Abstract
Background
Liver surgery for perihilar cholangiocarcinoma (PHC) is associated with postoperative mortality ranging from 5% to 18%. The aim of this study was to develop a preoperative risk score for postoperative mortality after liver resection for PHC, and to assess the effect of biliary drainage of the future liver remnant (FLR).
Study design
A consecutive series of 287 patients submitted to major liver resection for presumed PHC between 1997 and 2014 at two Western centers was analyzed; 228 patients (79%) underwent preoperative drainage for jaundice. FLR volumes were calculated with CT volumetry, and completeness of FLR drainage was assessed on imaging. Logistic regression was used to develop a mortality risk score.
Results
Postoperative mortality at 90-days was 14%, and was independently predicted by age (Odds ratio [OR] per 10 years 2.1), preoperative cholangitis (OR 4.1), FLR volume below 30% (OR 2.9), portal vein reconstruction (OR 2.3), and incomplete FLR drainage in patients with FLR volume below 50% (OR 2.8). The risk score showed good discrimination (AUC 0.75 after bootstrap validation), and ranking patients in tertiles identified three (low-intermediate-high) risk subgroups with predicted mortalities of 2%, 11%, and 37%. No postoperative mortality was observed in 33 undrained patients with FLR volumes above 50%, including 10 jaundiced patients (median bilirubin level 11 mg/dL).
Conclusions
The mortality risk score for patients with resectable PHC can be used for patient counseling and identification of modifiable risk factors, which include FLR volume, FLR drainage status, and preoperative cholangitis. We found no evidence to support preoperative biliary drainage in patients with an FLR volume above 50%.
Keywords: perihilar, cholangiocarcinoma, prediction, risk score, postoperative mortality, future liver remnant, volumetry, biliary drainage
INTRODUCTION
Perihilar cholangiocarcinoma (PHC) is the most common biliary cancer, with an annual incidence of 1-2 per 100.000 persons.(1) A resection with curative intent is indicated in the absence of metastatic and locally advanced disease. Complete resection of PHC requires a combined extrahepatic bile duct resection and liver resection;(2) however, liver resection for PHC is associated with a very high postoperative mortality rate, ranging from 5% to 18% in large series.(3-7)
Biliary obstruction from PHC has been identified as an important risk factor for postoperative mortality.(8-10) Decompression of the future liver remnant (FLR) with biliary drainage potentially decreases the risk of postoperative liver failure and mortality.(11) However, cholangitis caused by biliary instrumentation and contamination might in turn increase infectious complications and mortality.(3, 12) Recent studies have suggested that biliary drainage should be reserved only for patients with a small FLR volume, as stent related complications might outweigh the benefits of biliary decompression in subgroups with sufficient FLR volume. Kennedy et al showed higher postoperative mortality when patients with FLR’s above 30% were subjected to preoperative drainage.(13) Similarly, Farges et al found an adverse effect of preoperative drainage for left-sided hepatectomy (i.e. smaller volume resections).(5)
FLR volumetry has been used independently to anticipate the risks associated with liver resection for PHC,(14) but never has it been incorporated in risk assessment scores that fully account for patients’ risk profiles. Such preoperative risk scores have better accuracy than surgeons’ clinical anticipation,(15) and may be useful to guide decision-making regarding surgical therapy and preoperative management. Also, there is evidence that openly addressing the risk of a procedure increases patient safety.(16) The objective of this study was to develop a preoperative mortality risk score that predicts postoperative mortality after liver resection for PHC. Moreover, this study aimed to assess the effect of preoperative biliary drainage in subgroups with different FLR volumes.
METHODS
Patients
This study included only patients who underwent concomitant extrahepatic bile duct resection and major liver resection (i.e. resection of at least 3 liver segments) for presumed PHC. Approximately 10% of patients are typically found to have alternative diagnoses at postoperative pathology.(17, 18) These patients were included in the analyses, because information regarding the definitive pathology is unavailable at the time of preoperative shared decision-making. Consecutive patients, who underwent surgery between January 1997 and March 2014, were identified from prospective databases in the Academic Medical Center (AMC) in Amsterdam, the Netherlands, and the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, USA. Presumed PHC was defined as an apparent malignant biliary stricture, originating in the common hepatic duct, the hepatic duct confluence, or in the left or right hepatic duct.
Preoperative management and surgery
Preoperative biliary drainage was used in the majority of jaundiced patients, consisting of endoscopic stent placement, percutaneous transhepatic catheter placement, or both. The aim of biliary drainage was complete drainage of the FLR. Occasionally, stents decompressed the contra-lateral liver, either because they were placed before referral without consulting a hepatobiliary surgeon or inadvertently because endoscopic placement of stents into prespecified liver segments can be technically challenging. Moreover, stents placed in the FLR did not always provide complete decompression because of stent occlusion or isolated segmental bile ducts. Jaundiced patients underwent resection without drainage only if they were referred without stents already in place, and if the attending surgeon decided to omit preoperative drainage. Portal vein embolization was used since 2006 when CT volumetry indicated an FLR less than 30%.(13) Resection of the tumor was performed during exploratory laparotomy if no metastases were found beyond the hepatoduodenal ligament and if a complete, potentially curative resection was anticipated. Radical resection encompassed: excision of the liver hilum en bloc with (extended) hemihepatectomy, including the caudate lobe in most cases, complete lymphadenectomy of the hepatoduodenal ligament, and excision and reconstruction of the portal vein bifurcation when it was necessitated.
