Abstract
Purpose
To determine the effectiveness of different monitoring modalities to detect incident neovascularization associated with age-related macular degeneration (AMD).
Method
Secondary analyses compared the rates of detecting incident neovascular AMD in pre-scheduled office visits vs. office visits triggered by monitoring device or by symptom realization in a randomized trial evaluating home tele-monitoring device plus standard care (device arm) vs. standard care alone (SC).
Results
At pre-scheduled office visits, neovascular AMD was detected in 14/1927 visits (0.7%, 95% confidence interval [CI]: 0.4%-1.1%) and 14/1949 visits (0.7%, 95% CI: 0.3%-1.1%) in the device and SC arms, respectively. Thirty-seven participants with neovascular AMD were detected in 318 office visits (11.6%, 95% CI: 8.1%-15.2%) triggered by device or symptom realization and 17 neovascular AMD in 65 office visits (26%, 95% CI: 15.5%-36.8%) triggered by symptom realization in the device and SC arms, respectively. The home device strategy had a higher neovascular-AMD detection rate than pre-scheduled office visits (relative risk= 16.0 [95% CI: 8.8-29.3]). Neovascular AMD detected at triggered visits were associated with less vision loss from baseline in the device arm vs. SC arm (−3 letters vs −11.5 letters, respectively, p=0.03).
Conclusion
Tele-monitoring may alter our management of AMD patients and improve vision outcomes.
Keywords: age-related macular degeneration, incident choroidal neovascularization, tele-monitoring
Introduction
Treatment with intravitreal injections of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration (AMD) markedly reduces the risk of vision loss.1,2 The level of visual acuity at the time of treatment initiation strongly predicts vision outcomes at 12 and 24 months following treatment.3 Thus it is important to improve our ability to detect neovascular AMD early when there has been minimal visual acuity lost. The standard care for following patients with AMD and who are at risk of developing neovascular AMD traditionally has consisted of pre-scheduled office visits supplemented by additional visits when patients recognized new symptoms. Between scheduled visits patients have been encouraged to monitor their vision at home using monocular visual assessments, with or without an Amsler grid. The HOme Monitoring of the Eye (HOME) Study primary analyses demonstrated the efficacy of tele-monitoring with a home device in conjunction with standard care in detecting CNV-lesions early in persons at risk as 87% of eyes so monitored maintained visual acuity of 20/40 or better at the time of CNV-lesion detection.4 The HOME Study also provides an opportunity to explore through secondary analyses the effectiveness of the various monitoring modalities utilized in the trial in detecting neovascular AMD. This report compares the effectiveness of identifying new neovascular AMD and the vision outcomes at the time of neovascular AMD detection at pre-scheduled standard care office visits with triggered office visits precipitated by changes in visual function detected either by the home monitoring device or by participants’ recognition of new symptoms, with or without Amsler grid testing (triggered visits).
Methods
The detailed design of the HOME Study has been previously described.5 In brief, participants at risk for developing neovascular AMD (individuals with bilateral large drusen or advanced AMD in 1 eye and large drusen in the fellow eye) were followed by retinal specialists at 44 Age-Related Eye Disease Study 2 (AREDS2) clinical sites. The participants were randomly assigned to standard care alone (SC arm) or standard care plus home device monitoring (device arm). Major exclusion criteria included participants with best corrected visual acuity (BCVA) of less than 20/60, or an inability to operate or successfully pass an in office qualification test with the home monitoring device (ForeseeHome, Notal Vision Ltd, Tel Aviv, Israel). All study participants received instructions to monitor their vision function, which may have included an Amsler grid, and to call promptly for an office visit if and when new symptoms triggered their attention. Participants using the home monitoring device were notified by the clinic and scheduled promptly for a triggered office visit when the central monitoring center contacted the clinic to report changes on the home device testing. All participants were also encouraged to return for pre-scheduled office visits, according to the standard practice of the study clinician retina specialist, to be monitored for AMD progression.
Data were collected on the types and frequency of office visits, either pre-scheduled or triggered office visits, through review of the available medical records. At the initial visit in which neovascular AMD was noted in a study eye, the visit “type” was counted as the monitoring modality to diagnose neovascular AMD. Visit “type” was categorized as pre-scheduled visits for standard care visits recommended by the clinician or the annual study visits mandated for AREDS2 participants; or triggered visits that had to be initiated by the participants because of abnormalities detected by the device or by the participant. At any pre-scheduled office visit in which there was a clinical suspicion of neovascular AMD and at any triggered visits, standardized procedures for BCVA assessment, dilated fundus examination, stereoscopic color fundus photographs with fluorescein angiography and optical coherence tomography (OCT) imaging were performed. The presence or absence of neovascular AMD, in each study eye, at each office visit, was determined by the investigator.
