Abstract
Background and Purpose
Diabetes mellitus (DM) is a common metabolic disease among the middle-aged and older population, which leads to an increase of stroke incidence and poor stroke recovery. The present study was designed to investigate the impact of DM on brain damage and on ischemic brain repair after stroke in aging animals.
Methods
DM was induced in middle aged rats (13 months) by administration of nicotinamide (NTM) and streptozotocin (STZ). Rats with confirmed hyperglycemia status 30d after NTM-STZ injection and age matched Non-DM rats were subjected to embolic middle cerebral artery occlusion (MCAO).
Results
Middle aged rats subjected to NTM-STZ injection became hyperglycemic and developed cognitive deficits 2 months after induction of DM. Histopathological analysis revealed that there was sporadic vascular disruption including cerebral microvascular thrombosis, blood brain barrier (BBB) leakage, and loss of paravascular Aquaporin-4 in the hippocampi. Importantly, middle aged DM rats subjected to stroke had exacerbated sensorimotor and cognitive deficits compared to age-matched non-DM ischemic rats during stroke recovery. Compared to age-matched non-DM ischemic rats, DM ischemic rats exhibited aggravated neurovascular disruption in the bilateral hippocampi and white matter, suppressed stroke-induced neurogenesis and oligodendrogenesis, and impaired dendritic/spine plasticity. However, DM did not enlarge infarct volume.
Conclusions
Our data suggest that DM exacerbates neurovascular damage and hinders brain repair processes, which likely contribute to the impairment of stroke recovery.
Keywords: Diabetes, stroke, recovery
Introduction
Diabetes mellitus (DM), a common metabolic disease, affects more than 240 million people worldwide, and is anticipated to become more prevalent as the population ages1. Advanced age and DM are not only well established risk factors for stroke, but also prominent predictors of poor recovery for stroke patients2. Due to the risk of brain hemorrhage, patients with advanced age and DM are often excluded from tissue plasminogen activator (tPA) treatment, the only FDA approved treatment for acute ischemic stroke. Thus, it is imperative to understand the pathological manifestation of stroke in the aging DM brain and to develop therapies for this patient population.
DM potentiated long-term neurological impairments including cognitive decline have been well described in stroke patients3–5. Studies in young adult animals have shown that diabetes exacerbates stroke induced ischemic brain damage, and impairs neural circuit plasticity, leading to impairment of functional recovery6, 7. However, due to the lack of a clinically relevant model of stroke with comorbid DM during aging8, the effect of DM on ischemic brain repair processes in aged animals has not been extensively investigated. The present study was undertaken to develop a model of focal cerebral ischemia in the middle aged rat with DM and then to investigate brain repair processes after stroke. We found that DM exacerbated sensorimotor and cognitive deficits in the middle age rat during stroke recovery. DM potentiated neurovascular disruption and suppressed stroke-induced neurogenesis and white matter remodeling processes in both hemispheres, which likely contribute to worse functional recovery.
Material and Methods
All experimental procedures were approved by the Institutional Animal Care of Henry Ford Hospital. Rats were assigned randomly to DM induction and MCAO according to an online randomization tool (randomizer.org). All outcome measurements were performed by observers blinded to the experimental groups.
Induction of DM
Male Wistar rats (Charles River Laboratories) at 13 months of age (n=26) were subjected to intraperitoneal (IP) injection of 210 mg/kg of nicotinamide (NTM) and streptozotocin (STZ, 60 mg/kg). This NTM-STZ protocol causes partial destruction of pancreatic β-cells that leads to a decrease in blood insulin and a moderate increase in blood glucose in the rats9. This method of inducing T2DM is contrast to the massive β cell destruction induced by STZ alone, which leads to severe hyperglycemia and mimics type 1 DM, resulting from insulin deficiency10. These DM rats manifest metabolic defects such as glucose intolerance and significant but inadequate glucose-stimulated insulin secretion by the β-cells, which resemble the manifestations of insulin secretory dysfunction of human type 2 diabetes9–11. Non-fasting blood glucose concentration was assessed 2 days after NTM-STZ injection and weekly thereafter with a portable blood glucose reader. Animals with glucose concentrations >250 mg/dL were considered diabetics. Age-matched rats without NTM-STZ injection (n=24/group) were used as controls.
