Summary
We can improve human vision by correcting the optics of our lenses [1–3]. However, after the eye transduces the light, visual cortex has its own limitations that are challenging to correct [4]. Overcoming these limitations has typically involved innovative training regimes that improve vision across many days [5–7]. In the present study, we wanted to determine whether it is possible to immediately improve the precision of spatial vision with noninvasive direct-current stimulation. Previous work suggested that visual processing could be modulated with such stimulation [8–10]. However, the short duration and variability of such effects made it seem unlikely that spatial vision could be improved for more than several minutes [8, 11]. Here we show that visual acuity in the parafoveal belt can be immediately improved by delivering noninvasive direct current to visual cortex. Twenty minutes of anodal stimulation improved subjects’ vernier acuity by approximately 15%, and increased the amplitude of the earliest visually evoked potentials in lockstep with the behavioral effects. When we reversed the orientation of the electric field we impaired resolution and reduced the amplitude of visually evoked potentials. Next, we found that anodal stimulation improved acuity enough to be measureable with the relatively coarse Snellen test and that subjects with the poorest acuity benefitted the most from stimulation. Finally, we found that stimulation induced acuity improvements were accompanied by changes in contrast sensitivity at high spatial frequencies.
Keywords: direct-current stimulation, spatial vision, visual acuity, electrophysiology
Graphical Abstract
Results
In Experiment 1 we stimulated posterior visual cortex (i.e., occipitoparietal cortex) with transcranial direct-current stimulation (tDCS). We parametrically manipulated stimulation intensity to determine what dose might improve visual-spatial precision. All 20 subjects completed three anodal stimulation conditions at varying stimulation intensities (1.0 mA, 1.5 mA, 2.0 mA) on different days, order randomized across subjects. We stimulated either the left or right hemisphere (see Figure 1A, hemisphere counterbalanced), with stimuli presented in the stimulated visual field (i.e., contralateral) or the unstimulated visual field (i.e., ipsilateral), similar to approaches in neuropsychology [12]. We used 20 minutes of anodal tDCS in each condition because this duration and current flow direction has been shown to increase neuronal excitability [8, 13–16]. Subjects were blind to the stimulation conditions as verified with debriefing questionnaires (see Experimental Procedures).
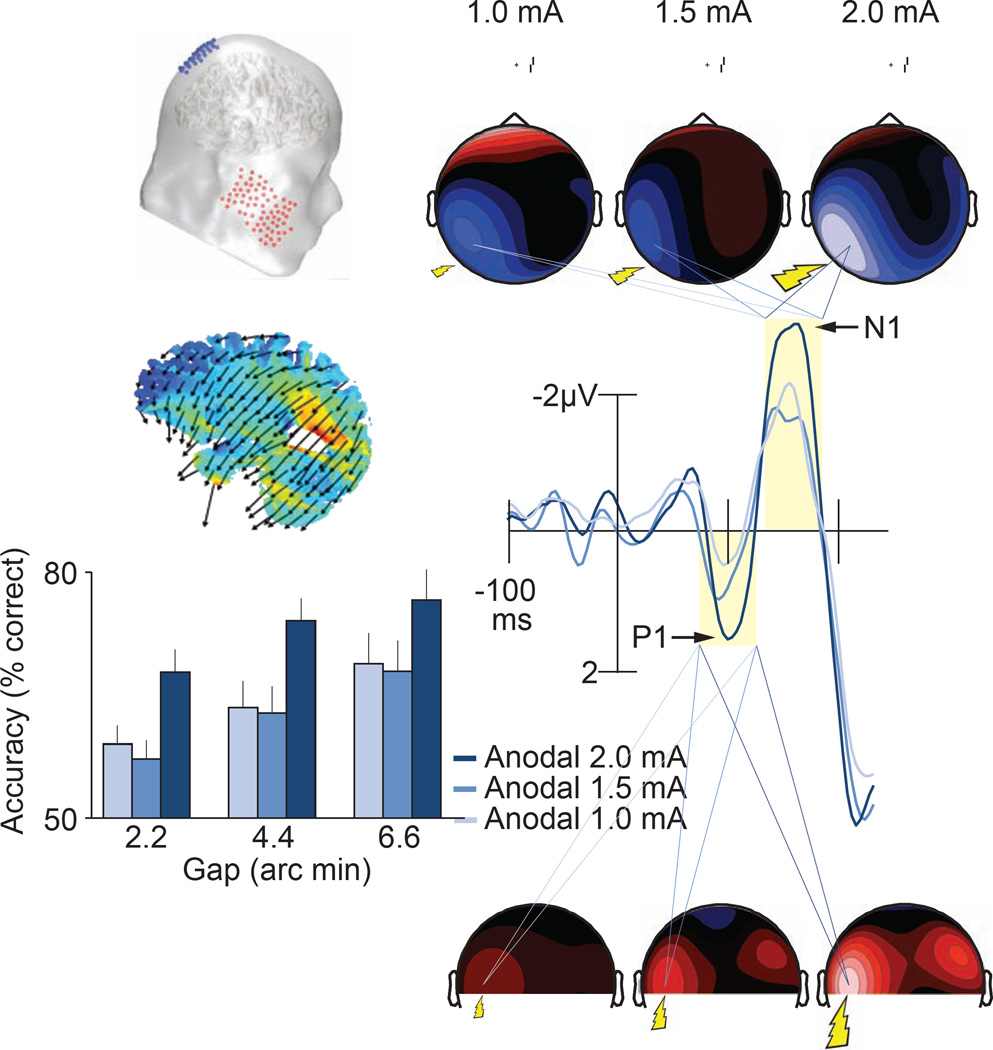
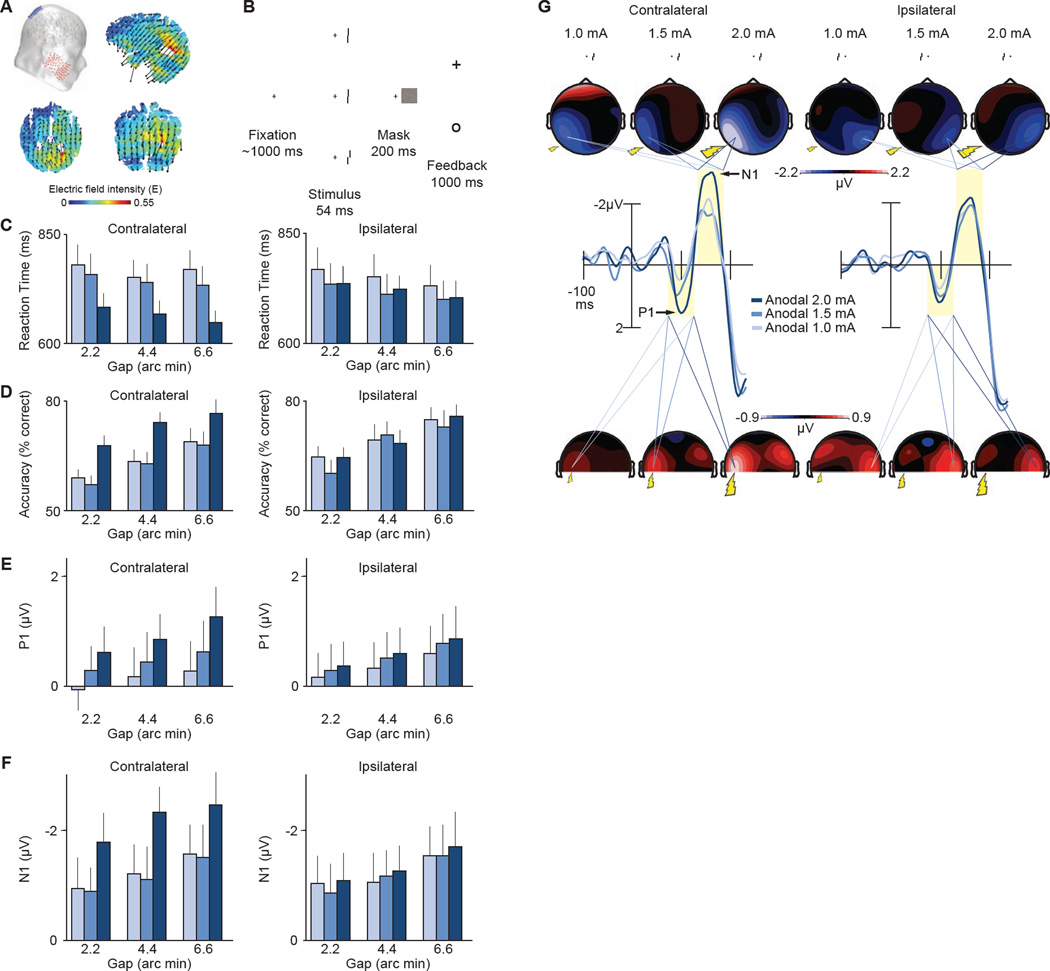
Figure 1. Current-flow model, task, and results from Experiment 1.
