Summary
Associative learning is an essential brain process where the contingency of different items increases after training. Associative learning has been found to occur in many brain regions [1-4]. However, there is no clear evidence that associative learning of visual features occurs in early visual areas, although a number of studies have indicated that learning of a single visual feature (perceptual learning) involves early visual areas [5-8]. Here, via decoded functional magnetic resonance imaging (fMRI) neurofeedback, termed “DecNef” [9], we tested whether associative learning of color and orientation can be created in early visual areas. During three days' training, DecNef induced fMRI signal patterns that corresponded to a specific target color (red) mostly in early visual areas while a vertical achromatic grating was physically presented to participants. As a result, participants came to perceive “red” significantly more frequently than “green” in an achromatic vertical grating. This effect was also observed 3 to 5 months after the training. These results suggest that long-term associative learning of the two different visual features such as color and orientation was created most likely in early visual areas. This newly extended technique that induces associative learning is called “A(ssociative)-DecNef” and may be used as an important tool for understanding and modifying brain functions, since associations are fundamental and ubiquitous functions in the brain.
Results and Discussion
The complete experiment consisted of four stages: retinotopic mapping [10], color classifier (decoder) construction, A-DecNef training, and post-test stages (see Supplemental Experimental Procedures). In the color classifier construction stage, we measured blood-oxygen-level dependent (BOLD) signal multi-voxel patterns in V1/V2 evoked by the presentation of red-black, green-black, and gray-black gratings of both vertical and horizontal orientations (Figure S1), and constructed a color classifier [11]. The outputs of the classifier represented the calculated likelihood of red color presented to the participants. The classifier's mean percentage of correct color classification was approximately 70%, which was significantly above chance (50%, t11=13.66, P<0.001 for red, t11=11.60, P<0.001 for green, one-sample t-test with Bonferroni correction).
The color classifier construction stage was followed by three days of the A-DecNef training stage (Figure 1). Participants were unknowingly trained to create an internal association between a physically-presented achromatic vertical grating and the neural activation for a specific target color (red), although no chromatic stimulus was presented in the display. Participants were asked to “Maintain your gaze at the fixation point at the center of the display. While the achromatic grating is being presented, try to somehow regulate your brain activity to make a to-be-presented solid gray disk as large as possible”. The participants were not informed that the size of the disk was proportional to the red likelihood. In short, participants were trained to induce the activation patterns for red in V1/V2 without having any real red stimulus presented. By pairing such activation patterns with a physically-presented vertical grating, we tested whether associative learning of red and vertical orientation occurred.
Figure 1. Procedure of a trial in the A-DecNef training stage.
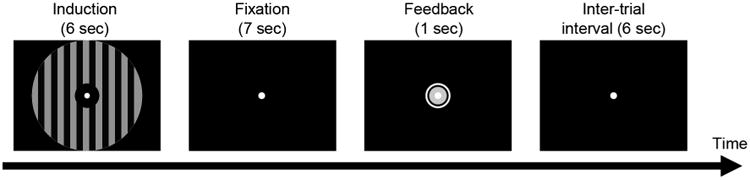
An achromatic (gray-black) vertical grating was presented during the induction period (6 sec), during which participants were trained to induce BOLD-signal multi-voxel patterns in V1/V2 corresponding to the color red, unbeknownst to participants. Immediately after the fixation period (7 sec), a feedback disk was presented for 1 sec. The disk size roughly represented how similar the BOLD-signal patterns in V1/V2 induced during the induction period were to the patterns evoked by the target color stimuli (red-black gratings) presented in the color classifier construction stage. See also Figure S1.
