Abstract
Humanin is the first newly discovered peptide encoded in the mitochondrial genome in over three decades. It is the first member of a novel class of mitochondrial derived peptides. This small, 24 amino acid peptide was initially discovered to have neuroprotective effects and subsequent experiments have shown that it is beneficial in a diverse number of disease models including stroke, cardiovascular disease, and cancer. Over a decade ago, our lab found that humanin bound IGFBP-3 and more recent studies have found it to decrease circulating IGF-I levels. In turn, IGF-I also seems to regulate humanin levels and in this review, we cover the known interaction between humanin and IGF-I. Although the exact mechanism for how humanin and IGF-I regulate each other still needs to be elucidated, it is clear that humanin is a new player in IGF-I signaling.
Keywords: Humanin, IGF-I, Growth Hormone, Mitochondria, Neuroprotection, Peptide
1. Introduction
Humanin (HN) is the first member of a new class of small signaling peptides that originate from the mitochondrial genome. First discovered as a neuroprotective molecule that protected neurons against amyloid-beta toxicity, it has additionally been shown to have anti-apoptotic and cytoprotective properties. Furthermore, HN is regulated by both insulin-like growth factor-I (IGF-I) and growth hormone (GH) and conversely HN has been shown to regulate IGF-I, possibly through its ability to bind insulin-like growth factor-binding protein 3 (IGFBP-3). In this review we discuss this newly emerging field of mitochondrial derived peptides and humanin’s biological role with a special focus on the interaction between humanin, IGF-I, and growth hormone.
2. The discovery of humanin
Small open reading frames (sORFs) and alternative open reading frames (altORFs) are ubiquitously present in all genomes, but their biological functions have not been thoroughly studied [1,2]. The translation of some of them have been confirmed [3], however, the coding capacity of the mitochondrial sORFs has long been overlooked. Previously, it was commonly accepted that the human mitochondrial DNA (mtDNA) was an extremely compact genome that only encoded 13 essential components of the electron transport chain and 24 structural RNAs required for their translation [4]. The mitochondrial genome displays exceptional economy of organization, with no introns and very few noncoding nucleotides between coding sequences [5]. Humanin is a sORF found in the mitochondrial genome about 10 years ago (Figure 1). The Nishimoto group identified the humanin cDNA sequence from a functional expression screen. Clones that protected cells from cell death induced by a mutant form of amyloid precursor protein (APP), a possible cause of Alzheimer’s disease, were compared and they all shared a 75 bp sORF. This sORF is identical to a region of the mitochondrial 16S rRNA and encodes a 24-amino acid peptide, which was called “humanin” in hopes that it would restore the humanity back to patients with Alzheimer’s disease [6,7]. Further experiments proved that the overexpression of humanin can suppress neuronal death caused by Familial Alzheimer’s Disease (FAD) proteins including APP, presenilin1 and 2 [6].
Figure 1. The human mitochondrial genome and the humanin ORF.
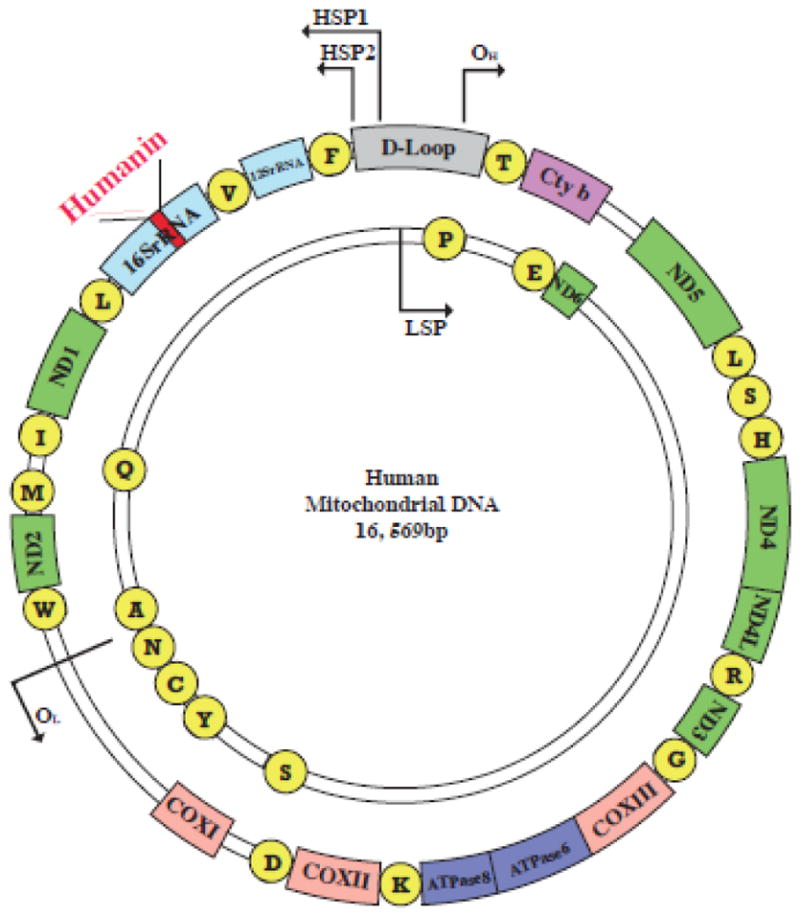
The human mitochondrial genome is compact (16,569 bp). Transcription can be initiated from one of two promoters (HSP1, HSP2) on the heavy strand or the single promoter (LSP) on the light strand. All of these promoters are located in the D-loop, a non-coding region acting as the control region. The genome encodes for 22 tRNAs (yellow circles), 2 rRNAs (dark blue) and 13 canonical protein-coding genes (green, NADH: ubiquinone oxidoreductase subunits 1–6; purple, cytochrome b; pink, cytochrome c oxidase subunits I–III; turquoise, ATPases 6 and 8). Humanin (red) sequence is within the 16S rRNA gene and transcribed from the heavy strand.