Middle cerebral artery occlusion (MCAO)
Rats with confirmed DM status at 1 month after DM induction (n=18) and age-matched Non-DM rats (n=16) were subjected to embolic MCAO, as previously described12. Briefly, blood clots from a donor rat were obtained 24h prior to MCAO. A modified PE-50 catheter carrying a single blood clot was introduced from the right external carotid artery into the lumen of the internal carotid artery until its tip reached the origin of the MCA. The clot was then delivered via the catheter. After that, the catheter was immediately withdrawn. Six DM rats and 4 non-DM rats died during or shortly (within 3d) after MCAO, and were excluded from the study.
Mitotic labeling of proliferating cells
Bromodeoxyuridine (BrdU, Sigma, St. Louis, MO) at a dose of 100 mg/kg was intraperitoneally (IP) administered to ischemic rats for 7 consecutive days starting 24h after stroke onset12.
Functional outcome
Modified neurological severity score (mNSS) and adhesive removal test were performed weekly starting 1 day after onset of MCAO12. Morris water maze test13 and odor recognition test14 were performed in the non-ischemic rats and before sacrifice in the ischemic rats. Please see Supplemental Data for details.
Histopathology and immunohistochemistry
Rats were sacrificed 35 days after MCAO. Infarct volume was measured on 7 hematoxylin and eosin (H&E) stained coronal brain sections as previously described12. DM rats without MCAO were sacrificed 65 days after NTM-STZ injection, whereas age-matched Non-DM control rats were sacrificed after the final behavioral tests.
Immunofluorescent staining was performed on brain coronal sections, according to our published protocols15. The following primary antibodies were used: mouse anti-BrdU (Dako) for proliferating cells, goat anti-doublecortin (DCX, Santa Cruz) for neuroblasts, rabbit anti-NG2 (Millipore) for oligodendrocyte progenitor cells (OPCs), mouse anti-adenomatous polyposis coli (APC, Abcam) for mature oligodendrocytes, mouse anti-phosphorylated neurofilament heavy chain (pNFH, Affinity Bioreagents) for axons and dendrites, rabbit anti-myelin basic protein (MBP, Millipore) for myelin, rabbit anti-glial fibrillary acidic protein (GFAP, Dako) for astrocytes, rabbit anti-aquaporin-4 (AQP4, Abcam), mouse anti-endothelial barrier antigen (EBA, Sternberger Monoclonals), and goat anti-fibrin/fibrinogen (Accurate Chemical& Scientific). The Golgi-Cox impregnation method along with the FD Rapid Golgi Stain kit (FD NeuroTechnologies) was used to identify Golgi-Cox impregnated dendrites and spines16. Please see Supplemental Data for details.
Statistics
Data were evaluated for normality using the Shapiro-Wilk test. Ranked data were used for behavioral data (adhesive removal test and mNSS) since the data were not normally distributed. The analysis of covariance with repeated measure (ANCOVA) was used to test the DM effect on functional recovery after stroke by considering possible interactions between groups (with and without DM) and various time points. A group by time interaction indicates that DM group effects vary among times of assessments. If such interaction was detected, a subgroup analysis was conducted. A significant effect was set if p-value is <0.05. Please see Supplemental Data for details on statistical tests and results on key behavioral data. This is a proof-of-concept study, and the analysis did not adjust for multiple endpoints. For histopathological data, ANOVA was used for analyzing differences between independent groups (e.g., DM with or without MCAO), while ANCOVA was used for analyzing for the effect of DM and stroke on neurogenesis and oligodendrogenesis, which were obtained from multiple brain regions per rat. The paired t-test or two-sample t-test was used if the main effect of DM or region was detected at a 0.05 level.
Results
DM rats exhibit impairments of cognitive function and neurovascular damage in the hippocampi
Significant (p<0.05) interactions were detected between the groups (e.g., DM presence/absence and/or MCAO presence/absence) and times on all the repeated Morris water maze tests and glucose levels (Fig. 1). However, the subgroup analysis did not show significant differences prior to the time of onset (e.g., DM and MCAO), indicating that a proper randomization with no group selection bias was observed. The NTM-STZ induced DM shares a number of features with human T2DM including hyperglycemia, glucose intolerance, and abnormalities of insulin secretion, and has been widely used to induce experimental T2DM in the rat10. To examine NTM-STZ induced morphological changes in pancreatic beta-cells, insulin staining was performed 65 days after NTM-STZ administration. Middle aged DM rats exhibited reduction of insulin producing pancreatic β-cells and disruptions in islet morphology compared to the age-matched rat without NTM-STZ injection (Fig. 1A). These rats developed persistent hyperglycemia starting 1 week after administration of NTM-STZ (Fig. 1B), which resembles the clinical manifestation of T2DM.
Figure 1. Pancreatic β-cells islet morphology, blood glucose level, and cognitive deficits.
