Abstract
The hypothalamus is a region of the anterior forebrain that controls basic aspects of vertebrate physiology, but the genes involved in its development are still poorly understood. Here, we investigate the function of the homeobox gene Rax/Rx in early hypothalamic development using a conditional targeted inactivation strategy in the mouse. We found that lack of Rax expression prior to embryonic day 8.5 (E8.5) caused a general underdevelopment of the hypothalamic neuroepithelium, while inactivation at later timepoints had little effect. The early absence of Rax impaired neurogenesis and prevented the expression of molecular markers of the dorsomedial hypothalamus, including neuropeptides Proopiomelanocortin and Somatostatin. Interestingly, the expression domains of genes expressed in the ventromedial hypothalamus and infundibulum invaded dorsal hypothalamic territory, showing that Rax is needed for the proper dorsoventral patterning of the developing medial hypothalamus. The phenotypes caused by the early loss of Rax are similar to those of eliminating the expression of the morphogen Sonic hedgehog (Shh) specifically from the hypothalamus. Consistent with this similarity in phenotypes, we observed that Shh and Rax are coexpressed in the rostral forebrain at late head fold stages and that loss of Rax caused a downregulation of Shh expression in the dorsomedial portion of the hypothalamus.
Introduction
The hypothalamus is a major control centre for basic physiological functions of all vertebrates, regulating body growth, reproduction, circadian rhythm, response to stress, food intake and metabolism. Traditionally considered to be part of the diencephalon, recent molecular neuroanatomical studies indicate that the hypothalamus is the rostralmost part of the forebrain, in the secondary prosencephalon1,2. Compared to regions like the spinal cord and the cerebral cortex, the study of hypothalamic development has been relatively neglected, owing to its difficult location and anatomical complexity1,3,4. At the end of gastrulation, the ventral character of the anterior forebrain - including the future hypothalamus - is induced by secreted proteins coming from the underlying prechordal mesoderm, including the morphogen Sonic hedgehog (Shh). Transcription factors expressed in the ventral forebrain, like Nkx2.1 and Six3, are then necessary to establish the hypothalamic character of the region3,4. Shh produced by the ventral forebrain is also necessary to stimulate proliferation and neuronal differentiation in the future hypothalamus5,6,7,8.
Rax, also known as Rx, encodes a paired-type, homeodomain-containing transcription factor expressed during early embryogenesis in the anterior neural plate and later in the retina, hypothalamus and pineal gland in various vertebrates9,10,11. Rax-null mice lack optic cups and all eye structures9, and rare hypomorphic mutations in Rax cause anophthalmia as well as brain abnormalities in humans12,13. While the roles of Rax in the retina and the eye have been extensively studied in several model organisms, its function in the ventral forebrain has received much less attention. Rax-null mice display severe craniofacial and forebrain abnormalities9,14, but the penetrance of these phenotypes is incomplete, since some Rax−/− embryos exhibit a grossly normal head morphology15. In these mildly-affected Rax−/− embryos, however, the ventral forebrain is always abnormal, displaying a thinning of the prospective hypothalamic neuroepithelium, lack of infundibulum (an evagination of the hypothalamic floor that gives rise to the posterior lobe of the pituitary) and absence of the mesenchyme adjacent to the ventral forebrain and future pituitary15,16. Using a conditional knockout approach in mice, Lu et al17 have removed Rax function from embryonic days (E) 9.0–9.5 onwards to show that it can function as a terminal differentiation gene in the hypothalamus, establishing the identity of some populations of neurons of the arcuate and ventromedial nuclei. However, Rax is expressed in the prospective hypothalamus since E7.59,10 and its function at earlier stages has never been studied in detail.
To gain insight on the role of Rax in the early stages of hypothalamic development, we analysed the effects of the conditional inactivation of Rax function at various timepoints and found that Rax is crucially important for hypothalamic development between E8.0 and E8.5. When Rax is eliminated at this timepoint, but not later, the developing dorsomedial hypothalamus looses its gene expression signature - including the expression of the morphogen Shh - and starts expressing genes that are characteristic of more ventral portions of the hypothalamus. Thus, Rax is essential for the early patterning of the hypothalamic territory.
Results
Here, we interpret our anatomical and gene expression results based on the updated prosomeric model of Puelles and Rubenstein2, in which the hypothalamus is viewed as the rostral-most region of the forebrain, with the telencephalon located dorsal to it. In this model, the hypothalamus is divided antero-posteriorly into terminal (THy) and penduncular (PHy) parts, and dorso-ventrally into alar and basal portions. The median portion of the THy can be seen as being part of an acroterminal domain (ATD), which comprises, among other structures, the optic chiasma, the suprachiasmatic nucleus (SCH), the median anterobasal area (ABasM), the arcuate nucleus (ARC), the median eminence (ME), the infundibulum (INF) and the median mamillary area (MnM)1,2. Here, to describe the expression patterns of genes and mutant phenotypes, we call the ATD territory that includes the future SCH and the ABasM/ARC as the dorsomedial terminal hypothalamus (THyDM) and the ATD territory that will give rise to the ME, INF and MnM as the ventromedial terminal hypothalamus (THyVM). The schematic representation in Figure 1A shows some anatomical and gene expression domains of the hypothalamus that are relevant for the present work. In relation to the nomenclature classically used in the literature, the THyDM corresponds to the “anterior” and the THyVM corresponds to the “posterior” hypothalamus. The updated prosomeric anatomical model is particularly suitable to interpret gene expression patterns and mutant phenotypes during hypothalamic development2,7,19. For a defence of the traditional view of hypothalamic organisation, see Bedont et al.4
Figure 1.
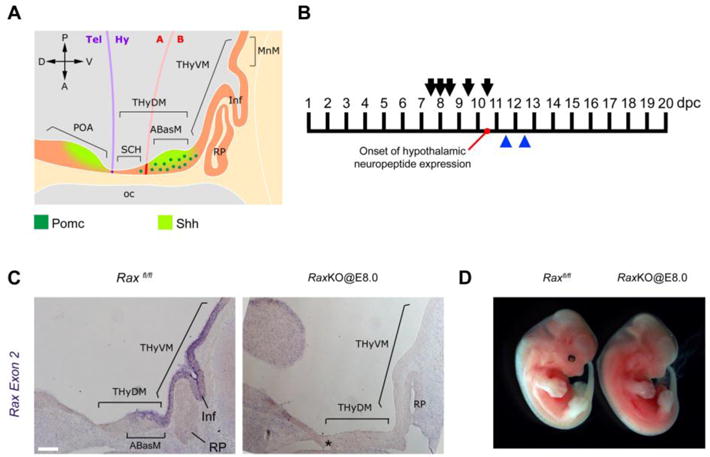
Conditional inactivation of the Rax gene. (A) Schematic of a sagittal section of a E11.5 mouse embryo showing anatomical features relevant for this study according to the updated prosomeric model. Pomc neurons and Shh-expressing regions are indicated. The future SCH, ABasM, INF and MnM are part of the acroterminal domain (ATD). See main text for abbreviations. (B) Timepoints of tamoxifen injection to pregnant females bearing Rax conditional mutant embryos (arrows) and embryo collection (blue arrowheads). Onset of hypothalamic neuropeptide expression occurs at 10.5 dpc (days post-coitum). (C) In situ hybridisation on midsagittal sections of E12.5 mouse embryos using probes for Rax exon 2. Left panel shows a control embryo while the right panel shows a Raxf/f; Cre-ER+ mutant embryo injected with tamoxifen at E8.0 (RaxKO@E8.0). The asterisk (*) indicates the presence of an extra RP in the mutant embryo. Scale bar 100 μm. (D) External morphology of control (left) and RaxKO@E8.0 (right) embryos collected at E12.5.
Temporal inactivation of Rax during mouse development. We employed a Cre/loxP strategy to study the temporal function of Rax during mouse ventral forebrain development. Mice with a targeted Rax locus in which exon 2 is flanked by loxP sites (“floxed”, Raxf/f 14) were crossed with mice harbouring a transgene that ubiquitously expresses a Cre recombinase fused to a mutated form of the ligand-binding domain of oestrogen receptor α (CAAG-CreERT18). In this way, tamoxifen injections to pregnant females at different timepoints could be used to delete the endogenous exon 2 of Rax in developing embryos18. Since exon 2 encodes the Rax homeodomain, the recombined allele is a loss-of-function Rax allele14. After tamoxifen injection to pregnant females, it takes 6–12 hours for DNA recombination to be complete in embryos18. For instance, tamoxifen injection to pregnant females carrying embryos at E8.0 should inactivate Rax around E8.5.
