Abstract
Objective
To examine the use, as well as postoperative and long-term oncologic outcomes of diverting loop ileostomy (DI) during primary debulking surgery (PDS) for ovarian cancer.
Methods
Patients with stage II–IV ovarian, fallopian tube, or primary peritoneal carcinoma who underwent colon resection during PDS from 1/2005–1/2014 were identified. Demographic and clinical data were analyzed.
Results
Of 331 patients, 320 (97%) had stage III/IV disease and 278 (84%) had disease of high-grade serous histology. Forty-four (13%) underwent a DI. There were no significant differences in age, comorbidity index, smoking status, serum albumin, or attending surgeon between the DI and non-DI groups. Operative time (OR=1.21; 95% CI, 1.03–1.42; p=.02) and length of rectosigmoid resection (OR=1.04; 95% CI, 1.01–1.08; p=.02) were predictors of DI on multivariable analysis. The overall anastomotic leak rate was 6%. A comparison of groups (DI vs non-DI) showed no significant differences in major complications (30% vs 23%; p=.41), anastomotic leak rate (5% vs 7%; p=.60), hospital length of stay (10 vs 9 days; p=.25), readmission rate (23% vs 17%; p=.33), or interval to postoperative chemotherapy (41 vs 40 days; p=.20), respectively. Ileostomy reversal was successful in 89% of patients. Median follow-up was 52.6 months. There were no differences in median progression-free (17.9 vs 18.6 months; p=.88) and overall survival (48.7 vs 63.8 months; p=.25) between the groups.
Conclusions
In patients undergoing PDS, those with longer operative time and greater length of rectosigmoid resection more commonly underwent DI. DI does not appear to compromise postoperative outcomes or long-term survival.
Keywords: diverting ileostomy, ovarian cancer, primary debulking surgery, rectosigmoid resection, anastomotic leak, postoperative outcomes
Introduction
Ovarian cancer is the most lethal gynecologic malignancy and fifth most common cause of cancer-related mortality in US women [1]. The majority of cases are diagnosed at an advanced stage, and because metastasis occurs by peritoneal seeding, disease often disseminates to the visceral peritoneum, cul-de-sac, and serosa of the rectosigmoid. Maximal tumor cytoreduction is one of the most important determinants of survival in ovarian cancer [2]. In order to achieve optimal debulking or complete gross resection of all visible disease, rectosigmoid and other large bowel resections are frequently performed.
Anastomotic leak (AL) is a life-threatening complication of large bowel resections. Reported rates of AL in the colorectal literature range from 1–19%, with rectosigmoid leaks being the most common [3]. Although less well studied, the reported incidence of AL in the gynecologic oncology literature ranges from 1.7% to 6.8% [4–9]. Morbidity can range from wound infection and intraabdominal abscesses to fecal peritonitis and septicemia, requiring intensive care unit (ICU) admission and reoperation. Mortality rates associated with AL are as high as 16% [10–13]. For ovarian cancer patients undergoing primary debulking surgery (PDS), this complication may also lead to the delayed administration of postoperative chemotherapy.
Diverting ileostomies (DIs) have been extensively studied as a protective measure in patients undergoing bowel resection. Several prospective trials in colorectal cancer have shown DIs to be associated with reduced morbidity from symptomatic leaks [14–16]. DIs, however, can be associated with their own morbidities, such as tissue necrosis, retraction, prolapse, stricture, high output leading to dehydration and renal failure, and complications related to surgical reversal [17–19].
Little has been published regarding the use of DIs at the time of PDS for ovarian cancer. Patient selection is challenging, largely due to the lack of well-established preoperative and intraoperative factors predictive of AL in this population. In patients with colorectal cancer, risk factors for AL include male gender, obesity, smoking and alcohol use, poor nutritional status, chronic steroid use, prior pelvic radiation, low preoperative serum albumin, long operative times, and distance of the anastomosis from the anal verge [20]. In ovarian cancer patients, serum albumin ≤3 g/dL and multiple bowel resections have been identified as potential factors associated with AL, although these findings have not been consistently reproduced [4, 7, 9].
The objective of this study was to describe the use of DIs at the time of PDS for ovarian cancer at a high-volume, comprehensive cytoreductive surgery program. We also sought to examine the associated postoperative and long-term oncologic outcomes in these patients.
Methods
Following Institutional Review Board (IRB) approval, all patients with International Federation of Gynecology and Obstetrics (FIGO) stage II to IV ovarian, fallopian tube, or primary peritoneal carcinoma who underwent large bowel resection during PDS at our institution between 1/2005 and 1/2014 were identified. Patients were excluded if they had received neoadjuvant chemotherapy prior to attempted PDS. Demographic, clinicopathologic, chemotherapy, and outcomes data were abstracted from medical records. Total intraoperative intravenous (IV) fluid was defined as the total volume of crystalloid, colloid, packed red blood cells, platelets, and fresh frozen plasma administered during surgery.
Patients were separated into two groups depending on the type of large bowel resection performed. Those who underwent rectosigmoid resection with or without additional resection of descending colon were placed into one group, while those who underwent ileocecal, ascending, transverse, or descending colon resection without rectosigmoid resection were placed in a second group. Details regarding length of bowel resection (measured in cm) were obtained from pathology reports. Adverse events were prospectively captured and graded according to the Memorial Sloan Kettering Cancer Center (MSK) institutional surgical secondary events grading system [21]. Age-Adjusted Charlson Comorbidity Index (AACCI) was calculated according to previously established criteria [22].
The date of first recurrence or disease progression was determined by computed tomography (CT) scan. The appearance of one or more new lesions on CT imaging resulting in initiation of a new chemotherapy regimen was considered a recurrence. Increased size of an existing lesion resulting in a change in treatment regimen was considered as progression. Progression-free survival (PFS) was defined as the time from PDS until recurrence, progression, death, or last follow-up. For patients alive without disease at the time of analysis or lost to follow-up, PFS was censored at the date of last follow-up. Overall survival (OS) was defined as the time from PDS until death or last follow-up. For patients alive at the time of analysis or lost to follow-up, OS was censored at the date of last documented vital status.
