Abstract
Background
Adolescence is a critical period for the development of alcohol use disorders; drinking habits are rather unstable and genetic influences, such as male sex and a positive Family History of alcoholism (FH), are often masked by environmental factors such as peer pressure.
Methods
We investigated how sex and FH modulate alcohol use in a sample of 18-19-year-olds from the Dresden Longitudinal Study on Alcohol use in Young Adults (D-LAYA). Adolescents reported their real-life drinking in a TimeLine Follow-Back (TLFB) interview. They subsequently completed a training and an experimental session of free-access intravenous Alcohol Self-Administration (i.v. ASA) using the computer-assisted alcohol infusion system in order to control for environmental cues as well as for biological differences in alcohol pharmacokinetics. During i.v. ASA, we assessed subjective alcohol effects at eight time points.
Results
Women reported significantly less real-life drinking than men and achieved significantly lower mean arterial Blood Alcohol Concentrations (aBACs) in the laboratory. At the same time, women reported greater sedation relative to men and rated negative effects as high as did men. A positive FH was associated with lower real-life drinking in men but not in women. In the laboratory, FH was not linked to i.v. ASA. Greater real-life drinking was significantly positively associated with higher mean aBACs in the laboratory, and all i.v. ASA indices were highly correlated across the two sessions.
Conclusions
We conclude that adolescent women chose lower aBACs because they experienced adverse alcohol effects, namely sedation and negative effects, at lower aBACs than men. A positive FH was not apparent as risk factor for drinking in our young sample. The i.v. ASA method demonstrated good external validity as well as test-retest reliability, the latter indicating that a separate training session is not required when employing the i.v. ASA paradigm.
Keywords: Computer- assisted Alcohol Infusion System (CAIS), intravenous Alcohol Self-Administration (i.v. ASA), subjective alcohol effects, subjective response to ethanol
Introduction
Two major genetically determined factors are known to affect the risk for alcohol use disorders, namely sex and Family History of alcoholism (FH). It was often reported that women drink less alcohol than men, tolerate lower amounts of alcohol, get intoxicated less frequently, and have a lower risk for alcohol use disorders (Erol and Karpyak, 2015). Underlying mechanisms include psychosocial and cultural factors associated with the female gender-role, such as considering excessive alcohol use to be a traditionally masculine behavior (Erol and Karpyak, 2015, DeVisser and McDonnell, 2012). Moreover, biological factors, including lower body weight and lower proportion of body water, increase the bioavailability of alcohol in women compared with men (Cederbaum, 2012). Therefore, women typically reach higher arterial Blood Alcohol Concentrations (aBACs) than men, when ingesting the same amount of alcohol (Erol and Karpyak, 2015). However, the literature on sex effects is inconsistent and many studies do not have enough power to analyze sex differences in alcohol consumption (Erol and Karpyak, 2015). Besides that, most studies using laboratory Alcohol Self-Administration (ASA) were conducted in adult samples. When sex was studied as secondary influential factor, women were found to achieve lower aBACs than men during oral ASA (Drobes et al., 2003), and to manifest a trend for lower peak aBACs in intravenous (i.v.) ASA (Hendershot et al., 2016). Such findings suggest that women prefer lower aBACs than men, when asked to produce pleasant alcohol effects, but this hypothesis has never been tested before.
Corresponding to the old observation that alcoholism runs in families, studies found a positive FH to be associated with an increased risk for alcohol use disorders (Grant, 1998). Underlying mechanisms include differences in reward-processing (Andrews et al., 2011) and impulsivity (Acheson et al., 2011). Such differences, however, do not necessarily result in increased drinking in college students (Elliott et al., 2012), implying no deterministic pathway from genotype to phenotype (Morean and Corbin, 2010). Similar to research on sex effects, studies examining FH effects on ASA in adolescents have not yet been reported.
As described above, sex and FH are broad descriptive factors, comprising several mechanisms possibly underlying alcohol use. One of those mechanisms is the subjective response to ethanol, which has also been found to differ by sex and FH (Morean et al., 2015). Moreover, the individual sensitivity for pleasant and unpleasant responses to fixed alcohol doses varies substantially and predicts future alcohol abuse (King et al., 2014). However, studies examining the connection between subjective responses and sex yielded inconsistent results. Whereas Luczak et al. (2002) reported lower subjective intoxication for women than men, Miller et al. (2009) reported opposite results, and Kerfoot et al. (2013) found no main effect of sex on subjective alcohol effects in an alcohol infusion study. Concerning FH, a positive FH was linked to a low level of response to alcohol (Quinn and Fromme, 2011; Schuckit, 2009), but Morean et al. (2015) reported stronger HIGH- (e.g. aggressiveness) effects for those with a positive compared with a negative FH.
According to previous research, evidence for genetically determined risk factors for alcohol use disorders may be difficult to find in adolescents, because they are masked by environmental factors including peer pressure (Steinberg et al., 1994). In line with that observation, Rose et al. (2001) found a decreasing impact of environmental factors on adolescent real-life drinking from age 16 to 18.5, while that of additive genetic effects increased.
A widely used approach to study alcohol consumption in the laboratory is oral ASA (Zimmermann et al., 2013). In these studies, subjects typically ingest standard drinks, which mimics naturalistic drinking, but is flawed by some unavoidable limitations due to high inter-individual variation in gastrointestinal absorption and distribution of alcohol (Ramchandani et al., 2009). Such pharmacokinetic differences yield a 2-3-fold variation in peak aBAC and make results of oral ASA difficult to compare across sexes. Therefore, we investigated how sex and FH modulate i.v. ASA, which ensures that the incremental aBAC following each “drink” is identical in all subjects (Plawecki et al., 2012; Ramchandani et al., 1999; Zimmermann et al., 2008). Compared to drinking self-reports, i.v. ASA tightly controls for environmental factors: (i) social influences on ASA are minimized, (ii) participants are blinded against the amount of administered alcohol, reducing the tendency to constrain their consumption to what they think is socially desirable; (iii) alcohol-associated Pavlovian cues such as brands and taste are removed; (iv) the act of drinking is replaced by pushing a button, reducing habitual components of behavior.