Pregnant females were injected with tamoxifen at E7.5, E8.0, E8.5, E9.5 and E10.5, and embryos were collected at E11.5 or E12.5 for analysis (Fig. 1B). Genomic PCR of embryo samples was used to identify embryos that were Raxf/f;CreER+ or Raxf/f;CreER−, with the latter being used as controls. At E11.5 and E12.5, sagittal sections of mouse embryos showed Rax expression in the subventricular zone of the ABasM domain of the THyDM, which originates neurons that populate the arcuate and the ventromedial nuclei19, as well as in the thin neuroepithelium of the THyVM, from which the infundibulum evaginates16,17,19 (Fig. 1C). In situ hybridisation with a Rax exon 2 probe showed that Cre recombination upon tamoxifen injection was very efficient at all time points, since Rax mRNA was not detected in Raxf/f; CreER+ embryos (Fig. 1C and Supplementary Fig. S1). As in Rax-null mice14,15, conditional inactivation of Rax by tamoxifen injections at E7.5 (referred here as RaxKO@E7.5) often caused lack of eyes and craniofacial defects associated with a delay in the closure of the neural folds (data not shown). In contrast, when Rax was inactivated at later time points, starting with tamoxifen injections at E8.0 (RaxKO@E8.0), head morphology of mutant embryos was much improved, without signs of aberrant neural fold closure (Fig. 1D). In histological sections, the morphology of the heads of RaxKO@E8.0 embryos analysed at E12.5 appeared normal, except for alterations in the hypothalamic region and adjacent tissues, namely i) a thinning of the neuroepithelium of the dorsomedial THy, including the ABasM; ii) lack of an evaginating infundibulum, a hypothalamic derivative that gives rise to the neural lobe of the pituitary; iv) a thinning of the mesenchyme located juxtaposed to the median THy and iii) multiple Rathke’s pouches, an invagination of the oral epithelium that gives rise to the anterior lobe of the pituitary (Fig. 1C; Fig. 2A–D). The formation of multiple Rathke’s pouches is evidenced both in histological staining of sections (Fig. 2A–B) as well as by immunohistochemistry against Lhx3, a specific pituitary marker (Fig. 2C–D). Apart from the ectopic Rathke’s pouches, the morphological phenotypes observed in RaxKO@8.0 embryos have been described previously in Rax-null embryos15,16.
Figure 2.
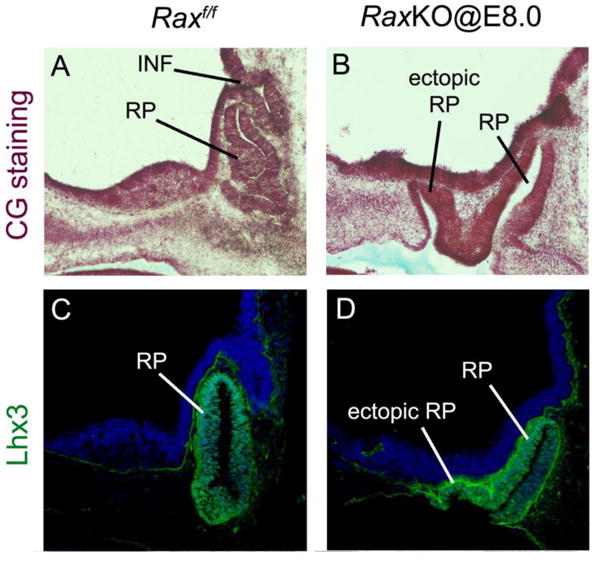
Ectopic Rathke’s pouches following Rax inactivation. Panels show sagittal cuts of the hypothalamic area of Raxf/f controls (A, C) and RaxKO@E8.0 (B, D) embryos subjected to either a Cajal-Gallego (CG) histological staining and collected at E12.5 (A–B) or E11.5 embryos subjected to Lhx3 immunofluorescence (C–D). Note the fomation of ectopic Rathke’s pouches (RP) in mutant embryos.
In RaxKO@E7.5 and RaxKO@E8.0 embryos analysed at E11.5, a defect on dorsomedial THy development was also evidenced by the strong reduction in the number of neurons expressing the mRNA for the neuropeptide gene Proopiomelanocortin (Pomc), one of the first hypothalamic neuropeptide markers to appear during development, at E10.520 (Fig. 3A–C). Interestingly, both the thickness of the dorsomedial THy neuroepithelium and Pomc expression were grossly normal when tamoxifen was injected at later time points (E8.5 through E10.5) (Fig. 3D–F). An exception was the lack of infundibulum, which was still evident in RaxKO@E9.5 embryos, although the phenotype was milder. These results indicate that Rax is necessary for Pomc expression and proper development of the median THy at around E8.5, when it is expected that Rax inactivation occurs following tamoxifen injection at E8.0; from this time onwards Rax expression does not seem to be essential in this region. Due to this finding, we concentrated our conditional inactivation experiments on RaxKO@E8.0 embryos.
Figure 3.
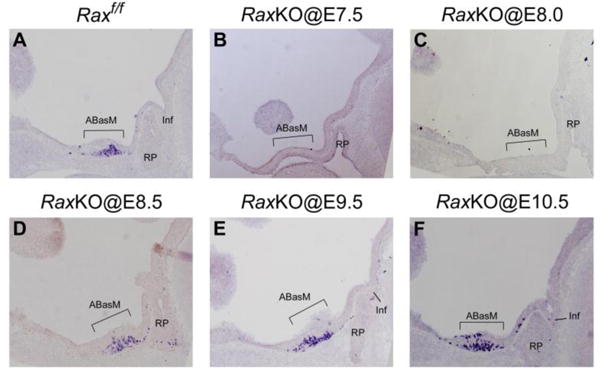
Temporal effect of Rax inactivation on Pomc expression and hypothalamic development. Panels show sagittal cuts of hypothalami of Raxf/f control (A); RaxKO@E7.5 (B); RaxKO@E8.0 (C); RaxKO@E8.5 (D); RaxKO@E9.5 (E); RaxKO@E10.5 (F) embryos collected at E11.5. All sections were subjected to in situ hybridisation against Pomc, which is expressed in the ABasM (brackets) of the THyDM at this stage. Note that Pomc expression and THyDM morphology are only affected when tamoxifen is injected at E7.5 and E8.0, but not later.
Rax is necessary for medial terminal hypothalamic gene expression. To better characterise the effects of the loss of Rax expression in the hypothalamus, we analysed mutant embryos for the expression of marker genes at E11.5 and E12.5. We observed that the inactivation of Rax in RaxKO@E8.0 embryos either supressed or greatly diminished the expression of markers of mature, post-mitotic neurons of the THyDM, like the Pomc peptide (detected by immunohistochemistry against a Pomc-derived peptide, adrenocorticotropic hormone, ACTH)20, the enzyme tyrosine hydroxylase (TH), a marker of dopaminergic neurons21, the neuropeptide somatostatin22 (Sst) and the transcription factor orthopedia23,24 (Otp), all of which are later expressed in neurons of the paraventricular, suprachiasmatic and/or arcuate nucleus of the mature hypothalamus25 (Fig. 4A–H; see also Supplementary information Fig. S1E–H). Expression of the LIM-homeodomain (LIM-HD)-containing protein Lhx1, which is expressed in the median alar domain of the THy fated to become the suprachiasmatic nuclei25,26 (SCH), was also absent in RaxKO@E8.0 embryos (Fig. 4I–J). Another LIM-HD protein, Isl1, which controls Pomc expression in the arcuate nucleus27, was also decreased in the dorsomedial THy of mutant embryos (Fig. 4K–L). We consistently observed that several Pomc or Sst-expressing cells remaining in RaxKO@E8.0 embryos left the neuroepithelium and relocated to the juxtaposed mesenchyme, a possible indication of aberrant cell adhesion properties in the absence of Rax (Fig. 5A). We also attempted to analyse RaxKO@E8.0 embryos at late time points, when hypothalamic nuclei are better defined, but we found that these embryos die after E12.5, as had previously been reported for the conditional inactivation of Rax by tamoxifen28.
Figure 4.
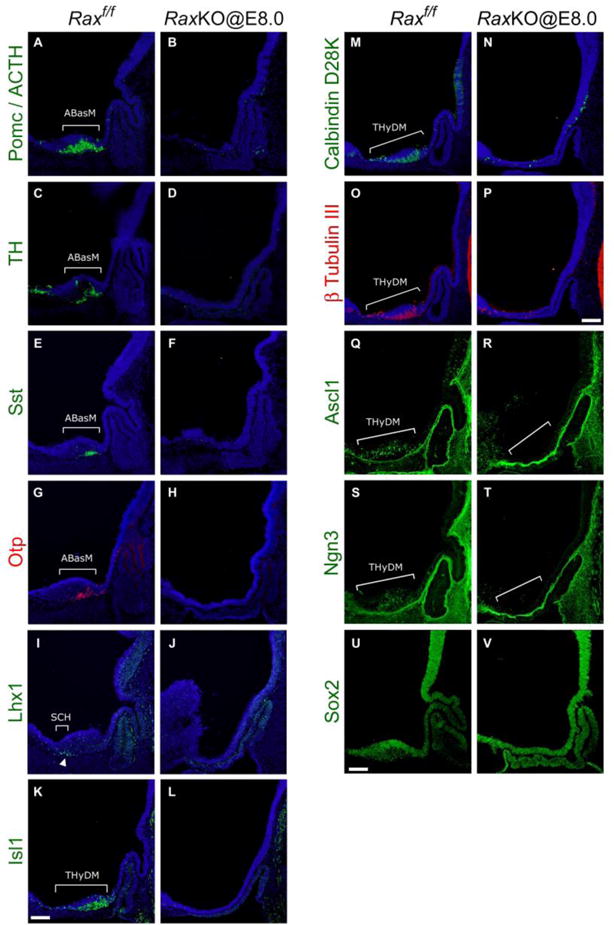
Early loss of Rax eliminates dorsomedial hypothalamic marker expression. Immunohistochemical analysis on midsagittal sections of control (left on each panel) and RaxKO@E8.0 (right on each panel) mouse embryos. Proteins detected were: (A–B) Pomc/ACTH, (C–D) TH, (E–F) Sst, (G–H) Otp, (I–J) Lhx1, (K–L) Isl1, (M–N) Calbindin, (O–P) β-tubulin III, (Q–R) Ascl1, (S–T) Ngn3 and (U–V) Sox2. Embryos were collected either at E12.5 (Pomc/ACTH, TH, Sst, Otp, Lhx1, Sox2) or E11.5 (Isl1, Calbindin, β-tubulin III, Ascl1, Ngn3). Brackets indicate gene expression domains in the SCH, ABasM and THyDM. Note that expression of all marker genes in these domains is greatly reduced or eliminated in mutant embryos. Scale bars 100 μm.
