Abstract
Objectives
Deep brain stimulation (DBS) is used for a variety of movement disorders, including Parkinson's disease. There are several theories regarding the biology and mechanisms of action of DBS. Previously, we observed an up‐regulation of neural progenitor cell proliferation in post‐mortem tissue suggesting that DBS can influence cellular plasticity in regions beyond the site of stimulation. We wanted to support these observations and investigate the relationship if any, between DBS, neural progenitor cells, and microglia.
Methods
We used naïve rats in this study for DBS electrode implantation, stimulation, and microlesions. We used immunohistochemistry techniques for labeling microglial and progenitor cells, and fluorescence microscopy for viewing and quantification of labeled cells.
Results
We present data that demonstrates a reciprocal relationship of microglia and neural precursor cells in the presence of acute high frequency stimulation. In our hands, stimulated animals demonstrate significantly lower numbers of activated microglia (p = 0.026) when compared to microlesion and sham animals. The subthalamic region surrounding the DBS stimulating electrode reveals a significant increase in the number of neural precursor cells expressing cell cycle markers, plasticity and precursor cell markers (Ki67; p = 0.0013, MCM2; p = 0.0002).
Interpretation
We conclude that in this animal model, acute DBS results in modest local progenitor cell proliferation and influenced the total number of activated microglia. This could be of clinical significance in patients with PD, as it is thought to progress via neuroinflammatory processes involving microglia, cytokines, and the complement system. Further studies are required to comprehend the behavior of microglia in different activation states and their ability to regulate adult neurogenesis under physiologic and pathologic conditions.
Keywords: Deep brain stimulation, microglia, neural precursor cell, Parkinson's disease, plasticity
Introduction
Deep brain stimulation (DBS) is becoming increasingly prevalent for addressing medication refractory symptoms in some neurodegenerative and neuropsychiatric disorders. It consists of electrode implantation into specific parts of the brain and delivery of local current to influence a neural network 1, 2. Current targets for patients with Parkinson's disease (PD) are subthalamic nucleus (STN) globus pallidus internus and ventral intermediate nucleus of the thalamus. Although clinical efficacy and usage of DBS have been investigated by many studies, the biology and mechanism(s) of action, specifically in the context of microglial cells and the neural stem cell compartment are not understood. Our preliminary results from human post‐mortem studies of PD‐DBS revealed up‐regulation of neural progenitor cells (NPC) in the electrical stimulation field 3. Despite demonstration that certain physiologic stimuli, environments, and physical activity can stimulate neural precursor cell genesis 4, 5, 6, it is unclear what cellular and molecular components facilitate the necessary neuropermissive environment. It has been recently described that it is possible to increase cell proliferation in the substantia nigra of 6‐OHDA microlesioned rats under conditions of enriched environments (physical activity) 7, 8. However, this proliferative response alone cannot explain the efficacy of DBS in all disease contexts.
Microglia normally exist in the central nervous system (CNS) in a quiescent, resting or “ramified” state with a round cell body and thin processes, constantly monitoring the physiologic environment 9. In the event of an injury, microglia proliferate rapidly and undergo significant morphological alterations 10, 11, 12, 13, 14. Initially, microglial cell bodies enlarge, their processes become thicker and begin to retract 15. Microglia appear amoeboid as they progress toward becoming more phagocytic and pleiomorphic 16, 17. Inflammatory processes in the CNS involving activated microglia 18 are believed to play a role in neuronal cell death in PD 19, 20, 21, 22. Initial microglial reaction is reparative, however uncontrolled, it can lead to a release of pro‐inflammatory and toxic factors 23. Anti‐inflammatory drugs and radiation 24 have been shown to repress microglial activation and exert neuroprotective effects in the CNS following injury 24, 25, 26.
Microglia have become candidates for modulation of neurogenesis in the injured and healthy brain. Up‐regulation of microglia correlates to the production of neurotoxic factors, and inversely to neurogenesis 27. There is evidence to show that local environmental cues (including microglia and secreted proteins) influence the functionality of NPCs. In a recent study by Mosher et al. 2012 27, it was reported that microglia were preferentially more densely populated in the neurogenic niches, and were present in close proximity with NPCs. Therefore, endogenous microglia and NPCs are well‐posed to interact. A better comprehension of the type of cell communication in the neurogenic niche and the ability of NPCs to modulate their environment indicated that NPCs maybe regulated by microglia, and in turn were capable of regulating microglia.