In our Dresden Longitudinal study on Alcohol use in Young Adults (D-LAYA), we employed ASA for the first time in adolescent subjects. Adolescents reported their real-life drinking (TLFB) and completed two sessions of i.v. ASA. As suggested by Zimmermann et al. (2009), the first session served as training in order to reduce behavioral noise attributable to user curiosity in the second session. Since our sample was considerably larger than the one in Zimmermann et al. (2009), we took the opportunity to re-examine the test-retest reliability of the i.v. ASA method. We hypothesized that male sex and a positive FH, would be associated with more alcohol consumption in real life and in the laboratory. Since participants were free to self-administer aBACs at which they felt comfortable, we expected no sex or FH differences in subjective pleasant and unpleasant alcohol effects.
Materials and Methods
The D-LAYA study (Clinical Trials NCT01063166) was reviewed and approved by the ethics committee of the Technische Universität Dresden, and fully complied with the Declaration of Helsinki.
Participants and recruitment
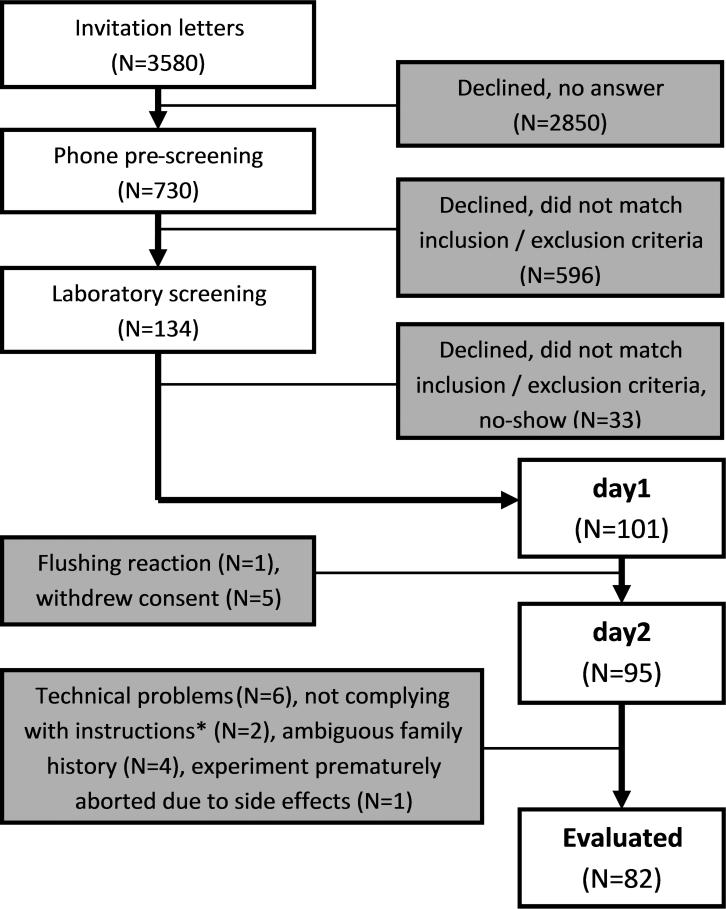
We mailed 3580 invitation letters to 18-year-old Dresden residents whose addresses were provided by the registration office. Respondents were called to provide study information and pre-check Family History of alcoholism (FH). Adolescents reporting a positive FH (FHP) were invited for laboratory screening, as well as the next respondent matching in sex and smoking status, but reporting a negative FH (FHN). Figure 1 presents the sample size in each recruitment step.
Figure 1.
Sample size in each step of the recruitment process. N=Number; *Two subjects reported having purposefully induced lower aBACs than they would have done at a party in order to shorten the time to sober up and be able to leave the lab earlier.
During the screening visit, participants received oral and written study information and provided written informed consent. To check inclusion and exclusion criteria, we obtained liver enzymes, a complete blood count, and interviewed participants using three instruments: the Munich Composite International Diagnostic Interview (M-CIDI; Lachner et al., 1998), the Diagnostisches Interview Psychischer Störungen (DIPS; Margraf, 1994), and the Family History Assessment Module (FHAM; Rice et al., 1995). Adolescents also reported their smoking status, completed column A (first five times of drinking) and C (time of most drinking) of the Self-Rating of the Effects of Alcohol (SRE; Schuckit et al., 1997), and the 45 day TimeLine Follow-Back interview (TLFB; Sobell and Sobell, 1992). We included 18-19-year-olds that had experienced at least one episode of drunkenness and reported having two or more alcoholic drinks per week during the last two months. Adolescents were excluded for any of the following reasons: any previous alcohol-related treatment; a medical disorder which would place them at risk if consuming alcohol, current or past substance dependence (except nicotine dependence); elevated aspartate transaminase, alanine-aminotransferase or gamma-glutamyl transferase; any severe current or past axis I disorder according to DSM-IV; a urine drug screen positive for cocaine, amphetamines, cannabinoids, benzodiazepines, opiates, barbiturates, ecstasy, antidepressants; pregnancy or breast-feeding, current desire to become pregnant; taking medication that might interact with alcohol; drinking alcohol on the test day or the day before.
The final sample consisted of 82 adolescents aged between 18-19 (M = 18.4, SD = 0.5), 35 women, and 38 FHP (see Figure 1 and Table 1).
Table 1.
Sample characteristics
| Men (N=47) |
Women (N=35) |
Total (N=82) |
||||
|---|---|---|---|---|---|---|
| FHP (N=17) | FHN (N=30) | FHP (N=21) | FHN (N=14) | FHP (N=38) | FHN (N=44) | |
| age | 18.5 (0.5) | 18.4 (0.5) | 18.3 (0.5) | 18.4 (0.5) | 18.4 (0.5) | 18.4 (0.5) |
| % smoking | 41.2 | 30.0 | 42.9 | 14.3 | 42.1 | 25.0 |
| AUDIT | 6.5 (1.8) | 8.4 (4.2) | 6.5 (5.0) | 6.4 (4.6) | 6.5 (3.9) | 7.8 (4.4) |
| BDI | 4.4 (5.0) | 3.6 (2.9) | 6.5 (5.1) | 4.2 (3.6) | 5.6 (5.1) | 3.8 (3.1) |
| SRE* | 4.7 (1.6) | 5.2 (2.9) | 3.6 (1.7) | 3.2 (1.3) | 4.1 (1.7) | 4.5 (2.7) |
| AEQ | 10.1 (3.2) | 11.0 (3.3) | 10.7 (4.4) | 9.6 (3.5) | 10.4 (3.9) | 10.6 (3.4) |
Note. Means, Standard deviations in brackets, and percentages of current regular smokers are presented. N=Number; FHP=Family History of alcoholism Positive; FHN=FH Negative; AUDIT=Alcohol Use Disorders Identification Test; BDI=Beck Depression Inventory; SRE=Self-Rating of the Effects of Alcohol (first five times of drinking); AEQ=Alcohol Expectancy Questionnaire
Women reported needing fewer drinks than men to experience the same alcohol effects (F(1,76)=8.7, p<0.01); There were no other significant effects of sex, FH or their interaction.
