Summary
It is suspected that primary central nervous system lymphoma (PCNSL) rates are increasing among immunocompetent people. We estimated PCNSL trends in incidence and survival among immunocompetent persons by excluding cases among human immunodeficiency virus (HIV)-infected persons and transplant recipients. PCNSL data were derived from 10 Surveillance, Epidemiology and End Results (SEER) cancer registries (1992–2011). HIV-infected cases had reported HIV infection or death due to HIV. Transplant recipient cases were estimated from the Transplant Cancer Match Study. We estimated PCNSL trends overall and among immunocompetent individuals, and survival by HIV status. A total of 4,158 PCNSLs were diagnosed (36% HIV-infected; 0.9% transplant recipients). HIV prevalence in PCNSL cases declined from 64.1% (1992–1996) to 12.7% (2007–2011), while the prevalence of transplant recipients remained low. General population PCNSL rates were strongly influenced by immunosuppressed cases, particularly in 20–39 year-old men. Among immunocompetent people, PCNSL rates in men and women aged 65+ years increased significantly (1.7% and 1.6%/year), but remained stable in other age groups. Five-year survival was poor, particularly among HIV-infected cases (9.0%). Among HIV-uninfected cases, 5-year survival increased from 19.1% (1992–1994) to 30.1% (2004–2006). In summary, PCNSL rates have increased among immunocompetent elderly adults, but not in younger individuals. Survival remains poor for both HIV-infected and HIV-uninfected PCNSL patients.
Keywords: lymphoma, central nervous system, incidence, survival, HIV, transplant
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare malignancy with an annual incidence rate of 7 cases per 1,000,000 people in the U.S. (O’Neill, et al 2013). The rates of PCNSL are strongly increased in immunosuppressed populations, particularly among people with human immunodeficiency virus (HIV) infection (Gibson, et al 2014). In addition, though poorly studied, rates of PCNSL in solid organ transplant recipients are probably elevated, as non-Hodgkin lymphoma (NHL) rates are significantly increased (Clarke, et al 2013). PCNSLs among immunocompetent and immunosuppressed individuals appear to have a different aetiology. Most notably, the majority of PCNSLs occurring in HIV-infected people and transplant recipients are associated with Epstein-Barr Virus (EBV), whereas EBV-related PCNSL cases are uncommon in immunocompetent individuals (Chimienti, et al 2009).
The HIV epidemic has strongly influenced the occurrence of PCNSL in the U.S. over the last several decades. In a prior study, we showed that during 1980–2007, 26.2% of PCNSLs in the U.S. occurred among HIV-infected people (Shiels, et al 2011). In the HIV population, occurrence of PCNSL has declined over time with effective treatment using highly active antiretroviral therapy (HAART) (Engels, et al 2008). In contrast, it has been suggested that rates among immunocompetent individuals have increased (Abrey 2009, Chimienti, et al 2009). Further, one study has shown improvements in survival over time in PCNSL cases who were presumed HIV-negative (Norden, et al 2011). However, given the contribution of cases occurring in immunosuppressed people to the population-level burden of PCNSL, it is not possible to adequately assess trends in immunocompetent PCNSL incidence and survival based on overall population data without first removing cases occurring among HIV-infected people and solid organ transplant recipients, the largest immunosuppressed populations in the U.S.
Prior studies using Surveillance, Epidemiology and End Results (SEER) data for the US have indirectly estimated PCNSL incidence and survival in immunocompetent people by excluding populations with higher HIV prevalence (i.e., San Francisco area residents, never married men and women, and <65 year-olds (O’Neill, et al 2013, Olson, et al 2002), and by excluding deaths due to HIV and other infectious diseases (Norden, et al 2011). However, these strategies do not fully remove the effect of HIV on PCNSL rates, and also do not account for the potential influence of transplant-related PCNSL on general population trends. Thus, the aims of this study were to quantify the fraction of PCNSLs that occurred in SEER areas among people who are HIV-infected or solid organ transplant recipients, to estimate trends in immunocompetent PCNSL after excluding these cases, and to compare PCNSL survival by HIV status.
Methods
Data sources
Data on PCNSLs were derived from 10 population-based SEER cancer registries in the U.S. (i.e., Connecticut, Hawaii, New Mexico, Utah, Atlanta, Detroit, Seattle-Puget Sound, Los Angeles, San Francisco-Oakland, San Jose-Monterey) that had information on HIV status for 1992–2011. NHL was defined using SEER site recoding based on the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) and the World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (2008) (Fritz, et al 2000, Swerdlow et al 2008). All NHLs with topography codes C70.0–C72.9 were classified as PCNSLs. Cases with unknown race/ethnicity, unknown age, or gender other than male/female were excluded (n=9).
