Summary
Descriptive epidemiological information on myeloproliferative neoplasms (MPNs) and myelodysplastic (MDS)/MPNs is largely derived from single institution and European population-based studies. Data obtained following adoption of the World Health Organization classification of haematopoietic neoplasms and JAK2 V617F mutation testing are sparse. Using population-based data, we comprehensively assessed subtype-specific MPN and MDS/MPN incidence rates (IRs), IR ratios (IRRs) and relative survival (RS) in the United States (2001-2012). IRs were highest for polycythaemia vera (PV) (IR=10.9) and essential thrombocythaemia (ET) (IR=9.6). Except for ET and mastocytosis, overall IRs were significantly higher among males (IRRs=1.4-2.3). All evaluable MPNs were associated with lower IRs among Hispanic whites than non-Hispanic whites (NHWs), with the exception of BCR-ABL1-positive chronic myeloid leukaemia (CML), chronic eosinophilic leukaemia (CEL) and juvenile myelomonocytic leukaemia. Except for CEL, Asians/Pacific Islanders had significantly lower MPN IRs than NHWs. ET, MPN-unclassifiable and CEL IRs were 18%, 19% and 60% higher, respectively, among blacks than NHWs. Five-year RS was more favourable for younger (<60 years) than older individuals and for women compared with men, except for PV at older ages. RS was highest (>90%) for younger PV and ET patients and lowest (<20%) for older chronic myelomonocytic leukaemia and atypical BCR-ABL1-negative CML patients. Varying MPN and MDS/MPN incidence patterns by subtype support distinct aetiologies and/or susceptible populations. Decreased survival rates as compared to that expected in the general population were associated with every MPN subtype, highlighting the need for new treatments, particularly among older individuals.
Keywords: Myeloproliferative neoplasm, MPNs, epidemiology, incidence, survival
Introduction
Myeloproliferative neoplasms (MPNs) are a group of stem cell disorders characterized by clonal myeloproliferation that is devoid of dyserythropoiesis, granulocytic dysplasia or monocytosis (Swerdlow, et al 2008). Closely related but distinct are the myelodysplastic (MDS)/MPNs, which are clonal haematopoietic neoplasms that share clinical, morphological and laboratory features not only with MPNs but also with myelodysplastic syndromes (Jaffe, et al 2001, Swerdlow, et al 2008).
Epidemiological studies of MPNs and MDS/MPNs in the United States (US) have been hampered by evolving disease classifications and non-reporting to cancer registries. In 2001, the World Health Organization (WHO) classification of tumours of haematopoietic and lymphoid tissues (Jaffe, et al 2001) built upon existing guidelines from the Polycythaemia Vera Study Group (Murphy, et al 1997) to define seven MPNs and four MDS/MPNs (Jaffe, et al 2001). While morphological and clinical features remained integral to the diagnosis of each MPN and MDS/MPN in 2001, the WHO classification incorporated genetic information into the diagnostic criteria of select entities. Most notably, the presence of BCR-ABL1 “unequivocally” confirmed a diagnosis of chronic myeloid leukaemia (CML), and one of the major diagnostic criteria for polycythaemia vera (PV) included the presence of a clonal genetic marker other than the BCR-ABL1 gene rearrangement. However, in 2001 no chromosomal or molecular markers specific for the BCR-ABL1-negative MPNs had been identified (Jaffe, et al 2001).
Classification of MPNs and MDS/MPNs further evolved with the introduction of the 2008 WHO classification (Swerdlow, et al 2008). Among the most important changes was the inclusion of the JAK2 V617F or other clonal genetic/molecular markers in the diagnostic criteria for the majority of BCR-ABL1-negative MPNs and MDS/MPNs (Kralovics, et al 2005). Other changes included decreasing the platelet count threshold for essential thrombocythaemia (ET), and changing the naming convention from chronic myeloproliferative disorders to MPNs, to better reflect the malignant nature of these entities (Swerdlow, et al 2008).
The International Classification of Diseases for Oncology (ICD-O) classification evolved in concert with the 2001 WHO classification. Prior to 2001 when the third edition of ICD-O (ICD-O-3) was adopted, several MPNs were not considered malignant and thus were not reportable to cancer registries in the US.
Population-based studies describing incidence of MPNs and MDS/MPNs limited to the current century are sparse (Rollison, et al 2008, Sant, et al 2010, Smith, et al 2011) and few include data subsequent to 2005 (Smith, et al 2011) when JAK2 V617F mutation testing became available. To gain insight into the patterns of occurrence of MPNs and MDS/MPNs by age, sex, race/ethnicity and calendar year; identify susceptible populations and provide a population-based assessment of patient survival, we used data from the Surveillance, Epidemiology and End Results (SEER) Program to describe incidence of MPNs and MDS/MPNs and patient survival in the US during the early 21st century.
Methods
A summary of the evolution of the MPNs and MDS/MPNs according to the WHO and ICD-O-3 classifications is detailed in Table I. We assessed all malignant cases of MPNs and MDS/MPNs diagnosed among residents of 18 cancer registry areas of the National Cancer Institute’s (NCI) SEER-18 Program during 2001-2012. An in-depth description of the SEER Program, including data quality and reliability methods, can be found in the Supplementary Text and at www.seer.cancer.gov. In brief, SEER-18 represents approximately 27.8% of the US population and includes the registries in eight states (Connecticut, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico and Utah); six metropolitan areas (Atlanta, Georgia; Detroit, Michigan; Los Angeles, San Francisco-Oakland and San Jose-Monterey, California; Seattle-Puget Sound, Washington); the areas of Greater California, Rural Georgia, Greater Georgia; and the Alaska Native Tumor Registry. For specified analyses by calendar year, we utilized SEER-17 (excluding Greater Georgia, which only officially entered the SEER program in 2010).
Table 1.
Evolution of the International Classification of Diseases for Oncology and World Health Organization classifications and associated disease codes of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms
| ICD code |
WHO (2001) |
WHO (2008) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Disease entity (ICD-O-3) | ICD-O | ICD-O-2 | ICD-O-3 | Disease entity | Category | Clonal marker | Disease entity | Category | Clonal marker |
| Chronic myeloid leukaemia, BCR- ABL1 positive |
~ | ~ | 9875/3 | Chronic myeloid leukaemia | CMPD | Yes (BCR-ABL1) | Chronic myeloid leukaemia, BCR- ABL1 positive |
MPN | Yes (BCR-ABL1) |
|
| |||||||||
| Chronic neutrophilic leukaemia | ~ | ~ | 9963/3 | Chronic neutrophilic leukaemia | CMPD | No | Chronic neutrophilic leukaemia | MPN | No |
|
| |||||||||
| Polycythaemia vera | 9950/1 | 9950/1 | 9950/3 | Polycythaemia vera | CMPD | Yes (NOS) | Polycythaemia vera | MPN | Yes (JAK2 V617F or other) |
|
| |||||||||
| Myelosclerosis with myeloid metaplasia | 9961/1 | 9961/1 | 9961/3 | Chronic idiopathic myelofibrosis | CMPD | No | Primary myelofibrosis | MPN | Yes (JAK2 V617F or other) |
|
| |||||||||
| Essential thrombocythaemia | 9962/1 | 9962/1 | 9962/3 | Essential thrombocythaemia | CMPD | No | Essential thrombocythaemia | MPN | Yes (JAK2 V617F or other) |
|
| |||||||||
| Hypereosinphilic syndrome | ~ | ~ | 9964/3 | Chronic eosinophilic leukaemia/hypereosinophilic syndrome |
CMPD | Yes (NOS) | Chronic eosinophilic leukaemia, NOS | MPN | Yes (NOS) |
|
| |||||||||
| Malignant mastocytosis | 9741/3 | 9741/3 | 9741/3 | Systemic mastocytosis with associated clonal, hematological non-mast cell lineage disease |
Mastocytosis | Yes (KIT D816V mutation) |
Mastocytosis: Systemic mastocytosis |
MPN | Yes (KIT D816V mutation) |
|
| |||||||||
| Aggressive systemic mastocytosis | Mastocytosis | Yes (KIT D816V mutation) |
|||||||
|
| |||||||||
| Mast cell sarcoma | 9740/3 | 9740/3 | 9740/3 | Mast cell sarcoma | Mastocytosis | No | Mastocytosis: Mast cell sarcoma | MPN | Yes (NOS) |
|
| |||||||||
| Mast cell leukaemia | ~ | ~ | 9742/3 | Mast cell leukaemia | Mastocytosis | Yes (KIT D816V mutation) |