General experimental methods
Participants underwent two identical sessions separated by at least 3 days (Range: 3-59, Median=11). The first day (day1) served as a training session (Zimmermann et al., 2009). Thereafter, a subsample completed two fMRI sessions which are reported elsewhere (Gan et al., 2015; Marxen et al., 2014). Participants reported to the lab at 1 p.m. and provided a urine sample for pregnancy testing (Alere medical pregnancy test, Köln, Germany) and urine drug screen (Nal von Minden Multi 12TF test, Moers, Germany). A brief history covering the time since the screening visit was obtained. At 1.30 p.m., we established an 18G i.v. line using an ante-cubital fossa vein of the non-dominant arm. During the next 45 minutes, participants worked on questionnaires, including the Brief Alcohol Expectancy Questionnaire (AEQ; Demmel & Hagen, 2002), Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 2001), and the Beck Depression Inventory (BDI; Hautzinger et al., 2000).
Throughout the experiment, participants sat in a comfortable arm chair facing a 32 inch video monitor at a viewing distance of 1.5m. We ensured that baseline aBAC was zero and participants completed subjective state ratings (0min). The i.v. ASA began at 2.15 p.m., and ended at 4.40 p.m. During each session, we obtained subjective state ratings at 10min, and every 20 minutes during i.v. ASA (45, 65, 85, 105,125, 145min), as well as 8 aBAC readings (10, 25, 45, 65, 85, 105, 125, 145min) using an Alcotest 6810med breathalyzer (Draeger Sicherheitstechnik, Lübeck, Germany). These data were entered into the CAIS software in real time to improve the individual pharmacokinetic model and adapt the prescribed infusion rates accordingly. The breathalyzer measured alcohol concentration in end-expiratory breath, which is closely related to arterial BAC during intravenous ethanol infusion (Lindberg et al., 2007). Since alcohol exposure is conventionally communicated as BAC, the breathalyzer applied the usual 1:2100 air/blood partition coefficient to approximate aBAC (mg%) from breath readings (mg ethanol/ liter of air). Due to the high cerebral perfusion index, aBAC provides a reliable estimate of brain alcohol exposure, which is the key factor driving both behavior and subjective alcohol effects.
During ASA, participants were free to watch sitcoms and use the bathroom. After the i.v. line was removed, participants were served a full meal. They could leave the lab as soon as their aBAC was below 45mg% if picked up by car (e.g. taxicab) or below 20mg% if they went home unaccompanied. Participants were paid 120€ for both i.v. ASA sessions and up to 40€ driving expense.
Measures
Family history of alcoholism
Participants were classified as FHP, if they had at least one first-degree biological relative fulfilling three or more lifetime DSM-IV alcohol dependence criteria, and as FHN, if none of their first-or second-degree relatives had been alcohol-dependent.
Real-life drinking
Self-reported real-life drinking was obtained at the first day using the TimeLine Follow-Back (TLFB) interview (Sobell and Sobell, 1992). Participants reported the number of alcoholic drinks they drank on each of the last 45 days in standard units of 12g pure alcohol. We extracted three TLFB variables: drinking days drinks per drinking day, and the number of binge days with five or more drinks for men and four or more for women (Courtney and Polich, 2009).
Free-access intravenous alcohol self-administration
The experimental methods were the same as described in Zimmermann et al. (2008). Briefly, the CAIS software was installed on the experimenter laptop which was connected to the participant's screen in the adjacent room. The screen informed participants about the experimental phase and whether the self-administration button was active. Infusion solutions were prepared by mixing 0.9% saline with 95% ethanol (Alkohol Konzentrat 95% Braun, Melsungen, Germany) giving a final concentration of 6.0% (v/v). For i.v. administration, we used two volumetric infusion pumps (Infusomat fms, BBraun, Melsungen, Germany). Participants’ age, gender, height, and weight were used as parameters for a Physiologically-Based PharmacoKinetic (PBPK) model (Plawecki et al., 2012). Pushing the button (Power Mate, Griffin Technology, Nashville, Tennessee) resulted in a linear increase in aBAC of 7.5mg% within 2.5 minutes, during which time the button was deactivated. Thereafter, the aBAC steadily declined by 1 mg%/min until the participant pushed the button, the infusion rate reached the minimum set rate (8 ml/h), or the experiment was over. An output of the aBAC system estimates every 30 seconds as well as entered breath readings were displayed to the experimenter, but not to the participant.
Participants were instructed to produce pleasant alcohol effects to the same extent as they usually would when drinking at a weekend party, but to avoid unpleasant effects. The experiment started with a priming phase, during which participants were prompted to push the button four times, resulting in an aBAC of 30mg% after 10 minutes. For the next 15 minutes, the button was inactivated, and the aBAC decreased linearly, reaching 15mg% at 25 minutes, at which time the voluntary free-access i.v. ASA phase began. During the next two hours, participants were free to request alcohol up to a safety limit of 120mg% at their discretion.
Subjective ratings
Subjective ratings were assessed in the following order: (1) stimulation: “Right now, I am experiencing stimulating alcohol effects, e.g. cheerful, excited, full of energy, zest for action...”; (2) sedation: “Right now, I am experiencing sedating alcohol effects, e.g. relaxed, tired, sluggish...”; (3) negative effects: “Right now, I am experiencing negative alcohol effects, e.g. nausea, dizziness, ringing in the ears...”; (4) alcohol desire: “I would like to consume more alcohol right now.”; (5) overall well-being: “Overall, I am feeling well right now.”; (6) drinks number: “Right now, I feel like I had ... drinks.”; (7) feeling drunk: “I am feeling drunk right now.”. The statements were programmed with Presentation (Neurobehavioral Systems, Albany, California), and presented on the video monitor. Subjects completed the rating using a mouse on visual analog scales anchored at 0 (not at all) and 100 (extremely); or by choosing a drinks number from 0-30.