Statistical analysis
Categorical variables were compared using the chi square test or the Fisher exact test. Median values for continuous variables were compared using the Mann-Whitney U test. Factors associated with DI formation were assessed with univariate and multivariable logistic regression. The Kaplan-Meier method was used to estimate survival outcomes. Survival distributions were compared using the logrank test, and factors associated with survival outcomes were analyzed using the Cox proportional hazards model. Median follow-up time was calculated using the Kaplan-Meier estimate of potential follow-up [23, 24]. Clinically significant variables and variables with a p value <.1 on univariate analysis were subsequently included in a multivariable analysis; all variables were tested for multicollinearity. Calculated p values were two-tailed, and p values <.05 were considered significant. Statistical analyses were performed using IBM SPSS for Windows, Version 22.0 (IBM Corporation, Armonk, NY).
Results
We identified 331 patients who met inclusion criteria. Demographic and clinicopathologic characteristics for the entire cohort are summarized in Table S1. Median age was 61 years (range, 26–91), and median serum albumin was 4.1 g/dL (range, 2.5–4.9). Almost all patients had stage IIIC or IV disease (95%), and the majority were diagnosed with disease of high-grade serous histology (84%). Optimal cytoreduction (residual disease ≤1 cm) was achieved in 91% of cases, with complete gross resection in 50% of the entire cohort. Eighty-five percent underwent rectosigmoid resection, and 36% underwent other colon resections. One bowel resection was performed in 77% of patients, and ≥2 bowel resections were performed in 23%. Median postoperative hospital length of stay (LOS) was 9 days (range, 3–69). Eighty patients (24%) had ≥1 grade 3–4 post-surgical complications. The 30- and 60-day readmission rates were 18% and 23%, respectively. Median time from surgery to administration of postoperative chemotherapy was 40 days (range, 9–115).
Of the 331 patients, 44 underwent primary intestinal diversion with loop ileostomy (13%). Preoperative and operative data are shown in Table 1. There were no significant differences in age, body mass index (BMI), smoking status, comorbidity index, serum albumin, or volume of ascites between those who underwent DI and those who did not (non-DI group). Diabetes (14% vs 4%), coronary artery disease (7% vs 1%), higher estimated blood loss (1.2 L vs 1.0 L), higher intraoperative IV fluid volume (9.9 L vs 7.7 L), and greater total length of colon resection (28.5 cm vs 20.4 cm) were significantly associated with DI formation on univariate analysis; however, these relationships were lost after adjusting for multiple factors on multivariable analysis (Table 2). Longer operative time (8.1 vs 6.2 h; p=.02) and greater length of rectosigmoid resection (20.5 cm vs 15.5 cm; p=.02) were significant predictors of DI formation on univariate and multivariable analysis. Of note, attending surgeon was not associated with DI formation on univariate or multivariable logistic regression models.
Table 1.
Preoperative and operative characteristics of patients who did and did not undergo a diverting ileostomy
| Variable | No DI (n=287) | DI (n=44) | p | ||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Age at surgery, yearsa (range) | 61 (26–89) |
63 (54–91) |
.11 | ||
| BMI, kg/m2a (range) | 26.0 (16.3–50.9) |
24.5 (19.1–42.7) |
.13 | ||
| Current smoker | |||||
| No | 270 | 94 | 41 | 93 | .74 |
| Yes | 17 | 6 | 3 | 7 | |
| Former smoker | |||||
| No | 180 | 63 | 27 | 61 | .86 |
| Yes | 107 | 37 | 17 | 39 | |
| Hypertension | |||||
| No | 192 | 67 | 31 | 70 | .64 |
| Yes | 95 | 33 | 13 | 30 | |
| Diabetes | |||||
| No | 275 | 96 | 38 | 86 | .01 |
| Yes | 12 | 4 | 6 | 14 | |
| Coronary artery disease | |||||
| No | 284 | 99 | 41 | 93 | .03 |
| Yes | 3 | 1 | 3 | 7 | |
| AACCI group | |||||
| Low (score 0–1) | 123 | 43 | 15 | 34 | .45 |
| Intermediate (score 2–3) | 134 | 47 | 25 | 57 | |
| High (score ≥ 4) | 30 | 10 | 4 | 9 | |
| Stage | |||||
| II | 8 | 3 | 3 | 7 | .16 |
| III | 205 | 71 | 26 | 59 | |
| IV | 74 | 26 | 15 | 34 | |
| Histology | |||||
| High-grade serous | 238 | 83 | 40 | 91 | .37 |
| Low-grade serous | 14 | 5 | 0 | 0 | |
| Endometrioid | 4 | 1 | 1 | 2 | |
| Clear Cell | 3 | 1 | 1 | 2 | |
| Mixed/Other | 28 | 10 | 2 | 5 | |
| Preoperative CA-125, U/mLa (range) |
480 (9–28503) |
612 (55–8816) |
.73 | ||
| Preoperative hemoglobin, g/dLa (range) |
12.5 (8.6–14.8) |
12.1 (8.4–14.9) |
.07 | ||
| Pre-operative albumin, g/dLa (range) |
4.1 (2.5–4.9) |
4.2 (2.5–4.9) |
.52 | ||
| Ascites, La (range) | 1.0 (0.0–13.0) |
0.8 (0.0–12.0) |
.45 | ||
| Estimated blood loss, La (range) | 1.0 (0.1–5.0) |
1.2 (0.5–6.0) |
.01 | ||
| Total intraoperative IV fluids, La (range) |
7.7 (1.3–23.7) |
9.9 (3.3–19.9) |
.04 | ||
| Operative time, h (range)a | 6.2 (2.1–14.9) |
8.1 (3.1–12.2) |
<.01 | ||
| Intraoperative complication | |||||
| No | 243 | 85 | 32 | 73 | .05 |
| Yes | 44 | 15 | 12 | 27 | |
| Colon resection, non-rectosigmoid/ descending |
|||||
| No | 187 | 65 | 26 | 59 | .43 |
| Yes | 100 | 35 | 18 | 41 | |
| Rectosigmoid/descending colon resection |
|||||
| No | 47 | 16 | 1 | 2 | .01 |
| Yes | 240 | 84 | 43 | 98 | |
| Length of rectosigmoid and descending colon resection, cma (range) |
15.5 (0.0–53.2) |
20.5 (0.0–54.8) |
<.01 | ||
| Total number of colon resections | |||||
| 1 | 229 | 80 | 27 | 61 | <.01 |
| 2 or more | 58 | 20 | 17 | 39 | |
| Total length of colon resection, cma (range) |
20.4 (1.5–108.5) |
28.5 (6.0–88.0) |
<.01 | ||
| Cytoreductive status | |||||
| Optimal | 263 | 92 | 39 | 89 | .57 |
| Suboptimal | 24 | 8 | 5 | 11 | |
| Residual disease | |||||
| 0 cm | 147 | 51 | 20 | 45 | .70 |
| 0.1–1 cm | 116 | 40 | 19 | 43 | |
| > 1 cm | 24 | 8 | 5 | 11 | |
DI, diverting ileostomy; AACCI, Age-Adjusted Charlson Comorbidity Index
Median
Table 2.
