Abstract
Background
Chronic intermittent ethanol vapor exposure (CIEV) has been used extensively to produce rodent models of alcohol dependence, but unlike other models of alcohol abuse, CIEV has not been assessed as a model of end-organ damage. The purpose of this study was to characterize the effects of CIEV on peripheral organ systems affected by alcohol abuse, including the liver, lungs and cardiovascular system.
Methods
Adult male Sprague-Dawley rats were exposed to daily CIEV for a period of 8 weeks (14HR ON/10HR OFF), producing blood alcohol levels of ~200 mg/dl. Controls were exposed to room air. After 8-weeks, echocardiography was performed to assess cardiac function. Indices of liver injury (alanine and aspartate aminotransferases (ALT and AST); cytochrome p450 2E1 (CYP2E1); alcohol dehydrogenase (ADH); Oil Red-O and triglyceride content; lipid peroxidation; inflammatory cytokine expression and macrophage infiltration), and lung inflammatory cell count, proinflammatory cytokine expression, and lipid peroxidation were measured.
Results
Left ventricular posterior wall thickness was significantly decreased, and systolic blood pressure was significantly elevated by CIEV compared to air controls. CIEV led to a significant increase in plasma ALT and triglycerides compared to room air controls. CIEV did not affect liver triglyceride content, lipid staining or peroxidation, but increased CYP2E1 and CCL2 protein expression, while decreasing ADH expression. CIEV significantly increased numbers of both PMNs and lymphocytes in the BALF, indicative of pulmonary inflammation. However, CIEV did not produce significant changes in lung mass, pulmonary lipid peroxidation, inflammatory cytokine expression, or edema.
Conclusions
These results show that CIEV produces hepatic, pulmonary and cardiovascular effects in rats similar to those found in other models of chronic alcohol administration. Alcohol vapor administration is a novel method of alcohol-induced tissue injury with high potential for widespread use in alcohol toxicology research.
Keywords: alcohol abuse, alcohol vapor, alcohol administration, liver disease, cardiovascular disease
INTRODUCTION
Chronic alcohol consumption remains the most common and costly form of drug abuse with approximately 7 percent of the adult U.S. population fulfilling the diagnostic criteria for alcohol abuse and/or alcoholism (Hasin, Stinson, Ogburn, & Grant, 2007). Alcohol abuse has significant multi-systemic pathophysiological consequences (Molina et al., 2003). Alcohol abuse is a major risk factor for the development of liver, cardiovascular, and pulmonary diseases (Kaphalia, Boroumand, Hyunsu, Kaphalia, & Calhoun, 2014; Kawano, 2010; Romero et al., 2014; Szabo, 2015; Wang, Zakhari, & Jung, 2010). Alcoholic liver disease (ALD) affects a large percentage of alcohol abusers, and remains a significant problem in the United States and globally (Nassir & Ibdah, 2014). The pathogenesis of ALD includes early onset steatosis and hepatitis, which can progress into more severe injury characterized by fibrosis and cirrhosis (Park, Lee, & Lee, 2014). Alcohol consumption is also correlated with increased incidence of cardiovascular disease and hypertension, as well as dyslipidemia (Kawano, 2010; Tang et al., 2012).
Several methods of alcohol administration have been used extensively in rodent models to study mechanisms of alcoholic liver, lung, and cardiovascular diseaseincluding the Lieber-DeCarli liquid ethanol diet, Tsukamoto-French intragastric feeding model, ethanol in the drinking water, and more recently, the NIAAA model, which combines chronic-plus-binge alcohol feeding to more closely mimic the drinking patterns of patients that develop alcoholic hepatitis (Bertola, Mathews, Ki, Wang, & Gao, 2013; Crestani et al., 2014; Kaphalia et al., 2014; Mathews, Xu, Wang, Bertola, & Gao, 2014; Romero et al., 2014; Takahashi et al., 2012). Although these classic models have been able to successfully produce peripheral tissue injury, they have several drawbacks. These include the rodents’ natural aversion to alcohol in the diet, difficulty in achieving elevated blood alcohol levels (BALs) comparable to those seen in clinical studies, technical difficulties, expensive equipment, and the requirement for second hits in order to induce injury, such as unsaturated fat in the diet or carbon tetrachloride administration (Tsukamoto, Machida, Dynnyk, & Mkrtchyan, 2009). Vapor inhalation is a method of alcohol administration that has been used extensively to produce alcohol dependence in rats and mice (Gilpin, Richardson, Cole, & Koob, 2008; McCool & Chappell, 2015). Alcohol vapor inhalation offers several advantages to the alcohol researcher, including the circumvention of rodents’ natural aversion to alcohol, and the reliable achievement of consistently high BALs with relative ease. Although this method of alcohol administration reliably increases alcohol-drinking and anxiety-like behavioral, its effects on other target organs known to be susceptible to alcohol-induced tissue injury have not been evaluated. The goal of this study was to characterize the effects of CIEV on the liver, lungs and cardiovascular system, peripheral organ systems affected by alcohol abuse. We demonstrated that CIEV produces many of the expected alcohol-mediated pathophysiological changes involved in hepatic, pulmonary and cardiovascular disease (Kaphalia et al., 2014; Kawano, 2010; Romero et al., 2014; Wakabayashi, 2013). These pathophysiological effects were achieved in the absence of second hits often used with other methods of alcohol administration (Tsukamoto et al., 2009). We propose that in addition to its value in neurobehavioral and addiction research, the CIEV model is a powerful tool for the study of alcohol-mediated peripheral tissue injury.