Analyses
The effectiveness of each monitoring modality in detecting CNV during the HOME Study period of July 2010 to April 2013 was calculated for all study participants. The relative risk (RR) and 95% confidence interval (CI) of the probability of identifying neovascular AMD at triggered office visits compared with pre-scheduled office visits for each monitoring group assignment were computed. Monitoring years were calculated for each participant from the time of enrollment to April 2, 2013 (the date of the data lock for the primary study analyses), while data from all participants were censored following incident neovascular AMD, death or dropping out of the study. The number of visits/per participant/year was calculated within each monitoring group by dividing the total number of office visits during the study period by the total monitoring years.
Results
From July 2010 to April 2013, only 28 of 1520 (2%) participants in the AREDS2-HOME Study did not have complete data, including 12 in the device arm and 16 in the SC arm, due to loss-to-follow-up (8), refusal for further follow-up (13) or death (7). During this study period, more office visits occurred in the device arm than the standard care arm, (2,245 vs. 2,014, respectively, Table 1), largely due to the addition of device prompted visits in this arm. The 263 visits triggered by the device represented 12% of the total office visits in the device arm. The majority of visits in each study arm were classified as pre-scheduled visits which represented 86% (1927 visits) and 97% (1949 visits) of all visits for the device and SC arms, respectively. Symptom realization resulted in relatively few visits, ≤3%, in each monitoring group (Table 1). The mean (SD) number of total visits/year was 2.3 (2.4) and 2.0 (2.0) in the device and SC arms, respectively, (p=0.002), while the median (interquartile range [IQR]) number of visits per year appeared similar at 1.8 (1.1-2.9) and 1.7 (0.8-2.6) in the device and SC arms, respectively. During this study period, as previously reported, a total of 82 eyes were diagnosed with incident CNV, 51 eyes in the device arm and 31 eyes in the SC arm.
Table 1.
Results of the Different Monitoring Modalities in Detecting Choroidal Neovascularization
| Enrolled participants | Device arm N=763 (100%) | Standard care arm N=757 (100%) | Total N=1520 | |
|---|---|---|---|---|
| Participants in current analysis | N (%) | 751 (98%)1 | 741 (98%)2 | 1492 (98%) |
| Total office visits3 | N (%) | 2245 (100%) | 2014 (100%) | 4259 (100%) |
| Device triggered visits | N (%) | 263 (12%) | NA | 263 (6%) |
| Symptom realization triggered visits | N (%) | 55 (2%) | 65 (3%) | 120 (3%) |
| Pre-scheduled office visits | N (%) | 1927 (86%) | 1949 (97%) | 3876 (91%) |
| Visits/year | Mean (SD) | 2.3 (2.4) | 2.0 (2.0) | 2.1 (2.2) |
| Median | 1.8 | 1.7 | 1.7 | |
| IQR | 1.1-2.9 | 0.8-2.6 | 0.9-2.7 | |
| New CNV lesions (TOTAL) | N (%) | 51 (100%) | 31 (100%) | 82 (100%) |
| Device triggered | N (%) | 26 (51%) | NA | 26 (32%) |
| Symptom triggered | 11 (22%) | 17 (55%) | 28 (34%) | |
| Pre-scheduled office | 14 (27%) | 14 (45%) | 28 (34%) | |
| Detection Rate of neovascular AMD4 | ||||
| Device triggered | Detection Rate % [95% CI] | 9.9% [6.3, 13.5] | NA | |
| Symptom realization | Detection Rate % [95% CI] | 20% [9.4, 30.6] | 26% [15.5, 36.8] | 23% [15.8, 30.9] |
| Combined device and symptom realization | Detection Rate % [95% CI] | 11.6% [8.1, 15.2] | NA | -- |
| Pre-scheduled | Detection Rate % [95% CI] | 0.7% [0.3, 1.1] | 0.7% [0.3, 1.1] | 0.7% [0.5, 1.0] |
| Proportion with Visual acuity ≥20/40 at detection of neovascular AMD5 | ||||
| Device Triggered Visits | N (%) | 22/23=96% | NA | NA |
| Symptom Triggered Visits | N (%) | 9/11=82% | 10/17=59% | 19/28=68% |
| At any visit between pre-scheduled visit (device and/or symptom) | N (%) | 31/34=91% | 10/17=59% | 41/51=80% |
| Pre-scheduled Visits | N(%) | 9/12=75% | 8/13=62% | 17/25=68% |
Twelve participants missing: 1 lost to follow-up, 10 refused further follow-up and 1 died
Sixteen participants missing: 7 lost to follow-up, 3 refused further follow-up, and 6 died.
