Abstract
Background and Purpose
The 70 kDa heat shock protein (Hsp70) protects brain cells in models of cerebral ischemia. Proteomic screening of mice subjected to middle cerebral artery occlusion (MCAO) identified dynamin as a major down-regulated protein in Hsp70-overexpressing mice (Hsp70Tg). Dynamin-1 is expressed in neurons and participates in neurotransmission, but also transports the death receptor Fas to the cell surface where it can be bound by its ligand and lead to apoptosis.
Methods
Mice were subjected to distal MCAO. Neuro-2a cells (N2a) were subjected to oxygen glucose deprivation (OGD). Hsp70Tg, Hsp70 deficient (Hsp70Ko) were compared to Wt. for histological and behavioral outcomes. Some mice and N2a cultures were given dynasore (Dyna), a dynamin inhibitor.
Results
Hsp70Tg mice had better outcomes, whereas Hsp70Ko mice had worse outcomes compared to Wt. This correlated to decreased and increased dynamin expression, respectively. Dynamin colocalized to neurons, and Fas, with higher Fas levels and increased caspase-8 expression. Hsp70 induction in N2a cells were protected from OGD while downregulating dynamin and Fas expression. Further, dynamin inhibition was neuroprotective.
Conclusions
Dynamin may facilitate Fas-mediated apoptotic death in the brain, and Hsp70 may protect by preventing this trafficking. Dynamin should be explored as a new therapeutic target for neuroprotection.
Keywords: Stroke, Apoptosis, Dynamin, Fas, neuroprotection
Introduction
The 70-kDa inducible Hsp (Hsp70) is known to protect the brain from stressful stimuli, including stroke. Hsp70 also interferes with cell death pathways such as apoptosis. Precise protective mechanisms are many, and protection by Hsp70 might lead to the identification of new therapeutic targets 1. To this end, we applied a proteomic approach to mice overexpressing Hsp70 (Hsp70Tg) subjected to experimental stroke and compared protein expression patterns to that of similarly injured wildtype (Wt) mice. The result of this analysis showed marked reduction of dynamin in the brains of Hsp70Tg mice (Supplemental Figure SI, Table).
Dynamin is a member of a family of guanine triphosphatase (GTPase) proteins largely known for their endocytic functions 2. Dynamin-1, the subject of this study, is exclusive to the brain 3. Recently, dynamin has been shown to translocate Fas protein from the Golgi apparatus to the cell surface where it can be bound by its ligand, FasL 4, and subsequently trigger one of several extrinsic apoptosis pathways leading to caspase-dependent cell death.
Ischemic stroke leads to cell death via several pathways, including apoptosis. The intrinsic apoptotic pathway occurs within mitochondria 5, whereas the extrinsic pathway is receptor-mediated 6. Extrinsic apoptosis involves the engagement of death receptors located on the plasma membrane. Receptor ligation causes activation of caspase-8, leading to activation of effector caspase-3 7. Following focal cerebral ischemia, the interaction between the Fas receptor and its ligand (FasL) initiates intracellular signaling cascades that ultimately terminate in caspase-dependent cell death after ischemic stroke.
Dynamin has not been studied extensively, and has not been studied in brain ischemia. In the central nervous system, dynamin has been associated with endocytic processes and synaptic transmission 3. Prior related work has shown that a similar protein in the same family, dynamin related protein-1 (DRP-1) may be important in brain ischemia due to its role in mitochondrial fission, and DRP-1 inhibition may be neuroprotective 8. However, to our knowledge, dynamin-1 has not been studied brain ischemia, nor has its potential connection to Hsp70 been explored.
Methods
All experimental procedures in animals were approved by the local IACUC, and were in accordance with NIH guidelines.
Animal models
Male Hsp70 transgenic (Hsp70Tg) and Hsp70 Knockout (Hsp70Ko) mice weighing 25–30g were produced from breeder mice originally generated by the Dillmann (UCSD) 9 and Pandita (Southwestern University) labs 10. Hemizygotic (Hsp70Tg) and homozygotic (Hsp70Ko) mice were compared to wildtype littermates (Wt).
Distal middle cerebral artery occlusion (dMCAO) was performed as described previously 11. Briefly, mice were anesthetized with isoflurane (5% for induction, 2% for maintenance via facemask) in a mixture of medical air/oxygen (3:1). The middle cerebral artery (MCA) was permanently occluded by short coagulation proximal to the olfactory branch.
At the end of experiments, mice were euthanized and transcardially perfused with normal saline and fixed in 4% PFA plus 20% sucrose, then frozen.
Infarct size
Infarct size was determined from cryosections (50μm) of brains of animals survived 14 d and stained with cresyl violet. Infarct volume was determined as previously described 11.
Behavior tests
Neurological assessments were performed as previously described 11. All studies were recorded using a video camera, and scoring was performed by 2 different investigators. Bederson score: The Bederson score was modified for use in mice, as previously reported 11 (grade 0=no observable neurological deficit; grade 1=unable to extend the contralateral forelimb; grade 2=flexion of the contralateral forelimb; grade 3=mild circling to the contralateral side; grade 4=severe circling; and grade 5=falling to the contralateral side). Ladder Test: Mice were coaxed onto a 40-rung ladder. The numbers of forelimb faults were counted. Fewer foot falls indicated improved sensorimotor function 12. Adhesive Removal (“Sticky Tape”) Test: 50mm2 (4mm diameter) adhesives were attached to the palm of each forepaw, and mice were observed for 2 min and scored for identifying the presence of and removal of the adhesive. Shorter removal times indicated improved neurological recovery. Elevated body swing test: To measure motor deficits, mice were suspended vertically by the tail with their heads elevated 3 inches above the test bench. A lateral swing was counted each time the animal moved its head >10 degrees away from the vertical axis 13.