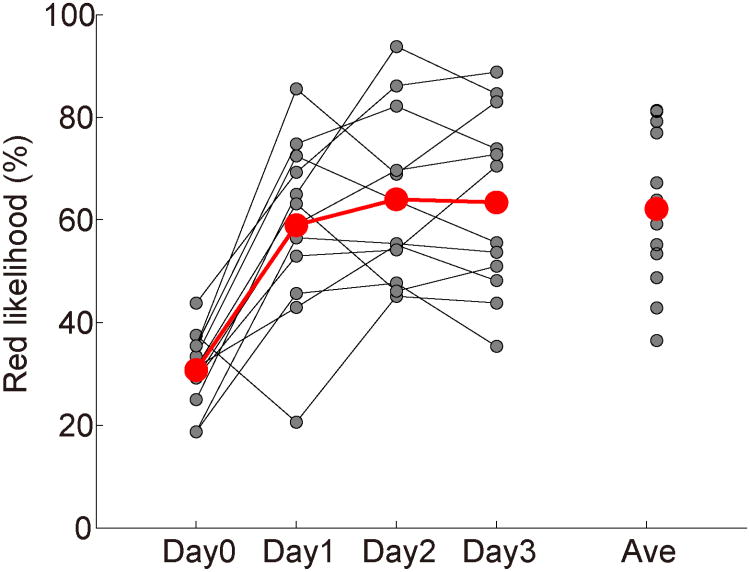
Figure 2 shows the red likelihood change due to A-DecNef training. The red likelihood on Day1, 2, and 3 were significantly higher than the red likelihood on Day0 (the red likelihood for the achromatic vertical grating during the color classifier construction stage). These results suggest that BOLD-signal patterns similar to those evoked by the red-black grating in V1/V2 were successfully induced during A-DecNef training. None of participants reported that they had the target color in mind during A-DecNef training (Table S1).
Figure 2. Change in the red likelihood by A-DecNef training (N = 12).
On Day0 the color classifier was constructed, whereas on Day1, 2, and 3 the A-DecNef training was conducted. Gray dots represent individual data and red dots represent the average across participants. The red likelihoods on Day1, 2, and 3 were significantly higher than on Day0 (P<0.001 for all three days and the average; t11=5.64 for Day 1; t11=7.25 for Day 2; t11=7.07 for Day 3; t11=7.42 for the average across the 3 days; paired t-test with Bonferroni correction). The average value is for Days 1-3.
Next, we tested whether associative learning between the color and orientation was indeed created as a result of A-DecNef. We conducted a psychophysical measurement during the post-test stage where a two-alternative-forced-choice task was performed to construct chromatic psychometric functions for each participant. The vertical, horizontal, and oblique gratings were used (Figure 3A). Assuming that A-DecNef training should have no effect on oblique stimuli, the oblique stimuli were used as control orientations for the vertical orientation. The color of the inner grating was tinted from green to red in 8 steps, passing through a neutral gray. Participants were instructed to judge whether the inner grating was red or green. Figures 3B and 3C show the mean red response% in the A-DecNef group, and the control group (see Supplemental Experimental Procedures) who did not participate in A-DecNef training, respectively. Results of a three-way mixed-design ANOVA (group as a between-participants factor, and orientation and color as within-participants factors) on the red response% indicated a significant main effect of color (F2.6,42.1=142.82, P<0.001; ε=0.38), and a significant three-way interaction (F7.1,113.5=2.18, P=0.040; ε=0.51). Testing for a simple interaction for each group showed that the interaction between orientation and color was significant for the A-DecNef group (F14,224=2.41, P=0.004), indicating that A-DecNef training resulted in an orientation-specific shift of the chromatic psychometric function. In contrast, a simple interaction between orientation and color was not significant for the control group (F14,224=1.32, P=0.20). Thus, it is suggested that A-DecNef training, which targeted V1/V2, created perception of a color associated with an orientation.
Figure 3. Stimuli and results of the post-test stage.
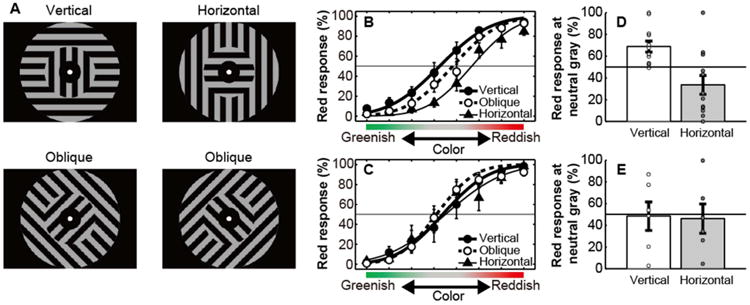
(A) Stimuli. The color in the inner gratings varied in 8 steps between a reddish tint (x=0.323, y=0.310, Y=17.9) and greenish tint (x=0.313, y=0.323, Y=17.9), passing through a neutral gray, while the outer grating was kept achromatic.
