Abstract
Major depressive disorder (MDD) is a common and debilitating psychiatric disorder. Traditional antidepressants are of limited efficacy and take weeks to months to yield full therapeutic effects. Thus, there is a clear need for effective rapid acting antidepressant medications. The N-methyl-D-aspartate receptor (NMDA-R) antagonist, ketamine, has received a great deal of attention over the last 20 years due to the discovery that a single sub-anesthetic dose leads to a rapid antidepressant effect in individuals with treatment-resistant depression. Animal and human research suggest that ketamine’s antidepressant effects are mediated by a glutamate surge that leads to a cascade of events which result in synaptogenesis and reversal of the negative effects of chronic stress and depression, particularly within the prefrontal cortex (PFC). Pre-clinical and clinical data have provided compelling insights into the mechanisms underlying the rapid acting antidepressant effects of ketamine. This review discusses stress-related neurobiology of depression and the safety, tolerability, and efficacy of ketamine for MDD, along with a review of ketamine’s mechanism of action and prospective predictors of treatment response. Research limitations and future clinical prospects are also discussed.
Keywords: Antidepressants, Depression, Treatment Resistance, Biological markers, Stress
Introduction
Major depressive disorder (MDD) is a common and debilitating psychiatric condition. Approximately 17% of the US population will meet diagnostic criteria for MDD within their lifetime.[1] MDD is the leading cause of worldwide disability among all psychiatric disorders;[2] it is a chronic condition that is associated with elevated risk of suicide, functional impairments, and a variety of socio-economic difficulties.
The Food and Drug Administration (FDA) has approved several drugs for the treatment of MDD, most of which target monoaminergic systems. Current approved medications are of limited efficacy. A significant proportion of patients do not show adequate response to available antidepressants and sustained remission is uncommon.[3] Moreover, it takes weeks or even months to gain the full therapeutic effects of traditional antidepressants.[4] There is a critical unmet need for antidepressants with a rapid onset of action, particularly in patients who do not respond to traditional antidepressants.
Surprising Antidepressant Effects of Ketamine
Ketamine is a glutamate N-methyl-D-aspartate receptor (NMDA-R) antagonist that has been used clinically since the 1960s, primarily as an anesthetic. Ketamine is most commonly administered intravenously, but can also be administered subcutaneously, intramuscularly, transdermally, intranasally, intrarectally, or orally. Route of administration substantially affects bioavailability, which is as high as 100% with intravenous and as low as 20% with oral administrations.[5] Ketamine is metabolized rapidly; it has a plasma redistribution half-life of 4 minutes and plasma terminal half-life of 2.5 hours.[6]
Ketamine has seen a recent surge in interest following findings that subanesthetic doses have rapid antidepressant effects.[7] An early study in treatment-refractory MDD patients revealed that a single subanesthetic dose of ketamine had a robust antidepressant effect within 4 hours.[7] Ketamine’s antidepressant effects have been replicated several times since (see [8] for a review), including four small placebo-controlled randomized controlled trials (RCTs).[7; 9–11] Meta-analyses have supported the robustness of ketamine’s rapid antidepressant effects relative to saline control and have shown it to be more effective than active placebo drugs with acute side effect profiles that optimize study drug blinding (i.e. midazolam).[12–16]
Ketamine’s antidepressant effects typically emerge about 4 hours after intravenous administration, well after the drug has been cleared from the bloodstream. Depressive symptoms usually return to baseline levels within 1 to 2 weeks.[17; 18] There is little data on ketamine’s optimal dosing, preferred route of administration, and the safety of repeated or chronic treatment. Recent primarily open-label studies have shown that smaller doses (e.g., 0.2 mg/kg[19]) and alternative routes of administration (e.g., intramuscular[20] or intranasal[21]) yield antidepressant effects that are comparable to the typical 0.5mg/kg intravenous dose. There is growing evidence that repeated administrations can extend ketamine’s antidepressant effects.[22; 23] Pilot data thus far suggests that up to six 0.5mg/kg intravenous infusions, administered three times per week for 2 weeks, are well tolerated and can prolong ketamine’s antidepressant effects.[23; 24]
Single infusion of ketamine is generally well tolerated. Ketamine does, however, cause transient side effects within the first 2 hours of treatment.[17; 22] The most common reported side effects of ketamine administration (0.5mg/kg) include transient perceptual disturbances, dissociation, dysphoria, euphoria, and anxiety; whereas the reported physical side effects include dizziness, nausea, and mild increase in blood pressure and heart rate. Given the short half-life of ketamine, these adverse effects abate within a few minutes of stopping ketamine infusion and generally fully remit within 2 hours.[16]
Studies to-date primarily used the racemic form of ketamine, which is composed of the enantiomers R-ketamine and S-ketamine, the latter has higher affinity to NMDA-R. A pilot clinical trial has recently shown rapid acting antidepressant effects using S-ketamine (a.k.a. esketamine) 0.2mg/kg and 0.4mg/kg administered intravenously over 40 min; these doses are believed to be equivalent to 0.25mg/kg and 0.5mg/kg of ketamine.[25] Intranasal administration of S-ketamine for the treatment of depression is being developed and was granted a fast track designation by the FDA (for a recent review of other NMDA-R modulating agents see Ref. [12])
Synaptic Plasticity and the Neurobiology of Depression
Synaptic plasticity refers to the process by which neurons and neural circuits are constantly regulating their excitability and connectivity. This is largely an adaptive process; neurons and circuits adjust to changing organismic (e.g., development and aging) and environmental circumstances (e.g., stress and learning).[26] Synaptic plasticity is accomplished by regulating synaptic strength, numbers, and density. Synaptic plasticity can be local or global.[27; 28] Long term potentiation (LTP)-like plasticity and long term depression (LTD)-like plasticity – also known as Hebbian plasticity – are two forms of local plasticity. Homeostatic plasticity – a type of global plasticity and form of synaptic scaling that modulates strength of neuronal connectivity – is particularly relevant to models of MDD.
