Abstract
Disruption in the expression and function of synaptic proteins, and ion channels in particular, is critical in the pathophysiology of human neuro-psychiatric and neuro-degenerative diseases. However, very little is known regarding the functional and pharmacological properties of native synaptic human ion channels, and their potential changes in pathological conditions. Recently, an electrophysiological technique has been enabled for studying the functional and pharmacological properties of ion channels present in crude membrane preparation obtained from post-mortem frozen brains. We here extend these studies by showing that also human synaptic ion channels can be studied in this way. Synaptosomes purified from different regions of rodent and human brain (control and Alzheimer’s) were characterised biochemically for enrichment of synaptic proteins, and expression of ion channel subunits. The same synaptosomes were also reconstituted in Xenopus oocytes, in which the functional and pharmacological properties of the native synaptic ion channels were characterised using the voltage clamp technique. We show that we can detect GABA, AMPA and NMDA receptors and modulate them pharmacologically with selective agonists, antagonists and allosteric modulators. Furthermore, changes in ion channel expression and function were detected in synaptic membranes from Alzheimer’s brains. Our present results demonstrate the possibility to investigate synaptic ion channels from healthy and pathological brains. This method of synaptosomes preparation and injection into oocytes is a significant improvement over the earlier method. It opens the way to directly testing, on native ion channels, the effects of novel drugs aimed at modulating important classes of synaptic targets.
Keywords: Human, synaptosomes, oocytes, ion channels, Alzheimer’s
Introduction
Ligand- and voltage-gated ion channels play essential roles in controlling cell excitability and, specifically in the nervous system, synaptic activity and plasticity (Voglis et al., 2006). Ion channels also represent important drug targets with several successfully marketed drugs targeting them as agonists, antagonists or allosteric modulators (Bagal et al., 2013). In the process of drug discovery we and others, have made extensive use of recombinant systems, where molecularly identified ion channel subunits are heterologously expressed in cell lines (Broad et al., 2006) or oocytes (Zwart et al., 2008; Zwart et al., 2014a) where their function and pharmacology can be evaluated. This has tremendous value for drug discovery in allowing high throughput screening of diverse compound libraries, as well as molecular dissection of drug action with the use, for example, of subunit chimeras or point mutations (Young et al., 2008).
One limitation of recombinant systems is that they are unlikely to replicate the structure and function of native ion channels with good fidelity since the subunit stoichiometry, accessory subunits, posttranslational modifications, and lipid environment are not completely known. This might affect, or even mislead, our understanding of drug effects in humans. Importantly, with the possible exception of human ion channel genetic mutations, recombinant ion channels do not reflect the pathological state occurring in tissues from human patients.
One way to circumvent some of the issues described above is to confirm the drug effects in native tissues, like primary neuronal cultures, brain slices or brain homogenates. While this is very useful, it is not uncommon that receptors and ion channels behave differently across species and, importantly, that the drugs themselves show species-specific pharmacology.
Human neuronal cells and tissues have been used, albeit in a limited way, to confirm drug effects on human native ion channels. This includes the use of human neuroblastoma cell lines (Carbone et al., 1990; Chini et al., 1992), the expanding area of human inducible pluripotent stem cell-derived neurons (Dage et al., 2014; Chatzidaki et al., 2015), human brain homogenates (Mitewa et al., 2013), and surgical brain specimens (Zwart et al., 2014b).
A novel approach, pioneered by Miledi and colleagues (Miledi et al., 2006), is based on the micro-transplantation into the cytosol of Xenopus oocytes of cellular membranes obtained from human frozen brains. These membranes undergo fusion with the plasma membranes of the oocytes, allowing the expression and the study of native human ion channels properties and pharmacology in normal and disease conditions (Palma et al., 2002; Bernareggi et al., 2007; Limon et al., 2012). We also used this approach recently to study the efficacy of novel antiepileptic agents at native AMPA receptors (Zwart et al., 2014b).
Most of the papers on human brain membranes micro-transplantation in oocytes utilized crude brain membrane preparations (Palma et al., 2002; Miledi et al., 2006; Bernareggi et al., 2007; Limon et al., 2012; Zwart et al., 2014b). It would be highly valuable if we could use this technique to study more specifically synaptic receptors and ion channels, since these are core to the emerging understanding of human synaptopathies (Selkoe, 2002; van Spronsen et al., 2010; Grant, 2012). There is at least one precedent where mammalian synaptosomes have been purified from rat cerebellum and successfully micro-transplanted into oocytes (Sanna et al., 1996).
Our aim was to expand this evaluation to synaptosomes from other rodent brain areas, as well as to explore its feasibility with human brain synaptosomes. Here we report on the successful recording of glutamatergic and GABAergic ion channel subtypes from rodent synaptosomes obtained from the prefrontal cortex, the hippocampus and the cerebellum, as well as, novel findings on human synaptosomes obtained from the cortex of control and Alzheimer’s brains.
Materials and Methods
Brain samples
Rat pre-frontal cortex, hippocampal and cerebellar brain samples were dissected from adult, female Wistar rats (Charles River, Margate, UK). Frozen fragments of human normal and AD cortex, hippocampus and cerebellum brain samples were obtained with ethical approval from the Oregon Alzheimer’s Disease Center, kept frozen at −80°C and utilised according to the Code of Ethics of the World Medical Association (Declaration of Helsinki), printed in the British Medical Journal (18 July 1964). Age, gender and post mortem interval (PMI) are listed in supplemental Table 1. All animal procedures were performed in accordance with the Animals (Scientific Procedures) Act 1986 and were approved by the Eli Lilly Animal Welfare Board.
Purification of rat and human brain membranes, synaptosomes and post-synaptic density (PSD)
Membrane samples were prepared according to the method developed and described by Miledi et al. (2006) and Eusebi et al. (2009), with minor modifications. Synaptosomes were purified with a sucrose gradient (w/wo osmotic shock) as described by Gray and Whittaker (1962) with minor modifications or with a Percoll gradient, as described by Dunkley et al. (2008) with minor changes. PSD was purified as described by Carlin et al. (1980) with minor modifications. In brief, pre-frontal cortex, hippocampal or cerebellar tissue was dissected from Wistar rats and homogenized ten times with a Teflon homogenizer in 9 mL/g of ice-cold 0.3 M sucrose containing 50 mM Tris-HCl (pH 7.4), 50mM EGTA, 50 mM EDTA and both protease (Roche) and phosphatase (Calbiochem) inhibitor cocktails. A scheme showing the protocol of membrane, synaptosome and PSD preparations is shown in supplemental figure S1. The homogenate was centrifuged at 1,500 g for 20 minutes at 4°C. The supernatant fraction (S1) was centrifuged at 16,000 g for 30 minutes at 4°C. The resulting pellet (P2) was split in two: one for total membranes and one for synaptosomes purification. For total membrane preparation, P2 was re-suspended in Tris 50 mM and centrifuged at 100000 g for 2 h at 4°C. For synaptosomes preparation, three different methods have been tested, described below.
Protocol A: Sucrose gradient with osmotic shock. P2 was re-suspended in 5 mM Tris-HCl (pH 8.0), homogenized three times with a Teflon homogenizer and left on ice for 45 minutes. The P2 fraction was homogenized again for ten times, re-suspended in sucrose to make a 34% (w/w) solution and layered onto a discontinuous sucrose density gradient consisting of, from bottom up, equal volume of sample, and buffer containing 0.85 M and 0.3 M sucrose, respectively. After centrifugation at 60,000 g for 2 hours with a Beckmann SW41Ti swing bucket rotor, the synaptosomal fraction (layer between 0.8 and 1.2 M sucrose) was collected and diluted with two volumes of ice-cold 50 mM TrisHCl (pH 7.4) and centrifuged at 48,000 g for 30 minutes. The resulting synaptosomal pellet was re-suspended in 50mM Tris. For PSD preparation, synaptosomes were extracted with 1.5% Triton for 30 min at 4°C, layered onto a buffer containing 0.85 M sucrose and 50 mM Tris pH 7.4 and centrifuged at 104,000 g for 1 h with a Beckmann SW41Ti swing bucket rotor. The final pellet (PSD) was re-suspended in Tris 50 mM.