Figure 5.
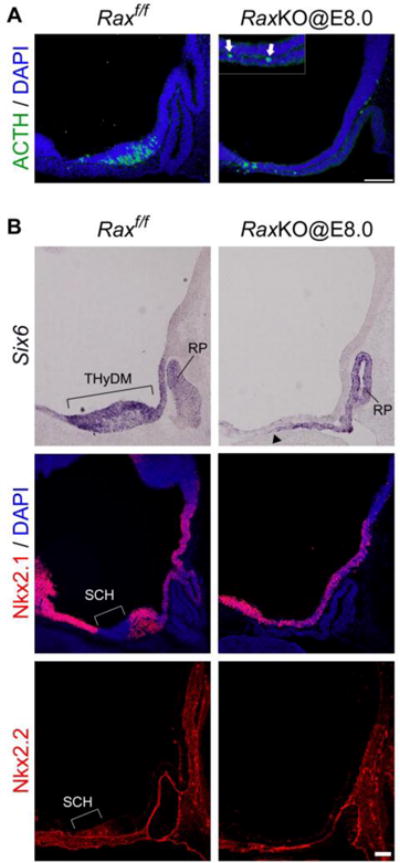
Effect of the loss of Rax function on hypothalamic transcription factors and neuron distribution. (A) Pomc/ACTH immunohistochemistry and DAPI staining of control (left) and RaxKO@E8.0 (right) embryos at E11.5. Several Pomc/ACTH neurons leave the thin neuroepithelium and relocate to the neighbouring oral ectoderm and mesenchyme, as shown in the inset of the right panel (arrows). (B) In situ hybridisation (Six6) and immunohistochemistry (Nkx2.1, Nkx2.2) analyses on midsagittal sections of control (left) and RaxKO@E8.0 (right) mouse embryos analysed at E12.5 (Six6, Nkx2.1) or E11.5 (Nkx2.2). In the control embryo, Six6 is expressed in the whole THyDM while Nkx2.1 is broadly expressed in the hypothalamus except for the alar area of the THyDM that corresponds to the future SCH, where Nkx2.2 is expressed. Elimination of Rax causes the loss of Six6 expression from the THyDM and loss of the characteristic gene expression (Nkx2.1−; Nkx2.2+) in the SCH domain. Note that Six6 expression in the Rathke’s pouch (RP) region extends dorsally in the oral ectoderm of the mutant (arrowhead; compare to Fig. 2). Scale bar 100 μm.
The general neuronal markers calbindin D28k and β-tubullin III were absent or diminished in the prospective hypothalamus of RaxKO@E8.0 embryos (Fig. 4M–P), while their expression was normal in other brain areas (data not shown). In line with this, the expression of the proneural basic helix-loop-helix (bHLH) transcription factors Ascl1 and Neurogenin 3 (Ngn3), which are necessary for proper hypothalamic neurogenesis29,30, was also greatly diminished in RaxKO@E8.0 embryos analysed at E11.5 (Fig. 4Q–T). Analysis of Ascl1 immunofluorescence in transversal cuts further show that the thinner hypothalamic neuroepithelium in RaxKO@E8.0 embryos exhibits reduced Ascl1 staining compared to controls, indicating reduced neurogenesis in the dorsomedial THy in these mutants (Supplemental information Figure S2C–D). Expression of the neural progenitor marker, Sox2, was not reduced by Rax elimination (Fig. 4U–V). These results indicate that absence of Rax function causes a generalised supression of neural differentiation in the dorsomedial THy.
Developmental defects in the median hypothalamus might reflect altered expression of transcription factors necessary for its early specification. We analysed the expression of transcription factors Six6 (Optx2) and Nkx2.1, whose expression begins in the prospective hypothalamic territory and persists at later stages of hypothalamic development5,19. Expression of Six6 was eliminated from the dorsomedial THy of RaxKO@E8.0 embryos (Fig. 5B), while Nkx2.1, which is present in a broader domain in the THy, was not reduced (Fig. 5B and Supplementary information Figure S1A–D). Nkx2.2 is expressed along the alar/basal separation line of the prospective hypothalamus, adjacent to the Nkx2.1 expression domain1. The lateral domains of Nkx2.2 were not altered in RaxKO@E8.0 embryos (data not shown), but its expression was lost from the medial-most region of the prospective hypothalamus, which is located in the alar part of the THy fated to become the suprachiasmatic nucleus26 (Fig. 5B). Instead of Nkx2.2, we observed Nkx2.1 expression in this region, indicating a loss of identity of the future suprachiasmatic nucleus, which is consistent with the lack of Lhx1 expression in this same area in mutants (Figs. 5B and 4I–J).
The thinning of the hypothalamic neuroepithelium and the loss of neuronal marker gene expression could be caused by reduced proliferation or increased cell death, or a combination of both. We checked for the expression of cleaved caspase-3 (Casp3), a marker of apoptosis, by immunofluorescence. Counting cells labelled by immunofluorescence in transversal slices of the hypothalamus region of control and RaxKO@8.0 embryos at stage E11.5 did not reveal significant differences in Casp3 staining (2.55 ± 0.75 labelled cells per slice in control vs 2.75 ± 0.25 in the mutant; P=0.8239). Proliferation was analysed by immunofluorescence against phospho-histone H3 (pHH3) in the hypothalami of E11.5 embryos, but again no significant difference was found (40.15 ± 4.85 labelled cells per slice in control vs 38.10 ± 5.90 in the mutant; P=0.8135). Supplementary information Figure S1A–B and E–F shows representative transversal sections of control and RaxKO@8.0 embryos at stage E11.5 stained for Casp3 and pHH3. These results indicate that the thinning of the hypothalamic neuroepithelium in RaxKO@8.0 is not caused by reduced proliferation or increased apoptosis.
Ventralisation of the dorsomedial terminal hypothalamus in Rax mutants. The loss of dorsomedial hypothalamic markers might indicate that this region assumes another identity in the absence of Rax function. Expression of Foxg1, a telencephalic marker present in the preoptic area dorsal to the alar hypothalamus5,6,7, extends a bit into the anterior hypothalamic region in RaxKO@E8.0 embryos analysed at E12.5 compared to control embryos (Fig. 6A–B; arrow). As for markers of the ventromedial THy, which include the infundibulum and the future mamillary nuclei, results varied. The expression boundaries of some markers, like Fgf8 and Rax itself (detected with a long riboprobe that hibridises to exon 3), stayed confined to the ventromedial hypothalamus (Fig. 6C–F), while the expression of Fgf10, Otx2 and Tbx3 were seen to extend from their ventral position into the dorsomedial hypothalamus in Rax mutants (Fig. 6G–L). Taken together, these results indicate that, when Rax expression is lost around E8.5, the dorsomedial THy is partially ventralised.
Figure 6.
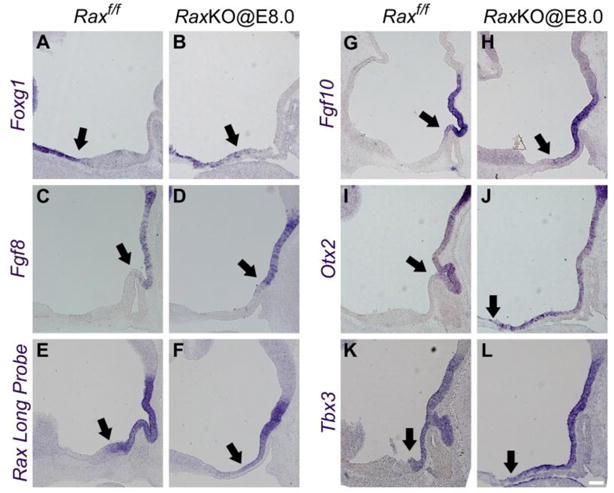
Loss of Rax ventralises the dorsomedial hypothalamic territory. In situ hybridisation analyses on midsagittal sections of control and RaxKO@E8.0 embryos with the following probes: (A–B) Foxg1, (C–D) Fgf8, (E–F) Rax long probe, (G–H) Fgf10, (I–J) Otx2 and (K–L) Tbx3. Embryos were analysed at E12.5 (Foxg1, Otx2, Tbx3) and E11.5 (Fgf8, Rax long probe, Fgf10). Arrows indicate the approximate boundaries of expression of the markers. Note that expression boundaries of Fgf10, Otx2 and Tbx3 extend towards the dorsomedial hypothalamus. Scale bars 100 μm.