In this study, we established a rodent model to investigate the effect of a microlesion vs. acute DBS on the interaction of microglia and NPCs in the context of electrical stimulation. We hypothesized that high frequency electrical stimulation (HFS) would activate molecular mechanisms decreasing the detrimental effects of activated microglia, perhaps through an up‐regulation of NPC proliferation 28, 29.
Material and Methods
All abbreviations used in the materials and methods are elaborated in Table 1.
Table 1.
List of Abbreviations Used.
| Abbreviation | Scientific name | Use/Definition |
|---|---|---|
| PBS | Phosphate buffered saline | Buffer solution |
| PFA | Paraformaldehyde | Used to |
| BrdU | Bromodeoxyuridine | Synthetic nucleoside, analog of thymidine used in the detection of proliferating cells in living tissues. |
| OCT | Optimal cutting temperature compound | This product is a formulation of water‐soluble glycols and resins and provides optimal conditions for cryostat sectioning at temperatures of −10 C (14 F) and below. |
| DAPI | (4′,6‐diamidino‐2‐phenylindole) | Fluorescent label that binds to DNA, used to cell staining and visualization via fluorescent microscopy. |
| MCM2 | Minichromosome maintenance complex | Part of a set of highly conserved proteins involved in the initiation of eukaryotic genome replication. |
| Ki67 | Also known as MKi67 | Nuclear protein associated with cell proliferation, and with rRNA transcription. Is active during all active phases of cell cycle. |
| Iba1 | Ionized calcium‐binding adapter molecule 1 | A calcium binding protein, specifically expressed in microglial cells. |
Experimental Animals
The University of Florida's Institutional Animal Care and Use Committee (IACUC) approved the study, and all procedures were in accordance with IACUC guidelines. Adult, male Sprague‐Dawley rats (Harlan Labs, 200–250 g) were used in these studies. In the final analysis, there were five rats in the stimulation group, four rats in the sham group, five rats in the microlesion group and five rats in the stimulation + BrdU group. Animals were housed with access to food and water ad libitum in a room, which was maintained at a constant temperature (20–22°C) on a 12 hours light‐dark cycle.
Surgical Procedures, Electrical Stimulation of the STN, and BrdU Administration
Rats were either unilaterally microlesioned, or implanted with stimulating electrodes. Animals were anesthetized using 5% isoflurane‐O2 mixture. Animals' heads were fixed to a stereotactic frame (Stoehling Instruments). Bregma was carefully delineated and marked. Bipolar stainless steel electrodes (Plastics One) (0.008 inch diameter; approximately 200 um) were implanted unilaterally in the STN in the following coordinates relative to bregma (AP −3.8 mm and ML +2.5 mm, DV −7.6 from dura), according to Paxinos and Watson (2005). In the stimulation group as well as the sham group, the electrode was secured using screws and dental acrylic. In the microlesion group, animals were not implanted with electrodes. The microlesion or microinjury was generated unilaterally using a sterile Hamilton needle (33 gauge, approximately 200 um) at the above stereotactic coordinates and withdrawn gently. Following surgeries, all animals were allowed to recover for two weeks. At the conclusion of experiments, the accuracy of electrode placement was confirmed histologically by standard Hemotoxylin & Eosin staining. The placement of the DBS lead was considered accurately targeted in the STN if the electrode tip could be confirmed by examination of post‐mortem sections.
High frequency electrical stimulation was performed after a two‐week recovery period at the following parameters: 130 Hz frequency, 50 usec pulse width, at an intensity of 50 uA for 1 hour daily, for two weeks postrecovery. The parameters have been described in the literature and were selected to be therapeutic but below the threshold intensity for contralateral forepaw dyskinesia 30. For HFS, animals were fixed to the stereotaxic frame and anesthetized. Stimulation was conducted using a World Precision Instruments Inc. stimulator, for one‐hour daily, for two weeks postrecovery. Microlesion and sham animals were also anesthetized, but not stimulated.
Bromodeoxyuridine (BrdU) (Sigma) was dissolved in saline and sterile filtered. Animals (n = 5) received a total of 50 mg/kg of BrdU intraperitoneally on the last three days of the stimulation paradigm.
Sacrifice
At the conclusion of the two‐week stimulation period and following the last BrdU injections, the animals were deeply anesthetized (pentobarbital, 60 mg/kg) and transcardially perfused with phosphate buffered saline (100 mL PBS), followed by 4% paraformaldehyde (100 mL PFA). The electrodes were carefully removed at this time, attempting to keep the tissue damage to a minimum, and the brains were removed from the skull and postfixed overnight in PFA and subsequently placed in 30% sucrose solution overnight in preparation for cryopreservation. Brains were then frozen in optimal cutting temperature (OCT) compound and stored at −80 C until analysis.