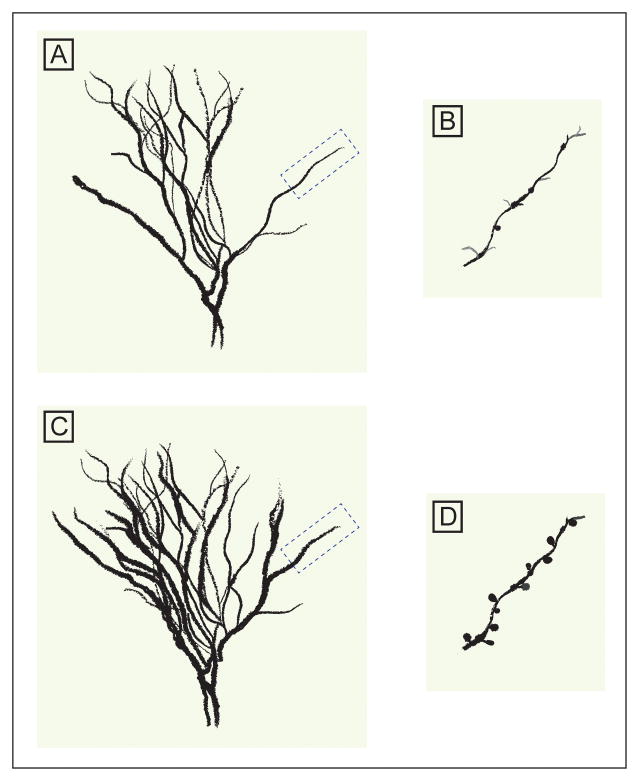
MDD is associated with prefrontal cortex (PFC) and hippocampal gray matter abnormalities[29] that are thought to be associated with synaptic downregulation as a consequence of neuronal excitotoxicity[30]. This downregulation is likely mediated by inflammatory cytokines and neurotrophins such as brain-derived neurotrophic factor (BDNF), among many other factors.[28; 31] Acute – psychologically circumscribed – stress promotes synaptic survival and strength and leads to behaviorally adaptive responses (e.g. enhanced working memory)[32]. In contrast, prolonged stress, a model of depression, is associated with neuronal atrophy and synaptic depression in the PFC and hippocampus.[33; 34] These changes are believed to be associated with stress-induced perturbations in the glutamatergic system.[8] A downstream effect of stress is prolonged excessive extracellular glutamate,[35] which is known to cause excitotoxicity, dysfunctional synaptic strength, reduced dendritic spine density, retraction of spines, and overall reduced dendritic branching within the PFC (Figure 1).[32; 36]
Figure 1. A schematic model depicting stress-induced neuronal atrophy and its normalization following ketamine treatment.
Chronic stress causes excess extracellular glutamate, and subsequent excitoxicity, leading to dendritic retraction, reduced dendritic arborization and spine density (A & B). Twenty-four hours post-treatment, subanesthetic dose of ketamine reverses the chronic stress-induced structural deficits culminating in rapid increases in spine density (C & D).