Protocol B: Sucrose gradient without osmotic shock. P2 was re-suspended immediately in 50 mM Tris-HCl (pH 7.4) and sucrose to make a 34% (w/w) solution before been layered onto discontinuous sucrose density gradients, without the 45 minutes osmotic shock step.
Protocol C: Percoll gradient. 2 ml (4–5 mg/mL of the S1 fraction were layered gently onto each of 12 discontinuous Percoll gradients. These comprised 2 mL each of 23%, 15%, 10% and 3% Percoll (v/v) in 0.32 M sucrose buffer. The tubes were centrifuged for 5 min at 31000 g and the fraction number 4 was carefully collected with a Pasteur pipette and pooled with the corresponding fractions from other tubes.
Membranes, synaptosomes and PSD aliquots were stored at −80°C until use. Protein content was determined using the Pierce BCA protein assay kit (Thermo Scientific).
Preparation of HEK293 NR1/NR2B membranes
HEK293 cells stably expressing GluN1/GluN2B were grown in bulk and about 1 gram of scraped cells were homogenized and membranes for oocyte injection were prepared using the protocol as described in (Zwart et al., 2014b) for brain tissues.
Western Blotting
2.5–5 micrograms of membranes, synaptosomes and PSD were added to an equal volume of 2× Laemmli sample buffer (Bio-Rad Laboratories) with 5% beta-mercaptoethanol and heated at 95°C for 3 min. Samples were then loaded into NuPageRTM Novex 4–12% Bis-Tris Midi Gel (Invitrogen) and run for approximately 1 hour at 200 V. Proteins were transferred to nitrocellulose membranes (Amersham Biosciences) for 2 h at 0.5 costant Ampere. Membranes were blocked with 5% non-fat dry milk in TBST (0.1% Tween 20) for 1 hour and then incubated overnight with the different primary antibodies (supplemental Table 2) diluted in TBST according to the antibody supplier data sheet. Membranes were then washed three times for 5 min with TBST and incubated with the appropriate anti-rabbit or anti-mouse secondary antibody diluted 1:40,000 for 1 hour (Sigma). After washing, Enhanced Chemiluminescence Detection Reagent (Amersham Biosciences) was applied for 5 min and images acquired with ImageQuant LAS 4000 (GE).
Xenopus Oocyte Studies
Xenopus oocytes (stages V–VI) were removed from sacrificed frogs and de-folliculated after treatment with collagenase type I (5 mg/mL calcium-free Barth’s solution) for 3 hours at room temperature. Unless otherwise stated, we injected 60 nL of membrane or synaptosome (1mg/mL) suspension per oocyte using a Drummond (Broomall, PA) variable volume micro-injector. In some experiments recombinant cDNAs encoding human NMDA receptor subunits GluN1 and GluN2B ligated in the pcDNA3.1 plasmid vector (InVitrogen, Carlsbad, CA) were injected in a 1:1 subunit ratio. Approximately 18.9 nL of the DNA mixture with a total concentration of 0.1 mg/mL was injected into the nuclei of the oocytes using a Nanoject Automatic Oocyte Injector (Drummond, Broomall, PA). After injection oocytes were incubated at 18°C in a modified Barth’s solution containing 88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.3 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, 15 mM HEPES, and 50 mg/L neomycin (pH 7.6 with NaOH; osmolarity 235 mOsm). Unless otherwise stated, experiments were performed on oocytes after 1–3 days of incubation for synaptosomes and 2–3 days for membranes and 3–5 days for cDNA injection.
Oocytes were placed in a recording chamber (internal diameter 3 mm), which was continuously perfused with a saline solution (115 mM NaCl, 2.5 mM KCl, 1.8 mM BaCl2, 30 µM glycine, 10 mM HEPES (pH 7.4) at a rate of approximately 10 mL/min. Dilutions of drugs in external saline were prepared immediately before the experiments and applied by switching between control and drug-containing saline using a BPS-8 solution exchange system (ALA Scientific Inc., Westbury, NY). Between responses, the oocytes were washed for 2 minutes. Oocytes were impaled by two microelectrodes filled with 3 M KCl (0.5–2.5 MΩ) and voltage-clamped using a Geneclamp 500B amplifier (Axon Instruments, Union City, CA) according to the methods described by Stühmer (1998). The external saline was clamped at ground potential by means of a virtual ground circuit using an Ag/AgCl reference electrode and a Pt/Ir current-passing electrode. The membrane potential was held at −100 mV. The current needed to keep the oocyte’s membrane at the holding potential was measured. Membrane currents were low-pass filtered (four-pole low-pass Bessel filter, −3 dB at 10 Hz), digitized (50 Hz), and stored on disc for offline computer analysis. Data are expressed as mean ± S.E.M. All experiments were performed at room temperature.
Drugs
BAPTA-AM, NMDA and (R,S)-AMPA hydrobromide, zolpidem, cyclothiazide, pregnenolone sulfate sodium salt, ifenprodil hemitartrate and (+)-MK801 maleate were obtained from Tocris (Bristol, U.K.); γ-aminobutyric acid (GABA) and (-)bicuculline methiodide were from Sigma-Aldrich (Poole, U.K.). Compound LY3130481 was synthesised internally at Eli Lilly and Company (Gardinier et al., 2016). Concentrated stock solutions of BAPTA-AM, zolpidem, cyclothiazide, pregnenolone sulfate, ifenprodil and MK801 were prepared in DMSO and stocks of NMDA, AMPA hydrobromide, GABA and bicuculline were prepared in distilled water. The stock solutions were stored in aliquots at −20 °C. Final dilutions in oocyte recording solution were made on the day of the experiments.
Statistical analysis
All analyses were performed using the Prism Graphpad software package. Concentration-inhibition curves were fitted according to the Hill equation. (GraphPad Software, San Diego, CA).
Results
Synaptosome purification: optimisation for TEVC
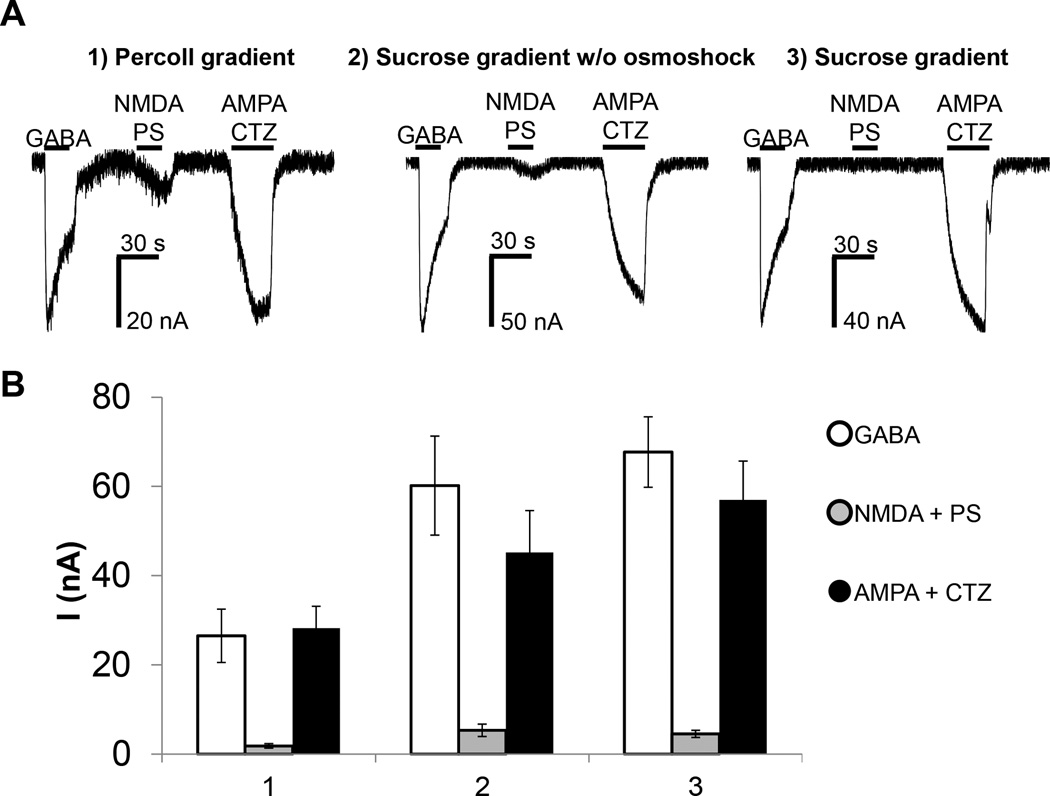
Three different protocols to purify synaptosomes (Protocol A, B and C, see methods) have been evaluated and optimised from the original description with the aim of identifying the one that allowed for the a) most efficient insertion of synaptosomes into the plasma membranes of Xenopus oocytes resulting in the largest ion channel currents, and b) the least toxicity for the oocytes. This optimisation includes the presence of high concentration of calcium chelating agents in the first steps of synaptosome purification and the re-suspension of synaptosomes in physiological buffer before injection into the oocytes. For each protocol, synaptosomes from rat PFC have been injected at the same concentration (1mg/mL), at the same volume (60 nL) the same day, in the same batch of cells, in order to avoid variability in oocytes batches. GABA, AMPA and NMDA currents were analysed by two microelectrode voltage clamp (TEVC). The best currents derived from synaptosomes purified using the sucrose gradients with osmotic shock (Fig 1). Therefore, for consistency, all subsequent experiments were performed on synaptosomes purified using Protocol A.