Immunohistochemistry, Microscopy, and Cell Counting
Frozen brains from all the groups were cut on a cryostat at 5 µm and mounted on SuperFrost Plus slides (Fisher).
Sections were immunostained with the following primary antibodies: MCM2 (Cell Signaling, 1:500), Ki67 (Novo Castra, 1:1000), Iba1 (Millipore, 1:500). Following block in serum for 1 hour, tissue was incubated in each of the antibodies overnight, at 4 C. Tween (0.1%) was added to the Tris buffer (0.1 M) during incubation to permeabilize cell membranes. Following primary incubations, sections were incubated in secondary antibodies (Alexa Fluor 488, or 594) for 1 hour. Slides were coverslipped using a Vectashield (Vector Labs) containing DAPI. A total of six sections of interest were analyzed per antibody using a Nikon fluorescent microscope. Only cells whose nuclei (stained with DAPI) were unambiguously associated with the given marker were scored.
For sections pre‐labeled with BrdU, slides were first incubated in HCl (1 N) for 30 min to break open the DNA to achieve a nuclear stain. The sections were then washed using borate buffer (0.1 M) three times. Following this, the sections were blocked in serum and treated with the antibody BrdU (Abcam, 1:100) as described above.
Microscopy and Statistical Analysis
Slides were viewed on a Nikon fluorescent microscope, equipped with a camera. The boundaries of the STN were delineated according to the Paxinos and Watson Rat Atlas (∼840 um thick). The caudal third ventricle (C3V) was defined as that tissue slice containing both the dorsal hippocampus (∼3.8–4.5 mm from bregma) and the third ventricle, following the Paxinos and Watson Rat Atlas. The total number of immunoreactive (IR) cells were estimated by using a 10 × 10 mm grid, superimposed on images taken at 20× magnification, using Adobe Photoshop®. Profile counts were done on every 25th section containing IR cells by an observer blinded to the animal treatment groups and to the identity of the sections (HK), and a total of six sections were counted per group. The contralateral side of the brain was used as control without treatment. Cell counts were obtained in a total of six sections on the contralateral (control) side as described above. For statistical analyses, Kruskal–Wallis, a nonparametric test was used.
Results
Microglial Phenotypes Around the Site of Electrode/Microlesion
In the region of the implanted stimulation electrodes in the STN, microglial morphology was studied using Iba1 immunolabeling. While both subtypes of microglia can be characterized by cytoplasmic staining, ramified microglia were clearly distinguishable from amoeboid microglia (Fig. 1a,c). The ramified cells have a smaller cell body when compared to the amoeboid cell type, which have a denser cell body. Furthermore, the ramified cells typically possess thick, radially projecting processes when compared to the activated cells, which have few to no processes.
Figure 1.
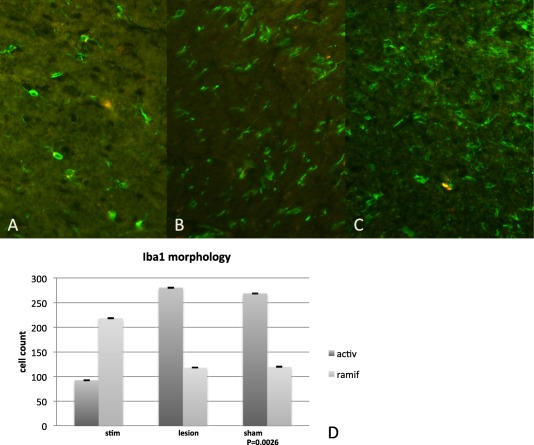
Morphology of microglia. a. The normal or “ramified/quiescent” state of microglia, with branching processes is showed. b. Shows a mildly activated state of microglia, where the processes are beginning to retract. c. Shows activated or “amoeboid” microglia, where processes are almost completely retracted and the cell bodies are round and macrophage‐like. d. Morphometric quantification of the number of Iba1 positive cells in the STN of HFS animals compared to microlesion and sham animals and is representative of the quantitative difference (p = 0.0026) between normal and activated microglia in animals that underwent HFS‐DBS and sham animals as well as animals that underwent microlesioning.