Accumulating molecular findings provide insight into the stress-related synaptic dysfunction that has been related to reduction in BDNF and/or to inhibition of the mammalian target of rapamycin complex 1 (mTORC1) in PFC regions. Depressive-like symptoms can be induced in animals by reducing BDNF or inhibiting mTORC1.[37; 38] Moreover, increasing BDNF or enhancing mTORC1 produce antidepressant-like effects in rodents.[37; 38] These data suggest that enhancement of BDNF and mTORC1 signaling are putative targets for antidepressant treatment. Monoaminergic antidepressants were found to increase BDNF and synaptogenesis following chronic, but not acute, treatment;[32; 37] this is consistent with the delayed antidepressant effect of traditional antidepressants. Rapid acting antidepressants, such as ketamine, were shown to rapidly increase BDNF and activate mTORC1 signaling, precipitating enhanced synaptogenesis and connectivity.[39–42]
The Antidepressant Mechanism of Action of Ketamine
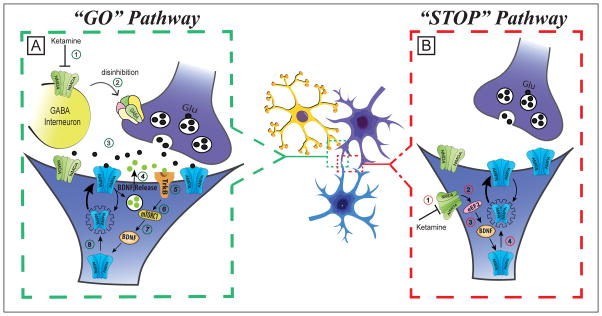
The rapid-acting antidepressant effects of ketamine are believed to be the result of a cascade of events, which include 1) blockade of interneuronal NMDA receptors,[43] 2) disinhibition of pyramidal cells leading to a glutamate surge,[44] 3) activation of the pro-synaptogenic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors,[45] 4) blockade of the excitotoxic extrasynaptic NMDA receptors,[41] and 5) activation of synaptogenic intracellular signaling, including mTORC1[39] and BDNF pathways (Figure 2).[46]
Figure 2. Mechanism of action of ketamine’s rapid antidepressant effects.
It is proposed that subanesthetic doses of ketamine will simultaneously activate the “GO”, and inhibits the “STOP”, pathways. The ketamine-induced alterations of both pathways converge to increase BDNF, protein synthesis, synaptic strength, and synaptogenesis. In this model, ketamine activates the “GO” pathway by A-1) preferentially blocking of NMDA receptors located on a subpopulation of interneurons, A-2) disinhibiting pyramidal neurons, A-3) generating a transient glutamate surge and AMPA receptors activation, A-4) stimulating BDNF release, A-5) activating TrkB receptors, A-6) stimulating the mTORC1 signaling, A-7) inducing BDNF translation, and A-8) increasing protein synthesis, AMPA cycling, and synaptogenesis. In parallel, ketamine blocks the “STOP” pathway by B-1) blocking extrasynaptic NMDA receptors, B-2) disinhibiting eEF2, B-3) inducing BDNF translation, and B-4) increasing protein synthesis, AMPA cycling, and synaptogenesis. Abbreviations: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA); brain derived neurotrophic factor (BDNF); mammalian target of rapamycin complex 1 (mTORC1); Tyrosine kinase B (TrkB); eukaryotic elongation factor 2 (eEF2); N-methyl-D-aspartate (NMDA); gamma-aminobutyric acid (GABA); glutamate (Glu).
Anesthetic doses of ketamine were found to decrease prefrontal glutamate neurotransmission.[44] Paradoxically, preclinical studies have reliably shown that subanesthetic doses of ketamine cause an increase in glutamate cycling and extracellular glutamate, particularly in the PFC.[44; 47–49] Increases in glutamate signaling are thought to be primarily due to the ketamine’s preferential blockade of NMDA receptors on a subpopulation of gamma-aminobutyric acid (GABA)ergic interneurons[43; 50] (for review see [51]). Inhibition of GABAergic interneurons lead to disinhibition of glutamatergic neurons and a glutamate surge in the PFC. This glutamate surge increases activation of AMPA receptors. Increased AMPA receptor activation, coupled with ketamine’s inhibition of extrasynaptic NMDA receptors, initiates and facilitates postsynaptic activation of neuroplasticity-related signaling pathways, including those involving BDNF and mTORC1.[39; 41] Activation of extrasynaptic NMDA receptors reduces synaptic strength (e.g., reduced dendritic arborization, decreased dendritic length, synaptic loss, and neuronal death).[52] As such, blockage of extrasynaptic NMDA receptors promotes synaptogenesis. Ketamine has been shown to enhance synaptogenesis at rest (independent of glutamate activation) through 1) inhibition of eukaryotic elongation factor 2 (eEF2) kinase, 2) de-suppression of eEF2, and 3) increased BDNF translation.[41] Additionally, selective genetic deletion of pyramidal neurons’ GluN2B subunits – which are abundant in extrasynaptic NMDA receptors – mimics the antidepressant and synaptic effects of ketamine.[53] Together, these neuroplasticity-related processes culminate in increases in AMPA receptors, synaptic strength, and synaptogenesis (Figure 2); leading to a rapid reversal of stress and depression-induced neuronal atrophy (Figure 1).
