Abstract
Neurotrophins are a family of growth factors playing key roles in the survival, development, and function of neurons. The neurotrophins BDNF and NT4 both bind to and activate TrkB receptors, however, they mediate distinct neuronal functions. The molecular mechanism of how TrkB activation by BDNF and NT4 leads to diverse outcomes is unknown. Here we report that BDNF and NT4 lead to differential endocytic sorting of TrkB receptors resulting in diverse biological functions in cultured cortical neurons. Fluorescent microscopy and surface biotinylation experiments showed that both neurotrophins stimulate internalization of TrkB with similar kinetics. Exposure to BDNF for 2–3 hours reduced the surface pool of TrkB receptors to half, whereas a longer treatment (4–5 hours) with NT4 was necessary to achieve a similar level of downregulation. Whereas BDNF and NT4 induced TrkB phosphorylation with similar intensities, BDNF induced more rapid ubiquitination and degradation of TrkB than NT4. Interestingly, TrkB receptor ubiquitination by these ligands have substantially different pH sensitivities, resulting in varying degrees of receptor ubiquitination at lower pH levels. Consequently, NT4 was capable of maintaining longer sustained downstream signaling activation that correlated with reduced TrkB ubiquitination at endosomal pH. Thus, by leading to altered endocytic trafficking itineraries for TrkB receptors, BDNF and NT4 elicit differential TrkB signaling in terms of duration, intensity, and specificity, which may contribute to their functional differences in vivo.
Keywords: BDNF, NT4, TrkB, trafficking, ubiquitination, degradation
Introduction
Neurotrophins play critical roles in nervous system development and function. Four neurotrophins have been identified in vertebrates: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3) and neurotrophin 4 (NT4). Neurotrophins bind to and activate the tropomyosin-related kinase receptors (Trks), which are receptor tyrosine kinases (RTK) that initiate downstream signaling pathways through Ras, Akt and Erk (Huang & Reichardt 2003, Chao et al. 2006). TrkB receptors, the most prominently expressed Trk isotype in the brain, binds two neurotrophins with similar affinities: BDNF and NT4 (Banfield et al. 2001, Naylor et al. 2002). These 2 neurotrophins are expressed throughout the brain, though the expression levels of NT4 are quite low (Patz & Wahle 2006, Timmusk et al. 1993). Despite similar potency for receptor activation, BDNF and NT4 can lead to different cellular responses. While BDNF has been established as a major regulator of synaptic plasticity as well as higher order cognitive functions, ranging from learning and memory to psychiatric disorders (Hall et al. 2000, Bramham & Messaoudi 2005, Chao et al. 2006, Chen et al. 2006, Patterson et al. 1996, Carvalho et al. 2008), the role of NT4 in synaptic transmission is less pronounced. Interestingly it has been shown that in certain cellular contexts, NT4 can act more potently than BDNF. For example, infusion of NT4 into the rat visual cortex was more efficient than BDNF in counteracting the effects of monocular deprivation (Lodovichi et al. 2000). NT4 also more potently supports survival of sensory neurons than BDNF; whereas two BDNF alleles are necessary to support nodosepetrosal neurons, a single NT4 allele is sufficient (Erickson et al. 1996). A genetic knock-in mouse study, where the BDNF gene was replaced by NT4, demonstrated that NT4 was more potent at inducing synaptic maturation and sensory neuron survival (Fan et al. 2000).
Modulation of endocytic trafficking has been established to be coupled with regulation of signaling specificity and intensity of RTK activity (Irannejad et al. 2015). Internalization of activated RTKs and subsequent targeting to the lysosome results in downregulation of the activated signaling receptor (Sommerfeld et al. 2000, Saxena et al. 2005, Knusel et al. 1997, Jullien et al. 2002). On the other hand, post-endocytic recycling of RTKs contributes to sustained signaling (Maxfield & McGraw 2004, Sorkin & von Zastrow 2009). Here, we hypothesized that the distinct biological outcomes achieved with BDNF and NT4 are due to differential sorting of TrkB receptors to distinct endocytic routes. Indeed we found that BDNF leads to more rapid TrkB ubiquitination and degradation than NT4. As a result, NT4 was capable of maintaining more sustained downstream signaling. Thus, these results suggest that the differential outcomes of TrkB-mediated signaling by these two neurotrophins are not associated with altered TrkB receptor activation, but rather rely on the duration of subsequent downstream signaling events.
Methods
Reagents and antibodies
Human recombinant BDNF and NT4 were obtained from PeproTech (Rocky Hill, NJ). Immunoprecipitations and immunocytochemistry were performed with the following antibodies: rabbit anti TrkB from Upstate or goat from R&D; rabbit SHP2 from Santa Cruz (SC-280); Flag and tubulin antibodies were purchased from Sigma. The following antibodies: TrkB, SHP2, EEA1 and Grb2 were purchased from BD Biosciences. The phosphotyrosine PY99, actin-HRP, Erk1, Erk2 were from Santa Cruz; tubulin from Sigma. Phospho-Erk, phosphor-Akt and Akt were from Cell Signaling. Alexa conjugated fluorescent secondary antibodies were from Molecular Probes. All other compounds were from Sigma-Aldrich. All reagents used to prepare primary neuronal cultures were purchased from Invitrogen, except glucose that was from Sigma.
Cell culture
Dissociated primary cultures of cortical neurons were prepared from timed-pregnant Sprague Dawley rats (Charles River Laboratories) at embryonic day 18 as described previously (Chen et al. 2005a). All animal procedures were approved by the Institutional Animal Care and Use Committees of Weill Cornell Medical College and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Embryos were dissected in Hanks Balanced solution (HBSS) supplemented with 0.37% glucose. Digestion was performed in the same medium supplemented with 0.05% trypsin for 10–15 minutes at 37°C. Neurons were mechanically dissociated and plated in plating medium (PM) (MEM containing 10% FBS, 1 mM pyruvate, 0.37% glucose, and 0.1 mg/ml of Primocin from Invivogen) for 24 hours. After one day in culture PM was replaced with Neurobasal supplemented with B-27, 0.5mM glutamine, 0.1 mg/ml Primocin and 2 μM Ara-C (Cytosine β-D-arabinofuranoside hydrochloride). Cells were grown on poly-D lysine-coated surfaces: on glass coverslips a density of 15x103 cells/cm2 was used for immunocytochemistry; on polystyrene dishes, a density of 76x103 cells/cm2 was used for biochemistry. Neurons were kept in a humidified incubator at 37°C and 5% CO2. 293 cells stably expressing TrkB were a kind gift of Moses Chao lab at NYU Medical center and were maintained in regular 293 media, DMEM (Invitrogen) containing 10% heat inactivated fetal bovine serum (GemCell), with 100 U/ml penicillin, 100 U/ml streptomycin (Pen/Strep) (Invitrogen), 2 mM glutamine (GlutaMAX from Invitrogen), supplemented with 250 μg/ml of Geneticin (Invitrogen).