Fig. 1.
Comparison of rat prefrontal cortex synaptosomes prepared with three different methods of purification and injected in Xenopus oocytes. A) Synaptosomes from rat prefrontal cortex purified with different methods, 1) Percoll gradient, 2) Sucrose gradient without osmotic shock and 3) Sucrose gradient with osmotic shock, respectively. The same amount of synaptosomes was injected in oocytes and the current amplitudes measured after stimulation with 1mM GABA, or 300 µM NMDA with 50 µM pregnenolone sulphate (PS), or 100 µM AMPA with 100 µM CTZ. B) Average of currents ± S.E.M measured from six different cells.
Biochemical characterisation of synaptic protein enrichment in rodent and human cortex
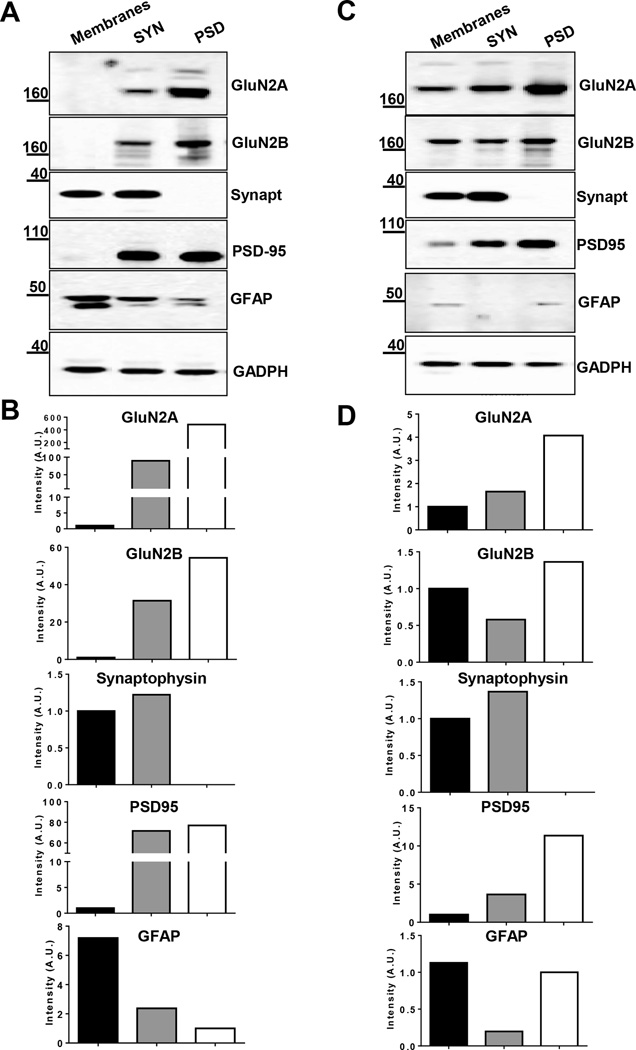
In order to confirm the enrichment of synaptic proteins, and decrease in contaminants, in the synaptosome fraction used for oocyte injection, we performed an extensive immunoblot analysis comparing three different subcellular fractions (total membranes, synaptosomes and PSD (Supplemental Fig S1) isolated from human cortex and rat prefrontal cortex (PFC), respectively. 5 µg of each fraction was subjected to SDS-PAGE, blotted onto nitrocellulose membranes and analysed for the presence of NMDA GluN2A and GluN2B subunits, synaptophysin (marker of the pre-synapse), PSD95 (marker of the post-synapse), and GFAP (marker of astroglia) (Fig. 2). A strong enrichment of GluN2A and 2B NMDA receptor subunits, as well as PSD95 was observed in both human and rat synaptosomes and PSD fractions compared to total membranes. In particular, the enrichment in synaptosomes versus total membranes of GluN2A is 3-fold stronger than for GluN2B, a result compatible with the reported extensive extra-synaptic distribution of the latter (Tovar et al., 1999; Traynelis et al., 2010). Synaptophysin was enriched in synaptosomes compared to total membranes, but absent in the PSD fraction, as expected for a pre-synaptic protein. The astroglia marker GFAP was also decreased in the synaptosome fraction, indicating a reduction in non-neuronal, non-synaptic, contaminants.
Fig. 2.
Biochemical characterisation of subcellular fractions from human cortex and rat prefrontal cortex. Immunoblot analysis of A–B) human cortex and C–D) rat PFC total membrane, synaptosome (SYN) and Post Synaptic Density (PSD) fraction for NMDA subunit GluN2A, GluN2B, for the pre-synaptic marker protein synaptophysin (Synapt), the post-synaptic marker PSD95 and for the astroglia GFAP. B–D) On the y-axis, the intensity of protein signal is indicated in Arbitrary Units (A.U.) normalised to GADPH. Black bar: total membranes; grey bar: synaptosomes; white bar: PSD.