Rax is necessary for Sonic hedgehog expression in the dorsomedial terminal hypothalamus after E10.5. The patterning of the neural tube depends on the morphogen Sonic hedgehog (Shh). At late head fold stages, Shh is expressed in the whole prospective hypothalamus but is later downregulated in the ventromedial THy, including the infundibulum31,32. Early inactivation of Shh signalling in the rostral forebrain causes a general reduction in proliferation, neurogenesis and regional gene expression in the hypothalamus5,6,7. As these phenotypes are similar to what we observed in our conditional Rax strategy, we checked for the expression of Shh in the mutants. When tamoxifen was injected at E8.0 (RaxKO@E8.0), we observed a complete loss of Shh expression from the midline of the dorsomedial THy of embryos analysed at E10.5 and E12.5 (Fig. 7A–P). In these mutants, Shh expression was still normal at more lateral levels of the hypothalamus and diencephalon, as well as in the ventral telencephalon, indicating that the effect of Rax loss on Shh expression is specific to the midline of the dorsal THy (Fig. 7E,H,K,N). In transversal slices, Shh immunostaining in RaxKO@E8.0 embryos is seen to be excluded from the thin hypothalamic neuroepithelium (Supplementary information Fig. S1E–H). Importantly, when tamoxifen was administered at E8.5 or later, Shh expression in the dorsomedial THy was normal, paralleling the effect of Rax loss on Pomc expression and general hypothalamic development (Fig. 7D and data not shown). Thus, the effect on median THy development and Shh expression is only obtained when Rax is inactivated upon tamoxifen injection at E8.0 but not later.
Figure 7.
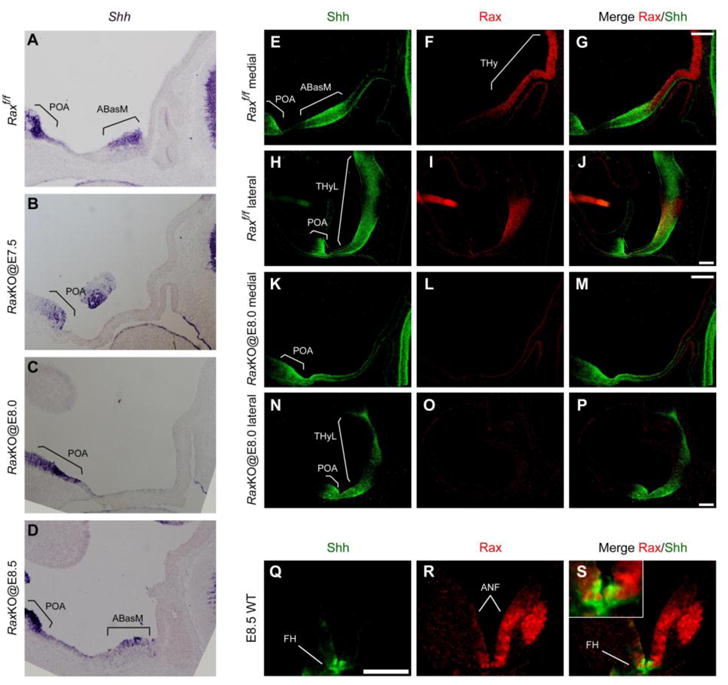
Rax is essential for anterior hypothalamic expression of Shh. (A–D) In situ hybridisation analysis of Shh mRNA expression on midsagittal hypothalamic sections of E11.5 in control (A) and RaxKO embryos injected with tamoxifen at E7.5 (B), E8.0 (C) and E8.5 (D). Note that Shh expression is lost upon Rax gene deletion when tamoxifen is injected at early (E7.5, E8.0) but not later (E8.5) stages. (E–P) Immunofluorescence detection of Shh (green) and Rax (red) expression on midsagittal and lateral sections of E10.5 control (E–J) and RaxKO@E8.0 (K–P) embryos. Shh expression is observed in the preoptic area (POA) of the telencephalon and in the ABasM (medial sections, E–G) and lateral THy (THyL, lateral sections, H–J) within the hypothalamus. Shh expression is lost from the ABasM in RaxKO@E8.0 embryos, but is mantained in the POA and THyL of the same embryos (H–J, N–P). (Q–S) Immunofluorescence detection of Shh (green) and Rax (red) expression on transversal sections of a wild-type E8.5 embryo. The merged image and inset (S) shows that the Shh expression domain, corresponding to the future ventral hypothalamus (FH), is contained within the broad domain of Rax expression in the anterior neural fold (ANF) at this stage. Scale bars 100 μm.
Rax is expressed in the anterior neural fold of the mouse at E7.5 and E8.5, and its expression becomes increasingly confined to the retina and hypothalamus as development progresses9,10. Consistent with this, fate mapping experiments with various Rax-Cre drivers indicate that Rax+ progenitors give rise to the forebrain region in general33,34 or more specifically to arcuate and ventromedial nuclei of the hypothalamus17. To check whether Rax and Shh are coexpressed at the late head fold stage, we performed double immunohistochemistry of transversal and cuts of wild-type E8.5 embryos. As shown previously by in situ hybridisation9,10, Rax protein is present in a broad area of the future forebrain (Fig. 7R) which includes the narrower, Shh expression domain in the midline of the rostral forebrain6,31,35 (Fig. 7Q,S). At stages E9.5 and E10.5, Rax and Shh still partially colocalise in the dorsomedial THy but not in the ventromedial THy, in which Shh is downregulated after E8.5 (Alvarez-Bolado et al., 2011; Supplementary information Fig. S3–S4). At stage E11.5, Rax is no longer expressed in most of the dorsomedial THy and does colocalise with Shh in the ventromedial THy either, although the markers still partially colocalise at lateral levels (Supplementary information Fig. S5).
Since E8.5 coincides with the onset stage of Shh expression in the ventral forebrain6,31, this result opened the possibility that the elimination of Rax in RaxKO@E8.0 embryos might be preventing Shh expression from starting in the future dorsomedial hypothalamus. To check whether the elimination of Rax causes a loss of Shh expression at these early developmental stages, we analysed gene expression in RaxKO@E8.0 embryos at E9.0, i.e. ~12 hours after the onset of Shh expression in the forebrain. However, no obvious changes in the expression of Shh mRNA or protein were observed in the future dorsomedial hypothalamus of RaxKO@E8.0 embryos compared to control embryos analysed at E9.0 (Fig. 8C–D, I–J). As excepted, the expression of Nkx2.1 was also unchanged in mutant embryos (Fig. 8G–H). Since Shh is no longer expressed in the dorsomedial hypothalamus of RaxKO@E8.0 embryos at E10.5 (Fig. 7E, K), this result indicates that Shh expression in these embryos starts normally but cannot be maintained in the dorsomedial THy in the absence of Rax expression. We also analysed the expression at E9.0 of Otx2, one of the ventromedial THy marker genes that invades dorsomedial territory upon Rax deletion. In control embryos, Otx2 expression is visibly absent from the prospective dorsomedial THy at E9.0 but, in RaxKO@E8.0 embryos, Otx2 is expressed continuously in the prospective hypothalamus (Fig. 8E–F). This indicates that the process of ventralisation of the dorsomedial THy in RaxKO@E8.0 embryos has already began as early as E9.0, before Shh expression is downregulated in mutant embryos.
Figure 8.
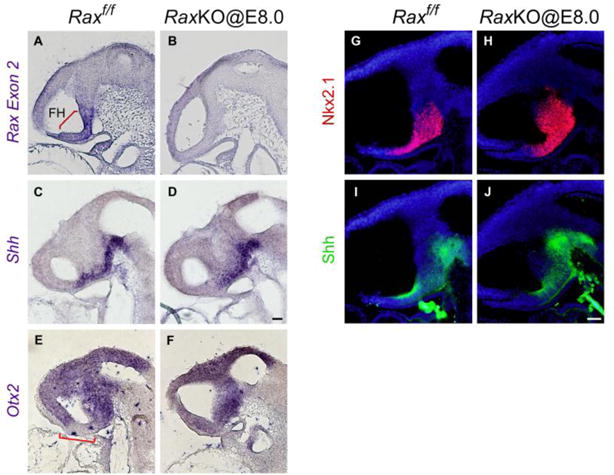
Early Rax inactivation does not impair the onset of Shh expression in the hypothalamus. (A–F) In situ hybridisation analysis using probes against Rax exon 2 (A–B), Shh (C–D) or Otx2 (E–F) on sagittal sections of Raxf/f (control, A, C) or RaxKO@E8.0 (B, D) E9.0 embryos. Inactivation of Rax was effective as shown by the loss of Rax exon 2 expression in the RaxKO@E8.0 embryo. Note that the expression of Shh in the RaxKO@E8.0 embryo does not differ from the control. In control embryos, Otx2 expression shows a gap in the prospective hypothalamus (bracket in E) which is absent in Rax mutant embryos, suggesting that the process of hypothalamic ventralisation is underway at this stage in these embryos. (G–J) Immunofluorescence analysis using antibodies against Nkx2.1 (G, H) or Shh (I, J) on sagittal sections of Raxf/f (control, G, I) or RaxKO@E8.0 (H, J) embryos at stage E9.0. Embryos are counterstained with DAPI (blue). The expression of Nkx2.1 and Shh is similar in both embryos. Scale bars: 20 μm.
Discussion
In this work, we used a conditional targeted inactivation strategy in mouse embryos and found that Rax expression is necessary before E8.5 to confer the proper morphology to the medial terminal hypothalamus, as well as for the expression of molecular markers typical for this region, including the morphogen Shh. Without Rax expression, the dorsomedial THy expresses molecular markers characteristic of more ventral portions, indicating that Rax functions in demarcating the boundaries of gene expression territories within the developing hypothalamus. The model in Figure 9 summarises these observations. Strikingly, elimination of Rax expression from E9.0 onwards did not cause a strong thinning of the hypothalamus or a decrease in Shh expression. Thus, Rax expression in the forebrain is critical for dorsomedial THy development around E8.0–E8.5; after this timepoint, hypothalamic patterning can proceed independently of Rax function.
Figure 9.