Factors associated with diverting ileostomy formation – univariate and multivariable logistic regression
| Variable | Univariate | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p | Adjusted OR (95% CI) |
p | |
| Age at surgery, years | 1.03 (1.0 – 1.06) | .05 | 1.03 (0.99 – 1.07) | .14 |
| BMI, kg/m2 | 0.96 (0.90 – 1.02) | .18 | ||
| Current smoker | ||||
| No | Referent | -- | ||
| Yes | 1.16 (0.33 – 4.14) | .82 | ||
| Former smoker | ||||
| No | Referent | -- | ||
| Yes | 1.06 (0.55 – 2.03) | .86 | ||
| Hypertension | ||||
| No | Referent | -- | ||
| Yes | 0.85 (0.42 – 1.69) | .64 | ||
| Diabetes | ||||
| No | Referent | -- | Referent | -- |
| Yes | 3.62 (1.28 – 10.21) |
.02 | 2.53 (0.76 – 8.40) | .13 |
| Coronary artery disease | ||||
| No | Referent | -- | Referent | -- |
| Yes | 6.93 (1.35 – 35.48) |
.02 | 3.10 (0.47 – 20.39) | .24 |
| AACCI group | ||||
| Low (score 0–1) | Referent | -- | ||
| Intermediate (score 2–3) | 1.53 (0.77 – 3.04) | .22 | ||
| High (score ≥ 4) | 1.09 (0.34 – 3.53) | .88 | ||
| Preoperative hemoglobin, g/dL | 0.80 (0.63 – 1.02) | .07 | 0.80 (0.61 – 1.07) | .13 |
| Preoperative albumin, g/dL | 1.16 (0.55 – 2.43) | .70 | ||
| Ascites, L | 0.97 (0.84 – 1.12) | .67 | ||
| Estimated blood loss, L | 1.46 (1.13 – 1.89) | <.01 | 1.07 (0.71 – 1.61) | .76 |
| Total intraoperative IV fluids, La | 1.09 (1.01 – 1.17) | .02 | ||
| Operative time, h | 1.33 (1.15 – 1.53) | <.01 | 1.21 (1.03 – 1.42) | .02 |
| Intraoperative complication | ||||
| No | Referent | -- | Referent | -- |
| Yes | 2.07 (0.99 – 4.33) | .05 | 0.82 (0.29 – 2.30) | .70 |
| Colon resection, non- rectosigmoid/descending |
||||
| No | Referent | -- | ||
| Yes | 1.30 (0.68 – 2.48) | .44 | ||
| Rectosigmoid/descending colon resectiona |
||||
| No | Referent | -- | ||
| Yes | 8.42 (1.13 – 62.67) |
.04 | ||
| Total number of colon resectionsa | ||||
| 1 | Referent | -- | ||
| 2 or more | 2.49 (1.27 – 4.87) | <.01 | ||
| Length of rectosigmoid and descending colon resection, cm |
1.05 (1.02 – 1.08) | <.01 | 1.04 (1.01 – 1.08) | .02 |
| Total length of colon resection, cm | 1.02 (1.00 – 1.04) | .01 | 1.00 (0.98 – 1.02) | .68 |
| Surgeonb | NS | NS | ||
BMI, body mass index; AACCI, Age-Adjusted Charlson Comorbidity Index; NS, non significant
Not included in multivariable analysis due to collinearity/confounding
Individual attending surgeons not listed, however OR was non significant for all 10 surgeons (range p=.12 to .99)
Distance of the anastomosis from the anal verge was only documented in 137 of 283 patients (48.4%) who underwent rectosigmoid resection. Thus, these data were not included in the statistical analysis of factors associated with ileostomy formation. For documented cases, median distance was 8 cm in the DI group (n=30/43; range, 3.5–12 cm) and 10 cm in the non-DI group (n=107/240; range, 5–18 cm). Nine patients in the DI group and 2 in the non-DI group had anastomoses within 6 cm of the anal verge.
Eight of 44 patients had documented indications for ileostomy formation, including: low rectosigmoid anastomosis (n=2), extensive colon resection (n=3), and anastomotic defect noted on intraoperative testing (n=3).
To evaluate short-term outcomes, we assessed postoperative hospital LOS, 30-day complication rates (including major complications, gastrointestinal [GI] complications, and complications related to surgical site infections), and readmission rates (Table 3). In patients with and without ileostomies, there were no significant differences in median hospital LOS (10 days vs 9 days; p=.25), complication rates (major complications 30% vs 23%; p=.41), 30- or 60-day readmission rates (23% vs 17%, p=.33; and 32% vs 22%, p=.13, respectively), or median time interval from surgery to the start of postoperative chemotherapy (41 days vs 40 days; p=.02). When assessing the impact of DI on surgical site infections, the rate of culture-positive wound infections was 7% in those who underwent DI compared with 13% in those who did not undergo DI. This finding was not statistically significant, possibly due to the small number of total wound infections. The rate of intra-abdominal abscess in patients who underwent DI versus those who did not was 20% versus 8%, respectively (p=.02). However, statistical significance was lost after adjusting for multiple preoperative and intraoperative factors on multivariable logistic regression.
Table 3.