Definitions
The primary endpoint of this study was postoperative mortality, defined as any cause of death within 90 days of resection.(19) Postoperative liver failure was defined as grade B or higher according to the International Study Group of Liver Surgery (i.e. impacting clinical management).(20)
FLR volume was assessed on post-hoc volumetry in patients with adequate preoperative CT or MRI imaging available. We chose to measure liver volumes directly and compute the remnant to total liver volume ratio.(21, 22) The body surface area, as used in the method described by Vauthey et al,(23) was not factored into these calculations because it often results in over- or underestimation of the total liver volume.(24) The total liver volume was retrospectively determined by semi-automated contouring of the liver on pre-operative scans, using integrated software (Mx-View v3.52, Philips Medical Systems, at AMC, and Scout™ v1.5.1.1, Pathfinder technologies, at MSKCC). Post-embolization volumes were used for patients undergoing preoperative portal vein embolization. This was repeated for the volume of the resected liver. The difference between total liver volume and resected volume was the FLR volume, which was subsequently categorized as small (<30%), intermediate (30-50%), or large (>50%); since PHC are small in size and arise from the biliary tree, tumor volume was not factored into these calculations. FLR drainage status was also assessed on pre-resection imaging, which was performed by an experienced HPB surgeon or fellow blinded to the outcome. Biliary dilatation in one or more segments of the FLR, either persisting after stent placement or occurring in patients with jaundice and no stent placement, was considered incomplete FLR drainage.(13) Preoperative cholangitis was considered present if, at any time in the preoperative course (before drainage, after drainage, or present at the time of surgery), the patient had an episode of fever, abdominal complaints, and leucocytosis requiring (additional) biliary drainage, as defined in clinical trials.(25, 26) Treatment with antibiotics alone was insufficient to diagnose cholangitis, since antibiotics are often prophylactic in nature or administered for other indications. The variable portal vein reconstruction was considered preoperatively available, because the necessity of a portal vein reconstruction is reliably assessable on preoperative CT images (sensitivity and specificity 89% and 92%, respectively).(27)
Statistical analysis
Univariable analysis consisted of Mann-Whitney U test for continuous variables and X2 tests for categorical variables. Variables with P <0.2 in univariable analysis were entered into multivariable analysis, which consisted of logistic regression. Subsequently, a backward stepwise selection method, with P <0.2 as condition to be retained in the model, was used to select the final model. Different effects of incomplete FLR drainage were expected in subgroups with different FLR volumes, based on two recent studies.(5, 13) Therefore, we not only included the variables incomplete FLR drainage and FLR volume in multivariable analysis, but also an interaction-term between these two variables. The interaction between the variables describes the additional effect if patients have both incomplete FLR drainage and FLR volume below 50%.(28, 29) Missing data were rare and handled with full-case analysis. We tested multiple imputation as an alternative method to handle missing data,(30) but the results were similar to full-case analysis.
A standard approach was used to develop the mortality risk score.(31) The number of points assigned to each risk factor equaled its regression coefficient in multivariable analysis. Points were divided by the risk factor with the smallest absolute number of points, and rounded to the nearest whole number. Predicted risks were then calculated for each patient by applying total point-scores to the logistic regression formula. Risk score tertiles were used to categorize patients into low-risk, intermediate-risk, and high-risk groups. We calculated the area under the curve (AUC) and 95% confidence intervals (CIs) to estimate how well the model discriminated between patients with and without postoperative mortality. Model validation was performed using bootstrap resampling to quantify the overfitting of our modeling strategy and predict future performance of the model. Statistical analyses were performed using SPSS v22 (SPSS Inc., Chicago, IL), and R, version 2.15.3, software packages (http://www.r-project.org/).
RESULTS
Patients
A total of 378 patients underwent resection of presumed PHC during the study period. Of these, 91 patients were excluded because they underwent an extrahepatic bile duct resection without a major liver resection (90-day mortality 1%). The remaining 287 patients underwent a combined extrahepatic bile duct resection and major liver resection, and were included in this study (n=151 from MSKCC and n=136 from AMC). Patient characteristics of the complete cohort are shown in Table 1; online Supplemental Table S1 compares the patient characteristics between centers. There were 184 men (64%); mean age was 62 years (range 29-87 years). Final pathologic diagnosis confirmed PHC in 258 patients (90%), but revealed other disease in 29 patients (10%), consisting of intraductal papillary neoplasm of the bile duct without invasive component in 6 (2%), and a benign stricture in 17 patients (6%). A malignancy other than the preoperatively suspected PHC was found in 6 patients (2%), consisting of gallbladder carcinoma (n=2), hepatocellular carcinoma (n=1), neuroendocrine tumor (n=1), sarcoma (n=1), and colorectal metastasis (n=1). Estimated median survival after resection of a malignancy was 37 months (range 0 – 178).