We used SEER data to classify cases by HIV status using two data sources. First, SEER registries recorded HIV serostatus at the time of cancer diagnosis (i.e., “HIV flag”) as part of the extent of disease field for individuals diagnosed with lymphoma (Clarke and Glaser 2004, Diamond, et al 2007). 51% of PCNSL cases had missing values for the HIV flag (n=2,102). We classified these cases as HIV flag-negative, because the presence of HIV infection is generally documented by physicians in medical records, and this flag has been shown to be highly sensitive at identifying HIV-infected NHL cases (Clarke and Glaser 2004, Diamond, et al 2007). Additionally, SEER captures HIV as an underlying cause of death from death certificates. Positive HIV status was determined by a positive HIV flag or HIV as the underlying cause of death. Of the 2,771 cases that were HIV-negative based on the HIV flag alone, 125 (4.5%) were re-classified based on cause of death. As PCNSL is an acquired immunodeficiency syndrome (AIDS)-defining condition (Centers for Disease Control and Prevention 1992), all PCNSL cases with HIV infection had an AIDS diagnosis.
As SEER registries do not include data on transplantation, we separately estimated the number of PCNSLs that occurred among transplant recipients. Data on PCNSLs occurring among solid organ transplant recipients during 1992–2011 were ascertained from the Transplant Cancer Match (TCM) Study (www.transplantmatch.cancer.gov) (Engels, et al 2011), a linkage of the U.S. Scientific Registry of Transplant Recipients (SRTR) and 16 cancer registries. The linkage was approved by the human subjects committees of the National Cancer Institute and, as required, participating cancer registries. A total of 147 PCNSLs linked to the SRTR; none of these cases was also HIV-infected.
Statistical Analysis
To estimate the number of PCNSLs in SEER that were diagnosed among transplant recipients, we multiplied SEER population counts by the incidence of PCNSL in transplant recipients, expressed as a fraction of the general population (i.e., defined as number of PCNSL cases that linked to the SRTR / general population counts for the cancer registry areas). These calculations were carried out in strata defined by calendar year (single years, 1992–2011), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic and other), sex and age (5-year groups from 0–4 to 85+ years).
We calculated the fraction of all PCNSLs that occurred among HIV-infected people and transplant recipients by calendar period and demographics. We derived the estimated number of immunocompetent PCNSL cases by subtracting HIV-infected and transplant recipient PCNSL cases from the total number of PCNSL cases. We then estimated sex- and age-stratified time trends in total PCNSL rates and immunocompetent PCNSL rates, age-standardized to the 2000 U.S. population. Annual per cent changes (APCs) in incidence rates were estimated with Joinpoint regression (Kim, et al 2000, http://surveillance.cancer.gov/joinpoint/.
We additionally estimated age-standardized survival curves for HIV-infected and HIV-uninfected PCNSL cases during 1992–2011, using the actuarial method in SEER*Stat, version 8.2.1 (http://seer.cancer.gov/seerstat/). To avoid biasing the survival estimates, we excluded cases that had been classified as HIV-infected based on cause of death alone. A sub-analysis was restricted to cases diagnosed in ≥1998, a time period when HAART was widely available. Further, we estimated 5-year survival rates by calendar year of diagnosis among HIV-uninfected PCNSL cases during 1992–2006. Cases from 2007–2011 were not included due to incomplete 5-year follow-up.
All statistical tests were two-sided with α=0.05.
Results
During 1992–2011, a total of 4,158 cases of PCNSL occurred during 718 million person-years in 10 SEER registry areas. Thirty per cent of PCNSLs were not further characterized by cell type or histology. Of the remaining 2,906 PCNSLs, 91.6% were B-cell (75.1% diffuse large B-cell), 1.5% were T-cell, and 6.9% were NHLs of unknown lineage. PCNSLs predominantly occurred in the brain (83.3%), with smaller fractions occurring in the meninges, spinal cord and other/unspecified sites. Though 84.2% of cases were microscopically-confirmed, this fraction differed substantially by HIV status (93.8% of HIV-uninfected cases and 67.3% of HIV-infected cases). HIV-infected cases were more likely to be diagnosed with only radiographic (26.5% vs. 4.4%) or clinical (4.6% vs. 0.8%) confirmation compared to HIV-uninfected cases.
Thirty-six per cent (n=1,512) of cases were HIV-infected and an estimated 0.9% (n=38) occurred in solid organ transplant recipients. Immunocompetent PCNSL cases were equally common in men and women, while cases occurring in HIV-positive people (91.7%) and transplant recipients (57.9%) were more likely to be men (Table I). In immunocompetent individuals, the median age at diagnosis was 67 years (estimated by taking the mid-point of each 5-year age category), compared to 37 years in HIV-infected people and 52 years in transplant recipients. While non-Hispanic white was the most common race/ethnicity in each group, larger fractions of cases were minorities in immunosuppressed populations.
Table I.