Mastocytosis: Mast cell leukaemia | MPN | Yes (KIT D816V mutation) |
|
| |||||||||
| Myeloproliferative disease, NOS* | ~ | ~ | 9975/1, 9975/3 |
Chronic myeloproliferative disease, unclassifiable |
CMPD | No | Myeloproliferative neoplasm, unclassifiable |
MPN | No |
|
| |||||||||
| Chronic myelomonocytic leukaemia, NOS |
~ | ~ | 9945/3 | Chronic myelomonocytic leukaemia | MDS/MPN | Yes (NOS) | Chronic myelomonocytic leukaemia | MDS/MPN | Yes (NOS) |
|
| |||||||||
| Atypical chronic myeloid leukaemia, BCR-ABL negative |
~ | ~ | 9876/3 | Atypical chronic myeloid leukaemia | MDS/MPN | No | Atypical chronic myeloid leukaemia, BCR-ABL1 negative | MDS/MPN | No |
|
| |||||||||
| Juvenile myelomonocytic leukaemia | ~ | ~ | 9946/3 | Juvenile myelomonocytic leukaemia | MDS/MPN | Yes (e.g., monosomy 7) |
Juvenile myelomonocytic leukaemia | MDS/MPN | Yes (PTPN11 or other) |
|
| |||||||||
| Myeloproliferative disease, NOS* | ~ | ~ | 9975/1, 9975/3 |
Myelodysplastic/ myeloproliferative neoplasm, unclassifiable |
MDS/MPN | No | Myelodysplastic/ myeloproliferative neoplasm, unclassifiable |
MDS/MPN | No |
|
| |||||||||
| Refractory anaemia with sideroblasts |
~ | 9982/1 | 9982/3 | Refractory anaemia with ringed sideroblasts |
MDS | No | Refractory anaemia with ring sideroblasts associated with marked thrombocytosis† |
MDS/MPN | No |
|
| |||||||||
| Refractory anaemia with ringed sideroblasts |
MDS | No | |||||||
|
| |||||||||
| Chronic myeloproliferative disease, NOS* |
9960/1 | 9960/1 | 9960/3 | ~ | ~ | ||||
|
| |||||||||
| Chronic myeloid leukaemia, NOS | 9863/3 | 9863/3 | 9863/3 | ~ | ~ | ||||
Abbreviations: CMPD, chronic myeloproliferative diseases; ICD-O, first edition of the International Classification of Diseases for Oncology (published 1975); ICD-O-2, second edition of the International Classification of Diseases for Oncology (published 1990); ICD-O-3, third edition of the International Classification of Diseases for Oncology (published 2000); MDS, Myelodysplastic syndromes; MDS/MPN, myelodysplastic/myeloproliferative neoplasms; MPN, Myeloproliferative neoplasms; NOS, not otherwise specified; SEER, Surveillance, Epidemiology and End Results; WHO (2001), World Health Organization Classification of Tumors of Haematopoietic and Lymphoid Tissues (2001 edition; Jaffe et al 2001); WHO (2008), World Health Organization Classification of Tumours of aHematopoietic and Lymphoid Tissues (2008 edition; Swerdlow et al 2008); ~, no code available.
ICD-O-3 histology code 9975 was originally associated with a behavior code of “/1” (neoplasm of uncertain and unknown behavior) and was not considered malignant (9975/3), and therefore reportable to the SEER Program, until 2010. In 2012, the code 9960/3 became obsolete and cases were instead coded to 9975/3. Therefore, in our analysis, 9975/3 and 9960/3 were considered jointly.
Provisional entity for which the WHO Working Group felt there was insufficient evidence to recognize as a distinct disease entity in 2008.
Disease classification
The SEER Program began utilizing the ICD-O-3 for coding information on tumour histology and topography in 2001 (Fritz, et al 2000). We included all MPNs and MDS/MPNs with an ICD-O-3 behaviour code of “/3” (malignant behaviour), as specified in Table I, and to the extent possible, categorized disease entities according to the 2008 WHO classification.
Incidence
We calculated incidence rates (IRs), IR ratios (IRRs) and associated 95% confidence intervals (CI) for each MPN and MDS/MPN entity using SEER*Stat, version 8.2.1 (http://seer.cancer.gov/seerstat/). All IRs were age-adjusted using the 2000 US population standard and expressed per one million person-years (PY). We assessed IRs overall and according to sex, race/ethnicity, calendar year of diagnosis and method of diagnostic confirmation. Age-specific IRs were calculated and depicted (plotted at the midpoint of the age group and at 93 years for the oldest age group) on a log-linear scale, as previously described (Devesa, et al 1995).
Delayed reporting
The SEER Program allows 22 months between the end of the diagnosis year and the time cancers are reported to NCI (Howlader, et al 2015). If case information becomes available after this period, the data are collected by the registries and reported to NCI in a subsequent data submission. The addition of cases after the standard 22-month delay is termed “reporting delay,” which may lead to initial underestimation of incidence rates (Clegg, et al 2002). We speculated that new cancer registry reporting requirements in the U.S. for some MPNs and MDS/MPNs in 2001 might be associated with delayed case ascertainment. Reporting delays related to changes in outpatient practice patterns also have been reported for melanoma (Clegg, et al 2002) and chronic lymphocytic leukaemia (CLL) (Dores, et al 2007). Similar to CLL, MPNs are often diagnosed in the outpatient setting and may not require histological confirmation to establish a diagnosis (Jaffe, et al 2001, Swerdlow, et al 2008). Additionally, because features of MPNs and MDS/MPNs overlap and evolve, precise disease classification may be difficult initially but become apparent over time. To evaluate whether IRs of MPNs were affected by reporting delays, using SEER-17 we calculated IRs for cases diagnosed during 2001-2002, 2003-2004, 2005-2006, 2007-2008, 2009-2010 and 2011-2012 using data from the November 2014 submission file and compared these IRs to those based on cases diagnosed during these time periods as reported in the November 2012, November 2010 and November 2008 data submission files. Because delayed reporting can only be assessed in retrospect, the determination of whether delayed reporting exists in recent years can only be made in the future by comparing incidence rates in 2016, for example, from a data file that will be submitted in 5-10 years and comparing those rates to those reported on the initial data file that included data from 2016.
Survival
We utilized the SEER*Stat Survival Session to estimate 5-year relative survival (RS) and 95% confidence intervals (CIs). RS provides a measure of the likelihood of survival from MPN or MDS/MPN in the absence of other causes of death by comparing the observed survival proportion among individuals with MPN or MDS/MPN with the survival of a similar (same sex, age, and race) hypothetical “cancer-free” U.S. population (Howlader, et al 2015). The actuarial or life table method is used to calculate the observed survival rate, with the assumption that cancer deaths represent a negligible proportion of all deaths. Therefore, RS represents the proportion of observed MPN and MDS/MPN survivors compared to the proportion of expected survivors in the population expressed as a percentage. We included all cases of MPNs and MDS/MPNs diagnosed in SEER-18 during 2001-2011 and actively followed for vital status through 2012. Among 40 810 individuals with MPNs and MDS/MPNs, we excluded individuals diagnosed by death certificate only (n=394), with unknown age or age not included in the expected survival table (n=26), alive with unknown survival time (n=124) or excluded from the Research Database (n=88). To minimize bias in survival estimates, we included patients with multiple primary cancers. Four individuals were excluded due to having had a prior diagnosis of MPN or MDS/MPN. Thus, the survival analysis was based on 40 174 individuals with MPNs or MDS/MPNs. We calculated RS for each disease entity overall and according to sex and age at diagnosis.
Results
Overall incidence
During 2001-2012, there were 31 904 cases of MPNs and 4 102 cases of MDS/MPNs diagnosed among residents of the 18 SEER registries (Table II). Age-adjusted IRs for MPNs were highest for PV (IR=10.9 per one million person-years) and ET (IR=9.6); intermediate for MPN-unclassifiable (IR=4.8), BCR-ABL1-positive CML (IR=3.3) and PMF (IR=3.1); and lowest for chronic neutrophilic leukaemia, chronic eosinophilic leukaemia and mastocytosis (IRs 0.1-0.4). Among the MDS/MPNs, rates were highest for chronic myelomonocytic leukaemia (CMML, IR=4.1) and very low for BCR-ABL1-negative CML and juvenile myelomonocytic leukaemia (IRs=0.1).
Table 2.