Table 1.
Patient Characteristics and Univariable Analysis
| Variable | All patients | No 90-day mortality | 90-day mortality | p Value |
|---|---|---|---|---|
| Age, mean ± SD | 62.7 ± 11.1 | 61.9 ± 10.9 | 67.4 ± 10.6 | 0.003 |
| Sex | 0.05 | |||
| Female | 105 (36.6) | 96 (38.9) | 9 (22.5) | |
| Male | 182 (63.4) | 151 (61.1) | 31 (77.5) | |
| Bismuth classification | 0.14 | |||
| Left or right duct | 30 (10.5) | 29 (11.7) | 1 (2.5) | |
| 1 | 11 (3.8) | 11 (4.5) | 0 (0) | |
| 2 | 20 (7.0) | 17 (6.9) | 3 (7.5) | |
| 3A – right | 102 (35.5) | 82 (33.2) | 20 (50.0) | |
| 3B – left | 72 (25.1) | 61 (24.7) | 11 (27.5) | |
| 4 | 52 (18.1) | 47 (19.0) | 5 (12.5) | |
| Lobar atrophy | 0.63 | |||
| None | 172 (59.9) | 146 (59.1) | 26 (65.0) | |
| Right | 51 (17.8) | 46 (18.6) | 5 (12.5) | |
| Left | 64 (22.3) | 55 (22.3) | 9 (22.5) | |
| Type of liver resection | 0.79 | |||
| Central hepatectomy | 3 (1.0) | 3 (1.2) | 0 (0) | |
| Left hemihepatectomy | 102 (35.5) | 90 (36.4) | 12 (30.0) | |
| Left extended hemihepatectomy | 36 (12.5) | 31 (12.6) | 5 (12.5) | |
| Right hemihepatectomy | 46 (16.0) | 40 (16.2) | 6 (15.0) | |
| Right extended hemihepatectomy | 100 (34.8) | 83 (33.6) | 17 (42.5) | |
| Portal vein reconstruction | 0.11 | |||
| No | 236 (82.2) | 207 (83.8) | 29 (72.5) | |
| Yes | 51 (17.8) | 40 (16.2) | 11 (27.5) | |
| FLR volume* | 0.003 | |||
| Large >50% | 115 (47.3) | 105 (50.5) | 10 (28.6) | |
| Intermediate 30-50% | 76 (31.3) | 66 (31.7) | 10 (28.6) | |
| Small <30% | 52 (21.4) | 37 (17.8) | 15 (42.9) | |
| Jaundice | 0.009 | |||
| No | 46 (16.0) | 45 (18.2) | 1 (2.5) | |
| Yes | 241 (84.0) | 202 (81.8) | 39 (97.5) | |
| FLR drainage* | 0.28 | |||
| Complete | 173 (71.2) | 150 (72.1) | 23 (65.7) | |
| Incomplete | 70 (28.8) | 58 (27.9) | 12 (34.3) | |
| Incomplete drainage + FLR below 50%* | 0.04 | |||
| No | 204 (84.0) | 179 (86.1) | 25 (71.4) | |
| Yes | 39 (16.0) | 29 (13.9) | 10 (28.6) | |
| Preoperative cholangitis* | 0.001 | |||
| No | 213 (76.3) | 193 (79.8) | 20 (54.1) | |
| Yes | 66 (23.7) | 49 (20.2) | 17 (45.9) | |
| Hospital, n (%) | 0.04 | |||
| MSKCC | 151 (52.6) | 136 (55.1) | 15 (37.5) | |
| AMC | 136 (47.4) | 111 (44.9) | 25 (62.5) | |
| Resection margin† | 0.55 | |||
| R0 | 204 (75.6) | 176 (76.2) | 28 (71.8) | |
| R1 | 66 (24.4) | 55 (23.8) | 11 (28.2) |
Data are presented as number (%), unless stated otherwise.
The variable cholangitis was missing in 8 patients; FLR assessment was missing in 44 patients.
The variable resection margin excludes 17 patients with benign disease on final postoperative pathology.
FLR, future liver remnant; IQR interquartile range; SD, standard deviation
FLR volumes
FLR volumetry was available for 243 patients (85%); six patients had undergone portal vein embolization. The median calculated FLR volume in all patients was 47% (range, 16-89%). Figure 1A presents the FLR volumes comparing right- and left-sided liver resections. Most patients who underwent a right-sided hepatectomy had a small FLR volume (50 of 127 patients; 39%) or intermediate FLR volume (66 of 127 patients; 52%). Most patients who underwent a left-sided hepatectomy had a large FLR volume (102 of 133 patients; 90%).
Figure 1.