Characteristics of PCNSL cases occurring among immunocompetent individuals, HIV-infected individuals and solid organ transplant recipients in 10 U.S. cancer registries, 1992–2011.
| Immunocompetent | HIV-infected | Transplant recipient | |
|---|---|---|---|
|
| |||
| N (%) | N (%) | N (%) | |
| Total | 2608 | 1512 | 38 |
| Median age, IQR* | 67 (52, 77) | 37 (32, 42) | 52 (42, 62) |
| Sex | |||
| Male | 1318 (50.5) | 1388 (91.7) | 22 (57.9) |
| Female | 1290 (49.5) | 124 (8.2) | 16 (42.1) |
| Race/ethnicity | |||
| Non-Hispanic white | 1798 (69.0) | 778 (51.5) | 19 (50.0) |
| Other | 810 (31.1) | 734 (48.5) | 19 (50.0) |
IQR: interquartile range
Median ages were estimated assigning the midpoint value to all cases in each 5-year category.
Table II presents the proportion of PCNSL cases occurring among HIV-infected people and transplant recipients by calendar period, gender, age and race/ethnicity. Over time, the fraction of cases occurring in HIV-infected people declined from 64.1% in 1992–1996 to 12.7% in 2007–2011. The prevalence of HIV was greatest in PCNSL cases who were male (50.9% vs. 8.7% in females), particularly men aged 20–39 years (88.5%) and non-Hispanic black males (84.6%). Even in the most recent time period (2007–2011), the prevalence of HIV was ≥50% among 20–39 year-old and non-Hispanic black men and women with PCNSL. The fraction of cases occurring among transplant recipients remained low, but increased from 0.4% (1992–1996) to 1.5% (2007–2011).
Table II.
Proportion of total PCNSL cases occurring among HIV-infected people and solid organ transplant recipients by sex, age, race and calendar period with data from 10 cancer registries, 1992–2011.
| Total % | 1992–1996 % | 1997–2001 % | 2002–2006 % | 2007–2011 % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| HIV+ | Transplant | HIV+ | Transplant | HIV+ | Transplant | HIV+ | Transplant | HIV+ | Transplant | |
| Males and Females | ||||||||||
| Total | 36.4 | 0.9 | 64.1 | 0.4 | 29.3 | 0.9 | 17.2 | 1.4 | 12.7 | 1.5 |
| Males | ||||||||||
| Total | 50.9 | 0.8 | 76.8 | 0.3 | 42.1 | 1.0 | 26.0 | 1.4 | 18.5 | 1.4 |
| Age (years) | ||||||||||
| 0–19 | 4.5 | 2.6 | 14.3 | 2.7 | 0.0 | 4.8 | 0.0 | 2.7 | 0.0 | 0.0 |
| 20–39 | 88.5 | 0.5 | 94.0 | 0.2 | 85.6 | 0.6 | 76.6 | 1.6 | 53.3 | 2.1 |
| 40–64 | 50.6 | 1.2 | 75.7 | 0.4 | 43.2 | 1.4 | 29.3 | 2.2 | 24.7 | 1.4 |
| 65+ | 1.8 | 0.6 | 3.0 | 0.0 | 1.4 | 0.5 | 1.6 | 0.2 | 1.5 | 1.3 |
| Race/ethnicity | ||||||||||
| Non-Hispanic white | 44.8 | 0.6 | 73.0 | 0.2 | 29.2 | 0.7 | 15.2 | 1.5 | 8.9 | 0.8 |
| Non-Hispanic black | 84.6 | 0.8 | 91.6 | 0.2 | 90.2 | 0.3 | 72.4 | 1.0 | 62.2 | 4.2 |
| Hispanic | 63.1 | 0.8 | 86.3 | 0.2 | 62.5 | 1.1 | 42.2 | 1.4 | 35.6 | 1.6 |
| Females | ||||||||||
| Total | 8.7 | 1.1 | 14.9 | 0.7 | 9.6 | 0.7 | 5.6 | 1.3 | 5.9 | 1.6 |
| Age (years) | ||||||||||
| 0–19 | 5.0 | 2.5 | 16.7 | 8.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 20–39 | 54.5 | 2.1 | 70.5 | 0.6 | 52.8 | 2.5 | 33.3 | 2.5 | 50.0 | 3.8 |
| 40–64 | 8.8 | 1.6 | 14.3 | 1.1 | 8.7 | 0.9 | 8.5 | 2.3 | 5.3 | 2.0 |
| 65+ | 0.7 | 0.6 | 0.6 | 0.3 | 0.6 | 0.1 | 0.5 | 0.6 | 0.9 | 1.1 |
| Race/ethnicity | ||||||||||
| Non-Hispanic white | 3.3 | 0.9 | 6.6 | 0.5 | 3.1 | 0.8 | 2.8 | 1.3 | 1.2 | 1.0 |
| Non-Hispanic black | 44.8 | 1.9 | 58.8 | 0.6 | 38.2 | 1.3 | 28.6 | 1.2 | 54.8 | 4.9 |