Western blotting and immunoprecipitation
Protein lysates for western blot and immunoprecipitation were prepared in RIPA buffer (150 mM NaCl, 50 mM Tris pH 8.0; 5 mM EDTA, 1% Triton X-100, 0.5% DOC, 0.1% SDS) containing protease and phosphatase inhibitors (2 μg/ml leupeptin, 2 μg/ml aprotinin, 1 mM sodium orthovanadate, 10 mM sodium fluoride, and 1 mM phenylmethylsulfonyl fluoride). Extracts were rotated at 4°C for 15 minutes and cleared by centrifugation (12,000xg for 15 min). For immunoprecipitation, antibody was added at 1:100 concentration a rotated for 2 hours at 4°C, followed by pull down with either protein A beads (Sigma) or protein G (Roche), overnight at 4°C. Lysates were washed 3–6 times in lysis buffer. Western blotting was performed using 10% BIS-TRIS pre-cast gels from Invitrogen. Protein samples were boiled with 4X LDS Nupage sample buffer from Invitrogen (NP0007) for 2 minutes before loading. Electrophoresis was done at 80mA using MES-SDS running buffer from Invitrogen. Transfer was done at 4°C, 100V for 90 minutes. PVDF membranes were blocked in 5% milk-TBS-T or 3% BSA-TBS-T if PY99 was being used in (0.1% Tween). Antibodies were probed over-night at 4°C.
Surface biotinylation assays
Cell surface biotinylation was used to specifically detect receptors present in the plasma membrane and to measure their proteolysis. Neurons were washed with ice-cold PBS supplemented with Ca2+ and Mg2+ (PBS++) and incubated with 300 μg/ml of sulfo-NHS-S-biotin (Pierce Chemical, Rockford, IL) for 20 min on ice with gentle agitation. Unreacted biotin was quenched and removed with 50mM Glycine in PBS++. Biotinylated cells were transferred to pre-warmed medium containing ligand for the indicated times, and then cells were immediately chilled on ice and lysed in RIPA buffer. For internalization assay, after neurotrophin treatment remaining surface-bound biotin was cleaved with glutathione solution containing: 50 mM glutathione in 75 mM NaCl, 10 mM EDTA, 1% BSA, 0.075 M NaOH. Biotinylated proteins were isolated from cell extracts by immobilization on high capacity streptavidin-conjugated Sepharose beads (Pierce Chemical, Rockford, IL). Washed beads were eluted with SDS sample buffer, and eluted proteins were resolved by SDS-PAGE.
Immunocytochemistry
DIV5-7 rat cortical neurons were serum-starved over-night in Neurobasal supplemented with 2% glucose. For endocytosis assays, receptors were live labeled with antibody feeding at 37°C. Cells were fixed in 3.7% formaldehyde solution (from EMS) for 15 min at RT. Unreacted formaldehyde was quenched with glycine. Permeabilization was done with 0.2% Triton-X with gentle agitation at RT for 6 minutes. Primary antibodies were incubated for 60 min at RT, followed by washes in PBS with gentle agitation. Secondary antibodies alexa-conjugated were incubated for 20 min at RT. Antibodies were prepared in blocking solution 3% BSA, 10% donkey serum in PBS. Quantification of internalization index was performed blind to treatment ((Inter /(Intern + Surface))x100. For endocytosis measured with EEA1 quantification was done by manually counting total TrkB puncta and TrkB puncta overlapping EEA1, using the manually count objects tool in Metamorph software.
Fluorescence Microscopy
Images were acquired on an inverted microscope, Nikon Eclipse TE2000-U, light source was PhotoFluor from Chroma, using Metamorph Software. Objective used was PlanApo 60xA/1.40 oil Nikon. Image quantification was performed using ImageJ and Metamorph software.
Statistical analyses
Statistical analyses were performed using GraphPad Prism V5.0 software. Statistical significance was considered at * p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001 between the means of a minimum of three groups was determined using Student’s t test or one-way ANOVA or 2-way ANOVA test as indicated in the figure legends. Results are expressed as the mean value ± SD. All experiments were performed with at least three independent biological replicates.
Results
NT4 leads to efficient endocytosis and targeting of TrkB receptor to the early endocytic compartment
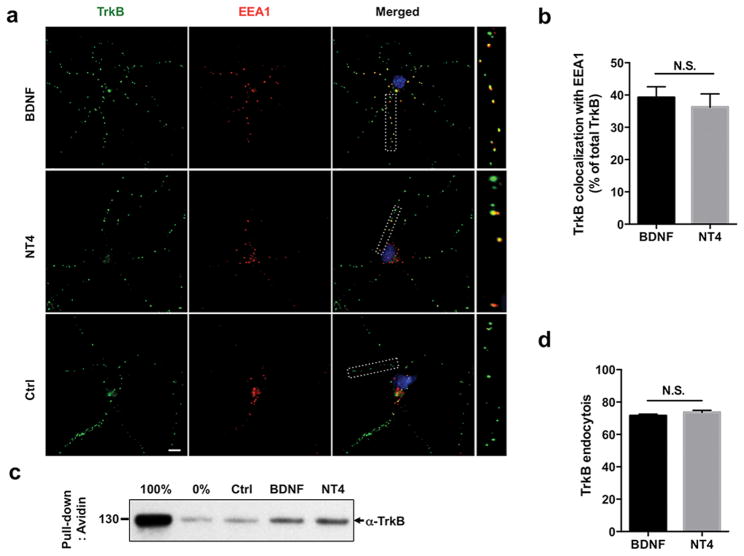
To determine how BNDF and NT4 may differentially modulate the endocytic trafficking of TrkB receptor, we first analyzed the initial endocytosis of TrkB upon ligand stimulation. While BDNF is known to induce endocytosis of TrkB in a clathrin-dependent manner to an early endocytic compartment (Zheng et al. 2008), the mechanisms of NT4-mediated TrkB trafficking is not known. To measure TrkB endocytosis and determine whether these neurotrophins target the receptor to similar endocytic compartments, we assessed by immunocytochemistry co-localization of activated TrkB with a marker for the early endosome EEA1 in cultured cortical neurons. Both BDNF and NT4 led to TrkB internalization that can be observed by the puncta-like structures corresponding to early endosomes (Fig. 1A). Quantification of TrkB co-localization with EEA1 revealed a comparable level of endocytosis at a 15 min time point, mediated by BDNF or NT4, suggesting that these ligands induce endocytosis through similar mechanisms (BDNF 39.2% ± 3.3%; NT4 35.7% ± 3.9%; n.s.: not significant) (Fig. 1A, B). A fraction of TrkB-positive puncta not co-localizing with EEA1 endosomes was located in distal neuronal processes, in agreement with previous studies showing that this marker is exclusively associated with endosomes of the somatodendritic compartment, and not with axonal endosomes (Wilson et al. 2000, Arevalo et al. 2006). To complement the immunocytochemical data, we measured TrkB internalization using a cleavable biotin assay (Saxena et al. 2005). In this assay, membrane proteins are surface biotinylated and after internalization has occurred, the remaining surface biotin is removed such that only internalized proteins are pulled down and analyzed by Western blots. In agreement with our previous observations, BDNF and NT4 elicit similar levels of TrkB endocytosis (Fig. 1C). Finally, using a fluorescent ratiometric assay (Cao et al. 1999, Tanowitz & von Zastrow 2003), we measured the surface-to-total TrkB ratio by quantifying fluorescence intensity of each fraction, and confirmed a comparable endocytosis rate induced by BDNF and NT4 (BDNF 72% ± 1.5%; NT4 74.3% ± 1.8%) (Fig. 1 D).