Activated microglia were identified around the site of the stimulation electrode and were readily recognized by their typical amoeboid morphology, with round cell bodies, with few to no ramifications or processes. This type of a morphological change from quiescent/ramified morphology to the amoeboid/macrophage‐type is typically seen in microglia after infection, traumatic injury and/or neurodegenerative disease. As we moved away from the site of stimulation (1 mm or more), the number of activated microglia dramatically decreased and there was a predominance of ramified or normal microglia (Fig. 1a–c). Quantitative analysis showed that in sham and microlesion‐only animals, there was an increase in the density of activated microglia, when compared to quiescent microglia (Fig. 1d). The number of Iba1 positive amoeboid cells was significantly greater in the sham and microlesion only groups (p = 0.0026) when compared to the stimulated group.
Cell Proliferation Around the Site of Electrical Stimulation in DBS Animals
BrdU
BrdU was administered to label dividing cells after the stimulation paradigm in a subset of animals after applying STN‐DBS at similar parameters to those used in patients (130 Hz, 2.5 V, 60 usec) for 1 hour everyday under a state of general anesthesia. These animals were subsequently sacrificed the last day after the BrdU injection paradigm. Animals that received HFS‐DBS showed an increase in BrdU‐positive cells in the region immediately surrounding the site of electrical stimulation (Fig. 2a) and in an increase in the third ventricle region (Fig. 2b), confirming our previous observations from post‐mortem human tissue 3. The maximum number of labeled cells was observed 5–7 days poststimulation.
Figure 2.
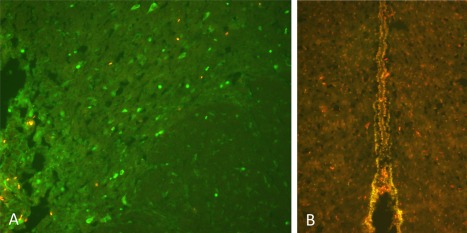
a. BrdU labeling of proliferative cells immediately surrounding the site of HFS‐DBS in the STN. b. Colabeling of Sox2 (green) and BrdU (red) positive cells around the third ventricle in STN‐HFS animals.
Quantitative Analysis of Proliferation Positive Cells in Post‐DBS Animals
MCM2
For purposes of quantifying changes in proliferative cells in DBS and non‐DBS animals, an experienced analyst (HK) who was blinded to the experimental groups evaluated the number of MCM2 positive cells around the site of the lead tip in stimulated, sham, as well as microlesion animals when compared to the contralateral (untreated) side (Fig. 3a–c). MCM2 was significantly increased (p = 0.0002) in sections from HFS‐DBS animals, indicating NPC proliferation as a result of electrical stimulation (Fig. 3d).
Figure 3.
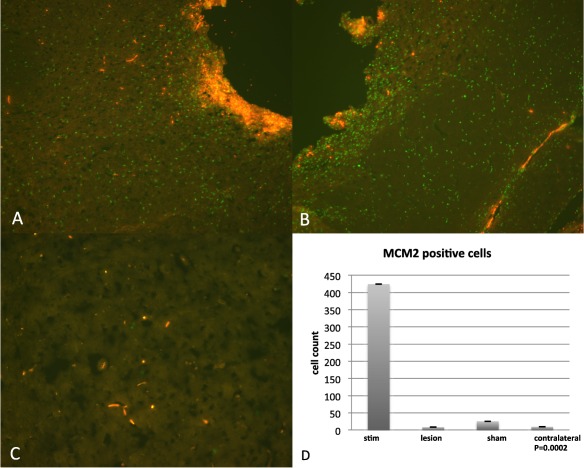
a,b. MCM2 positive (green) cells immediately surrounding electrode tip in STN‐HFS animals (orange is auto‐fluorescent blood). c. Contralateral side to HFS‐STN demonstrating almost no MCM2 positive (green) cells in the STN. d. Graph showing quantification of the number of MCM2 positive cells in the STN of HFS animals compared to microlesion, and sham animals. Also shown is STN in contralateral side of an HFS animal. Values in graph represent mean number of MCM2 positive cells per group of animals, p = 0.0002.
Correlation Between Amoeboid Microglia Density and Progenitor Cell Proliferation in STN
Ki67 and Iba1
To further confirm our findings and correlate the microglial phenotype to progenitor cell proliferation, we quantified proliferative cells (Iba1 positive; green, Ki67 positive; red) in the region immediately surrounding the site of electrical stimulation, sham or microlesion (Fig. 4a–c). The number of Ki67 positive cells was fewer in the sham and microlesion groups when compared to the stimulated group (p = 0.0013; Fig. 4d). Conversely, the number of activated microglial cells was greater in the sham and microlesion groups when compared to the stimulated group (from Fig. 1d).