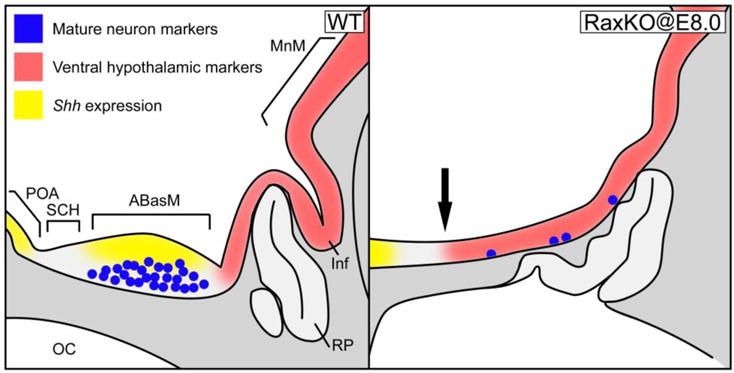
Model of hypothalamic phenotype in Rax conditional knockout embryos. Schematics of gene expression territories at E12.5 in sagital cuts of a wild-type embryo (left) and a RaxKO@E8.0 embryo (right). In the embryo lacking Rax, the expression of Shh (yellow) in the ABasM is abolished, and the formation of mature neurons expressing dorsomedial hypothalamic markers (Pomc, Otp, TH, Sst; blue dots) is also abolished or greatly reduced. At the same time, the expression of ventromedial hypothalamic markers (Fgf10, Otx2, Tbx3, red) extends into dorsomedial territory (arrow).
Neurogenesis and neural differentiation are impaired in RaxKO@E8.0 embryos, as evidenced by the thinning of the medial hypothalamic neuroepithelium, reduced expression of proneural genes Ascl1 and Ngn3, which are necessary for the generation of hypothalamic neurons27,29,30 and the reduced expression of mature neuronal markers. Expression of markers for distinct THy nuclei are reduced, including the SCH (Six6, Lhx1, Nkx2.2)26,45,46, anterior hypothalamic area (Sst, TH) and ARC (Isl1, Pomc, Otp, TH, Sst)27,21,22. Overall, these effects resemble those observed in mouse models lacking Shh expression in the prospective hypothalamus at early stages. Thus, inactivation of Shh at E10.0 leads to a thinning of the hypothalamus and loss of hypothalamic markers Lhx1 and Pomc5; Shh inactivation at E8.5 leads to diminished cell proliferation, reduced size and absence of hypothalamic markers6 and Shh inactivation at E9.0 causes a thinning of the neuroepithelium, reduction in the expression of Ascl147 and loss of hypothalamic markers like Six67. Nkx2.1 is still expressed in the thinner hypothalamus of these conditional Shh knockout models6,7, indicating that general specification of the hypothalamic anlage does not require neuroepithelial expression of Shh, as we observed for Rax.
The similarity of the effects of the lack of Rax and Shh on hypothalamic development suggest that at least part of the hypothalamic aberrations observed in RaxKO@E8.0 embryos are caused by the lack of Shh expression. However, a word of caution is needed at this point. First, although hypothalamic Shh mutants show decreased cell proliferation in the hypothalamus (Szabo et al., 2009; Zhao et al., 2012), we could not observe this same effect in RaxKO@E8.0 embryos. This might be due to the fact that, while the mutant models described in the literature lack Shh in the whole hypothalamus, RaxKO@E8.0 embryos lack Shh only in the midline of the dorsomedial THy, which might cause a smaller effect on cell proliferation while still exhibiting a strong negative effect on neurogenesis and neuronal differentiation. Second, the effects of Rax deletion on gene expression begin before Shh is downregulated in any noticeable way. Thus, while Shh expression is apparently normal at E9.0 in the future dorsomedial THy of RaxKO@E8.0 embryos, the ventromedial marker Otx2 is already ectopically expressed in the prospective dorsomedial THy in these same embryos. In conclusion, it seems that the absence of Rax alters gene expression in the dorsomedial THy in such a way as to prevent it from maintaining Shh expression at E10.5 onwards, which in its turn might contribute to the underdevelopment of this hypothalamic region.
In parallel with the loss of dorsomedial THy gene expression, inactivation of Rax at E8.5 causes ventromedial THy marker gene expression (Otx2, Tbx3, Fgf10) to invade dorsomedial territory (Fig. 9). This ventralisation effect is evident since very early on, as Otx2 expression is already ectopically expressed in dorsomedial THy territory at E9.0. A partial ventralisation of the dorsomedial THy has also been reported for embryos lacking hypothalamic Shh, in which Fgf10 expression extends from the ventromedial into the dorsomedial hypothalamus7. Members of the Bmp and Fgf families secreted by the ventromedial hypothalamus play a role in the induction and development of Rathke’s pouch48. Consistent with this, the expansion of ventromedial hypothalamic markers in RaxKO@E8.0 embryos is accompanied by the induction of ectopic Rathke’s pouches in the oral ectoderm juxtaposed to the hypothalamic neuroepithelium. Extra Rathke’s pouches are also present in hypothalamic Shh mutants, along with an expansion of Fgf10 expression7, and mutants lacking the homeobox gene Vax1 display an ectopic site of Fgf10 expression and develop extra Rathke’s pouches49. Hence, the expansion of Fgf10 observed in Rax and Shh mutants may account for the formation of supernumerary primordia of the anterior pituitary. The dorsal expansion of ventromedial THy markers may also be linked to the lack of Shh expression in RaxKO@E8.0 embryos. During normal development, the transcriptional repressor Tbx3 sequesters Sox2 and prevents it from activating Shh expression in the ventromedial THy50. Thus, the dorsal expansion of Tbx3 expression in RaxKO@E8.0 embryos might also contribute to the Shh downregulation in the dorsomedial THy that occurs between E9.0 and E10.5.
In contrast to its effects in the dorsomedial THy, we could not detect any change in gene expression of the ventromedial THy of RaxKO@E8.0 embryos, even though Rax is strongly expressed in this region throughout early embryogenesis. What we consistently observed was a lack of evagination of the infundibulum, as observed in Rax-null embryos15. Interestingly, the cells that undergo the morphogenetic movements that form the optic cup and infundibulum need to express Rax, i.e. this gene functions cell autonomously in these structures16. Thus, it would seem that Rax is not required to specify the identity of the ventromedial hypothalamus but rather to endow the cells in this region with their typical morphogenetic behaviour, likely by controlling genes related to cell movement and adhesion. In this regard, it is interesting that some Pomc and Sst-expressing cells in RaxKO@E8.0 embryos migrate out of the hypothalamic neuroepithelium, suggesting changes in cell adhesion properties of Rax-deficient cells.
The role of Rax in early hypothalamic development differs from recent reports on the conditional inactivation of the gene at stages later that E8.5. Lu et al17 used a Shh-CreGFP transgene to inactivate Rax from progenitors of the ventromedial nucleus of the hypothalamus (VMH) starting at E10.0, and observed that the fate of many VMH neurons was respecified, as evidenced by ectopic formation of GABAergic neurons at E14.517. The same authors also used a Six3-Cre transgene to inactivate Rax in the prospective hypothalamus starting at E9.0–E9.5 and observed a reduction in hypothalamic markers at birth, including Pomc17. More recently, Salvatierra et al51 used a Foxd1-Cre transgenic line to eliminate Rax starting at E9.5. Defects in general hypothalamic development were not reported, but the differentiation of tanycytes lining the third ventricle was disturbed51. Although the experiments are not completely comparable due to differences in the stages analysed, one can envisage several successive roles of Rax in hypothalamic development. First, Rax expression in the future ventral forebrain at around E8.5 is necessary for the specification and growth of the dorsomedial hypothalamic territory, an effect that might be mediated by Shh. Later, as the hypothalamic neuronal progenitors give rise to the different neurons that coalesce into distinct nuclei, Rax is necessary for confering the identity of some neurons of the VMH and perhaps the arcuate17. In parallel, Rax expression in the ventral walls of the third ventricle, which starts during embryogenesis and persists in this area until adulthood, is needed for the differentiation and function of tanycytes51,52.
According to the updated prosomeric model, the midline of the rostral part of the ventral forebrain - the acroterminal domain (ATD) - is topologically and molecularly different from the floor plate tissue that occupies the midline of the ventral diencephalon, hindbrain and spinal cord1,2. The ATD includes the SCH, optic chiasm and optic cups, ABasM, ARC, median eminence and infundibulum, structures that are all affected in Rax mutants. Thus, Rax can be viewed as responsible for the fine patterning within the ATD and differentiates this region from the floor plate, since Rax is not expressed in the midline of the caudal central nervous system.
In conclusion, Rax is necessary for the specification of the dorsomedial THy at late head fold stages of mouse development, while in the ventromedial THy it mostly functions in the coordination of morphogenetic movements. Future characterisation of the gene targets of Rax in the acroterminal domain of the hypothalamus shall shed light on the molecular mechanisms at play during the development of this important and still incompletely studied subdivision of the forebrain.
Methods
Mice
Mice were housed in ventilated cages under controlled temperature and photoperiod (12-h light/12-h dark cycle). All mouse procedures followed the Guide for the Care and Use of Laboratory Animals54 and in agreement with the INGEBI-CONICET institutional animal care and use committee. Mice carrying the RaxloxP allele14 were obtained from Mark Lewandoski (Center for Cancer Research, National Cancer Institute, Frederick, MD) and maintained as homozygotes on a mixed background. CAAG-CreERT mice18 were obtained from The Jackson Laboratory (B6.Cg-Tg[CAG-cre/Esr1]5Amc/J) and were intercrossed with RaxloxP/loxP mice to generate the TAM-inducible Rax conditional knockout strain CAG-CreERT.RaxloxP/loxP (Rax-cKO). Genotyping was performed by PCR using genomic DNA extracted from ear biopsies or embryos. Primers used were RxFlox1: 5′-AGGAGCTCCAGGAGCTCGAAAGAGC-3′; RxFlox2: 5′-GGACGTGCTTCTCCTTGCTCCTTGG-3′; Cre2: 5′-GCATAACCAGTGAAACAGCATTGCTG-3′; Cre6: 5′-AAAATTTGCCTGCATTACCG-3′.