In a separate, independent screen by the Reed lab, they discovered the mechanism by which HN acts as an anti-apoptotic molecule [8]. HN was cloned as a Bax binding partner from a yeast two-hybrid screen and the interaction was confirmed by co-immunoprecipitation. Although other Bcl-2 family proteins such as Bcl-2 and Bcl-B share structural similarity with Bax, they did not interact with HN. Furthermore, HN could suppress apoptosis in a Bax-dependent way by preventing Bax translocation from cytosol to mitochondria, but apoptosis induced by Bax-independent stimulus such as necrosis induction was not suppressed by HN [8].
At almost the same time, a study by our group shed light on how HN, insulin-like growth factor, and apoptosis could be related. We cloned HN from another yeast two-hybrid screen when searching for IGFBP-3 interacting proteins [9]. The affinity and specificity of binding between the two molecules were determined both in vitro and in vivo. IGF-binding proteins are a group of proteins that regulate IGF-I bioavailability by acting as carriers. In particular, IGFBP-3, the most abundant one, accounts for 80% of all IGF binding and can participate in regulation of cell survival both dependently or independently of IGF-I [10]. The levels of IGFBP-3 are up-regulated by pro-apoptotic signals and IGFBP-3 has been shown to induce apoptosis [11]. On the other hand, IGF-I is known to have an anti-apoptotic effect and can protect against Aβ-induced neuronal death in an IGFBP-3 sensitive fashion, as 10nM IGFBP-3 is sufficient to completely abrogate IGF-I mediated protection [12]. Similar to IGF-I, HN is described as a circulating factor that promotes cell survival. HN administration attenuated hormone deprivation-induced apoptosis of testicular germ cells, whereas IGFBP-3 treatment had opposing effects [13]. HN specifically blocked IGFBP-3-induced cell death through its interaction with the C-terminal domain of IGFBP-3, but did not compete with IGFBP-IGF-I binding. Surprisingly, IGFBP-3 enhanced HN protection against Aβ1-43 toxicity in mouse primary neurons, suggesting that IGFBP-3 synergized with HN [9].
Humanin is the first protein encoding sORF found in the mitochondrial genome. This finding is important since it implies the existence of a group of novel mitochondrial derived peptides (MDPs) and we have published the discovery of another MDP, MOTS-c [14,15]. Moreover, HN itself can acts as a signal peptide sequence and is secreted out of the cell with HN levels being measurable in plasma, cerebrospinal fluid (CSF), and seminal fluid [16]. This secretory feature allows HN to perform a more diverse signaling function, and most importantly, allows HN to signal from the mitochondria to the cell to the rest of the organism.
3. The structure of humanin
Human HN is a 24- or 21-amino acid peptide depending on if the mRNA is translated in the cytoplasm or mitochondria, respectively. It is not yet clear where the exact site of translation is, but both synthesized forms of HN show anti-apoptotic effects [8]. A recent study from Parharkova et al. suggests that at least in rats, translation occurs within the mitochondria [17]. Because humanin is relatively short, systematic single amino acid substitution has been performed on each residue to study the importance of each amino acid (summarized in Figure 2). Residues 3-19 were found to be the “core domain” for HN’s neuro-protective ability [18]. Furthermore, this region may also directly bind to Aβ, thus preventing Aβ from self-aggregating [19]. Modification of the “core domain” yielded potent HN analogues: replacement of Ser14 with glycine gave rise to an enhanced form of HN (S14G/HNG) with increased potency over 1,000-fold [20]. Replacement of Ser14 by D-form serine also improved HN’s function [21]. With regards to the IGFBP-3 binding ability, Phe6 and Lys21 were identified as essential sites. Substitution of Phe6 by alanine completely negated the interaction between HN and IGFBP-3, whereas Lys21 to alanine conversion only prevented binding at lower concentrations of IGFBP-3 [9]. HNG-F6A, is another HN analogue with Ser14 to glycine and Phe6 to alanine modifications. As a non-IGFBP-3 binding peptide with more potent cyto-protective function, HNG-F6A has been shown to regulate insulin action in β-cells and stimulate glucose-stimulated insulin secretion [22]. Moreover, HNG-F6A treatment prevented endothelial dysfunction and atherosclerosis progression by down-regulating oxidative stress and apoptosis in the developing plaque [23]. The amino acid residues Leu9, Leu10, Leu11, Pro19 and Val20 were found to be critical for humanin secretion. Other HN analogues have also been characterized, including HN-C8P (abolished binding to Bax), HN-S7A (no self dimerization) and HN-L12A (HN antagonist) and these HN analogues are able to inhibit cyclophosphamide-induced germ cell apoptosis with the exception of HN-L12A [24].
Figure 2. Function of specific amino acid residues within humanin.
Mutational analysis has revealed critical amino acid residues for humanin actions. Important amino acids are marked with their function as noted in the key.
4. The mechanism of humanin action
HN interacts with multiple other proteins intracellularly to inhibit apoptosis. Bax and IGFBP-3 were the first two HN binding partners identified. It has long been established that HN can bind Bax and prevent Bax translocation to the mitochondria [8]. Recently, a study also demonstrated that HN, by interacting with IGFBP-3, interfered with the binding of importin-β1 to IGFBP-3 in vitro, thereby inhibiting IGFBP-3 dependent cell death [25]. BH3 interacting-domain death agonist (Bid) and its truncated form (tBid), as well as the extra long isoform of Bim (BimEL) were identified HN binding partners [26,27]. A V-set and transmembrane domain containing two like (VSTM2L) protein has been shown to co-localize with HN in distinct brain regions and primary neurons, where it acts as an antagonist of HN [28]. Actinin-4 and M-phase phosphoprotein 8 (MPP8) have also been identified as HN interacting proteins, however, the biological consequences of their interactions with HN have not been elucidated [29].