MATERIALS AND METHODS
Animals
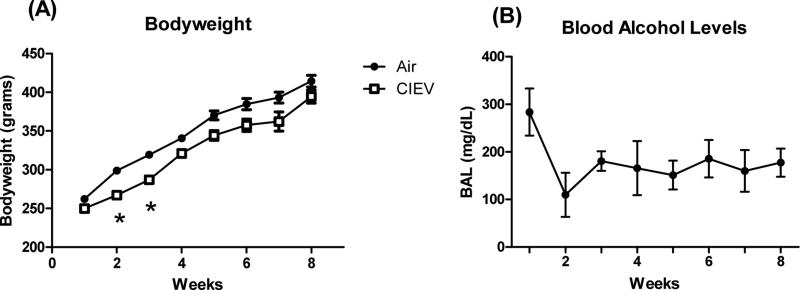
Adult male Sprague-Dawley rats weighing 200-250 grams were purchased from Harlan Laboratories (Indianapolis, IN). All experimental procedures were approved by Louisiana State University Health Sciences Center's Institutional Animal Care and Use Committee. Rats (n=10) were exposed to chronic intermittent ethanol vapor (CIEV) daily for 8 weeks (14HR ON/10HR OFF) in vapor chambers (La Jolla Alcohol Research, Inc., La Jolla, CA) to produce blood-alcohol levels (BALs) between 150-200 mg/dl. Initial alcohol vapor settings were determined from previous experiments, as described in Gilpin, et al., 2008. Each week, blood was collected and BAL analyzed (Analox Instruments, Huntington Beach, CA). Vapor settings were then adjusted to maintain stable BALs of 150-200mg/dl (Figure 1B). Control rats were exposed to ambient room air for the duration of the study (n=9). Rats in both groups were fed a standard chow diet. After 8 weeks of exposure, rats were weighed, and terminal procedures including blood pressure measurements, and tissue collection were performed. For lung wet/dry weight analysis, a section of lung (~100mg) was weighed, then dried for 24 hours at 60°C, and reweighed.
Figure 1. Bodyweight Progression and Blood Alcohol Levels (BALs).
(A) Bodyweight gain was lower at 2 and 3 weeks, but returned to Air control levels afterward. (B) BAL progression over the course of the 8 week study.
Echocardiography
Left ventricular (LV) function was assessed at the end of the 8 week study by echocardiography (VEVO 770, VisualSonics; Toronto, CA) under sedation (1-1.5% isoflurane). M-mode tracings of the LV short axis were obtained to determine LV end-diastolic and end-systolic (LVEDD and LVESD, respectively), anterior and posterior wall thickness, and heart rate. Fractional shortening (%FS) was calculated as %FS=(LVEDD-LVESD/LVEDD)*100.
Blood Pressure
At the end of the 8-week protocol, mean arterial blood pressure was determined by aortic catheterization. Rats were anesthetized with 2-2.5% isoflurane and ventilated. A catheter (Scisense; Ithaca, NY) was introduced into the right carotid artery and advanced into the aorta. Pressure signals were recorded using the Advantage PV System (Model FY897B Scisense, Ontario, CA). Data were acquired using an iWorx 308T data acquisition system with Labscribe software (iWorx; Dover, NH).
Plasma and Tissue Biochemical Assays
Blood samples were obtained prior to sacrifice, and plasma separated and stored for analysis. Plasma alanine and aspartate aminotransferase (ALT and AST) activity was determined by a commercial kit (Bio Scientific #3460-08, Austin, TX) according to the manufacturer's instructions. Plasma and hepatic triglycerides were determined using a colorimetric assay kit based on the enzymatic digestion of triglycerides to glycerol and free fatty acids by lipoprotein lipase (Cayman #10010303, Ann Arbor, MI). Lipid peroxidation in lung, liver, and LV tissues was assessed by measurement of thiobarbituric acid reactive substances (TBARS; Cayman #10009055; Ann Arbor, MI) according to the manufacturer's instructions.
Histology
Liver and lung were excised at necropsy. Livers were sectioned and samples were snap-frozen or fixed. Liver sections were fixed in 4% paraformaldehyde and lungs were perfusion-fixed through the main stem bronchus with Z-fix (Anatech Ltd.; Battle Creek, MI). Fixed liver and lung tissues were paraffin-embedded, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). Snap frozen livers were sectioned at 5 μm and stained with Oil Red O. Images were obtained with a microscope equipped with a digital color camera at 20X magnification (Olympus DP72, Center Valley, PA). For all images and image analyses, the data collectors were blinded to the study groups.
Immunohistochemistry
Liver sections were fixed in 4% paraformaldehyde, paraffin-embedded, and sectioned at 5 μm. Sectioned livers were cleared with xylene and dehydrated using an ethanol gradient. Endogenous peroxidase was blocked with a methanol peroxide solution. Antigen retrieval was performed using 10 mM citric acid (pH 6) in a steamer for 15 min. Tissues were blocked in 1% bovine serum albumin (BSA) and exposed to primary antibodies at a 1:100 dilution in 1% BSA for 1 hr at room temperature. Anti-HAM56 was used for the visualization of macrophages (#45018; Cambridge, UK). Following incubation with primary antibody, slides were stained with an ABC staining kit (#2023; Santa Cruz Biotechnology, Inc.; Dallas, TX).