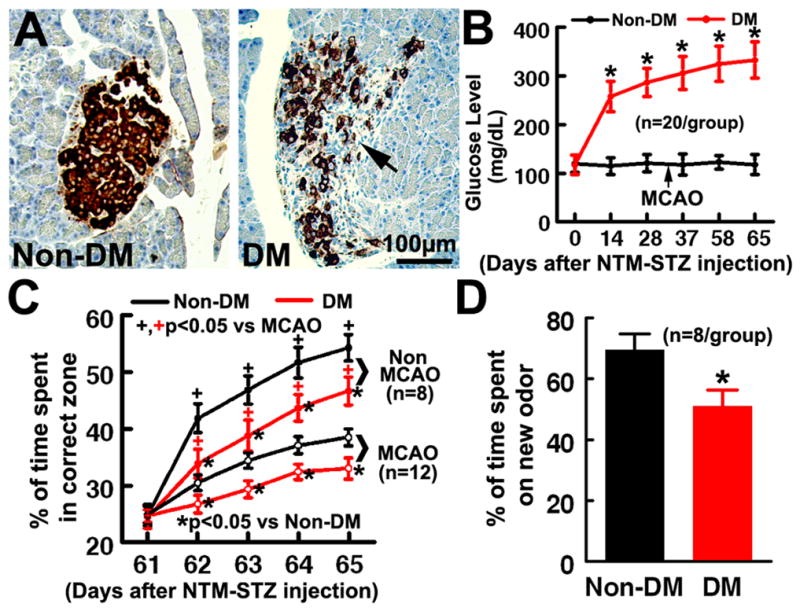
Insulin immunoreactivity within the pancreas of middle-aged non-DM and DM rats (A). Insulin+ cells occupied most of the central region of the β-cell islets (brown) in the pancreas of middle-aged non-DM rats, whereas decreased insulin+ cells and distortion of islet shape (A, arrow) were evident in middle-aged rats after NTM-STZ administration. B shows glucose levels in rats with or without induction of DM. DM rats exhibited significant cognitive deficits measured by the Morris water maze test (C) and odor recognition test (D) 2 months after induction of DM. *P< 0.05 as compared with the Non-DM rats. ANCOVA for panels B and C. ANOVA/two-sample t-test for panel D.
Analysis of the Morris water maze test revealed that DM rats spent significantly less time in the correct quadrant 2 months after administration of NTM-STZ compared with non-DM rats (Fig. 1C). In addition, DM rats also spent much less time on a novel object measured by the odor recognition test 2 months after induction of DM (Fig. 1D). These data indicate that DM induces impairment of cognitive function.
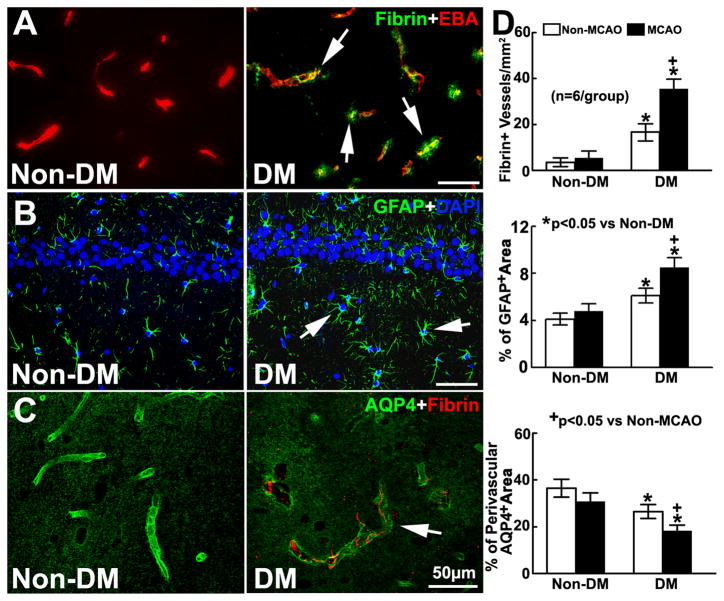
Immunofluorescent analysis of brain tissues showed that microvascular thrombosis measured by fibrin/fibrinogen immunoreactive microvessels, and BBB leakage assessed by extravascular fibrin deposition were localized to the hippocampi of DM rats (Fig. 2A). Although GFAP+ astrocytes were activated, paravascular AQP4 immunoreactivity was substantially reduced (Fig. 2B&C). These data indicate that DM induces neurovascular damage in the hippocampus.
Figure 2. Vascular damage in the DM brain.