Since HN is a secretory protein, it participates in diverse extracellular signaling events in addition to its intracellular functions. Ying et al. demonstrated that both HN and Aβ42 activate the G protein-coupled formylpeptide-like-1 receptor (FPRL1) [30]. FPRs are known to be activated by bacterial or mitochondrial formyl peptides and activate MAP kinases (MAPKs). Both HN and Aβ42 were able to chemoattract monocytes and activate phagocytic cells [30]. Although Aβ42 induced neuronal death, HN prevented apoptosis potentially by competing with Aβ42 for binding to FPRL1. This finding was confirmed by an independent study searching for ligands for orphan G-coupled receptors. Using Chinese hamster ovary (CHO) cells transfected with the FPRL1 and FPRL2 receptors, it was found that HN directly interacts with these two receptors [31]. Nevertheless, Hashimoto et al. found that STAT3 phosphorylation, but not the FPRL1 receptor is required for HN’s ability to prevent cell apoptosis against neurotoxicity in F11 hybrid neuronal cells [32]. HN induced STAT3 phosphorylation was further shown to be important in pancreatic beta-cell survival and function [33]. A later study on chemotherapy-induced germ cell apoptosis also demonstrated involvement of STAT3 signaling in HN mediated protection [24]. In addition, the improvement on insulin sensitivity by HN is dependent on hypothalamic STAT3 stimulation [34]. Taken together, STAT3 activation is essential for many of the HN effects, suggesting that the HN receptor may belong to the cytokine receptor family. Consistent with this notion, Hashimoto et al. found that HN requires a tripartite cytokine-like receptor complex consists of the ciliary neurotrophic factor (CNTF) receptor, the IL-27 receptor WSX1, and glycoprotein (gp) 130 (IL6ST) to prevent neuronal loss [35]. Activation of this complex will up-regulate the Janus kinase (JAK) 2 and STAT-3 pathways. As for additional signaling pathways associated with HN, Akt-1 phosphorylation was enhanced in primary mouse neurons in response to HNG administration, indicating increased insulin/IGF-I signaling [36]. However, in mouse heart, an increase in phospho-AMPK was observed after HNG injection in vivo [37]. It is also reported that HN inhibits APP induced c-Jun N-terminal kinase (JNK) activation as an alternative strategy to rescue neurons from apoptosis [38].
5. The regulation of humanin
Being an essential cyto-protective factor, HN is up-regulated as a part of the stress response. For example, ApoE-deficient mice fed with a high-cholesterol diet had elevated levels of HN in the endothelial cells [23], but the underlying molecular mechanism is not understood. Pigmented villonodular synovitis (PVNS) is a joint disease associated with chronic inflammation and HN expression was increased markedly in mitochondria and siderosomes in cells derived from PVNS patients [39]. Muscles from patients with chronic progressive external ophthalmoplegia or mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS) also had increased HN expression compared to the muscles of those in control subjects [40]. The authors suggested that the induction of endogenous HN production in MELAS patients may be a compensatory mechanism for the defects in energy production in the affected muscle fibers; while further progressive defects may ultimately lead to degenerative ragged-red fibers. The hypothesis that up-regulation of HN is necessary to help cope with cellular stress was further supported by the findings of increased intracellular levels of HN after myocardial ischemia and reperfusion (MI-R) in mouse cardiac tissue [37]. Regarding the conditions that suppress HN expression, age is the primary factor that negatively influences the circulating HN levels in human and rodents. Additionally, rats have a significant loss of humanin in brain and skeletal muscle as they age [34]. These findings hinted that HN may be an anti-aging peptide. As for the degradation and recycling of HN, a tripartite motif protein TRIM11 was found to bind HN and down-regulate HN levels [41]. TRIM11 has a RING finger domain that functions as ubiquitin E3 ligase. Taken together, it is highly likely that TRIM11 targets HN for proteasomal pathway by ubiquitination.
6. The role of humanin in age-related pathologies
The HN ORF was discovered from the protected region of an AD brain, which inspired researchers to focus on the effects of HN on neurodegenerative diseases. Both in vivo and in vitro experiments supported the protective role of HN in AD-related pathology. It was demonstrated that HN could antagonize neurotoxicity induced by a wide range of FAD genes, including mutant APP, Presenilin (PS)1, PS2, and other Aβ peptides (Aβ 1-42 and Aβ 25-35) in vitro [6]. HN does not inhibit secretion of Aβ peptides but mediates its effects via activation of Jak2/STAT3 pro-survival pathway [32]. It stabilizes mitochondrial potential and prevents release of cytochrome c [42]. Moreover, HNG (a potent HN analogue) was shown to disaggregate Aβ fibrils in vitro [43]. As for in vivo evidence, intra-cerebro-ventricular injection prevented impairment of spatial working memory caused by Aβ25-35 [44]. In addition, intraperitoneal injection of HNG also ameliorated behavioral deficits induced by Aβ25-35 by reducing neuro-inflammation and apoptosis [45]. HN treatment also had beneficial effects in different AD mouse models, including APPswe/PS1dE9 mice, APPswe/tauP310L/PS-1M146V triple transgenic mice and Tg2576 mice [46–48]. The role of HN was also studied in other neurological diseases, such as stroke. HN treatment has been shown to reduce infarct volume, prevent neuronal cell death and improve neurological function in an ischemia and reperfusion (I/R) mouse model [49]. The neuro-protective effect of HN on I/R injury was confirmed by several independent studies [50,51].
Besides neurological disorders, cardiovascular disease (CVD) is another age-related disease that has become a major focus of HN research. HN treated mice demonstrated attenuated myocardial I/R injury, as shown by decreased infarct size and improved cardiac output [37]. Furthermore, lower HN levels are associated with coronary endothelial dysfunction [52]. Consistent with this observation, HN treatment prevented endothelial cell apoptosis caused by Ox-LDL-induced oxidative stress [53] and preserved endothelial function in ApoE deficient mice on a high cholesterol diet by enhancing eNOS activity [23].