Figure 4.
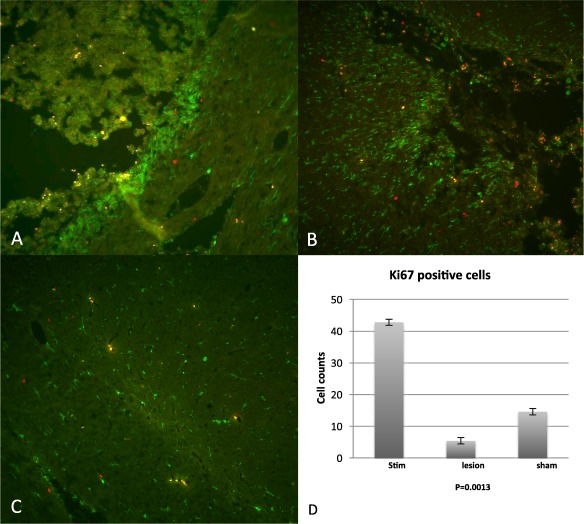
a,b. Ki67 (red) and Iba1 (green) labeled cells in two representative HF‐STN animals. Iba1 positive cells immediately surrounding the electrode site demonstrate amoeboid morphology, whereas immediately adjacent to the site of stimulation, cells demonstrate normal distribution and ramified morphology. c. Bottom panel shows normal, ramified Iba1 microglia in the contralateral STN from HFS animals. d. Graph representing the quantification of Ki67 positive cells in the STN in stimulated animals vs. microlesion and sham animals, clearly demonstrating a significant increase in Ki67 positive cells in the stimulated group alone, p = 0.0013.
Discussion
In the CNS, resident microglia comprise approximately 5–20% of glia. In their resting state, microglia are typically ramified and their fine processes intertwine into their surrounding cells. As “sentinels” of the brain, microglia are able to switch from resting to an activated state on injury. The resulting morphological change prepares them for phagocytosis, however, recent evidence indicates that microglia may also be involved in regulation of neurogenesis as well as in migration 31, 32. In the present study, we sought to delineate changes in microglial phenotypes in the presence and absence of high frequency electrical stimulation, and in the context of progenitor cell proliferation. Our findings show the presence of amoeboid and ramified microglia in the STN of stimulated and also in sham and microlesion animals. A significant increase in the density of amoeboid microglia was observed in the STN of the sham and microlesion only animals, and the presence of more progenitor cells in stimulated animals. These findings support the recent observation from human studies of the DBS Brain Tissue Network that there is an up‐regulation of progenitor cells around the DBS electrode, and around the third ventricle. Furthermore, our observations correlate with the recent demonstration in human postmortem PD tissue that there is an elevation of activated microglia in the STN when compared to HF‐DBS tissue 33. This study by Pienaar et al. presents the novel hypothesis that DBS might influence the neuroinflammatory response in PD patients 33. Pienaar et al. demonstrated that in human post‐mortem subjects, the number of activated microglia was reduced in the STN of the STN‐DBS cohort when compared to non PD DBS cases. Our study lends support to this finding. It has been previously reported that new glial cells can be generated in a rat lesion model (6‐OHDA) as a result of increased cellular plasticity in an enriched environment 34. Further, Steiner et al. showed that a unilateral ablative STN lesion can induce transient changes in the plasticity of the substantia nigra, by inducing the increase in proliferative cells, a proportion of which were identified as microglia 35. Another study in a rat model of stroke revealed that electrical stimulation of the relevant region (cortex) suppressed microglial activation, whereas in control animals there was rampant microglial activation as was confirmed by Iba1 staining 36. These findings further strengthen our current observations.