Data analysis
CAIS data from the priming interval were excluded. CAIS aBAC estimates during the voluntary i.v. ASA interval (241 in total) were optimized to fit the measured aBACs, providing a continuous and more reliable representation of instantaneous aBAC than individual breath readings. Therefore, these estimates were used to calculate two aggregated outcome variables: mean aBAC (mg%) and maximum aBAC (mg%). Further we analyzed the number of alcohol requests made by the subject. Hypotheses were tested using day2 data only, because day1 served as a training session. The i.v. ASA measures max aBAC, mean aBAC, and alcohol requests were strongly correlated (all r>.87, p-values<.001; see Table 2), indicating high internal consistency. To avoid multiple testing with highly related outcome variables, we report results for mean aBAC only, because (i) compared to maximum aBAC, it is a more integrative measure, and (ii) compared to alcohol requests, it is a continuous and normally distributed variable, thus more appropriate for ANOVAs.
Table 2.
Pearson correlations TLFB and intravenous Alcohol Self-Administration (i.v. ASA) measures
| N=82 | TLFB drinking days | TLFB binge days | TLFB drinks per drinking day | i.v. ASA maximum aBAC | i.v. ASA mean aBAC |
|---|---|---|---|---|---|
| binge days | .69*** | ||||
| drinks per drinking day | .14 | .63*** | |||
| maximum aBAC | .22 | .36** | .33** | ||
| mean aBAC | .24* | .35** | .30** | .97*** | |
| alcohol requests | .19 | .36*** | .39*** | .91*** | .88*** |
Note. N=Number; TLFB=TimeLine Follow-Back over the last 45 days, binge days=number of days with five or more drinks for men and four or more for women; aBAC=arterial Blood Alcohol Concentration
p<.05
p<.01
p<.001.
Subjective ratings were measured at eight time points. Due to technical problems there was an unsystematic pattern of missing data: 1.2%- 4.3% of all ratings, respectively. We removed data of the first measurement (0min) for stimulation, sedation, negative effects, estimated drinks number, and feeling drunk, because they consisted of zero values only, which caused a zero-inflation as well as a violation of the homogeneity of variance assumption.
For analyses and graphics we used R version 3.2.2 (R Core Team; http://www.R-project.org/). For testing sex (women vs. men) and FH (FHP vs. FHN) differences in age and questionnaire scores, type III ANOVAs (Anova, Manova; package car) were used, as well as chi-square tests for smoking status (chisq.test, package: stats). Further, we used (M)ANOVAs for testing sex and FH effects on real-life drinking (type III) as well as i.v. ASA (type II as there was no interaction; Langsrud, 2003). Internal consistency and test-retest reliability of the i.v. ASA measures were analyzed using correlations (cor.test, package: stats), we computed paired significance tests for correlation differences (paired.r, package: psych), and used t-tests for testing differences between day1 and day2 (t.test, package: stats). For all analyses we used the conventional p-value threshold of 5% to assess significance. Time course analyses of the i.v. ASA measures and subjective ratings were conducted with mixed-effects models (lmer, package: lme4). Given the large number of observations, the t-statistics for the contrasts approximate the normal distribution, and |t-values| > 2.0 were interpreted as significant (Kliegl, et al., 2010). In all models, we tested the effects of time (linear and quadratic), sex (women −0.5 vs. men 0.5), and FH (FHN −0.5 vs. FHP 0.5) on aBACs or subjective ratings, using the maximum random effects structure.
Results
Sample characteristics
Table 1 provides an overview on the prevalence of sex and FH in our sample as well as participants’ age, smoking status, AUDIT, BDI, AEQ, and SRE scores. In the SRE items covering the subjects’ first five drinking episodes, adolescent women reported needing fewer drinks to experience alcohol effects (F(1,76)=8.7, p<0.01, see Table 1) than men. There were no other significant effects of sex, FH or sex × FH interactions.
Effects of sex and family history of alcoholism on real-life drinking
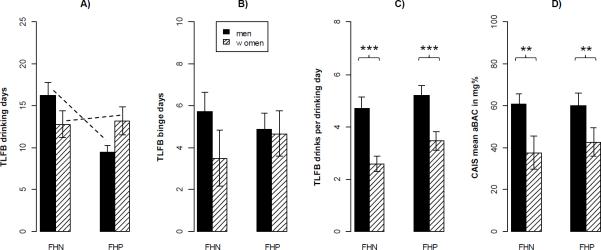
As depicted in Figure 2 A-C, women reported significantly less real-life drinking than men, and FHP men less than FHN men.
Figure 2.
Effects of Family History of alcoholism (FH) and sex on real-life drinking measured by TimeLine Follow-Back interview over the last 45 days (A-C), and on laboratory intravenous Alcohol Self-Administration measured by the mean arterial Blood Alcohol Concentration (aBAC; D). Means and standard errors of the mean (error bars) in each group are displayed. Asterisks denote significant sex main effects in univariate ANOVAs (**p<.01,***p<.001), and dashed lines denote a significant sex × FH interaction (p<.05).
Overall, we found significant main effects of sex (F(3, 76)=9.1, p<.001) and FH (F(3, 76)=3.0, p<.05) on real-life drinking using a MANOVA. The corresponding univariate models revealed that women reported fewer drinks per drinking day (F(1, 78)=20.0, p<.001) than men. Further, a significant sex × FH interaction on drinking days (F(1, 78)=5.0, p<.05) indicated that FHP men reported fewer drinking days than FHN men, whereas there was no such difference in women. After controlling for SRE scores or smoking status, the overall main effect of FH failed to reach significance (p=.09), whereas all other effects remained unchanged.
Effects of sex and family history of alcoholism on laboratory alcohol self-administration
We found that women preferred lower mean aBACs than men, but there was no effect of FH on laboratory i.v. ASA (Figure 2D). The main effect of sex (F(1,78)=10.0, p<.01), remained significant (p-values <.05) after controlling for SRE scores, smoking status, TLFB drinking days or TLFB drinks per drinking day. There was still no significant effect of FH when including the interactions of these control variables.
Figure 3A shows the time courses of aBAC for women and men. ABAC estimates significantly increased over time (timelinear Estimate=1580.1, SE=189.4, t=8.3), but the increase gradually slowed over time, as indicated by a negative quadratic term (timequadratic Estimate=−873.7, SE=121.6, t=−7.2). In line with the above described ANOVAs, aBAC estimates were generally lower for women than men (sex Estimate=20.4, SE=6.5, t=3.2), and the quadratic time effect was smaller for women than men (timequadratic × sex Estimate=−556.5, SE=243.1, t=−2.3) whereas the interaction between sex and time (linear) failed to reach significance (t=1.9). Again, there were no effects of FH.