A, Our visual cortex transcranial direct-current stimulation (tDCS) montage with the anode over sites P1 or P2 and cathode over the left or right cheek, respectively. The schematic shows the P2 (anodal) and right cheek (reference) configuration. Saggital, axial, and coronal maps centered on the gravity center of the induced electric field show current flow through the brain. Arrows show orientation of the electric field. Warmer colors show greater electric field magnitude. B, The vernier acuity task requiring subjects to judge the relative position of two line segments in the periphery while maintaining central fixation. Subjects indicated with one of two buttons on a gamepad whether the upper line was offset to the left or right of the lower line. Mean correct reaction time (RT) (C) and accuracy (D) across offset size (2.2, 4.4, 6.6 arc min), and tDCS intensity level (1.0, 1.5, 2.0 mA). Data are binned according to the location of vernier stimuli with respect to tDCS application (contralateral, ipsilateral). For example, contralateral data include trials with left visual field stimuli following right hemisphere tDCS, and trials with right visual field stimuli following left hemisphere tDCS. See also Figure S1 for accuracy across the entire experimental session and see Figure S2 for how this performance is related to improvements in contrast sensitivity. Mean amplitude of the P1 (E) and N1 (F) event-related potentials (ERPs) as in C–D. G, Waveforms time-locked to the onset of the vernier stimuli and related topographical maps across tDCS and laterality conditions. Shaded regions show the analysis windows for the P1 (75–125 ms) and N1 (140–190 ms) component amplitudes. Topographies show data collapsed across vernier stimulus locations and the hemispheres of tDCS application using a method that preserved the electrode location relative to the location of the stimuli. All contralateral signals are projected onto the left hemisphere (contralateral) and ipsilateral signals projected onto the right hemisphere (ipsilateral). See Table S1 for the results of the statistical analyses in their entirety.
Immediately following stimulation, subjects performed a lateralized version of the vernier acuity task (Figure 1B). Subjects judged the relative position of two abutting vertical lines, and indicated whether the upper line was displaced to the left or right of the lower line, displaced by 2.2, 4.4, or 6.6 arc minutes randomized across trials, with the lines centered 5° to the left or right of fixation [17–19] (i.e., in the parafoveal belt, see Experimental Procedures for details). This combination of eccentricity and displacement was sufficient to bring subjects’ performance below ceiling and reveal stimulation benefits if they exist.
Experiment 1 showed that 20 minutes of anodal stimulation at 2.0 mA improved vernier acuity relative to performance following 1.0 and 1.5 mA of stimulation. Figure 1C–D shows that we found significant main effects of intensity on RT (p = 0.01) and accuracy (p = 0.04) across task difficulty levels (2.2 – 6.6 arc minutes) (see Table S1 in Supplemental Information for statistical details). These main effects were due to 2.0 mA resulting in faster RTs and higher accuracy than 1.0 mA (RT, p = 0.01; accuracy, p = 0.04) and 1.5 mA of tDCS (RT, p = 0.02; accuracy, p = 0.03). However, this was only true for stimuli presented contralateral to the stimulating electrode. For ipsilateral stimuli, the stimulation intensity had no significant behavioral effects (RT, p = 0.64; accuracy, p = 0.91). These lateralized improvements did not differ between subjects who received left versus right hemisphere tDCS (no stimulation location × intensity interactions on RT, contra, p = 0.93; ipsi, p = 0.25; or accuracy, contra, p = 0.79; ipsi, p = 0.95). These findings show that increasing the excitability of the visual cortex with anodal direct current can improve behavioral measures of vernier acuity in the parafovea.
By recording the subjects’ event-related potentials (ERPs), we verified that stimulation was changing how the brain processed the visual information at the earliest measureable points in processing. Figure 1E–G shows that the stimulation enhanced the early visual waveforms related to sensory processing (i.e., the visual P1 and N1) [20]. The P1 and N1 amplitudes elicited by stimuli contralateral to the stimulation increased with intensity (main effect of intensity on P1, p = 0.05; N1, p = 0.01), with the strongest enhancements following 2.0 mA relative to 1.0 mA intensity (P1, p = 0.04; N1, p = 0.01). No stimulation-induced changes to P1 and N1 amplitudes were observed for ipsilaterally presented stimuli (P1, p = 0.61; N1, p = 0.90), or between lower stimulation intensities (1.0 mA versus 1.5 mA, P1 contra, p = 0.22; P1 ipsi, p = 0.50; N1 contra, p = 0.76; N1 ipsi, p = 0.91). Thus, the subjects’ visual P1 and N1 components exhibited amplitude changes that mirrored the behavioral effects, demonstrating that tDCS changed the earliest stages of processing in the visual system.
Our results from Experiment 1 show that the benefits of anodal stimulation over visual cortex were only evident when we used 2.0 mA of tDCS for 20 minutes, not the lower levels of intensity. In our subsequent experiments, we focus on using this level of current in our active stimulation conditions.
Next, we reversed the direction of current flow in Experiment 2 (Figure 2A). All subjects completed a sham baseline condition and an active 2.0 mA cathodal stimulation condition on different days, with order counterbalanced across subjects. Cathodal stimulation has been shown to decrease the excitability of cortex in humans and animal models [13, 21]. Thus, 20 minutes of cathodal stimulation should decrease vernier acuity and reduce the amplitude of the visual ERPs relative to the sham baseline.