7. The overlapping functions of IGF-I and humanin
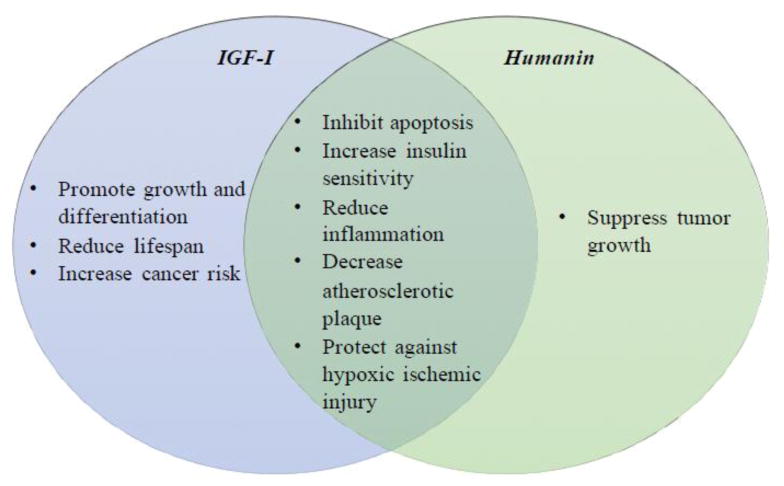
Humanin and IGF-I have both overlapping and distinct biological functions (Figure 3). First of all, like IGF-I, humanin is secreted from cells and is found in the plasma, and humanin expression is detected in multiple tissues including the skeletal muscle, liver, fat, and hypothalamus [34]. Furthermore, IGFBP-3 binds to both [9,54]1. Similar to the IGF-I signaling pathway, humanin activates multiple downstream signaling pathways including ERK1/2 and JAK2-STAT3 by binding to plasma membrane receptors [30,35]. In stroke models, both molecules have beneficial effects. In middle cerebral artery occlusion (MCAO) mice and oxygen-glucose deprivation in primary cortical neurons, humanin induces PI3K/Akt activation similar to IGF-I stimulation, which mediates humanin’s protective effect against hypoxia/ischemia reperfusion injury [50]. Similarly, IGF-I protects against hypoxic ischemic injury [55] and increased levels of bioavailable IGF-I in the CSF protected rat brains from ischemic insult [56]. Both molecules also inhibit neuronal apoptosis induced by familial Alzheimer’s Disease genes and similar to humanin, IGF-I was found to enhance brain Aβ clearance in rats and the Tg2576 mice [12] [47,48,57]. Both signaling pathways control cell death, metabolism, and transcription. Humanin has similar beneficial effects on metabolism and cell death as IGF-I. For example, IGF-I and humanin showed similar protective effects in ApoE-null mice fed with a high-fat diet. Both IGF-I and humanin decrease atherosclerotic plaque size, reduce vascular oxidative stress and apoptosis, and decrease vascular inflammatory responses by reducing the expression of proinflammatory cytokines [58,59]. Both also have cardioprotective effects in murine models of myocardial ischemia reperfusion as well [53,60] [23].
Figure 3.
The unique and overlapping functions of IGF-I and humanin
In contrast to those similar beneficial effects between IGF-I and humanin, the role of IGF-I and humanin in cancer seems to be opposite. Meta-regression analysis showed that high levels of circulating IGF-I are correlated with the increased risk of cancer, and the correlation varies between cancer sites [61]. In addition, IGF-I receptor expression and activation play a crucial role in cancer development [62]. On the other hand, HNG suppresses melanoma lung metastasis in mice, and HNG with a chemotherapeutic agent, cyclophosphamide, further suppresses the lung metastasis [63]. In addition, HNG alone and HNG with bortezomib showed suppression of tumor growth and an increase in apoptosis in mice with human neuroblastoma or medulloblastoma tumor xenografts [64]. These studies together not only reveal the potential of HN as an anti-cancer therapeutic, but also find HN to be protective against chemotherapy side-effects. HNG treatment also prevented chemotherapy-induced bone growth impairment and infertility [65]. The correlation between the level of circulating humanin and the development of cancer is still unclear. Even though humanin has multiple protective effects in physiological and pathological conditions, like IGF-I, the circulating humanin level declines with age in mice, rats, and humans [34,66]. Given the numerous functions of humanin and their overlap with IGF-I, we will discuss the functional interaction of humanin with IGF-I below.
8. Regulation of humanin by IGF-I
GH/IGF-I signaling has been well characterized in the aging process [67]. Emerging evidence suggests that mitochondrial factors including mtDNA and mitochondrial derived peptides could play a key role in regulation of aging and aging-related diseases. Although the regulation of endogenous humanin is still unclear, it has been reported that circulating humanin levels decline with age [34]. A recent paper from our lab explored the possible interactions of the GH/IGF-I signaling pathway and humanin in genetically modified mouse models in the GH/IGF-I axis and humans [68]. These mouse models have a unique GH and IGF-I profile, and their corresponding humanin levels suggest that IGF-I negatively regulates circulating humanin levels, but not GH per se. GH-transgenic mice (GH-Tg) have a 70% reduction in plasma humanin levels and increased levels of GH and IGF-I. They exhibit increased body size, various symptoms of premature aging, and a 50% decrease in lifespan compared to control [69]. In contrast, Ames dwarf (Prop-1df) mice have a 40% increase in plasma humanin levels and have nondetectable levels of GH and IGF-I. This strain has a decreased body size, delayed symptoms of aging, and an increased lifespan [70]. Liver-specific IGF-I deficient (LID) mice, which have a 45% increase in plasma humanin level, display elevated levels of GH and a 75% reduction of IGF-I and exhibit normal growth and lifespan [71]. IGFBP-3 knockout (BP3KO) mice, which showed a 70% decrease in plasma humanin levels, exhibit no change in GH and no difference in body weight, but show reduced lifespan and enhanced IGF-I activity due to an increase in the free IGF-I [72]. Direct treatment of rhGH and rhIGF-I in male C57BL/6 mice caused a reduction in humanin levels in plasma, further suggesting that GH inhibits humanin levels via IGF-I [68]. In humans, plasma samples from GH-deficient children who were being evaluated for their short stature had humanin levels that were negatively correlated to IGF-I levels [68]. Furthermore, plasma samples from an Ecuadorian cohort with GH receptor deficiency, very low levels of IGF-I, and extreme short stature showed that humanin levels were 80% elevated compared to normal matched Ecuadorian relatives [73]. These results suggest that humanin levels are directly down-regulated by IGF-I (Figure 4). Notably, humanin and IGF-I levels simultaneously decrease with age. This could be due to the age-dependent accumulation of mitochondrial DNA damage as well as the impairment of mitochondria quality control in mice and humans [74]. In contrast to the in vivo experiments, the regulation of humanin in response to IGF-I in vitro is different. According to another study where rat humanin levels were measured in testicular cells, the endogenous humanin level increased in response to GH and IGF-I in cultured Leydig cells from 10- and 40-day-old rats, but not from 60-day-old rats. This may suggest a developmental regulation of humanin in response to IGF-I [75]. In this study, humanin promoted the survival of Leydig cells in culture, and humanin cooperatively with IGF-I enhanced the rate of steroidogenesis by Leydig cells from 10- and 40-day-old rats, but not from 60-day-old rats [75]. Humanin’s effect on steroidogenesis may be due to the increased survival of IGF-I responding cells in the culture rather than an enhanced rate of steroidogenesis. Differential receptor expression patterns of these aged cells may also explain the differential action of humanin on these cells, but further studies are required to clarify this point of view.