Figure 1. BDNF and NT4-induced endocytosis of TrkB receptor.
a) Rat cortical neurons were live fed with TrkB antibody on ice, and subsequently treated with 50 ng/ml of BDNF or NT4 for 15 min. Co-localization of TrkB antibody with the early endocytic marker EEA1 was analyzed. b) Quantification of percent TrkB co-localizing with EEA1 upon BDNF or NT4 treatment (BDNF 39.2% ± 3.3%; NT4 35.7% ± 3.9%; n.s= non-significant, Student's t test). Scale bar: 10 μm c) Biochemical internalization assay with HEK293 cells stably expressing TrkB receptor. Cells were surface biotinylated with cleavable sulfo-NHS-S-biotin that is cell impermeable. After a 50 ng/ml of BDNF/NT4 treatment and endocytosis had occurred for 15 min, remaining surface biotin was cleaved with a reducing agent, and avidin was used to pull down internalized proteins. Precipitates were run on Western blot and probed with TrkB antibody depicting the internalized pool only. As a positive control, biotin was not cleaved and as a negative control, biotin was cleaved without the incubation step. d) Endocytosis assay of TrkB receptor in rat cortical neurons in which the endogenous surface pool of TrkB was labeled using a live fed antibody on ice. After surface labeling, neurons were incubated at 37°C for an initial 10 min for temperature-adaptation and a further 15 min with BDNF or NT4, 50 ng/ml. After internalization, cells were fixed and TrkB receptor remaining on the surface was labeled with saturating concentrations of secondary antibody conjugated with alexa488, followed by permeabilization, after which the internalized pool was labeled with secondary antibody conjugated with alexa568. Control neurons were incubated without neurotrophins. Quantification of internalized TrkB (BDNF 72% ± 1.5%; NT4 74.3% ± 1.8%, Student's t test). Image quantification was done using ImageJ software.
BDNF induces more efficient ubiquitination of TrkB than NT4
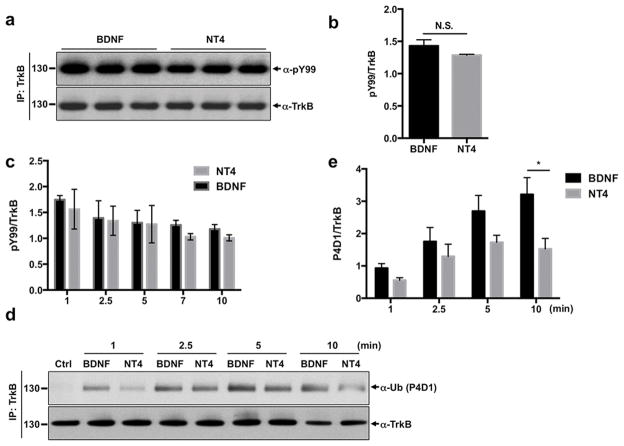
Ligand-dependent induction of RTK phosphorylation modulates the sorting of receptors through the endocytic pathway (Goh & Sorkin 2013). We asked whether BDNF and NT4 induced differential TrkB phosphorylation while in the early endosome compartment. Previous studies comparing TrkB activation with BDNF and NT4 utilized cell lines transfected with the receptor (Yuen & Mobley 1999). However studying ectopically expressed RTKs poses limitations since overexpression itself can promote receptor dimerization and activation in a ligand-independent manner. Thus, we analyzed the time course of endogenous TrkB phosphorylation in cortical neuronal cultures. We determined that BDNF and NT4 induced phosphorylation of TrkB receptor with similar kinetics and intensity (Fig. 2A–C).
Figure 2. BDNF induces more efficient ubiquitination of TrkB than NT4.
a) DIV5 rat cortical neurons were treated for 15 min with 50 ng/ml of BDNF or NT4. Neurons were subsequently lysed and TrkB was immunoprecipitated with a specific antibody. A phosphotyrosine antibody (PY99) was used to measure TrkB phosphorylation. BDNF and NT4 induce similar phosphorylation of TrkB receptor. Representative western blot was shown with PY99 antibody of immunoprecipiated TrkB. b) Phosphorylation levels were normalized to total TrkB levels and relative quantification is shown. (BDNF ratio 1.4 ± 0.09; NT4 ratio 1.3 ± 0.01, Student's t test). c) Time course of phosphorylation of TrkB receptor. Quantification was done using ImageJ software. Average of three independent experiments. d) Cortical neurons were treated for indicated times with 50 ng/ml of BDNF, NT4 or left untreated. TrkB receptor was immunoprecipitated and ubiquitination was analyzed by western blot with an ubiquitin antibody (P4D1). e) Quantification of four independent experiments is shown. *p < 0.05 between BDNF and NT4 groups, Student's t test.