Age-adjusted incidence rates and incidence rate ratios of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms, overall and according to gender, SEER-18, 2001-2012*
| Total |
Males |
Females |
Male-to-female |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ICD-O-3 code | N | Median age (years) |
IR | N | IR | N | IR | IRR (95% CI) | P-value | |
| Myeloproliferative neoplasms | † | 31,904 | 32.4 | 16,332 | 36.9 | 15,572 | 28.8 | 1.28 (1.26-1.31) | <0.001 | |
| Chronic myeloid leukaemia, BCR-ABL1 positive | 9875 | 3,266 | 54 | 3.3 | 1,820 | 3.8 | 1,446 | 2.7 | 1.40 (1.30-1.50) | <0.001 |
| Chronic neutrophilic leukaemia | 9963 | 62 | 71 | 0.1 | 42 | 0.1 | 20 | 0.04 | 2.80 (1.59-5.05) | <0.001 |
| Polycythaemia vera | 9950 | 10,812 | 65 | 10.9 | 6,238 | 13.7 | 4,574 | 8.4 | 1.64 (1.57-1.70) | <0.001 |
| Primary myelofibrosis | 9961 | 2,988 | 70 | 3.1 | 1,764 | 4.1 | 1,224 | 2.3 | 1.82 (1.69-1.96) | <0.001 |
| Essential thrombocythaemia | 9962 | 9,394 | 68 | 9.6 | 3,665 | 8.5 | 5,729 | 10.6 | 0.80 (0.77-0.83) | <0.001 |
| Chronic eosinophilic leukaemia, NOS | 9964 | 375 | 57 | 0.4 | 228 | 0.5 | 147 | 0.3 | 1.73 (1.39-2.14) | <0.001 |
| Mastocytosis | 9740-9742 | 382 | 55 | 0.4 | 192 | 0.4 | 190 | 0.4 | 1.14 (0.92-1.40) | >0.05 |
| Myeloproliferative neoplasm, unclassifiable | 9960, 9975¥ | 4,625 | 73 | 4.8 | 2,383 | 5.8 | 2,242 | 4.1 | 1.42 (1.33-1.50) | <0.001 |
| Myelodysplastic syndromes/myeloproliferative neoplasms | § | 4,102 | 4.3 | 2,559 | 6.4 | 1,543 | 2.8 | 2.30 (2.16-2.45) | <0.001 | |
| Chronic myelomonocytic leukaemia | 9945 | 3,874 | 76 | 4.1 | 2,411 | 6.1 | 1,463 | 2.6 | 2.31 (2.16-2.47) | <0.001 |
| Atypical chronic myeloid leukaemia, BCR-ABL1 negative | 9876 | 133 | 69 | 0.1 | 81 | 0.2 | 52 | 0.1 | 2.05 (1.42-2.98) | <0.001 |
| Juvenile myelomonocytic leukaemia | 9946 | 95 | <1 | 0.1 | 67 | 0.1 | 28 | 0.1 | 2.30 (1.46-3.72) | <0.001 |
| Other (ICD-O-3 classification only) | ||||||||||
| Chronic myeloid leukaemia, NOS | 9863** | 8,906 | 61 | 9.0 | 5,036 | 11.2 | 3,870 | 7.2 | 1.56 (1.49-1.63) | <0.001 |
Abbreviations: CI, confidence interval; ICD-O-3, International Classification of Diseasae for Oncology, third edition; IR, incidence rate; IRR, IR ratio; N, number of cases; NOS, not otherwise specified; SEER-18, 18 cancer registry areas of the Surveillance, Epidemiology and End Results Program.
SEER-18 includes the registries in eight states (Connecticut, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, and Utah), six metropolitan areas (Atlanta, Georgia; Detroit, Michigan; Los Angeles, San Francisco-Oakland, and San Jose-Monterey, California; Seattle-Puget Sound, Washington), the areas of Greater California (includes California Cancer Registry regions in Central California, Sacramento, Tri-County, Desert Sierra, Northern California, San Diego/Imperial, Orange County), Rural Georgia, Greater Georgia (entire state excluding Clayton, Cobb, DeKalb, Fulton, Glascock, Greene, Gwinnett, Hancock, Jasper, Jefferson, Morgan, Putnam, Taliaferro, Warren and Washington Counties), and the Alaska Native Tumor Registry. All incidence rates are age-adjusted to the 2000 US standard population and expressed per 1,000,000 person-years. Incidence rate ratios are based on unrounded rates. All ICD-O-3 codes have an associated behaviour codes of “/3” (malignant behaviour).
Includes ICD-O-3 morphology codes 9740-9742, 9875, 9950, 9960-9964, 9975. We did not include the provisional entity of “refractory anemia with ring sideroblasts associated with marked thrombocytosis” (9982/3) because in 2008 the WHO considered that there was insufficient evidence to recognize this as an MPN.
ICD-O-3 code 9960/3 (chronic myeloproliferative disease, NOS) became obsolete for cases diagnosed after 12/31/2009 and was replaced with ICD-O-3 code 9975/3 (myeloproliferative disease, NOS) beginning with cases diagnosed 1/1/2010 to present. Therefore, we combined ICD-O-3 codes 9960/3 and 9975/3 and considered these cases as “myeloproliferative neoplasm, unclassifiable” within the category of myeloproliferative neoplasms. The 2008 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues includes 9975/3 in the category of “myeloproliferative neoplasms” and “myelodysplastic syndromes/myeloproliferative neoplasms” as detailed in Table 1. To avoid redundancy, we included cases coded to 9975/3 only under the category of myeloproliferative neoplasms.
Includes ICD-O-3 morphology codes 9876, 9945, 9946.
While the WHO classification does not include the entity chronic myeloid leukaemia, NOS (9863/3), this entity is included in ICD-O-3 and a large number of cases were coded accordingly. We evaluated chronic myeloid leukaemia, NOS separately because of the possibility that this disease category included cases of BCR-ABL1-positive and BCR-ABL1-negative atypical CML.
Incidence rates by age and sex
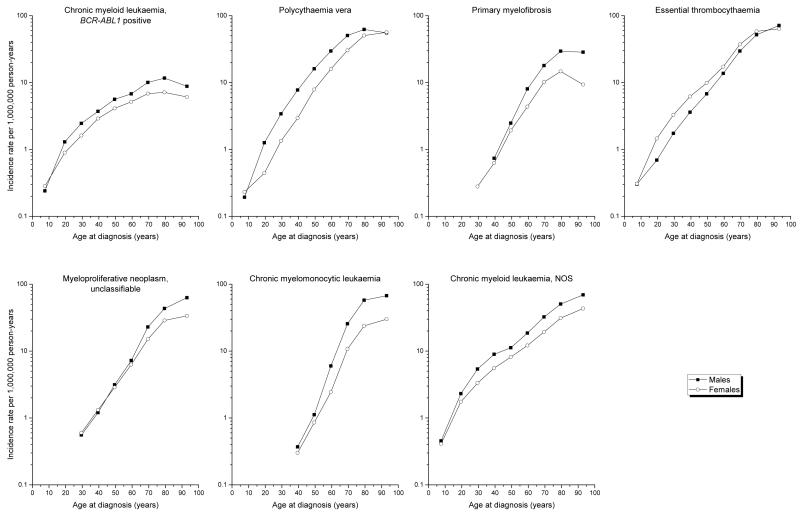
The overall IR of each MPN and MDS/MPN subtype generally was significantly higher among males than females, with IRs ranging from >14% to >100% higher among males (Table II). ET was the only entity with significantly lower IR among males than females, (male-to-female IRR=0.80; 95% CI 0.77-0.83). Among the more common entities, BCR-ABL1-positive CML (IRR=1.40; 95% CI 1.30-1.50), PV (IRR=1.64; 95% CI 1.57-1.70) and CML-not otherwise specified (NOS) (IRR=1.56; 95% CI 1.49-1.63) predominated among males across nearly the entire age spectrum (Figure 1). PMF, MPN-unclassifiable and CMML occurred rarely among the youngest age groups, and the male predominance was most apparent only after mid- to later-life. The female predominance for ET was most notable at ages <60 years. Among males and females, rates increased exponentially with increasing age until the oldest age group for most entities shown, with the pace most rapid for CMML and least rapid for BCR-ABL1-positive CML.
Figure 1. Age-specific incidence rates of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms according to sex, SEER-18, 2001-2012.
Per SEER Program convention, IRs based on fewer than 16 cases were omitted from the figure (Howlader, et al 2015).
Incidence rates by race/ethnicity
All specified MPNs, MDS/MPNs, and CML-NOS rates were lower among Hispanic whites than non-Hispanic whites (Table III). With the exception of chronic eosinophilic leukaemia, which had a similar incidence among non-Hispanic whites (IRR=0.75; 95% CI 0.51-1.07) and Asians/Pacific Islanders (APIs) (IRR=1.12; 95% CI 0.77-1.60), all other MPN IRs were significantly lower among APIs. In contrast, the IRs of ET, chronic eosinophilic leukaemia, MPN-unclassifiable and CML-NOS were 18%, 60%, 19% and 8% higher among blacks than non-Hispanic whites; BCR-ABL1-positive CML occurred approximately equally (IRR=0.91; 95% CI 0.81-1.03); and only PV (IRR 0.61; 95% CI 0.57-0.66), PMF (IRR 0.73; 95% CI 0.63-0.84), mastocytosis and CMML IRs were significantly lower among blacks than non-Hispanic whites.
Table III.