Figure 4. Schematic diagram of GH, IGF-I, and Humanin regulation.
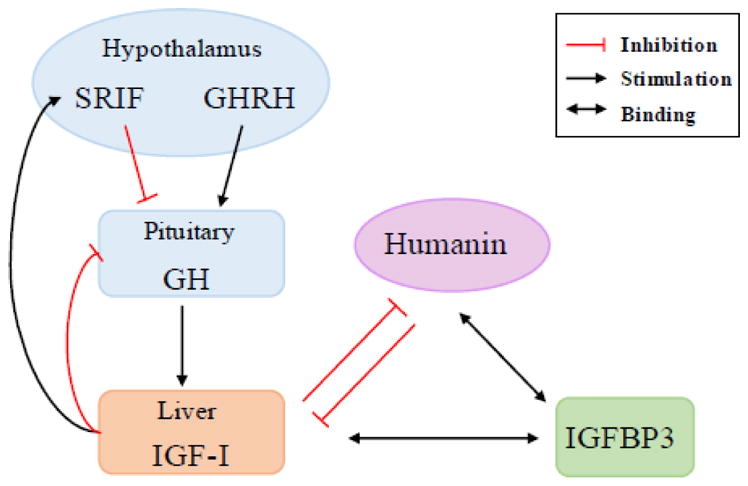
GHRH stimulates pituitary GH release which stimulates IGF-I production mainly in the liver. IGF-I forms negative feedback loop to GH release by stimulating SRIF, which inhibits pituitary GH release, and by inhibiting pituitary GH release directly. IGF-I suppresses humanin level in the plasma, and vice versa humanin suppresses circulating IGF-I level. Both IGF-I and humanin binds to IGFBP-3. Somatotropin release-inhibiting factor (SRIF); GH-releasing hormone (GHRH)
9. IGFBP-3 and humanin pharmacokinetics
HNGF6A is unique in that it does not bind to IGFBP-3 but retains humanin’s cytoprotective effects. A pharmacokinetic profile study of HNG and HNGF6A in IGFBP-3 knock out (IGFBP-3 KO) mice and their wild type littermates showed that circulating IGFBP-3 appeared to modulate HN levels, including peak levels and clearance rates of injected HN analogues. WT mice treated with either HNG or HNGF6A had a higher peak HN plasma level compared to the IGFBP-3 KO mice treated with HNG. Clearance of HN analogues was slower in the two groups characterized by absence of HN and IGFBP-3 interaction: WT mice injected with HNGF6A and IGFBP-3 KO mice injected with HNG, compared to WT mice given HNG [76]. These results suggest that HNG bound to IGFBP-3 resulted in a higher peak level, but more rapid clearance from plasma.
10. Regulation of IGF-I and IGFBP-3 levels by humanin
The effects of humanin analogues on plasma IGFBP-3 and IGF-I levels were examined in mice [76]. According to the study, HNG decreased the plasma IGFBP-3 levels over time, decreasing to 80% of baseline after 3hrs. In contrast, an IGFBP3-nonbinding analogue, HNGF6A, did not change the plasma IGFBP-3 levels. Similarly, plasma IGF-I levels were differentially regulated by HNG and HNGF6A. IGF-I levels decreased approximately 30% when HNG was administered whereas they remained unchanged by HNGF6A in wild type mice and also remained unchanged when HNG was injected in IGFBP-3 KO mice. These results support the view that humanin’s binding affinity to IGFBP-3 leads to the enhanced clearance and reduced circulating levels of IGF-I and IGFBP-3. As humanin does not inhibit the binding affinity between IGF-I and IGFBP-3, HN’s binding to IGFBP-3 may alter the stability of the ternary complexes [76] [54]. Furthermore, a study demonstrating that humanin enhances cyclophosphamide (CP)-induced suppression of lung melanoma metastases showed that administration of HNG with CP suppressed plasma IGF-I levels compared to CP alone [63]. These results suggest that the reduction of IGF-I mediated by humanin may cooperatively enhance the tumor suppressive effect of CP.
11. Conclusion
As the new field of mitochondrial derived peptides emerges, the role of these peptides as signaling molecules becomes more clear. Although the exact mechanism by which IGF-I regulates humanin and vice-versa occurs is still under investigation, future studies will determine these details. Humanin’s overlapping function with IGF-I as well as its apparent control by and control of IGF-I/GH suggests that humanin is a new emerging player in IGF-I/GH signaling.
Highlights.
Humanin is a new player in the regulation of the GH-IGF axis
IGFBP-3 interacts with Humanin to influence both IGF-I and Humanin bioavailability
Humanin is the first member of a novel class of peptides encoded within small open reading frames in the mitochondrial genome
Humanin has also been shown to have neuroprotective and cytoprotective effects
Humanin decreases circulating IGF-I levels in mouse studies, having a diet-restriction-mimetic effect
Acknowledgments
This review was supported by a Glenn Foundation Award and NIH grants to PC (1P01AG034906, 1R01GM 090311, 1R01ES 020812), and an Ellison/AFAR post-doctoral fellowship to KY and SJK.