Ubiquitination is a protein modification that plays a pivotal role on receptor sorting to lysosomes (Goh & Sorkin 2013). Ubiquitinated receptors are recognized by the multisubunit complexes ESCRT-I, II and III, and sorted to multivesicular bodies, that later mature into lysosomes (Hurley 2008, Piper et al. 2014). Thus, differential regulation of receptor ubiquitination can lead to either higher or lower protein degradation rates. Similar kinetics of TrkB phosphorylation and internalization upon BDNF and NT4 treatment suggested that TrkB ubiquitination might follow a similar pattern. Thus, we measured the time course of TrkB receptor ubiquitination when stimulated with BDNF or NT4. Unexpectedly, these two ligands display a marked difference in ubiquitination patterns. While both neurotrophins induce ubiquitination of TrkB in a time-dependent manner, BDNF exhibited more potent and more rapid TrkB ubiquitination than NT4 (Fig. 2D, E). BDNF shows a trend toward higher TrkB ubiquitination compared to NT4 as early as one minute after neurotrophin treatment, and the difference becomes significant at later time point (10 min). BDNF was more potent than NT4 in stimulating ubiquitination of TrkB both at 50 ng/ml and 10 ng/ml doses (Fig. S1).
BDNF and NT4 exhibit different pH sensitivities for TrkB activation in neurons
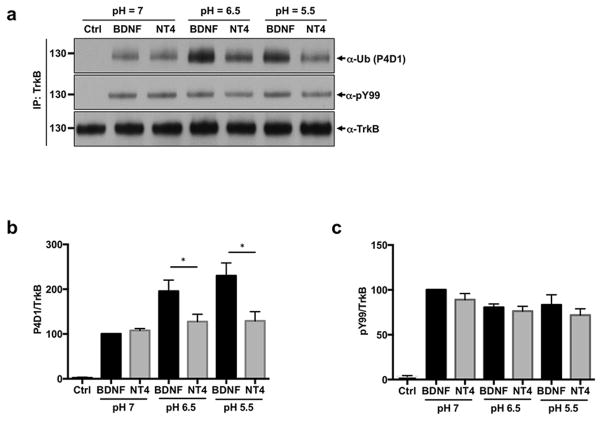
Our observation of differential TrkB ubiquitination is surprising considering the endocytosis results, which suggests that TrkB is sorted at the same rate to a similar endocytic compartment upon BDNF or NT4 stimulation. Thus, we sought to investigate the mechanisms mediating this differential ubiquitination. Recent studies have shown that NT3 and NGF exhibit different pH sensitivities for TrkA activation in PC12 cells. Whereas at certain ligand concentrations both can efficiently activate TrkA at pH=7, NT3 was unable to activate downstream signaling due to unstable interaction with TrkA within the acidic condition (Harrington et al. 2011). Thus, we examined the efficiency of NT4-mediated TrkB activation and ubiquitination at lower pHs in cortical neurons that mimic the environment of the early endosome (pH 5.5 and 6.5) (Fig. 3A–C). We found that BDNF induced greater ubiquitination of TrkB receptors at lower pHs as compared with NT4. Interestingly, the phosphorylation levels of TrkB by BDNF and NT4 was similar at all pHs tested, suggesting that these neurotrophins differ specifically in the mechanisms of TrkB ubiquitination (Fig. 3A, C).
Figure 3. BDNF and NT4 exhibit different pH sensitivities for TrkB activation in neurons.
DIV5 rat cortical neurons were stimulated with 50 ng/ml BDNF or NT4 for 15 min in Neurobasal buffered at pH 7.0, pH 6.5, or pH 5.5. Neurons were subsequently lysed and TrkB was immunoprecipitated with a specific antibody. A phosphotyrosine antibody (PY99) and a anti-ubiquitin antibody (P4D1) were used to measure TrkB phosphorylation and ubiquitination. a) Representative western blot with PY99 antibody and P4D1 antibody of immunoprecipiated TrkB. b) Ubiqutination levels were normalized to total TrkB levels and relative quantification is shown. *p < 0.05 between BDNF and NT4 groups, Student's t test. c) Phosphorylation levels were normalized to total TrkB levels and relative quantification is shown.
BDNF, but not NT4, targets TrkB receptor efficiently to the degradative pathway
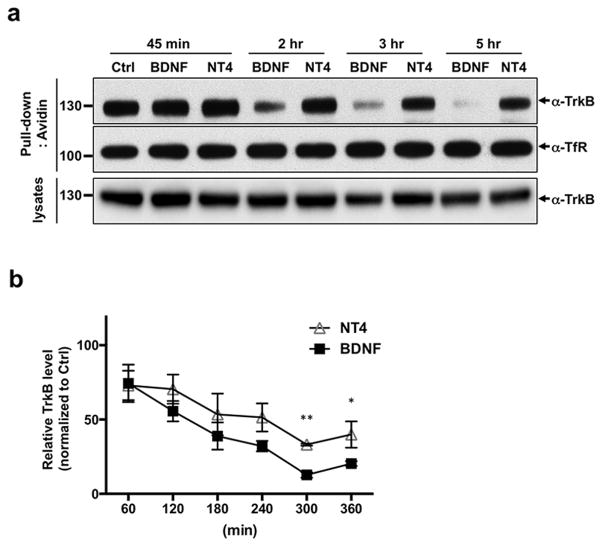
Considering the role of ubiquitination in sorting cargo to the degradative pathway, we questioned whether BDNF and NT4 induced different rates of TrkB receptor degradation. It has been previously shown that BDNF leads to effective downregulation of TrkB receptors in cultured cerebellar granule neurons (Sommerfeld et al. 2000); however, whether NT4 can induce TrkB degradation was not known. Considering that BDNF promotes greater initial TrkB ubiquitination (Fig. 2D, E), we hypothesized that sorting to the degradative pathway would be more efficient with BDNF than with NT4. To test this, we analyzed the time course of BDNF and NT4-induced TrkB degradation using a surface biotinylation degradation assay (Chen et al. 2005b). After biotinylation, cultured cortical neurons were treated with BDNF or NT4 for indicated times periods, lysed, and precipitated with streptavidin beads. Resulting pull-down samples were immunoblotted with TrkB antibodies, which assess the proteolysis of the endocytosed Trk receptors. Exposure of neurons to BDNF or NT4 for one hour reduced the TrkB protein levels to 75% compared to control, demonstrating that NT4 treatment also leads to TrkB downregulation (Fig. 4A, B). After 4 hours of BDNF treatment, TrkB protein levels were reduced to 30% of vehicle-treated neurons. At the same time point, cells treated with NT4 only had a reduction of TrkB levels to 60% compared to control. This difference was more evident at a 5-hour treatment when BDNF treatment led to a reduction to less than 15% of controls, whereas in cells treated with NT4 almost half of receptors were still available (reduction to 40%). Thus, we found that BDNF induced faster ubiquitination of TrkB receptors which led to enhanced downregulation of this receptor, whereas NT4 does not.