(B) Mean (±SEM) chromatic psychometric functions for the vertical (black circles), oblique (white circles), and horizontal (black triangles) gratings for the A-DecNef group (N=12).
(C) Mean (±SEM) chromatic psychometric functions for the vertical (black circles), oblique (white circles), and horizontal (black triangles) gratings for the control group (N=6).
(D, E) Individual and mean (±SEM) red response% for the vertical and horizontal gratings at the neutral gray for the A-DecNef group (D) and for the control group (E). See also Figures S2 and S4.
We further tested whether A-DecNef successfully created the orientation-specific color perception in the A-DecNef group. If A-DecNef successfully associates the vertical orientation with red BOLD-signal patterns, we should observe the following two aspects in the A-DecNef group. First, the point of subjective equality (PSE) to produce 50% red responses (therefore 50% green as well) for the vertical orientation should be significantly more greenish in terms of the tinted color level in the inner gratings than for the other orientations. Second, the red responses% should be significantly higher than chance when the vertical orientation was at the neutral gray, which is defined as the PSE for the control oblique orientation.
First, a cumulative Gaussian function was fitted to the data from each individual participant, and the PSE for each orientation was defined as the stimulus level with the 50% red response. We applied one-way repeated measures ANOVA (orientation as a within-participants factor) to the PSE for each orientation. The ANOVA showed that a main effect of orientation on the PSE was significant (F1.3,14.2=7.28, P=0.013; ε=0.65). The PSE for the vertical orientation was significantly different from those for the horizontal (t11=2.87, P=0.015) and the oblique orientation (t11=2.77, P=0.018), indicating a significant shift of PSE for the orientation used in A-DecNef training.
Next, we obtained the red response% for the vertical orientation at the neutral gray. Perception of the neutral gray for the oblique (control) grating was not significantly affected by A-DecNef, because two-way mixed-design ANOVA (group as a between-participants factor, and color as a within-participants factor) on the chromatic psychometric function of the oblique grating showed no significant effect of group (F1,16=2.51, P=0.133). The red response% for the vertical orientation was defined as the fitted value of a cumulative Gaussian function at this neutral gray. The red response% for the vertical grating was significantly higher than chance (t11=3.70, P=0.004). These results suggest that A-DecNef created red perception in an achromatic vertical grating.
Is the effect of associative learning temporary or long-lasting? To test this, the chromatic psychometric functions for vertical, horizontal, and oblique orientations for the A-DecNef group were measured after 3-5 months and compared with those of the control group. The results of detailed analyses (Figure S2, Table S2) indicate that psychometric functions were significantly different between the groups and that the vertical grating continued to be perceived as reddish in the A-DecNef group even several months later, and that the A-DecNef group showed significant differences on the PSE between vertical and other orientations even after 3-5 months. These results show that the association between orientation and color is long-lasting, as has been reported for other types of associative learning [12, 13].
It is worth noting that the green perception tends to increase for the horizontal gratings after A-DecNef training. In Figure 3B, the PSE for the horizontal grating was significantly different from that for the oblique grating (t11=2.25, P=0.046), whereas in Figure 3D, there was the tendency that the red (or green) responses% for the horizontal grating at the neutral gray was lower (or higher) than chance (t11=-1.89, P=0.086). Three to five months later (Figure S2A), the same tendency was observed. The PSEs for the horizontal and oblique gratings were significantly different from each other (t8=5.56, P<0.001), and the red (or green) responses% was significantly lower (or higher) than chance (t8=-7.84, P<0.001).