A shows the double immunofluorescent images of fibrin (green) with EBA (red) in the ipsilateral hippocampus in ischemic rats with and without induction of DM. B and C are the immunofluorescent images of GFAP+ astrocytes (green, B) and AQP4 immunoreactivity (green, C) within the ipsilateral hippocampus. Bar graphs in D show DM rats with and without MCAO exhibited significant increases of fibrin deposition and GFAP immunoreactivity, and reductions of paravascular AQP4 immunoreactivity in the hippocampus, respectively. ANOVA.
DM exacerbates sensorimotor and cognitive function during stroke recovery
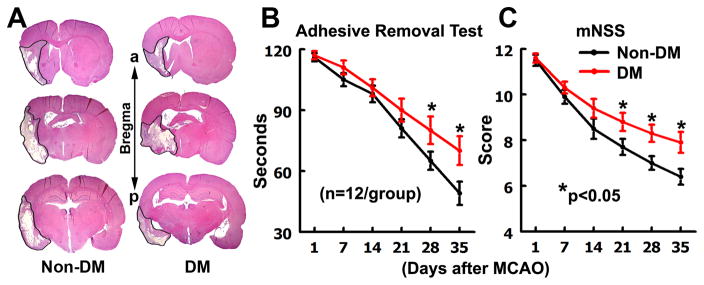
We then subjected DM rats to MCAO one month after the NTM-STZ injection. Significant (p<0.05) interactions were detected between the groups (e.g., DM presence/absence and/or MCAO presence/absence) and the time on functional tests. The subgroup analysis showed that rats in the non-DM and DM groups exhibited severe neurological deficits measured by adhesive removal test and mNSS 24 hours after MCAO and there were no significant differences between the two groups (Fig. 3B&C), indicating that the initial sensorimotor deficits are comparable. Non-DM ischemic rats exhibited spontaneous functional improvement during stroke recovery (Fig. 3B&C). However, DM ischemic rats exhibited significant impairment of spontaneous functional recovery measured by mNSS at 21, 28, and 35 days, and by the adhesive removal test at 28 and 35 days after MCAO compared to non-DM rats (Fig. 3B&C). Moreover, Morris water maze analysis showed that DM ischemic rats exhibited aggravated spatial learning deficits compared to age-matched non-DM ischemic rats one month after MCAO (Fig. 1C). These data indicate that DM impairs spontaneous functional improvement during stroke recovery.
Figure 3. Infarction and neurological functional outcome.
A shows H&E stained coronal brain sections from representative non-DM and DM rats after MCAO. a: anterior. p: posterior. B&C show the neurological functional outcome measured by adhesive removal test (B), and modified neurological severity score (C) in middle aged Non-DM and DM rats after MCAO. *P< 0.05 as compared with the Non-DM rats. ANCOVA.
DM exacerbates microvascular damage during stroke recovery
Infarct volume measured 35 days after MCAO revealed that DM rats had 27.7±2.4% infarct compared to the contralateral hemisphere (n=8) while non-DM rats had infarction of 27.1±2.6% (n=8), which was not statistically significant (p>0.05).
However, compared to non-DM ischemic rats, DM ischemic rats exhibited a significant increase in density of fibrin/fibrinogen immunoreactive vessels in the bilateral hippocampi, which was concurrent with aggravated astrocyte activation and reduction of paravascular AQP4 reactivity (Fig. 2D). Additionally, DM ischemic rats exhibited significantly (p<0.05) increased density of fibrin/fibrinogen immunoreactive vessels (44.3±3.6/mm2) in the ipsilateral peri-infarct regions, including the corpus callosum and striatum, but did not exhibit increased density of fibrin/fibrinogen immunoreactive vessels in the corresponding contralateral regions (6.4±1.9/mm2) compared to the non-DM ischemic rats (17.5±1.7/mm2at ipsilateral, 4.9±1.4/mm2 at contralateral). These data indicate that DM intensifies the neurovascular disruption after stroke.