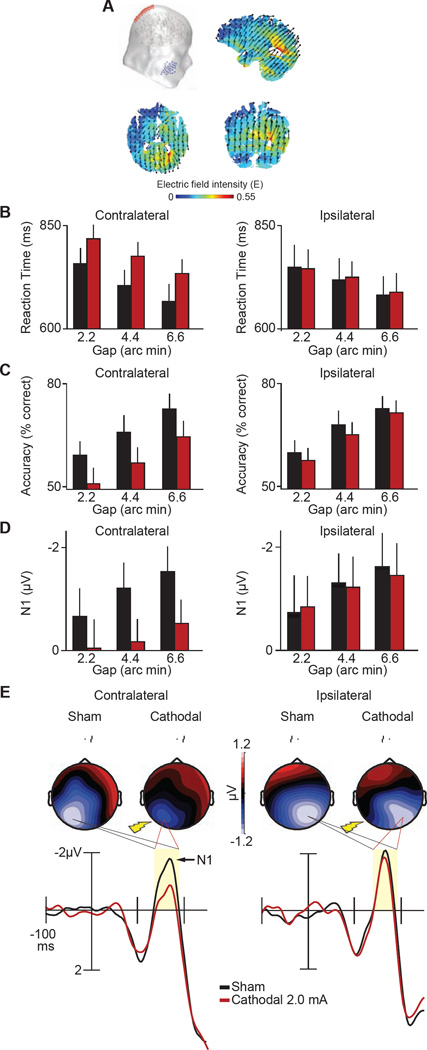
Figure 2. Current-flow model and results from Experiment 2.
A, Our visual cortex transcranial direct-current stimulation (tDCS) montage used a cathodal electrode over P1 or P2 sites (International 10–20 System) paired with an anodal electrode over the left or right cheek, respectively. The schematic here shows the P2 (cathodal) and right cheek (reference) configuration. Saggital, axial, and coronal maps centered on the gravity center of the induced electric field show current flow through the brain. Arrows denote the orientation of the electric field, and warmer colors denote greater electric field magnitude. Mean correct reaction time (RT) (B) and accuracy (C) for vernier offset discrimination as a function of gap offset size (2.2, 4.4, 6.6 arc min), and tDCS condition (sham, black; 2.0 mA cathodal, red). Data are sorted by the location of the vernier stimuli with respect to tDCS application (contralateral, ipsilateral) as in Figure 1. D, Mean N1 amplitude as in B–C. E, Waveforms time-locked to the onset of the vernier stimuli and related topographies across tDCS and laterality conditions. Arrow shows N1 component. Shaded regions show the analysis window for the N1 component amplitude (140–190 ms). Topographies show all contralateral and ipsilateral signals as described in Figure 1G. See Table S1 for the results of the statistical analyses in their entirety.
Experiment 2 demonstrated that we could push behavior and the subjects’ visual ERPs in the opposite direction with cathodal tDCS. Figure 2B–C shows that relative to the sham baseline condition, 20 minutes of 2.0 mA cathodal tDCS significantly slowed RTs (p = 0.03) and reduced accuracy (p = 0.04) across displacements (i.e., 2.2 to 6.6 arc minutes) (see Table S1 in Supplemental Information for statistical details). These behavioral changes in vernier acuity were specific to the visual field contralateral to the tDCS. No such changes in task performance were observed for ipsilaterally presented stimuli (RT, p = 0.95; accuracy, p = 0.48).
Figure 2D–E shows that cathodal stimulation reduced the amplitude of the visual N1 component relative to sham, but this time largely spared the amplitude of the preceding P1 component. Paralleling the behavior, we found a spatially mapped pattern of ERP results. Only N1 amplitudes elicited by stimuli appearing contralateral to tDCS were significantly reduced (contra, p = 0.03; ipsi, p = 0.87). No effects were seen in the P1 amplitude following stimulation (contra, p = 0.23; ipsi, p = 0.89). The findings of Experiment 2 demonstrate that by reversing the current flow, we can flip the behavioral and electrophysiological effects that we observed in Experiment 1.