Figure 4. Time course degradation of TrkB receptor mediated by BDNF and NT4.
Cortical neurons were surface biotinylated with a cell impermeable sulfo-biotin. Subsequently neurons were incubated for the indicated times with 50 ng/ml of BDNF or NT4. A) Avidin pull-down precipitates were analyzed by western blot with a TrkB antibody and a transferrin receptor antibody. TrkB receptors in lysates were shown with a TrkB antibody. b) Quantification of TrkB degradation as percentage of the untreated control; represents an average of 5 independent experiments. *p < 0.05, **p < 0.01 between BDNF and NT4 groups, Student's t test.
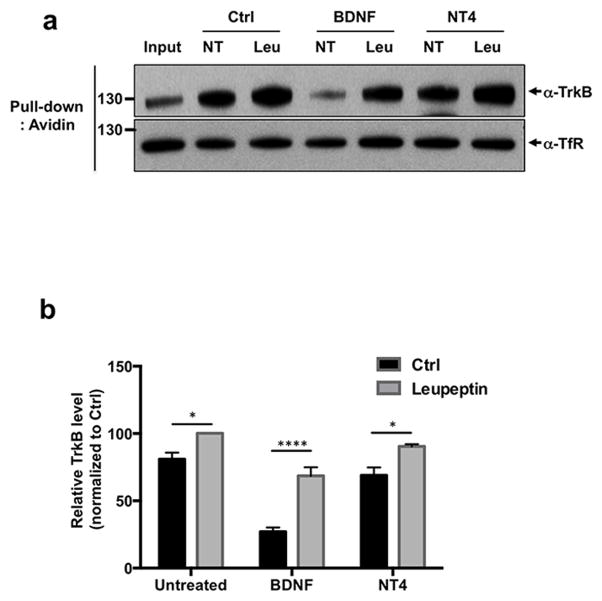
Lysosomal-dependent degradation of TrkB mediated by BDNF or NT4
The differences observed in TrkB downregulation suggest that BDNF and NT4 induce receptor degradation through different mechanisms. Previous studies have suggested that BDNF induced TrkB degradation is proteasome-dependent (Sommerfeld et al. 2000). Subsequent studies with TrkA showed that both the proteasome system and the lysosome play a sequence coordinated role in the degradation of this receptor (Geetha & Wooten 2008). Extensive studies analyzing RTK downregulation have highlighted the relevance of the lysosome for this pathway (Katzmann et al. 2002). To test if neurotrophin-mediated downregulation is lysosomal-dependent we performed the surface biotinylation degradation assay in the presence of a lysosome inhibitor, leupeptin (Fig. 5A, B). Given the unstable nature of this inhibitor in aqueous solutions we tested degradation at a 3–4 hour time point. Treatment with 50 ng/ml with BDNF led to a degradation index of an average of 27.0 ± 3.2% that was significantly different than cortical neurons treated with the same concentration of BDNF but in the presence of leupeptin 68.5 ± 6.4 % (p < 0.0001). In the case of NT4, treatment with 50 ng/ml led to a degradation index of 68.9 ± 5.8 %, whereas in the presence of NT4 and leupeptin it was 90.3 ± 1.7% (p < 0.05). Thus, in both cases leupeptin significantly inhibited neurotrophin-induced TrkB degradation, however, in the case of BDNF this effect was much more pronounced. In fact, at such time point, even in the absence of neurotrophins and comparing the untreated group with and without leupeptin, we observed that surface biotinylation alone led to a degradation of 20% of TrkB, as can see by the quantification showing that after 3 hour incubation period, the untreated control had a degradation index of 80.8 ± 4.8%. This most likely represents endogenous protein turnover. Considering that leupeptin only blocked 20% of NT4-mediated degradation whereas it blocked 40% of BDNF, this raises the possibility that the degradation observed upon NT4 treatment might rely on mechanisms independent of the lysosome.
Figure 5. Lysosomal-dependent degradation of TrkB by BDNF or NT4.
A surface biotinylation degradation assay was performed with cortical neurons treated for 3–4 hours with 50 ng/ml BDNF, NT4 or untreated in the presence or absence of 100 μg/ml leupeptin. a) Representative western blot with TrkB antibody. b) Quantification of an average of three independent experiments. 50 ng/ml with BDNF alone led to a degradation index of 27.0 ± 3.2%M; BDNF and leupeptin 68.5 ± 6.4 % (****p < 0.0001, Student's t test). 50 ng/ml of NT4 led to a degradation index of 68.9 ± 5.8 %, NT4 and leupeptin 90.3 ± 1.7% (*p < 0.05, Student's t test).
All these studies suggest that NT4 sorts TrkB receptor away from the lysosomal-dependent degradation pathway. The differential sorting most likely occurs after the early endosome where NT4 might promote TrkB sorting to the recycling endosome and a fraction of it might be degraded via the proteasome system.
NT4, but not BDNF, induces sustained signaling
Endocytic sorting of RTKs away from the degradative pathway can have a significant on biological outcomes, as it results in attenuation of intracellular signaling cascades. Studies with the EGFR have shown that TGFα, which promotes recycling of the receptor rather than degradation, is a more potent mitogen than EGF, which efficiently sorts EGFR to the lysosomal-degradation pathway (Chung et al. 2010). As NT4 maintains a stable pool of activated TrkB receptor at prolonged time points, we expected that this ligand would also maintain active downstream signaling cascades, as compared to BDNF. To test this, we treated cortical neurons with BDNF or NT4 (for 10 min, 2 and 5 hours) and then analyzed the activation of the two major signaling pathways MAPK, through Erk1/Erk2 phosphorylation and Akt (Fig. 6A, C). Whereas BDNF and NT4 induced similar level of Akt and Erk1/Erk2 activation at 10 min and 2-hour after treatment, we found that after a 5-hour treatment, NT4 led to significantly higher phoshphorylation of Akt and Erk1/Erk2 as compared with BDNF, most likely due to the amount of TrkB receptors that are still available at this time point. Interestingly, pretreatment of lysosome inhibitor, leupeptin extended duration of BDNF-induced Akt and Erk1/Erk2 phosphorylation, suggesting that the stability of TrkB receptors is a main determinant for sustained downstream signaling (Fig. 6B, C).