Our study shows that high frequency stimulation at clinically relevant parameters drives progenitor proliferation around the site of electrical stimulation and also suppresses microglial activation. While the activation state of microglia is likely to be regulated by numerous molecules, there is significant evidence accumulating that depending on their state of activation, microglia can either be supportive or detrimental for adult neurogenesis in the healthy, as well as the injured brain. Recent imaging studies have demonstrated that patients with idiopathic PD have an increase in neuroinflammation in the basal ganglia, striatum, and frontal and temporal cortical regions. This has been corroborated in post‐mortem tissue, where activated microglial cells are present surrounding dopaminergic neurons, which are impaired in the SN, suggesting neuroinflammation. It is hypothesized from these results that microglial activation likely occurs at an early stage of the disease either before (or in parallel with) the important loss of dopaminergic neurons 37, 38. There is now a preponderance of evidence in the literature suggesting that microglia, depending on their state of activation can either be beneficial or detrimental to adult neurogenesis, in both the diseased/injured and healthy brain 39. Therefore, it is relevant to tease out the differences in activation states of microglia and the regulation of adult neurogenesis in physiologic as well as in pathologic conditions. It is possible that the functional role of microglia in neurogenesis depends on the location of the microglial population, and hence the protein expression of these cells but this remains unknown 39. Early studies have provided evidence that inflammation and microglial activation can be detrimental for adult neurogenesis, however, recent experimental evidence shows their involvement to be complex and not necessarily detrimental 39. Whether the role and function of microglia in neurogenesis is dependent on differences in environmental cues and whether microglial function determines neurogenic potential are important future questions, which still need to be addressed. One must be cautious and distinguish between neurogenesis in the niche regions when compared to other parts of the adult CNS such as the SN or STN, where cellular plasticity, but not neurogenesis occurs. Further, our results have to be viewed in the context of the acute stimulation paradigm, which is significantly different than the constant stimulation that patients with PD DBS undergo. There is new enthusiasm in the field that various types of electrical stimulation (electroconvulsive therapy, transcranial magnetic stimulation, DBS, vagal nerve stimulation, epidural stimulation, and transcranial direct current stimulation) are effective for a variety of disorders. The reason for the success of this relatively new method of treatment has been attributed to the following potential mechanisms: alteration of cortical excitability 40, modulation of the brain inflammatory response 31, permeability of the blood‐brain barrier 41, brain perfusion and neuronal apoptosis 42, 43, and enhancement of neural plasticity 44. Neural plasticity could include synapse formation, dendritic architecture, and also neurogenesis 45, 46. Exogenous electrical fields have been shown to influence directional migration and differentiation of NPCs 47, 48 in vitro, and it is likely that these results are similar in in vivo systems, but the results and mechanisms are yet to be elucidated. While our observations support the concept of neural stimulation inducing changes in local cellular plasticity by driving NPC proliferation, the BrdU incorporating compartment could not be further characterized, likely because of the developmental stage of the cells (antigen markers of neural cells are not yet expressed). Further cell culture studies and time course models will likely resolve this problem. Furthermore, determining whether signaling via the Wnt/ß‐catenin pathway is responsible for the NPC proliferation was beyond the scope of this study.
In summary, we present a rat model that further supports observations from human subjects and expands into a comparison of electrical stimulation and microlesion therapy. We present animal data that reveal a reciprocal relationship of microglia and neural precursor cells (NPC) in the presence of acute high frequency stimulation (HFS). These observations add to our understanding of the biologic changes that co‐exist during DBS and microlesion therapy.
Authorship Statements
Dr. Vedam‐Mai designed, performed, supervised experiments conducted at University of Florida and prepared the figures. Dr. Vedam‐Mai also collated the data and composed the manuscript. Massoud Baradaran‐Shoraka performed the experiments pertaining to animal surgeries, immunohistochemistry, and prepared the figures. Dr. Reynolds provided scientific advice and resources for all experiments performed. Dr. Okun provided the conception of the paper, critical revisions, and overall supervision for this project. All authors approved the final version of the manuscript.
Comments
A previous study reported on proliferating cells in the brains of humans who were treated with Deep Brain Stimulation (DBS) (PMID: 24594681). In the present animal study the authors explored this observation in more detail and found that DBS may result “in modest local progenitor cell proliferation and influenced the total number of activated microglia” around the active DBS electrodes or ventricular wall. This is surprising given that independent postmortem brain tissue analysis of more than 40 human subjects who were treated with DBS did not report of any of these changes (PMID: 24947418 [a review] or 26011773). However, one has to keep an open mind and think of how these findings can turn into a better understanding of the basic mechanism(s) of DBS functioning and patient treatment.
Martin Kronenbuerger, MD
Baltimore, MD, USA
Comments not included in the Early View version of this paper.
Acknowledgements
Funding for this study came from the University of Florida Foundation and the NPF Center for Excellence at the University of Florida. Dr. Okun's DBS research was supported by NIH R01 NR14852.
Dr. Reynolds and Dr. Okun share senior authorship.