In vitro models
Neuro-2a cells (N2a), were purchased from the American Type Culture Collection (ATCC). Cultures were grown and maintained in DMEM (Cellgro) supplemented with 10% Fetal bovine Serum Defined (FBS, Hyclone). Under humidified 5%, CO2 95% air atmosphere and at 37°C, cells were plated in 25cm2 cell culture flasks (Corning). Media was changed 3 d after seeding, and split twice a week. For experiments, cells were plated on 12-well dishes (1×106 cells/well).
Cells were exposed to oxygen glucose deprivation (OGD) by placing in an anoxia chamber (O2 tension <0.001%, Coy Laboratories) for 2 h at 37°C. Media was removed and replaced with balanced salt solution (BSS0) lacking serum or glucose, or oxygen. Control cultures were incubated at 37°C with balanced salt solution containing 5.5mM glucose (BSS5.5). After of OGD, glucose was added to each well to a final concentration of 5.5mM, and plates were returned to normoxia.
Cell death and viability were assessed by morphological assessment by light microscopy, vital staining (trypan blue; Sigma) and the tetrazolium dye MTT 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT; Sigma) assay.
17-N-allylamino-17-demethoxygeldanamycin (17-AAG) and Dynasore administration
After control or OGD exposure, N2a cultures were treated with 60μM 17-AAG (Sigma) to induce Hsp70, or 30μM dynasore (Sigma), a dynamin inhibitor. Each compound was solubilized in 0.1% dimethyl sulfoxide (DMSO, Sigma) and dimethylformamide (DMF, Sigma) and diluted in PBS.
Intracerebroventricular (ICV) injections took place 30 min after dMCAO or sham surgeries. Anesthetized mice were placed in a stereotaxic frame and a total volume 2μl of dynasore (0.3 mg/ml dose) or vehicle (0.1% DMF in PBS) was injected into the right lateral cerebral ventricle (stereotaxic coordinates: 1mm caudal to bregma, 1.3mm lateral to sagittal suture, and 2mm in depth) at a speed of 0.5μl/min via a burr hole. The needle was left place for 5 min to allow drug diffusion into tissue before it was removed, and the burr hole filled with bone wax.
Co-immunoprecipitation (Co-ip), Membrane fractionation and Immunoblotting
For immunoblots, ipsilateral hemispheres or N2a cells were homogenized and solubilized in RIPA lysis buffer (Sigma) with protease inhibitors mixture (ROCHE). Co-ip was performed as previously described by our lab 14. Brain lysates were incubated with 2.5mg of mouse anti-Hsp70 (Stressgen) or an IgG isotype control (2.5mg normal mouse IgG, Santa Cruz), and the protein A/G PLUS-Agarose was collected. To detect Fas in the cell membrane, a Subcellular Protein Fractionation Kit (Thermo) was used according to the manufacturer’s instructions to separate and isolate membrane from tissue samples. 20μg protein samples were subjected to 10% SDS-polyacrylamide gel electrophoresis, then transferred to polyvinylidinene fluoride (PVDF) membranes (Millipore), and probed for the protein of interest by incubation with mouse anti-Hsp70 (1:1000; Stressgen) or dynamin-1 (1:1000; Santa Cruz) and rabbit anti-caspase-8 (1:1000; Santa Cruz) or Fas (1:1000; Santa Cruz) antibodies, followed by a horseradish peroxidase (HRP) conjugated secondary antibody. Blots were visualized using the ECL system (Amersham) according to the manufacturer’s directions, and imaged using LAS-4000 (Fuji).
Flow cytometry
After OGD, 1×106 N2a cells were washed once in PBS and blocked with Flow Cytometry Staining Buffer (eBioscience) for 10 min. Samples were stained with mouse anti-Fas (1:1000, Santa Cruz), followed by Alexa Fluor 488-conjugated goat anti-mouse IgG (1:1000, Invitrogen). Mouse IgG-PE (Santa Cruz) was used as an isotype control. N2a cells were sorted on FACSCalibur (BD Biosceinces), and data were analyzed using CellQuest (BD Biosciences).
Immunohistochemistry
3 d after dMCAO, brain sections (10μm thick) were incubated with primary antibodies against mouse anti-dynamin-1 (1:1000; Santa Cruz) and rabbit anti-caspase-8 (1:500, Santa Cruz) or Fas (1:200, Santa Cruz), followed by secondary biotinylated antibodies, and 3,3′-diaminobenzidine (Vector Laboratories), and counterstained with cresyl violet.
Brain sections were colabeled with antibodies against dynamin-1 plus cell markers for neurons (MAP-2), astrocytes (GFAP), microglia (CD11b), or Fas plus dynamin-1 in order to determine which cell populations expressed dynamin-1, followed by a fluorescent secondary antibody.
TUNEL staining
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL, ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit, Millpore) was used according to the manufacturer’s instructions to assess DNA fragmentation in neuron and described previously 15. Numbers of TUNEL-positive cells in the ipsilateral hemisphere was normalized the numbers of cells counted from brains of sham animals.