Postoperative outcomes associated with diverting ileostomy
| Variable | No DI (n=287) |
DI (n=44) |
p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Median postoperative length of stay, days (range) | 9 (3–69) |
10 (5–30) |
.25 | ||
| Major (Grade 3/4/5) postoperative complications | |||||
| 0 | 220 | 77 | 31 | 70 | .41 |
| 1–2 | 63 | 22 | 13 | 30 | |
| 3 or more | 4 | 1 | 0 | 0 | |
| Gastrointestinal complications | |||||
| No | 212 | 74 | 32 | 72.7 | .87 |
| Yes | 75 | 26 | 12 | 27.3 | |
| Anastomotic leak | |||||
| No | 268 | 93 | 42 | 95.5 | .60 |
| Yes | 19 | 7 | 2 | 4.5 | |
| Intra-abdominal abscess | |||||
| No | 264 | 92 | 35 | 79.5 | .02a |
| Yes | 23 | 8 | 9 | 20.5 | |
| Wound infection | |||||
| No | 250 | 87 | 41 | 93.2 | .25 |
| Yes | 37 | 13 | 3 | 6.8 | |
| Readmission within 30 days of surgery | |||||
| No | 239 | 83 | 34 | 77.3 | .33 |
| Yes | 48 | 17 | 10 | 22.7 | |
| Readmission within 60 days of surgery | |||||
| No | 225 | 78 | 30 | 68.2 | .13 |
| Yes | 62 | 22 | 14 | 31.8 | |
| Median time from surgery to start of chemotherapy, days (range) |
40 (9–115) |
41 (11–75) |
.20 | ||
| Number of postoperative chemotherapy cycles | |||||
| 0–4 | 10 | 3.6 | 2 | 4.8 | .66 |
| 5 or more | 269 | 96.4 | 40 | 95.2 | |
DI, diverting ileostomy
Non-significant on multivariable analysis
With regards to ALs, the leak rate for the entire cohort was 6% (n=21): 4.5% (n=2) in the DI group and 7% (n=19) in the non-DI group. We attempted to identify risk factors associated with AL by assessing multiple variables (including age, BMI, smoking status, history of smoking, co-morbidities, prior pelvic radiation, American Society of Anesthesiologists [ASA] score, preoperative serum albumin, total number of large bowel resections, total length of large bowel resection, estimated blood loss, intraoperative IV fluid administration, operative time, and presence of operative complications). Median serum albumin was 4.1 g/dL in both patients with AL and those without AL (with AL, range 3.2–4.6; without AL, range 2.5–4.9), and >1 large bowel resection was performed in 24% of those with AL compared with 23% of those without AL. None of the abovementioned variables were significantly associated with AL. The inability to identify statically significant risk factors for AL may be due to the low number of leaks in our cohort. Incomplete data regarding the distance of the anastomosis from the anal verge precluded the use of this variable for statistical analysis of risk factors associated with AL. Of the available data, 3 patients with AL and 8 patients without AL had anastomoses within 6 cm of the anal verge.
Of the 2 patients who underwent DI and experienced AL (n=2/44, 4.5%; Figure S1), one presented 19 days after PDS with abnormal vaginal discharge and a collection on CT scan and was treated with IR-guided drainage, while the other was found to have sepsis 5 days after surgery, requiring ICU admission and operative intervention for gross abdominal fecal contamination. Of the 19 patients in the non-DI group who experienced AL (n=19/287, 7%), 12 (63%) were treated with reoperation and proximal diversion (ileostomy n=8, colostomy n=4), 5 (26%) were managed with IR-guided drainage and endoscopic stent placement, and 1 (5%) was treated solely with IR-guided drainage. One patient required exploratory laparotomy; however, dense adhesions precluded access to the bowel, and thus, diversion could not be performed. The median time to chemotherapy administration in patients who developed AL was 55 days (range, 43–115), compared with only 38 days (range, 9–93) for those who did not develop AL (p<.01).
In the DI group, there were 10 readmissions within 30 days after surgery (Figure S2). Three readmissions were ileostomy-related—2 due to high ostomy output and dehydration and the other for appliance leakage causing severe contact dermatitis. Reasons for readmission in the remaining 7 patients were as follows: intra-abdominal abscess (3), anastomotic leak (1), failure to thrive (1), pulmonary embolism (1), and diabetic hypoglycemia (1). The 4 admissions between days 31–60 after surgery were due to small bowel obstruction distant from the ileostomy site (2), intra-abdominal abscess (1), and pulmonary embolism (1). Two patients developed parastomal hernias that were addressed at the time of elective ileostomy reversal. No other ileostomy complications, such as necrosis, retraction or prolapse with incarceration, were noted.
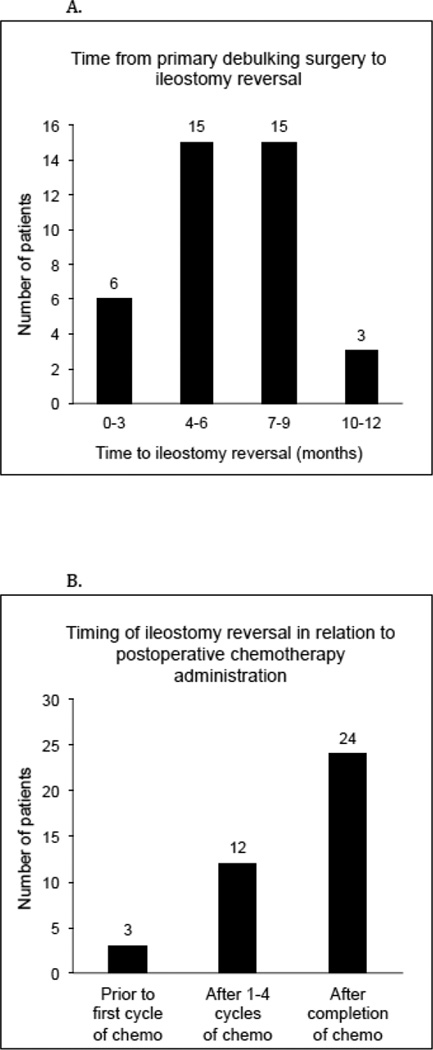
Among the 44 patients who underwent DI formation at the time of PDS, 39 (89%) had successful ileostomy reversals; the remaining 5 patients did not undergo re-establishment of bowel continuity prior to their deaths (4 were deceased within 10 months of surgery and one had an OS of 43.6 months). For those who had their ileostomies reversed, the median time from initial surgery to reversal was 182 days (range, 42–347). The majority (77%) underwent reversal between 4–9 months, although 6 patients (15%) had their ileostomies reversed within 3 months of debulking surgery (Figure 1A). Post ileostomy reversal, 3 patients (n=3/39, 8%) experienced major complications. All 3 were diagnosed with intra-abdominal abscesses. Two were managed conservatively with IR-guided drainage and one required surgical intervention. One additional patient was diagnosed with a stricture at the site of her prior large bowel anastomosis, which became symptomatic after ileostomy closure. The 30-day readmission rate was 13% (n=5). Four patients were readmitted for the reasons cited previously, and one was readmitted for nausea and vomiting.