A-DecNef training was specifically based on BOLD-signal patterns in V1/V2. However, we thought that it was necessary to test whether similar neural activities in different areas other than V1/V2 have occurred by A-DecNef and contributed to the association between the color and orientation. If A-DecNef training induced color-specific brain activities in areas other than V1/V2, then BOLD-signal patterns in those areas should in turn properly predict the red likelihood in V1/V2 on a trial-by-trial basis. The results of a searchlight analysis [14] that explored the whole brain utilizing a L1 regularized least square regressor (Supplemental Experimental Procedures) indicated the following results. First, during the color classifier construction stage in which participants were presented with the chromatic gratings, the colors were accurately classified based on BOLD-signal patterns in V1/V2 and in ventral areas including V4 (Figure 4A). Second, during the color classifier construction stage, the red likelihood of V1/V2 was best predicted by BOLD-signal patterns in V1/V2 and moderately by those in ventral areas including V4 (Figure 4B). However, during the A-DecNef training, the BOLD-signal patterns in areas other than V1/V2 including V4 were very poor predictors of the red likelihood of V1/V2 (Figure 4C). These results collectively indicate the following points: the result of the color classification accuracy (Figure 4A) indicates the ability of the classifier to extract chromatic information in V4 when there is chromatic information in V4. Furthermore, multi-voxel patterns in V4 can predict chromatic information in V1/V2 by the regressor during the color classifier constructions stage (Figure 4B). Thus, the result that the same regressor did not predict chromatic information in V1/V2 from multi-voxel patterns in V4 during the A-DecNef training (Figure 4C) suggests that there was little chromatic information in V4 and other areas during the A-DecNef training. These results further suggest that the A-DecNef predominantly modified BOLD-signal patterns and the red likelihood in early visual areas.
Figure 4. Accuracy of the color classifier and predictability of the red likelihood in V1/V2 using a searchlight analysis [14]. Color coded voxels correspond to P<0.05.
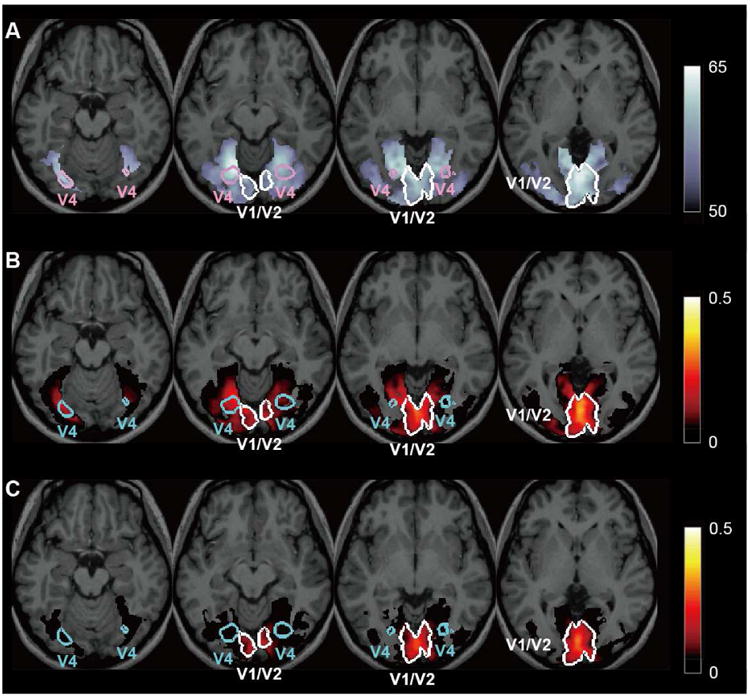
(A) Distribution map of the color classifier accuracy during the color classifier construction stage. The accuracy was computed by moving a sphere ROI across the whole brain. The classifier has the ability to extract information for the red likelihood in both V1/V2 and ventral areas including V4. The color scale bar indicates the accuracy (%).
(B) Distribution map of the predictability of the red likelihood of V1/V2 during the color classifier construction stage. The predictability was the highest in V1/V2 and to a moderate degree in ventral areas including V4. The color scale bar indicates a coefficient of determination between the V1/V2 red likelihood predicted by BOLD-signal patterns within an ROI and the actual V1/V2 red likelihood (based on the V1/V2 BOLD-signal patterns) in the searchlight analysis. See Supplemental Experimental Procedures for technical details.