Represents visits (all types) at HOME study clinical centers plus non-study treating physicians not in AREDS2 clinical centers
Proportion of visits in which new CNV lesions were diagnosed
Percent is out of total that had ≥20/40 at baseline.
Effectiveness of each monitoring modality to detect neovascular AMD and change in visual acuity from baseline at neovascular AMD detection
In the device arm, 14 eyes were diagnosed with CNV during 1927 pre-scheduled office visits (0.7% detection rate, 95%CI: 0.3%, 1.1%). At the visit at which these CNV lesions were detected, the median [IQR] change in vision from baseline was −8.5 (−11.0,−2.0) letters. However, 37 eyes had incident CNV lesions detected during 318 triggered office visits (11.6% detection rate, 95% CI: 8.1%, 51.2%). These 37 eyes had a median (IQR) visual acuity loss of −3.0 (−10.0,−1.0) letters from baseline at time of incident neovascular AMD detection which included the eyes triggered by the device. [−3.0 (−8.0, 1.0)] and those prompted by symptom recognition [−7.0 (16,−3.0)]. Further breakdown of the detection rate by symptoms vs. device alerts is reported in Table 1.
In the SC arm, 14 eyes were diagnosed with neovascular AMD during 1949 pre-scheduled office visits, (0.7 % detection rate, 95%CI: 0.3%, 1.1%). These eyes experienced a median (IQR) loss of −8.0 (−10.0,−5.0) letters from baseline at neovascular AMD diagnosis. Seventeen eyes were found to have CNV lesions identified during 65 triggered office visits prompted by new symptoms realized by the participant, resulting in detection rate of 26% with 95% CI: 15.5%, 36.8%. However, the median (IQR) change in vision was a loss of −11.5 (−26.0,−3.5) letters from baseline for these triggered visits. The ratio between the number of CNV lesions identified in triggered visits in the device arm (37) and the SC arm (17) was 2.2, while the number of CNV lesions detected in pre-scheduled office visits was identical in both arms (14). The ratio between the number of CNV lesions identified in the device arm during triggered visits (37) to pre-scheduled visits (14) was 2.6, while the participants in the device arm used only one sixth (318/1927) of visits to achieved that. In the SC arm, triggered visits identified slightly more CNV lesions (17 vs. 14) than a 30 times larger number of pre-scheduled visits. It should be noted that participants with home monitoring device had 5.6 times as many false positive unscheduled visits as those in the SC arm.
In the device arm, 9 of the 12 (75%) eyes diagnosed with neovascular AMD during pre-scheduled office visits, and 31 of the 34 (91%) eyes diagnosed with neovascular AMD during triggered visits, which was consisted of 22 of 23 (96%) events triggered by device alerts and 9 of 11 (82%) events triggered by symptoms realization in the device group, maintained vision of 20/40 or better (Table 1). In the SC arm, 8 of the 13 (62%) eyes diagnosed with neovascular AMD during pre-scheduled office visits, and 10 of the 17 (59%) eyes diagnosed with neovascular AMD during triggered visits maintained vision of 20/40 or better. When comparing the percentage of eyes maintaining VA of 20/40 or better by detection modality between the monitoring groups of device vs. standard care, there was no significant difference identified between those detected during pre-scheduled visits (75% vs. 62%, p=0.39) while a significant difference between monitoring groups was identified for events detected during triggered visits (91% vs. 59%, p=0.01). No difference was identified for vision loss of 15 letters or more at time of neovascular AMD detection during pre-scheduled visits for eyes in the device vs. standard care group (7.1% vs 7.1%, respectively); however, fewer participants suffered this level of vision loss at triggered visits in the device arm than in the standard care arm (13.5% vs 35.3%, p=0.07).
When comparing the detection rates of the different monitoring modalities within each study arm, the home monitoring strategy that incorporates the home device plus symptom recognition resulted in a substantial increase in the detection rate of neovascular AMD when compared to pre-scheduled visits alone in that arm (RR= 16.0, 95% CI: 7.3, 35.3 in the device arm). The rate of detection of neovascular AMD was greatest when participants recognized new symptoms and presented promptly for an examination in the SC arm (RR=36.4, 95% CI: 18.8, 60.6) when compared to pre-scheduled visits alone in that arm. However, when comparing vision change from baseline at the time of neovascular AMD diagnosis by triggered visits in each arm, device arm participants with neovascular AMD experience less vision loss from study entry than the SC arm participants with neovascular AMD (−3 letters vs −11.5 letters, respectively, p=0.03). The results with the four combinations of detection rates and visual acuity changes are summarized in Figure 1
Figure 1.
