implant HCRU shed some light on these previously conflicting results. It is intuitive that patients with increased pharmaceutical payments, opioid payments, prescriptions filled, and office visits reflect a decreased level of pain relief from SCS. Thus, our observed trend of increased post-implant HCRU with longer pain-to-SCS time may suggest a decreased efficacy of SCS with longer delays, as Kumar et al. observed13,16,18. Regardless of efficacy, post-implant HCRU is an important factor for treating physicians to consider when deciding where in the treatment continuum to offer SCS to patients with chronic pain.
Given the results of our study demonstrating that earlier intervention with SCS in patients with chronic pain results in lower post-implant HCRU, we believe that it is important to raise the awareness of SCS therapy's role in treating chronic pain among physicians. As Kumar et al. showed, non-implanting physicians took considerably longer before considering SCS as treatment for chronic pain syndromes than implanting physicians18. Having physicians consider SCS earlier in the treatment continuum may reduce the burden of chronic pain on the U.S. healthcare economy by decreasing HCRU by chronic pain patients. Additionally, SCS implantation is itself a costly procedure with Lad et al.25 and Kumar et al.26 both reporting the total cost of permanent SCS implantation to be approximately $58,000 in 2006 and 2007, respectively. Earlier SCS implantation may work to offset the initial cost of SCS by decreased post-implant HCRU. Given the growing body of evidence on the benefits of timely SCS implantation, it is important that treating physicians avoid unnecessary delays in implantation.
Our study analyzed a large, nationwide cohort, which reduced bias from differences in practice at different centers and in different regions of the U.S. Despite its strengths, the study's limitations must also be acknowledged. First, there were a relatively small number of subjects in the study considering only 762 of patients met all inclusion/exclusion criteria of the 7,527 patients in the database who underwent permanent SCS implantation for chronic pain within the specified timeframe. . A majority of patients were excluded due to inadequate post-implant follow-up time or insufficient insurance enrollment period prior to implantation. The small number of subjects could reduce the statistical power of the study and the ability to detect significant trends. Second, there were various clinical factors that were not assessed prior to SCS implantation, such as differences in patient history, pain complexity, and prior HCRU. Third, we excluded patients with more than approximately 3.2 years of pain-to-SCS time, due to the 5 year window of MarketScan data used in this study. Fourth, the subset of the population in MarketScan contained patients or dependents of people with commercial insurance, which may not have been representative of the entire chronic pain population. Finally, our study was retrospective and nonrandomized, leaving it susceptible to the effects of other confounding variables. Despite this, our study offers valuable evidence to support the timely implementation of SCS to reduce HCRU in chronic pain patients. Our results are especially significant given the challenges in conducting a prospective, randomized trial to assess the effect of pain-to-SCS time on post-implant HCRU.
Supplementary Material
Acknowledgments
Funding Statement: NIH KM1 CA 156687 provided funding for the study.
Footnotes
Authorship Statement: Frank Petraglia prepared the manuscript draft with important intellectual input from Dr. Shivanand Lad, Dr. Ashwini Sharan, Dr. Peter Staats, Dr. Steven Cook, Dr. Alexander Kent, Dr. Nirav Dalal and Edward Karst. Dr. Shivanand Lad, Dr. Alexander Kent, Dr. Nirav Dalal, Dr. Peter Staats, Dr. Ashwini Sharan, Edward Karst and Frank Petraglia designed and conducted the study including data collection, analysis and interpretation. All authors approved the final manuscript.
Conflict of Interest Statement: Shivanand Lad, MD, PhD, has consulted for or received grant support from Medtronic Inc., Boston Scientific and St. Jude Medical. He serves as Director of the Duke Neuro-Outcomes Center which has received research funding from NIH KM1 CA 156687. Alexander R. Kent, PhD, Nirav Dalal, MS, MBA, and Edward Karst, MS are employees at St. Jude Medical. Ashwini Sharan, MD, has consulted for Medtronic and St. Jude Medical. Peter Staats, MD, MBA has consulted for and/or received research support from St. Jude Medical, Medtronic, Boston Scientific, Grunenthal, Spinal Modulation, Bioness, Vertos Medical, and Saluda Medical. The remaining authors report no conflicts of interest.