Age-adjusted incidence rates and incidence rate ratios of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms according to race/ethnicity, SEER-18, 2001-2012*
| Whites |
Hispanics |
Blacks |
APIs |
Hispanics:whites |
Blacks:whites |
APIs:whites |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | IR | N | IR | N | IR | N | IR | IRR (95% CI) | P-value | IRR (95% CI) | P-value | IRR (95% CI) | P-value | |
| Myeloproliferative neoplasms | 23,428 | 34.3 | 2,551 | 22.9 | 3,010 | 31.0 | 2,064 | 23.9 | 0.67 (0.64-0.70) | <0.001 | 0.90 (0.87-0.94) | <0.001 | 0.70 (0.66-0.73) | <0.001 |
| Chronic myeloid leukaemia, BCR-ABL1 positive | 2,148 | 3.3 | 444 | 3.0 | 336 | 3.0 | 222 | 2.4 | 0.92 (0.82-1.02) | >0.05 | 0.91 (0.81-1.03) | >0.05 | 0.71 (0.61-0.82) | <0.001 |
| Chronic neutrophilic leukaemia | 45 | 0.1 | 5 | ~ | 7 | ~ | 4 | ~ | ~ | ~ | ~ | |||
| Polycythaemia vera | 8,408 | 12.3 | 782 | 7.2 | 736 | 7.5 | 660 | 7.5 | 0.59 (0.54-0.64) | <0.001 | 0.61 (0.57-0.66) | <0.001 | 0.61 (0.56-0.66) | <0.001 |
| Primary myelofibrosis | 2,287 | 3.3 | 207 | 2.2 | 226 | 2.4 | 206 | 2.4 | 0.69 (0.59-0.80) | <0.001 | 0.73 (0.63-0.84) | <0.001 | 0.73 (0.63-0.85) | <0.001 |
| Essential thrombocythaemia | 6,641 | 9.7 | 717 | 6.4 | 1,108 | 11.5 | 633 | 7.4 | 0.66 (0.61-0.72) | <0.001 | 1.18 (1.11-1.26) | <0.001 | 0.76 (0.70-0.83) | <0.001 |
| Chronic eosinophilic leukaemia, NOS | 230 | 0.4 | 41 | 0.3 | 61 | 0.6 | 37 | 0.4 | 0.75 (0.51-1.07) | >0.05 | 1.60 (1.18-2.15) | <0.01 | 1.12 (0.77-1.60) | >0.05 |
| Mastocytosis | 325 | 0.5 | 24 | 0.2 | 16 | 0.2 | 8 | ~ | 0.40 (0.24-0.62) | <0.001 | 0.34 (0.19-0.56) | <0.001 | ~ | |
| Myeloproliferative neoplasm, unclassifiable | 3,344 | 4.8 | 331 | 3.5 | 520 | 5.7 | 294 | 3.6 | 0.72 (0.64-0.81) | <0.001 | 1.19 (1.08-1.31) | <0.001 | 0.75 (0.67-0.85) | <0.001 |
| Myelodysplastic/myeloproliferative neoplasms | 3,276 | 4.7 | 288 | 3.2 | 269 | 3.3 | 223 | 2.9 | 0.68 (0.60-0.77) | <0.001 | 0.70 (0.61-0.79) | <0.001 | 0.62 (0.53-0.71) | <0.001 |
| Chronic myelomonocytic leukaemia | 3,130 | 4.4 | 252 | 3.0 | 248 | 3.1 | 204 | 2.7 | 0.68 (0.59-0.77) | <0.001 | 0.69 (0.60-0.79) | <0.001 | 0.60 (0.52-0.70) | <0.001 |
| Atypical chronic myeloid leukaemia, BCR-ABL1 negative | 95 | 0.1 | 12 | ~ | 12 | ~ | 10 | ~ | ~ | ~ | ~ | |||
| Juvenile myelomonocytic leukaemia | 51 | 0.1 | 24 | 0.1 | 9 | ~ | 9 | ~ | 0.75 (0.44-1.31) | >0.05 | ~ | ~ | ||
| Other (ICD-O-3 classification only) | ||||||||||||||
| Chronic myeloid leukaemia, NOS | 6,068 | 9.1 | 1,031 | 8.0 | 1,013 | 9.8 | 568 | 6.2 | 0.88 (0.82-0.95) | <0.001 | 1.08 (1.01-1.16) | <0.05 | 0.68 (0.63-0.75) | <0.001 |
Abbreviations: APIs, Asians/Pacific Islanders; CI, confidence interval; Hispanics, Hispanic whites; IR, incidence rate; IRR, IR ratio; N, number of cases; NOS, not otherwise specified; SEER-18, 18 cancer registry areas of the Surveillance, Epidemiology and End Results Program; Whites, non-Hispanic whites; ~, IRs not specified for <16 cases, and IRR cannot be calculated.
All incidence rates are age-adjusted to the 2000 US standard population and expressed per 1,000,000 person-years. IRRs are based on unrounded rates. Due to the small number of cases, the table excludes information on American Indians/Alaskan natives (AIANs) and unspecfied race/ethnicity (R/E NOS). IRs are not calculated for R/E NOS. Number of cases and IRs among AIAN and R/E NOS are as follows: chronic myeloid leukaemia, BCR-ABL1 positive (AIAN, N=35, IR 2.7; R/E NOS, N=81); chronic neutrophilic leukaemia, NOS (AIAN, N=0; R/E NOS, N<5); polycythaemia vera (AIAN, N=44, IR 4.2; R/E NOS, N=182); primary myelofibrosis (AIAN, N=14; R/E NOS, N=48); essential thrombocythaemia (AIAN, N=54, IR 5.9; R/E NOS, N=241); chronic eosinophilic leukaemia, NOS (AIAN, N<5; R/E NOS, N<5); mastocytosis (AIAN, N<5; R/E NOS, N=7); myeloproliferative neoplasm, unclassifiable (AIAN, N=8; R/E NOS, N=128); chronic myelomonocytic leukaemia, NOS (AIAN, N=9; R/E NOS, N=31); atypical chronic myeloid leukaemia, BCR-ABL1 negative (AIAN, N<5; R/E NOS, N=0); juvenile myelomonocytic leukaemia (AIAN, N<5; R/E NOS, N=0); chronic myeloid leukaemia, NOS (AIAN, N=59, IR 5.0; R/E NOS, N=167).
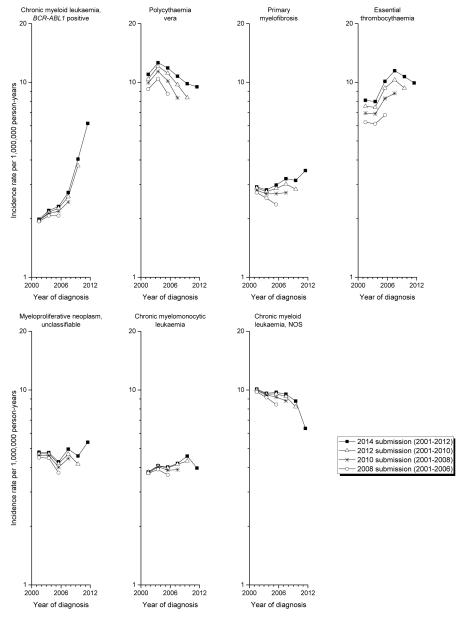
Delayed reporting and other temporal trends
While the follow-up interval was short and limited to 2001-2012, some findings were notable. The incidence of PV peaked in 2003-2004 and progressively decreased thereafter (Figure 2). In contrast, IRs for ET markedly increased after 2003-2004, with a suggestion of decrease after 2008. BCR-ABL1-positive CML IRs increased progressively over the study period, whereas CML-NOS rates decreased. PMF, CMML and MPN-unclassifiable IRs remained relatively stable over time, although rising IRs are suggested beginning in 2011-2012 and additional follow-up will be needed to clarify this observation. Delayed reporting was most evident for PV and ET, with 18-36% (PV) and 15-49% (ET) significantly higher IRs reported in the 2014 data file compared to data files submitted in prior years (Figure 2, Supplementary Table 1). In contrast, BCR-ABL1-positive CML and CMML rates did not show any significant delayed reporting effects (BCR-ABL1-positive CML: 3-12%; CMML: 2-10%).
Figure 2. Age-adjusted incidence rates of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms, according to year of SEER submission file, SEER-17, 2001-2012.
The six 2-year calendar periods reflecting year of diagnosis include 2001–2002, 2003–2004, 2005–2006, 2007–2008, 2009–2010, and 2011–2012.