Tamoxifen injections
Tamoxifen (T5648, Sigma) was diluted in sesame oil (S3547, Sigma) at 10 mg/mL by sonication. 1 mL-aliquots were frozen and thawed for 10 minutes at 37°C right before injection. Pregnant females were injected intraperitoneally with 4 mg tamoxifen/30 g of body weight18.
Embryo collection and embedding. Pregnant mouse females at 8.5–12.5 days post coitum (dpc) were sacrificed and embryos were collected and washed in cold RNAse-free PBS. Embryos were fixed overnight (for ISH) or 30 min to 2 h (for IHC) in 4% PFA-PBS at 4°C. Samples were then washed in cold PBS and cryoproctected with RNAse-free, 10% sucrose-PBS overnight. Tails and posterior limbs were cut in order to obtain tissue for genotyping. Tissues were then stabilised in 10% sucrose-10% gelatin-PBS at 37°C for 30 min prior to freezing. Gelatin blocks containing the tissues were stuck in the proper orientation on cork sheets with Tissue-Tek OCT compound (Sakura Finetek). Gelatin blocks were snap-frozen in 2-Methylbutane (Sigma, M32631) at −65°C and stored at −80°C for up to 1 year. 16–20 μm slices were cut in a cryostat (Leica CM 1850) and mounted on microscope slides (Fisherbrand Superfrost Plus, 12-550-15). Sections were then left to air-dry overnight and stored at −20°C for up to one year until use.
Immunohistochemistry. Immunohistochemistry (IHC) was performed as described27. Primary antibodies used were: guinea pig anti-Rax (1:1000, kind gift by Takahisa Furukawa28); rabbit anti-rACTH (1:1000, National Hormone and Peptide Program); mouse anti-Isl1 (1:2000, 40.2D6, Developmental Studies Hybridoma Bank, University of Iowa); mouse anti-Shh (1:1000, 5E1, Developmental Studies Hybridoma Bank, University of Iowa); mouse anti-Lhx3 (1:100, 67.4E12, Developmental Studies Hybridoma Bank, University of Iowa); rabbit anti-calbindin D28K (1:5000, CB38, Swant); mouse anti-β-tubulin III (1:1000, MMS-435P, Covance); rabbit anti-TH (1:1000, 657012, Calbiochem); goat anti-SST (1:150, sc-7819, Santa Cruz Biotechnology); goat anti-Sox2 (1:500, sc-17320, Santa Cruz Biotechnology); mouse anti-Nkx2.2 (1:1000, 74.5A5, Developmental Studies Hybridoma Bank, University of Iowa); rabbit anti-Nkx2.1 (1:1000, ab40880, Abcam); mouse anti-Mash1 (1:200, 556604, BD Pharmingen); mouse anti-Ngn3 (1:1000, F25A1B3, Developmental Studies Hybridoma Bank, University of Iowa); rabbit anti-Otp (1:1000, kind gift by Flora Vaccarino); goat anti-Lhx1 (1:500, sc-19341, Santa Cruz Biotechnology); rabbit anti-cleaved Caspase-3 (1:1000, Asp175, Cell Signaling); rabbit anti-phospho-Histone H3 (1:1000, ab5176, Abcam). Secondary antibodies were either anti-mouse or anti-rabbit AlexaFluor 555 (1:1000, Invitrogen); anti-mouse or anti-rabbit AlexaFluor 488 (1:1000, Invitrogen); anti-guinea pig-Cy3 (1:200, Milipore); anti-goat AlexaFluor 488 (1:500; Jackson ImmunoResearch Labs). Depending on the marker antigen, immunohistochemistry experiments were repeated in 2 to 10 different embryos, with consistent results.
To estimate apoptosis and cell proliferation, one series of slides spanning the dorso-ventral axis of E11.5 embryos (controls and RaxKO@E8.0 embryos, n=2) was immunofluorescently labelled with cleaved Caspase-3 and phospho-Histone H3 antibodies. The number of labelled cells in the midline region was counted and the number of labelled cells was compared using two-way Student’s t test to determine the significance between groups.
Histological stains. Tissues were stained using the Cajal-Gallego trichrome. Sections were washed in water and stained in acetic-fuchsin-solution (10 mL distilled water; 1 drop glacial acetic acid; 10 drops of Ziehl’s fuchsin) for 2 min. Slides were washed in water and incubated in acetic-formol solution (10 mL distilled water; 2 drops of formaldehyde, 1 drop of glacial acetic acid) for 5 min. After washing with water, slides were quickly counterstained with picro-indigo-carmine stain, dehydrated with xylene and mounted with Permount (Fischer Scientific).
Riboprobes
Primers were designed to PCR-amplify specific fragments of genes from mouse cDNA, which were then cloned into the pGEM-T easy vector (Promega). Primers used were: Six6 (F: 5′-CCGGAATTCAAGGGAGAGGTGCTGCGTGTGC-3′; R: 5′-CCGCTCGAGGAGGCAGGCACGGAGACATTGC-3′), Fgf10 (F: 5′-TGCTCTTTTTGGTGTCTTCGT-3′, R: 5′-GGGAGCTCCTTTTCCATTCAA-3′; 490 bp), RxLong (F: 5′-GGGGCTAGCATGCACCTGCCGGGCTGCGCG-3′, R: 5′-GGGGAATTCCTAGAGGGCTTGCCAGGGCTT-3′; 1029 bp), RxEx2 (F: 5′-AGCTACTAGGCCCTGCTAC-3′, R: 5′-GTCGGTTCTGGAACCATAC-3′; 275 bp), Lhx2 (F: 5′-GCGTGGACAAGACGTCAG-3′, R: 5′-CCTAAGGCACGTGGCAGT-3′; 552 bp), Foxg1 (F: 5′-CCTCACCTTCATGGACCG-3′, R: 5′-GTTCTCAAGGCCTGCGTC-3′; 945 bp), Tbx3 (F: 5′-TACTGAAACCGACTTCCAGGAG-3′, R: 5′-TCAGTTTGTGGAAAGTGACGAC-3′; 920 bp) and Otx2 (F: 5′-TCCAGCTCGGGAAGTGAG-3′, R: 5′-AGGCCATGACCTTCCCTC-3′; 875bp). Other plasmids used contained a 600-bp fragment of Pomc exon 3, a 730-bp fragment of Fgf855 and a full-length Shh cDNA (1300 bp). Antisense riboprobes were synthesised with a DIG labelling mix (Roche) using the digested vector as a template and SP6 (Roche) or T7 (Promega) RNA polymerases.
In situ hybridisation of frozen mouse sections. In situ hybridisation (ISH) on mouse embryo cryosections was performed as described56, except that, after washing the riboprobe and blocking with heat-inactivated normal goat serum (HINGS), slides were incubated with an alkaline phosphatase-coupled anti-DIG antibody (1:3500, Roche) in a buffer containing B1 buffer (0.1 M Tris-HCl [pH=7.5], 150 mM NaCl, 0.1% Triton X-100) and 1% HINGS overnight at 4°C. Next, slides were washed three times in B1 and two times in B2 (Tris-HCl 0.1 M [pH=9.5], 100 mM NaCl, 50 mM MgCl2, 0.1% Tween-20). Colour development was done at room temperature in 10 mL of B2 containing 200 μL of NBT/BCIP solution (Roche Applied Science) for 6–20 hours. Slides are then washed twice in 0.01 M EDTA/PBS, fixed with 4% paraformaldehyde/PBS, washed twice in 0.02 M Tris-HCl (pH=7.5) and left to air dry overnight. Slides were then dehydrated in xylene and mounted with Permount (Fisher Scientific). Depending on the marker gene, in situ hybridisation experiments were repeated in 3 to 9 different embryos, with consistent results.
Supplementary Material
Supplementary Figure S1. Analysis of hypothalamic gene expression in Rax mutants. Immunofluorescence analysis on transversal sections of E11.5 control (left on each panel) and RaxKO@E8.0 (right on each panel) mouse embryos. Two anatomical levels are presented; dorsal and ventral. Proteins detected were: (A–D) Nkx2.1 (green), (E–H) Pomc/ACTH (red) and Shh (green). All slices are stained with DAPI (blue). Note that the hypothalamic midline of mutant embryos is thinner than that of controls and lacks expression of ACTH and Shh (brackets), while Nkx2.1 expression is not altered. Shh is still expressed at lateral levels in mutant embryos.
Supplementary Figure S2. Apoptosis, proliferation and neurogenesis in Rax mutants. Immunofluorescence analysis on representative transversal sections of E11.5 control (left on each panel) and RaxKO@E8.0 (right on each panel) mouse embryos. Markers analysed are: (A–B) cleaved-caspase 3 (Casp3, red), (C–D) Ascl1 (green) and phospho-histone H3 (pHH3, red). All slices are stained with DAPI (blue). Note that the thin hypothalamic midline of mutant embryos (brackets) mostly lacks expression of the proneural gene Ascl1. Casp3 staining is almost absent from both control and mutant embryos, while pHH3 is abundantly detected in the hypothalamic ventricular zone of both embryos.