Biochemical characterisation of synaptic ion channels in human and rat brain
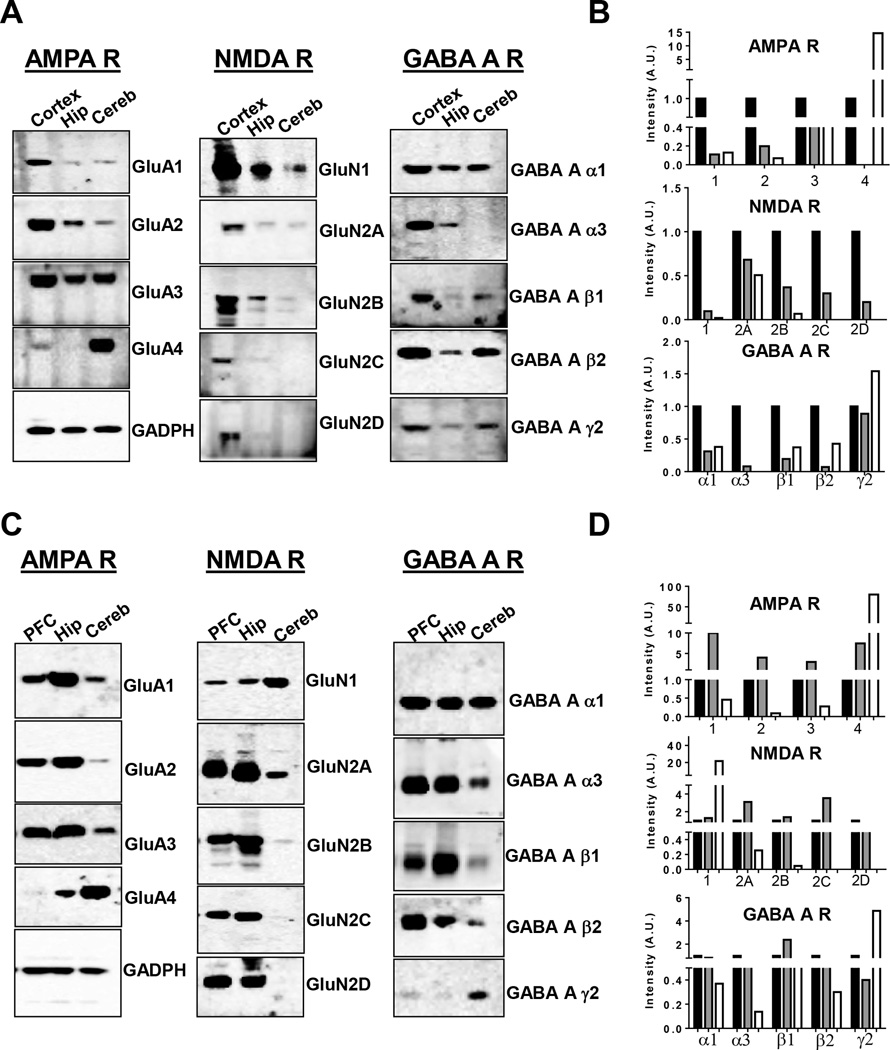
Immunohistochemical studies of the anatomical distribution of AMPA, NMDA and GABAA receptor subunits in rodent brain have been previously performed by several groups (Day et al., 1995; Wenzel et al., 1995; Pirker et al., 2000), but a head to head, semi-quantitative, comparison of the synaptic distribution in human and rat brain has not been previously reported, to the best of our knowledge. The proteome integrity of the human tissue used was assessed by evaluation of the HUSPIR ratio (Bayes et al., 2014) in purified synaptosomes. For each human brain region the HUSPIR ratio, which is the ratio between the full-length (Band 1) and degraded (Band 2) NR2B protein signal, was >1 (supplemental Fig S2). Fig 3 summarises the western blot results for human (A and B) and rat (C and D) brain regions. GluA1-4 AMPA subunits were found to be differentially distributed in the three different brain regions. GluA1 and GluA2 were particularly enriched in human cortex synaptosomes whereas in the rat they were expressed more in hippocampal synaptosomes. GluA3 was widely distributed in all the brain regions, both in human and rat brain. Interestingly, GluA4 was selectively enriched both in the human and rat cerebellar synaptosomes, although in the rat a lower level was found also in the hippocampus. The GluN1 NMDA receptor subunit was found in synaptosomes from all tested regions of the human and rat brain. However, the relative expression of GluN1 was higher in the cortex and in the cerebellum in human and rat, respectively. The GluN2A subunit was enriched in human cortex and rat PFC and hippocampus, with little but detectable presence in the cerebellum, whereas the GluN2B subunit was restricted to the forebrain in both species. Both GluN2C and 2D were expressed in the cortex of both human and rats, but they were expressed in the hippocampus only in rats. Surprisingly, we observed GluN2C only in synaptosomes from the human cortex and rat forebrain, while Wenzel et al. (1995) reported a selective enrichment of GluN2C in the rat cerebellum. Regarding the anatomical distribution of synaptic GABAA receptor subunit, we observed a widely and rather similar distribution of alpha 1 in both human and rat brain. The alpha 3 subunit was expressed in the forebrain of both species, but expressed in the cerebellum only in rats. The beta 1 subunit was expressed mainly in human cortex and rat hippocampus, while the beta 2 subunit was expressed broadly in both human and rat brain. Interestingly, the gamma 2 subunit was broadly expressed in human brain synaptosomes, but enriched in rat cerebellum.
Fig. 3.
Biochemical characterisation of synaptosomes prepared from human cortex and rat prefrontal cortex and human and rat hippocampus and cerebellum. Immunoblot analysis of A–B) human synaptosomes and C–D) rat synaptosomes for: AMPA (GluA1-2-3-4), NMDA (GluN1-2A-2B-2C-2D) and GABAA (α1–3; β1–2; γ2) receptor subunits. The same amount of protein was loaded in each well (a representative GADPH is shown). B–D) On the y-axis, the intensity of protein signal is indicated in Arbitrary Units (A.U.), normalised to GADPH. Black bar: human cortex (B) or rat prefrontal cortex (D); grey bar: hippocampus; white bar: cerebellum.
Time course of expression and calcium dependency of insertion of native channels into the plasma membrane of Xenopus oocytes
The time course of incorporation of GABA and NMDA ion channels was evaluated by recording ion currents evoked by application of 1 mM GABA and 300 µM NMDA at different times after the injection of oocytes with rat PFC synaptosomes (Supplemental Fig S3 A–B). Consistent with previous findings with total membranes (Bernareggi et al., 2007) the responses were detectable within 5 hours after the injection and peaked between 16–42 hours. In order to assess if the fusion of synaptosomes with plasma membrane of the oocytes is Ca2+ dependent, oocytes were injected with rat PFC synaptosomes and incubated in Barth’s solution containing 100 µM BAPTA-AM, a cell permeable Ca2+ chelator, for 5 hours before recording (Supplemental Fig S3 C–D). In oocytes treated with BAPTA-AM significantly smaller GABA and NMDA-evoked responses were measured, suggesting that Ca2+ is important for the fusion of synaptosomes with the oocyte plasma membrane.
Pharmacological characterisation of GABAA, AMPA and NMDA currents from rat prefrontal cortex synaptosomes
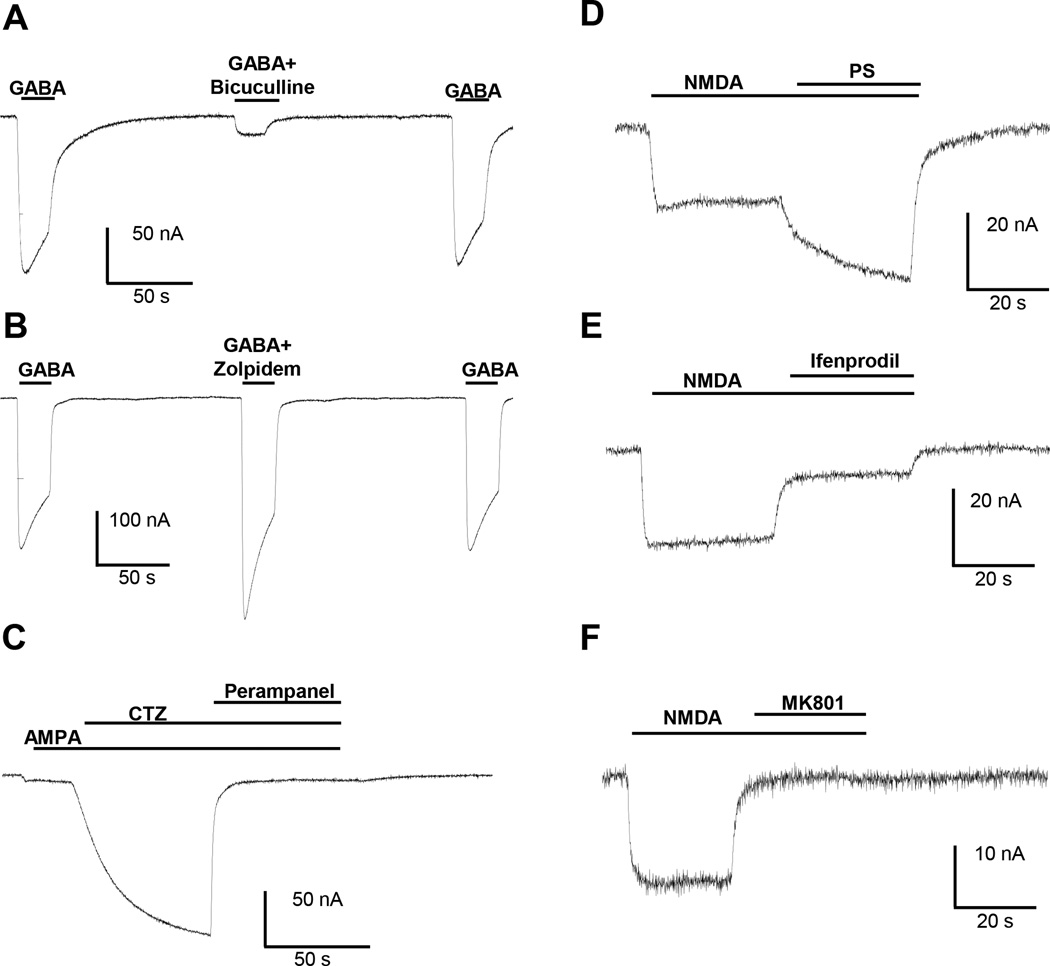
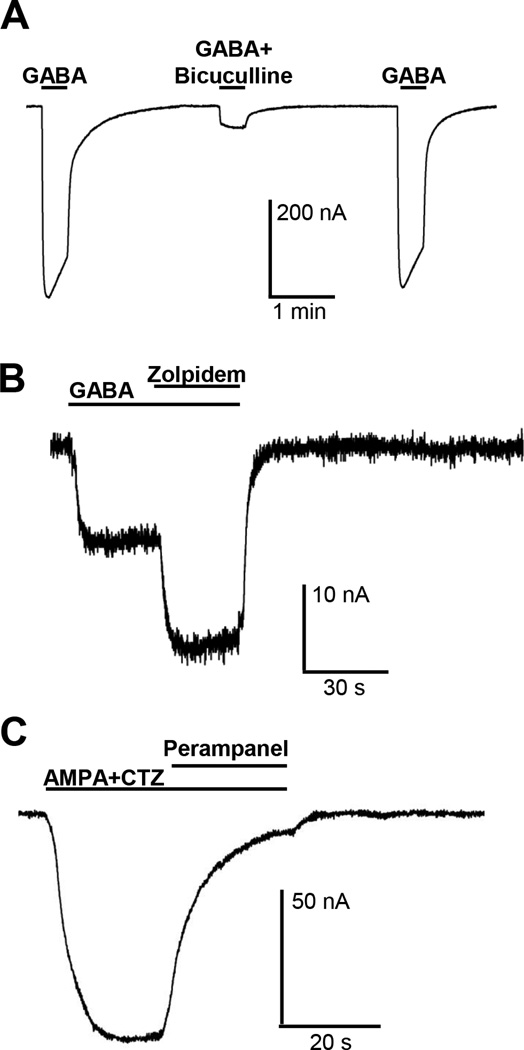