Convergent evidence support the hypothesis that AMPA receptor activation and mTOR and BDNF signaling mediate ketamine induced synaptogenesis and ketamine’s antidepressant effects.[39; 45; 46; 54; 55] Administration of AMPA antagonists block ketamine’s antidepressant effect[55] in a dose dependent fashion.[56] However, it is not yet known whether AMPA receptor activation contribute to the synaptic and antidepressant effects through postsynaptic depolarization that is required for activation of synaptic NMDA receptors, or through direct activation of intracellular downstream pathways. Blockage of mTOR signaling blocks ketamine induced synaptogenesis and ketamine’s antidepressant-like effects.[39] Ketamine fails to exert an antidepressant effect or to induce PFC synaptogenesis in rodents with impaired BDNF transmission.[46; 57]
Administration of ketamine to rodents has recently been shown to inhibit brain glycogen synthase kinase-3 (GSK-3), a mechanism also shared by lithium.[58] Ketamine increases the phosphorylation of GSK-3 and rodents with a knock out mutation that blocks the phosphorylation of GSK-3 do not respond to ketamine in a behavioral model of depression.[58] Moreover, the synaptogenesis and antidepressant-like effects of a subtherapeutic dose of ketamine were rescued when given together with a subtherapeutic dose of lithium or other GSK-3 inhibitors.[59; 60] These effects involved rapid activation of the mTOR signaling pathway, increased synaptic spine density/diameter and increased synaptic strength in the medial prefrontal cortex (mPFC) layer 5 pyramidal neurons and antidepressant responses that persisted for up to 1 week in the animal model of depression.[59] Although the presented model focused on the well-studied NMDA based ketamine’s pathways, other promising complementary mechanisms have been proposed and await supportive evidence. These proposed mechanisms include the theoretical possibility that the observed rapid acting antidepressant effects could be mediated by ketamine’s effect on intracellular lysosomes, on the opioid system, or the nicotinic receptors (see [61–63]).
Biomarkers of Ketamine’s Antidepressant Effects
Animal studies have shown that ketamine promotes the rapid development of new synapses in the mPFC.[39] Ketamine-enhanced synaptogenesis persists for up to 7 days after administration, long after the drug has been fully metabolized.[39; 64] This parallels the timeline of antidepressant effects, and animal research has provided evidence that these changes in neuroplasticity underlie ketamine’s antidepressant properties.[40; 65] Positron emission tomography (PET) studies demonstrate that low dose ketamine acutely increases brain metabolism, particularly within the PFC.[66–68] Given the direct relation between glutamate cycling and neural energy consumption,[69] these studies also provide indirect evidence of ketamine induced alterations in glutamate neurotransmission.
As described above, synaptic connectivity is affected following depression and prolonged stress and these synaptic abnormalities are rapidly reversed by ketamine treatment.[70] Therefore, neuroimaging studies of the effects of ketamine on brain circuitry and connectivity may play a critical role in unraveling its underlying mechanism as well as provide unique insight into normal brain functioning. A pilot MDD study recently identified abnormal reduction in caudate activation, as measured by functional magnetic resonance imaging (fMRI), in response to images of happy faces. Ketamine treatment increased caudate activation, however, no correlation was found between caudate activation and response to treatment.[71] Following ketamine treatment, higher antidepressant response was associated with increased connectivity between the caudate and several brain regions, including mPFC.[71]
Using magnetoencephalography (MEG), Cornwell and colleagues showed a significant increase in somatosensory evoked fields, a putative measure of synaptic connectivity and cortical excitability, 6 hours after drug administration in depressed patients who responded to ketamine, but not in non-responders.[72] Lazzaro and colleagues used transcranial magnetic stimulation to show that low dose ketamine increased cortico-spinal excitability (i.e., enhanced motor evoked potentials and decreased motor threshold) in healthy subjects after drug administration.[73] A recent sleep study used electroencephalography (EEG) to show that a subanesthetic dose of ketamine increased sleep slow oscillations, a marker of synaptic plasticity, among depressed patients the night after ketamine administration.[74] This same study showed that plasma BDNF increased among all patients 4 hours following ketamine administration, and that BDNF increases correlated with enhanced synaptic strength in the responders group.[74] A more recent study using a sample of patients diagnosed with MDD showed that plasma BDNF increased more among responders compared to non-responders following ketamine administration.[75] However, an earlier study failed to detect a significant relation between serum BDNF and the antidepressant effects of ketamine.[76]
Prospective Predictors of Ketamine’s Antidepressant Effects
Investigators have utilized a variety of experimental methods to investigate prospective predictors of ketamine’s antidepressant effects. Hippocampal volume reduction in MDD has been related to glutamate dysregulation and treatment resistance to traditional antidepressants.[77] In contrast, comparable to other glutamate-modulating agents,[78] ketamine was found in a pilot study to exert its highest antidepressant effects in patients with reduced hippocampal volume as measured by magnetic resonance imaging (MRI).[79]