Conflicts of Interest: There are no conflicts of interest for Dr. Vedam‐Mai, Massoud Baradaran‐Shoraka and Dr. Reynolds. Dr. Okun reports grants from National Institutes of Health, Medtronic, Abbvie, ANS/St. Jude, Michael J. Fox Foundation, and grants from Bachmann‐Strauss Foundation, outside the submitted work.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
References
- 1. Vedam‐Mai V, van Battum EY, Kamphuis W et al. Deep brain stimulation and the role of astrocytes. Mol Psychiatry 2012;17:124–131, 115. doi: 10.1038/mp.2011.61. [DOI] [PubMed] [Google Scholar]
- 2. Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord 2002;17(Suppl. 3):S69–72. Epub 2002/04/12. [DOI] [PubMed] [Google Scholar]
- 3. Vedam‐Mai V, Gardner B, Okun MS et al. Increased precursor cell proliferation after deep brain stimulation for Parkinson's disease: a human study. PLoS One 2014;9:e88770. doi: 10.1371/journal.pone.0088770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 5. Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia 2004;46:41–52. doi: 10.1002/glia.10337. [DOI] [PubMed] [Google Scholar]
- 6. van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long‐term potentiation in mice. Proc Natl Acad Sci USA 1999;96:13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tajiri N, Yasuhara T, Shingo T et al. Exercise exerts neuroprotective effects on Parkinson's disease model of rats. Brain Res 2010;1310:200–207. doi: 10.1016/j.brainres.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 8. Anastasia A, Torre L, de Erausquin GA, Masco DH. Enriched environment protects the nigrostriatal dopaminergic system and induces astroglial reaction in the 6‐OHDA rat model of Parkinson's disease. J Neurochem 2009;109:755–765. doi: 10.1111/j.1471-4159.2009.06001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 10. Niquet J, Ben‐Ari Y, Represa A. Glial reaction after seizure induced hippocampal lesion: immunohistochemical characterization of proliferating glial cells. J Neurocytol 1994;23:641–656. [DOI] [PubMed] [Google Scholar]
- 11. Huttmann K, Sadgrove M, Wallraff A et al. Seizures preferentially stimulate proliferation of radial glia‐like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur J Neurosci 2003;18:2769–2778. [DOI] [PubMed] [Google Scholar]
- 12. Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20:269–287. [DOI] [PubMed] [Google Scholar]
- 13. Gehrmann J, Banati RB, Wiessner C, Hossmann KA, Kreutzberg GW. Reactive microglia in cerebral ischaemia: an early mediator of tissue damage? Neuropathol Appl Neurobiol 1995;21:277–289. [DOI] [PubMed] [Google Scholar]
- 14. Davalos D, Grutzendler J, Yang G et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 15. Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996;19:312–318. [DOI] [PubMed] [Google Scholar]
- 16. Thomas WE. Brain macrophages: evaluation of microglia and their functions. Brain Res Brain Res Rev 1992;17:61–74. [DOI] [PubMed] [Google Scholar]
- 17. Boche D, Perry VH, Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol 2013;39:3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- 18. Peters A, Josephson K, Vincent SL. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anat Rec 1991;229:384–398. doi: 10.1002/ar.1092290311. [DOI] [PubMed] [Google Scholar]
- 19. Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci USA 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borges K, Gearing M, McDermott DL et al. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol 2003;182:21–34. [DOI] [PubMed] [Google Scholar]
- 21. Ziv Y, Avidan H, Pluchino S, Martino G, Schwartz M. Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. Proc Natl Acad Sci USA 2006;103:13174–13179. doi: 10.1073/pnas.0603747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ziv Y, Ron N, Butovsky O et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 23. Blank T, Prinz M. Microglia as modulators of cognition and neuropsychiatric disorders. Glia 2013;61:62–70. doi: 10.1002/glia.22372. [DOI] [PubMed] [Google Scholar]
- 24. Wang N, Mi X, Gao B et al. Minocycline inhibits brain inflammation and attenuates spontaneous recurrent seizures following pilocarpine‐induced status epilepticus. Neuroscience 2015;287:144–56. doi: 10.1016/j.neuroscience.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 25. Li B, Mahmood A, Lu D et al. Simvastatin attenuates microglial cells and astrocyte activation and decreases interleukin‐1beta level after traumatic brain injury. Neurosurgery 2009;65:179–185; discussion 85–86. doi: 10.1227/01.NEU.0000346272.76537.DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hua K, Schindler MK, McQuail JA, Forbes ME, Riddle DR. Regionally distinct responses of microglia and glial progenitor cells to whole brain irradiation in adult and aging rats. PLoS One 2012;7:e52728. doi: 10.1371/journal.pone.0052728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mosher KI, Andres RH, Fukuhara T et al. Neural progenitor cells regulate microglia functions and activity. Nat Neurosci 2012;15:1485–1487. doi: 10.1038/nn.