Figure 1.
Timing of ileostomy reversal. (A) Interval from primary debulking surgery to ileostomy reversal. (B) Timing of ileostomy reversal in relation to postoperative chemotherapy administration.
In consultation with their treatment teams, 3 patients (8%) elected to undergo ileostomy closure prior to starting postoperative chemotherapy, 12 (31%) had their ileostomies reversed after 1–4 cycles of chemotherapy, and 24 (62%) underwent reversal after completion of chemotherapy (Figure 1B). Eleven (31%) of 36 patients who remained diverted during postoperative chemotherapy required additional IV hydration (either by home or outpatient IV hydration up to 3 times per week) while on treatment. Only one patient experienced significant dehydration and failure to thrive, electing to undergo ileostomy closure after 2 cycles of chemotherapy. There was no difference in the number of platinum-based chemotherapy cycles completed between the DI and non-DI groups (95% vs 96% completed 5 or more cycles, respectively, p=.66).
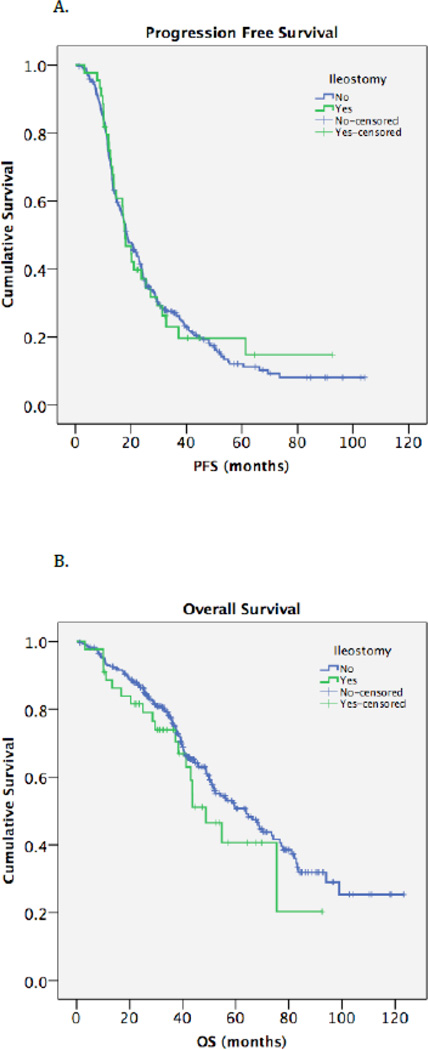
Median follow-up time was 52.6 months (range, 0.7–123.2). For the entire cohort, median PFS was 18.4 months (95% CI, 16.4–20.5) and median OS was 59.6 months (95% CI, 48.6–70.6). DI was not significantly associated with PFS or OS (Table 4, Figures 2A and 2B). To further assess for prognostic significance, age, BMI, smoking status, comorbidities, stage, histology, preoperative CA-125 and serum albumin, volume of ascites, DI, residual disease, time from surgery to the start of chemotherapy, and number of chemotherapy cycles completed were analyzed using Cox proportional hazards models. After adjusting for the aforementioned factors, 3 remained significantly associated with an increased rate of recurrence and death: histology, residual disease >1 cm (PFS: 0 cm referent; 0.1–1 cm, HR=1.29, 95% CI 0.99–1.68, p=.06; >1 cm, HR=2.51, 95% CI 1.59–3.97, p<.01; and OS: 0 cm referent; 0.1–1 cm, HR=1.27, 95% CI 0.86–1.87, p=.24; >1 cm, HR=2.43, 95% CI 1.43–4.13, p<.01), and fewer number of chemotherapy cycles completed (PFS: 0–4 cycles referent; >5 cycles, HR=0.48, 95% CI 0.23–0.99, p=.04; and OS: 0–4 cycles referent; >5 cycles HR=0.19, 95% CI 0.08–0.42, p<.01). These data are summarized in Table S2. Again, DI was not associated with an increased hazard ratio for progression or death.
Table 4.
Progression-free survival (PFS) and overall survival (OS) in patients who did and did not undergo a diverting ileostomy
| Median PFS (mo) |
95% CI | p | Median OS (mo) | 95% CI | p | |
|---|---|---|---|---|---|---|
| Entire cohort | 18.4 | 16.4 – 20.5 | -- | 59.6 | 48.6 – 70.6 | -- |
| Ileostomy | ||||||
| No | 18.6 | 16.1 – 21.2 | .88 | 63.8 | 52.2 – 75.3 | .25 |
| Yes | 17.9 | 14.1 – 21.8 | 48.7 | 34.5 – 63.0 | ||
| Median follow-up |
52.6 months (range, 0.7–123.2) | |||||
Figure 2.
Kaplan-Meier survival curves for progression-free survival (A) and overall survival (B) in patients who underwent diverting ileostomy and those who did not.
Discussion
The primary treatment of advanced ovarian cancer involves maximal tumor cytoreduction, as optimal tumor debulking to minimal residual disease followed by platinum-based chemotherapy affords patients the greatest survival advantage. In order to accomplish this, large bowel resections are often necessary. DIs are commonly used to abate the high morbidity and mortality associated with ALs. However, DIs are associated with inherent complications and require a second operation for reversal. They can also have profound psychological effects and a significant impact on quality of life [25, 26]. The current study highlights the challenges in determining appropriate indications for DI and comprehensively addresses the impact of DIs in the context of PDS for ovarian cancer.
Only 2 factors were found to be significantly associated with the decision to perform a DI during PDS for advanced ovarian cancer—longer operative time and increased length of rectosigmoid resection. Notably, individual attending surgeon was not associated with ileostomy formation on univariate or multivariable analysis. The importance of operative time and length of rectosigmoid resection, therefore, reflect the viewpoints of a group of high-volume gynecologic oncology surgeons. It is reasonable to assume that operative time reflects the complexity of the surgical resection. With regard to length of rectosigmoid resection, this may represent a surrogate for anastomotic tension; however, further prospective evaluation would be needed to clarify this.