To determine the neurotransmitter sensitivity of the transplanted receptors, we applied different concentrations of GABA to the oocytes and we obtained concentration-response curves for rat prefrontal cortex, hippocampus and cerebellum (Supplemental Figure S4). Application of 100 µM of the GABAA receptor agonist GABA induced large inward currents that decayed to baseline upon washout of GABA. These currents were inhibited by 85% upon co-application of 80 µM of the GABAA receptor antagonist bicuculline (Straughan et al., 1971). Inhibition by bicuculline was reversed within 2 minutes of washout of the drug (Fig. 4A). Figure 4B shows that inward currents evoked by 100 µM GABA were potentiated by 144% by the GABAA receptor modulator zolpidem (Arbilla et al., 1985) and this potentiating effect of zolpidem was completely reversed within two minutes of washout of the drug.
Fig. 4.
Rat prefrontal cortex synaptosomes. A) 20 s co-application of 100 µM GABA and 80 µM bicuculline resulted in a large inhibition of 100 µM GABA-induced responses in oocytes injected with rat prefrontal cortex synaptosomes (N=12; 85% inhibition; paired T-test ****<0.0001). B) 20 s co-application of 100 µ µM GABA and 1 µM zolpidem resulted in a significant potentiation of the 100 µM GABA-induced response in oocytes injected with rat prefrontal cortex synaptosomes (N=3; 144% potentiation; paired T-test *<0.05) . C) 60 s co-application of 100 µM AMPA and 30 µM CTZ resulted in a large potentiation of AMPA-induced responses in oocytes injected with rat prefrontal cortex synaptosomes (N=16; 1800% potentiation; paired T-test ****<0.0001). The potentiated AMPA response is blocked by application of 30 µM perampanel (N=7; 97% inhibition; paired T-test ***<0.001). D) 30 s co-application of 300 µM NMDA and 50 µM pregnenolone sulphate (PS) resulted in a large potentiation of 300 µM NMDA-induced response in oocytes injected with rat prefrontal cortex synaptosomes (N=4; 177% potentiation; paired T-test *<0.05). E) 30 s co-application of 300 µM NMDA with 10 µM Ifenprodil resulted in a significant inhibition of the 300 µM NMDA-induced response (N=5; 75% inhibition; paired T-test **<0.01). F) 30 s co-application of 300 µM NMDA and 1 µM MK801 resulted in a significant inhibition of 300 µM NMDA-induced response (N=4; 100% inhibition; paired T-test ***<0.001).
In contrast to GABA, application of 100 µM agonist AMPA produced very weak ion current in oocytes injected with synaptosomes from rat prefrontal cortex (Fig. 4C). In order to detect AMPA receptor activity reliably, AMPA was routinely co-applied with 30 µM cyclothiazide, a positive allosteric modulator of AMPA receptors (Bertolino et al., 1993). Ion currents induced with the combination of 100 µM AMPA and 30 µM cyclothiazide were almost completely blocked by 30 µM of perampanel, an antagonist of AMPA receptors (Zwart et al., 2014b).
One batch of rat PFC synaptosomes gave small, but detectable inward currents upon application of 300 µM of NMDA. These currents were potentiated to 177% of control upon co-application of 300 µM of NMDA with 50 µM of pregnenolone sulfate, a positive allosteric potentiator of NMDA receptors (Malayev et al., 2002) (PS; Fig 4D). NMDA-induced inward currents were inhibited by 75% by 10 µM ifenprodil, a selective GluN2B antagonist (Williams, 1993) and by 100% by 1 µM MK801, a selective and non-competitive NMDA receptor antagonist (Huettner et al., 1987). The pharmacology of GABAA, AMPA and NMDA receptors were studied also in rat hippocampus (Supplemental Fig S5) and rat cerebellum (Supplemental Fig S6) synaptosomes.
Effect of TARP gamma-8-selective AMPA receptor antagonist in oocytes injected with rat hippocampal and cerebellar synaptosomes
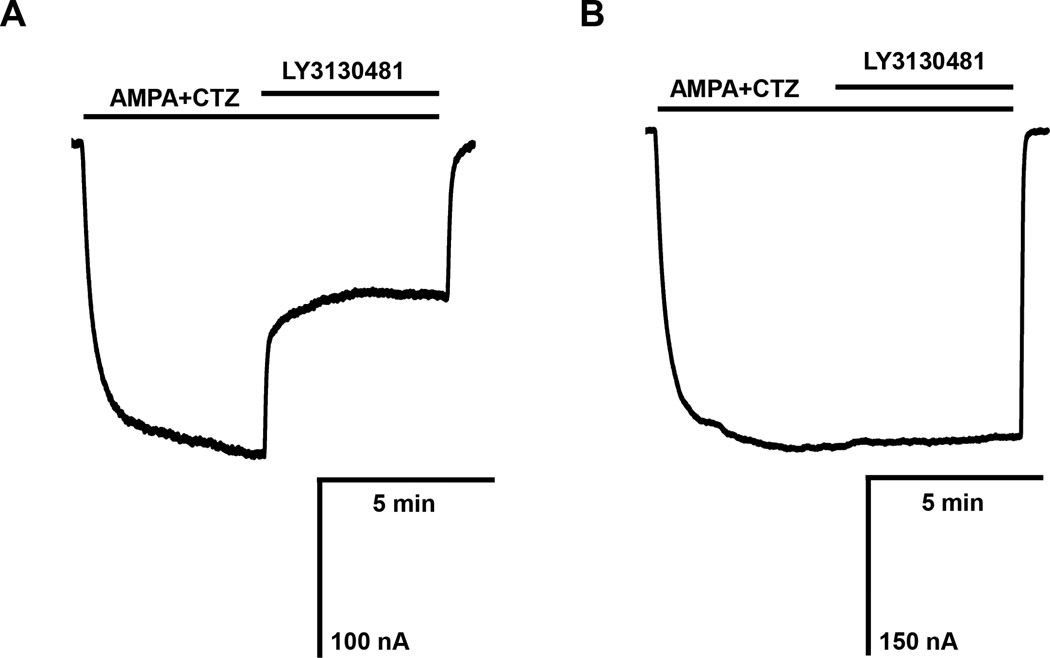
A possible complication of this approach might be the loss of protein-protein complexes during the preparation procedure of synaptosomes. To address this point, we studied the effect of a novel TARP gamma-8-dependent AMPA receptor antagonist, LY3130481 (Gardinier et al., 2016; Kato et al., 2015; Maher et al., 2016). Transmembrane AMPA Receptor Regulatory Proteins (TARPs) are a family of scaffolding proteins that regulate AMPA receptor trafficking and function. TARP proteins have distinct regional expression in the brain. TARP gamma-8 is the predominant subtype expressed in the hippocampus, whereas its expression in the cerebellum is very low. To demonstrate that reconstituted synaptic ion channels in the oocyte membrane retain accessory proteins necessary for their function, we tested the effect of LY3130481 in oocytes injected with rat hippocampal and cerebellar synaptosomes (Fig. 5). Compound LY3130481 at 10 µM inhibited responses induced by 100 µM AMPA and 30 µM cyclothiazide by 55.0 ± 6.8%, whereas it left similar responses in oocytes injected with cerebellar synaptosomes unaffected.