Footnotes
Conflict of interest
Pinchas Cohen is a consultant and stockholder of Cohbar Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Basrai MA, Hieter P, Boeke JD. Small open reading frames: beautiful needles in the haystack. Genome Research. 1997 doi: 10.1101/gr.7.8.768. [DOI] [PubMed] [Google Scholar]
- 2.Vanderperre B, Lucier JF, Bissonnette C, Motard J, Tremblay G, Vanderperre S, et al. Direct detection of alternative open reading frames translation products in human significantly expands the proteome. PLoS ONE. 2013;8:e70698. doi: 10.1371/journal.pone.0070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews SJ, Rothnagel JA. Emerging evidence for functional peptides encoded by short open reading frames. Nat Rev Genet. 2014;15:193–204. doi: 10.1038/nrg3520. [DOI] [PubMed] [Google Scholar]
- 4.Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 5.Taanman J-W. The mitochondrial genome: structure, transcription, translation and replication. Biochimica Et Biophysica Acta (BBA) - Bioenergetics. 1999;1410:103–123. doi: 10.1016/S0005-2728(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc Natl Acad Sci USA. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto Y, Ito Y, Niikura T, Shao Z, Hata M, Oyama F, et al. Mechanisms of neuroprotection by a novel rescue factor humanin from Swedish mutant amyloid precursor protein. Biochem Biophys Res Commun. 2001;283:460–468. doi: 10.1006/bbrc.2001.4765. [DOI] [PubMed] [Google Scholar]
- 8.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- 9.Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, et al. Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci USA. 2003;100:13042–13047. doi: 10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firth SM, Baxter RC. Cellular Actions of the Insulin-Like Growth Factor Binding Proteins. Endocrine Reviews. 2013;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 11.Rajah R, Valentinis B, Cohen P. Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-beta1 on programmed cell death through a p53- and IGF-independent mechanism. 1997;272:12181–12188. doi: 10.1074/jbc.272.18.12181. [DOI] [PubMed] [Google Scholar]
- 12.Niikura T, Hashimoto Y, Okamoto T, Abe Y, Yasukawa T, Kawasumi M, et al. Insulin-like growth factor I (IGF-I) protects cells from apoptosis by Alzheimer’s V642I mutant amyloid precursor protein through IGF-I receptor in an IGF-binding protein-sensitive manner. J Neurosci. 2001;21:1902–1910. doi: 10.1523/JNEUROSCI.21-06-01902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lue Y, Swerdloff R, Liu Q, Mehta H, Hikim AS, Lee KW, et al. Opposing roles of insulin-like growth factor binding protein 3 and humanin in the regulation of testicular germ cell apoptosis. Endocrinology. 2010;151:350–357. doi: 10.1210/en.2009-0577. [DOI] [PubMed] [Google Scholar]
- 14.Yen K, Lee C, Mehta H, Cohen P. The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol. 2013;50:R11–9. doi: 10.1530/JME-12-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. The Mitochondrial-Derived Peptide MOTS-c Promotes Metabolic Homeostasis and Reduces Obesity and Insulin Resistance. Cell Metab. 2015;21:443–454. doi: 10.1016/j.cmet.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong Z, Tas E, Muzumdar R. Humanin and age-related diseases: a new link? Front Endocrinol (Lausanne) 2014;5:210. doi: 10.3389/fendo.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paharkova V, Alvarez G, Nakamura H, Cohen P, Lee K-W. Rat Humanin is encoded and translated in mitochondria and is localized to the mitochondrial compartment where it regulates ROS production. Molecular and Cellular Endocrinology. 2015 doi: 10.1016/j.mce.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Yamagishi Y, Hashimoto Y, Niikura T, Nishimoto I. Identification of essential amino acids in Humanin, a neuroprotective factor against Alzheimer’s disease-relevant insults. Peptides. 2003;24:585–595. doi: 10.1016/s0196-9781(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 19.Maftei M, Tian X, Manea M, Exner TE, Schwanzar D, von Arnim CAF, et al. Interaction structure of the complex between neuroprotective factor humanin and Alzheimer’s β-amyloid peptide revealed by affinity mass spectrometry and molecular modeling. J Pept Sci. 2012;18:373–382. doi: 10.1002/psc.2404. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto Y, Niikura T, Ito Y, Sudo H, Hata M, Arakawa E, et al. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer’s disease-relevant insults. J Neurosci. 2001;21:9235–9245. doi: 10.1523/JNEUROSCI.21-23-09235.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terashita K, Hashimoto Y, Niikura T, Tajima H, Yamagishi Y, Ishizaka M, et al. Two serine residues distinctly regulate the rescue function of Humanin, an inhibiting factor of Alzheimer’s disease-related neurotoxicity: functional potentiation by isomerization and dimerization. Journal of Neurochemistry. 2003;85:1521–1538. doi: 10.1046/j.1471-4159.2003.01797.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuliawat R, Klein L, Gong Z, Nicoletta-Gentile M, Nemkal A, Cui L, et al. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the β cell. Faseb J. 2013;27:4890–4898. doi: 10.1096/fj.13-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh YK, Bachar AR, Zacharias DG, Kim SG, Wan J, Cobb LJ, et al. Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice. Atherosclerosis. 2011;219:65–73. doi: 10.1016/j.atherosclerosis.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia Y, Ohanyan A, Lue YH, Swerdloff RS, Liu PY, Cohen P, et al. The effects of humanin and its analogues on male germ cell apoptosis induced by chemotherapeutic drugs. Apoptosis. 2015;20:551–561. doi: 10.1007/s10495-015-1105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Njomen E, Evans HG, Gedara SH, Heyl DL. Humanin Peptide Binds to Insulin-Like Growth Factor-Binding Protein 3 (IGFBP3) and Regulates Its Interaction with Importin-β. Protein Pept Lett. 2015;22:869–876. doi: 10.2174/0929866522666150728114955. [DOI] [PubMed] [Google Scholar]