(C) Distribution map of the predictability of the red likelihood of V1/V2 during the A-DecNef training stage. Not much significant predictability was found outside V1/V2. The color scale bar indicates a coefficient of determination, as in (B). See Supplemental Experimental Procedures for technical details.
See also Figure S3.
We also examined A-DecNef related brain activation by a conventional GLM analysis (Figure S3A) to test whether V1/V2 is the main locus of the A-DecNef. While we found significant activation in nine areas during A-DecNef training stage, the activation in these areas was negligibly correlated with the perceptual change except for V1/V2 (Figure S3B). Note that the searchlight analysis was based on classification performance on “red” vs “green”, whereas the GLM analysis was conducted on BOLD signal amplitudes, which could be caused by multiple factors. Considering these differential aspects of the analyses, it is suggested that during the A-DecNef training stage, activations not only involved in red perception but also in the training procedure occurred.
These results together suggest that early visual areas, rather than the higher areas including V4, are most likely to be the main locus of the associative learning of the orientation and color in the current study. However, we need to say that we cannot completely reject the possibility of the contribution of other areas to the current associative learning. Future studies to test whether induced associative learning transfers between eyes or between retinal locations may be helpful to clarify the roles of the higher visual areas in the current associative learning.
There are neurons that respond to a specific combination of color and orientation in V1 [15-18]. Does this invariably indicate that associative learning of color and orientation should occur in V1? The answer is negative. For example, it is well known that there are neurons in V1 that best respond to a specific orientation. However, this does not necessarily mean that learning of the orientation occurs in V1. In a similar manner of logic, the existence of neurons that respond best to a specific combination of orientation and color does not necessarily indicate that learning to associate a specific combination of color and orientation occurs in V1. There has been no study, to our knowledge, that indicates that neurons responding to a specific combination of color and orientation were learned to induce perception of the color when the orientation is presented as a cue.
What is a possible neural mechanism for the current associative learning? As mentioned above, A-DecNef training resulted in not only reddish perception on an achromatic vertical grating but also greenish perception on an achromatic horizontal grating. Simple pairing of a vertical orientation and red color would not produce greenish perception on the horizontal grating [19]. Because of the nature of A-DecNef training which uses the red-vs-green output of the classifier, it is likely that the A-DecNef training created a neural state that biases toward a ‘more likely to be red, not green’ state as opposed to an absolute red state. We therefore suggest that A-DecNef changed the balance of mutual inhibition between the neuronal populations that process red and green (Figure S4, see [18, 20]). This suggests that for the current associative learning to occur, mere activation of population for red paired with a vertical grating is not sufficient. The neurofeedback training that leads to the ‘more likely to be red, not green’ state, which is perhaps due to changes in balance of mutual inhibition between neuronal populations for red and green, may be necessary. That may be why associative learning between color and orientation as a result of pairing of a real colored stimulus with an achromatic grating has never been reported. Future studies have to test the validity of the model. One good way is to use a grating in a different color (e.g., blue) during the DecNef training to test shifts in psychometric functions after association of a given color and achromatic grating.
One may wonder whether the McCollough effect [19] is compelling evidence of associative learning in early visual areas. In some cases, the McCollough effect has been discussed as a possible manifestation of associative learning of orientation and color in early visual areas. However, there is no clear evidence for such a claim. First, it is a matter of great controversy whether the McCollough effect occurs in early visual areas [21-24]. In addition to the studies that advocate the V1 origin hypothesis of the effect [25, 26], there are a substantial number of studies that are against the view. One fMRI study found BOLD activity changes in multiple areas and concluded that the McCollough effect is created through top-down processing from a high-cognitive area [27]. Another fMRI study found that the left anterior portion of the color-selective area in the ventral occipital cortex, presumably V4 alpha, was significantly activated in association with the McCollough effect [28]. Second, in the McCollough effect the induced color is complementary to the exposed color. This indicates that in contrast to the associative learning in the present study, the McCollough effect is not due to a simple form of association, but rather reflects the complexity of the underlying neural mechanism including adaptation processes [29]. Some studies have suggested that the McCollough effect is not due to associative learning [30].