Statistical Analysis
All studies were randomized and assessments carried out by investigators blinded to experimental conditions. All data were analyzed with standard statistical methods (T-test, Systat Software, Inc). Data were presented as the mean ± SE. Differences between the two groups were compared using an unpaired t test, and multiple comparisons were performed using one-way ANOVA followed by Bonferroni’s post hoc test. P ≤0.05 was considered significant.
Results
Hsp70 protects against experimental stroke
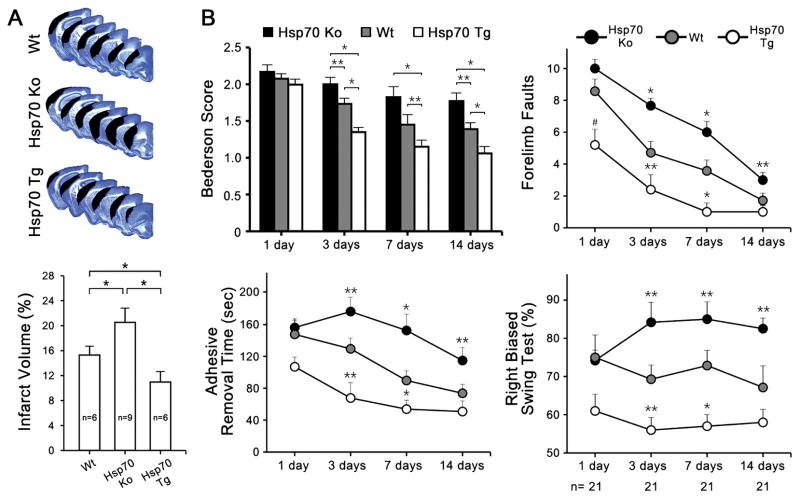
Consistent with previously published reports 16, Hsp70 overexpression led to improved neurological outcomes, whereas Hsp70 deficiency led to worsened outcomes compared to Wt. Infarct size 14 d post dMCAO from Wt, Hsp70Ko, and Tg mice were compared. Infarct volumes quantified from cresyl violet stained sections was significantly smaller in the Hsp70Tg mice compared with Wt and Hsp70Ko mice (Figure 1A). Motor function was also improved among Hsp70Tg mice and worsened in Hsp70Ko mice (Figure 1B). Behavioral indices among sham controls did not reveal any baseline differences due to gene manipulation (data not shown).
Figure 1.
Hsp70 reduces ischemic brain injury and improves neurological function. A, Representative cresyl violet-stained brain sections showed a smaller infarct in a Hsp transgenic brain (Hsp70Tg) compared to wildtype (Wt) and larger infarct in a Hsp knockout (Hsp70Ko). Infarct size among Hsp70Tg mice was reduced compared to Wt mice, and Hsp70Ko mice had larger infarcts. B, For 4 separate behavioral tests, Hsp70Ko mice performed worse than Wt and Hsp70Tg mice. Graphs show results from the Bederson, ladder, adhesive tape removal (time to remove tape from forelimbs), and elevated body swing tests (n=6–9/group, *P<0.01, **P<0.05).
Hsp70 overexpression decreases and interacts with dynamin-1
Using our in vitro model, we induced Hsp70 in N2a cells using 17-AAG as we previously reported 17. 17-AAG treatment led to decreased cell death after OGD (Supplemental Figure SIIA, B) and 2-fold higher Hsp70 induction and 2.3-fold lower dynamin-1 expression relative to controls (Supplemental Figure SIIC).
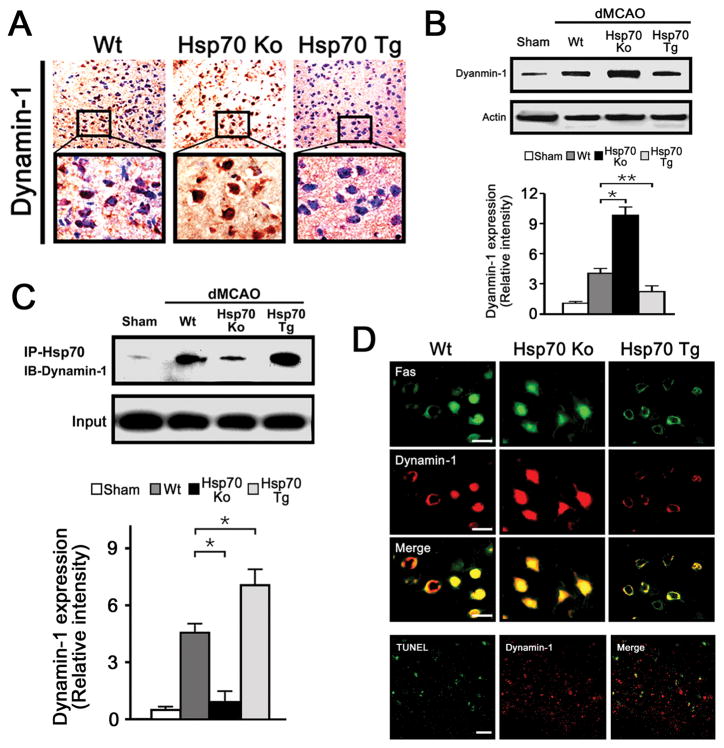
Consistent with observations from the proteomic analysis, Hsp70Tg mice showed reduced dynamin-1 levels compared to Ko and Wt mice after brain ischemia, while Hsp70Ko mice showed the highest dynamin-1 levels (Figure 2A, B). This was seen especially within cells of the ischemic border zone. Double labeling showed that dynamin-1 was present in neurons but not astrocytes or microglia under non injury conditions. In ischemic brains, dynamin-1, Fas, and Hsp70 were expressed in neurons (Supplemental Figure SIII).