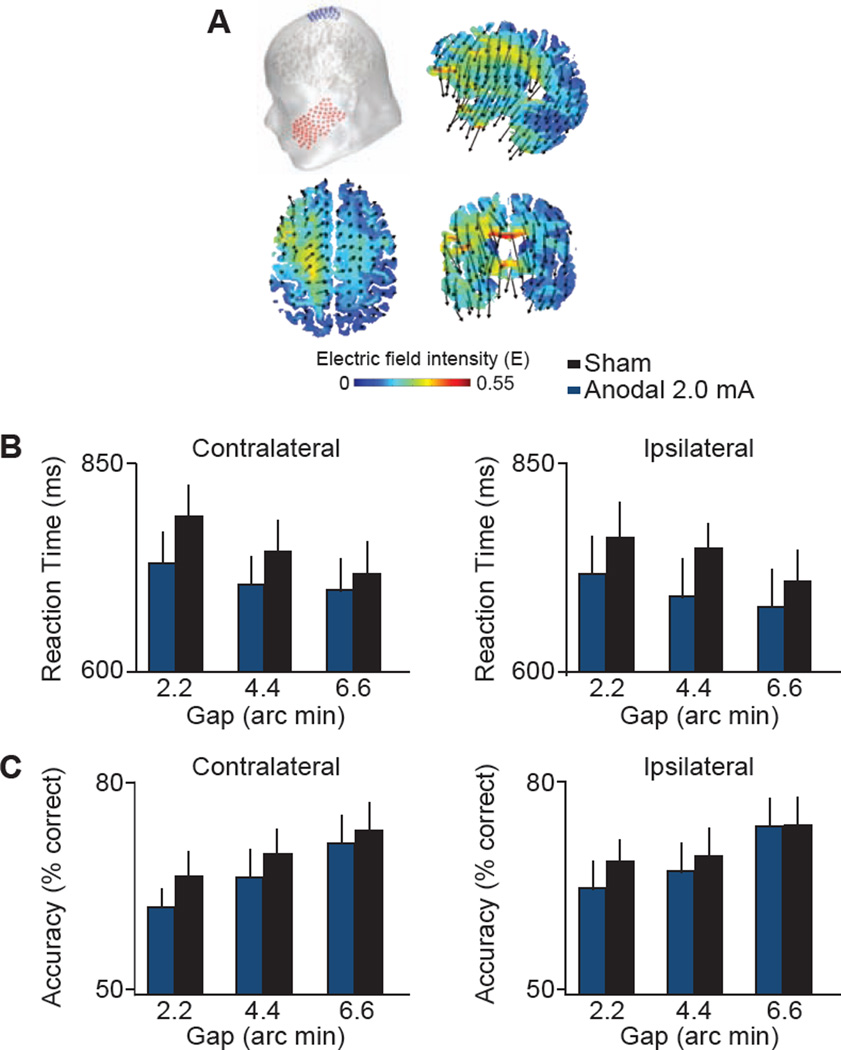
If the results from Experiments 1–2 are not unique to tDCS applied to visual cortex, but can be produced using any lateralized tDCS montage due to nonspecific effects such as arousal or demand characteristics, then we should find a similar pattern of results when stimulating over a different brain region. In Experiment 3, we applied 20 minutes of sham and 2.0 mA anodal tDCS over the left or right hemispheres of motor cortex (see Figure 3A), with hemisphere counterbalanced across 20 subjects, prior to having the subjects complete the vernier acuity task. These subjects showed a completely different pattern of behavioral effects compared to tDCS of visual cortex. Figure 3B–C shows that subjects simply responded faster and less accurately regardless of the location of stimuli in the visual field (see Supplemental Results for details). Whereas the results of Experiments 1–2 rule out attentional, eye movement, and pupil dilation accounts of the tDCS effects (see Supplemental Discussion for details), the results of Experiment 3 show that only tDCS of visual cortex improves performance in the vernier task.
Figure 3. Current-flow model and results from Experiment 3.
A, Our motor cortex transcranial direct-current stimulation (tDCS) montage used an anodal electrode over C1 or C2 sites (International 10-20 System) paired with a cathodal electrode over the left or right cheek, respectively. The schematic shows the C1 (anodal) and left cheek (cathodal) configuration. Saggital, axial, and coronal maps centered on the gravity center of the induced electric field show current flow through the brain. Arrows denote the orientation of the electric field, and warmer colors denote greater electric field magnitude. Mean correct reaction time (RT) (B) and accuracy (C) for the vernier stimuli as a function of gap offset size (2.2, 4.4, 6.6 arc min), and tDCS condition (sham, 2.0 mA anodal). Data are sorted by laterality (contralateral, ipsilateral) as in Figures 1–2.
If the anodal stimulation of Experiment 1 is truly improving subjects’ visuo-spatial resolution, then we should improve real-world acuity outside the laboratory. In Experiment 4, we examined the impact of 20 minutes of 2.0 mA anodal tDCS of visual cortex on the performance of the Snellen chart for visual acuity, a common exam in clinical practice. All 20 subjects completed the Snellen test before and after a sham tDCS testing day, and before and after an anodal tDCS testing day. The order of stimulation and the hemisphere stimulated (left or right) were counterbalanced across subjects.