Salvadore and colleagues conducted a series of MEG studies to advance this issue. The first such study showed that decreased pretreatment pregenual anterior cingulate cortex (ACC) activity during an affective face viewing task was associated with an attenuated antidepressant response to ketamine.[80] Conversely, decreased pretreatment pregenual ACC activity during a working memory (updating) task was associated with better response to ketamine.[81] Similarly, poorer beta desynchronization between the pregenual and subgenual ACC during the working memory task was associated with greater antidepressant response. Poorer coherence, a measure of functional connectivity, between the pregenual ACC and amygdala during the working memory task was also associated with a greater antidepressant response to ketamine.[81] Taken together, these findings suggest that hyperactivity of the pregenual ACC during emotional processing and hypoactivity or hypoconnectivity of the pregenual ACC during cognitive processing are associated with greater antidepressant response to ketamine. In general, the ACC can be divided into cognitive and affective subdivisions, with dorsal sections generally dedicated to cognitive functions and ventral sections – including the pregenual ACC – dedicated to affective functions.[82] As such, one interpretation of Salvadore and colleagues’ findings is that ketamine may act to strengthen synaptic connectivity in brain regions regulating ACC and amygdala activity.[83; 84]
Few studies have utilized magnetic resonance spectroscopy (MRS) to assay glutamate neurotransmitter concentration among depressed patients treated with ketamine. One study showed that depressed patients with lower pretreatment PFC (glutamate+glutamine)/glutamate ratios reported a greater antidepressant response to ketamine.[85] Yet this study failed to find a significant association between total PFC glutamate concentrations and treatment response.[85] Another study failed to find significant correlations between occipital glutamate, glutamine, or GABA concentrations and treatment response.[11] Recently, a preliminary MRS study found rapid increases in the total level of GABA and Glx (an MRS signal comprising glutamate+glutamine) in the medial prefrontal cortex of eight depressed patients.[86]
Conclusions and Future Directions
A single subanesthetic dose of ketamine has rapid antidepressant effects. These effects emerge approximately 4 hours after drug administration, suggesting ketamine’s antidepressant effects are not a direct effect of the drug itself, but rather a downstream effect of the acute reaction of the brain to the drug. Ketamine’s antidepressant effects are typically sustained for 1–2 weeks, long after the drug has been fully metabolized. Clinical data suggest that ketamine is well tolerated and side effects typically abate within 1–2 hours after drug administration. Although there is promising evidence that multiple doses of ketamine can enhance or prolong the drug’s antidepressant effects, these data are in their infancy. Side effects and inconvenience associated with the current standard administration (0.5mg/kg intravenous) could potentially be mitigated with alternative routes of administration or smaller doses; both of which appear to be associated with antidepressant effects.[21] There is an urgent need for dose response studies, including investigations that use more convenient routes of administration or multiple doses. Researchers should also continue investigating alternative means for extending ketamine’s antidepressant effects. Given the putative mechanisms of action, it is plausible that other agents that enhance prefrontal plasticity might extend, enhance, or even replace ketamine’s antidepressant effects.
In summary, preclinical and clinical data suggest that low doses of ketamine initiate brain plasticity processes and increases prefrontal connectivity, thus reversing potential effects of chronic stress and depression. These antidepressant effects are likely mediated by a glutamate surge in the PFC, increased AMPA receptor activation, and increased AMPA cycling; the latter of which is itself mediated by neuroplasticity-related signaling pathways, particularly BDNF and mTORC1. Data have also shown that ketamine’s at rest blockage of NMDA receptors, presumably extrasynaptic, may facilitate synaptogenesis. These processes result in new spine growth, improved spine density, and dendritic branching; all of which enhance local and global neural connectivity. Insights into nearly all of these processes were ascertained from animal research. There is need for research with human subjects, particularly in depressed subjects, to determine if these candidate mechanisms of action mediate ketamine’s antidepressant effects in depressed patients. There is also a lack of clinical research focused on prospectively predicting which MDD patients will benefit from ketamine. While several pilot studies provided initial evidence of potential biomarkers of ketamine’s antidepressant effects, it is important to underscore the preliminary nature of these studies and the need for replication prior to making any firm conclusions. Clinical studies should integrate pre-treatment behavioral and biological assessments to better characterize the probability of treatment response. This, coupled with the aforementioned future direction, may allow for targeted dosing modifications or treatment augmentation strategies.
There is growing evidence for ketamine’s efficacy for a variety of other psychiatric conditions, including: bipolar disorder,[87] posttraumatic stress disorder,[88; 89] obsessive-compulsive disorder,[90; 91] and substance abuse/dependence.[92] Ketamine has also been shown to rapidly reduce acute suicidality.[19; 93] Controlled research is needed to determine if ketamine’s clinical effects are reliable for these other conditions. Although there is much to be learned about ketamine’s antidepressant effects, it is even more unclear how ketamine affects other psychiatric conditions. It is likely that the glutamate surge, prefrontal synaptogenesis, and prefrontal connectivity are implicated in ketamine’s psychiatric effects for non-depressive disorders, but these ideas are largely hypothetical at present.