Figure 6. Sustained signaling mediated by BDNF or NT4.
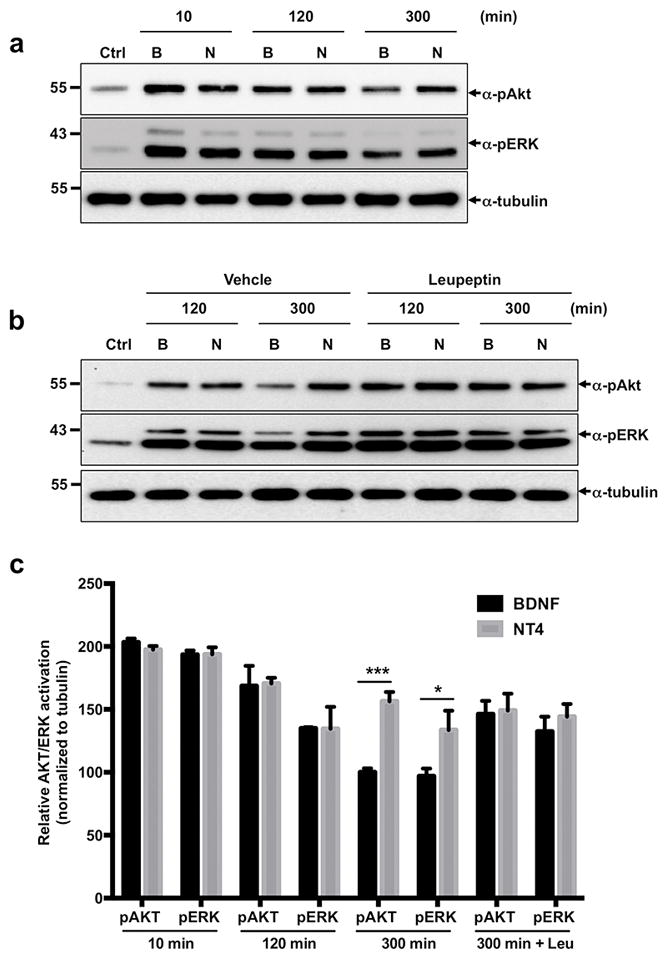
a) Cortical neurons were treated for indicated times with 50 ng/ml of BDNF, NT4 or left untreated. Lysates were immunobloted for phoshpo-Akt (pAkt) and phospho-Erk (pErk). b) Cortical neurons were pre-treated with 100 μg/ml leupeptin for 1 hr. Then neurons were stimulated with 50 ng/ml of BDNF, NT4 or left untreated in the presence or absence of leupeptin for indicated times. Lysates were immunobloted for phoshpo-Akt (pAkt) and phospho-Erk (pErk). c) Quantification represents phosphorylation of Akt and Erk normalized to tubulin. Average of three independent experiments (*p<0.05, **p<0.01, Student's t test).
Discussion
Our studies reveal that the endocytic trafficking of TrkB receptors is differentially modulated by its two cognate neurotrophins. BDNF induces rapid activation and ubiquitination of TrkB receptor, which is stable even at acidic pHs, leading to efficient downregulation of TrkB receptors, as well as signaling attenuation. On the other hand, NT4-mediated ubiquitination of TrkB is unstable at the lower pHs, suggesting that the NT4/TrkB interaction does not persist in the early endosome. As a result, the receptor is less efficiently sorted to the degradative pathway leading to enhanced activation of downstream signaling.
Previous studies analyzing BDNF-induced TrkB degradation in cerebellar neurons showed a t1/2 of 1–2 hours (Sommerfeld et al. 2000). In cortical neurons we observed that exposure to BDNF for 2–3 hours reduced the surface pool of TrkB receptors to half, whereas a longer treatment (4–5 hours) with NT4 was necessary to achieve a similar level of downregulation. We observed a similar time-course with hippocampal neurons (data not shown) suggesting that in the forebrain, TrkB receptors follow a slower degradation curve. It should be noted that the cortical cultures used include diverse cell types that express TrkB receptors that differ from those in cerebellar neuronal cultures, which may contribute to these differential kinetics.
We then asked if the mechanisms mediating TrkB degradation were similar with BDNF or NT4 activation. To test this we performed the same degradation assay, in the presence or absence of a lysosomal inhibitor, leupeptin. Interestingly, leupeptin was much more efficient in blocking BDNF induced degradation than NT4 induced degradation. One possibility for this difference might be due to the time point analyzed, at which BDNF induces significantly higher degradation (73% degradation of TrkB) than NT4 (32% degradation of TrkB). On the other hand, it is possible that NT4 might sort TrkB to different degradation pathways such as the proteasome. Indeed, previous studies in cerebellar granular neurons have suggested that TrkB follows a proteasomal dependent degradation pathway (Sommerfeld et al. 2000). Subsequent studies with TrkA showed that both the proteasome system and the lysosome play a sequence coordinated role in the degradation of this receptor (Geetha & Wooten 2008). It is possible that NT4 would preferentially sort TrkB receptors to an alternate compartment that would be mostly associated with the proteasome whereas BDNF efficiently targets the receptor to lysosomal degradation.