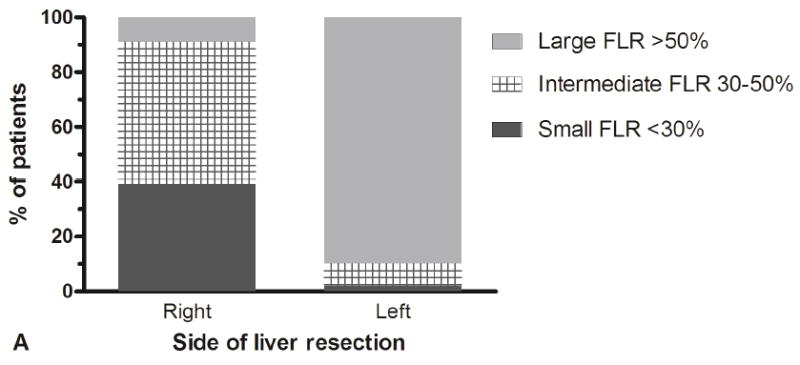
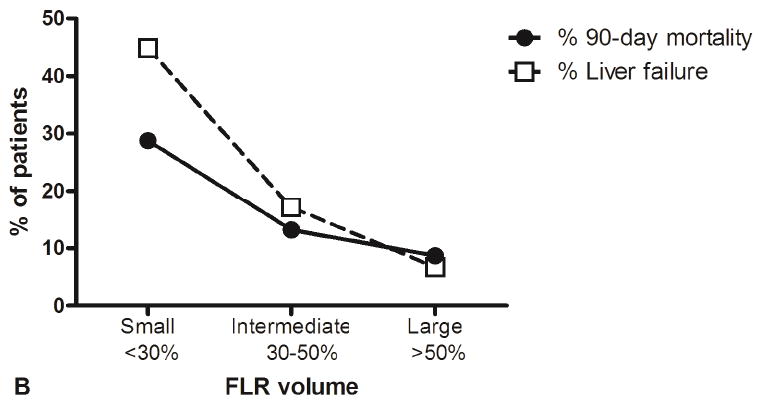
FLR volumes and associated outcomes in the patient cohort. (A) Distribution of FLR volumes among patients who underwent a right- or left-sided liver resection, and (B) 90-day mortality and liver failure rates according to the FLR volumes.
Preoperative drainage
Jaundice was observed in 241 patients (84%); the median bilirubin level of jaundiced patients at presentation was 9.5 mg/dL (range 3.0 – 37.9). Preoperative biliary drainage was used to treat jaundice in 228 patients (79%), which included endoscopic stent placement (n=130), percutaneous transhepatic catheter placement (n=27), or both (n=71). The median time between first stent placement and laparotomy was 60 days (range, 7 – 262); the outliers being explained by some patients who at first received a stent for suspicion of a benign stricture that was later diagnosed as malignant. Among patients who underwent biliary drainage, the bilirubin level decreased to a median of 1.2 mg/dL at laparotomy (range, 0.2 – 11.6). Preoperative cholangitis was observed in 66 of 228 patients (29%) after biliary drainage. All patients developing cholangitis underwent revisional drainage procedures prior to surgery. Cholangitis was observed in 0 out of 58 patients without preoperative drainage.
Among the 243 patients with available FLR volumetry, 70 patients (29%) had incomplete drainage of the FLR segments at the time of surgery: 26 patients had stents or catheters draining segments other than the FLR, 20 patients had isolated segmental bile ducts that remained obstructed after stents or catheter placement, and 12 patients with jaundice underwent no preoperative drainage.
Postoperative mortality and liver failure
Postoperative mortality at 90-days was 14% (n=40/287); 10% at MSKCC (n=15/151) and 18% at AMC (n=25/136). The rate of postoperative mortality remained constant during the study period; it was 14% between 1997 to 2005 (n=18/129) and 14% between 2006 to 2014 (n=22/158). A total of 46 patients (16%) developed postoperative liver failure with a mortality rate of 72%. Among the 40 patients who died within 90 days, 33 (83%) had developed liver failure, which was often accompanied by an infectious complication or sepsis (n=22/33); the others died from causes unrelated to liver failure: sepsis (n=5), aspiration (n=1), and myocardial infarction (n=1). One patient had benign disease on final pathologic diagnosis.
Univariable analysis
Table 1 details the univariable analyses, and Figure 1B illustrates the association between FLR volume and postoperative mortality. Incomplete FLR drainage was not associated with postoperative mortality in the complete cohort (P=0.28), but the effect of incomplete FLR drainage was different in subgroup analysis of patients with different FLR volumes, as shown in Figure 2. Incomplete FLR drainage was associated with postoperative mortality only in patients with a small or intermediate FLR (Table 1; P=0.04). By contrast, patients with large FLR volume had comparable postoperative mortality with complete and incomplete FLR drainage (Figure 2).
Figure 2.