| Hispanic | 12.6 | 0.7 | 25.0 | 0.0 | 16.0 | 0.5 | 6.7 | 0.5 | 5.4 | 1.3 |
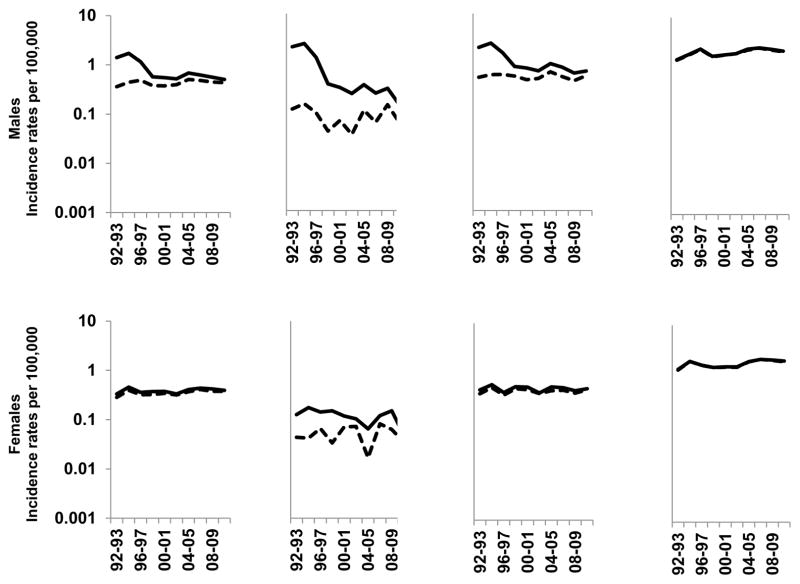
Figure 1 presents age-standardized incidence rates during 1992–2011 for total and immunocompetent PCNSL (i.e., total – immunosuppressed cases) by gender and age group. Supplemental Table 1 presents the corresponding APCs for these calendar trends. Immunosuppressed cases have had a profound impact on PCNSL rates in men. Total PCNSL rates among men declined steeply 30.6%/year (95% confidence interval [CI] -47.9, -7.5) during 1995–1998, with non-significant trends prior to and following this time period. However, when immunosuppressed cases were excluded, the overall incidence was lower, no significant changes in the APC were detected, and there was no longer a significant trend over 1992–2011 (APC: 0.72%; 95%CI -0.32–1.77). Similar patterns were observed for 20–39 and 40–59 year-old men. However, among men aged ≥65 years old, PCNSL rates increased over time, both overall (APC: 1.73%; 95%CI 0.29–3.20) and after excluding immunosuppressed people (APC: 1.68%; 95%CI 0.33–3.05). Immunosuppressed individuals contributed very little to total PCNSL trends among women. No significant trends in APCs were observed for total or immunocompetent PCNSL among 20–39 year-old or 40–59 year-old women during 1992–2011. However, rates significantly increased among 65+ year-old women, both overall (APC: 1.86%; 95%CI 0.34–3.40) and without immunosuppressed cases (APC: 1.56%; 95%CI 0.06–3.08).
Figure 1.
Age-standardized incidence rates of PCNSL overall and among immunocompetent individuals (i.e., excluding HIV-infected people and transplant recipients) in 10 SEER registries, 1992–2011. Solid lines indicate overall rates and dashed lines indicate immunompetent rates.
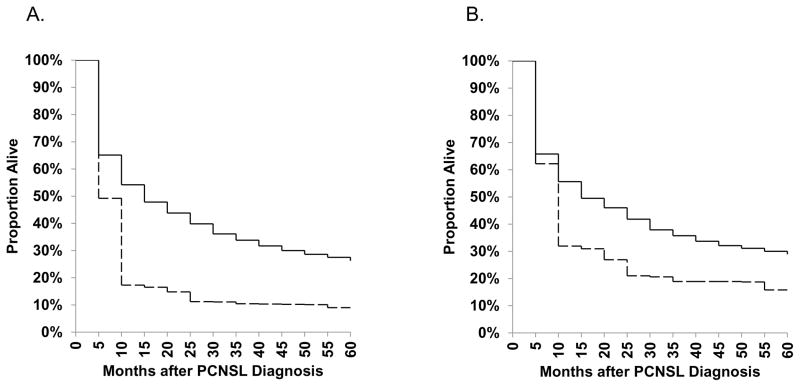
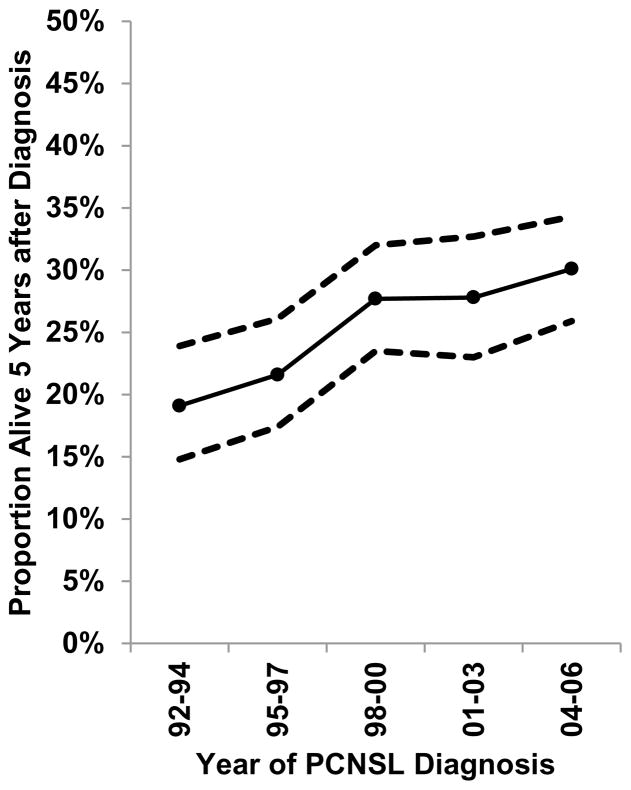
During 1992–2011, survival was poor for PCNSL cases, with just 9.0% (95%CI 3.3–18.3%) of HIV-infected cases and 26.2% (95%CI 24.4–28.1%) of HIV-uninfected cases alive five years after diagnosis (Figure 2). When restricted to cases diagnosed in 1998–2011, a time period when HAART was widely available, 5-year survival only improved slightly to 15.8% (95%CI 6.6–28.4%) in HIV-infected cases and 28.9% (95%CI 26.6–31.2%) in HIV-uninfected cases. We were unable to estimate 5-year survival among HIV-infected individuals in more recent time periods because of the small number of people under follow-up. For HIV-uninfected PCNSL cases, 5-year survival estimates improved from 19.1% (95%CI 14.8–23.9) for cases diagnosed during 1992–1994, to 30.1% (95%CI 25.9–34.3) during 2004–2006 (Figure 3).
Figure 2.
Age-standardized survival estimates for PCNSL cases diagnosed in 10 SEER registries during A) 1992–2011 and B) 1998–2011. Solid lines represent HIV-uninfected cases and dashed lines represent HIV-infected cases.
Figure 3.
Age-standardized 5-year survival estimates for HIV-uninfected PCNSLs by 3-year categories of calendar year of diagnosis in 10 SEER registries during 1992–2005. Points represent estimates and dashed lines represent 95% confidence intervals.
Discussion