Western Blot Analysis
Frozen liver sections (~50mg) were homogenized in RIPA buffer in the presence of a protease inhibitor cocktail (Bio-Rad; Hercules, CA). Protein concentration was determined by Bradford assay. Proteins (50 μg) were separated by SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes. Membranes were probed with primary antibodies diluted in 5% BSA against CYP2E1 (Abcam #19140; 1:1000 dilution), ADH (Abcam #108197), and β-actin (Santa Cruz #47778) overnight at 4 °C, and then goat-anti rabbit secondary antibody (Abcam #97051; 1:1000 dilution) for 2 hours at room temperature. Membranes were digitally imaged using a GelLogic 2200Pro machine (Carestream; Rochester, NY), and quantified with Carestream Molecular Imaging Software.
Lung Immune Cell Counts
Bronchoalveolar lavage was performed as previously described (Quinton et al., 2005). Lungs were excised and lavaged with 10 ml of PBS containing 1% dextrose. Bronchoalveolar lavage fluid (BALF) was collected, centrifuged, and the pellet was resuspended in phosphate-buffered saline. The cell suspension was diluted 1:1 with methylene blue in 3% acetic acid and counted on a hemocytometer. Total numbers of cells were calculated, along with the number of macrophages, lymphocytes, and polymorphonuclear neutrophils.
Real-Time PCR
Gene expression of inflammatory cytokines in lung and liver tissues was assessed by quantitative real-time PCR. RNA was extracted from lung and liver using RNeasy Mini-Kit (#74104; Qiagen; Hilden, Germany) and reverse transcribed into cDNA (#170-8841; Bio-Rad; Hercules, CA) for real-time analysis with SYBR green master mix (#172-5121; Bio-Rad; Hercules, CA). Primers for TNF-α, IL-1β, IL-6, and CCL2 were purchased from Integrated DNA Technologies (Coralville, IA). Gene expression was analyzed using the ΔΔCq method and normalized to RPS13 Cq values.
Statistics
All data are expressed as mean ± SEM. Differences between groups were determined by 1-way ANOVA. For bodyweight analysis, 2-way ANOVA was performed followed by repeated measures post-hoc analysis. A p-value of less than 0.05 was considered statistically significant. Data processing and statistical analyses were performed using Microsoft Excel and Graphpad 5.0 (Prism, San Diego, CA).
RESULTS
Effects of CIEV on Morphological Parameters
CIEV slowed bodyweight gain compared to air-exposed controls through the first 2 weeks, but later returned to control levels (Figure 1). CIEV significantly decreased LV mass (normalized to tibial length), but had no significant effects on right ventricular (LV and RV), lung, or liver mass, or lung wet/dry weight ratios (Table 1). There were no significant differences in tibial lengths between groups.
Table 1.
Morphological Parameters.
| Parameter | Air | CIEV |
|---|---|---|
| LV/Tibia (mg/mm) | 22.8±0.5 | 20.9±0.7* |
| RV/Tibia (mg/mm) | 8.9±0.4 | 9.3±0.5 |
| Liver/Tibia (mg/mm) | 321±10 | 316±13 |
| Lung/Tibia (mg/mm) | 43.0±2.0 | 40.2±1.1 |
| Lung Wet/Dry Weight | 4.9±0.6 | 5.3±0.8 |
CIEV decreased left ventricular (LV) mass compared to Air control. CIEV did not affect RV, liver, or lung weights or lung wet/dry weight ratios. There were no significant differences in tibial lengths between groups (not shown).
p<0.05.
CIEV elevated markers of liver injury
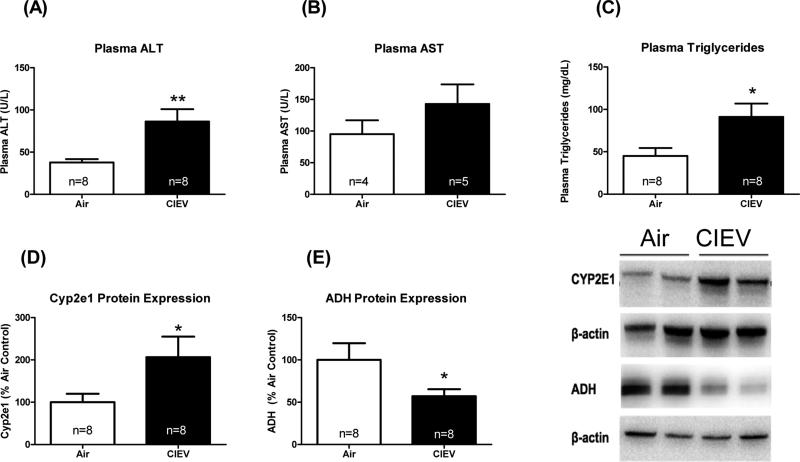
CIEV significantly increased plasma ALT activity (Figure 2A; p=0.003), an indicator of liver injury (Liu et. al. 2014), but did not significantly affect plasma AST activity (Figure 2B). CIEV produced a significant increase in plasma triglycerides (Figure 2C; p=0.02). CIEV also increased hepatic CYP2E1 protein expression (Figure 2D; p=0.04), and decreased ADH expression (Figure 2E; p=0.03).
Figure 2. CIEV elevated plasma ALT and triglycerides, and altered alcohol metabolizing enzymes.