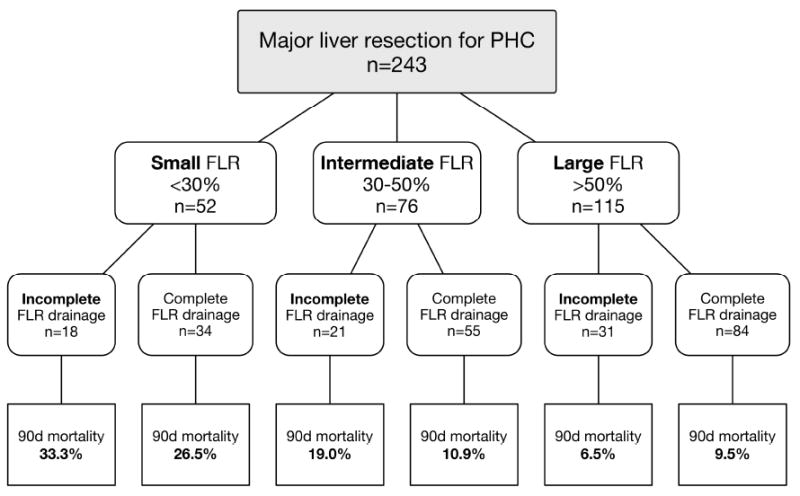
Breakdown of postoperative mortality according to FLR volume, showing incomplete FLR drainage as an adverse risk factor in patients with a small or intermediate FLR, but not in patients with a large FLR. Preoperative drainage was used in 100% and 97.1% in the small FLR group; in 90.5% and 78.2% in the intermediate FLR group; in 75.0% and 72.6% in the large FLR group (enumerating patients with incomplete and complete FLR drainage, respectively). FLR Future liver remnant; 90d mortality postoperative 90-day mortality.
Multivariable analysis
Multivariable analysis is shown in Table 2. Five variables were selected in the final model, including age, preoperative cholangitis, FLR volume below 30%, portal vein reconstruction, and incomplete FLR drainage in patients with FLR volume below 50%. The treating hospital was not associated with postoperative mortality after adjustment in multivariable analysis.
Table 2.
Multivariable Analysis of Postoperative Mortality
| Risk factor | Full model | Selected model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age (per 10 years) | 2.1 | 1.4-3.3 | 0.001 | 2.1 | 1.4-3.3 | 0.001 |
| Male sex | 1.6 | 0.6-4.1 | 0.34 | - | - | - |
| Jaundice | 3.1 | 0.4-26.8 | 0.30 | - | - | - |
| Preoperative cholangitis | 3.5 | 1.5-8.5 | 0.005 | 4.1 | 1.8-9.4 | 0.001 |
| Incomplete drainage | 0.7 | 0.1-3.6 | 0.64 | - | - | - |
| Incomplete drainage + FLR below 50% | 3.8 | 0.7-23.2 | 0.15 | 2.8 | 1.1-7.5 | 0.04 |
| FLR below 30% | 2.5 | 1.1-6.1 | 0.04 | 2.9 | 1.2-6.9 | 0.02 |
| Portal vein reconstruction | 2.1 | 0.8-5.6 | 0.13 | 2.3 | 0.9-5.8 | 0.09 |
| Treating hospital (MSKCC vs AMC) | 1.4 | 0.6-3.2 | 0.46 | - | - | - |
AMC; Academic Medical Center Amsterdam; CI, confidence interval; FLR, future liver remnant; OR, odds ratio; MSKCC, Memorial Sloan Kettering Cancer Center
Mortality risk score
The derived preoperative risk score to predict postoperative mortality yielded a total score ranging from 0-9. (Table 3) Predicted risks for each point score are presented in Table 4. The predicted risk was 2% in the low-risk tertile (0-2 points); 11% in the intermediate-risk tertile (3 or 4 points); and 37% in the high-risk tertile (≥5 points).
Table 3.
Developed Mortality Risk Score to Predict 90-day Postoperative Mortality After Liver Resection for Perihilar Cholangiocarcinoma
| Risk factor | Points |
|---|---|
| Age, y | |
| <50 | 0 |
| 50-59 | 1 |
| 60-69 | 2 |
| 70-79 | 3 |
| ≥80 | 4 |
| Preoperative cholangitis | 2 |
| FLR below 30% | 1 |
| Incomplete drainage + FLR below 50% | 1 |
| Portal vein reconstruction | 1 |
FLR, future liver remnant
Table 4.
Risks Predicted by the Mortality Risk Score
| Group | Point total | N* | Predicted risk of postoperative mortality, % |
|---|---|---|---|
| Low-risk | 0 | 4 | 1 |
| 1 | 23 | 1 | |
| 2 | 45 | 3 | |
| Intermediate-risk | 3 | 60 | 6 |
| 4 | 55 | 14 | |
| High-risk | 5 | 34 | 28 |
| 6 | 16 | 47 | |
| 7 | 5 | 67 | |
| 8 | 1 | 82 | |
| 9 | 0 | N/A |
N represents the number of patients in the study for each point score; 44 patients were not classified because the future liver remnant (FLR) volume was missing.