In recent decades, population-based estimates of PCNSL incidence and survival have been strongly influenced by cases occurring among HIV-infected individuals. In the presence of HIV, it is difficult to discern trends in immunocompetent PCNSL, particularly given the dramatic temporal changes in HIV-related PCNSL risk. Though studies have suggested that the incidence of PCNSL among immunocompetent people has increased over time, and that survival has improved, no prior study has separated out cases occurring among HIV-infected people and transplant recipients. For the first time, we show that the rates of PCNSL in the immunocompetent U.S. population increased significantly during 1992–2011 among both men and women aged 65 years and older, while rates in younger age groups have remained unchanged. We also show that though 5-year survival in HIV-uninfected PCNSL cases has increased slightly over time, it remains poor.
NHL risk is elevated strongly in HIV-infected people and solid organ transplant recipients due to the immunosuppression experienced by these populations (Clarke, et al 2013, Gibson, et al 2014). In the setting of immunosuppression, most PCNSLs are caused by the loss of immune surveillance of EBV, resulting in EBV-driven lymphoproliferation (Gloghini, et al 2013). At the beginning of the AIDS epidemic, PCNSL rates in HIV-infected people were elevated 5,000-fold over the general population, but have declined with widespread treatment of HIV infection with HAART to a 50-fold increase in the most recent era (Engels, et al 2006, Gibson, et al 2014). These strong elevations have contributed substantially to population estimates of PCNSL. Indeed, we demonstrate that more than 1 in 3 cases in US SEER areas during the most recent 20-year period were HIV-infected. These estimates far exceed the 6% HIV prevalence estimated among all NHLs during 1992–2009 (Shiels, et al 2013), and are somewhat higher than our earlier estimate of 26.2% HIV prevalence among U.S. PCNSL cases during 1980–2007 (Shiels, et al 2011), probably due to the evaluation of different time periods and geographic areas. HIV prevalence was greatest among young and non-Hispanic black men, reflecting characteristics of the U.S. HIV-infected population (Centers for Disease Control and Prevention 2013), and declined over time, reflecting a decrease in PCNSL risk with HAART (Engels, et al 2008).
Solid organ transplant recipients take medications that suppress the immune system to prevent organ rejection. CNS involvement has been reported to occur in up to 15% of NHL cases occurring in this population (Buell, et al 2005), and associated with poor survival (Evens, et al 2013). The fraction of PCNSL cases occurring among transplant recipients increased slightly during 1992–2011, but remained very low (i.e., 0.9% over the entire time period). Transplant-related PCNSL is not well-studied. The increase in the fraction of PCNSL cases occurring among transplant recipients is partly due to the overall decline in the total number of PCNSL cases in the general population. Furthermore, there has been an increase in the number of people living with a transplant (Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) 2014). Finally, PCNSL incidence among US transplant recipients has increased, for unclear reasons, from 0.003 per 100,000 person-years during 1992–1996 to 0.008 per 100,000 person-years during 2007–2011 (data not shown).