Investigations of ketamine’s antidepressant mechanism of action have aided in the development of alternative models of depression and paved the way for the development of novel pharmacological antidepressant agents. Drugs that target neuroplasticity-related signaling pathways and synaptogenesis hold particular promise for MDD and other psychiatric disorders associated with chronic stress.
Footnotes
Conflict of Interest: Dr. Abdallah has served on advisory boards for Genentech. He is an employee of the Yale School of Medicine and has received funding from the National Institutes of Health, the Brain and Behavioral Research Foundation, the VA National Center for PTSD, the Department of Defense, the American Psychiatric Foundation, and the Robert E. Leet and Clara Guthrie Patterson Trust. Dr. Sanacora has received consulting fees form AstraZeneca, Avanier Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly & Co., Hoffman La-Roche, Merck, Navigen, Naurex, Noven Pharmaceuticals, Servier Pharmaceuticals, Takeda, Teva and Vistagen therapeutics over the last 24 months. He has also received additional research contracts from AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co., Johnson & Johnson, Hoffman La-Roche, Merck & Co., Naurex and Servier over the last 24 months. Free medication was provided to Dr. Sanacora for an NIH sponsored study by Sanofi-Aventis. In addition he holds shares in BioHaven Pharmaceuticals Holding Company and is a co-inventor on a US patent (#8,778,979) held by Yale University. Dr. Krystal is a consultant for AbbVie, Inc., Amgen, Astellas Pharma Global Development, Inc., AstraZeneca Pharmaceuticals, Biomedisyn Corporation, Bristol-Myers Squibb, Eli Lilly and Company, Euthymics Bioscience, Inc., Neurovance, Inc., FORUM Pharmaceuticals, Janssen Research & Development, Lundbeck Research USA, Novartis Pharma AG, Otsuka America Pharmaceutical, Inc., Sage Therapeutics, Inc., Sunovion Pharmaceuticals, Inc., and Takeda Industries; is on the Scientific Advisory Board for Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Inc., Naurex, Inc., and Pfizer; is a stockholder in Biohaven Medical Sciences; holds stock options in Mnemosyne Pharmaceuticals, Inc.; holds patents for Dopamine and Noradrenergic Reuptake Inhibitors in Treatment of Schizophrenia, U.S. Patent No. 5,447,948 (issued Sep 5, 1995), and Glutamate Modulating Agents in the Treatment of Mental Disorders, U.S. Patent No. 8,778,979 (issued Jul 15, 2014); and filed a patent for Intranasal Administration of Ketamine to Treat Depression. U.S. Application No. 14/197,767 (filed on Mar 5, 2014); U.S. application or Patent Cooperation Treaty international application No. 14/306,382 (filed on Jun 17, 2014). All other authors declare no conflict of interest.
Contributor Information
Chadi G. Abdallah, Email: chadi.abdallah@yale.edu.
Thomas G. Adams, Email: thomas.adamsjr@yale.edu.
Benjamin Kelmendi, Email: ben.kelmendi@yale.edu.
Irina Esterlis, Email: irena.esterlis@yale.edu.
Gerard Sanacora, Email: gerard.sanacora@yale.edu.
John H. Krystal, Email: john.krystal@yale.edu.