N/A, not applicable
Model performance
Predictive accuracy (discrimination) of the mortality risk score was measured by calculating the AUC, which was 0.79 (95% CI 0.71 – 0.87). Bootstrap validation of the model revealed minimal evidence of model overfit: the AUC after bootstrap validation was 0.75. Predictive accuracy remained good after categorizing patients into low-, intermediate-, and high-risk groups (AUC 0.76, 95% CI 0.68 – 0.84). Also, the predictive accuracy remained constant during the study period; the AUC was 0.80 before 2006 and 0.78 after 2006.
Effect of drainage in patients with large FLR
Since incomplete FLR drainage was not a risk factor in patients with large FLR volume above 50% as described above, we assessed the effect of biliary drainage in this subgroup in Figure 3. Jaundice at presentation was observed in 92 of 115 patients (80%) with large FLR, and 82 of these patients underwent preoperative biliary drainage with stent or catheter placement. Postoperative mortality in the drained subgroup was 12% (n=10/82), and the major determinant was preoperative cholangitis developing after preoperative drainage (20% vs. 8%). By contrast, postoperative mortality among the 10 undrained jaundiced patients was 0%. The median bilirubin level in these patients was 11.0 mg/dL (range 3.0 – 24.7); median FLR volume was 71% (range 55 – 77); 9 patients underwent left-sided hepatectomy and 1 right-sided hepatectomy. Postoperative mortality in 23 patients with large FLR and no jaundice was also 0%.
Figure 3.
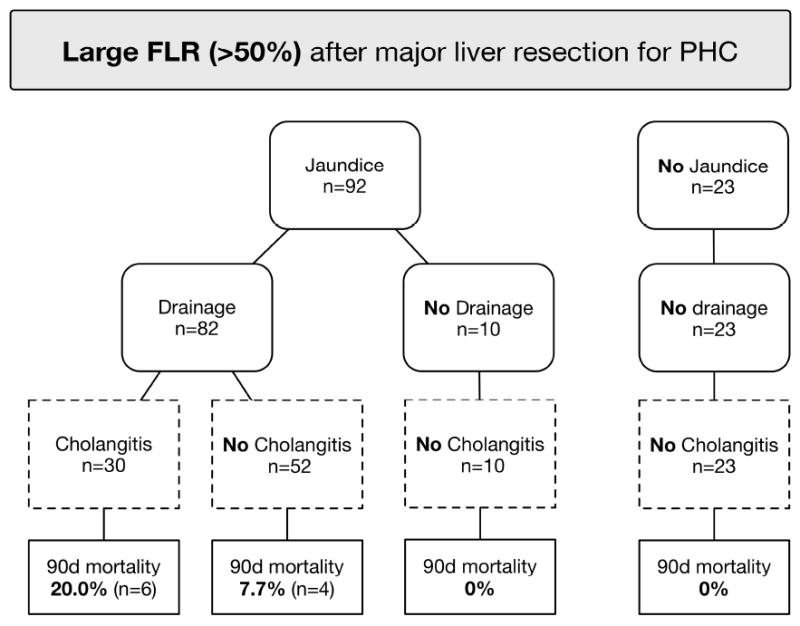
Breakdown of 115 patients with a large FLR (>50%) showing the effect of biliary drainage on cholangitis and associated mortality. FLR Future liver remnant; 90d mortality postoperative 90-day mortality.
DISCUSSION
Principal findings
This study found a 90-day postoperative mortality rate of 14% after major liver resection for PHC. We developed a mortality risk score based on five preoperative risk factors: age, preoperative cholangitis, FLR volume below 30%, portal vein reconstruction, and incomplete FLR drainage in patients with FLR volume below 50%. Incomplete drainage of the FLR was not a risk factor in patients with a large FLR above 50%.
Mortality risk score
The preoperatively applicable mortality risk score had good predictive accuracy (AUC 0.75 after bootstrap validation), and resulted in similar-sized groups with a low risk, intermediate risk, and high risk for postoperative mortality. This is the first risk score reported for patients submitted to liver resection for PHC. Patients with five or more points in the risk score have a high risk of postoperative mortality that may be considered unacceptable. A major strength of the risk score is that it allows preoperative modification of risk factors with the potential to reduce the risk of postoperative mortality. Modifiable risk factors in the score include FLR volume, FLR drainage status, and preoperative cholangitis.