Thirty-day postoperative outcomes were similar between patients who underwent DI and those who did not. We did not observe any significant differences in hospital LOS, rate of major complications or GI complications (including diarrhea, ileus, small/large bowel obstruction, GI bleed, and bowel ischemia) between the two groups, which is consistent with the colorectal literature [27]. Many colorectal cancer studies have found that diverting stomas decrease the incidence of ALs [14–16], although the body of literature as a whole is somewhat conflicting and, therefore, this association is still widely debated. The rate of AL in this study was slightly lower in those who were diverted compared with those who were not (4.5% vs 7%, respectively), although the difference was not statistically significant. This suggests that DIs did not drastically decrease the rate of AL in our patient population, possibly because we have yet to correctly identify those who are at highest risk for this complication, our sample size was too small to detect a statistically significant difference in AL rate, or because DIs do not decrease the incidence of ALs in ovarian cancer patients undergoing PDS. It is also difficult to draw any conclusions about the effects of DI on AL-associated morbidity due to the extremely low incidence of leaks in our DI group.
Thirty- and 60-day readmission rates were also similar between those with and without DIs. Recently, Glasgow et al. reported on 53 patients with gynecologic malignancies who underwent DI formation [28]. The authors cited a 34% 30-day readmission rate, which is higher than the 60-day readmission rate of 16–21% published in the colorectal literature [17, 29], as well as our rate of 23%. This can be explained by differing patient populations. The study by Glasgow included gynecologic oncology patients who required fecal diversion for any reason, including small bowel obstruction (45.3%), bowel perforation (13.2%), treatment of AL (15.1%), PDS (18.9%), and other (7.6%). In comparison, our study consisted only of patients newly diagnosed with ovarian cancer, those healthy enough to undergo extensive cytoreductive surgery, and those who did not require urgent surgery, and were therefore generally less likely to suffer complications.
Dehydration is cited as the most common ileostomy-related complication, accounting for 40–47% of all readmissions [17, 29, 30]. Decreased small bowel transit time and reduced colonic water absorption associated with ileostomies can result in significant fluid and electrolyte abnormalities. In our study, only 4.5% of patients with ileostomies were readmitted for dehydration secondary to high ostomy output, accounting for 20% of all 30-day readmissions. This rate may be lower than those of previously published reports as a result of differences in patient population as well as extensive patient education regarding hydration and titration of anti-diarrheal medications, aggressive surveillance by nursing staff, close follow-up, and servicing a well-educated population. Several prospective studies have successfully employed educational interventions and “ileostomy pathways,” resulting in decreased hospital LOS, reduced stoma-related complications, and improved quality of life [31–33]. However, 2 systematic reviews on this topic demonstrated conflicting results: one concluded that structured patient education programs reduced hospital costs and improved quality of life [34], while the other found the use of educational interventions for new ostomates have limited evidence for improvement in clinical outcomes [35]. Both highlighted the heterogeneity of the interventions and study designs used and stated that additional research is needed before any definitive conclusions can be made. Thus, until further data are available, we should continue to provide comprehensive education, counseling, and follow-up care to this patient population.
Platinum-based chemotherapy is a crucial part of primary treatment for newly diagnosed ovarian cancer. Timely initiation of chemotherapy is essential in order to avoid recurrence or progression of disease after cytoreductive surgery. Importantly, DIs were not associated with any delay in the initiation of chemotherapy treatment. Although the readmission rate for dehydration secondary to high ileostomy output was only 4.5% and none of these patients required routine IV hydration, we found that a much larger proportion of these patients (30.5%) required additional outpatient or home IV hydration, up to 3 times per week, while being treated with chemotherapy. It is possible that chemotherapy-related dehydration is augmented in DI patients compared to non-DI patients; however, we did not evaluate the use of IV hydration in patients without DIs undergoing chemotherapy.
Ileostomy closure was feasible in 88.6% of patients, and major complication and readmission rates were lower than those described in colorectal surgery [19, 36]. Three patients underwent ileostomy reversal between 42 and 49 days after surgery, prior to starting chemotherapy treatment. Given that most symptomatic ALs are diagnosed within the first 46 days after bowel resection [37–40] (between 5–23 days in our cohort) and patients with ileostomies appear to experience increased dehydration while being treated with chemotherapy, ileostomy closure prior to beginning primary chemotherapy could be considered in a highly select group of patients. However, further investigation regarding the optimal timing of DI reversal is warranted.
Long-term oncologic outcomes related to DI formation were evaluated in this study. Similar to limited previously published data in ovarian cancer patients [9], we did not observe any statistically significant difference in median PFS or OS between those who underwent DI and those who did not. This is an important finding, as it supports the oncologic safety of the procedure when it is deemed necessary by the operating surgeon. There was a small, although statistically non-significant difference in median OS between the two groups, with a slightly shorter OS in the DI group (63.8 months vs 48.7 months). This is likely a reflection of poor tumor biology, resulting in greater initial tumor burden, more extensive PDS with the need for protective ileostomy formation, and more aggressive disease, ultimately leading to decreased survival.
Although DIs have the potential to decrease the morbidity and mortality associated with AL, the major problem remains the identification of patients with ovarian cancer undergoing cytoreductive surgery who are at greatest risk for AL. It is these patients in whom the benefits of diversion outweigh the risks, including psychological impact, effect on quality of life, complications associated with DI, and the need for additional surgery for reversal. As such, careful consideration must be exercised when deciding whether to perform a DI.
There are several limitations to this study, including its retrospective nature. This study was performed at a comprehensive cancer care center, and thus, the results may not be generalizable to all surgical practices. We were unable to evaluate distance of the anastomosis from the anal verge as a risk factor for DI or AL due to unavailable data. With regards to postoperative outcomes, we were unable to fully assess whether patients were evaluated or admitted for complications at other hospitals. Additionally, we evaluated the need for routine IV hydration in DI patients prior to and during platinum-based chemotherapy treatment but did not assess the need for IV hydration while receiving chemotherapy in non-DI patients. Chemotherapy itself can cause dehydration, so the association between DIs and exacerbation of chemotherapy-related dehydration remains only a hypothesis. The major strength of this study lies in its clinical relevance and contribution to an important topic for which limited data exist. ALs can lead to catastrophic sequelae, and understanding the implications of management strategies such as DI are crucial to improving care.