In order to understand the trends in PCNSL incidence in the immunocompetent population, immunosuppressed cases, particularly among younger men, must first be excluded. For the first time, we showed that, after the removal of immunocompromised cases, overall rates of immunocompetent PCNSL did not increase during 1992–2011. In contrast, rates among men and women aged 65+ years have increased 1.7% and 1.6% per year, respectively. By age group, trends in PCNSL differed by sex, due to the disproportionate impact of HIV on general population rates in men. However, after removing immunocompromised cases, trends were similar among males and females. Though prior studies have suggested an increase in immunocompetent PCNSL over time, they have relied on surrogates by excluding groups of individuals with higher HIV prevalence.(Norden, et al 2011, O’Neill, et al 2013, Olson, et al 2002) These approaches are generally inadequate to fully remove HIV-infected cases. In our data, 33.1% of remaining PCNSL cases were HIV-infected after excluding the San Francisco registry, 13.1% after excluding never married men and women, and 8.0% after excluding deaths due to HIV and other infectious and parasitic diseases. As seen in our study, restricting analyses to cases aged 65+ years adequately selects only immunocompetent individuals, as the HIV prevalence in this age group is quite low, but of course this method cannot be used to study trends in PCNSL incidence among younger immunocompetent people.
It is unclear why PCNSL rates in older immunocompetent people have increased over time. These increasing trends do not reflect patterns observed in the U.S. for other NHL types. Though there was a well-documented decades-long increase in NHL rates, we have previously shown that rates among HIV-uninfected people in the U.S. have plateaued since 2004 (Shiels, et al 2013). It has been hypothesized that rates may have risen due to increases in detection through heightened surveillance, improved imaging techniques or more frequent diagnosis procedures (Abrey 2009). If this were the case, a similar increase in glioma might be expected (Olson, et al 2002); however, glioma rates for people aged 65+ years have remained stable over this calendar period (Surveillance Epidemiology and End Results (SEER) Program 2014). Restricting to microscopically confirmed cases did not notably alter CNS lymphoma trends in the oldest age group (men: 1.5% increase per year, p-value=0.07; women: 1.7% per year, p-value=0.03), suggesting that secular trends in confirmation have not driven the increases in incidence rate. Others have suggested that the increasing use of immunosuppressive medications to treat autoimmune conditions in the elderly may be contributing to rising PCNSL rates (Villano, et al 2011).
Survival after a PCNSL diagnosis was very poor, particularly among HIV-infected people. Poorer survival among HIV-infected patients may reflect severe immunosuppression, more aggressive lymphomas, or less frequent treatment (Bayraktar, et al 2011). Some studies have shown that HAART improves overall survival of PCNSL patients, and the National Comprehensive Cancer Network recommends the consideration of HAART for HIV-infected patients (Bayraktar, et al 2011, National Comprehensive Cancer Network 2015). Of note, current national guidelines recommend treatment with HAART for all HIV-infected people ( Panel on Antiretroviral Guidelines for Adults and Adolescents 2015). We observed a modest increase in survival among HIV-infected people during 1998–2011, though we were unable to restrict the analysis to a more recent period due to a limited number of cases. Among immunocompetent individuals, high-dose methotrexate is the recommended first-line treatment, however there is controversy regarding the optimal induction and consolidation regimens, leading to substantial variation in clinical practice (Hoang-Xuan, et al 2015, National Comprehensive Cancer Network 2015). Five-year survival in HIV-uninfected patients improved from 19% to 30% over the ten years from 1992–94 and 2004–06, probably reflecting improvements in treatment and falling within the range reported by recent clinical studies (22–56%) (Ferreri 2011). In spite of this progress, outcomes for PCNSL remain poor, and clinical trial enrolment is recommended when feasible (National Comprehensive Cancer Network 2015).