References
- 1.Kennedy J, Roll JM, Schraudner T, Murphy S, McPherson S. Prevalence of persistent pain in the U.S. Adult population: new data from the 2010 national health interview survey. J Pain. 2014 Oct;15(10):979–84. doi: 10.1016/j.jpain.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, Castel LD, Kalsbeek WD, Carey TS. The rising prevalence of chronic low back pain. Arch Intern Med. 2009 Feb 9;169(3):251–8. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012 Aug;13(8):715–24. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008 Oct;9(7):803–12. doi: 10.1111/j.1526-4637.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine (US) Committee on Advancing Pain Research . Care, Education, and Research. National Academies Press (US); Washington (DC): 2011. Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention. [PubMed] [Google Scholar]
- 6.Simpson EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J. Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation. Health Technol Assess. 2009 Mar;13(17):iii, ix–x, 1–154. doi: 10.3310/hta13170. [DOI] [PubMed] [Google Scholar]
- 7.Taylor RJ, Taylor RS. Spinal cord stimulation for failed back surgery syndrome: a decision-analytic model and cost-effectiveness analysis. Int J Technol Assess Health Care. 2005;21(3):351–8. doi: 10.1017/s0266462305050464. Summer. [DOI] [PubMed] [Google Scholar]
- 8.Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O'Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, North RB. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007 Nov;132(1-2):179–88. doi: 10.1016/j.pain.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 9.North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98–106. doi: 10.1227/01.neu.0000144839.65524.e0. [DOI] [PubMed] [Google Scholar]
- 10.Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006 Feb;10(2):91–101. doi: 10.1016/j.ejpain.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Börjesson M, Andrell P, Lundberg D, Mannheimer C. Spinal cord stimulation in severe angina pectoris--a systematic review based on the Swedish Council on Technology assessment in health care report on long-standing pain. Pain. 2008 Dec;140(3):501–8. doi: 10.1016/j.pain.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 12.de Vos CC, Meier K, Zaalberg PB, Nijhuis HJ, Duyvendak W, Vesper J, Enggaard TP, Lenders MW. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomised clinical trial. Pain. 2014 Aug 29;:S0304–3959(14)00390-X. doi: 10.1016/j.pain.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Kumar K, Rizvi S. Cost-effectiveness of spinal cord stimulation therapy in management of chronic pain. Pain Med. 2013 Nov;14(11):1631–49. doi: 10.1111/pme.12146. [DOI] [PubMed] [Google Scholar]
- 14.Kumar K, Toth C, Nath RK, Laing P. Epidural spinal cord stimulation for treatment of chronic pain--some predictors of success. A 15-year experience. Surg Neurol. 1998 Aug;50(2):110–20. doi: 10.1016/s0090-3019(98)00012-3. [DOI] [PubMed] [Google Scholar]
- 15.Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015 Feb 17;162(4):276–86. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 16.Kumar K, Wilson JR. Factors affecting spinal cord stimulation outcome in chronic benign pain with suggestions to improve success rate. Acta Neurochir Suppl. 2007;97(Pt 1):91–9. doi: 10.1007/978-3-211-33079-1_12. [DOI] [PubMed] [Google Scholar]
- 17.The British Pain Society Spinal Cord Stimulation for the Management of Pain: Recommendations for Best Clinical Practice. http://www.britishpainsociety.org/book_scs_main.pdf.
- 18.Kumar K, Rizvi S, Nguyen R, Abbas M, Bishop S, Murthy V. Impact of Wait times on Spinal Cord Stimulation Therapy Outcomes. Pain Pract. 2014 Nov;14(8):709–20. doi: 10.1111/papr.12126. [DOI] [PubMed] [Google Scholar]
- 19.Veizi E, Hayek S. Spinal cord stimulation efficacy: review of 5 years experience from an Academic Center Database. [abstract]. AAPM 2012 Annual Meeting Abstracts. Pain Med. 2012;13:291. [Google Scholar]
- 20.Neuromodulation Therapy Access Coalition Position Statement on Spinal Cord Neurostimulation. 2008 http://www.painmed.org/files/position-statement-on-spinal-cord-neurostimulation.pdf.
- 21.De Ridder D, Vancamp T, Lenders MW, De Vos CC, Vanneste S. Is Preoperative Pain Duration Important in Spinal Cord Stimulation? A Comparison Between Tonic and Burst Stimulation. Neuromodulation. 2015 Jan;18(1):17–7. doi: 10.1111/ner.12253. [DOI] [PubMed] [Google Scholar]
- 22.Taylor RS, Desai MJ, Rigoard P, Taylor RJ. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta-regression analysis. Pain Pract. 2014 Jul;14(6):489–505. doi: 10.1111/papr.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burchiel KJ, Anderson VC, Wilson BJ, Denison DB, Olson KA, Shatin D. Prognostic factors of spinal cord stimulation for chronic back and leg pain. Neurosurgery. 1995 Jun;36(6):1101–10. doi: 10.1227/00006123-199506000-00006. discussion 1110-1. [DOI] [PubMed] [Google Scholar]
- 24.Fiume D, Sherkat S, Callovini GM, Parziale G, Gazzeri G. Treatment of the failed back surgery syndrome due to lumbo-sacral epidural fibrosis. Acta Neurochir Suppl. 1995;64:116–8. doi: 10.1007/978-3-7091-9419-5_25. [DOI] [PubMed] [Google Scholar]
- 25.Lad SP, Kalanithi PS, Arrigo RT, Patil CG, Nathan JK, Boakye M, Henderson JM. A socioeconomic survey of spinal cord stimulation (SCS) surgery. Neuromodulation. 2010 Oct;13(4):265–8. doi: 10.1111/j.1525-1403.2010.00292.x. discussion 269. [DOI] [PubMed] [Google Scholar]
- 26.Kumar K, Bishop S. Financial impact of spinal cord stimulation on the healthcare budget: a comparative analysis of costs in Canada and the United States. J Neurosurg Spine. 2009 Jun;10(6):564–73. doi: 10.3171/2009.2.SPINE0865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.