DM suppresses stroke-induced neurogenesis
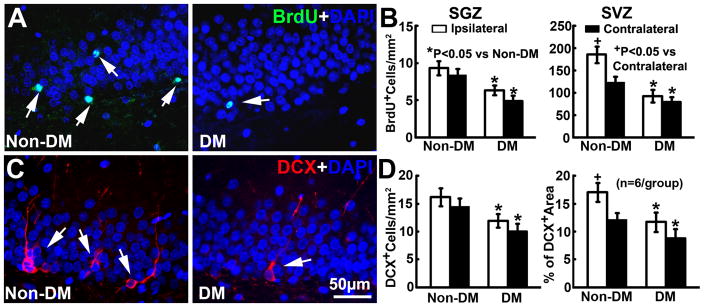
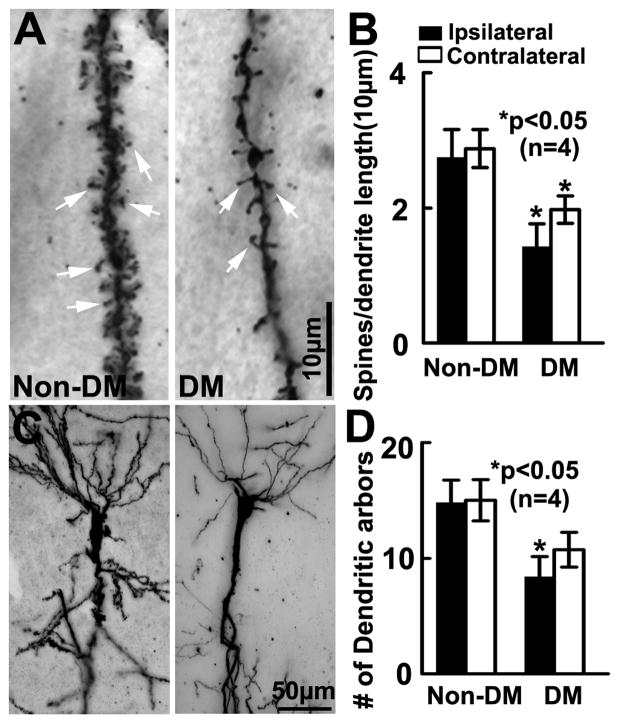
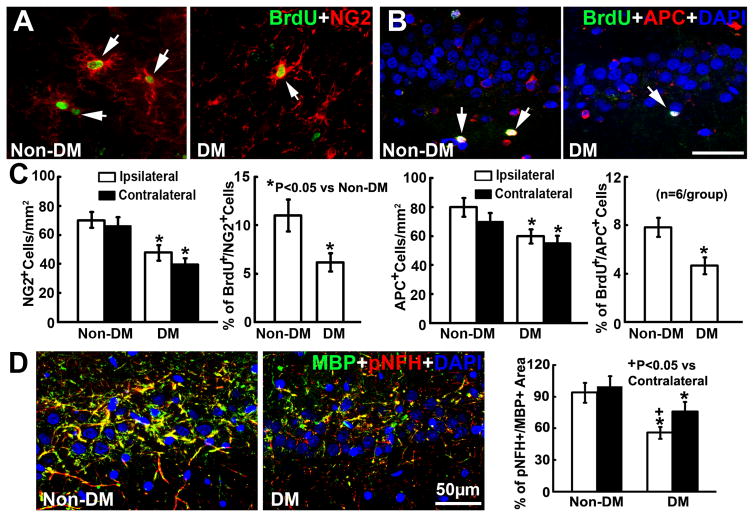
We and others have demonstrated that stroke induces neurogenesis in the subventricular zone (SVZ) of the lateral ventricle15, 17, 18. Consistent with published studies, ischemic non-DM rats showed a significant increase in the number of BrdU+ neural progenitor cells (Fig. 4B) and DCX+ neuroblasts in the ipsilateral SVZ (Fig. 4D). In contrast, DM ischemic rats exhibited significant reductions of BrdU+ cells and DCX+ neuroblasts in the SVZ and the subgranular zone (SGZ) of the dentate gyrus of both hemispheres (Fig. 4A–D). Furthermore, Golgi-Cox staining showed that DM ischemic rats exhibited marked reductions of bilateral spine density (Fig. 5A&B) and ipsilateral basal dendritic arborization (Fig. 5C&D) of pyramidal neurons in the CA1 compared to that in non-DM ischemic rats. These data suggest that DM suppresses stroke-induced neurogenesis and exacerbates dendritic spine loss.
Figure 4. Neurogenesis.
A shows the immunofluorescent images of BrdU+(green) cells within the SGZ of the ipsilateral dentate gyrus. Ischemic DM rats exhibited significant reductions of BrdU+ cell density in the bilateral SGZ and the SVZ (B). C shows the immunofluorescent images of DCX+(red) neuroblasts within the SGZ of the ipsilateral dentate gyrus. Ischemic DM rats exhibited marked bilateral reductions of DCX+ neuroblasts (D). ANCOVA.
Figure 5. Dendritic spines and arborization.