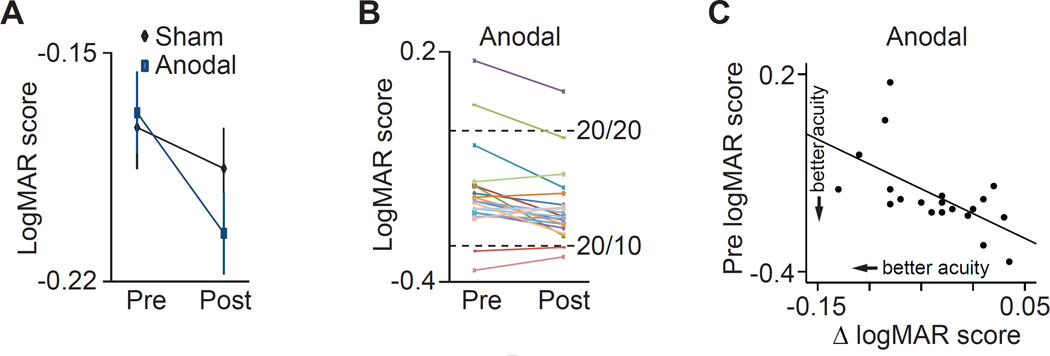
Figure 4A–C shows that subjects scored higher on the Snellen test of central visual acuity after the lateral application of anodal tDCS compared with before anodal tDCS (pre versus post percent change, mean ± SEM, 21.8 ± 9.2%; main effect of time on the logarithm of the minimal angle of resolution or logMAR score, p = 0.003). Our sham condition allowed us to control for practice effects. We found that despite mild trend-level practice effects in the sham condition (mean ± SEM, 7.2 ± 5.7%; main effect of time on logMAR score, p = 0.06), the anodal tDCS induced significantly greater behavioral gains relative to the sham condition, evidenced by a stimulation × time interaction on logMAR score (p = 0.04). Accounting for practice effects, the stimulation boosted subjects’ logMAR score by approximately 14.6%. An individual subject analysis showed that all but two subjects had 20/20 vision (i.e., logMAR = 0) or better in both eyes, consistent with previous work with young adults [22] (see Supplemental Information for results with these two subjects excluded). Despite the excellent vision of our subjects, the majority of subjects improved with tDCS (14 out of 20), correctly reading an additional 1.2 letters on average (accounting for practice effects) after the anodal stimulation. Individuals with the poorest eyesight experienced the largest stimulation-induced gains, evidenced by a significant subject-wise correlation (p = 0.005). If our findings generalize to individuals with clinical grade vision deficits, the findings of Experiment 4 suggest that those with the largest deficits may show the largest benefit of anodal tDCS. However, the true therapeutic utility of this tDCS protocol will require clinical research with patient populations.
Figure 4. Results from Experiment 4.
A, Mean logMAR scores from the Snellen eye chart before (pre) and after (post) the sham and anodal visual cortex tDCS at 2.0 mA intensity. B, Individual subject data shows the change in logMAR score before and after anodal tDCS. C, Scatter plot shows the relationship between a subject’s score on the Snellen test before anodal stimulation (pre logMAR score) and their improvement following anodal stimulation (i.e., post minus pre logMAR score). Smaller logMAR scores reflect better performance (i.e., more letters read correctly). See Table S1 for the results of the statistical analyses in their entirety.
Finally, in Experiment 5 we tested whether the improvements in acuity following tDCS co-occurred with improvements in contrast sensitivity given the close link between these visual functions [23–25]. Experiment 5 was identical to Experiment 4, except that we used a two interval forced choice task with a Bayesian adaptive procedure to estimate subjects’ contrast sensitivity function across a range of spatial frequencies (0.5 to 20 cycles per degree, cpd) (see Experimental Procedures for details). We found that anodal stimulation had an effect on mean contrast sensitivity, but only for contralaterally presented grating stimuli at high spatial frequencies (see Figure S1 and Table S1 in Supplemental Information). This was demonstrated by an interaction between time and stimulation for contralateral (p = 0.001), but not ipsilateral stimuli (p = 0.65). Parsing this interaction revealed an improvement across time (pre versus post) in the anodal condition (p = 0.001), but not the sham (p = 0.56). Further, within the anodal condition, we observed a time × frequency interaction (p = 0.01), driven by changes at spatial frequencies greater than 7 cpd (ps < 0.03). These results converge with those from Experiment 1 by showing that occipitoparietal tDCS can have a hemifield-selective influence on the high spatial frequency contrast sensitivity thresholds in the parafovea. These results add to previous work [11, 26] and confirm overlapping function between contrast sensitivity and visual acuity [23–25].
Discussion
Our demonstration that anodal tDCS of occipitoparietal visual cortex improves spatial vision may seem counterintuitive because this type of stimulation does not target the specific neurons that code for the line segments in the vernier acuity task or the letters on the Snellen chart. One might expect tDCS to increase both the noise and the signal to the same degree given anodal stimulation increases baseline-firing rates in the absence of stimuli [21]. However, the present effects of tDCS could be accounted for in at least two ways. First, tDCS may have increased the gain for stimuli presented contralateral to the stimulation, consistent with our observation that the vernier stimuli elicited more vigorous electrophysiological responses following anodal stimulation, and attenuated responses following cathodal stimulation. Second, the broad stimulating effects of anodal tDCS may have increased stochastic resonance. By increasing the baseline level of activity in the visual system under the anodal electrode, the signal-to-noise ratio may have been effectively increased because the addition of higher amplitude white noise to this nonlinear neural system can improve processing [27]. In contrast, cathodal stimulation reduces activity levels, and the intrinsic stochastic resonance in the system, reducing the signal-to-noise ratio. Stochastic resonance has been shown to make a variety of neural systems more sensitive signal processors and our anodal tDCS protocol may induce just such beneficial noise. We believe that these competing explanations can be distinguished by experiments that combine direct-current stimulation with recordings from neurons in animal models.