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song S, Song S, Cao C et al. Hippocampal neurogenesis and the brain repair response to brief stereotaxic insertion of a microneedle. Stem Cells Int 2013;2013:205878. doi: 10.1155/2013/205878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cicchetti F, Barker RA. The glial response to intracerebrally delivered therapies for neurodegenerative disorders: is this a critical issue? Front Pharmacol 2014;5:139. doi: 10.3389/fphar.2014.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spieles‐Engemann AL, Behbehani MM, Collier TJ et al. Stimulation of the rat subthalamic nucleus is neuroprotective following significant nigral dopamine neuron loss. Neurobiol Dis 2010;39:105–115. doi: 10.1016/j.nbd.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ekdahl CT. Microglial activation—tuning and pruning adult neurogenesis. Front Pharmacol 2012;3:41. doi: 10.3389/fphar.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pienaar IS, Lee CH, Elson JL et al. Deep‐brain stimulation associates with improved microvascular integrity in the subthalamic nucleus in Parkinson's disease. Neurobiol Dis 2015;74:392–405. doi: 10.1016/j.nbd.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 34. Steiner B, Winter C, Hosman K et al. Enriched environment induces cellular plasticity in the adult substantia nigra and improves motor behavior function in the 6‐OHDA rat model of Parkinson's disease. Exp Neurol 2006;199:291–300. doi: 10.1016/j.expneurol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 35. Steiner B, Kupsch A, Siebert E et al. Unilateral lesion of the subthalamic nucleus transiently provokes bilateral subacute glial cell proliferation in the adult rat substantia nigra. Neurosci Lett 2008;430:103–108. doi: 10.1016/j.neulet.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 36. Baba T, Kameda M, Yasuhara T et al. Electrical stimulation of the cerebral cortex exerts antiapoptotic, angiogenic, and anti‐inflammatory effects in ischemic stroke rats through phosphoinositide 3‐kinase/Akt signaling pathway. Stroke 2009;40:e598–605. doi: 10.1161/STROKEAHA.109.563627. [DOI] [PubMed] [Google Scholar]
- 37. Gerhard A, Pavese N, Hotton G et al. In vivo imaging of microglial activation with [11C](R)‐PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 38. McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Mov Disord 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 39. Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 40. Ludemann‐Podubecka J, Bosl K, Rothhardt S, Verheyden G, Nowak DA. Transcranial direct current stimulation for motor recovery of upper limb function after stroke. Neurosci Biobehav Rev 2014;47:245–259. doi: 10.1016/j.neubiorev.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 41. Levi H, Schoknecht K, Prager O et al. Stimulation of the sphenopalatine ganglion induces reperfusion and blood‐brain barrier protection in the photothrombotic stroke model. PLoS One 2012;7:e39636. doi: 10.1371/journal.pone.0039636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Borsody MK, Yamada C, Bielawski D et al. Effects of noninvasive facial nerve stimulation in the dog middle cerebral artery occlusion model of ischemic stroke. Stroke 2014;45:1102–1107. doi: 10.1161/STROKEAHA.113.003243. [DOI] [PubMed] [Google Scholar]
- 43. Wang J, Dong WW, Zhang WH, Zheng J, Wang X. Electrical stimulation of cerebellar fastigial nucleus: mechanism of neuroprotection and prospects for clinical application against cerebral ischemia. CNS Neurosci Ther 2014;20:710–716. doi: 10.1111/cns.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boggio PS, Valasek CA, Campanha C et al. Non‐invasive brain stimulation to assess and modulate neuroplasticity in Alzheimer's disease. Neuropsychol Rehabil 2011;21:703–716. doi: 10.1080/09602011.2011.617943. [DOI] [PubMed] [Google Scholar]
- 45. Lendvai B, Stern EA, Chen B, Svoboda K. Experience‐dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- 46. Nithianantharajah J, Hannan AJ. Enriched environments, experience‐dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 47. Huang YJ, Wu HC, Tai NH, Wang TW. Carbon nanotube rope with electrical stimulation promotes the differentiation and maturity of neural stem cells. Small 2012;8:2869–2877. doi: 10.1002/smll.201200715. [DOI] [PubMed] [Google Scholar]
- 48. Huang Y, Li Y, Chen J, Zhou H, Tan S. Electrical stimulation elicits neural stem cells activation: new perspectives in CNS repair. Front Hum Neurosci 2015;9:586. doi: 10.3389/fnhum.2015.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]