Method of diagnosis according to time period
In the 2001 and 2008 WHO classifications, the presence of a clonal marker was a major diagnostic criteria for PV, and bone marrow biopsy became nonessential if other criteria were fulfilled (Jaffe, et al 2001, Swerdlow, et al 2008). In contrast, although clonal markers were incorporated into the diagnostic algorithms of other MPNs, bone marrow biopsy remained an essential component of diagnosis. To assess the effect of JAK2 V617F mutation testing introduced in 2005, we evaluated the method of diagnostic confirmation for each entity prior to 2005, early use of JAK2 V617F testing during 2005-2008, and broader use of JAK2 V617F mutation testing in 2009-2012 (Supplementary Figure 1 and Supplementary Table 2). Because information on JAK2 V617F mutation testing is not available in the SEER Program prior to 2010, we utilized calendar year as a surrogate. The percentage of cases microscopically confirmed over the three time periods decreased, not only for PV, from 61.2% to 54.0% to 50.3%, but also for ET, from 83.2% to 70.9% to 60.1%, during 2001-2004 to 2005-2008 to 2009-2012, respectively. There was a notable rise in the fraction of chronic neutrophilic leukaemia, PV and ET cases diagnosed by laboratory test/marker study or clinical means over time with an associated decrease in the percentage of cases diagnosed by microscopic confirmation. Of note, however, is the limited number of cases of chronic neutrophilic leukaemia available for this analysis.
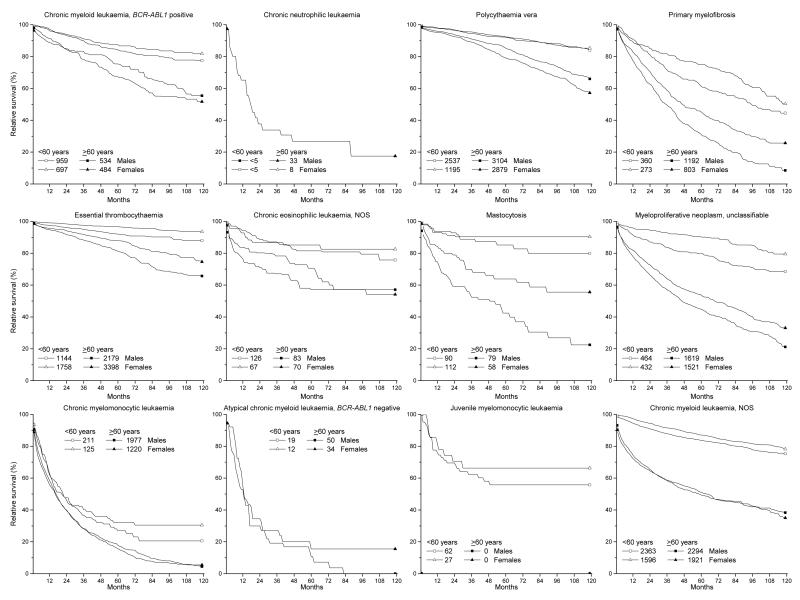
Relative survival
Among males and females, for all evaluable MPNs, 5-year RS was more favourable for those <60 years of age at diagnosis than for those diagnosed at older ages (≥60 years) (Figure 3, Supplementary Table 3). Patients with PV or ET had the most favourable RS among both sexes and age groups, generally 92.0%-96.7% among those <60 years and 79.1%-87.9% among those ≥60 years. Survival among the younger age group with PV was similar for males and females (IRR 1.00) and only slightly, but significantly, better for older males than females (male-to-female IRR=1.08, 95% CI 1.03-1.13). Generally, 5-year RS was significantly better for females than males at all ages, except for PV, BCR-ABL1-positive CML and CML-NOS among males ≥60 years. Based on small numbers, patients with chronic neutrophilic leukaemia, CMML or BCR-ABL1-negative atypical CML had the least favourable 5-year RS (<35%).
Figure 3. Relative survival of patients with myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms according to sex and age at diagnosis, SEER-18, 2001-2012.
Individuals were diagnosed 2001-2011 and followed through 2012. Survival is presented by sex and age (<60 years vs. ≥60 years) at diagnosis. Survival rates based on fewer than 25 cases (total) were omitted from the figure. The total number of cases among each sex/age group is indicated within the legend.
Discussion
This is the largest population-based study of MPNs and MDS/MPNs in the US that comprehensively describes incidence patterns and patient survival by disease subtypes, and one of the few to include more than five years of data from the JAK2 V617F diagnostic era. Disease heterogeneity across MPNs and MDS/MPNs was evident based on distinct age, sex and racial/ethnic incidence patterns, as has similarly been described for other myeloid and lymphoproliferative neoplasms (Dores, et al 2012, Morton, et al 2006). In this first assessment of delayed reporting, we found significant delayed reporting for PV and ET entities with new cancer registry reporting requirements in the U.S. in 2001, demonstrating that IRs were previously underestimated. The decrease in microscopic confirmation for PV and ET cases over time suggests that diagnoses are increasingly reliant on clonal markers/clinical diagnosis and, for ET, that fewer cases are diagnosed utilizing WHO criteria. Further, the decline in PV incidence after 2004 may have been influenced by the availability of JAK2 V617F mutation testing facilitating the exclusion of cases of secondary erythrocytosis. The joint availability of molecular testing and effective targeted therapies is likely to increase awareness of the diagnoses of MPNs. BCR-ABL1 testing and imatinib were available throughout the study period and probably facilitated case finding by and reporting to cancer registries, possibly accounting for the rise in incidence of BCR-ABL1-positive CML and the accompanying decline in CML, NOS. Ruxolitinib, a JAK inhibitor, was initially approved by the US Food and Drug Administration in November 2011 and, as such, its availability would probably not have influenced our results. Lastly, we found that 5-year RS varied considerably by MPN subtype, with poorer survival among males, with a few exceptions, and among those diagnosed at older ages.
Population-based studies in the 21st century
Population-based studies describing incidence of MPNs and MDS/MPNs limited to the current century are sparse (Rollison, et al 2008, Sant, et al 2010, Smith, et al 2011) and few include data subsequent to 2005 (Smith, et al 2011). Smith et al (2011) described incidences for CML, PMF, chronic MPNs and CMML during 2004-2009 in the Haematologic Malignancy Research Network and found age and gender to be determinants of these and other haematological diseases. While they noted a male predominance across most myeloid neoplasms, chronic MPNs were associated with a significant female excess (Smith, et al 2011). Given the differences in disease groupings and calendar years in prior studies, as described by others (Moulard, et al 2014, Titmarsh, et al 2014), comparison with our findings is limited. In the European HAEMACARE project (2000-2002), a clear male predominance was noted for CML, whereas the incidence was nearly equal for other MPNs considered as a group (Sant, et al 2010). The authors also found less geographic variation across Europe for CML than other MPNs, citing more stable diagnostic and classification criteria over time for the former, similar to the disease classification changes we describe in the US. Using data from SEER and the North American Association of Central Cancer Registries, Rollison et al (2008) described the incidence and 3-year RS of chronic myeloproliferative disorders (MPNs, excluding CML, considered in aggregate) diagnosed 2001-2004 in the US, with information by MPN subtypes limited to overall IRs. Increasing age, male sex, and white race were noted to be risk factors for these chronic myeloproliferative disorders (Rollison, et al 2008). We were able to assess MPNs and MDS/MPNs by subtype, and found age-, sex-, and racial/ethnic differences in IRs and reported 5-year patient survival by gender and age. Our findings suggest important aetiological, susceptibility and/or biological differences across subtypes that are obscured when disease categories are considered in aggregate.
Race-ethnicity
Population-based data describing the incidence of MPN and MDS/MPN subtypes among different racial/ethnic groups has not been previously reported. We report lower IRs of all evaluable MPNs and MDS/MPNs among Hispanic whites compared to non-Hispanic whites. Similarly, with the exception of chronic eosinophilic leukaemia, APIs had significantly lower incidence of MPNs and MDS/MPNs compared to non-Hispanic whites. Although subtype-specific incidence data by racial/ethnic subgroups were not previously available, it is notable that JAK2 V617F mutations have been reported with generally similar frequencies among Asian populations with MPNs as in other populations (Ha, et al 2012, Xu, et al 2012). We observed greater variation in IRs across MPN and MDS/MPN subtypes among blacks than other racial/ethnic groups when compared to non-Hispanic whites. Interestingly, ET was associated with a female predominance among non-Hispanic whites, white Hispanics, blacks and APIs, suggesting shared gender-specific risk factor(s) across these racial/ethnic groups. The diverse incidence patterns we observed for MPNs and MDS/MPNs support inherent differences in susceptibility.