CIEV elevated plasma ALT activity (A), but not AST activity (B). CIEV increased plasma triglycerides (C). Hepatic expression of CYP2E1 (D) was elevated in CIEV animals and expression of ADH was decreased (E). Values are mean±SEM of room air (open bars) and CIEV (solid bars) rats. *p<0.05.
CIEV increased markers of hepatic inflammation, but did not cause hepatic steatosis
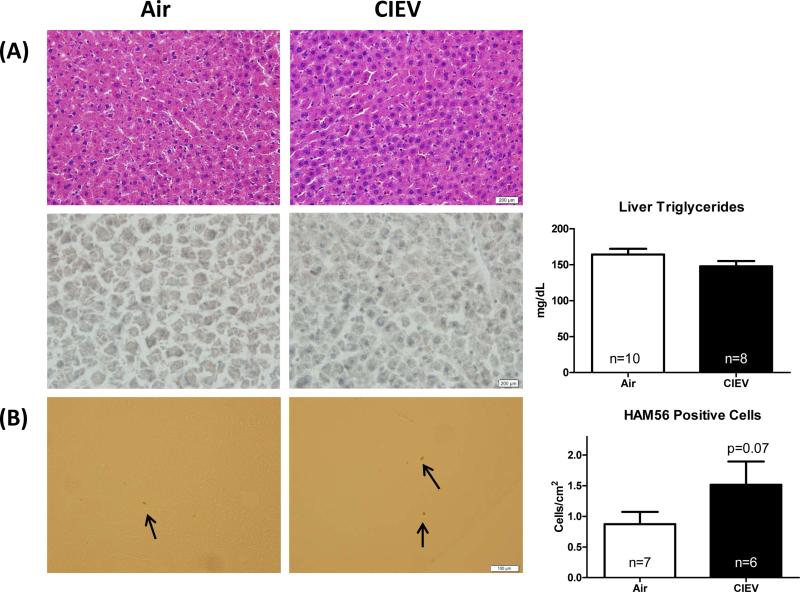
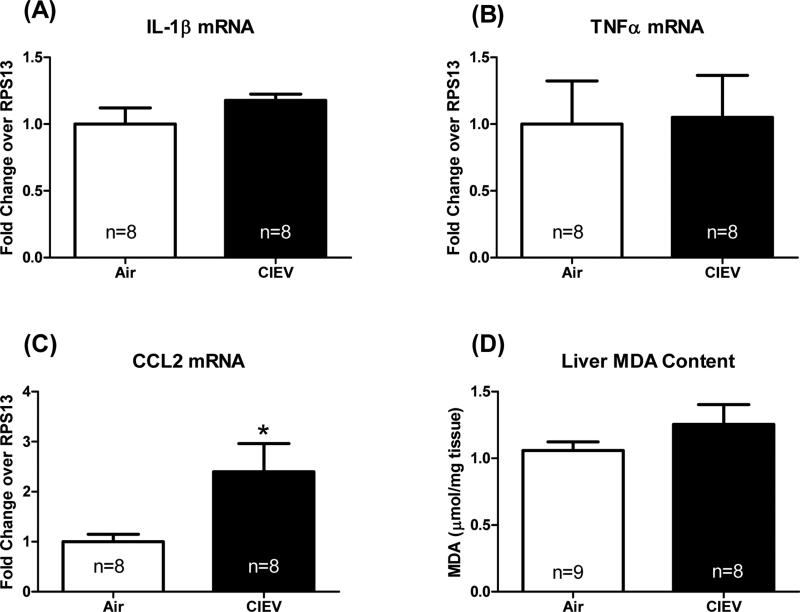
CIEV did not alter liver triglyceride content (Figure 3A). These results were confirmed using histological staining for H&E and Oil Red O (Figure 3A). CIEV caused an increase in hepatic macrophage infiltration (Figure 3B), however, this difference failed to reach statistical significance (p=0.07). While CIEV did not affect hepatic mRNA levels of inflammatory cytokines IL-1β or TNF-α (Figure 4A and 4B), it did increase CCL2 mRNA (Figure 4C; p=0.01). CIEV did not significantly increase lipid peroxidation as assessed by malondialdehyde (MDA) content (Figure 4D).
Figure 3. CIEV did not cause hepatic steatosis.
(A) Representative H&E and Oil Red O staining of liver sections from Air (left) and CIEV (right) animals (20x). CIEV did not affect liver triglyceride content. (B) Immunohistochemical staining for macrophages (anti-HAM56; 40x; arrows mark positive staining). CIEV did not significantly increase hepatic macrophage infiltration (p=0.07). Values are mean±SEM of room air (open bars) and CIEV (solid bars) animals.
Figure 4. CIEV increased hepatic CCL2 expression, but not lipid peroxidation.
CIEV did not affect hepatic IL-1β (A) or TNF-α (B) expression, but did significantly increase CCL2 expression (C). CIEV significantly increase hepatic lipid peroxidation (D). Values are mean±SEM of room air (open bars) and CIEV (solid bars) animals. *p<0.05.