A major strength of this study is that it provides the first population-based estimates of PCNSL incidence among immunocompetent people (i.e., HIV-uninfected, non-transplant recipients). Unlike prior studies that utilized indirect measures to remove HIV-infected cases, we were able to directly remove these cases using HIV variables collected by registries. In addition, we were able to utilize data from the largest study of cancer in solid organ transplant recipients to estimate the number of PCNSLs in transplant recipients. A limitation of this study is that the HIV flag collected by the registries was missing in about half of all PCNSLs. Previous studies, albeit in single registry areas, reported that the HIV flag has a sensitivity of >90% (Clarke and Glaser 2004, Diamond, et al 2007), so we assumed that the flag was probably present when PCNSLs were HIV-infected. Additionally, we were unable to remove HIV-infected individuals and transplant recipients from the denominator of the immunocompetent incidence rates, because HIV was not a reportable condition in all registry areas for the full time period of interest. However, given the low prevalence of HIV and transplant recipients in the U.S. general population, incidence rates for immunocompetent PCNSL are only slightly underestimated (Shiels, et al 2013). Transplant recipients were included in the survival estimates in HIV-uninfected individuals but, given the uncommon occurrence of transplant-related PCNSL in this population, this should not have affected those estimates. Though we refer to the HIV-uninfected non-transplant recipient population as “immunocompetent,” we acknowledge that there are other immunosuppressed populations that were not excluded, such as bone marrow transplant recipients and individuals with congenital immunosuppression, though these individuals are unlikely to have had a large impact on the immunocompetent estimates presented here. In addition, we could not adjust for treatment in our survival estimates, as the ascertainment of chemotherapy treatment is not complete in cancer registries. Finally, our estimates may not be representative of the whole US; the SEER Program registries only cover 19% of the U.S. population and comprises a mixture of high- and low- HIV prevalence regions.
In conclusion, PCNSLs arising in HIV-infected people have had a profound impact on general population rates, particularly among young men. For the first time, we have shown that PCNSL rates among young immunocompetent individuals were stable overall during 1992–2011, but rates increased among the elderly. The cause of increasing rates is unknown, and studies on the aetiology and treatment of PCNSL in the elderly are needed. Despite improvements in survival over time, prognosis remains poor, and future clinical trials are needed in order to continue to make advances in PCNSL survival.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the National Cancer Institute. The authors report no conflicts of interest. MSS and EAE designed the study; EAE, CAC, LN and KP contributed to the acquisition of the data; MSS and RMP analysed the data; and MSS and EAE drafted the paper. All authors contributed to the interpretation of the data, critically revised the paper and approved of the submitted and final versions of the paper.
The authors gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (Monica Lin), the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California, Colorado (Jack Finch), Connecticut (Lou Gonsalves), Georgia (Rana Bayakly), Hawaii (Brenda Hernandez), Iowa (Charles Lynch), Illinois (Lori Koch), Michigan (Glenn Copeland), New Jersey (Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Texas, and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). We also thank analysts at Information Management Services for programming support (David Castenson, Matthew Chaloux, Michael Curry, Ruth Parsons).
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors.
The SRTR is currently operated under contract number HHSH250201000018C (Health Resources and Services Administration) by the Minneapolis Medical Research Foundation, Minneapolis, MN. During the initial period when registry linkages were performed, the SRTR was managed by Arbor Research Collaborative for Health in Ann Arbor, MI (contract HHSH234200537009C). The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201000024C), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (HSN261201000032C and N01-PC-35143), New Jersey (HHSN261201300021I, N01-PC-2013-00021), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN2612013000171). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP003875-01), Illinois (5U58DP003883-03), Maryland (U58DP12-1205 3919-03), Michigan (5U58DP003921-03), New Jersey (5U58/DP003931-02), New York (U58DP003879), North Carolina (U58DP000832) and Texas (5U58DP000824-04). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, Massachusetts (Massachusetts Cancer Prevention and Control Cooperative Agreement 5458DP003920), New Jersey (Memorandum of Agreement 5-100-046-4220-496-6140), New York (including the Cancer Surveillance Initiative), Texas, Utah, and Washington, as well as the University of Utah and Fred Hutchinson Cancer Research Center in Seattle, WA.
References
- Abrey LE. Primary central nervous system lymphoma. Curr Opin Neurol. 2009;22:675–680. doi: 10.1097/WCO.0b013e328332533b. [DOI] [PubMed] [Google Scholar]
- Bayraktar S, Bayraktar UD, Ramos JC, Stefanovic A, Lossos IS. Primary CNS lymphoma in HIV positive and negative patients: comparison of clinical characteristics, outcome and prognostic factors. J Neurooncol. 2011;101:257–265. doi: 10.1007/s11060-010-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell JF, Gross TG, Hanaway MJ, Trofe J, Roy-Chaudhury P, First MR, Woodle ES. Posttransplant lymphoproliferative disorder: significance of central nervous system involvement. Transplant Proc. 2005;37:954–955. doi: 10.1016/j.transproceed.2004.12.130. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV Surveillance Report, 2011. Vol. 23. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; Atlanta, GA: 2013. http://www.cdc.gov/hiv/pdf/statistics_2011_hiv_surveillance_report_vol_23.pdf. [Google Scholar]