The color-orientation association in the present study is also distinguished from the anti-McCollough effect [31] in which the exposed color rather than the complementary color is perceived in a configuration similar to the McCollough effect [31]. First, the anti-McCollough effect shows 100% interocular transfer, suggesting that it takes place in higher areas than in V1. Second, the anti-McCollough effect lasts shorter than a day, whereas the color-orientation association in the present study lasts at least 3 to 5 months. These lines of evidence indicate that neither the McCollough effect nor anti-McCollough effect has provided any clear evidence that associative learning occurs in early visual areas.
To summarize, using A-DecNef, we created long-lasting associative learning of color and orientation in early visual areas. These results suggest that early visual areas are most likely to be the main locus of the associative learning, although we cannot completely reject the possibility of the contribution of other areas to the associative learning.
Experimental procedures
A total of 18 participants were employed in the study. See Supplemental Experimental Procedures for more details.
Supplementary Material
Highlights.
There was no evidence for associative learning of features in early visual areas
Neurofeedback induced signals for red paired with an achromatic vertical grating
Such paired presentations led to associative learning of color and orientation
The results show that early visual areas may create associative learning
Acknowledgments
We thank Drs. Ben Seymour, Naotsugu Tsuchiya and Ryota Kanai for critical discussions and comments on an early draft. We also thank Dr. Hiroshi Ban for technical assistance, and Drs. Jonathan Dobres and Aaron Berard for editing. This study is the result of a contract with the National Institute of Information and Communications Technology entitled, ‘Development of network dynamics modeling methods for human brain data simulation systems’. KS was partially supported by JSPS, TW by NIH R01EY019466, R01AG031941, YS by R01MH091801, NSF BCS 1539717, MK by Japan MIC “Novel and Innovative Brain R&D”. There is a potential financial conflict of interest; the authors are the inventors of patents related to the neurofeedback method used in this study, while the original assignee of the patents is ATR to which the authors are affiliated.
Footnotes
Author contributions: K.A., K.S., M.K., Y.S., and T.W. conceived and designed the experiments. K.A. performed experiments and analyzed the data. K.A., K.S., M.K., Y.S., and T.W. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–1407. doi: 10.1016/s0896-6273(00)80658-3. [DOI] [PubMed] [Google Scholar]
- 2.Petrides M. Deficits on conditional associative-learning tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1985;23:601–614. doi: 10.1016/0028-3932(85)90062-4. [DOI] [PubMed] [Google Scholar]
- 3.McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- 4.Henke K, Buck A, Weber B, Wieser HG. Human hippocampus establishes associations in memory. Hippocampus. 1997;7:249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 5.Hua T, Bao P, Huang CB, Wang Z, Xu J, Zhou Y, Lu ZL. Perceptual learning improves contrast sensitivity of V1 neurons in cats. Curr Biol. 2010;20:887–894. doi: 10.1016/j.cub.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Piech V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nat Neurosci. 2004;7:651–657. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yotsumoto Y, Watanabe T, Sasaki Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron. 2008;57:827–833. doi: 10.1016/j.neuron.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yotsumoto Y, Sasaki Y, Chan P, Vasios CE, Bonmassar G, Ito N, Nanez JE, Sr, Shimojo S, Watanabe T. Location-specific cortical activation changes during sleep after training for perceptual learning. Curr Biol. 2009;19:1278–1282. doi: 10.1016/j.cub.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibata K, Watanabe T, Sasaki Y, Kawato M. Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science. 2011;334:1413–1415. doi: 10.1126/science.1212003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wandell BA, Winawer J. Imaging retinotopic maps in the human brain. Vision Research. 