Even though NT4 is widely expressed in the brain, little is known about NT4-mediated signaling. In large part, this is due to the subtle phenotypes observed in the NT4 null mice in comparison with the BDNF null mice that has severe developmental defects and is lethal (Erickson et al. 1996, Ernfors et al. 1994). However, interestingly the complete TrkB null mouse shows neuronal death in the hippocampus whereas the BDNF null does not, suggesting a key role for NT4-mediated TrkB activation (Minichiello & Klein 1996, Alcantara et al. 1997). The lack of major developmental abnormalities in the CNS of NT4 null mice is most likely due to compensation by BDNF, given the overlapping expression of these two neurotrophins and the levels at which they are expressed. Studies comparing the levels of both neurotrophins in the rat brain with quantitative PCR suggest that the levels of NT4 are half of BDNF in the adult (Patz & Wahle 2006). In addition BDNF is released in an activity-dependent manner whereas NT4 has been reported to be sorted to the constitutive secreted pathway (Griesbeck et al. 1999, Hibbert et al. 2006, Mowla et al. 1999), suggesting that the levels of BDNF can be upregulated in the absence of NT4. There are several studies also suggesting that NT4 is a more potent trophic factor than BDNF. Replacement of BDNF with NT4 in the BDNF locus not only rescued the major abnormalities of the BDNF null mouse but also supported the survival of more vestibular neurons than in wild type mice. In addition, both synaptic maturation and function were enhanced (Fan et al. 2000). A similar effect was observed on the survival of nodose-petrosal neurons, whereas a single NT4 allele is sufficient to support survival of these cells, while two functional BDNF alleles were necessary to achieve the same effect (Erickson et al. 1996). Our data showing that NT4 activation of TrkB does not lead to significant down-regulation of the receptor thus ensuring sustained signaling, presents the first molecular evidence that could explain those findings. Moreover, we show that prolonged NT4 treatment leads to significantly higher sustained phosphorylation of Akt and MAPK pathway than BDNF treatment, supporting the idea that NT4 is a more potent neurotrophin.
Our studies show that minor differences in the initial activation and trafficking steps of TrkB receptors are critical for long-term signaling. It remains unclear how NT4, which binds TrkB with similar affinity as BDNF, might sort this receptor do alternate trafficking destinations that are less efficiently coupled to degradation. Surface plasmogen studies measuring the affinity of these neurotrophins showed that upon acidic wash TrkB uncoupled from NT4 much more efficiently than it did from BDNF (Naylor et al. 2002). Efficient ligand/receptor uncoupling has been associated with increased EGFR recycling and decreased degradation (Alwan et al. 2003). EGF, TGFα and E4T are all ligands for EGFR capable of inducing similar phosphorylation and internalization of the receptor, however only EGF induces efficient recruitment of the E3 ligase Cbl, ubiquitination and degradation of EGFR in the endosomal acidic compartment. In contrast, TGFα and E4T, rapidly uncouple from EGFR at acidic pHs and triggered more efficient recycling of EGFR (Roepstorff et al. 2009, Alwan et al. 2003). Interestingly studies have shown that TGFα is a more potent mitogen than EGF, demonstrating how critical modulation of endocytic trafficking is, to achieve specific biological outcomes (Alwan et al. 2003, Roepstorff et al. 2009). In our studies we observed that NT4-induced ubiquitination of TrkB receptor was pH-sensitive whereas BDNF-induced ubiquitination of TrkB was stable even at acidic pHs. Together this suggests that BDNF-TrkB binding is stable even in the endosome, facilitating the recruitment of an E3 ligase followed by efficient degradation, whereas, NT4 may quickly uncouple from TrkB, favoring recycling and consequently sustained signaling. A recent study demonstrated that loss of function in endosomal Na+/H+ Exchanger 6 (NHE6) induces over-acidification of the endosomal compartment that may lead to the attenuated BDNF signaling and reduced TrkB level in NHE6 mutant neurons (Ouyang et al. 2013). Our current observation of enhanced TrkB ubiquitination by BDNF treatment in low pH condition provides a plausible explanation on how BDNF signaling is impaired in over-acidified endosomes of NHE6 mutant neurons. In all, the present study sheds light into the mechanisms mediating the postendocytic fate of TrkB receptors in the CNS in which the ligand (NT4 vs BDNF) plays a critical role in determining sorting of the receptors to the degradative pathway. In this context, these findings may have implications for clinical efforts to use NT4 and BDNF as therapeutic agents. Our studies suggest that NT4 may be the preferable TrkB ligand as there would be less downregulation of TrkB receptors with prolonged systemic treatments, as has been implicated for BDNF in previous clinical trials (Thoenen, 2001; Thoenen and Sendtner, 2002).
Supplementary Material
Acknowledgments
We acknowledge support from the National Institutes of Health grants MH079513 (F.S.L.) and NS052819 (F.S.L.), Brain and Behavior Research Foundation (F.S.L.) and Gulbenkian PhD Programe in Biomedicine (C.C.P.), Fundacao para Ciencia e Tecnologia (C.C.P.),
Abbreviations
- Trk
Tropomyosin Related Kinase
- BDNF
Brain-Derived Neurotrophic Factor
- NT4
Neurotrophin-4
- NGF
nerve growth factor
- RTK
receptor tyrosine kinases
- EGFR
Epidermal growth factor receptor
- TGFα
Transforming growth factor α
- MAPK
Mitogen Activated Protein Kinase
Footnotes
Competing Financial Interests
The authors declare no competing financial interests.