Preoperative drainage
The merits of preoperative biliary drainage have long been a topic of debate for both perihilar and distal biliary obstruction. It is used as standard treatment in many centers, since it has been shown to improve liver function and regeneration capacity.(11) Also, patients are often referred to specialty centers with drains already in place. Nonetheless, preoperative biliary drainage has a high morbidity rate, which was exemplified by a Dutch multi-center trial that showed an increased rate of perioperative complications after preoperative drainage for cancer of the pancreatic head.(25) In patients with PHC, a recent French multi-center study found that preoperative drainage was beneficial for right-sided hepatectomy but not for left-sided hepatectomy.(5) Preoperative drainage for left-sided hepatectomy was associated with increased postoperative mortality, presumably because drainage-related complications outweighed the benefits of biliary decompression in this subgroup. The difference in outcomes in that study was attributed to a smaller parenchymal sacrifice after left- compared to right-sided hepatectomy. However, the side of liver resection in PHC is an inaccurate surrogate for the resected volume because atrophy of the tumor-involved liver with contralateral hypertrophy of the FLR is common. Moreover, extended resections are not considered when right- and left-sided resections are compared. Using patient-level volumetry to accurately determine resected and remnant liver volumes, we showed that 10% of patients submitted to left-sided liver resections had a liver remnant below 50%. Furthermore, 9% of patients submitted to right-sided liver resections had a hypertrophic large liver remnant above 50. We found that incomplete drainage of the FLR (i.e. persistent biliary dilatation on imaging) was a risk factor in patients with a small or intermediate FLR volume below 50%. By contrast, incomplete FLR drainage was not an adverse factor in patients with a large FLR volume above 50%. The risk of 90-day mortality in patients with a large FLR was rather determined by preoperative cholangitis developing after preoperative drainage. No postoperative mortality was observed in 33 undrained patients with large FLR volumes, including 10 jaundiced patients.
Limitations
This retrospective study has several limitations. Firstly, a low number of events was associated with statistical uncertainty in multivariable analysis, as reflected by the relatively wide confidence intervals. Although a larger study population would allow for more definitive conclusions, the present report combines the two largest published Western single-center series. Secondly, the risk score has not yet been externally validated, which would be desirable. As a substitute, internal validation with bootstrap resampling revealed minimal evidence of model overfit. Thirdly, other described risk factors for postoperative mortality, such as intra-operative blood loss and blood transfusion,(3, 4) could have further improved the mortality risk score. These factors, however, were intentionally excluded because they are not available preoperatively. Fourthly, completeness of FLR drainage was classified by assessing biliary dilatation on imaging. As an alternative, bilirubin levels have often been used in previous studies as a measure of biliary obstruction, but bilirubin levels may not be sensitive to (partial) obstruction in the FLR when stents drain the contralateral resection liver. Finally, some included patients were shown to have other disease than PHC at final postoperative pathology. These patients were intentionally included in the analysis because they were suspected to have PHC at preoperative staging. This perspective is important, since about 80% of patients undergo surgery without preoperative proof of malignancy,(32) and about 10% have a different diagnosis at final postoperative pathology.(17, 18)
Mortality rates
The observed 14% all cause postoperative 90-day mortality rate is comparable to other large Western series of liver resection for PHC. The French multicenter study reported a 90-day mortality of 11% in 366 patients,(5) and a recent Italian multicenter study reported a 90-day mortality of 10% in 376 patients.(4) Another study included 224 patients who underwent liver resection from centers in Europe and USA, and reported a 90-day mortality of 16%.(6) Several European centers reported higher postoperative mortality rates up to 18%.(7, 33) On the other hand, several Asian centers published lower postoperative mortality rates. For example, a Japanese group reported a 90-day postoperative mortality of 5% in 574 patients.(3) The actual range may be even wider due to publication bias.(34)
The infrequent usage of portal vein embolization in the early years of this study may have contributed to the postoperative mortality rate. In response, both centers have started to use portal vein embolization as a standard procedure if the anticipated FLR is below 30% since 2006. Nonetheless, mortality rates in the study have not decreased over the years, signifying that other risk factors equally contribute to mortality. The difference in postoperative mortality of 10% to 18% between the two centers in the study is substantial, but multivariable analysis failed to demonstrate an independent association. At AMC Amsterdam it has been common practice to achieve complete drainage of the FLR segments prior to hepatectomy, partly because the vast majority of patients are referred with endoscopic stents already in situ. One hypothesis is that this drainage policy comes at the cost of a higher rate of cholangitis due to malfunctioning stents, which could explain the higher mortality rate at AMC.
Clinical recommendations
FLR volumetry should be used to guide decision-making for biliary drainage and portal vein embolization, since volumes reflect atrophy of the tumor-involved liver and contralateral hypertrophy. Volume enhancement with portal vein embolization is recommended in patients with an FLR volume below 30%,(35-37) and should be preceded by complete biliary drainage of the non-embolized lobes to facilitate regeneration.(38) Some authors have suggested to use portal vein embolization also for an FLR between 30% to 50%,(39, 40) but there is currently insufficient evidence to support this recommendation. We found no significant difference in mortality between the group with an FLR between 30% to 50% and the group with an FLR above 50% in this study, while portal vein embolization prolongs time to surgery with 2-6 weeks.
Cholangitis should be avoided or resolved prior to surgery, since it is strongly associated with postoperative mortality.(3, 12) Cholangitis is less often reported after percutaneous drainage (~10%) than after endoscopic drainage (40-60%),(41, 42) but this difference remains to be confirmed in a randomized controlled trial that is currently underway.(26) Based on the results of this study and the study by Farges et al,(5) selective use of preoperative biliary drainage may reduce the rate of cholangitis and related mortality. Patients with jaundice and FLR volumes below 50% may benefit from preoperative drainage, because the risk of an undrained small FLR is more important than the risk of inflammatory complications. However, preoperative drainage should be avoided in patients with a large FLR volume above 50%. Preoperative drainage carries a risk of cholangitis and related mortality, while biliary decompression has shown no benefit in these patients because the FLR is large enough to regenerate sufficiently.