Golgi-Cox staining of dendritic spines (A, arrows) and dendrites (C) in the hippocampal CA1 of Non-DM and DM rats 35d after MCAO. DM rats exhibited significant bilateral reduction of spine density (B) and ipsilateral basal dendritic arborization (D) compared to Non DM rats. ANCOVA.
DM suppresses oligodendrogenesis and aggravates white matter damage after stroke
In addition to neurogenesis, we found that the DM ischemic rats exhibited profound bilateral reductions of NG2+ OPCs and APC+ oligodendrocytes in the hippocampus and subcortical regions compared to the non-DM ischemic rats (Fig. 6A–C). Double immunofluorescent staining revealed that DM ischemic rats exhibited significant reductions of BrdU+/NG2+ OPCs and BrdU+/APC+ oligodendrocytes in the hippocampus and subcortical regions (Fig. 6A–C). DM ischemic rats also exhibited significant bilateral reductions of pNFH and MBP immunoreactive density in the hippocampus and subcortical white matter structures compared to the non-DM ischemic rats (Fig. 6D). However, the loss of pNFH and MBP immunoreactive density was more pronounced in the ipsilateral hippocampus and peri-infarct white matter, where there was BBB leakage. Collectively, these data suggest that DM impairs oligodendrogenesis and aggravates white matter damage in the ischemic brain.
Figure 6. Oligodendrogenesis.
The double immunofluorescent images of BrdU+(green)/NG2+(red) OPCs (A) and BrdU+(green)/APC+(red) oligodendrocytes (B) within the ipsilateral hippocampus. Bar graphs in C show that compared to ischemic non-DM rats, ischemic DM rats exhibited marked bilateral reductions of NG2+ OPCs and APC+oligodendrocytes, and the ipsilateral reduction of proliferating OPCs (BrdU+/NG2+ cells) and newly generated oligodendrocytes (BrdU+/APC+ cells). D shows the double immunofluorescent images of MBP+(green)/pNFH+(red) myelinated axon within the ipsilateral hippocampus acquired from the adjacent coronal sections from B. The bar graph in D shows that ischemic DM rats exhibited significant bilateral reductions of myelinated axon density in the hippocampus and subcortical white matter structures. ANCOVA.
Discussion
The present study demonstrated that middle aged rats with DM induced by NTM-STZ administration exhibited cognitive impairment, and DM rats subjected to stroke exhibited exacerbated sensorimotor and cognitive deficits during stroke recovery. This DM model appears to parallel the clinical conditions observed in diabetic patients who suffer accelerated cognitive impairment during aging and impaired neurological recovery after stroke19, 5. We also demonstrated that middle age DM ischemic rats exhibited aggravated neurovascular disruption in the hippocampus and white matter and impaired brain repair processes, which likely contribute to exacerbated impairments of functional recovery after stroke induced by DM.
The prevalence of cognitive dysfunction is high in diabetic patients, especially among the elderly19. Given the multi-faceted nature of the metabolic disease, several structural and functional abnormalities present in the CNS following disturbances of glucose homoeostasis, including micro/macro vascular complications, hyperglycemia-associated oxidative stress and neurotoxicity, defects in neural insulin and amyloid metabolism, and decreased neuroplasticity capacity, which have been implicated in the development of cognitive abnormalities20. However, the causal neuropathological mechanisms underpinning cognitive impairment associated with DM remain to be investigated. In a mouse model of type 1 DM, a recent study shows that DM downregulated vascular endothelial growth factor (VEGF) and its receptor VEGFR2 in the hippocampus where vascular disruption and reduction of dendritic spine density were detected, which were associated with cognitive impairment 21. Intraventricular infusion of VEGF restored cognitive function, vascular integrity, and spine density in the hippocampus 21. These data suggest that the VEGF signaling pathway plays an important role in DM-induced cognitive impairment 21. In addition, we have recently demonstrated that DM induced cognitive deficits are highly correlated with the impairment of the glymphatic system, a brain wide extracellular fluid exchange pathway driven by cerebrovascular pulsation and astrocytic AQP4 water channels that line paravascular space22. The impairment of the glymphatic pathway following vascular disruption and loss of AQP4 water channels leads to the paravascular accumulation of waste metabolic products, including amyloid-beta, a characteristic feature that predicts the progression of cognitive decline and the development of Alzheimer’s disease22, 23. Our current data reveal that DM induced cognitive deficits were associated with hippocampi microvascular disruption, astrocyte activation, and loss of paravascular AQP4 immunoreactivity. The hippocampus is involved with learning and memory, which is highly susceptible to changes in oxygen and blood supply24. Together with published studies, the present data suggest that DM-induced hippocampal neurovascular disruption along with an impairment of the glymphatic system may contribute to the development of cognitive deficits.