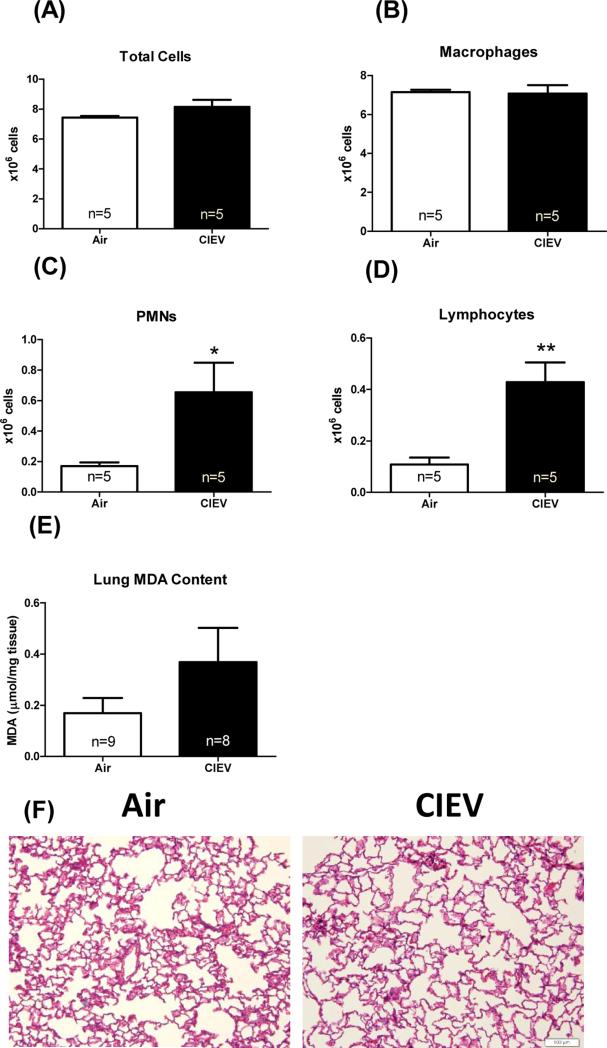
CIEV led to pulmonary inflammatory cell infiltration, but not significant injury
CIEV did not affect lung wet weights, wet/dry weight, (Table 1), or lipid peroxidation (Figure 5E). However, CIEV did cause significant increases in both PMNs (Figure 5C; p=0.01) and lymphocytes (Figure 5D;; p=0.002) in bronchoalveolar lavage fluid. Despite these increases in inflammatory cells, there were no differences in the mRNA expression of IL-6, CCL-2, IL-1β or TNF-α (data not shown).
Figure 5. CIEV caused pulmonary inflammatory cell infiltration, but not injury.
CIEV did not affect total cell (A) or macrophage count (B), but did significantly increase PMNs (C) and lymphocytes (D) in the BALF. CIEV did not significantly increase lipid peroxidation in the lung (E). Representative H&E staining of lung sections from Air control (left) and CIEV (right) animals (F).
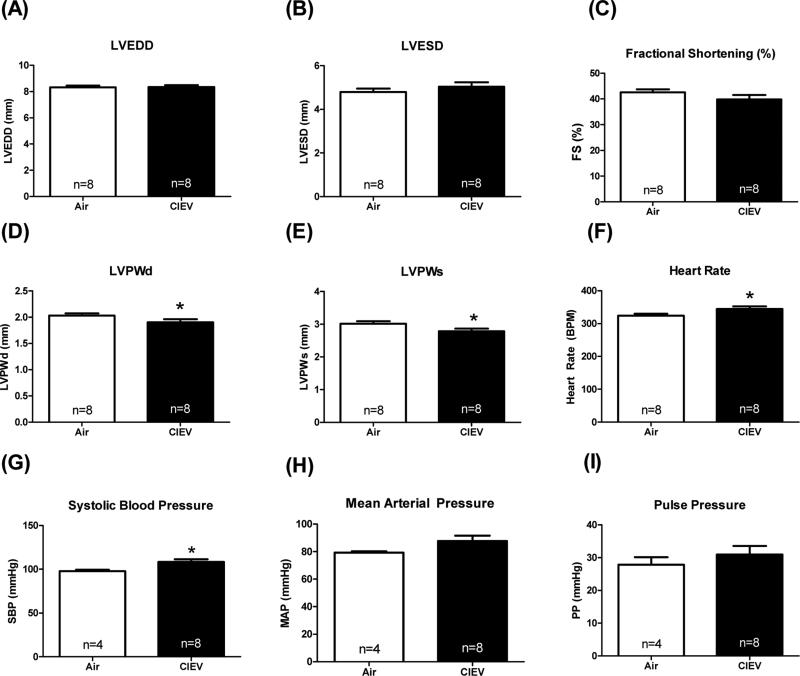
CIEV decreased LV wall thickness and increased systolic blood pressure
Finally, we determined the effects of CIEV on LV function using echocardiography. CIEV did not significantly alter LV chamber dilation (LVEDD and LVESD, Figure 6A and 6B) or systolic function (%fractional shortening, Figure 6C). However, CIEV did significantly reduce posterior wall thickness at diastole (Figure 6D; LVPWd; p=0.047) and systole (Figure 6E; LVPWs; p=0.03), and increased heart rate (Figure 6F; p=0.03). Prolonged alcohol exposure has been shown to produce hypertension in rats, so we assessed blood pressure in each group (Crestani et al., 2014). Our results showed that CIEV increased systolic blood pressure (Figure 6G; p=0.02), but did not significantly increase mean arterial pressure (p=0.08) or pulse pressure (Figure 6H and 6I).
Figure 6. CIEV reduced cardiac wall thickness and increased heart rate.