References
- Alcantara S, Frisen J, del Rio JA, Soriano E, Barbacid M, Silos-Santiago I. TrkB signaling is required for postnatal survival of CNS neurons and protects hippocampal and motor neurons from axotomy-induced cell death. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:3623–3633. doi: 10.1523/JNEUROSCI.17-10-03623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan HA, van Zoelen EJ, van Leeuwen JE. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J Biol Chem. 2003;278:35781–35790. doi: 10.1074/jbc.M301326200. [DOI] [PubMed] [Google Scholar]
- Arevalo JC, Waite J, Rajagopal R, Beyna M, Chen ZY, Lee FS, Chao MV. Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron. 2006;50:549–559. doi: 10.1016/j.neuron.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Banfield MJ, Naylor RL, Robertson AG, Allen SJ, Dawbarn D, Brady RL. Specificity in Trk receptor:neurotrophin interactions: the crystal structure of TrkB-d5 in complex with neurotrophin-4/5. Structure. 2001;9:1191–1199. doi: 10.1016/s0969-2126(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. British journal of pharmacology. 2008;153(Suppl 1):S310–324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Tanowitz M, Lee FS. A novel endocytic recycling signal distinguishes biological responses of Trk neurotrophin receptors. Molecular biology of the cell. 2005a;16:5761–5772. doi: 10.1091/mbc.E05-07-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Tanowitz M, Lee FS. A novel endocytic recycling signal distinguishes biological responses of Trk neurotrophin receptors. Mol Biol Cell. 2005b;16:5761–5772. doi: 10.1091/mbc.E05-07-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung I, Akita R, Vandlen R, Toomre D, Schlessinger J, Mellman I. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature. 2010;464:783–787. doi: 10.1038/nature08827. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, Katz DM. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J Neurosci. 1996;16:5361–5371. doi: 10.1523/JNEUROSCI.16-17-05361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Fan G, Egles C, Sun Y, Minichiello L, Renger JJ, Klein R, Liu G, Jaenisch R. Knocking the NT4 gene into the BDNF locus rescues BDNF deficient mice and reveals distinct NT4 and BDNF activities. Nat Neurosci. 2000;3:350–357. doi: 10.1038/73921. [DOI] [PubMed] [Google Scholar]
- Geetha T, Wooten MW. TrkA receptor endolysosomal degradation is both ubiquitin and proteasome dependent. Traffic. 2008;9:1146–1156. doi: 10.1111/j.1600-0854.2008.00751.x. [DOI] [PubMed] [Google Scholar]
- Goh LK, Sorkin A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb Perspect Biol. 2013;5:a017459. doi: 10.1101/cshperspect.a017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbeck O, Canossa M, Campana G, Gartner A, Hoener MC, Nawa H, Kolbeck R, Thoenen H. Are there differences between the secretion characteristics of NGF and BDNF? Implications for the modulatory role of neurotrophins in activity-dependent neuronal plasticity. Microsc Res Tech. 1999;45:262–275. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<262::AID-JEMT10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Harrington AW, St Hillaire C, Zweifel LS, Glebova NO, Philippidou P, Halegoua S, Ginty DD. Recruitment of Actin Modifiers to TrkA Endosomes Governs Retrograde NGF Signaling and Survival. Cell. 2011;146:421–434. doi: 10.1016/j.cell.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbert AP, Kramer BM, Miller FD, Kaplan DR. The localization, trafficking and retrograde transport of BDNF bound to p75NTR in sympathetic neurons. Mol Cell Neurosci. 2006;32:387–402. doi: 10.1016/j.mcn.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol. 2008;20:4–11. doi: 10.1016/j.ceb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, Tsvetanova NG, Lobingier BT, von Zastrow M. Effects of endocytosis on receptor-mediated signaling. Curr Opin Cell Biol. 2015;35:137–143. doi: 10.1016/j.ceb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien J, Guili V, Reichardt LF, Rudkin BB. Molecular kinetics of nerve growth factor receptor trafficking and activation. J Biol Chem. 2002;277:38700–38708. doi: 10.1074/jbc.M202348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Knusel B, Gao H, Okazaki T, Yoshida T, Mori N, Hefti F, Kaplan DR. Ligand-induced down-regulation of Trk messenger RNA, protein and tyrosine phosphorylation in rat cortical neurons. Neuroscience. 1997;78:851–862. doi: 10.1016/s0306-4522(96)00616-1. [DOI] [PubMed] [Google Scholar]
- Lodovichi C, Berardi N, Pizzorusso T, Maffei L. Effects of neurotrophins on cortical plasticity: same or different? J Neurosci. 2000;20:2155–2165. doi: 10.1523/JNEUROSCI.20-06-02155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Klein R. TrkB and TrkC neurotrophin receptors cooperate in promoting survival of hippocampal and cerebellar granule neurons. Genes & development. 1996;10:2849–2858. doi: 10.1101/gad.10.22.2849. [DOI] [PubMed] [Google Scholar]
- Mowla SJ, Pareek S, Farhadi HF, Petrecca K, Fawcett JP, Seidah NG, Morris SJ, Sossin WS, Murphy RA. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J Neurosci. 1999;19:2069–2080. doi: 10.1523/JNEUROSCI.19-06-02069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor RL, Robertson AG, Allen SJ, et al. A discrete domain of the human TrkB receptor defines the binding sites for BDNF and NT-4. Biochem Biophys Res Commun. 2002;291:501–507. doi: 10.1006/bbrc.2002.6468. [DOI] [PubMed] [Google Scholar]
- Ouyang Q, Lizarraga SB, Schmidt M, Yang U, Gong J, Ellisor D, Kauer JA, Morrow EM. Christianson syndrome protein NHE6 modulates TrkB endosomal signaling required for neuronal circuit development. Neuron. 2013;80:97–112. doi: 10.1016/j.neuron.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Patz S, Wahle P. Developmental changes of neurotrophin mRNA expression in the layers of rat visual cortex. Eur J Neurosci. 2006;24:2453–2460. doi: 10.1111/j.1460-9568.2006.05126.x. [DOI] [PubMed] [Google Scholar]
- Piper RC, Dikic I, Lukacs GL. Ubiquitin-dependent sorting in endocytosis. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepstorff K, Grandal MV, Henriksen L, Knudsen SL, Lerdrup M, Grovdal L, Willumsen BM, van Deurs B. Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic. 2009;10:1115–1127. doi: 10.1111/j.1600-0854.2009.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Howe CL, Cosgaya JM, Steiner P, Hirling H, Chan JR, Weis J, Kruttgen A. Differential endocytic sorting of p75NTR and TrkA in response to NGF: a role for late endosomes in TrkA trafficking. Mol Cell Neurosci. 2005;28:571–587. doi: 10.1016/j.mcn.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Sommerfeld MT, Schweigreiter R, Barde YA, Hoppe E. Down-regulation of the neurotrophin receptor TrkB following ligand binding. Evidence for an involvement of the proteasome and differential regulation of TrkA and TrkB. J Biol Chem. 2000;275:8982–8990. doi: 10.1074/jbc.275.12.8982. [DOI] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz M, von Zastrow M. A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J Biol Chem. 2003;278:45978–45986. doi: 10.1074/jbc.M304504200. [DOI] [PubMed] [Google Scholar]
- Timmusk T, Belluardo N, Metsis M, Persson H. Widespread and developmentally regulated expression of neurotrophin-4 mRNA in rat brain and peripheral tissues. The European journal of neuroscience. 1993;5:605–613. doi: 10.1111/j.1460-9568.1993.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Wilson JM, de Hoop M, Zorzi N, Toh BH, Dotti CG, Parton RG. EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells, and fibroblasts. Molecular biology of the cell. 2000;11:2657–2671. doi: 10.1091/mbc.11.8.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EC, Mobley WC. Early BDNF, NT-3, and NT-4 signaling events. Exp Neurol. 1999;159:297–308. doi: 10.1006/exnr.1999.7148. [DOI] [PubMed] [Google Scholar]
- Zheng J, Shen WH, Lu TJ, et al. Clathrin-dependent endocytosis is required for TrkB-dependent Akt-mediated neuronal protection and dendritic growth. J Biol Chem. 2008;283:13280–13288. doi: 10.1074/jbc.M709930200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.