In summary, our study demonstrates that in ovarian cancer patients, DIs performed during PDS have acceptably low associated morbidity, high reversal rates, and do not appear to compromise postoperative or long-term oncologic outcomes. However, the optimal use of DIs has yet to be achieved. Since there are limited data regarding DIs in gynecologic oncology, our assumptions and management decisions have largely been extrapolated from the colorectal cancer literature. Further research is necessary to define which patients will benefit most from DI formation and to tailor surgical interventions and postoperative care specifically to our ovarian cancer population.
Supplementary Material
Highlights.
Longer OR time and greater length of rectosigmoid resection were associated with DI formation
DI formation did not appear to compromise postoperative or oncologic outcomes
Further research identifying risk factors for anastomotic leak is imperative
Acknowledgments
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement The authors declare that there are no financial or commercial conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016 Jan;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002 Mar 1;20(5):1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 3.Phitayakorn R, Delaney CP, Reynolds HL, Champagne BJ, Heriot AG, Neary P, et al. Standardized algorithms for management of anastomotic leaks and related abdominal and pelvic abscesses after colorectal surgery. World J Surg. 2008 Jun;32(6):1147–1156. doi: 10.1007/s00268-008-9468-1. [DOI] [PubMed] [Google Scholar]
- 4.Obermair A, Hagenauer S, Tamandl D, Clayton RD, Nicklin JL, Perrin LC, et al. Safety and efficacy of low anterior en bloc resection as part of cytoreductive surgery for patients with ovarian cancer. Gynecol Oncol. 2001 Oct;83(1):115–120. doi: 10.1006/gyno.2001.6353. [DOI] [PubMed] [Google Scholar]
- 5.Mourton SM, Temple LK, Abu-Rustum NR, Gemignani ML, Sonoda Y, Bochner BH, et al. Morbidity of rectosigmoid resection and primary anastomosis in patients undergoing primary cytoreductive surgery for advanced epithelial ovarian cancer. Gynecol Oncol. 2005 Dec;99(3):608–614. doi: 10.1016/j.ygyno.2005.07.112. [DOI] [PubMed] [Google Scholar]
- 6.Tebes SJ, Cardosi R, Hoffman MS. Colorectal resection in patients with ovarian and primary peritoneal carcinoma. Am J Obstet Gynecol. 2006 Aug;195(2):585–589. doi: 10.1016/j.ajog.2006.03.079. discussion 9–90. [DOI] [PubMed] [Google Scholar]
- 7.Richardson DL, Mariani A, Cliby WA. Risk factors for anastomotic leak after recto-sigmoid resection for ovarian cancer. Gynecol Oncol. 2006 Nov;103(2):667–672. doi: 10.1016/j.ygyno.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Peiretti M, Bristow RE, Zapardiel I, Gerardi M, Zanagnolo V, Biffi R, et al. Rectosigmoid resection at the time of primary cytoreduction for advanced ovarian cancer. A multi-center analysis of surgical and oncological outcomes. Gynecol Oncol. 2012 Aug;126(2):220–223. doi: 10.1016/j.ygyno.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Kalogera E, Dowdy SC, Mariani A, Weaver AL, Aletti G, Bakkum-Gamez JN, et al. Multiple large bowel resections: potential risk factor for anastomotic leak. Gynecol Oncol. 2013 Jul;130(1):213–218. doi: 10.1016/j.ygyno.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Docherty JG, McGregor JR, Akyol AM, Murray GD, Galloway DJ. Comparison of manually constructed and stapled anastomoses in colorectal surgery. West of Scotland and Highland Anastomosis Study Group. Ann Surg. 1995 Feb;221(2):176–184. doi: 10.1097/00000658-199502000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alves A, Panis Y, Trancart D, Regimbeau JM, Pocard M, Valleur P. Factors associated with clinically significant anastomotic leakage after large bowel resection: multivariate analysis of 707 patients. World J Surg. 2002 Apr;26(4):499–502. doi: 10.1007/s00268-001-0256-4. [DOI] [PubMed] [Google Scholar]
- 12.Matthiessen P, Hallbook O, Andersson M, Rutegard J, Sjodahl R. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis. 2004 Nov;6(6):462–469. doi: 10.1111/j.1463-1318.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 13.McArdle CS, McMillan DC, Hole DJ. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg. 2005 Sep;92(9):1150–1154. doi: 10.1002/bjs.5054. [DOI] [PubMed] [Google Scholar]
- 14.Huser N, Michalski CW, Erkan M, Schuster T, Rosenberg R, Kleeff J, et al. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg. 2008 Jul;248(1):52–60. doi: 10.1097/SLA.0b013e318176bf65. [DOI] [PubMed] [Google Scholar]
- 15.Tan WS, Tang CL, Shi L, Eu KW. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg. 2009 May;96(5):462–472. doi: 10.1002/bjs.6594. [DOI] [PubMed] [Google Scholar]
- 16.Montedori A, Cirocchi R, Farinella E, Sciannameo F, Abraha I. Covering ileo- or colostomy in anterior resection for rectal carcinoma. Cochrane Database Syst Rev. 2010;(5):CD006878. doi: 10.1002/14651858.CD006878.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Phatak UR, Kao LS, You YN, Rodriguez-Bigas MA, Skibber JM, Feig BW, et al. Impact of ileostomy-related complications on the multidisciplinary treatment of rectal cancer. Ann Surg Oncol. 2014 Feb;21(2):507–512. doi: 10.1245/s10434-013-3287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilney HS, Sains PS, Lovegrove RE, Reese GE, Heriot AG, Tekkis PP. Comparison of outcomes following ileostomy versus colostomy for defunctioning colorectal anastomoses. World J Surg. 2007 May;31(5):1142–1151. doi: 10.1007/s00268-006-0218-y. [DOI] [PubMed] [Google Scholar]