Comorbid DM in stroke aggravates ischemic brain damage and impairs neurological functional recovery in stroke patients3, 4. The present study suggests that brain repair processes exacerbated by DM likely lead to impairment of functional outcome because DM did not enlarge infarction. Stroke induces a cascade of interactive remodeling events including neurogenesis, oligodendrogenesis, and rewiring of lost neuronal circuits, all of which contribute to stroke recovery18. DM and hyperglycemia reduce neurogenesis in the adult brain, and negatively impact cognitive function25, 26. However, the impact of DM on stroke-induced brain remodeling processes in aging brain has not been investigated. Our data demonstrated that DM suppressed the ipsilateral neurogenesis induced by stroke and also decreased contralateral neurogenesis in the SVZ and the SGZ of the dentate gyrus. Moreover, the spine number and dendritic arborization in the ipsilateral CA1 pyramidal neurons were drastically reduced in the DM rat subjected MCAO, indicating an impairment of dendritic plasticity. Stroke-induced neuroblasts are required for brain repair processes and functional recovery27. New neurons generated in the hippocampal regions of the adult rodent connect to resident neurons in the hippocampal regions and form neuronal circuitry, which mediates learning and memory function28, 29. Thus, neurogenesis suppressed by DM likely impairs hippocampal synaptic plasticity and dendritic remodeling in ischemic brain, leading to cognitive decline after stroke. However, additional experiments are warranted to determine whether stroke exacerbates DM induced olfactory learning and memory deficits and the potential contribution of neurogenesis to olfactory function.
White matter damage, associated with vascular disruption, is an important brain structure substrate that contributes to neurobehavioral impairment in stroke patients30. Experimental data from young adult animals have demonstrated that DM worsens stroke induced vascular disruption and subsequent damage to peri-infarct neuronal circuits, which leads to impairment of stroke recovery31. Here, we found that the observed microvascular disruption in the hippocampus and white matter structure in the ischemic DM brain were closely associated with myelinated axonal loss, which is consistent with previous studies and further validates our recent neuroimaging observations showing that DM rats suffer persistent vascular disruption that hinders white matter reorganization after stroke31–33. In addition to neurogenesis, neural progenitor cells within the SVZ and SGZ after brain injury generate OPCs that are recruited to the injured site where they differentiate into myelinating oligodendrocytes and contribute to neurological functional recovery34. Thus, reduction of stroke-induced oligodendrogenesis in the ipsilateral hippocampus and peri-infarct white matter by DM may underlie DM-induced white matter damage after stroke.
A caveat of the present study is that the contralateral hemisphere was used as control, which could mask the effects of stroke induced bilateral remodeling processes. Future experiments are warranted to include the naïve or sham operated animals as control for investigating the relative contribution of DM and stroke to bilateral brain remodeling processes.
In conclusion, our data suggest that DM induces cognitive decline in middle aged animals, which is associated with hippocampal neurovascular disruption. Furthermore, DM exacerbates neurovascular damage and inhibits brain interactive remodeling processes, leading to impairment of neurological functional recovery and cognitive decline in middle aged rats after stroke, which mimics the pathological manifestations observed in stroke patients with DM.
Supplementary Material
Acknowledgments
The authors wish to thank Cynthia Roberts, Min Wei, Qing-e Lu, Sutapa Santra for technical assistance.
Funding Sources: This work was supported by the National Institutes of Health grants, RO1 NS 079612 (ZGZ), RO1 NS 075156 (ZGZ), and RO1 AG037506 (MC).
Footnotes
Disclosures: None.