Fig. 5.
Effect of 10 µM of the TARP gamma-8-selective antagonist LY3130481 on AMPA responses in oocytes injected with rat hippocampal (A) and rat cerebellar synaptosomes (B). AMPA receptors were activated by co-applying 100 µM AMPA and 30 µM CTZ and after 5 minutes, 10 µM LY3130481 was applied with AMPA and CTZ. Consistent with the high expression of TARP gamma-8 in the hippocampus and the low expression of TARP gamma-8 in the cerebellum, LY3130481 inhibited the AMPA receptor-mediated responses in oocytes injected with rat hippocampal synaptosomes by 55.0 ± 6.8 % (n=3) and did not inhibit AMPA receptors in oocytes injected with B) rat cerebellar synaptosomes (n=3).
Pharmacological characterisation of GABAA and AMPA currents from human cortical synaptosomes
As with the rodent synaptosomes, the injection of human synaptosomes into Xenopus oocytes also led to the reconstitution of native human synaptic GABAA and AMPA receptors in the plasma membranes of the oocytes. Figure 6A shows that 1 mM GABA evokes large inward currents that were reversibly blocked by the co-application of 80 µM bicuculline, with an inhibition of 89%. Zolpidem, a positive allosteric modulator of GABAA receptors, potentiated ion currents evoked by a low concentration of GABA (30 µM) to 212% (Fig. 6B). Upon co-application of the AMPA receptor agonist AMPA with the AMPA receptor allosteric modulator cyclothiazide large inward currents were observed that were nearly completely blocked by the AMPA receptor antagonist perampanel (Fig. 6C). Application of NMDA to oocytes injected with human synaptosomes did not evoke ion currents that were large enough to be characterized pharmacologically in more detail.
Fig. 6.
Human cortex synaptosomes. A) 20 s co-application of 1 mM GABA and 80 µM bicuculline resulted in a large inhibition of the GABA-induced response in oocytes injected with human cortex synaptosomes (N=3; 89% inhibition). B) 20 s co-application of 30 µM GABA and 1 µM zolpidem resulted in a large potentiation of GABA-induced response (N=3; 212% potentiation). C) AMPA receptor-mediated responses evoked by co-applying 100 µM AMPA and 100 µM CTZ were largely blocked by 30 µM of the AMPA receptor antagonist perampanel (N=3; 91% inhibition).
Studies on recombinant NMDA receptors
The poor expression of NMDA receptors after injection of either rat or human synaptosomes was puzzling. In order to ensure our protocols were suitable to record NMDA currents and that membrane microinjection could work also for NMDA receptors, we used two approaches. First we injected oocytes with plasmids containing recombinant human GluN1 and GluN2B subunits. Second, we purified and microinjected membranes from HEK293 cells overexpressing human GluN1/GluN2B NMDA receptors (Supplemental Fig S7). In both cases, robust NMDA currents with amplitudes ranging from a few hundreds of nA to over a µA were measurable in the oocytes. This suggests that NMDA receptors are either expressed at a lower overall density in native synaptosomes, or that they are particularly vulnerable to some form of “inactivation” during synaptosome preparation and microinjection. However, as shown before (Fig 3 and supplemental Figure 1) the actual NMDA subunit proteins were readily detectable in western blots from synaptosomes, and were not significantly degraded.
Pharmacological and biochemical study of synaptic proteins and ion channels from human cortical control and AD brain
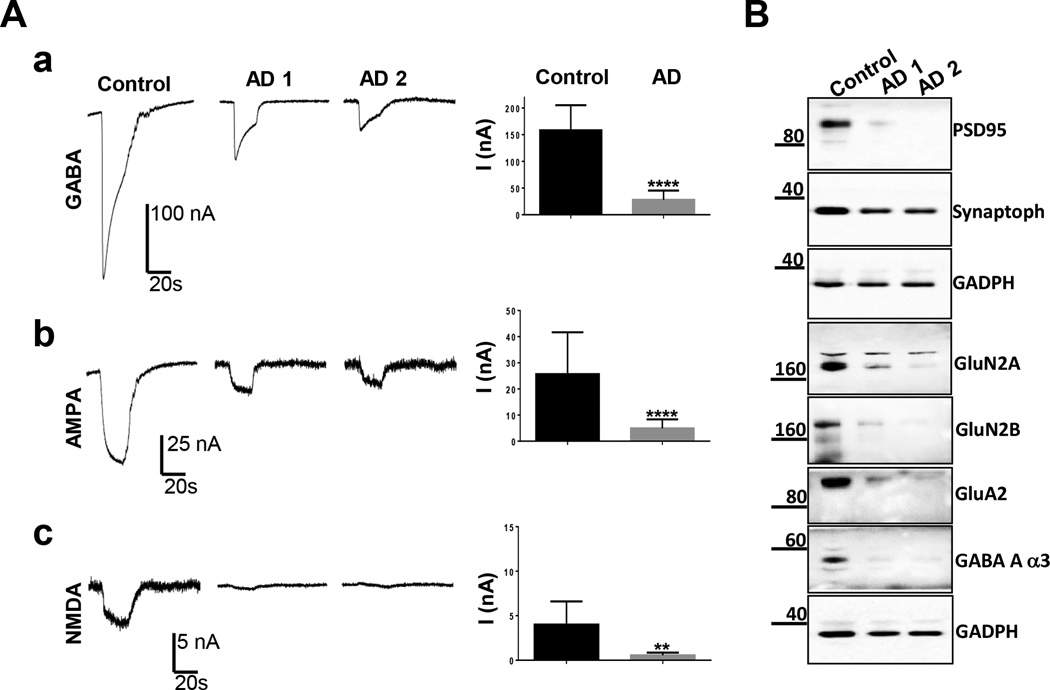
Alzheimer’s disease (AD) is characterised by loss of synaptic markers and degeneration of excitatory and inhibitory pathways (Selkoe et al., 2002). Previous studies revealed a significant decrease in the amplitude of GABA and glutamate currents in oocytes transplanted with total membranes from AD brains (Miledi et al., 2004; Bernareggi et al., 2007; Limon et al., 2012). Here, we decided to focus on synaptic ion channels, and therefore measured GABA, AMPA and NMDA protein levels and functional currents in oocytes injected with human control and AD synaptosomes (Figure 7). As expected, we observed a reduction in the protein levels of pre- (synaptophysin) and post-synaptic markers (PSD95) in synaptosomes purified from the cortex of two cases of AD (Braak stage 4), with a more pronounced loss for PSD95 (Fig 7B). NMDA GluN2A and 2B, AMPA GluA2 and GABA alpha3 receptor subunits were also significantly reduced (Fig 7B). We also found that the currents induced by GABA, AMPA and NMDA were significantly reduced, in parallel to the reduction in protein levels, in the AD samples (Fig 7A). While these data are from only a few individuals, and certainly need confirmation in a larger number of patients, the nice correlation between biochemistry and electrophysiology is promising.