Figure 2.
A, Dynamin-1 is increased more markedly in Hsp70Ko mice 3 d post insult, compared to Wt mice, whereas expression is reduced in Hsp70Tg brains (n=3/group, scale bar=50 μm). B, Immunoblots show dynamin-1 from Wt, Hsp70Ko and Hsp70Tg mouse brains 1d after dMCAO. Relative intensities of protein normalized to β-actin show that dynamin-1 was increased in Hsp70Ko compared to Wt and Hsp70Tg (n=3/group, *P<0.01, **P<0.05). C, 1 d post ischemia, brain lysates were immunoprecipitated using anti-Hsp70. Immunoblots (IB) of the immunoprecipitate (IP) showed significant levels of dynamin-1, indicating that Hsp70 associates with dynamin-1 (n=3/group, *P<0.01). Relative intensities of the immunoblots showed that Hsp70 highly associated with dynamin-1 in Hsp70Tg. D, 3 days post dMCAO, dynamin-1 (red) and Fas (green) colocalized (Merge, yellow). Fas and dynamin-1 were reduced in Hsp70Tg brains and increased in Hsp70Ko brains compared to Wt (n=3/group, scale bar=10 μm). In ischemic brain, dynamin-1 also colcalized with TUNEL-positive cells in Wt mice.
To determine whether there is any relationship between Hsp70, dynamin-1 and Fas, co-immunoprecipitation studies showed that Hsp70 associated with dynamin-1 (Figure 2C). The highest Hsp70-dynamin-1 associations were seen in the Tg mice, and the least in the Ko mice. Colabeling for dynamin-1 and Fas showed that both colocalized to neuron-like cells and were the highest in Hsp70Ko brains and lowest in the Tg brains (Figure 2D). Further, several dynamin positive cells were TUNEL positive (Figure 2D).
Hsp70 attenuates Fas-mediated apoptotic death
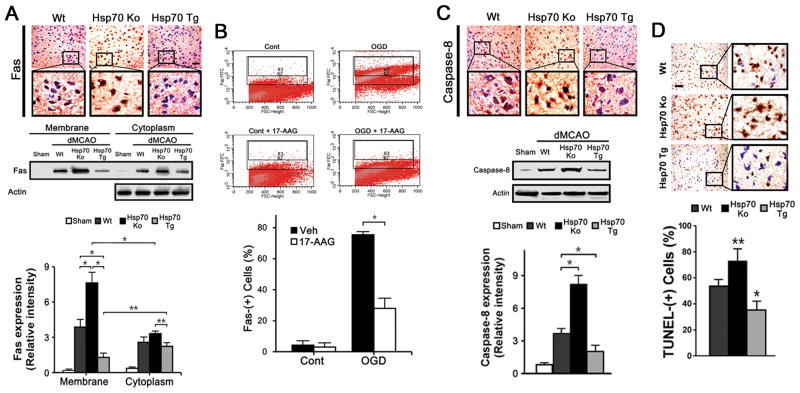
To explore a link between Hsp70 and Fas mediated cell death, dMCAO exposed brains from Wt, Hsp70Ko and Hsp70Tg mice were stained for Fas. Fas positive cells were increased by brain ischemia, and brains of Hsp70Ko mice expressed more Fas compared to either Wt or Hsp70Tg. Since Fas must be expressed on the cell’s surface in order to trigger cell death, immunoblots of membrane fractions showed that membrane bound Fas was significantly decreased in the brain of Hsp70Tg mice, whereas Hsp70Ko mice had 4-fold higher Fas expression (Figure 3A). Further, N2a cells exposed to OGD showed increased Fas positive cells, and treatment with 17-AAG decreased these numbers by about 42% (Figure 3B). The percentage of Fas+ cells were calculated as follows. R2 demarcated the FITC signal threshold for stained cells, excluding baseline FITC levels in unstained controls. R3 excludes FITC levels from non-specific staining in control samples (secondary antibody only control). R3 thereby conservatively estimates the population of Fas-expressing cells within a given sample.
Figure 3.
A, 3 d post dMCAO, Fas staining was more intense after dMCAO in Hsp70Ko, but decreased in Hsp70Tg (n=3/group, scale bar=50 μm). 1 d post dMCAO, ischemic brain tissues were fractionated, and subjected to immunoblotting. Fas from membrane and cytosolic fractions increased in both fractions following dMCAO, but Fas was most increased amongst Hsp70Ko, and the least in Hsp70Tg. Fas was also higher in the membrane fraction amongst Wt and Hsp70Ko, compared to the cytosolic fraction, but decreased in Hsp70Tg (n=3/group, *P<0.01). B, Hsp70 induced in N2a cells with 17-AAG and subjected to OGD show fewer Fas positive cells by quantitative FACS compared to controls. (n=3/group, *P<0.01). C, 3d post dMCAO, caspase-8 staining was more intense after dMCAO in Hsp70Ko, but decreased in Hsp70Tg (n=3/group, scale bar=50 μm). Immunoblots show caspase-8 expression 1day after dMCAO. Caspase-8 was decreased in Hsp70Tg compared to Wt and Hsp70Ko (n=3/group, *P<0.01). D, 3 d post dMCAO, Hsp70Ko showed increased numbers of TUNEL-positive cells in the ischemic cortex when compared to Hsp70Tg. The percentage of TUNEL-positive cells normalized to total cell numbers acquired from sham show fewer TUNEL-positive cells in samples from Hsp70Tg compared to Wt or Hsp70Ko (n=5/group, *P<0.01, **P<0.05, scale bar=50μm)
Since caspase-8 is activated downstream of Fas, but not during intrinsic (mitochondria) apoptosis, we assayed for caspase-8. Caspase-8 was the most decreased amongst ischemic Hsp70Tg brain samples compared to either Wt or Hsp70Ko mice, whereas capase-8 expression was highest amongst Hsp70Ko (Figure 3C). Increased dynamin-1, Fas and caspase-8 protein also corresponded to an increased number of TUNEL-positive injured neurons, particularly in Hsp70Ko mice. Hsp70Tg mice showed significantly reduced numbers of TUNEL-positive cells by ~20–25% (Wt vs Tg) and ~35–40% (Ko vs Tg) respectively (Figure 3D). These data suggest that dynamin contributes to Fas mediated cell death in experimental stroke, and that Hsp70 may protect by inhibiting dynamin expression and preventing surface expression of Fas.