The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
References
- 1.Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lukovits TG, Mazzone TM, Gorelick TM. Diabetes mellitus and cerebrovascular disease. Neuroepidemiology. 1999;18:1–14. doi: 10.1159/000026190. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen H, Nakayama H, Raaschou HO, Olsen TS. Stroke in patients with diabetes. The Copenhagen stroke study. Stroke. 1994;25:1977–1984. doi: 10.1161/01.str.25.10.1977. [DOI] [PubMed] [Google Scholar]
- 4.Newman GC, Bang H, Hussain SI, Toole JF. Association of diabetes, homocysteine, and hdl with cognition and disability after stroke. Neurology. 2007;69:2054–2062. doi: 10.1212/01.wnl.0000280457.29680.9c. [DOI] [PubMed] [Google Scholar]
- 5.Mizrahi EH, Waitzman A, Blumstein T, Arad M, Adunsky A. Diabetes mellitus predicts cognitive impairment in patients with ischemic stroke. Am J Alzheimers Dis Other Demen. 2010;25:362–366. doi: 10.1177/1533317510365343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sweetnam D, Holmes A, Tennant KA, Zamani A, Walle M, Jones P, et al. Diabetes impairs cortical plasticity and functional recovery following ischemic stroke. J Neurosci. 2012;32:5132–5143. doi: 10.1523/JNEUROSCI.5075-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: Links to cognition and depression. Neurosci Biobehav Rev. 2013;37:1346–1362. doi: 10.1016/j.neubiorev.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szkudelski T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp Biol Med (Maywood) 2012;237:481–490. doi: 10.1258/ebm.2012.011372. [DOI] [PubMed] [Google Scholar]
- 10.Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, et al. Experimental niddm: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47:224–229. doi: 10.2337/diab.47.2.224. [DOI] [PubMed] [Google Scholar]
- 11.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Zhang Z, Zhang RL, Cui Y, LaPointe MC, Silver B, et al. Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain Res. 2006;1118:192–198. doi: 10.1016/j.brainres.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 13.Choi SH, Woodlee MT, Hong JJ, Schallert T. A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J Neurosci Methods. 2006;156:182–193. doi: 10.1016/j.jneumeth.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Spinetta MJ, Woodlee MT, Feinberg LM, Stroud C, Schallert K, Cormack LK, et al. Alcohol-induced retrograde memory impairment in rats: Prevention by caffeine. Psychopharmacology (Berl) 2008;201:361–371. doi: 10.1007/s00213-008-1294-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhang RL, Chopp M, Roberts C, Wei M, Wang X, Liu X, et al. Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoS One. 2012;7:e48141. doi: 10.1371/journal.pone.0048141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueno Y, Chopp M, Zhang L, Buller B, Liu Z, Lehman NL, et al. Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke. Stroke. 2012;43:2221–2228. doi: 10.1161/STROKEAHA.111.646224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 18.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: Underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 20.Qiu C, Sigurdsson S, Zhang Q, Jonsdottir MK, Kjartansson O, Eiriksdottir G, et al. Diabetes, markers of brain pathology and cognitive function: The age, gene/environment susceptibility-reykjavik study. Ann Neurol. 2014;75:138–146. doi: 10.1002/ana.24063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor SL, Trudeau D, Arnold B, Wang J, Gerrow K, Summerfeldt K, et al. Vegf can protect against blood brain barrier dysfunction, dendritic spine loss and spatial memory impairment in an experimental model of diabetes. Neurobiol Dis. 2015;78:1–11. doi: 10.1016/j.nbd.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates csf flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardy J, Selkoe DJ. The amyloid hypothesis of alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- 25.Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang WJ, Tan YF, Yue JT, Vranic M, Wojtowicz JM. Impairment of hippocampal neurogenesis in streptozotocin-treated diabetic rats. Acta Neurol Scand. 2008;117:205–210. doi: 10.1111/j.1600-0404.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- 27.Sun C, Sun H, Wu S, Lee CC, Akamatsu Y, Wang RK, et al. Conditional ablation of neuroprogenitor cells in adult mice impedes recovery of poststroke cognitive function and reduces synaptic connectivity in the perforant pathway. J Neurosci. 2013;33:17314–17325. doi: 10.1523/JNEUROSCI.2129-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. Gaba regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.JSR-F, Sa-Roriz TM, Rosset I, Camozzato AL, Santos AC, Chaves ML, et al. (pre)diabetes, brain aging, and cognition. Biochim Biophys Acta. 2009;1792:432–443. doi: 10.1016/j.bbadis.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Reeson P, Tennant KA, Gerrow K, Wang J, Weiser Novak S, Thompson K, et al. Delayed inhibition of vegf signaling after stroke attenuates blood-brain barrier breakdown and improves functional recovery in a comorbidity-dependent manner. J Neurosci. 2015;35:5128–5143. doi: 10.1523/JNEUROSCI.2810-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prakash R, Li W, Qu Z, Johnson MA, Fagan SC, Ergul A. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: Relevance to stroke recovery. Stroke. 2013;44:2875–2882. doi: 10.1161/STROKEAHA.113.001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding G, Yan T, Chen J, Chopp M, Li L, Li Q, et al. Persistent cerebrovascular damage after stroke in type two diabetic rats measured by magnetic resonance imaging. Stroke. 2015;46:507–512. doi: 10.1161/STROKEAHA.114.007538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.