Sex
Excluding ET, which predominated among females, and mastocytosis, which occurred approximately equally among males and females, all other MPNs and MDS/MPNs were associated with significantly higher overall IRs among males. The gender disparities for PV, ET, and PMF are similar to those reported in a recent systematic review and meta-analysis of MPNs (Titmarsh, et al 2014) and a review of European studies (Moulard, et al 2014), but differ from the higher PV rates reported among women than men in Malmo City, Sweden between 1980-1984 (Berglund and Zettervall 1992). Among the European studies, ET was noted to predominate among women in most, but not all studies (Maynadie, et al 2011). With the exception of ET and cancers of the anus, gallbladder, breast and thyroid, few haematopoietic (Dores, et al 2012, Morton, et al 2006, Smith, et al 2011) and solid tumours (Cook, et al 2009) demonstrate a female proclivity. Possible explanations for the gender differences include hormonal influences, occupational exposures, lifestyle factors or other health conditions (Cook, et al 2009, Smith, et al 2011). The gender disparity for ET was most prominent in young adulthood and midlife, supporting a potential role for hormonal influences in disease initiation/progression. As recently reviewed, some male predominant occupations including agricultural workers and other rural sector workers have been found to have increased risk of MPNs, whereas no association has been found for professional, administrative, and clerical occupations (Anderson, et al 2012). Distinct aetiologies for MPN subtypes are supported by the association of ET, but not PV, with body mass index, physical activity and adult onset diabetes, whereas PV, but not ET, has been linked with smoking (Leal, et al 2013). Although these entities are rare, subtype-specific analytical studies are needed to clarify subtype-specific risk factors (Kroll, et al 2012, Murphy, et al 2013).
Age
Age at relevant exposure, duration of exposure and disease latency influence age-specific patterns. Accumulating DNA damage, immune senescence, autoimmunity, and chronic inflammation have been suggested as causes for increasing cancer incidence with aging (Boren and Gershwin 2004, Coussens and Werb 2002). The rate of rise in incidence of BCR-ABL1 positive CML with advancing age was less prominent than in other MPNs, and the flattening IR pattern is reminiscent of that observed for acute myeloid leukaemia subtypes with associated cytogenetic abnormalities (e.g., t(8:21), inv(16), t(15;17)) (Dores, et al 2012). The slowing in IRs with aging may reflect a change in disease susceptibility or less intensive testing among older individuals. PMF, CMML and MPN-unclassifiable occurred rarely prior to age 25 years, in contrast to PV, ET and CML. While the majority of MPNs occur in a sporadic fashion, childhood MPNs have been reported (Niemeyer, et al 1997, Teofili, et al 2007), and studies have suggested a role for shared susceptibility genes and familial inheritance patterns for some (Landgren, et al 2008, Ranjan, et al 2013), but not all MPNs (Bjorkholm, et al 2013). As postulated for other infant leukaemias (Linet, et al 2013), in utero exposures and/or maternal/paternal factors may be most relevant for juvenile myelomoncytic leukaemia given the early age at onset (median age <1 year).
Survival
With the exception of CML (Brunner, et al 2013, Pulte, et al 2013), population-based studies describing survival of MPNs in the US are sparse (Price, et al 2014, Rollison, et al 2008), in contrast to reports emanating from European countries (Barbui, et al 2011, Cervantes, et al 2012, Hultcrantz, et al 2012, Maynadie, et al 2013, Maynadie, et al 2011, Osca-Gelis, et al 2014, Phekoo, et al 2006, Sant, et al 2014). This is the first US population-based study to describe 5-year RS of MPN and MDS/MPN by individual subtype, sex and age for cases diagnosed in the modern diagnostic and treatment era. We found the most favourable 5-year RS for PV and ET, as also noted by others (Hultcrantz, et al 2012, Maynadie, et al 2013, Mesa, et al 1999, Phekoo, et al 2006, Rollison, et al 2008). While some studies have reported normal life expectancy with ET (Abdulkarim, et al 2010, Passamonti, et al 2008, Passamonti, et al 2004, Rozman, et al 1991), our findings of decreased 5-year RS for ET and PV, confirm clinical and population-based reports from Europe (Gruppo Italiano Studio Policitemia 1995, Hultcrantz, et al 2012, Passamonti, et al 2004) and the US (Price, et al 2014, Wolanskyj, et al 2006).
Our study is among the first to demonstrate the absence of gender disparity in RS among patients <60 years with PV. We confirm findings from a SEER-Medicare study (2000-2005) that older males with PV have more favourable survival than older women, and that the converse association is noted for ET (Price, et al 2014). We extend these results by demonstrating that the survival advantage for older males with PV and older (as well as younger) females with ET persists for more than a decade. Older age is an established risk factor for thrombosis in PV and ET and a negative prognostic indicator in PMF (Tefferi and Pardanani 2015). Older age remains a significant risk factor for thrombosis in ET, after adjusting for risk factors included in the International Prognostic Score for thrombosis in the World Health Organization - Essential Thrombocythaemia (IPSET-thrombosis) (Barbui, et al 2012). In PMF, age is an independent risk factor for survival, after adjusting for risk factors included in the Dynamic International Prognostic Scoring System (anaemia, leucocytosis, peripheral blood blasts and constitutional symptoms) (Passamonti, et al 2010). In addition, older age is a poor prognostic feature in the Sokal and Hasford scoring systems used in BCR-ABL1-positive CML (Hasford, et al 1998, Sokal, et al 1984). Consistent with these prognostic scoring systems, we describe consistently worse survival among older individuals (≥60 years) for all evaluable MPNs and MDS/MPNs compared to younger individuals. Limited to a paediatric population, survival of juvenile myelomonocytic leukaemia is dependent on prognostic features (Emanuel 2008), and a 45% 5-year overall survival was reported in a population-based study (1990-1999) in the United Kingdom (Passmore, et al 2003). We report slightly more favourable 55.7% 5-year RS in our study, notably similar to that reported in the SEER Program for acute myelomonocytic leukaemia among the youngest age groups (Dores, et al 2012).
Strengths and limitations
Among the strengths of this descriptive epidemiological population-based study is the large number of cases that allowed us to calculate IRs and RS for individual MPNs and MDS/MPNs during 2001-2012. We had sufficient cases to assess gender differences by age and to provide a detailed assessment of IRs by racial/ethnic groups not previously reported. We cannot exclude the possibility of under-ascertainment or underreporting of cases, which has been documented to occur in myeloid neoplasms (Craig, et al 2012), particularly among minority populations or among the elderly where diagnostic evaluation might not be as aggressively sought as in younger individuals. In addition, the overlap in clinical, laboratory, morphological and molecular features of MPNs and MDS/MPNs may present diagnostic challenges, despite the availability of clonal markers in the 21st century (Jaffe, et al 2001, Swerdlow, et al 2008). MPNs and MDS/MPNs also have a potential to transform to myelofibrosis (Kreft, et al 2005), myelodysplastic syndrome, or acute leukaemia, with clinical, morphological and molecular features that can evolve over time. Not surprisingly, there is known inter-observer variability in establishing MPN diagnoses (Alvarez-Larran, et al 2014), and a potential for disease misclassification in our study and those by other investigators (Mehta, et al 2014, Moulard, et al 2014, Sant, et al 2010, Titmarsh, et al 2014) lacking a centralized pathology and clinical review. Our assessment of delayed reporting was an effort to address potential misclassification through correction and updating of diagnoses. We used calendar year as a surrogate for JAK2 V617F mutation status because information on this clonal marker was not available in the SEER database prior to 2011. However, by including cases from the most recent decade, we were able to maximize the number of cases diagnosed during an era when molecular testing was available. Lastly, because information on chemotherapy is not publicly available in the SEER database, our survival analyses did not include treatment information.
Summary
In summary, diverse MPNs and MDS/MPNs incidence patterns support variable aetiologies and/or susceptibility by age, sex and race/ethnicity. While changes in classification schemes and misclassification across entities may have influenced our results, these high quality data from the SEER Program reflect diagnoses established in the general US population. We found that microscopic confirmation of PV and ET decreased over the study period, and note that the forthcoming WHO classification describes an increased role for histological confirmation in new diagnostic algorithms, including PV (Tefferi and Pardanani 2015). As MPN diagnoses are facilitated by genetic testing, improved classification schemes (Tefferi, et al 2014) and increased awareness of reporting requirements to cancer registries (Selinger and Ma 2009), more timely reporting and a decrease in disease misclassification (based on adherence to diagnostic criteria) is expected. Given the major role of molecular diagnostics in the MPNs and MDS/MPNs, strong consideration should be given to expanded collection of data on molecular markers by cancer registries (Polednek 2011). Information on molecular markers would further enrich population-based analyses and potentially unveil additional aetiological and susceptibility clues. Lastly, differences in patient survival by age suggest that those ≥60 years may benefit most from inclusion in clinical trials.
Supplementary Material
Supplementary figure. Frequency of method of diagnosis of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms diagnosed in SEER-18 according to calendar period, 2001-2012. Categories include: [Microscopic] microscopically confirmed (positive histology, positive cytology, positive histology and positive immunophenotyping and/or positive genetic studies, positive microscopic confirmation with method unspecified); [Lab test] laboratory test/marker study; [Clinical] clinical diagnosis only (direct visualization without microscopic confirmation, radiography without microscopic confirmation, clinical diagnosis only); and [Not specified] other method of diagnosis/unknown.
Acknowledgements
This work was supported by the Oklahoma City Veterans Affairs Health Care System in Oklahoma City; and the Intramural Research Program, National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Footnotes
The content of this publication does not necessarily reflect the views or polices of the Department of Veterans Affairs or the Department of Health and Human Services.