Dynamin inhibition is protective
To determine functional significance of dynamin, OGD-exposed N2a cells were treated with dynasore (Dyna), a dynamin inhibitor which interferes with its GTPase activity 18. This led to improved cell viability and reduced cell death (Figure 4A, B), and 3.4-fold lower dynamin-1 expression relative to Veh groups (Figure 4C), and decreased numbers of Fas positive N2a cells as demonstrated by flow cytometry (Figure 4D).
Figure 4.
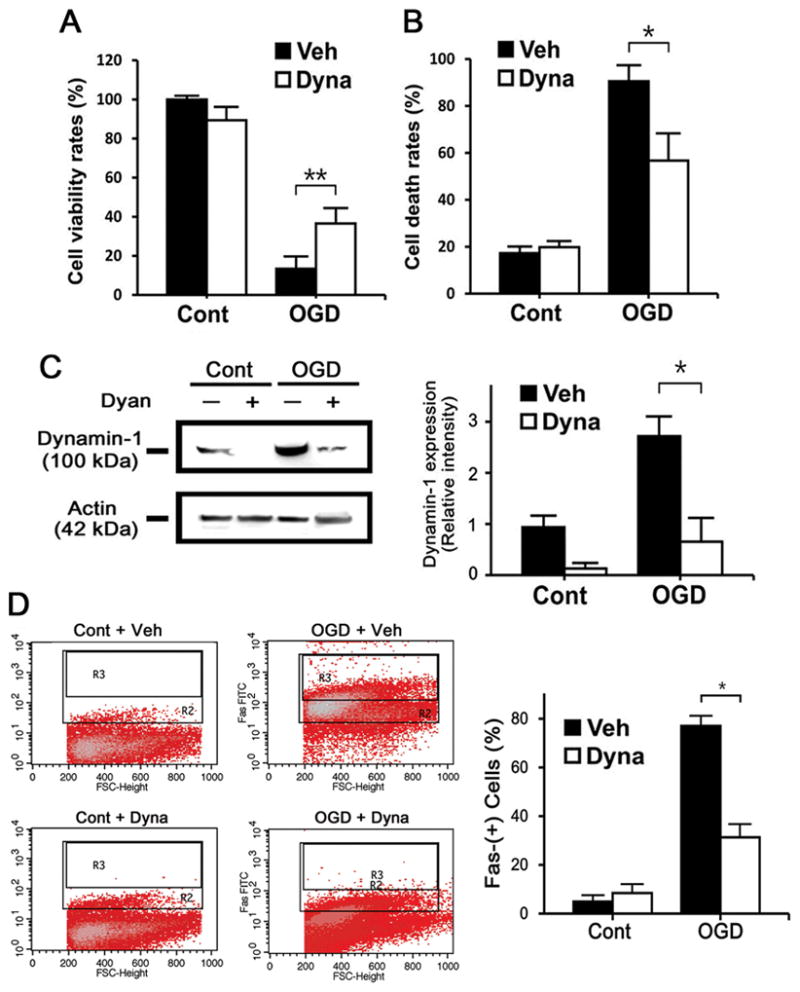
Dynamin inhibition is neuroprotective. Cultures of N2a cells were subjected to OGD and treated with dynasore (Dyna) and vehicle (Veh), a dynamin inhibitor. MTT (A) and trypan blue stains (B) showed increased cell viability and decreased cell death, respectively with Dyna treatment cells (n=3/group; *P<0.01, **P<0.05). C, Immunoblots of OGD-exposed N2a cells show increased dynamin-1 expression, whereas treatment with Dyna reduced this (n=3/group; *P<0.01). D, Fas labeled N2a cells were then subjected to FACS. Dyna treatment decreased number of Fas positive cells (n=3/group; *P<0.01, **P<0.05).
At the in vivo level, we first established that Dyna could engage target tissues in the brain. Texas red-conjugated Dyna (courtesy of Dr. Nick Cairns, Combinix, Inc. Mountain View, CA) was injected ICV into uninjured animals. Fluorescent signals were observed in brain tissues showing that Dyna did in fact travel to and bind to brain cells (Supplemental Figure SIV). Dyna administration did not appear to cause any seizures or obvious neurological impairment in uninjured mice.