References
- 1.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Collins PY, Patel V, Joestl SS, et al. Grand challenges in global mental health. Nature. 2011;475(7354):27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Katz MM, Tekell JL, Bowden CL, et al. Onset and early behavioral effects of pharmacologically different antidepressants and placebo in depression. Neuropsychopharmacology. 2004;29(3):566–79. doi: 10.1038/sj.npp.1300341. [DOI] [PubMed] [Google Scholar]
- 5.Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings) CNS Neurosci Ther. 2013;19(6):370–80. doi: 10.1111/cns.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao X, Venkata SL, Moaddel R, et al. Simultaneous population pharmacokinetic modelling of ketamine and three major metabolites in patients with treatment-resistant bipolar depression. Br J Clin Pharmacol. 2012;74(2):304–14. doi: 10.1111/j.1365-2125.2012.04198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 8.Abdallah CG, Averill LA, Krystal JH. Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Ann N Y Acad Sci. 2015;1344:66–77. doi: 10.1111/nyas.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134–42. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 11.Valentine GW, Mason GF, Gomez R, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191(2):122–7. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry. 2015;172(10):950–66. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 13.Caddy C, Giaroli G, White TP, et al. Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy. Ther Adv Psychopharmacol. 2014;4(2):75–99. doi: 10.1177/2045125313507739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fond G, Loundou A, Rabu C, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl) 2014;231(18):3663–76. doi: 10.1007/s00213-014-3664-5. [DOI] [PubMed] [Google Scholar]
- 15.McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693–704. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- 16.Romeo B, Choucha W, Fossati P, Rotge JY. Meta-analysis of short- and mid-term efficacy of ketamine in unipolar and bipolar depression. Psychiatry Res. 2015;230(2):682–8. doi: 10.1016/j.psychres.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 17.Aan Het Rot M, Zarate CA, Jr, Charney DS, Mathew SJ. Ketamine for Depression: Where Do We Go from Here? Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim L, Diaz Granados N, Jolkovsky L, et al. A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012;32(4):551–7. doi: 10.1097/JCP.0b013e31825d70d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14(8):1127–31. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 20.Chilukuri H, Reddy NP, Pathapati RM, et al. Acute antidepressant effects of intramuscular versus intravenous ketamine. Indian J Psychol Med. 2014;36(1):71–6. doi: 10.4103/0253-7176.127258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapidus KA, Levitch CF, Perez AM, et al. A Randomized Controlled Trial of Intranasal Ketamine in Major Depressive Disorder. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blier P, Zigman D, Blier J. On the safety and benefits of repeated intravenous injections of ketamine for depression. Biol Psychiatry. 2012;72(4):e11–2. doi: 10.1016/j.biopsych.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 23.Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74(4):250–6. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh JB, Fedgchin M, Daly D, et al. 144 - A Double-Blind, Randomized, Placebo-Controlled, Parallel Group, Dose Frequency Study of Intravenous Ketamine in Patients with Treatment-Resistant Depression. Society of Biological Psychiatry; New York, NY: 2014. [DOI] [PubMed] [Google Scholar]
- 25.Singh JB, Fedgchin M, Daly E, et al. Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33(1):18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 27.Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4(1):a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- 29.Kempton MJ, Salvador Z, Munafo MR, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Archives of General Psychiatry. 2011;68(7):675–90. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 30.Kassem MS, Lagopoulos J, Stait-Gardner T, et al. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol. 2013;47(2):645–61. doi: 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- 31.Beattie EC, Stellwagen D, Morishita W, et al. Control of synaptic strength by glial TNFalpha. Science. 2002;295(5563):2282–5. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 32.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13(1):22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang HJ, Voleti B, Hajszan T, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18(9):1413–7. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuen EY, Wei J, Liu W, et al. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73(5):962–77. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60(5):1650–7. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- 36.Krystal JH, Sanacora G, Duman RS. Rapid-Acting Glutamatergic Antidepressants: The Path to Ketamine and Beyond. Biol Psychiatry. 2013;73(12):1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Ota KT, Liu RJ, Voleti B, et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med. 2014 doi: 10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li N, Liu RJ, Dwyer JM, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69(8):754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia LS, Comim CM, Valvassori SS, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):140–4. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 43.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(43):11496–500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maeng S, Zarate CA, Jr, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–52. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 46.Liu RJ, Lee FS, Li XY, et al. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71(11):996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chowdhury GM, Behar KL, Cho W, et al. (1)H-[(1)(3)C]-nuclear magnetic resonance spectroscopy measures of ketamine’s effect on amino acid neurotransmitter metabolism. Biol Psychiatry. 2012;71(11):1022–5. doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abekawa T, Honda M, Ito K, Koyama T. Effects of NRA0045, a novel potent antagonist at dopamine D4, 5-HT2A, and alpha1 adrenaline receptors, and NRA0160, a selective D4 receptor antagonist, on phencyclidine-induced behavior and glutamate release in rats. Psychopharmacology. 2003;169(3–4):247–56. doi: 10.1007/s00213-003-1517-8. [DOI] [PubMed] [Google Scholar]