Figure 3.
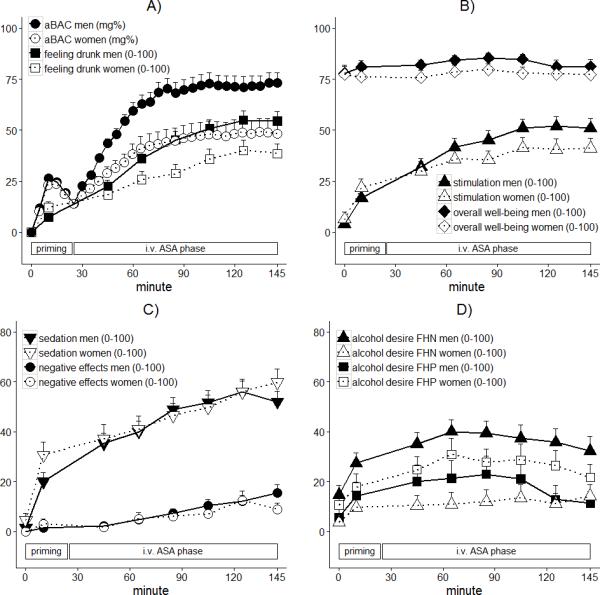
Effects of time and sex on CAIS arterial Blood Alcohol Concentration (aBAC) estimates and subjective ratings of alcohol effects (A-C), as well as effects of time, sex, and Family History of alcoholism (FH) on subjective alcohol desire (D). Means and standard errors (upper error bars) for each measure during priming and intravenous Alcohol Self-Administration (i.v. ASA) phase are displayed. Note that for reasons of clarity, the graph of aBACs does only include every 10th estimate.
Effects of sex and family history on subjective ratings
With respect to time, we found that all subjective ratings, except overall well-being, linearly increased over time (t-values>2.8). Further, there were quadratic time effects on all ratings, except negative effects, because the strongest rate of increase occurred at the beginning of the experiment (|t-values|>2.3), paralleling the increase in aBACs. With respect to sex (see Figure 3A-C), women had a lower estimated drinks number (sex Estimate=1.6, SE=0.5, t=2.9). Further, the linear increase in stimulation, feeling drunk, and drinks number was lower for women than men (t-values>2.1), and women's sedation ratings at 10min and at the end (145min) were higher than those of men (timequadratic × sex Estimate =−105.0, SE=43.6, t=−2.4). The time course of all other ratings, namely overall well-being, negative effects, and alcohol desire did not differ between sexes. With respect to FH (see Figure 3D), we found a significant sex × FH interaction on alcohol desire. FHP men reported lower alcohol desire (sex × FH Estimate=−29.3, SE=9.5, t=−3.1) than FHN men, while the opposite relation was found for women.
In separate models, we tested whether significant sex effects on subjective ratings were mediated by individual aBACs. After including the linear and quadratic aBAC main effects to the models predicting stimulation, estimated drinks number, and feeling drunk the linear aBAC effect was highly significant (t-values>5.2). In the updated model for stimulation, the sex difference over time failed to reach significance (t=1.6), implying that self-induced aBACs mediated the sex effect on stimulation. For estimated drinks number as well as feeling drunk, sex effects remained significant (t-values>2.2), but were substantially reduced (reduction in sex Estimate=24% for drinks number; reduction in sex × time Estimate=17% for drinks number and 28% for feeling drunk). Thus, sex effects on drinks number and feeling drunk were partly mediated by self-induced aBACs.
External validity of laboratory alcohol self-administration
We found a positive association between self-reported real-life drinking and laboratory ASA (see Table 2). Mean aBAC during i.v. ASA correlated positively with drinking days, binge days, and average number of drinks per drinking day (r(80)=.24-.35, p-values<.05). Moreover, we compared binge drinking in real life to that during laboratory i.v. ASA (reaching an aBAC of 80mg% or above; Courtney and Polich, 2009). In the TLFB, all but 6 participants reported binge drinking within the last 45 days. None of those 6 achieved maximum aBACs above 80mg% during i.v. ASA, whereas all others did.
Test-retest reliability of laboratory alcohol self-administration
We found a high test-retest reliability of the i.v. ASA measures across both i.v. ASA sessions. A paired t-test revealed no systematic mean aBAC difference between day1 and day2. Further, all correlations between day1 and day2 measures were significant, positive, and strong, including mean aBAC with r(80)=.60 (p<.001). Correlations between i.v. ASA and TLFB real-life drinking were generally stronger at day2 (r(80)=.20-.39) than day1 (r(82)=.17-.29, but only the correlation between alcohol requests and TLFB drinks per drinking day was significantly higher at day2 than day1 (t=2.51, p=.01, uncorrected for multiple testing).
When repeating the preceding analyses with day1 i.v. ASA measures, results remained the same with one exception: the interaction between sex and time (linear) on aBAC estimates reached significance (t=3.1).
Side effects
One woman of our final sample spontaneously reported nausea during the last 15 minutes of i.v. ASA, and two men vomited after the infusion was terminated. Otherwise, only minor alcohol-related side effects, such as tiredness, occurred.
Discussion
Two known genetically determined risk factors for AUDs, namely sex and FH, were tested in late adolescents. In that age group, male sex, but not positive FH, was associated with more alcohol intake.
Sex effect on alcohol intake
Our finding that women reported lower real-life drinking than men and induced lower aBACs in the laboratory corresponds to the broad literature reporting sex- and gender-related effects on alcohol intake (Erol and Karpyak, 2015). In our study, the sex effect on i.v. ASA remained robust after controlling for individual SRE scores, smoking status, or real-life drinking, suggesting that adolescent women prefer lower brain alcohol exposures than men. Certainly, the sex effect on i.v. ASA cannot be explained by sex differences in pharmacokinetics or distribution of alcohol. Instead, the sex effect can be explained by the following three options: 1) women selected lower aBACs than men due to sex differences in alcohol-induced subjective states; 2) they purposefully induced lower aBACs secondary to some unmeasured value system associated with the female gender-role; 3) a combination of 1 and 2. Our data support a role for sex differences in alcohol-induced unpleasant subjective states, because women experienced the same level of negative effects and more sedation at lower aBACs than men. Further, testing a subset of our sample at constant aBACs, Marxen et al. (2014) found larger increases in brain perfusion for women than men, which implies that the same brain alcohol exposure exerts stronger physiological effects in women than men. In line with this concept, Mick et al. (2015) reported that the alcohol-induced impairment relative to aBAC was lower in girls than boys aged 11-15, but higher in girls than boys aged 16-17 years, when examining adolescents who were admitted to in-patient pediatric care due to alcohol bingeing. A three-way interaction between sex, aBAC, and adverse alcohol-effects, might therefore represent a previously unknown pharmacodynamic factor explaining why, in our sample, adolescent women drank less alcohol in real life.