CIEV did not significantly alter left ventricular (LV) chamber dimension (A and B) or cardiac function (C), as assessed by ultrasound echocardiography. CIEV decreased LV wall thickness (D and E) and increased heart rate (F). Systolic blood pressure was increased by CIEV (G), whereas mean arterial pressure and pulse pressures were not significantly different than air-exposed controls (H and I; assessed by pressure catheterization). *p<0.05; LVEDD=LV end diastolic diameter; LVESD=LV end systolic diameter; FS=fractional shortening; PWd=posterior wall at diastole; PWs=posterior wall at systole; SBP=systolic blood pressure; MAP=mean arterial pressure; PP=pulse pressure.
DISCUSSION
Chronic intermittent ethanol vapor (CIEV) inhalation has been used extensively in neurobehavioral research to produce alcohol dependence and withdrawal symptoms (Becker, 2012; Gilpin et al., 2008; Macey, Schulteis, Heinrichs, & Koob, 1996). The effect of CIEV on other tissues has not been thoroughly investigated. The present study shows strong evidence that this route of ethanol exposure results in relevant alcohol-induced organ injury in the liver, lungs, and cardiovascular system.
The most well characterized organ system in terms of alcohol abuse is the liver. The pathogenesis of alcoholic liver disease (ALD) is characterized by early onset steatosis and hepatitis (Orman, Odena, & Bataller, 2013). Steatosis is characterized by the deposition of excess lipids in hepatocytes. In this study, we did not detect significant changes in liver triglycerides due to CIEV. This may be due to the lack of an added insult, such as a high-fat diet, and/or due to the relatively short duration of CIEV exposure. The second key feature in early onset ALD is hepatitis, or inflammation of the liver, which is characterized by increases in hepatic leukocytes, such as macrophages. Although not statistically significant, we did observe a trend toward greater hepatic macrophage infiltration in CIEV animals. It is possible that if the duration of CIEV was increased, significant macrophage infiltration could occur. Furthermore, CIEV increased hepatic CCL2 expression, which has been associated with alcoholic liver disease severity in clinical populations (Degre et al., 2012). CCL2 (or MCP-1) is a chemokine expressed by infiltrating inflammatory cells, hepatocytes, and stellate cells (Degre et al., 2012). CIEV increased hepatic CYP2E1 expression, a hallmark of alcohol-induced liver injury (Leung & Nieto, 2013). CYP2E1 is an inducible enzyme belonging to the cytochrome p450 family, and has been heavily implicated in ALD. With prolonged alcohol abuse, CYP2E1 plays a major role in hepatic alcohol metabolism. The enzymatic activity of CYP2E1 produces a large amount of reactive oxygen species as byproducts, which are responsible for a large majority of the oxidative stress and tissue injury observed in ALD. Concomitant with this increase in CYP2E1 expression, our results also showed that alcohol vapor decreased expression of ADH, the major hepatic alcohol metabolizing enzyme. These results are similar to those found in mice on a high-fat diet orally administered ethanol for 6 weeks. In these animals, ethanol administration increased hepatic CYP2E1 activity and decreased ADH activity (Lee et al., 2013). Studies on liver samples from human subjects suggest that decreases in hepatic ADH activity due to alcohol consumption are an indirect consequence of alcohol-induced liver damage (Vidal, Perez, Morancho, Pinto, & Richart, 1990). Our findings warrant further studies to assess the functional significance of this decrease in ADH expression.
Chronic heavy alcohol consumption is associated with increased prevalence of cardiovascular disease, including hypertension, dyslipidemia, and dilated cardiomyopathy (Kawano, 2010; Piano, 2002; Shen et al., 2014). Previous studies in our laboratory have shown that CIEV for 2 weeks increased left ventricular collagen expression, which was associated with an altered matrix-metalloproteinase (MMP) to tissue inhibitor of MMPs (TIMPs) ratio favoring pro-fibrotic signaling (El Hajj et al., 2014). Chronic alcohol diets have been shown to decrease cardiac mass, wall thickness, and function (Lang & Korzick, 2014; Steiner, Pruznak, Navaratnarajah, & Lang, 2015). Consistent with these studies, we found that CIEV decreased LV mass and wall thickness, increased heart rate, and increased systolic blood pressure. Elevated blood pressure is a major risk factor for the development of heart failure and myocardial infarction (Baradaran, Nasri, & Rafieian-Kopaei, 2014; Drazner, 2011). Our blood pressure measurements were performed in anesthetized rats, which is known to suppress cardiac function and blood pressurethus, our results may not fully reflect the extent of alcohol-induced changes in blood pressure. Even with the effects of anesthesia, systolic blood pressure was significantly increased in animals exposed to CIEV. Our results also demonstrate that 8 weeks of CIEV increased plasma triglycerides despite animals being in a nutritionally complete chow diet. Elevations in plasma triglycerides increase the risk of cardiovascular disease, especially coronary events (Stauffer, Weisenfluh, & Morrison, 2013). In addition, triglycerides, in the form of very low density lipoproteins (VLDL), promote atherogenesis in the vasculature through several mechanisms, including endothelial dysfunction, inflammation, and thrombosis (Tenenbaum, Klempfner, & Fisman, 2014). Taken together, the increase in blood pressure and the rise in circulating triglycerides suggest that CIEV may be used as a model to examine the mechanisms underlying alcohol-induced cardiovascular disease.