- 26.Luciano F, Zhai D, Zhu X, Bailly-Maitre B, Ricci JE, Satterthwait AC, et al. Cytoprotective peptide humanin binds and inhibits proapoptotic Bcl-2/Bax family protein. BimEL. 2005;280:15825–15835. doi: 10.1074/jbc.M413062200. [DOI] [PubMed] [Google Scholar]
- 27.Zhai D, Luciano F, Zhu X, Guo B, Satterthwait AC, Reed JC. Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. 2005;280:15815–15824. doi: 10.1074/jbc.M411902200. [DOI] [PubMed] [Google Scholar]
- 28.Rossini L, Hashimoto Y, Suzuki H, Kurita M, Gianfriddo M, Scali C, et al. VSTM2L is a novel secreted antagonist of the neuroprotective peptide Humanin. Faseb J. 2011;25:1983–2000. doi: 10.1096/fj.10-163535. [DOI] [PubMed] [Google Scholar]
- 29.Maximov VV, Martynenko AV, Arman IP, Tarantul VZ. Humanin binds MPP8: mapping interaction sites of the peptide and protein. J Pept Sci. 2013;19:301–307. doi: 10.1002/psc.2500. [DOI] [PubMed] [Google Scholar]
- 30.Ying G, Iribarren P, Zhou Y, Gong W, Zhang N, Yu Z-X, et al. Humanin a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. Journal of Immunology. 2004;172:7078–7085. doi: 10.4049/jimmunol.172.11.7078. [DOI] [PubMed] [Google Scholar]
- 31.Harada M, Habata Y, Hosoya M, Nishi K, Fujii R, Kobayashi M, et al. N-Formylated humanin activates both formyl peptide receptor-like 1 and 2. Biochem Biophys Res Commun. 2004;324:255–261. doi: 10.1016/j.bbrc.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto YY, Suzuki HH, Aiso S, Niikura T, Nishimoto I, Matsuoka MM. Involvement of tyrosine kinases and STAT3 in Humanin-mediated neuroprotection. Life Sci. 2005;77:3092–3104. doi: 10.1016/j.lfs.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 33.Hoang PT, Park P, Cobb LJ, Paharkova-Vatchkova V, Hakimi M, Cohen P, et al. The neurosurvival factor Humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metab Clin Exp. 2010;59:343–349. doi: 10.1016/j.metabol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muzumdar RH, Huffman DM, Atzmon G, Buettner C, Cobb LJ, Fishman S, et al. Humanin: a novel central regulator of peripheral insulin action. PLoS ONE. 2009;4:e6334. doi: 10.1371/journal.pone.0006334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Molecular Biology of the Cell. 2009;20:2864–2873. doi: 10.1091/mbc.E09-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X, Chua CC, Gao J, Chua KW, Wang H, Hamdy RC, et al. Neuroprotective effect of humanin on cerebral ischemia/reperfusion injury is mediated by a PI3K/Akt pathway. Brain Research. 2008;1227:12–18. doi: 10.1016/j.brainres.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muzumdar RH, Huffman DM, Calvert JW, Jha S, Weinberg Y, Cui L, et al. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler Thromb Vasc Biol. 2010;30:1940–1948. doi: 10.1161/ATVBAHA.110.205997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashimoto Y, Tsuji O, Niikura T, Yamagishi Y, Ishizaka M, Kawasumi M, et al. Involvement of c-Jun N-terminal kinase in amyloid precursor protein-mediated neuronal cell death. Journal of Neurochemistry. 2003;84:864–877. doi: 10.1046/j.1471-4159.2003.01585.x. [DOI] [PubMed] [Google Scholar]
- 39.Ijiri K, Tsuruga H, Sakakima H, Tomita K, Taniguchi N, Shimoonoda K, et al. Increased expression of humanin peptide in diffuse-type pigmented villonodular synovitis: implication of its mitochondrial abnormality. Ann Rheum Dis. 2005;64:816–823. doi: 10.1136/ard.2004.025445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kariya S, Hirano M, Furiya Y, Sugie K, Ueno S. Humanin detected in skeletal muscles of MELAS patients: a possible new therapeutic agent. Acta Neuropathol. 2005;109:367–372. doi: 10.1007/s00401-004-0965-5. [DOI] [PubMed] [Google Scholar]
- 41.Niikura T, Hashimoto Y, Tajima H, Ishizaka M, Yamagishi Y, Kawasumi M, et al. A tripartite motif protein TRIM11 binds, destabilizes Humanin, a neuroprotective peptide against Alzheimer’s disease-relevant insults. Eur J Neurosci. 2003;17:1150–1158. doi: 10.1046/j.1460-9568.2003.02553.x. [DOI] [PubMed] [Google Scholar]
- 42.Jin H, Liu T, Wang WX, Xu JH, Yang PB, Lu HX, et al. Protective effects of [Gly14]-Humanin on beta-amyloid-induced PC12 cell death by preventing mitochondrial dysfunction. Neurochemistry International. 2010;56:417–423. doi: 10.1016/j.neuint.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Du Y, Bai M, Xi Y, Li Z, Miao J. S14G-humanin inhibits Aβ1-42 fibril formation, disaggregates preformed fibrils, and protects against Aβ-induced cytotoxicity in vitro. J Pept Sci. 2013 doi: 10.1002/psc.2484. [DOI] [PubMed] [Google Scholar]
- 44.Tajima H, Kawasumi M, Chiba T, Yamada M, Yamashita K, Nawa M, et al. A humanin derivative, S14G-HN, prevents amyloid-beta-induced memory impairment in mice. Journal of Neuroscience Research. 2005;79:714–723. doi: 10.1002/jnr.20391. [DOI] [PubMed] [Google Scholar]
- 45.Miao J, Zhang W, Li Z. 7, S14G-Humanin ameliorates Abeta25-35-induced behavioral deficits by reducing neuroinflammatory responses and apoptosis in mice. Neuropeptides. 2008;42:557–567. doi: 10.1016/j.npep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Zhang W, Li Z, Hao J, Zhang Z, Liu L, et al. S14G-humanin improves cognitive deficits and reduces amyloid pathology in the middle-aged APPswe/PS1dE9 mice. Pharmacology Biochemistry and Behavior. 2012;100:361–369. doi: 10.1016/j.pbb.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Niikura T, Sidahmed E, Hirata-Fukae C, Aisen PS, Matsuoka Y. A humanin derivative reduces amyloid beta accumulation and ameliorates memory deficit in triple transgenic mice. PLoS ONE. 2011;6:e16259. doi: 10.1371/journal.pone.0016259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park TY, Kim SH, Shin YC, Lee NH, Lee RKC, Shim JH, et al. Amelioration of neurodegenerative diseases by cell death-induced cytoplasmic delivery of humanin. J Control Release. 2013;166:307–315. doi: 10.1016/j.jconrel.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 49.Xu X, Chua CC, Gao J, Hamdy RC, Chua BHL. Humanin is a novel neuroprotective agent against stroke. Stroke. 2006;37:2613–2619. doi: 10.1161/01.STR.0000242772.94277.1f. [DOI] [PubMed] [Google Scholar]