Importantly, there seemed to be no sex differences in pleasant alcohol effects. Women had lower ratings of stimulation than men, paralleling their lower aBACs, and in fact, the sex difference in stimulation was fully mediated by self-induced aBACs. Unfortunately, the ASA method is inappropriate for testing direct relationships between aBACs and subjective alcohol effects, because participants manipulate their aBAC as a tool to control subjective effects, which feeds back to behavior in a closed loop. For that reason, additional studies investigating negative subjective alcohol effects at constant aBACs are warranted.
Alternatively, adolescent women may have purposefully induced lower aBACs in response to gender-related societal pressures and expectations. DeVisser and McDonnell (2012) described gender specific double-standards for alcohol intake in English students aged 18-25. In their study, drinking was linked to traditionally masculine traits such as risk taking and aggression. The belief that women should drink less alcohol than men was rationalized based on feminine attributes, such as concerns with physical appearance or vulnerability to unwanted sex. Differences in drinking motives are another potential mechanism underlying the sex effect on i.v. ASA. Unfortunately, we can only speculate regarding our subjects’ gender-related motivations, and on whether they might be specific to late adolescence. Lastly, Erol and Karpyak (2015) summarized how mood and emotions influence heavy drinking. They reported that women tend to drink more often in response to negative emotions, whereas men typically drink to enhance positive emotions. If that is true, our laboratory setting could have specifically promoted men's drinking, because all adolescents reported high overall well-being before i.v. ASA.
Family history of alcoholism and alcohol intake
The present results are in contrast with our earlier finding that FHP adults self-infuse to higher aBACs than FHN (Zimmermann et al., 2009). In fact, a positive FH in men was associated with less frequent real-life drinking, and FHP men also reported less alcohol desire during i.v. ASA. The consistency between drinking behavior and desire to consume alcohol illustrates that subjective alcohol effects in the laboratory can reflect differences in real-life drinking. To determine if a FH effect on i.v. ASA was absent because it was masked by real-life substance intake, we tested interactions between FH and either SRE scores, smoking status, or real-life drinking. Still, the FH null-effect on mean aBAC remained.
There are several speculative mechanisms explaining our FH null-effect. First of all, drinking during adolescence is rather unstable. Consistently, several studies examining college students also reported FH null-effects, and instead of FH, familial depression predicted drinking problems (MacDonald et al., 1991). Further, Müller et al. (2015) found no differences between FHP and FHN adolescents in drinking, when testing 14-year-old subjects of the IMAGEN study. Comparing these studies with those reporting FH effects (for a review see Schuckit et al., 2009), suggests that FH becomes more influential with age, which was also supported by Rose et al. (2001). Besides young age, there are other aspects in which our sample differs from the general population. Seventy-eight percent had an educational level that qualified them for University, which is more than in the respective German population (43% in 2011; Statistisches Bundesamt, 2012). In Germany, higher qualification is an indicator of higher parental education and socioeconomic status which were both found to be linked to lower drinking in adolescents (Saraceno et al., 2009). Moreover, we excluded adolescents with psychiatric disorders such as depression or anxiety, although it is well-established that the genetic risk for alcohol dependence increases with the number of externalizing and internalizing symptoms (Lieberman, 2000).
Last but not least, subjects with a positive FH are a heterogeneous group. Since our analyses were based on group means, it remains possible that a subgroup of young FHP adults drank much more whereas another subgroup drank much less alcohol because they were aware of their own risk for drinking problems (Haller and Chassin, 2010). However, visual inspection of the TLFB histograms indicated no evidence for a bimodal distribution in the FHP group; the histograms for both FH groups were practically identical. However, we cannot rule out the possibility that some FHP participants were motivated to drink more or less due to awareness of their risk. Besides that, there are several risk genes for alcoholism, often identified with small effect sizes (Heath et al., 2011), and it remains unclear which of those genes were actually present in our sample. In the context of other data suggesting a functional role of XRCC5 in the development of alcohol use disorders we genotyped our participants for the rs28701 polymorphism. Although XRCC5 affected individual maximum aBACs in an allele-dose-dependent manner, FHP and FHN subjects did not significantly differ in the numbers of XRCC5 gene variants (Juraeva et al., 2015). Such findings argue against the use of FH as a proxy for specific genetic risk in scientific studies, although FH remains a valid and useful clinical indicator.
Methodological aspects of intravenous alcohol self-administration
Laboratory self-infusion was positively linked to real-life drinking, indicating that i.v. ASA is a valid tool for measuring drinking behavior. However, correlations were rather weak, which implies that i.v. ASA captures different aspects of drinking than the TLFB. In fact, i.v. ASA is measured in a highly controlled laboratory setting and integrates alcohol liking, wanting, and tolerance. The TLFB, on the other side, is a retrospective self-report measure of real-life drinking which can be affected by numerous additional factors, including alcohol price, social interaction, and peer pressure.
Unlike Zimmermann et al. (2008), we found strong positive correlations between day1 and day2 self-infusion behavior. Since adolescents are less consistent in decision-making than adults (Littlefield et al., 2010) our results indicate substantial test-retest reliability of the i.v. ASA method. Correspondingly, when repeating our day2 i.v. ASA analyses with data from day1, we came to the same conclusions. Therefore, we suggest that a separate training session is not required for i.v. ASA studies with large sample sizes and detailed instructions.
To summarize, the present study was the first applying ASA to adolescents. Our findings suggest no role for FH as a risk factor for drinking in that age group, but imply that young women preferentially self-infuse alcohol to lower aBACs than men when asked to produce pleasant alcohol effects. Whether our findings pertain to adolescents only, will be determined in the second wave of our D-LAYA study, in which we are testing the same participants three years later.