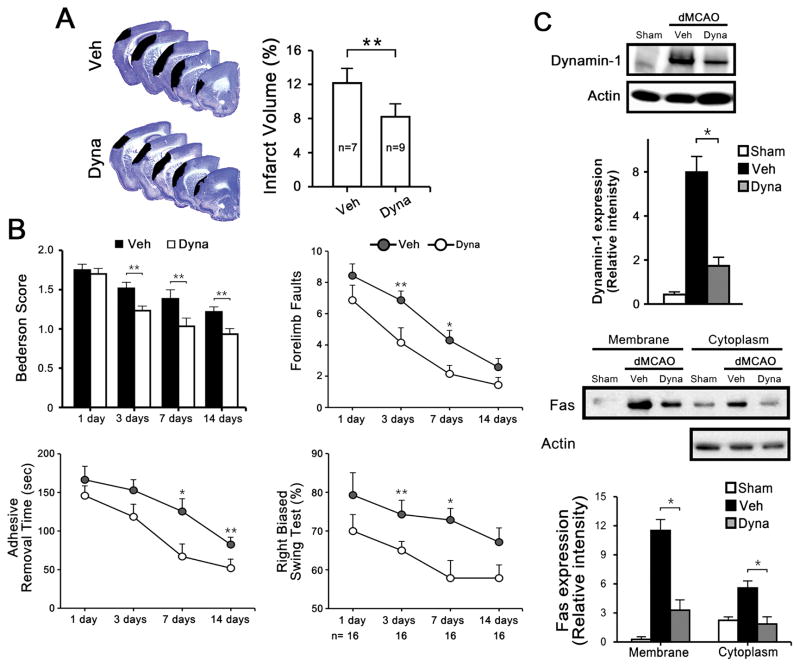
Dyna treatment in dMCAO mice led to smaller strokes compared to vehicle (Figure 5A) treated controls, with improved motor function (Figure 5B). Dynamin-1 expression was also decreased with Dyna treatment. To determine whether dynamin inhibition might prevent Fas translocation to the cell surface, immunoblots of cytoplasmic and membrane fractions showed that dMCAO led to increased expression of Fas in the membrane fraction, and Dyna treatment decreased this (Figure 5C).
Figure 5.
Intracerebroventricular (ICV) injection of Dyna protects against dMCAO. 14 d post dMCAO, cresyl violet-stained brain sections show smaller infarcts in mice treated with Dyna compared to Veh treated mice. A, Infarct size amongst mice treated with Dyna was reduced by compared to mice treated with Veh. B, Bederson, Ladder test, adhesive removal, and body swing tests show that mice given Dyna performed better than Veh treated mice (n=7–9/group, *P<0.01, **P<0.05). C, Dynamin inhibition attenuated the amount of Fas present on the cell surface in experimental stroke. Immunoblots of brain samples show decreased dynamin-1 expression at 3 d following Dyna treatment after dMCAO (n=3/group, *P<0.01). Immunoblots of Fas from membrane and cytosolic fractions of brain tissue 3 d after MCAO show increased membrane Fas in Veh treated mice compared, and this increase was reduced by Dyna treatment (n=4/group, *P<0.01).
To explore any synergistic actions of Hsp70 and dynamin inhibition, we administered Dyna to Hsp70Tg mice exposed to dMCAO. However, Dyna did not lead to any further lesion size reduction or improvement in neurological function (Supplemental Figure SV).
Discussion
Hsp70Tg mice exposed to stroke led to the identification of dynamin as a markedly down regulated protein. To our knowledge, this is the first report of Dynamin in brain ischemia. We show that dynamin increases after stroke, along with Fas and caspase-8, and these proteins are decreased in mice overexpressing Hsp70, while the opposite was observed in Hsp70 deficiency. Further, we show that the proportion of membrane bound Fas is increased after stroke, but is reduced by Hsp70Tg overexpression. This is consistent with prior work that showed that dynamin trafficks Fas to the cell’s surface 4, and is in line with our hypothesis that Hsp70 prevents this trafficking (Figure 6). We also demonstrate for the first time that inhibition of dynamin improves outcome from experimental stroke.
Figure 6.
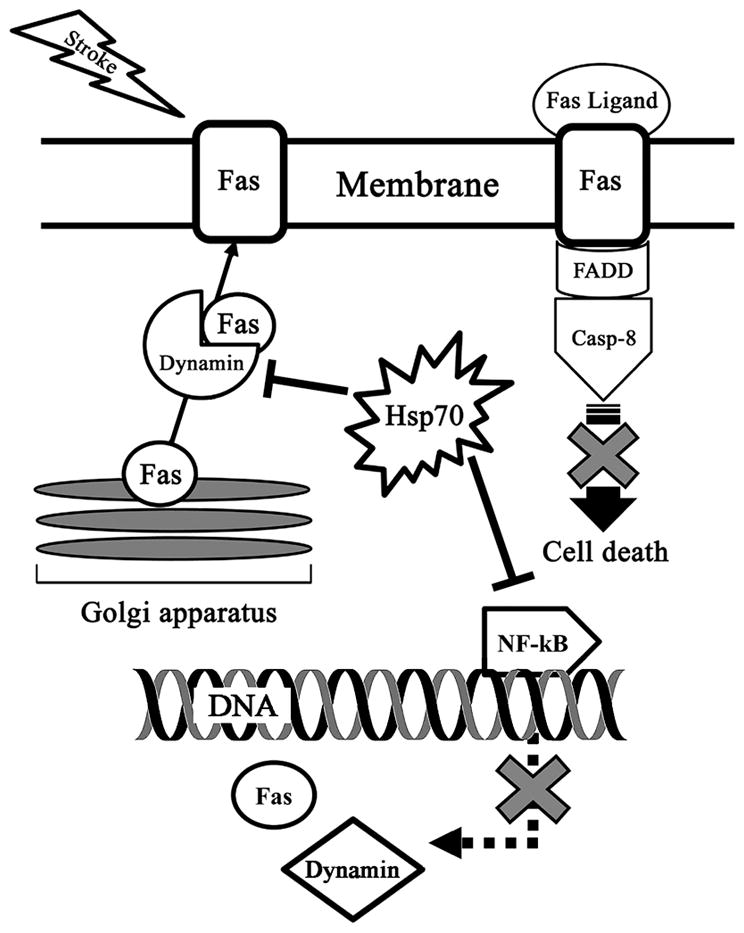
Proposed dynamin and Fas interactions during stroke. Stroke leads to increased membrane Fas expression, presumably due to its trafficking from the Golgi apparatus by dynamin. Fas ligand, also increased following stroke, binds Fas and activates caspase-8, which then leads to cell death through apoptosis. Hsp70 may reduce both Fas and dynamin expression, but also prevents Fas membrane expression.