Authorship:
Contributions: S.A.S., S.S.D., L.M.M., R.E.C., M.S.L. and G.M.D. conceived and designed research; G.M.D. performed statistical analysis; S.A.S., L.M.M., S.S.D., D.P.C., R.E.C., M.S.L. and G.M.D analysed and interpreted data; S.A.S. and G.M.D. wrote the manuscript; and S.A.S., S.S.D., L.M.M., D.P.C., R.E.C., M.S.L. and G.M.D. critically reviewed and edited the manuscript for important intellectual content.
Conflict of interest disclosure: The authors declare no competing financial interests.
References
- Abdulkarim K, Ridell B, Johansson P, Kutti J, Safai-Kutti S, Andreasson B. The impact of peripheral blood values and bone marrow findings on prognosis for patients with essential thrombocythemia and polycythemia vera. Eur J Haematol. 2010;86:148–155. doi: 10.1111/j.1600-0609.2010.01548.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Larran A, Ancochea A, Garcia M, Climent F, Garcia-Pallarols F, Angona A, Senin A, Barranco C, Martinez-Aviles L, Serrano S, Bellosillo B, Besses C. WHO-histological criteria for myeloproliferative neoplasms: reproducibility, diagnostic accuracy and correlation with gene mutations and clinical outcomes. Br J Haematol. 2014;166:911–919. doi: 10.1111/bjh.12990. [DOI] [PubMed] [Google Scholar]
- Anderson LA, Duncombe AS, Hughes M, Mills ME, Wilson JC, McMullin MF. Environmental, lifestyle, and familial/ethnic factors associated with myeloproliferative neoplasms. Am J Hematol. 2012;87:175–182. doi: 10.1002/ajh.22212. [DOI] [PubMed] [Google Scholar]
- Barbui T, Thiele J, Passamonti F, Rumi E, Boveri E, Ruggeri M, Rodeghiero F, d'Amore ES, Randi ML, Bertozzi I, Marino F, Vannucchi AM, Antonioli E, Carrai V, Gisslinger H, Buxhofer-Ausch V, Mullauer L, Carobbio A, Gianatti A, Gangat N, Hanson CA, Tefferi A. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: an international study. J Clin Oncol. 2011;29:3179–3184. doi: 10.1200/JCO.2010.34.5298. [DOI] [PubMed] [Google Scholar]
- Barbui T, Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E, Ruggeri M, Rodeghiero F, Randi ML, Bertozzi I, Gisslinger H, Buxhofer-Ausch V, De Stefano V, Betti S, Rambaldi A, Vannucchi AM, Tefferi A. Development and validation of an International Prognostic Score of thrombosis in World Health Organization-essential thrombocythemia (IPSET-thrombosis) Blood. 2012;120:5128–5133. doi: 10.1182/blood-2012-07-444067. quiz 5252. [DOI] [PubMed] [Google Scholar]
- Berglund S, Zettervall O. Incidence of polycythemia vera in a defined population. Eur J Haematol. 1992;48:20–26. doi: 10.1111/j.1600-0609.1992.tb01788.x. [DOI] [PubMed] [Google Scholar]
- Bjorkholm M, Kristinsson SY, Landgren O, Goldin LR. No familial aggregation in chronic myeloid leukemia. Blood. 2013;122:460–461. doi: 10.1182/blood-2013-05-501312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boren E, Gershwin ME. Inflamm-aging: autoimmunity, and the immune-risk phenotype. Autoimmun Rev. 2004;3:401–406. doi: 10.1016/j.autrev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Brunner AM, Campigotto F, Sadrzadeh H, Drapkin BJ, Chen YB, Neuberg DS, Fathi AT. Trends in all-cause mortality among patients with chronic myeloid leukemia: a Surveillance, Epidemiology, and End Results database analysis. Cancer. 2013;119:2620–2629. doi: 10.1002/cncr.28106. [DOI] [PubMed] [Google Scholar]
- Cervantes F, Dupriez B, Passamonti F, Vannucchi AM, Morra E, Reilly JT, Demory JL, Rumi E, Guglielmelli P, Roncoroni E, Tefferi A, Pereira A. Improving survival trends in primary myelofibrosis: an international study. J Clin Oncol. 2012;30:2981–2987. doi: 10.1200/JCO.2012.42.0240. [DOI] [PubMed] [Google Scholar]
- Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94:1537–1545. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, Devesa SS, McGlynn KA. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18:1174–1182. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig BM, Rollison DE, List AF, Cogle CR. Underreporting of myeloid malignancies by United States cancer registries. Cancer Epidemiol Biomarkers Prev. 2012;21:474–481. doi: 10.1158/1055-9965.EPI-11-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–304. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- Dores GM, Anderson WF, Curtis RE, Landgren O, Ostroumova E, Bluhm EC, Rabkin CS, Devesa SS, Linet MS. Chronic lymphocytic leukaemia and small lymphocytic lymphoma: overview of the descriptive epidemiology. Br J Haematol. 2007;139:809–819. doi: 10.1111/j.1365-2141.2007.06856.x. [DOI] [PubMed] [Google Scholar]
- Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012;119:34–43. doi: 10.1182/blood-2011-04-347872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel PD. Juvenile myelomonocytic leukemia and chronic myelomonocytic leukemia. Leukemia. 2008;22:1335–1342. doi: 10.1038/leu.2008.162. [DOI] [PubMed] [Google Scholar]
- Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, editors. International Classification of Diseases for Oncology. World Health Organization; Geneva, Switzerland: 2000. [Google Scholar]
- Gruppo Italiano Studio Policitemia Polycythemia vera: the natural history of 1213 patients followed for 20 years. Ann Intern Med. 1995;123:656–664. doi: 10.7326/0003-4819-123-9-199511010-00003. [DOI] [PubMed] [Google Scholar]
- Ha JS, Kim YK, Jung SI, Jung HR, Chung IS. Correlations between Janus kinase 2 V617F allele burdens and clinicohematologic parameters in myeloproliferative neoplasms. Ann Lab Med. 2012;32:385–391. doi: 10.3343/alm.2012.32.6.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, Alimena G, Steegmann JL, Ansari H. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90:850–858. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975-2012. National Cancer Institute; Bethesda, MD: 2015. http://seer.cancer.gov/csr/1975_2012/ </csr/1975_2012/>, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. [Google Scholar]
- Hultcrantz M, Kristinsson SY, Andersson TM, Landgren O, Eloranta S, Derolf AR, Dickman PW, Bjorkholm M. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30:2995–3001. doi: 10.1200/JCO.2012.42.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe ES, Harris NL, Stein H, Vardiman JW. Pathology and genetics of haematopoietic and lymphoid tissues. IARC Press; Lyon: 2001. World Health Organization classification of tumours. [Google Scholar]
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Kreft A, Buche G, Ghalibafian M, Buhr T, Fischer T, Kirkpatrick CJ. The incidence of myelofibrosis in essential thrombocythaemia, polycythaemia vera and chronic idiopathic myelofibrosis: a retrospective evaluation of sequential bone marrow biopsies. Acta Haematol. 2005;113:137–143. doi: 10.1159/000083452. [DOI] [PubMed] [Google Scholar]
- Kroll ME, Murphy F, Pirie K, Reeves GK, Green J, Beral V. Alcohol drinking, tobacco smoking and subtypes of haematological malignancy in the UK Million Women Study. Br J Cancer. 2012;107:879–887. doi: 10.1038/bjc.2012.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren O, Goldin LR, Kristinsson SY, Helgadottir EA, Samuelsson J, Bjorkholm M. Increased risks of polycythemia vera, essential thrombocythemia, and myelofibrosis among 24,577 first-degree relatives of 11,039 patients with myeloproliferative neoplasms in Sweden. Blood. 2008;112:2199–2204. doi: 10.1182/blood-2008-03-143602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal AD, Thompson CA, Wang AH, Vierkant RA, Habermann TM, Ross JA, Mesa RA, Virnig BA, Cerhan JR. Anthropometric, medical history and lifestyle risk factors for myeloproliferative neoplasms in the Iowa Women's Health Study cohort. Int J Cancer. 2013;134:1741–1750. doi: 10.1002/ijc.28492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linet MS, Dores GM, Kim CJ, Devesa SS, Morton LM. Epidemiology and Hereditary Aspects of Acute Leukemia. In: Wiernik P, Dutcher J GJ, Kyle R, editors. Neoplastic Diseases of the Blood. Springer; New York, NY, USA: 2013. [Google Scholar]