- 19.Chow A, Tilney HS, Paraskeva P, Jeyarajah S, Zacharakis E, Purkayastha S. The morbidity surrounding reversal of defunctioning ileostomies: a systematic review of 48 studies including 6,107 cases. Int J Colorectal Dis. 2009 Jun;24(6):711–723. doi: 10.1007/s00384-009-0660-z. [DOI] [PubMed] [Google Scholar]
- 20.Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg. 2009 Feb;208(2):269–278. doi: 10.1016/j.jamcollsurg.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Strong VE, Selby LV, Sovel M, Disa JJ, Hoskins W, Dematteo R, et al. Development and assessment of Memorial Sloan Kettering Cancer Center's Surgical Secondary Events grading system. Ann Surg Oncol. 2015 Apr;22(4):1061–1067. doi: 10.1245/s10434-014-4141-4. PubMed PMID: 25319579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suidan RS, Leitao MM, Jr, Zivanovic O, Gardner GJ, Long Roche KC, Sonoda Y, et al. Predictive value of the Age-Adjusted Charlson Comorbidity Index on perioperative complications and survival in patients undergoing primary debulking surgery for advanced epithelial ovarian cancer. Gynecol Oncol. 2015 Aug;138(2):246–251. doi: 10.1016/j.ygyno.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996 Aug;17(4):343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 24.Clark TG, Bradburn MJ, Love SB, Altman DG. Survival analysis part I: basic concepts and first analyses. Br J Cancer. 2003 Jul 21;89(2):232–238. doi: 10.1038/sj.bjc.6601118. PubMed PMID: 12865907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canova C, Giorato E, Roveron G, Turrini P, Zanotti R. Validation of a stoma-specific quality of life questionnaire in a sample of patients with colostomy or ileostomy. Colorectal Dis. 2013 Nov;15(11):e692–e698. doi: 10.1111/codi.12324. [DOI] [PubMed] [Google Scholar]
- 26.Vonk-Klaassen SM, de Vocht HM, den Ouden ME, Eddes EH, Schuurmans MJ. Ostomy-related problems and their impact on quality of life of colorectal cancer ostomates: a systematic review. Qual Life Res. 2016 Jan;25(1):125–133. doi: 10.1007/s11136-015-1050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hignett S, Parmar CD, Lewis W, Makin CA, Walsh CJ. Ileostomy formation does not prolong hospital length of stay after open anterior resection when performed within an enhanced recovery programme. Colorectal Dis. 2011 Oct;13(10):1180–1183. doi: 10.1111/j.1463-1318.2010.02381.x. [DOI] [PubMed] [Google Scholar]
- 28.Glasgow MA, Shields K, Vogel RI, Teoh D, Argenta PA. Postoperative readmissions following ileostomy formation among patients with a gynecologic malignancy. Gynecol Oncol. 2014 Sep;134(3):561–565. doi: 10.1016/j.ygyno.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messaris E, Sehgal R, Deiling S, Koltun WA, Stewart D, McKenna K, et al. Dehydration is the most common indication for readmission after diverting ileostomy creation. Dis Colon Rectum. 2012 Feb;55(2):175–180. doi: 10.1097/DCR.0b013e31823d0ec5. [DOI] [PubMed] [Google Scholar]
- 30.Hayden DM, Pinzon MC, Francescatti AB, Edquist SC, Malczewski MR, Jolley JM, et al. Hospital readmission for fluid and electrolyte abnormalities following ileostomy construction: preventable or unpredictable? J Gastrointest Surg. 2013 Feb;17(2):298–303. doi: 10.1007/s11605-012-2073-5. [DOI] [PubMed] [Google Scholar]
- 31.Nagle D, Pare T, Keenan E, Marcet K, Tizio S, Poylin V. Ileostomy pathway virtually eliminates readmissions for dehydration in new ostomates. Dis Colon Rectum. 2012 Dec;55(12):1266–1272. doi: 10.1097/DCR.0b013e31827080c1. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhri S, Brown L, Hassan I, Horgan AF. Preoperative intensive, community-based vs. traditional stoma education: a randomized, controlled trial. Dis Colon Rectum. 2005 Mar;48(3):504–509. doi: 10.1007/s10350-004-0897-0. [DOI] [PubMed] [Google Scholar]
- 33.Younis J, Salerno G, Fanto D, Hadjipavlou M, Chellar D, Trickett JP. Focused preoperative patient stoma education, prior to ileostomy formation after anterior resection, contributes to a reduction in delayed discharge within the enhanced recovery programme. Int J Colorectal Dis. 2012 Jan;27(1):43–47. doi: 10.1007/s00384-011-1252-2. [DOI] [PubMed] [Google Scholar]
- 34.Danielsen AK, Burcharth J, Rosenberg J. Patient education has a positive effect in patients with a stoma: a systematic review. Colorectal Dis. 2013 Jun;15(6):e276–e283. doi: 10.1111/codi.12197. [DOI] [PubMed] [Google Scholar]
- 35.Phatak UR, Li LT, Karanjawala B, Chang GJ, Kao LS. Systematic review of educational interventions for ostomates. Dis Colon Rectum. 2014 Apr;57(4):529–537. doi: 10.1097/DCR.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 36.Wong KS, Remzi FH, Gorgun E, Arrigain S, Church JM, Preen M, et al. Loop ileostomy closure after restorative proctocolectomy: outcome in 1,504 patients. Dis Colon Rectum. 2005 Feb;48(2):243–250. doi: 10.1007/s10350-004-0771-0. [DOI] [PubMed] [Google Scholar]
- 37.Kanellos I, Vasiliadis K, Angelopoulos S, Tsachalis T, Pramateftakis MG, Mantzoros I, et al. Anastomotic leakage following anterior resection for rectal cancer. Tech Coloproctol. 2004 Nov;8(Suppl 1):s79–s81. doi: 10.1007/s10151-004-0119-8. [DOI] [PubMed] [Google Scholar]
- 38.Alves A, Panis Y, Pocard M, Regimbeau JM, Valleur P. Management of anastomotic leakage after nondiverted large bowel resection. J Am Coll Surg. 1999 Dec;189(6):554–559. doi: 10.1016/s1072-7515(99)00207-0. [DOI] [PubMed] [Google Scholar]
- 39.Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it's later than you think. Ann Surg. 2007 Feb;245(2):254–258. doi: 10.1097/01.sla.0000225083.27182.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caulfield H, Hyman NH. Anastomotic leak after low anterior resection: a spectrum of clinical entities. JAMA Surg. 2013 Feb;148(2):177–182. doi: 10.1001/jamasurgery.2013.413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.