Hsp70 has previously been shown to interfere with many aspects of the intrinsic apoptotic pathway by inhibiting caspase activation, preventing mitochondrial release of cytochrome c, or increasing the anti-apoptotic protein Bcl-2 19. Less has been studied with respect to the extrinsic, or receptor mediated apoptotic pathways. Death receptors include Fas, which initiates cell death with the binding of Fas by its ligand FasL, and leads to caspase-8 activation and apoptosis 20. Fas activation has been documented in ischemic stroke and related pathologies 21. Fas and its ligand have been documented in the brain following ischemia 22, and several studies, including some from our lab, have shown that interrupting this pathway is protective 22, 23.
In neurological disease, dynamin-1 deficiency has been linked to defects in γ-amino butyric acid (GABA) transmission and epilepsy 2. However, intact dynamin has also been shown to have negative consequences. Dynamin has been linked to Alzheimer’s disease pathology 24 while its inhibition or deficiency led to decreased neuronal degeneration 25. Dynamin has been shown to traffick Fas protein from the Golgi apparatus through the Trans Golgi network (TGN) to the cell surface where it can be bound by FasL4, and may suggest an additional role in brain cell death and degeneration. There are scant reports of dynamin in the ischemia literature. The most widely studied appears to be dynamin related protein-1 (Drp1) and its role in mitochondrial fission. In cardiac and renal ischemia, Drp1 inhibition has been shown to be cytoprotective by preventing mitochondrial apoptosis 26, 27. There are a few reports characterizing Drp1 in experimental stroke 28, and Drp1 inhibition decreased apoptosis 29.
The relationship of dynamin to Hsp70 is unknown, but the present data indicate an inverse relationship between Hsp70 and dynamin in the ischemic brain. Hsp70 overexpression attenuated increases in dynamin after experimental stroke, while its deficiency did the opposite. Transgenic mice overexpressing Hsp70 had decreased Fas, caspase-8, and membrane-associated Fas. Based on our own experiments and reports in the literature, we postulate two potential mechanisms for this relationship. Hsp70 may regulate dynamin at the transcriptional level. Dynamin’s promoter region contains a sequence similar to those recognized by NF-kB 30, as does Fas 31. Hsp70 is known to inhibit NF-kB’s transcriptional activity 16, and may disrupt dynamin at the transcriptional level. However, it is also possible that Hsp70 may directly inhibit dynamin-dependent Fas-translocation by containing dynamin in the cytosol through specific chaperone interactions.
Pharmacologic inhibition of dynamin can also protect against stroke. Dynamin inhibition led to protection in our stroke models, and reduced Fas expression on the cell surface. However, it should be noted that we administered dynasore intracerebroventricularly shortly after stroke onset. While this paradigm was designed as a proof of concept study, future studies should further address the optimal timing and dosing of dynamin inhibitors.
Conclusion
We reveal a previously unknown mechanism of protection by Hsp70 in the ischemic brain and identify dynamin as a potential therapeutic target. Future studies may focus on more precise interactions between Hsp70 and dynamin, and the development of dynamin inhibitors.
Supplementary Material
Acknowledgments
Sources of Funding
This study was funded by grants from the National Institutes of Health (NS40516), Department of Defense and the Veteran’s Merit Award (I01 BX000589) to MY, American Heart Association Western States Affiliate Postdoctoral Fellowship (13POST14810019) to JYK and National Research Foundation of Korea grant from the Korean government (NRF-2014R1A2A2A01006556) to JEL. Grants to MY and JYK were administered by the Northern California Institute for Research and Education, and supported by resources of the Veterans Affairs Medical Center, San Francisco, California.
Footnotes
Disclosures
None.