- Maynadie M, Girodon F, Manivet-Janoray I, Mounier M, Mugneret F, Bailly F, Favre B, Caillot D, Petrella T, Flesch M, Carli PM. Twenty-five years of epidemiological recording on myeloid malignancies: data from the specialized registry of hematologic malignancies of Cote d'Or (Burgundy, France) Haematologica. 2011;96:55–61. doi: 10.3324/haematol.2010.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynadie M, De Angelis R, Marcos-Gragera R, Visser O, Allemani C, Tereanu C, Capocaccia R, Giacomin A, Lutz JM, Martos C, Sankila R, Johannesen TB, Simonetti A, Sant M, Group HW. Survival of European patients diagnosed with myeloid malignancies: a HAEMACARE study. Haematologica. 2013;98:230–238. doi: 10.3324/haematol.2012.064014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55:595–600. doi: 10.3109/10428194.2013.813500. [DOI] [PubMed] [Google Scholar]
- Mesa RA, Silverstein MN, Jacobsen SJ, Wollan PC, Tefferi A. Population-based incidence and survival figures in essential thrombocythemia and agnogenic myeloid metaplasia: an Olmsted County Study, 1976-1995. Am J Hematol. 1999;61:10–15. doi: 10.1002/(sici)1096-8652(199905)61:1<10::aid-ajh3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulard O, Mehta J, Fryzek J, Olivares R, Iqbal U, Mesa RA. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol. 2014;92:289–297. doi: 10.1111/ejh.12256. [DOI] [PubMed] [Google Scholar]
- Murphy F, Kroll ME, Pirie K, Reeves G, Green J, Beral V. Body size in relation to incidence of subtypes of haematological malignancy in the prospective Million Women Study. Br J Cancer. 2013;108:2390–2398. doi: 10.1038/bjc.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S, Peterson P, Iland H, Laszlo J. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol. 1997;34:29–39. [PubMed] [Google Scholar]
- Niemeyer CM, Arico M, Basso G, Biondi A, Cantu Rajnoldi A, Creutzig U, Haas O, Harbott J, Hasle H, Kerndrup G, Locatelli F, Mann G, Stollmann-Gibbels B, van't Veer-Korthof ET, van Wering E, Zimmermann M. Chronic myelomonocytic leukemia in childhood: a retrospective analysis of 110 cases. European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS) Blood. 1997;89:3534–3543. [PubMed] [Google Scholar]
- Osca-Gelis G, Puig-Vives M, Saez M, Gallardo D, Lloveras N, Guardia R, Marcos-Gragera R. Is survival in myeloid malignancies really improving? A retrospective 15-year population-based study. Leuk Lymphoma. 2015;56:896–902. doi: 10.3109/10428194.2014.947610. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Rumi E, Pungolino E, Malabarba L, Bertazzoni P, Valentini M, Orlandi E, Arcaini L, Brusamolino E, Pascutto C, Cazzola M, Morra E, Lazzarino M. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117:755–761. doi: 10.1016/j.amjmed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Rumi E, Arcaini L, Boveri E, Elena C, Pietra D, Boggi S, Astori C, Bernasconi P, Varettoni M, Brusamolino E, Pascutto C, Lazzarino M. Prognostic factors for thrombosis, myelofibrosis, and leukemia in essential thrombocythemia: a study of 605 patients. Haematologica. 2008;93:1645–1651. doi: 10.3324/haematol.13346. [DOI] [PubMed] [Google Scholar]
- Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, Guglielmelli P, Pungolino E, Caramella M, Maffioli M, Pascutto C, Lazzarino M, Cazzola M, Tefferi A. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2010;115:1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- Passmore SJ, Chessells JM, Kempski H, Hann IM, Brownbill PA, Stiller CA. Paediatric myelodysplastic syndromes and juvenile myelomonocytic leukaemia in the UK: a population-based study of incidence and survival. Br J Haematol. 2003;121:758–767. doi: 10.1046/j.1365-2141.2003.04361.x. [DOI] [PubMed] [Google Scholar]
- Phekoo KJ, Richards MA, Moller H, Schey SA, South Thames Haematology Specialist, C. The incidence and outcome of myeloid malignancies in 2,112 adult patients in southeast England. Haematologica. 2006;91:1400–1404. [PubMed] [Google Scholar]
- Polednek AP. US death rates from myeloproliferative neoplasms, and implications for cancer surveillance. J Registry Management. 2011;38:87–92. [PubMed] [Google Scholar]
- Price GL, Davis KL, Karve S, Pohl G, Walgren RA. Survival patterns in United States (US) medicare enrollees with non-CML myeloproliferative neoplasms (MPN) PLoS One. 2014;9:e90299. doi: 10.1371/journal.pone.0090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulte D, Barnes B, Jansen L, Eisemann N, Emrich K, Gondos A, Hentschel S, Holleczek B, Kraywinkel K, Brenner H. Population level survival of patients with chronic myelocytic leukemia in Germany compared to the US in the early 21st century. J Hematol Oncol. 2013;6:70. doi: 10.1186/1756-8722-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan A, Penninga E, Jelsig AM, Hasselbalch HC, Bjerrum OW. Inheritance of the chronic myeloproliferative neoplasms. A systematic review. Clin Genet. 2013;83:99–107. doi: 10.1111/cge.12044. [DOI] [PubMed] [Google Scholar]
- Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, Edwards BK, List AF. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- Rozman C, Giralt M, Feliu E, Rubio D, Cortes MT. Life expectancy of patients with chronic nonleukemic myeloproliferative disorders. Cancer. 1991;67:2658–2663. doi: 10.1002/1097-0142(19910515)67:10<2658::aid-cncr2820671042>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, Marcos-Gragera R, Maynadie M, Simonetti A, Lutz JM, Berrino F, HAEMACARE Working Group Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116:3724–3734. doi: 10.1182/blood-2010-05-282632. [DOI] [PubMed] [Google Scholar]
- Sant M, Minicozzi P, Mounier M, Anderson LA, Brenner H, Holleczek B, Marcos-Gragera R, Maynadie M, Monnereau A, Osca-Gelis G, Visser O, De Angelis R. Survival for haematological malignancies in Europe between 1997 and 2008 by region and age: results of EUROCARE-5, a population-based study. Lancet Oncol. 2014;15:931–942. doi: 10.1016/S1470-2045(14)70282-7. [DOI] [PubMed] [Google Scholar]
- Selinger HA, Ma X. Jakking up tumor registry reporting of the myeloproliferative neoplasms. Am J Hematol. 2009;84:124–126. doi: 10.1002/ajh.21333. [DOI] [PubMed] [Google Scholar]
- Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105:1684–1692. doi: 10.1038/bjc.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, Tso CY, Braun TJ, Clarkson BD, Cervantes F. Prognostic discrimination in "good-risk" chronic granulocytic leukemia. Blood. 1984;63:789–799. [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumors of Haematopoetic and Lymphoid Tissue. IARC Press; Lyon, France: 2008. [Google Scholar]
- Tefferi A, Pardanani A. Myeloproliferative Neoplasms: A Contemporary Review. JAMA Oncol. 2015;1:97–105. doi: 10.1001/jamaoncol.2015.89. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Thiele J, Vannucchi AM, Barbui T. An overview on CALR and CSF3R mutations and a proposal for revision of WHO diagnostic criteria for myeloproliferative neoplasms. Leukemia. 2014;28:1407–1413. doi: 10.1038/leu.2014.35. [DOI] [PubMed] [Google Scholar]
- Teofili L, Giona F, Martini M, Cenci T, Guidi F, Torti L, Palumbo G, Amendola A, Foa R, Larocca LM. Markers of myeloproliferative diseases in childhood polycythemia vera and essential thrombocythemia. J Clin Oncol. 2007;25:1048–1053. doi: 10.1200/JCO.2006.08.6884. [DOI] [PubMed] [Google Scholar]
- Titmarsh GJ, Duncombe AS, McMullin MF, O'Rorke M, Mesa R, De Vocht F, Horan S, Fritschi L, Clarke M, Anderson LA. How common are myeloproliferative neoplasms? A systematic review and meta-analysis. Am J Hematol. 2014;89:581–587. doi: 10.1002/ajh.23690. [DOI] [PubMed] [Google Scholar]
- Wolanskyj AP, Schwager SM, McClure RF, Larson DR, Tefferi A. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc. 2006;81:159–166. doi: 10.4065/81.2.159. [DOI] [PubMed] [Google Scholar]
- Xu Z, Gale RP, Zhang Y, Qin T, Chen H, Zhang P, Zhang T, Liu L, Qu S, Xiao Z. Unique features of primary myelofibrosis in Chinese. Blood. 2012;119:2469–2473. doi: 10.1182/blood-2011-11-389866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure. Frequency of method of diagnosis of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms diagnosed in SEER-18 according to calendar period, 2001-2012. Categories include: [Microscopic] microscopically confirmed (positive histology, positive cytology, positive histology and positive immunophenotyping and/or positive genetic studies, positive microscopic confirmation with method unspecified); [Lab test] laboratory test/marker study; [Clinical] clinical diagnosis only (direct visualization without microscopic confirmation, radiography without microscopic confirmation, clinical diagnosis only); and [Not specified] other method of diagnosis/unknown.