Acknowledgements
We thank Maike Neumann and Stefanie Reißmann for their outstanding assistance in recruitment, data acquisition and digitization, as well as Maik Spreer and Cornelius Groß for data acquisition. Further, we acknowledge Ute Böttner, Luise Olbricht, and Lucia Hämmerl who excelled at quality checking, reorganizing and digitizing the data. We would also like to thank Daniel Schad, and Sandra Wittleder for their extraordinary helpful comments on the statistical analyses. We are grateful for the ongoing efforts of Victor Vitvitskiy in the software maintenance of CAIS. Finally, we appreciate Michael Schmidt's prompt technical support.
Funding
The Dresden Longitudinal Study on Alcohol use in Young Adults (D-LAYA) was funded by the National Institute of Alcohol Abuse and Alcoholism (NIAAA), National Institutes of Health (NIH), grants P60 AA007611, and 1U01AA017900-01. Additional support came from the Bundesministerium für Bildung und Forschung (BMBF), grants 01EV0711 and 01ZX1311H and by the Deutsche Forschungsgemeinschaft (DFG), FOR 1617 grants SM 80/7-1 and ZI1119/4-1. The present work does not represent the DFGs, NIHs or NIAAAs official opinion. The author USZ declares that over the past three years, he has received money from the Sächsische Landesärztekammer, Gewerkschaft für Erziehung und Wissenschaft, Parkkrankenhaus Leipzig, ABW Wissenschaftsverlag, Servier, Janssen, and Lundbeck.
Footnotes
Disclosure
All other authors: EJ, GG, IM, CSe, AM, CSo, MHP, SOC, and MMS, declare no conflict of interest.
REFERENCES
- Acheson A, Richard DM, Mathias CW, Dougherty DM. Adults with a family history of alcohol related problems are more impulsive on measures of response initiation and response inhibition. Drug Alcohol Depend. 2011;117:198–203. doi: 10.1016/j.drugalcdep.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Stevens MC, O'Malley S, Book GA, Reynolds B, Pearlson GD. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT The alcohol use disorders identification test Guidelines for use in primary care. 2nd edn. World Health Organization, Department of Mental Health and Substance Dependence; Geneva: 2001. [Google Scholar]
- Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667–685. doi: 10.1016/j.cld.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmel R, Hagen J. Faktorenstruktur und psychometrische Eigenschaften einer gekürzten deutschsprachigen Version des Alcohol Expectancy Questionnaire (Brief AEQ G). Zeitschrift für Differentielle und Diagnostische Psychologie. 2002;23(2):205–216. [Google Scholar]
- DeVisser RO, McDonnell EJ. ‘That's OK. He's a guy’: a mixed-methods study of gender double-standards for alcohol use. Psychol Health. 2012;27:618–639. doi: 10.1080/08870446.2011.617444. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. A clinical laboratory paradigm for evaluating medication effects on alcohol consumption: naltrexone and nalmefene. Neuropsychopharmacology. 2003;28:755–764. doi: 10.1038/sj.npp.1300101. [DOI] [PubMed] [Google Scholar]
- Elliott JC, Carey KB, Bonafide KE. Does family history of alcohol problems influence college and university drinking or substance use? A meta-analytical review. Addiction. 2012;107:1774–1785. doi: 10.1111/j.1360-0443.2012.03903.x. [DOI] [PubMed] [Google Scholar]
- Erol A, Karpyak VM. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. 2015 doi: 10.1016/j.drugalcdep.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Gan G, Sterzer P, Marxen M, Zimmermann US, Smolka MN. Neural and Behavioral Correlates of Alcohol-Induced Aggression Under Provocation. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Alcohol Health Res World. 1998;22:144–147. [PMC free article] [PubMed] [Google Scholar]
- Haller MM, Chassin L. The Reciprocal Influences of Perceived Risk for Alcoholism and Alcohol Use Over Time: Evidence for Aversive Transmission of Parental Alcoholism. J Stud Alcohol Drugs. 2010;71:588–596. doi: 10.15288/jsad.2010.71.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzinger M, Bailer M, Worall H, Keller F. Testhandbuch. 3rd edn Huber; Bern: 2000. Beck-Depressions-Inventar : (BDI). [Google Scholar]
- Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA, McEvoy BP, Schrage AJ, Grant JD, Chou Y-L, Zhu R, Henders AK, Medland SE, Gordon SD, Nelson EC, Agrawal A, Nyholt DR, Bucholz KK, Madden PAF, Montgomery GW. A quantitative-trait genome-wide association study of alcoholism risk in the community: Findings and implications. Biol Psychiatry. 2011;70:513–518. doi: 10.1016/j.biopsych.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Claus ED, Ramchandani VA. Associations of OPRM1 A118G and alcohol sensitivity with intravenous alcohol self-administration in young adults. Addict Biol. 2016;21:125–135. doi: 10.1111/adb.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraeva D, Treutlein J, Scholz H, Frank J, Degenhardt F, Cichon S, Ridinger M, Mattheisen M, Witt SH, Lang M, Sommer WH, Hoffmann P, Herms S, Wodarz N, Soyka M, Zill P, Maier W, Jünger E, Gaebel W, Dahmen N, Scherbaum N, Schmäl C, Steffens M, Lucae S, Ising M, Smolka MN, Zimmermann US, Müller-Myhsok B, Nöthen MM, Mann K, Kiefer F, Spanagel R, Brors B, Rietschel M. XRCC5 as a risk gene for alcohol dependence: Evidence from a genome-wide gene-set-based analysis and follow-up studies in drosophila and humans. Neuropsychopharmacology. 2015;40:361–371. doi: 10.1038/npp.2014.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot K, Pittman B, Ralevski E, Limoncelli D, Koretski J, Newcomb J, Arias AJ, Petrakis IL. Effects of family history of alcohol dependence on the subjective response to alcohol using the intravenous alcohol clamp. Alcohol Clin Exp Res. 2013;37:2011–2018. doi: 10.1111/acer.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D. Alcohol Challenge Responses Predict Future Alcohol Use Disorder Symptoms: A 6-Year Prospective Study. Biological Psychiatry. 2014;75(10):798–806. doi: 10.1016/j.biopsych.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegl R, Masson MEJ, Richter EM. A linear mixed model analysis of masked repetition priming. Visual Cognition. 2010;18(5):655–681. [Google Scholar]