Supplementary Figure S3. Rax and Shh expression in wild-type embryos at E9.5. Immunofluorescence analysis on transversal sections of E9.5 wild-type embryos for the expression of Rax (red) and Shh (green). From top to bottom, rows of images are shown from dorsal to ventral levels. DAPI staining is also shown (blue). Note that, at this stage, Rax and Shh are coexpressed at the hypothalamic midline at dorsal levels (A–J), while at ventral levels (K–T) Shh is reduced in the hypothalamic midline (arrowheads in panels L and Q).
Supplementary Figure S4. Rax and Shh expression in wild-type embryos at E10.5. Immunofluorescence analysis on transversal sections of E10.5 wild-type embryos for the expression of Rax (red) and Shh (green). From top to bottom, rows of images are shown from dorsal to ventral levels. DAPI staining is also shown (blue). Note that Rax and Shh are still mostly coexpressed at this stage in the hypothalamic midline at dorsal levels (A–J). At more ventral levels (K–Y) Shh is absent from the hypothalamic midline, which strongly expresses Rax, including the infundibulum (Inf in panel U).
Supplementary Figure S5. Rax and Shh expression in wild-type embryos at E11.5. Immunofluorescence analysis on transversal sections of E11.5 wild-type embryos for the expression of Rax (red) and Shh (green). From top to bottom, rows of images are shown from dorsal to ventral levels. DAPI staining is also shown (blue). Note that, at this stage, Rax and Shh are no longer coexpressed at dorsalmost levels (A–E) and, at more ventral levels (F–T), coexpression between the two proteins is limited to bilateral stripes in the lateral wall of the hypothalamus. Inf: infundibulum.
Highlights.
-
-
Lack of Rax expression prior to embryonic day 8.5 (E8.5) caused a general underdevelopment of the hypothalamic neuroepithelium, while inactivation at later timepoints had little effect.
-
-
The early absence of Rax impaired neurogenesis and prevented the expression of molecular markers of the dorsomedial hypothalamus.
-
-
Rax is needed for the proper dorsoventral patterning of the developing medial hypothalamus.
-
-
The early loss of Rax causes phenotypes similar to those found when eliminating the expression of Sonic hedgehog (Shh) from the hypothalamus
-
-
Shh and Rax are coexpressed in the rostral forebrain at late head fold stages and that loss of Rax caused a downregulation of Shh expression in the dorsomedial portion of the hypothalamus.
Acknowledgments
The authors are greatly indebted to Mark Lewandoski (Center for Cancer Research, National Cancer Institute, Frederick, MD) for Rax floxed mice, Guillermo Lanuza (Instituto de Investigaciones Bioquímicas de Buenos Aires, CONICET, Argentina) for in situ hybridisation probes, Flora Vaccarino (Yale School of Medicine, New Haven, CT) for the anti-Otp antibody, Takahisha Furukawa (Osaka Bioscience Institute, Osaka, Japan) for the anti-Rax antibody, Gladys Hermida (Department of Biodiversity and Experimental Biology, University of Buenos Aires, Argentina) for help with histological stains and Jesica Unger for technical assistance. This work was supported by National Institutes of Health Grant R01 DK068400 (M.J.L. and M.R.), and Agencia Nacional de Promoción Científica y Tecnológica, Argentina PICT 2013-2171 (F.S.J.S.) and PICT 2013-2832 (M.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
D.P.O, M.R. and F.S.J.S. conceived the project and designed the experiments. D.P.O. performed the experiments with some help from S.N. and F.S.J.S.. M.J.L. assisted with experimental design. D.P.O. and F.S.J.S. wrote the manuscript with input from M.R. All authors reviewed the manuscript.
Conflict of Interest: The authors declare no competing financial interests
References
- 1.Puelles L, Martinez-de-la-Torre M, Bardet S, Rubenstein JLR. Hypothalamus. In: Watson C, Paxino G, Puelles L, editors. The Mouse Nervous System. London: Academic Press; 2012. pp. 221–312. [Google Scholar]
- 2.Puelles L, Rubenstein JL. A new scenario of hypothalamic organization: rationale of new hypotheses introduced in the updated prosomeric model. Front Neuroanat. 2015;9:27. doi: 10.3389/fnana.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearson CA, Placzek M. Development of the medial hypothalamus: forming a functional hypothalamic-neurohypophyseal interface. Curr Top Dev Biol. 2013;106:49–88. doi: 10.1016/B978-0-12-416021-7.00002-X. [DOI] [PubMed] [Google Scholar]
- 4.Bedont JL, Newman EA, Blackshaw S. Patterning, specification, and differentiation in the developing hypothalamus. Wiley Interdiscip Rev Dev Biol. 2015;4:445–468. doi: 10.1002/wdev.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimogori T, et al. A genomic atlas of mouse hypothalamic development. Nat Neurosci. 2010;13:767–775. doi: 10.1038/nn.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szabó NE, et al. Role of neuroepithelial Sonic hedgehog in hypothalamic patterning. J Neurosci. 2009;29:6989–7002. doi: 10.1523/JNEUROSCI.1089-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haddad-Tóvolli R, Paul FA, Zhang Y, Zhou X, Theil T, Puelles L, Blaess S, Alvarez-Bolado G. Differential requirements for Gli2 and Gli3 in the regional specification of the mouse hypothalamus. Front Neuroanat. 2015;9:34. doi: 10.3389/fnana.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaess S, Szabó N, Haddad-Tóvolli R, Zhou X, Álvarez-Bolado G. Sonic hedgehog signaling in the development of the mouse hypothalamus. Front Neuroanat. 2015;8:156. doi: 10.3389/fnana.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa T, Kozak CA, Cepko CL. rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc Natl Acad Sci USA. 1997;94:3088–3093. doi: 10.1073/pnas.94.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muranishi Y, Terada K, Furukawa T. An essential role for Rax in retina and neuroendocrine system development. Dev Growth Differ. 2012;54:341–348. doi: 10.1111/j.1440-169X.2012.01337.x. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Rodriguez J, et al. Mutational screening of CHX10, GDF6, OTX2, RAX and SOX2 genes in 50 unrelated microphthalmia-anophthalmia-coloboma (MAC) spectrum cases. Br J Ophthalmol. 2010;94:1100–1104. doi: 10.1136/bjo.2009.173500. [DOI] [PubMed] [Google Scholar]
- 13.Abouzeid H, et al. RAX and anophthalmia in humans: evidence of brain anomalies. Mol Vis. 2012;18:1449–1456. [PMC free article] [PubMed] [Google Scholar]
- 14.Voronina VA, Kozlov S, Mathers PH, Lewandoski M. Conditional alleles for activation and inactivation of the mouse Rx homeobox gene. Genesis. 2005;41:160–164. doi: 10.1002/gene.20109. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Mathers PH, Jamrich M. Function of Rx, but not Pax6, is essential for the formation of retinal progenitor cells in mice. Genesis. 2000;28:135–142. [PubMed] [Google Scholar]
- 16.Medina-Martinez O, et al. Cell-autonomous requirement for rx function in the mammalian retina and posterior pituitary. PLoS One. 2009;4:e4513. doi: 10.1371/journal.pone.0004513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu F, et al. Rax is a selector gene for mediobasal hypothalamic cell types. J Neurosci. 2013;33:259–272. doi: 10.1523/JNEUROSCI.0913-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 19.Ferrán JL, Puelles L, Rubenstein JL. Molecular codes defining rostrocaudal domains in the embryonic mouse hypothalamus. Front Neuroanat. 2015;9:46. doi: 10.3389/fnana.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santangelo AM, et al. Ancient exaptation of a CORE-SINE retroposon into a highly conserved mammalian neuronal enhancer of the proopiomelanocortin gene. PLoS Genet. 2007;3:1813–1826. doi: 10.1371/journal.pgen.0030166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marín F, Herrero MT, Vyas S, Puelles L. Ontogeny of tyrosine hydroxylase mRNA expression in mid- and forebrain: neuromeric pattern and novel positive regions. Dev Dyn. 2005;234:709–717. doi: 10.1002/dvdy.20467. [DOI] [PubMed] [Google Scholar]
- 22.Morales-Delgado N, et al. Topography of Somatostatin Gene Expression Relative to Molecular Progenitor Domains during Ontogeny of the Mouse Hypothalamus. Front Neuroanat. 2011;5:10. doi: 10.3389/fnana.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acampora D, et al. Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 1999;13:2787–2800. doi: 10.1101/gad.13.21.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Lufkin T. The murine Otp homeobox gene plays an essential role in the specification of neuronal cell lineages in the developing hypothalamus. Dev Biol. 2000;227:432–449. doi: 10.1006/dbio.2000.9902. [DOI] [PubMed] [Google Scholar]
- 25.Díaz C, Morales-Delgado N, Puelles L. Ontogenesis of peptidergic neurons within the genoarchitectonic map of the mouse hypothalamus. Front Neuroanat. 2015;8:162. doi: 10.3389/fnana.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanDunk C, Hunter LA, Gray PA. Development, maturation, and necessity of transcription factors in the mouse suprachiasmatic nucleus. J Neurosci. 2011;31:6457–6467. doi: 10.1523/JNEUROSCI.5385-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasif S, et al. Islet 1 specifies the identity of hypothalamic melanocortin neurons and is critical for normal food intake and adiposity in adulthood. Proc Natl Acad Sci USA. 2015;112:E1861–E1870. doi: 10.1073/pnas.1500672112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muranishi Y, et al. An essential role for RAX homeoprotein and NOTCH-HES signaling in Otx2 expression in embryonic retinal photoreceptor cell fate determination. J Neurosci. 2011;31:16792–16807. doi: 10.1523/JNEUROSCI.3109-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNay DE, Pelling M, Claxton S, Guillemot F, Ang SL. Mash1 is required for generic and subtype differentiation of hypothalamic neuroendocrine cells. Mol Endocrinol. 2006;20:1623–1632. doi: 10.1210/me.2005-0518. [DOI] [PubMed] [Google Scholar]