- 50.Xu X, Chua KW, Chua CC, Liu CF, Hamdy RC, Chua BHL. Synergistic protective effects of humanin and necrostatin-1 on hypoxia and ischemia/reperfusion injury. Brain Research. 2010;1355:189–194. doi: 10.1016/j.brainres.2010.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao ST, Huang XT, Zhang C, Ke Y. Humanin protects cortical neurons from ischemia and reperfusion injury by the increased activity of superoxide dismutase. Neurochem Res. 2012;37:153–160. doi: 10.1007/s11064-011-0593-0. [DOI] [PubMed] [Google Scholar]
- 52.Widmer RJ, Flammer AJ, Herrmann J, Rodriguez-Porcel M, Wan J, Cohen P, et al. Circulating humanin levels are associated with preserved coronary endothelial function. Am J Physiol Heart Circ Physiol. 2013;304:H393–7. doi: 10.1152/ajpheart.00765.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, et al. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res. 2010;88:360–366. doi: 10.1093/cvr/cvq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res. 2003;13:113–170. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 55.Gluckman P, Klempt N, Guan J, Mallard C, Sirimanne E, Dragunow M, et al. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun. 1992;182:593–599. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- 56.Loddick SA, Liu XJ, Lu ZX, Liu C, Behan DP, Chalmers DC, et al. Displacement of insulin-like growth factors from their binding proteins as a potential treatment for stroke. Proc Natl Acad Sci USA. 1998;95:1894–1898. doi: 10.1073/pnas.95.4.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med. 2002;8:1390–1397. doi: 10.1038/nm793. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, Urbieta-Caceres VH, Eirin A, Bell CC, Crane JA, Tang H, et al. Humanin prevents intra-renal microvascular remodeling and inflammation in hypercholesterolemic ApoE deficient mice. Life Sci. 2012;91:199–206. doi: 10.1016/j.lfs.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sukhanov S, Higashi Y, Shai S-Y, Vaughn C, Mohler J, Li Y, et al. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 60.Buerke M, Murohara T, Skurk C, Nuss C, Tomaselli K, Lefer AM. Cardioprotective effect of insulin-like growth factor I in myocardial ischemia followed by reperfusion. Proc Natl Acad Sci USA. 1995;92:8031–8035. doi: 10.1073/pnas.92.17.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 62.Werner H. Tumor suppressors govern insulin-like growth factor signaling pathways: implications in metabolism and cancer. Oncogene. 2012;31:2703–2714. doi: 10.1038/onc.2011.447. [DOI] [PubMed] [Google Scholar]
- 63.Lue Y, Swerdloff R, Wan J, Xiao J, French S, Atienza V, et al. The Potent Humanin Analogue (HNG) Protects Germ Cells and Leucocytes while Enhancing Chemotherapy-induced Suppression of Cancer Metastases in Male Mice. 2015 doi: 10.1210/en.2015-1542. en.2015–1542–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eriksson E, Wickström M, Perup LS, Johnsen JI, Eksborg S, Kogner P, et al. Protective role of humanin on bortezomib-induced bone growth impairment in anticancer treatment. Journal of the National Cancer Institute. 2014;106:djt459. doi: 10.1093/jnci/djt459. [DOI] [PubMed] [Google Scholar]
- 65.Cohen P. New role for the mitochondrial peptide humanin: protective agent against chemotherapy-induced side effects. Journal of the National Cancer Institute. 2014;106:dju006–dju006. doi: 10.1093/jnci/dju006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zadik Z, Chalew SA, McCarter RJ, Meistas M, Kowarski AA. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab. 1985;60:513–516. doi: 10.1210/jcem-60-3-513. [DOI] [PubMed] [Google Scholar]
- 67.Narasimhan SD, Yen K, Tissenbaum HA. Converging pathways in lifespan regulation. Curr Biol. 2009;19:R657–66. doi: 10.1016/j.cub.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee C, Wan J, Miyazaki B, Fang Y, Guevara-Aguirre J, Yen K, et al. IGF-I regulates the age-dependent signaling peptide humanin. Aging Cell. 2014;13:958–961. doi: 10.1111/acel.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78:210–216. doi: 10.1159/000073704. [DOI] [PubMed] [Google Scholar]
- 70.Bartke A, Masternak M, Al-Regaiey K, Bonkowski M. Effects of dietary restriction on the expression of insulin-signaling-related genes in long-lived mutant mice. Interdisciplinary Topics in Gerontology. 2007;35:69–82. doi: 10.1159/000096556. [DOI] [PubMed] [Google Scholar]
- 71.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yakar S, Rosen CJ, Bouxsein ML, Sun H, Mejia W, Kawashima Y, et al. Serum complexes of insulin-like growth factor-1 modulate skeletal integrity and carbohydrate metabolism. Faseb J. 2009;23:709–719. doi: 10.1096/fj.08-118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng C-W, et al. Growth Hormone Receptor Deficiency Is Associated with a Major Reduction in Pro-Aging Signaling. Cancer, and Diabetes in Humans, Science Translational Medicine. 2011;3:70ra13–70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chistiakov DA, Sobenin IA, Revin VV, Orekhov AN, Bobryshev YV. Mitochondrial aging and age-related dysfunction of mitochondria. BioMed Research International. 2014;2014:238463–7. doi: 10.1155/2014/238463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colón E, Strand ML, Carlsson-Skwirut C, Wahlgren A, Svechnikov KV, Cohen P, et al. Anti-apoptotic factor humanin is expressed in the testis and prevents cell-death in leydig cells during the first wave of spermatogenesis. Journal of Cellular Physiology. 2006;208:373–385. doi: 10.1002/jcp.20672. [DOI] [PubMed] [Google Scholar]
- 76.Chin YP, Keni J, Wan J, Mehta H, Anene F, Jia Y, et al. Pharmacokinetics and tissue distribution of humanin and its analogues in male rodents. Endocrinology. 2013;154:3739–3744. doi: 10.1210/en.2012-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]