Because the lungs are the primary site of exposure in the CIEV model, we were interested in determining the effects of CIEV on lung injury, inflammation, and histology. Rats fed chronic Lieber-DeCarli diets show signs of pulmonary inflammation and oxidative stress (Kaphalia et al., 2014; Romero et al., 2014). CIEV significantly increased the infiltration of both PMNs and lymphocytes into the BALF, indicative of pulmonary inflammation. We also observed trends towards increased lipid peroxidation in lungs of CIEV animals, although these results were not statistically significant. We did not observe pulmonary edema or changes in total lung weights, suggesting that the effects found in the liver and cardiovascular system were not due to changes in pulmonary function. Whether lungs from CIEV exposed rats show alterations in the pulmonary circulation and/or impaired immune response to infectious challenges remains to be investigated.
In conclusion, our results show that CIEV, a method of alcohol administration typically used in neurobehavioral research, produces pathophysiological effects in the liver, lungs and cardiovascular system reflective of chronic alcohol-induced disease observed in both basic and clinical research. However, it is important to note the limitations of this approach. In terms of using the vapor model for research on alcoholic liver disease, it is important for the researcher to consider that alcohol vapor does not pass through the gastrointestinal tract, thus bypassing any effects of alcohol on the gut-liver axis (Szabo, 2015). With these limitations in mind, we propose that the CIEV model of alcohol exposure may be a useful preclinical alternative to standard ingestion models for studying the biomedical consequences of chronic alcohol abuse.
Acknowledgements
Funding provided by: NIH/NIAAA 1R21AA022690-01; NIH/NIAAA 5-T32-AA007577-14; NIH/NIAAA 110350146G; and a pilot grant from the LSUHSC Alcohol and Drug Abuse Center of Excellence.
Footnotes
Disclosures
NWG is a consultant for Glauser Life Sciences.
References
- Baradaran A, Nasri H, Rafieian-Kopaei M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J Res Med Sci. 2014;19(4):358–367. [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res. 2012;34(4):448–458. [PMC free article] [PubMed] [Google Scholar]
- Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc. 2013;8(3):627–637. doi: 10.1038/nprot.2013.032. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani CC, Lopes da Silva A, Scopinho AA, Ruginsk SG, Uchoa ET, Correa FM, Elias LL, Antunes-Rodrigues J, Resstel LB. Cardiovascular alterations at different stages of hypertension development during ethanol consumption: time-course of vascular and autonomic changes. Toxicol Appl Pharmacol. 2014;280(2):245–255. doi: 10.1016/j.taap.2014.08.012. doi: 10.1016/j.taap.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Degre D, Lemmers A, Gustot T, Ouziel R, Trepo E, Demetter P, Verset L, Quertinmont E, Vercruysse V, Le Moine O, Devière J, Moreno C. Hepatic expression of CCL2 in alcoholic liver disease is associated with disease severity and neutrophil infiltrates. Clin Exp Immunol. 2012;169(3):302–310. doi: 10.1111/j.1365-2249.2012.04609.x. doi: 10.1111/j.1365-2249.2012.04609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123(3):327–334. doi: 10.1161/CIRCULATIONAHA.108.845792. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- El Hajj EC, El Hajj MC, Voloshenyuk TG, Mouton AJ, Khoutorova E, Molina PE, Gilpin NW, Gardner JD. Alcohol modulation of cardiac matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs favors collagen accumulation. Alcohol Clin Exp Res. 2014;38(2):448–456. doi: 10.1111/acer.12239. doi: 10.1111/acer.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 2008 doi: 10.1002/0471142301.ns0929s44. Chapter 9, Unit 9 29. doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Kaphalia L, Boroumand N, Hyunsu J, Kaphalia BS, Calhoun WJ. Ethanol metabolism, oxidative stress, and endoplasmic reticulum stress responses in the lungs of hepatic alcohol dehydrogenase deficient deer mice after chronic ethanol feeding. Toxicol Appl Pharmacol. 2014;277(2):109–117. doi: 10.1016/j.taap.2014.02.018. doi: 10.1016/j.taap.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y. Physio-pathological effects of alcohol on the cardiovascular system: its role in hypertension and cardiovascular disease. Hypertens Res. 2010;33(3):181–191. doi: 10.1038/hr.2009.226. doi: 10.1038/hr.2009.226. [DOI] [PubMed] [Google Scholar]