2011;51:718–737. doi: 10.1016/j.visres.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita O, Sato MA, Yoshioka T, Tong F, Kamitani Y. Sparse estimation automatically selects voxels relevant for the decoding of fMRI activity patterns. NeuroImage. 2008;42:1414–1429. doi: 10.1016/j.neuroimage.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakai K, Miyashita Y. Neural organization for the long-term memory of paired associates. Nature. 1991;354:152–155. doi: 10.1038/354152a0. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman HS, Fleshler M, Jensen P. Stimulus Aspects of Aversive Controls: The Retention of Conditioned Suppression. J Exp Anal Behav. 1963;6:575–583. doi: 10.1901/jeab.1963.6-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3863–3868. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conway BR, Livingstone MS. Spatial and temporal properties of cone signals in alert macaque primary visual cortex. J Neurosci. 2006;26:10826–10846. doi: 10.1523/JNEUROSCI.2091-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson EN, Hawken MJ, Shapley R. The orientation selectivity of color-responsive neurons in macaque V1. J Neurosci. 2008;28:8096–8106. doi: 10.1523/JNEUROSCI.1404-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubel DH, Livingstone MS. Color and contrast sensitivity in the lateral geniculate body and primary visual cortex of the macaque monkey. J Neurosci. 1990;10:2223–2237. doi: 10.1523/JNEUROSCI.10-07-02223.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seymour K, Clifford CW, Logothetis NK, Bartels A. Coding and binding of color and form in visual cortex. Cereb Cortex. 2010;20:1946–1954. doi: 10.1093/cercor/bhp265. [DOI] [PubMed] [Google Scholar]
- 19.McCollough C. Color Adaptation of Edge-Detectors in the Human Visual System. Science. 1965;149:1115–1116. doi: 10.1126/science.149.3688.1115. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Qiu J, Zhang Y, Han S, Fang F. Misbinding of color and motion in human visual cortex. Curr Biol. 2014;24:1354–1360. doi: 10.1016/j.cub.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Humphrey GK, James TW, Gati JS, Menon RS, Goodale MA. Perception of the Mccollough Effect Correlates with Activity in Extrastriate Cortex: A Functional Magnetic Resonance Imaging Study. Psychological Science. 1999;10:444–448. [Google Scholar]
- 22.Uhlarik J, Pringle R, Brigell M. Color aftereffects contingent on perceptual organization. Perception & Psychophysics. 1977;22:506–510. [Google Scholar]
- 23.Vidyasagar TR. Orientation specific colour adaptation at a binocular site. Nature. 1976;261:39–40. doi: 10.1038/261039a0. [DOI] [PubMed] [Google Scholar]
- 24.Grossberg S, Hwang S, Mingolla E. Thalamocortical dynamics of the McCollough effect: boundary-surface alignment through perceptual learning. Vision research. 2002;42:1259–1286. doi: 10.1016/s0042-6989(02)00055-x. [DOI] [PubMed] [Google Scholar]
- 25.Vul E, MacLeod DIA. Contingent aftereffects distinguish conscious and preconscious color processing. Nature Neuroscience. 2006;9:873–874. doi: 10.1038/nn1723. [DOI] [PubMed] [Google Scholar]
- 26.Humphrey GK, Goodale MA. Probing unconscious visual processing with the McCollough effect. Conscious Cogn. 1998;7:494–519. doi: 10.1006/ccog.1998.0369. [DOI] [PubMed] [Google Scholar]
- 27.Barnes J, Howard RJ, Senior C, Brammer M, Bullmore ET, Simmons A, David AS. The functional anatomy of the McCollough contingent colour after-effect. Neuroreport. 1999;10:195–199. doi: 10.1097/00001756-199901180-00037. [DOI] [PubMed] [Google Scholar]
- 28.Morita T, Kochiyama T, Okada T, Yonekura Y, Matsumura M, Sadato N. The neural substrates of conscious color perception demonstrated using fMRI. Neuroimage. 2004;21:1665–1673. doi: 10.1016/j.neuroimage.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Skowbo D. Are McCollough effects conditioned responses? Psychol Bull. 1984;96:215–226. [PubMed] [Google Scholar]
- 30.Byth W, McMahon D, King DJ. Cholinergic agents and the McCollough effect. Perception. 2000;29:461–480. doi: 10.1068/p2892. [DOI] [PubMed] [Google Scholar]
- 31.Sheth BR, Shimojo S. Adapting to an aftereffect. J Vis. 2008;8:21–10. doi: 10.1167/8.3.29. 29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.