- Lindberg L, Brauer S, Wollmer P, Goldberg L, Jones AW, Olsson SG. Breath alcohol concentration determined with a new analyzer using free exhalation predicts almost precisely the arterial blood alcohol concentration. Forensic Sci Int. 2007;168(2-3):200–207. doi: 10.1016/j.forsciint.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Lachner G, Wittchen HU, Perkonigg A, Holly A, Schuster P, Wunderlich U, Türk D, Garczynski E, Pfister H. Structure, content and reliability of the Munich-Composite International Diagnostic Interview (M-CIDI) substance use sections. Eur Addict Res. 1998;4:28–41. doi: 10.1159/000018922. [DOI] [PubMed] [Google Scholar]
- Langsrud Ø . ANOVA for unbalanced data: Use Type II instead of Type III sums of squares. Statistics and Computing. 2003;13:163–167. [Google Scholar]
- Lieberman DZ. Children of alcoholics: An update. Curr Opin Pediatr. 2000;12:336–340. doi: 10.1097/00008480-200008000-00009. [DOI] [PubMed] [Google Scholar]
- Littlefield AK, Sher KJ, Steinley D. Developmental trajectories of impulsivity and their association with alcohol use and related outcomes during emerging and young adulthood I. Alcohol Clin Exp Res. 2010;34:1409–1416. doi: 10.1111/j.1530-0277.2010.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak SE, Elvine-Kreis B, Shea SH, Carr LG, Wall TL. Genetic risk for alcoholism relates to level of response to alcohol in Asian-American men and women. Journal of Studies on Alcohol. 2002;63(1):74–82. [PubMed] [Google Scholar]
- MacDonald R, Fleming MF, Barry KL. Risk factors associated with alcohol abuse in college students. American Journal of Drug and Alcohol Abuse. 1991;4:439–449. doi: 10.3109/00952999109001603. [DOI] [PubMed] [Google Scholar]
- Margraf J. Mini DIPS: Diagnostisches Kurz-Interview bei psychischen Störungen. Springer; Berlin: 1994. [Google Scholar]
- Marxen M, Gan G, Schwarz D, Mennigen E, Pilhatsch M, Zimmermann US, Guenther M, Smolka MN. Acute effects of alcohol on brain perfusion monitored with arterial spin labeling magnetic resonance imaging in young adults. J Cereb Blood Flow Metab. 2014;34:472–479. doi: 10.1038/jcbfm.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick I, Gross C, Lachnit A, Kalkbrenner M, Hoppe L, Reichert J, Zimmermann US. Alcohol-induced impairment in adolescents admitted to inpatient treatment after heavy episodic drinking: effects of age and gender. Journal of Studies on Alcohol and Drugs. 2015;76(3):493–497. doi: 10.15288/jsad.2015.76.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Weafer J, Fillmore MT. Gender Differences in Alcohol Impairment of Simulated Driving Performance and Driving-Related Skills. Alcohol and Alcoholism. 2009;44(6):586–593. doi: 10.1093/alcalc/agp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: A critical review of the literature. Alcohol Clin Exp Res. 2010;34:385–395. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR, Treat TA. Differences in subjective response to alcohol by gender, family history, heavy episodic drinking, and cigarette use: refining and broadening the scope of measurement. Journal of Studies on Alcohol and Drugs. 2015;76(2):287–295. doi: 10.15288/jsad.2015.76.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller KU, Gan G, Banaschewski T, Barker GJ, Bokde ALW, Büchel C, Conrod P, Fauth-Bühler M, Flor H, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Lawrence C, Loth E, Mann K, Martinot J-L, Nees F, Paus T, Pausova Z, Rietschel M, Ströhle A, Struve M, Schumann G, Smolka MN, IMAGEN Consortium No differences in ventral striatum responsivity between adolescents with a positive family history of alcoholism and controls. Addict Biol. 2015;20:534–545. doi: 10.1111/adb.12136. [DOI] [PubMed] [Google Scholar]
- Plawecki MH, Zimmermann US, Vitvitskiy V, Doerschuk PC, Crabb D, O'Connor S. Alcohol exposure rate control through physiologically based pharmacokinetic modeling. Alcohol Clin Exp Res. 2012;36:1042–1049. doi: 10.1111/j.1530-0277.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective Response to Alcohol Challenge: A Quantitative Review. Alcoholism: Clinical and Experimental Research. 2011;35(10):1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li T-K, O'Connor S. A Physiologically-Based Pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999;23:617–623. [PubMed] [Google Scholar]
- Ramchandani VA, Plawecki M, Li T-K, O'Connor S. Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcoholism: Clinical and Experimental Research. 2009;33:938–944. doi: 10.1111/j.1530-0277.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: regional residency moderates longitudinal influences on alcohol use. Alcohol Clin Exp Res. 2001;25:637–643. [PubMed] [Google Scholar]
- Saraceno L, Munafó M, Heron J, Craddock N, van den Bree MBM. Genetic and non-genetic influences on the development of co-occurring alcohol problem use and internalizing symptomatology in adolescence: A review. Addiction. 2009;104:1100–1121. doi: 10.1111/j.1360-0443.2009.02571.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. Journal of Substance Abuse Treatment. 2009;36(1):S5–14. [PubMed] [Google Scholar]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, Bierut L, Goate A, Foroud T. A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res. 2001;25:323–329. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Tipp JE. The Self-Rating of the Effects of Alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction. 1997;92:979–988. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption. Humana Press Inc; New York, NY: 1992. pp. 41–72. [Google Scholar]
- Steinberg L, Fletcher A, Darling N. Parental monitoring and peer influences on adolescent substance use. Pediatrics. 1994;93:1060–1064. [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Laucht M, Vitvitskiy V, Plawecki MH, Mann KF, O'Connor S. Offspring of parents with an alcohol use disorder prefer higher levels of brain alcohol exposure in experiments involving computer-assisted self-infusion of ethanol (CASE). Psychopharmacology. 2009;202:689–697. doi: 10.1007/s00213-008-1349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Vitvitskyi V, Plawecki MH, Mann KF, O'Connor S. Development and pilot validation of computer-assisted self-infusion of ethanol (CASE): A new method to study alcohol self-administration in humans. Alcohol Clin Exp Res. 2008;32:1321–1328. doi: 10.1111/j.1530-0277.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, O'Connor S, Ramchandani VA. Modeling alcohol self-administration in the human laboratory. Curr Top Behav Neurosci. 2013;2013:315–353. doi: 10.1007/7854_2011_149. [DOI] [PubMed] [Google Scholar]