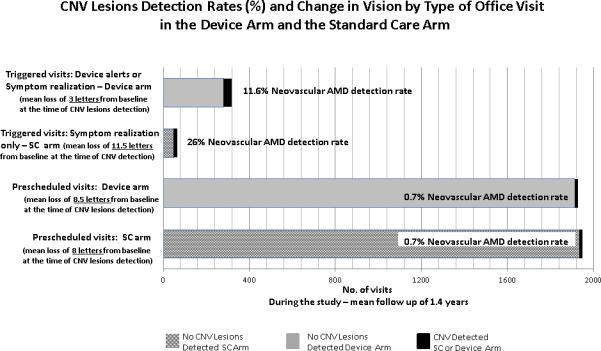
Triggered visits: initiated because of changes either detected by the device (compared with baseline) or participants have notified the clinical center in advance of a pre-scheduled office visit that new symptoms were noted (symptom realization).
Pre-scheduled visit: initiated by the physician as part of routine care or as a required in office AREDS2 study visit
SC: Standard Care Arm (hatched bars)
Device Arm (solid bars) which consists of home device monitoring plus standard care. neovascular AMD detected: (solid dark bars, either device arm or standard care arm)
Discussion
The standard care of AMD patients at risk of neovascular AMD has traditionally consisted of periodic pre-scheduled office visits of variable frequency to monitor for disease progression along with additional visits prompted by patients recognizing and acting upon new symptoms. The optimal frequency for performing office visits to monitor for disease progression in these patients in the absence of new symptoms remains unknown. However, the American Academy of Ophthalmology, through the Preferred Practice Pattern for Retina,6 recommends “office exams at 6 to 24 month intervals for asymptomatic patients at risk of CNV or prompt examination for new symptoms suggestive of CNV.” On average, each of the HOME study participants was examined twice per year, largely in the context of pre-scheduled office visits. The effectiveness of these pre-scheduled visits to detect new CNV lesions was low, as only 0.7% of all pre-scheduled visits in each study arm resulted in detection of incident neovascular AMD. However, the strategy combining home device monitoring with self-monitoring (such as Amsler grid) for monocular visual changes, increased the rate of detection of neovascular AMD by a factor of 16 fold while in the SC arm, symptom realization increased the rate of detection of neovascular AMD by a factor of about 30 fold. However, the visual acuity at the time of detection of neovascular AMD showed less visual acuity loss compared with baseline in those randomized to the home device monitoring arm.
Monitoring the large population of persons at risk of AMD progression and vision impairment with in office examinations poses a large burden for these patients, their family, physicians and the health care system in general. Recognizing the low detection rate of incident neovascular AMD at pre-scheduled office visits makes their utility for this purpose questionable. However, despite the low yield, 27% of all the neovascular AMD detected in the device arm were detected at these pre-scheduled visits and were missed between the pre-scheduled visits despite the availability of the home device monitoring in conjunctions with self-monitoring. The pre-scheduled office visits, at least at some minimal frequency, may also be important for the physician to have the opportunity to educate the patient on symptoms of disease progression and to emphasize the need for prompt notification upon symptom recognition. The physician can also educate the patient about modifiable risk factors such as cigarette smoking and use of nutritional supplements that have been proven to reduce the risk of progression to late AMD. These office exams also provide an opportunity to diagnose and monitor other ocular diseases such as cataract and glaucoma which have increased prevalence with advancing age.
A key limitation of the home monitoring device is the exclusion of persons with AMD who were not able to use the technology or to establish the crucial reproducible baseline values for future comparisons. During follow-up some participants discontinued the use of the home monitoring device if the device cannot re-establish a reproducible baseline following any device initiated false alerts. As commonly seen in the management of any chronic condition, compliance with device usage may decrease in some participants even to the point of abandoning device monitoring. Despite these caveats, participants assigned to the device arm had a substantial increase in detection of neovascular AMD relative to pre-scheduled visits with marked preservation of visual acuity at time of neovascular AMD detection. The utility of the device is not known for those individuals who are monitored more frequently such as patients receiving monthly intravitreal injections of anti-vascular endothelial growth factor in their fellow eye. To fully explore the utility for this sub-set of individuals, further studies will be required.