Here we found tDCS effects on spatial vision that last for over an hour, with large, immediate improvements in acuity (see Figure S2 in Supplemental Information). How do we reconcile our results with previous work that found small effects of tDCS on the visual system that disappeared within 10 minutes of the end of stimulation [11, 28]? We see the electrode montage as the largest difference between the present and previous work. We stimulated occipitoparietal cortex using a distant cheek location for the other electrode. Previous work placed one electrode over the occipital pole or over V5 and the other at the top of the head (Cz), resulting in modest effects that lasted only a few minutes after stimulation ceased, possibly due to opposing effects induced by the electrode at Cz [21]. Because the cathodal and anodal electrodes push and pull activity levels in opposite directions, we will need experiments to verify that the electrode placements in previous work result in the short and counterintuitive effects compared to the effects observed here.
To increase the applicability of the occipitoparietal tDCS protocol as a potential therapeutic treatment, it will be necessary for future work to more extensively explore the tDCS parameter space. Lengthening stimulation duration, increasing the focality of current flow, and conducting repeated stimulation sessions with optimal spacing intervals [29], are methods that may generate longer lasting improvements in spatial vision. Finally, our psychophysical manipulations tested vernier acuity at locations in the parafoveal belt using a brief stimulus presentation. It will be useful for future work to determine whether occipitoparietal stimulation influences processing in the fovea (< 0.06°) and periphery (> 5°) with prolonged viewing durations, while tracking eye movements. Forthcoming work that addresses the above questions will significantly improve our understanding of our occipitoparietal tDCS method and determine its value for applications outside the laboratory.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01-EY019882, R01-EY025275, P30-EY08126, T32-EY007135, F31-MH102042). We thank the reviewers and Randolph Blake for helpful comments. We thank Kevin Dieter for technical assistance in designing the psychophysical procedure for Experiment 5.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Experimental procedures, supplemental results and discussion, one table, and two figures are available on the Current Biology website.
Author Contributions
R.M.G.R., W.X., and L.J.M. conducted the experiments and the analyses. R.M.G.R. and G.F.W designed the experiments. R.M.G.R. and G.F.W. wrote the paper. All authors approved the final version of this report.
Conflict of interest statement: The authors have no competing financial interests.
References
- 1.Hamill MB, Koch DD. Refractive surgery - the cutting edge. Nature Medicine. 1996;2:640–741. doi: 10.1038/nm0796-740. [DOI] [PubMed] [Google Scholar]
- 2.Palmer SE. Vision science: Photons to phenomenology. Cambridge, MA: Bradford Books/MIT Press; 1999. [Google Scholar]
- 3.Dolgin E. The myopia boom. Nature. 2015;519:276–278. doi: 10.1038/519276a. [DOI] [PubMed] [Google Scholar]
- 4.Dutton GN. Cognitive vision, its disorders and differential diagnosis in adults and children: knowing where and what things are. Eye. 2003;17:289–304. doi: 10.1038/sj.eye.6700344. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DE, Vogel EK, Awh E. Precision in visual working memory reaches a stable plateau when individual item limits are exceeded. Journal of Neuroscience. 2011;31:1128–1138. doi: 10.1523/JNEUROSCI.4125-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Deveau J, Ozer DJ, Seitz AR. Improved vision and on-field performance in baseball through perceptual learning. Current Biology. 2014;24:R146–R147. doi: 10.1016/j.cub.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagi D. Perceptual learning in vision research. Vision Research. 2011;51:1552–1566. doi: 10.1016/j.visres.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Antal A, Varga ET, Kincses TZ, Nitsche MA, Paulus W. Oscillatory brain activity and transcranial direct current stimulation in humans. NeuroReport. 2004;15:1307–1310. doi: 10.1097/01.wnr.0000127460.08361.84. [DOI] [PubMed] [Google Scholar]
- 9.Plow EB, Obretenova SN, Jackson ML, Merabet LB. Temporal profile of functional visual rehabilitative outcomes modulated by transcranial direct current stimulation. Neuromodulation. 2012;15:367–373. doi: 10.1111/j.1525-1403.2012.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiegel DP, Byblow WD, Hess RF, Thompson B. Anodal transcranial direct current stimulation transiently improves contrast sensitivity and normalizes visual cortex activation in individuals with amblyopia. Neurorehabilitation and Neural Repair. 2013;27:760–769. doi: 10.1177/1545968313491006. [DOI] [PubMed] [Google Scholar]