- Lang CH, Korzick DH. Chronic alcohol consumption disrupts myocardial protein balance and function in aged, but not adult, female F344 rats. Am J Physiol Regul Integr Comp Physiol. 2014;306(1):R23–33. doi: 10.1152/ajpregu.00414.2013. doi: 10.1152/ajpregu.00414.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HI, McGregor RA, Choi MS, Seo KI, Jung UJ, Yeo J, Kim MJ, Lee MK. Low doses of curcumin protect alcohol-induced liver damage by modulation of the alcohol metabolic pathway, CYP2E1 and AMPK. Life Sci. 2013;93(18-19):693–699. doi: 10.1016/j.lfs.2013.09.014. doi: 10.1016/j.lfs.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. 2013;58(2):395–398. doi: 10.1016/j.jhep.2012.08.018. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13(2):163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Mathews S, Xu M, Wang H, Bertola A, Gao B. Animals models of gastrointestinal and liver diseases. Animal models of alcohol-induced liver disease: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;306(10):G819–823. doi: 10.1152/ajpgi.00041.2014. doi: 10.1152/ajpgi.00041.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Chronic intermittent ethanol inhalation increases ethanol self-administration in both C57BL/6J and DBA/2J mice. Alcohol. 2015;49(2):111–120. doi: 10.1016/j.alcohol.2015.01.003. doi: 10.1016/j.alcohol.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, Hoek JB, Nelson S, Guidot DM, Lang CH, Wands JR, Crawford JM. Mechanisms of alcohol-induced tissue injury. Alcohol Clin Exp Res. 2003;27(3):563–575. doi: 10.1097/01.ALC.0000057946.57330.F7. doi: 10.1097/01.ALC.0000057946.57330.F7. [DOI] [PubMed] [Google Scholar]
- Nassir F, Ibdah JA. Role of mitochondria in alcoholic liver disease. World J Gastroenterol. 2014;20(9):2136–2142. doi: 10.3748/wjg.v20.i9.2136. doi: 10.3748/wjg.v20.i9.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28(Suppl 1):77–84. doi: 10.1111/jgh.12030. doi: 10.1111/jgh.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BJ, Lee YJ, Lee HR. Chronic liver inflammation: clinical implications beyond alcoholic liver disease. World J Gastroenterol. 2014;20(9):2168–2175. doi: 10.3748/wjg.v20.i9.2168. doi: 10.3748/wjg.v20.i9.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano MR. Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest. 2002;121(5):1638–1650. doi: 10.1378/chest.121.5.1638. [DOI] [PubMed] [Google Scholar]
- Quinton LJ, Nelson S, Zhang P, Happel KI, Gamble L, Bagby GJ. Effects of systemic and local CXC chemokine administration on the ethanol-induced suppression of pulmonary neutrophil recruitment. Alcohol Clin Exp Res. 2005;29(7):1198–1205. doi: 10.1097/01.alc.0000171927.66130.aa. [DOI] [PubMed] [Google Scholar]
- Romero F, Shah D, Duong M, Stafstrom W, Hoek JB, Kallen CB, Lang CH, Summer R. Chronic alcohol ingestion in rats alters lung metabolism, promotes lipid accumulation, and impairs alveolar macrophage functions. Am J Respir Cell Mol Biol. 2014;51(6):840–849. doi: 10.1165/rcmb.2014-0127OC. doi: 10.1165/rcmb.2014-0127OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shen Z, Munker S, Wang C, Xu L, Ye H, Chen H, Xu G, Zhang H, Chen L, Yu C, Li Y. Association between alcohol intake, overweight, and serum lipid levels and the risk analysis associated with the development of dyslipidemia. J Clin Lipidol. 2014;8(3):273–278. doi: 10.1016/j.jacl.2014.02.003. doi: 10.1016/j.jacl.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Stauffer ME, Weisenfluh L, Morrison A. Association between triglycerides and cardiovascular events in primary populations: a meta-regression analysis and synthesis of evidence. Vasc Health Risk Manag. 2013;9:671–680. doi: 10.2147/VHRM.S52713. doi: 10.2147/VHRM.S52713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JL, Pruznak AM, Navaratnarajah M, Lang CH. Alcohol Differentially Alters Extracellular Matrix and Adhesion Molecule Expression in Skeletal Muscle and Heart. Alcohol Clin Exp Res. 2015;39(8):1330–1340. doi: 10.1111/acer.12771. doi: 10.1111/acer.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148(1):30–36. doi: 10.1053/j.gastro.2014.10.042. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Satake N, Yamashita H, Tamura A, Sasaki M, Matsui-Yuasa I, Tabuchi M, Akahoshi Y, Terada M, Kojima-Yuasa A. Ecklonia cava polyphenol protects the liver against ethanol-induced injury in rats. Biochim Biophys Acta. 2012;1820(7):978–988. doi: 10.1016/j.bbagen.2012.02.008. doi: 10.1016/j.bbagen.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Tang Y, Gao C, Xing M, Li Y, Zhu L, Wang D, Yang X, Liu L, Yao P. Quercetin prevents ethanol-induced dyslipidemia and mitochondrial oxidative damage. Food Chem Toxicol. 2012;50(5):1194–1200. doi: 10.1016/j.fct.2012.02.008. doi: 10.1016/j.fct.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Tenenbaum A, Klempfner R, Fisman EZ. Hypertriglyceridemia: a too long unfairly neglected major cardiovascular risk factor. Cardiovasc Diabetol. 2014;13:159. doi: 10.1186/s12933-014-0159-y. doi: 10.1186/s12933-014-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, Machida K, Dynnyk A, Mkrtchyan H. “Second hit” models of alcoholic liver disease. Semin Liver Dis. 2009;29(2):178–187. doi: 10.1055/s-0029-1214373. doi: 10.1055/s-0029-1214373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal F, Perez J, Morancho J, Pinto B, Richart C. Hepatic alcohol dehydrogenase activity in alcoholic subjects with and without liver disease. Gut. 1990;31(6):707–711. doi: 10.1136/gut.31.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi I. Alcohol intake and triglycerides/high-density lipoprotein cholesterol ratio in men with hypertension. Am J Hypertens. 2013;26(7):888–895. doi: 10.1093/ajh/hpt033. doi: 10.1093/ajh/hpt033. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16(11):1304–1313. doi: 10.3748/wjg.v16.i11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]