References
- 1.Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004;16:53–61. doi: 10.1097/00008506-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling gtpase. Nat Rev Mol Cell Biol. 2012;13:75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lou X, Fan F, Messa M, Raimondi A, Wu Y, Looger LL, et al. Reduced release probability prevents vesicle depletion and transmission failure at dynamin mutant synapses. Proc Natl Acad Sci U S A. 2012;109:E515–523. doi: 10.1073/pnas.1121626109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivanov VN, Ronai Z, Hei TK. Opposite roles of fap-1 and dynamin in the regulation of fas (cd95) translocation to the cell surface and susceptibility to fas ligand-mediated apoptosis. The Journal of biological chemistry. 2006;281:1840–1852. doi: 10.1074/jbc.M509866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galluzzi L, Morselli E, Kepp O, Kroemer G. Targeting post-mitochondrial effectors of apoptosis for neuroprotection. Biochim Biophys Acta. 2009;1787:402–413. doi: 10.1016/j.bbabio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Qin ZH. Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis. 2010;15:1382–1402. doi: 10.1007/s10495-010-0481-0. [DOI] [PubMed] [Google Scholar]
- 7.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 8.Grohm J, Kim SW, Mamrak U, Tobaben S, Cassidy-Stone A, Nunnari J, et al. Inhibition of drp1 provides neuroprotection in vitro and in vivo. Cell death and differentiation. 2012;19:1446–1458. doi: 10.1038/cdd.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kd heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt CR, Dix DJ, Sharma GG, Pandita RK, Gupta A, Funk M, et al. Genomic instability and enhanced radiosensitivity in hsp70.1- and hsp70.3-deficient mice. Mol Cell Biol. 2004;24:899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawabori M, Kacimi R, Kauppinen T, Calosing C, Kim JY, Hsieh CL, et al. Triggering receptor expressed on myeloid cells 2 (trem2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J Neurosci. 2015;35:3384–3396. doi: 10.1523/JNEUROSCI.2620-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann J, Blum R, Kovalchuk Y, Adelsberger H, Kuner R, Durand GM, et al. Distinct roles of galpha(q) and galpha11 for purkinje cell signaling and motor behavior. J Neurosci. 2004;24:5119–5130. doi: 10.1523/JNEUROSCI.4193-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borlongan CV, Sanberg PR. Elevated body swing test: A new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J Neurosci. 1995;15:5372–5378. doi: 10.1523/JNEUROSCI.15-07-05372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JY, Yenari MA, Lee JE. Regulation of inflammatory transcription factors by heat shock protein 70 in primary cultured astrocytes exposed to oxygen-glucose deprivation. Neuroscience. 2015;286:272–280. doi: 10.1016/j.neuroscience.2014.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JY, Ho H, Kim N, Liu J, Tu CL, Yenari MA, et al. Calcium-sensing receptor (casr) as a novel target for ischemic neuroprotection. Ann Clin Transl Neurol. 2014;1:851–866. doi: 10.1002/acn3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kda heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008;28:53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]
- 17.Kacimi R, Yenari MA. Pharmacologic heat shock protein 70 induction confers cytoprotection against inflammation in gliovascular cells. Glia. 2015;63:1200–1212. doi: 10.1002/glia.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Kelly S, Zhang ZJ, Zhao H, Xu L, Giffard RG, Sapolsky RM, et al. Gene transfer of hsp72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: Influence of bcl-2. Ann Neurol. 2002;52:160–167. doi: 10.1002/ana.10264. [DOI] [PubMed] [Google Scholar]
- 20.Love S. Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:267–282. doi: 10.1016/S0278-5846(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 21.Padosch SA, Popp E, Vogel P, Bottiger BW. Altered protein expression levels of fas/cd95 and fas ligand in differentially vulnerable brain areas in rats after global cerebral ischemia. Neurosci Lett. 2003;338:247–251. doi: 10.1016/s0304-3940(02)01408-8. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Kim JY, Koike MA, Yoon YJ, Tang XN, Ma H, et al. Fasl shedding is reduced by hypothermia in experimental stroke. J Neurochem. 2008;106:541–550. doi: 10.1111/j.1471-4159.2008.05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blankenberg FG, Kalinyak J, Liu L, Koike M, Cheng D, Goris ML, et al. 99mtc-hynic-annexin v spect imaging of acute stroke and its response to neuroprotective therapy with anti-fas ligand antibody. Eur J Nucl Med Mol Imaging. 2006;33:566–574. doi: 10.1007/s00259-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhu L, Su M, Lucast L, Liu L, Netzer WJ, Gandy SE, et al. Dynamin 1 regulates amyloid generation through modulation of bace-1. PLoS One. 2012;7:e45033. doi: 10.1371/journal.pone.0045033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, et al. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 27.Qi X, Qvit N, Su YC, Mochly-Rosen D. A novel drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci. 2013;126:789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, Tian F, Kurata T, Morimoto N, Abe K. Dynamic changes of mitochondrial fusion and fission proteins after transient cerebral ischemia in mice. J Neurosci Res. 2012;90:1183–1189. doi: 10.1002/jnr.23016. [DOI] [PubMed] [Google Scholar]
- 29.Zhang N, Wang S, Li Y, Che L, Zhao Q. A selective inhibitor of drp1, mdivi-1, acts against cerebral ischemia/reperfusion injury via an anti-apoptotic pathway in rats. Neurosci Lett. 2013;535:104–109. doi: 10.1016/j.neulet.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 30.Yoo J, Lee SS, Jeong MJ, Lee KI, Kwon BM, Kim SH, et al. Characterization of the mouse dynamin i gene promoter and identification of sequences that direct expression in neuronal cells. Biochem J. 2000;351(Pt 3):661–668. [PMC free article] [PubMed] [Google Scholar]
- 31.Liu F, Bardhan K, Yang D, Thangaraju M, Ganapathy V, Waller JL, et al. Nf-kappab directly regulates fas transcription to modulate fas-mediated apoptosis and tumor suppression. The Journal of biological chemistry. 2012;287:25530–25540. doi: 10.1074/jbc.M112.356279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.