The home monitoring device, particularly when used at least twice per week as recommended, as an adjunct to self-monitoring, could potentially allow a reduction in the number of pre-scheduled office visits for some patients at risk of AMD progression, reducing the burden on physicians, patients, families, and payers. Recent guidelines of the European Society of Retina Specialists recommend use of the home monitoring device for the management of AMD 7 and the potential impact of home monitoring was raised in an editorial.8 A cost-benefit analysis of the monitoring device strategy, a subject of a future report, will be helpful to assess how this novel home management modality may best fit into the management of persons at high risk of AMD progression.
Summary statement.
Office visits triggered by home tele-monitoring or patient's symptoms resulted in 16-fold higher effectiveness in the detection rate of wet AMD compared to the detection rate during routine office visits. Furthermore, visual acuity of patients detected following home tele-monitoring triggered visit was significantly better than those triggered by self-monitoring alone
Acknowledgments
The manuscript was drafted by the investigators with collaboration and input from the sponsors: Gidi Benyamini and Yair Alster assisted in manuscript preparation.
The study is supported by Notal Vision Ltd through a clinical trial agreement with the National Eye Institute, National Institutes of Health, Bethesda, Maryland (CTA no.: CTA-00833); and a service agreement with EMMES Corporation. The Age-Related Eye Disease Study 2 study is supported by the intramural program funds and contracts from the National Eye Institute, National Institutes of Health (contract nos.: HHS-N-260-2005-00007-C, N01-EY-5-0007).
Footnotes
FINANCIAL DISCLOSURES
Emily Y. Chew, Traci E. Clemons, Molly Harrington, Alexander Brucker: None
Susan B. Bressler: Consultant - GlaxoSmithKline; Grant support – Boehringer Ingelheim Pharma, EMMES Corporation, Jaeb Center, Genentech, Lumenis, Inc., Notal Vision Ltd., Novartis Corporation, Regeneron Pharmaceuticals Inc., ThromboGenics NV; Lecturer - providers of continuing medical education materials. Dr. S. Bressler is supported by a Physician-Scientist grant from Research to Prevent Blindness, Inc., New York, New York.
Michael J Elman: Consultant – Notal Vision Ltd., Genentech Roche; Grant Support – EMMES Corporation, Jaeb Center, Genentech, Merck; Lecturer - Genentech
Judy E. Kim: Consultant-Allergan; Honoraria-Novartis, Bayer; Research Support: Notal Vision, Optos
Richard Garfinkel: Consultant-Notal Vision; Shareholder of Covalent Medical
Jeffrey Heier: Consultant/Advisor: Allergan, Avalanche, Bayer, Forsight Labs, Genentech, ,Kanghong, Neurotech, Notal Vision, Novarits, Ohr Pharmaceuticals, Regeneron. He also received research funding from Alcon, Allergan, Genentech, Lpath, Notal Vision, Ohr Pharmaceuticals, Ophthotech, and Regeneron, Sanofi/Genzyme.
David Boyer: Consultant/Advisor Alcon, Allergan, Genetech, Bayer, Regeneron, Novartis, Ohr, Thrombogenics, GlaxoSmithKline, Aeripo, Allegro, Notal Vision
References
- 1.Brown DM, Kaiser PK, Michels, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 2.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Ying GS, Huang J, Maguire MG, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120:122–129. doi: 10.1016/j.ophtha.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chew EY, Clemons TE, Bressler SB, et al. AREDS2-HOME Study Research Group. Randomized trial of a home monitoring system for early detection of choroidal neovascularization. Home Monitoring of the Eye (HOME) study. Ophthalmology. 2014;121:535–544. doi: 10.1016/j.ophtha.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chew EY, Clemons TE, Bressler SB, Elman MJ, Danis RP, Domalpally A, Heier JS, Kim JE, Garfinkel RA. Randomized trial of the ForeseeHome monitoring device for early detection of neovascular age-related macular degeneration. The HOme Monitoring of the Eye (HOME) study design - HOME Study report number 1. Contemp Clin Trials. 2014;37:294–300. doi: 10.1016/j.cct.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Ophthalmology Retina/Vitreous Panel . Preferred Practice Pattern® Guidelines. Age-Related Macular Degeneration. American Academy of Ophthalmology; San Francisco, CA: 2014. Available at www.aao.org/ppp. [Google Scholar]
- 7.Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, Schlingemann R, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA) Br J Ophthalmol. 2014;98:1144–1167. doi: 10.1136/bjophthalmol-2014-305702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han D. The ForeSeeHome Device and the HOME Study. A milestone in the self-detection of neovascular age-related macular degeneration. JAMA Ophthalmology. doi: 10.1001/jamaophthalmol.2014.1405. Published online July 24, 2014. [DOI] [PubMed] [Google Scholar]