- Chimienti E, Spina M, Vaccher E, Tirelli U. Management of immunocompetent patients with primary central nervous system lymphoma. Clin Lymphoma Myeloma. 2009;9:353–364. doi: 10.3816/CLM.2009.n.070. [DOI] [PubMed] [Google Scholar]
- Clarke CA, Glaser SL. Population-based surveillance of HIV-associated cancers: utility of cancer registry data. J Acquir Immune Defic Syndr. 2004;36:1083–1091. doi: 10.1097/00126334-200408150-00012. [DOI] [PubMed] [Google Scholar]
- Clarke CA, Morton LM, Lynch C, Pfeiffer RM, Hall EC, Gibson TM, Weisenburger DD, Martinez-Maza O, Hussain SK, Yang J, Chang ET, Engels EA. Risk of lymphoma subtypes after solid organ transplantation in the United States. Br J Cancer. 2013;109:280–288. doi: 10.1038/bjc.2013.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond C, Taylor TH, Im T, Wallace M, Saven A, Anton-Culver H. How valid is using cancer registries’ data to identify acquired immunodeficiency syndrome-related non-Hodgkin’s lymphoma? Cancer Causes Control. 2007:18. doi: 10.1007/s10552-006-0096-5. [DOI] [PubMed] [Google Scholar]
- Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, Biggar RJ. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, Grigg R, Hylton T, Pawlish KS, McNeel TS, Goedert JJ. Cancer risk in people infected with human immunodeficiency virus in the United States. International Journal of Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evens AM, Choquet S, Kroll-Desrosiers AR, Jagadeesh D, Smith SM, Morschhauser F, Leblond V, Roy R, Barton B, Gordon LI, Gandhi MK, Dierickx D, Schiff D, Habermann TM, Trappe R. Primary CNS posttransplant lymphoproliferative disease (PTLD): an international report of 84 cases in the modern era. Am J Transplant. 2013;13:1512–1522. doi: 10.1111/ajt.12211. [DOI] [PubMed] [Google Scholar]
- Ferreri AJ. How I treat primary CNS lymphoma. Blood. 2011;118:510–522. doi: 10.1182/blood-2011-03-321349. [DOI] [PubMed] [Google Scholar]
- Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, editors. International Classification of Diseases for Oncology. World Health Organization; Geneva: 2000. [Google Scholar]
- Gibson TM, Morton LM, Shiels MS, Clarke CA, Engels EA. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. AIDS. 2014;28:2313–2318. doi: 10.1097/QAD.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloghini A, Dolcetti R, Carbone A. Lymphomas occurring specifically in HIV-infected patients: from pathogenesis to pathology. Semin Cancer Biol. 2013;23:457–467. doi: 10.1016/j.semcancer.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Hoang-Xuan K, Bessell E, Bromberg J, Hottinger AF, Preusser M, Ruda R, Schlegel U, Siegal T, Soussain C, Abacioglu U, Cassoux N, Deckert M, Dirven CM, Ferreri AJ, Graus F, Henriksson R, Herrlinger U, Taphoorn M, Soffietti R, Weller M European Association for Neuro-Oncology Task Force on Primary CNS Lymphoma. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015;16:e322–332. doi: 10.1016/S1470-2045(15)00076-5. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. NCCN Guidelines Version 1.2015 Central Nervous System Cancers. National Comprehensive Cancer Network; Fort Washington: 2015. pp. 23–25. [DOI] [PubMed] [Google Scholar]
- Norden AD, Drappatz J, Wen PY, Claus EB. Survival among patients with primary central nervous system lymphoma, 1973–2004. J Neurooncol. 2011;101:487–493. doi: 10.1007/s11060-010-0269-7. [DOI] [PubMed] [Google Scholar]
- O’Neill BP, Decker PA, Tieu C, Cerhan JR. The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkin’s lymphoma. Am J Hematol. 2013;88:997–1000. doi: 10.1002/ajh.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JE, Janney CA, Rao RD, Cerhan JR, Kurtin PJ, Schiff D, Kaplan RS, O’Neill BP. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: a surveillance, epidemiology, and end results analysis. Cancer. 2002;95:1504–1510. doi: 10.1002/cncr.10851. [DOI] [PubMed] [Google Scholar]
- Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2012 Annual Data Report. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR), Department of Health and Human Services, Health Resources and Services Administration; Rockville, MD: 2014. [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2015. [Accessed 10 March 2016]. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- Shiels MS, Pfeiffer RM, Hall HI, Li J, Goedert JJ, Morton LM, Hartge P, Engels EA. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980–2007. JAMA. 2011;305:1450–1459. doi: 10.1001/jama.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Engels EA, Linet MS, Clarke CA, Li J, Hall HI, Hartge P, Morton LM. The epidemic of non-Hodgkin lymphoma in the United States: disentangling the effect of HIV, 1992–2009. Cancer Epidemiol Biomarkers Prev. 2013;22:1069–1078. doi: 10.1158/1055-9965.EPI-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveillance Epidemiology and End Results (SEER) Program. Incidence - SEER 13 Regs Research Data, Nov 2013 Sub (1992–2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total US, 1969–2012 Counties. National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; Rockville, MD: 2014. [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO Classification of Tumours of Haematopoietic andLymphoid Tissues. 4. International Agency for Research on Cancer Press; Lyon, France: 2008. [Google Scholar]
- Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105:1414–1418. doi: 10.1038/bjc.2011.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.