- 11.Antal A, Nitsche MA, Paulus W. External modulation of visual perception in humans. Neuroreport. 2001;16:3553–3555. doi: 10.1097/00001756-200111160-00036. [DOI] [PubMed] [Google Scholar]
- 12.Lavidor M, Walsh V. The nature of foveal representation. Nature Reviews Neuroscience. 2004;5:729–735. doi: 10.1038/nrn1498. [DOI] [PubMed] [Google Scholar]
- 13.Reinhart RMG, Woodman GF. Causal control of medial-frontal cortex governs electrophysiological and behavioral indices of performance monitoring and learning. Journal of Neuroscience. 2014;34:4214–4227. doi: 10.1523/JNEUROSCI.5421-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reinhart RMG, Woodman GF. Enhancing long-term memory with stimulation tunes visual attention in one trial. Proceedings of the National Academy of Sciences of the USA. 2015;112:625–630. doi: 10.1073/pnas.1417259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinhart RMG, Zhu J, Park S, Woodman GF. Medial-frontal stimulation enhances learning in schizophrenia by restoring prediction-error signaling. Journal of Neuroscience. 2015;35:12232–12240. doi: 10.1523/JNEUROSCI.1717-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinhart RMG, Zhu J, Park S, Woodman GF. Synchronizing theta oscillations with direct-current stimulation strengthens adaptive control in the human brain. Proceedings of the National Academy of Sciences of the USA. 2015;112:9448–9453. doi: 10.1073/pnas.1504196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker TY, Bryan GB. Errors of observation. In: Stroughton Ha., editor. Proceedings of the Optical Convention. London: 1912. [Google Scholar]
- 18.Berry RN. Quantitative relations among vernier, real depth, and stereoscopic depth acuities. Journal of Experiment al Psychology. 1948;38:708–721. doi: 10.1037/h0057362. [DOI] [PubMed] [Google Scholar]
- 19.Westheimer G, McKee SP. Spatial configurations for visual hyperacuity. Vision Research. 1977;17:941–947. doi: 10.1016/0042-6989(77)90069-4. [DOI] [PubMed] [Google Scholar]
- 20.Luck SJ, Hillyard SA. Electrophysiological evidence for parallel and serial processing during visual search. Perception & Psychophysics. 1990;48:603–617. doi: 10.3758/bf03211606. [DOI] [PubMed] [Google Scholar]
- 21.Bindman LJ, Lippold OCJ, Redfearn JWT. Long lasting changes in the level of the electrical activity of the cerebral cortex produced by polarizing currents. Nature. 1962;196:584–585. doi: 10.1038/196584a0. [DOI] [PubMed] [Google Scholar]
- 22.Li RW, Brown B, Edwards MH, Ngo CV, Chat SW, Levi DM. Reduced sampling efficiency causes degraded Vernier hyperacuity with normal aging: Vernier acuity in position noise. Scientific Reports. 2012;2:300. doi: 10.1038/srep00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkins AS, Szlyk JP, Ardickas Z, Alexander KR, Wilensky JT. Comparison of contrast sensitivity, visual acuity, and Humphrey Visual Field testing in patients with glaucoma. Journal of Glaucoma. 2003;12:134–138. [PubMed] [Google Scholar]
- 24.Hashemi H, Khabazkhoob M, Jafarzadehpur E, Emamian MH, Shariati M, Fotouhi A. Contrast sensitivity evaluation in a population-based study in Shahroud, Iran. Ophthalmology. 2012;119:541–546. doi: 10.1016/j.ophtha.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 25.Kromer R, Serbecic N, Krastel H, Beutelspacher SC. Comparison of VEP with contrast sensitivity and other measurements of central visual function. Acta Ophthalmologica. 2014;92:e141–e146. doi: 10.1111/aos.12176. [DOI] [PubMed] [Google Scholar]
- 26.Kraft A, Roehmel J, Olma MC, Schmidt S, Irlbacher K, Brandt SA. Transcranial direct current stimulation affects visual perception measured by threshold perimetry. Experimental Brain Research. 2010;207:283–290. doi: 10.1007/s00221-010-2453-6. [DOI] [PubMed] [Google Scholar]
- 27.McDonnell MD, Ward LM. The benefits of noise in neural systems: bridging theory and experiment. Nature Reviews Neuroscience. 2011;12:415–426. doi: 10.1038/nrn3061. [DOI] [PubMed] [Google Scholar]
- 28.Antal A, Nitsche MA, Kruse W, Kincses TZ, Hoffman KP, Paulus W. Direct current stimulation over V5 enhances visuo-motor coordination by improving motion perception in humans. Journal of Cognitive Neuroscience. 2004;16:521–527. doi: 10.1162/089892904323057263. [DOI] [PubMed] [Google Scholar]
- 29.Goldsworthy MR, Pitcher JB, Ridding MC. Spaced noninvasive brain stimulation: Prospects for inducing long-lasting human cortical plasticity. Neurorehabilitation and Neural Repair. 2015;29:714–721. doi: 10.1177/1545968314562649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.