Fig. 7.
Human AD cortex synaptosomes. A) Sample currents elicited by a) 1 mM GABA, b) 100 µM AMPA + 100 µM CTZ, and c) 300 µM NMDA + 30 µM pregnenolone sulfate (PS) in oocytes injected with synaptosomes from control and two AD cortex brains (AD1 and AD2). The AD group (n=2 cases, 21 cells for a), 16 cells for b) and 9 cells for c) gave smaller responses than the control group (n=1 case, 10 cells for a-b) and 6 cells for c). Paired T-test, P**<0.01; P****< 0.0001. B) Western blots of differential expression of synaptic markers in control versus human AD cortex (Case 1 and 2).
Discussion
We have used a combination of biochemical and electrophysiological techniques to show that synaptosomes isolated from both rodent and human brain can be successfully used to reconstitute native synaptic ion channels into the plasma membranes of Xenopus oocytes. Ion currents mediated by native, synaptic, GABA, AMPA and NMDA receptors have been recorded and their pharmacology evaluated. Our present results expand on the previously reported use of total human brain membranes in a series of papers by Miledi and colleagues (Palma et al., 2002; Miledi et al., 2006; Bernareggi et al., 2007; Limon et al., 2012) and also by ourselves (Zwart et al., 2014b) and will most likely lead to a better understanding of native human synaptic ion channels under normal and pathological conditions.
The more reliable ion currents measurable in the synaptosomal preparations were those activated by GABA. We could easily generate GABA dose-response curves in synaptosomes and compare their properties in different regions (Supplemental Figure S4) or total membranes (not shown). Overall our results with synaptosomes do not differ significantly from those reported previously with total human membranes (Miledi et al., 2004). The data were also similar to a previous report, utilising the same micro-transplantation technique, but with rat cerebellar synaptosomes (Sanna et al., 2006). Because we identified multiple GABA receptor subunits in the synaptosomal membranes biochemically, it is likely that GABA currents were mediated by a mixture of GABA receptor subtypes. One future goal is to try to modulate the currents from different synaptosomes with more selective GABA receptor modulators and try to better define their regional selectivity in human brain.
Glutamate receptors were more difficult to study in the synaptosomal preparations. While AMPA activated currents were measurable most of the time, they were relatively small and, therefore, were routinely investigated in the presence of the positive allosteric modulator cyclothiazide (Zwart et al., 2014b). This, nevertheless, allowed us to demonstrate that rat and human synaptic AMPA currents were blocked by perampanel, a novel anti-epileptic drug, in all regions tested. Furthermore, using the novel TARP gamma-8 selective AMPA receptor antagonist, LY3130481, we demonstrated that AMPA currents were selectively inhibited in the hippocampusbut not in cerebellum, consistent with the known regional expression of TARP gamma 8 in the brain. This finding also confirmed that reconstituted synaptic ion channels in oocyte membranes maintain the integrity of complexes with accessory proteins that are required for their function. While synaptosomal NMDA activated currents were even weaker than the AMPA-activated currents, we managed to get large enough currents to evaluate their pharmacology in the rat PFC. In this case, we showed that the currents were potentiated by pregnenolone sulfate, a GluN2A and 2B preferring potentiator (Malayev et al., 2002), and significantly blocked by the GluN2B selective antagonist ifenprodil (Williams et al., 1993). The two results in combination suggest a major contribution of the GluN2B subunit to these synaptic currents. However, in other regions, the NMDA currents were too weak to allow pharmacological studies. The presence of protease and phosphatase inhibitors in the buffers did not help. Likewise, increasing the holding potential, removing Mg2+ and/or adding glycine did not help. It is possible that a combination of low expression together with a specific susceptibility to metabolic degradation in post-mortem brain contribute to the lack of a robust current. On the other hand, we showed that our recording system is efficient for the study of both recombinant NMDA receptors, as well as of membranes purified from a cell line overexpressing recombinant NMDA receptors and micro-transplanted into the oocytes.
Despite these challenges, we decided to biochemically and electrophysiologically profile the expression of the various channel classes in synaptosomes purified from human control and Alzheimer’s brain. In the few individuals we studied, we identified a striking correlation between the level of ion channel protein expression and size of the functional currents. GABAA, AMPA and NMDA subunits were all expressed at much lower levels in Alzheimer’s versus control synaptosomes. In parallel, ion currents through these channels were significantly reduced, or absent, in the Alzheimer’s samples. Similar findings were reported by using total brain membranes (Miledi et al., 2004) but, to the best of our knowledge, this is the first direct functional evidence of dramatic ion channel loss in the surviving synapses from Alzheimer’s brains.
Supplementary Material
Acknowledgments
We would like to thank Drs Eleonora Palma, Marcia Roy and Seth Grant for early suggestions and for sharing protocols. RZ, ES, KMG, WP and JR are permanent employees of Eli Lilly and Company, FM is a post-doc funded by Eli Lilly and Co., GMS was an Erasmus student training in ES laboratory, and GM a visiting PhD student from the University of Florence. We would also like to thank Donna Farley (Eli Lilly, Indianapolis) for help with obtaining human brain tissues from the Oregon Alzheimer’s Disease Center (supported by NIH grant P30AG008017).
Abbreviations
- GABA
γ-Aminobutyric acid
- AMPA
(RS)-α-Amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid
- NMDA
N-Methyl-D-aspartic acid
- TARP
Transmembrane AMPA Receptor Regulatory Protein
Footnotes
Conflicts of interest: The authors have no conflict of interest to declare.
Supporting information
Refer to JNC’s web site for supplementary material.