- 49.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281(5381):1349–52. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 50.Neymotin SA, Lazarewicz MT, Sherif M, et al. Ketamine disrupts theta modulation of gamma in a computer model of hippocampus. J Neurosci. 2011;31(32):11733–43. doi: 10.1523/JNEUROSCI.0501-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37(1):4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11(10):682–96. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller OH, Yang L, Wang CC, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife. 2014;3:e03581. doi: 10.7554/eLife.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224(1):107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 55.Zhou W, Wang N, Yang C, et al. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry. 2014;29(7):419–23. doi: 10.1016/j.eurpsy.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Koike H, Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res. 2014;271:111–5. doi: 10.1016/j.bbr.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 57.Lepack AE, Fuchikami M, Dwyer JM, et al. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2015;18(1) doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16(11):1068–70. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu RJ, Fuchikami M, Dwyer JM, et al. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology. 2013;38(11):2268–77. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghasemi M, Raza M, Dehpour AR. NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test. J Psychopharmacol. 2010;24(4):585–94. doi: 10.1177/0269881109104845. [DOI] [PubMed] [Google Scholar]
- 61.Lester HA, Lavis LD, Dougherty DA. Ketamine Inside Neurons? Am J Psychiatry. 2015;172(11):1064–6. doi: 10.1176/appi.ajp.2015.14121537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moaddel R, Abdrakhmanova G, Kozak J, et al. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in alpha7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698(1–3):228–34. doi: 10.1016/j.ejphar.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams NR, Schatzberg AF. NMDA antagonist treatment of depression. Curr Opin Neurobiol. 2016;36:112–7. doi: 10.1016/j.conb.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Duman RS, Aghajanian GK. Neurobiology of rapid acting antidepressants: role of BDNF and GSK-3beta. Neuropsychopharmacology. 2014;39(1):233. doi: 10.1038/npp.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends in neurosciences. 2011 doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Breier A, Malhotra AK, Pinals DA, et al. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. The American journal of psychiatry. 1997;154(6):805–11. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- 67.Vollenweider FX, Leenders KL, Oye I, et al. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 1997;7(1):25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- 68.Vollenweider FX, Leenders KL, Scharfetter C, et al. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG) European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 1997;7(1):9–24. doi: 10.1016/s0924-977x(96)00039-9. [DOI] [PubMed] [Google Scholar]
- 69.Hyder F, Rothman DL, Bennett MR. Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc Natl Acad Sci U S A. 2013;110(9):3549–54. doi: 10.1073/pnas.1214912110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murrough JW, Collins KA, Fields J, et al. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry. 2015;5:e509. doi: 10.1038/tp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cornwell BR, Salvadore G, Furey M, et al. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry. 2012;72(7):555–61. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Lazzaro V, Oliviero A, Profice P, et al. Ketamine increases human motor cortex excitability to transcranial magnetic stimulation. J Physiol. 2003;547(Pt 2):485–96. doi: 10.1113/jphysiol.2002.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duncan WC, Sarasso S, Ferrarelli F, et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2013;16(2):301–11. doi: 10.1017/S1461145712000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haile CN, Murrough JW, Iosifescu DV, et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. 2014;17(2):331–6. doi: 10.1017/S1461145713001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Machado-Vieira R, Yuan P, Brutsche N, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009;70(12):1662–6. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abdallah CG, Jackowski A, Sato JR, et al. Prefrontal cortical GABA abnormalities are associated with reduced hippocampal volume in major depressive disorder. Eur Neuropsychopharmacol. 2015;25(8):1082–90. doi: 10.1016/j.euroneuro.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abdallah CG, Coplan JD, Jackowski A, et al. A pilot study of hippocampal volume and N-acetylaspartate (NAA) as response biomarkers in riluzole-treated patients with GAD. Eur Neuropsychopharmacol. 2013;23(4):276–84. doi: 10.1016/j.euroneuro.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abdallah CG, Salas R, Jackowski A, et al. Hippocampal volume and the rapid antidepressant effect of ketamine. J Psychopharmacol. 2015;29(5):591–5. doi: 10.1177/0269881114544776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salvadore G, Cornwell BR, Colon-Rosario V, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65(4):289–95. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salvadore G, Cornwell BR, Sambataro F, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35(7):1415–22. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 83.Fuchikami M, Thomas A, Liu R, et al. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc Natl Acad Sci U S A. 2015;112(26):8106–11. doi: 10.1073/pnas.1414728112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu RJ, Ota KT, Dutheil S, et al. Ketamine Strengthens CRF-Activated Amygdala Inputs to Basal Dendrites in mPFC Layer V Pyramidal Cells in the Prelimbic but not Infralimbic Subregion, A Key Suppressor of Stress Responses. Neuropsychopharmacology. 2015;40(9):2066–75. doi: 10.1038/npp.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salvadore G, van der Veen JW, Zhang Y, et al. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2011:1–10. doi: 10.1017/S1461145711001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Milak MS, Proper CJ, Mulhern ST, et al. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.D’Andrea D, Andrew Sewell R. Transient Resolution of Treatment-Resistant Posttraumatic Stress Disorder Following Ketamine Infusion. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 89.Feder A, Parides MK, Murrough JW, et al. Efficacy of Intravenous Ketamine for Treatment of Chronic Posttraumatic Stress Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.62. [DOI] [PubMed] [Google Scholar]
- 90.Bloch MH, Wasylink S, Landeros-Weisenberger A, et al. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2012;72(11):964–70. doi: 10.1016/j.biopsych.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rodriguez CI, Kegeles LS, Levinson A, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38(12):2475–83. doi: 10.1038/npp.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dakwar E, Levin F, Foltin RW, et al. The Effects of Subanesthetic Ketamine Infusions on Motivation to Quit and Cue-Induced Craving in Cocaine-Dependent Research Volunteers. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66(5):522–6. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]