- 30.Pelling M, et al. Differential requirements for neurogenin 3 in the development of POMC and NPY neurons in the hypothalamus. Dev Biol. 2011;349:406–416. doi: 10.1016/j.ydbio.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez-Bolado G, Paul FA, Blaess S. Sonic hedgehog lineage in the mouse hypothalamus: from progenitor domains to hypothalamic regions. Neural Dev. 2012;7:4. doi: 10.1186/1749-8104-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning L, et al. Regional morphogenesis in the hypothalamus: a BMP-Tbx2 pathway coordinates fate and proliferation through Shh downregulation. Dev Cell. 2006;11:873–885. doi: 10.1016/j.devcel.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 33.Klimova L, Lachova J, Machon O, Sedlacek R, Kozmik Z. Generation of mRx-Cre transgenic mouse line for efficient conditional gene deletion in early retinal progenitors. PLoS One. 2013;8:e63029. doi: 10.1371/journal.pone.0063029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pak T, Yoo S, Miranda-Angulo AL, Wang H, Blackshaw S. Rax-CreERT2 knock-in mice: a tool for selective conditional gene deletion in progenitor cells radial glia of the retina hypothalamus. PLoS One. 2014;9:e90381. doi: 10.1371/journal.pone.0090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geng X, et al. Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev Cell. 2008;15:236–247. doi: 10.1016/j.devcel.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuang J, Mathers P, Raymond P. Expression of three Rx homeobox genes in embryonic and adult zebrafish. Mech Dev. 1999;84:195–198. doi: 10.1016/s0925-4773(99)00077-5. [DOI] [PubMed] [Google Scholar]
- 37.Dickmeis T, et al. Glucocorticoids play a key role in circadian cell cycle rhythms. PLoS Biol. 2007;5:e78. doi: 10.1371/journal.pbio.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapsimali M, Caneparo L, Houart C, Wilson SW. Inhibition of Wnt/Axin/beta-catenin pathway activity promotes ventral CNS midline tissue to adopt hypothalamic rather than floorplate identity. Development. 2004;131:5923–5933. doi: 10.1242/dev.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loosli F, et al. Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep. 2003;4:894–899. doi: 10.1038/sj.embor.embor919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rojas-Muñoz A, Dahm R, Nüsslein-Volhard C. chokh/rx3 specifies the retinal pigment epithelium fate independently of eye morphogenesis. Dev Biol. 2005;288:348–362. doi: 10.1016/j.ydbio.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 41.Stigloher C, et al. Segregation of telencephalic and eye-field identities inside the zebrafish forebrain territory is controlled by Rx3. Development. 2006;133:2925–2935. doi: 10.1242/dev.02450. [DOI] [PubMed] [Google Scholar]
- 42.de Souza FS, Bumaschny VF, Low MJ, Rubinstein M. Subfunctionalization of expression and peptide domains following the ancient duplication of the proopiomelanocortin gene in teleost fishes. Mol Biol Evol. 2005;22:2417–2427. doi: 10.1093/molbev/msi236. [DOI] [PubMed] [Google Scholar]
- 43.Song Y, Golling G, Thacker TL, Cone RD. Agouti-related protein (AGRP) is conserved and regulated by metabolic state in the zebrafish, Danio rerio. Endocrine. 2003;22:257–265. doi: 10.1385/ENDO:22:3:257. [DOI] [PubMed] [Google Scholar]
- 44.Ekker SC, et al. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5:944–955. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- 45.Clark DD, Gorman MR, Hatori M, Meadows JD, Panda S, Mellon PL. Aberrant development of the suprachiasmatic nucleus and circadian rhythms in mice lacking the homeodomain protein Six6. J Biol Rhythms. 2013;28:15–25. doi: 10.1177/0748730412468084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bedont JL, et al. Lhx1 controls terminal differentiation and circadian function of the suprachiasmatic nucleus. Cell Rep. 2014;7:609–622. doi: 10.1016/j.celrep.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zevallos SE. PhD Thesis. 2011. The role of Sonic hedgehog in the hypothalamus. (Paper 357). Publicly accessible Penn Dissertations. [Google Scholar]
- 48.Davis SW, et al. Pituitary gland development and disease: from stem cell to hormone production. Curr Top Dev Biol. 2013;106:1–47. doi: 10.1016/B978-0-12-416021-7.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bharti K, Gasper M, Bertuzzi S, Arnheiter H. Lack of the ventral anterior homeodomain transcription factor VAX1 leads to induction of a second pituitary. Development. 2011;138:873–878. doi: 10.1242/dev.056465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trowe MO, Zhao L, Weiss AC, Christoffels V, Epstein DJ, Kispert A. Inhibition of Sox2-dependent activation of Shh in the ventral diencephalon by Tbx3 is required for formation of the neurohypophysis. Development. 2013;140:2299–2309. doi: 10.1242/dev.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salvatierra J, et al. The LIM homeodomain factor Lhx2 is required for hypothalamic tanycyte specification and differentiation. J Neurosci. 2014;34:16809–1620. doi: 10.1523/JNEUROSCI.1711-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miranda-Angulo AL, Byerly MS, Mesa J, Wang H, Blackshaw S. Rax regulates hypothalamic tanycyte differentiation and barrier function in mice. J Comp Neurol. 2014;522:876–899. doi: 10.1002/cne.23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tessmar-Raible K, et al. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell. 2007;129:1389–13400. doi: 10.1016/j.cell.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 54.Committee on Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda) 1996. (DHHS Publ No (NIH) 85-23). [Google Scholar]
- 55.Mahmood R, et al. A role for FGF-8 in the initiation and maintenance of vertebrate limb bud outgrowth. Curr Biol. 1995;5:797–806. doi: 10.1016/s0960-9822(95)00157-6. [DOI] [PubMed] [Google Scholar]
- 56.Kamm GB, López-Leal R, Lorenzo JR, Franchini LF. A fast-evolving human NPAS3 enhancer gained reporter expression in the developing forebrain of transgenic mice. Philos Trans R Soc Lond B Biol Sci. 2013;368:20130019. doi: 10.1098/rstb.2013.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Analysis of hypothalamic gene expression in Rax mutants. Immunofluorescence analysis on transversal sections of E11.5 control (left on each panel) and RaxKO@E8.0 (right on each panel) mouse embryos. Two anatomical levels are presented; dorsal and ventral. Proteins detected were: (A–D) Nkx2.1 (green), (E–H) Pomc/ACTH (red) and Shh (green). All slices are stained with DAPI (blue). Note that the hypothalamic midline of mutant embryos is thinner than that of controls and lacks expression of ACTH and Shh (brackets), while Nkx2.1 expression is not altered. Shh is still expressed at lateral levels in mutant embryos.
Supplementary Figure S2. Apoptosis, proliferation and neurogenesis in Rax mutants. Immunofluorescence analysis on representative transversal sections of E11.5 control (left on each panel) and RaxKO@E8.0 (right on each panel) mouse embryos. Markers analysed are: (A–B) cleaved-caspase 3 (Casp3, red), (C–D) Ascl1 (green) and phospho-histone H3 (pHH3, red). All slices are stained with DAPI (blue). Note that the thin hypothalamic midline of mutant embryos (brackets) mostly lacks expression of the proneural gene Ascl1. Casp3 staining is almost absent from both control and mutant embryos, while pHH3 is abundantly detected in the hypothalamic ventricular zone of both embryos.
Supplementary Figure S3. Rax and Shh expression in wild-type embryos at E9.5. Immunofluorescence analysis on transversal sections of E9.5 wild-type embryos for the expression of Rax (red) and Shh (green). From top to bottom, rows of images are shown from dorsal to ventral levels. DAPI staining is also shown (blue). Note that, at this stage, Rax and Shh are coexpressed at the hypothalamic midline at dorsal levels (A–J), while at ventral levels (K–T) Shh is reduced in the hypothalamic midline (arrowheads in panels L and Q).
Supplementary Figure S4. Rax and Shh expression in wild-type embryos at E10.5. Immunofluorescence analysis on transversal sections of E10.5 wild-type embryos for the expression of Rax (red) and Shh (green). From top to bottom, rows of images are shown from dorsal to ventral levels. DAPI staining is also shown (blue). Note that Rax and Shh are still mostly coexpressed at this stage in the hypothalamic midline at dorsal levels (A–J). At more ventral levels (K–Y) Shh is absent from the hypothalamic midline, which strongly expresses Rax, including the infundibulum (Inf in panel U).
Supplementary Figure S5. Rax and Shh expression in wild-type embryos at E11.5. Immunofluorescence analysis on transversal sections of E11.5 wild-type embryos for the expression of Rax (red) and Shh (green). From top to bottom, rows of images are shown from dorsal to ventral levels. DAPI staining is also shown (blue). Note that, at this stage, Rax and Shh are no longer coexpressed at dorsalmost levels (A–E) and, at more ventral levels (F–T), coexpression between the two proteins is limited to bilateral stripes in the lateral wall of the hypothalamus. Inf: infundibulum.