References
- Arbilla S, Depoortere H, George P, Langer SZ. Pharmacological profile of the imidazopyridine zolpidem at benzodiazepine receptors and electrocorticogram in rats. Naunyn Schmiedebergs Arch. Pharmacol. 1985;330:248–251. doi: 10.1007/BF00572441. [DOI] [PubMed] [Google Scholar]
- Bagal SK, Brown AD, Cox PJ, Omoto K, Owen RM, Pryde DC, Sidders B, Skerratt SE, Stevens EB, Storer RI, Swain NA. Ion channels as therapeutic targets: a drug discovery perspective (2013) J. Med. Chem. 2013;56:593–624. doi: 10.1021/jm3011433. [DOI] [PubMed] [Google Scholar]
- Bayés À, Collins MO, Galtrey CM, Simonnet C, Roy M, Croning MDR, Gou G, van de Lagemaat LN, Mildward D, Whittle IR, Smith C, Choudhary JS, Grant SGN. Human post-mortem synapse proteome integrity screening for proteomic studies of postsynaptic complexes. Mol. Brain. 2014;7:88. doi: 10.1186/s13041-014-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernareggi A, Duenas Z, Reyes-Ruiz JM, Ruzzier F, Miledi R. Properties of glutamate receptors of Alzheimer’s disease brain transplanted to frog oocytes. Proc. Natl. Acad. Sci. (USA) 2007;104:2956–2960. doi: 10.1073/pnas.0611513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino M, Baraldi M, Parenti C, Braghiroli D, DiBella M, Vicini S, Costa E. Modulation of AMPA/kainate receptors by analogues of diazoxide and cyclothiazide in thin slices of rat hippocampus. Receptors and Channels. 1993;1:267–278. [PubMed] [Google Scholar]
- Broad LM, Zwart R, Pearson KH, Lee M, Wallace L, McPhie GI, Emkey R, Hollinshead SP, Dell CP, Baker SR, Sher E. Identification and pharmacological profile of a new class of selective nicotinic acetylcholine receptor potentiators. J. Pharmacol. Exp. Ther. 2006;318:1108–1117. doi: 10.1124/jpet.106.104505. [DOI] [PubMed] [Google Scholar]
- Carbone E, Sher E, Clementi F. Calcium currents in human neuroblastoma IMR32 cells: kinetics, permeability and pharmacology. Plügers. Arch. (Eur. J. Physiol.) 1990;416:170–179. doi: 10.1007/BF00370239. [DOI] [PubMed] [Google Scholar]
- Carlin RK, Grab JD, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J. Cell Biol. 1980;86:831–843. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzidaki A, Fouillet A, Li J, Dage J, Millar NS, Sher E, Ursu D. Pharmacological characterisation of nicotinic acetylcholine receptors expressed in human iPSC-derived neurons. PLoS ONE. 2015;10:e0125116. doi: 10.1371/journal.pone.0125116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Clementi F, Hukovic N, Sher E. Neuronal-type alpha-bungarotoxin receptors and the alpha-5 nicotinic receptor subunit gene are expressed in neuronal and non-neuronal human cell lines. Proc. Natl. Acad. Sci. (USA) 1992;89:1572–1576. doi: 10.1073/pnas.89.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dage JL, Colvin EM, Fouillet A, Langron E, Roell WC, Li J, Mathur SX, Mogg AJ, Schmitt MG, Felder CC, Merchant KM, Isaac J, Broad LM, Sher E, Ursu D. Pharmacological characterisation of ligand- and voltage-gated ion channels expressed in human iPSC-derived forebrain neurons. Psychopharmacology. 2014;231:1105–1124. doi: 10.1007/s00213-013-3384-2. [DOI] [PubMed] [Google Scholar]
- Day NC, Williams TL, Ince PG, Kamboj RK, Lodge D, Shaw PJ. Distribution of AMPA-selective glutamate receptor subunits in the human hippocampus and cerebellum. Mol. Brain Res. 1995;31:17–32. doi: 10.1016/0169-328x(95)00021-j. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Robinson PJ. A rapid Percoll gradient procedure for preparation of synaptosomes. Nat. Protoc. 2008;3:1718–1728. doi: 10.1038/nprot.2008.171. [DOI] [PubMed] [Google Scholar]
- Eusebi F, Palma E, Amici M, Miledi R. Microtransplanation of ligand-gated receptor-channels from fresh or frozen nervous tissue into Xenopus oocytes: a potent tool for expanding functional information. Prog. Neurobiol. 2009;88:32–40. doi: 10.1016/j.pneurobio.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Gardinier KM, Gernert DL, Porter WJ, Reel JK, Ornstein PL, Spinazze P, Stevens FC, Hahn P, Hollinshead SP, Mayhugh D, Schkeryantz J, Khilevich A, De Frutos O, Gleason SD, Kato AS, Luffer-Atlas D, Desai PV, Swanson S, Burris KD, Ding C, Heinz BA, Need AB, Barth VN, Stephenson GA, Diseroad BA, Woods TA, Yu H, Bredt D, Witkin JM. The Discovery of The First α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid (AMPA) Receptor Antagonist Dependent Upon Transmembrane AMPA Receptor Regulatory Protein (TARP) Gamma-8. J Med Chem. 2016 doi: 10.1021/acs.jmedchem.6b00125. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Grant SGN. Synaptopathies: diseases of the synaptome. Curr. Opin Neurobiol. 2012;22:522–529. doi: 10.1016/j.conb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Gray EG, Whittaker VP. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J. Anat. 1962;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc. Natl. Acad. Sci. (USA) 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Ding C, Gardinier KM, Gernert DL, Yu H, Zwart R, Wang H, Qian Y, Pasqui F, Sher E, Burris K, Isaac JTR, Witkin JM, Bredt DS, Nisenbaum ES. Discovery of a forebrain-specific AMPA receptor antagonist: Electrophysiological characterization of a TARP gamma-8-dependent AMPA receptor antagonist. Soc. Neurosc. Abstr 207.17. 2015 [Google Scholar]
- Limon A, Reyes-Ruiz JM, Miledi R. Loss of functional GABAA receptors in the Alzheimer diseased brain. Proc. Natl. Acad. Sci. (USA) 2012;109:10071–10076. doi: 10.1073/pnas.1204606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher MP, Wu N, Ravula S, Ameriks MK, Liu C, Lord B, Wyatt RM, Matta JA, Dugovic C, Yun S, Ver Donck L, Steckler T, Wickenden AD, Carruthers NI. and Lovenberg TW. Discovery and characterization of AMPA receptor modulators selective for TARP-γ8. JPET. 2016 doi: 10.1124/jpet.115.231712. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Malayev A, Gibbs TT, Farb DH. Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. Br. J. Pharmacol. 2002;135:901–909. doi: 10.1038/sj.bjp.0704543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R, Duenas Z, Martinez-Torres A, Kawas CH, Eusebi F. Microtransplantation of functional receptors and channels from the Alzheimer’s brain to frog oocytes. Proc. Natl. Acad. Sci. (USA) 2004;101:1760–1763. doi: 10.1073/pnas.0308224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R, Palma E, Eusebi F. Microtransplantation of neurotransmitter receptors from cells to Xenopus oocyte membranes: new procedure for ion channel studies. Meth. Mol. Biol. 2006;322:347–355. doi: 10.1007/978-1-59745-000-3_24. [DOI] [PubMed] [Google Scholar]
- Mitewa S, Kirkcaldie MTK, Dickson TC, Vickers JC. Altered synapses and gliotransmission in Alzheimer’s disease and AD model mice. Neurobiol. of Aging. 2013;34:2341–2351. doi: 10.1016/j.neurobiolaging.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Palma E, Esposito V, Mileo AM, Di Gennaro G, Quarato P, Giangaspero F, Scoppetta C, Onorati P, Trettel F, Miledi R, Eusebi F. Expression of human epileptic temporal lobe neurotransmitter receptors in Xenopus oocytes: an innovative approach to study epilepsy. Proc. Natl. Acad. Sci. (USA) 2002;99:15078–15083. doi: 10.1073/pnas.232574499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Sanna E, Motzo C, Murgia A, Amato F, Deserra T, Biggio G. Expression of native GABAA receptors in Xenopus oocytes injected with rat brain synaptosomes. J. Neurochem. 1996;67:2212–2214. doi: 10.1046/j.1471-4159.1996.67052212.x. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Straughan DW, Neal MJ, Simmonds MA, Collins GG, Hill RG. Evaluation of bicuculline as a GABA antagonist. Nature. 1971;233:352–354. doi: 10.1038/233352a0. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro . J. Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SI, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr. Neurol. Neurosci. Rep. 2010;10:207–214. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglis G, Tavernarakis N. The role of synaptic ion channels in synaptic plasticity. EMBO Reports. 2006;7:1104–1110. doi: 10.1038/sj.embor.7400830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Scheurer L, Kunzi R, Fritschy JM. Distribution of NMDA receptor subunit proteins NR2A, 2B, 2C and 2D in rat brain. Neuroreport. 1995;7:45–48. [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-Methyl-D-Aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol. Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, Millar NS. Potentiation of α7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc. Natl. Acad. Sci. (USA) 2008;105:14686–14691. doi: 10.1073/pnas.0804372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, Broad LM, Williams AC, Zhang D, Ding C, Heinz BA, Sher E. Sazetidine-A is a potent and selective agonist at native and recombinant α4β2 nicotinic acetylcholine receptors. Mol. Pharmacol. 2008;73:1838–1843. doi: 10.1124/mol.108.045104. [DOI] [PubMed] [Google Scholar]
- Zwart R, Strotton M, Ching J, Astles PC, Sher E. Unique pharmacology of heteromeric α7β2 nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Eur. J. Pharmacol. 2014a;726:77–86. doi: 10.1016/j.ejphar.2014.01.031. [DOI] [PubMed] [Google Scholar]
- Zwart R, Sher E, Ping X, Jin X, Sims JR, Jr, Chappell AS, Gleason SD, Hahn PJ, Gardinier K, Gernert DL, Hobbs J, Smith JL, Valli SN, Witkin JM. Perampanel, an antagonist of α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid receptors, for the treatment of epilepsy: studies in human epileptic brain and nonepileptic brain and in rodent models. J. Pharmacol. Exp. Ther. 2014b;351:124–133. doi: 10.1124/jpet.114.212779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.