Age(3) and the need for portal vein reconstruction(6) are non-modifiable risk factors of postoperative mortality. Especially age was identified as a strong risk factor. According to the proposed mortality risk score, resection in patients aged 80 years or older with additional risk factors is associated with high risks, and may thus be considered contra-indicated.
CONCLUSIONS
This study has shown that patients undergoing major liver resection for PHC can be stratified into low-, intermediate-, and high-risk of postoperative mortality based on preoperatively available risk factors. The proposed mortality risk score may be used for decision-making regarding the benefits and harm of surgery. FLR volume, FLR drainage status, and preoperative cholangitis are to some extent modifiable risk factors. Portal vein embolization is recommended if the FLR is below 30%. Complete preoperative drainage of the FLR segments is associated with lower postoperative mortality in patients with an FLR volume below 50%. By contrast, we found no evidence to support preoperative biliary drainage in the presence of an FLR volume above 50%; for these patients the risk of cholangitis and related mortality developing after drainage seems to outweigh the questionable benefit of biliary decompression.
Supplementary Material
Acknowledgments
Support: Dr Wiggers was funded by the Academic Medical Center Foundation and Dr Groot Koerkamp was funded by the Dutch Cancer Society (DCS), grant number UVA 2011-4973. This study was supported in part by NIH/NCI P30 CA008748 (Cancer Center Support Grant).
Footnotes
Presented at the European-African Hepatobiliary Association 11th Annual Congress, Manchester, United Kingdom, April 2015.
Disclosure Information: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ito F, Agni R, Rettammel RJ, et al. Resection of hilar cholangiocarcinoma: concomitant liver resection decreases hepatic recurrence. Ann Surg. 2008 Aug;248(2):273–9. doi: 10.1097/SLA.0b013e31817f2bfd. [DOI] [PubMed] [Google Scholar]
- 3.Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013 Jul;258(1):129–40. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]
- 4.Nuzzo G, Giuliante F, Ardito F, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012 Jan;147(1):26–34. doi: 10.1001/archsurg.2011.771. [DOI] [PubMed] [Google Scholar]
- 5.Farges O, Regimbeau JM, Fuks D, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013 Jan;100(2):274–83. doi: 10.1002/bjs.8950. [DOI] [PubMed] [Google Scholar]
- 6.de Jong MC, Marques H, Clary BM, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer. 2012 Oct 1;118(19):4737–47. doi: 10.1002/cncr.27492. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser GM, Paul A, Sgourakis G, et al. Novel prognostic scoring system after surgery for Klatskin tumor. Am Surg. 2013 Jan;79(1):90–5. [PubMed] [Google Scholar]
- 8.Blamey SL, Fearon KC, Gilmour WH, et al. Prediction of risk in biliary surgery. Br J Surg. 1983 Sep;70(9):535–8. doi: 10.1002/bjs.1800700910. [DOI] [PubMed] [Google Scholar]
- 9.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000 Jul;191(1):38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 10.Dixon JM, Armstrong CP, Duffy SW, Davies GC. Factors affecting morbidity and mortality after surgery for obstructive jaundice: a review of 373 patients. Gut. 1983 Sep;24(9):845–52. doi: 10.1136/gut.24.9.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iacono C, Ruzzenente A, Campagnaro T, et al. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: highlights and drawbacks. Ann Surg. 2013 Feb;257(2):191–204. doi: 10.1097/SLA.0b013e31826f4b0e. [DOI] [PubMed] [Google Scholar]
- 12.Sakata J, Shirai Y, Tsuchiya Y, et al. Preoperative cholangitis independently increases in-hospital mortality after combined major hepatic and bile duct resection for hilar cholangiocarcinoma. Langenbecks Arch Surg. 2009 Nov;394(6):1065–72. doi: 10.1007/s00423-009-0464-1. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy TJ, Yopp A, Qin Y, et al. Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB (Oxford) 2009 Aug;11(5):445–51. doi: 10.1111/j.1477-2574.2009.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoup M, Gonen M, D’Angelica M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7(3):325–30. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 15.Farges O, Vibert E, Cosse C, et al. “Surgeons’ Intuition” Versus “Prognostic Models”. Annals of Surgery. 2014 Nov;260(5):923–30. doi: 10.1097/SLA.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 16.Weldon S-M, Korkiakangas T, Bezemer J, Kneebone R. Communication in the operating theatre. Br J Surg. 2013 Dec;100(13):1677–88. doi: 10.1002/bjs.9332. [DOI] [PubMed] [Google Scholar]
- 17.Corvera CU, Blumgart LH, Darvishian F, et al. Clinical and pathologic features of proximal biliary strictures masquerading as hilar cholangiocarcinoma. J Am Coll Surg. 2005 Dec;201(6):862–9. doi: 10.1016/j.jamcollsurg.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Erdogan D, Kloek JJ, ten Kate FJW, et al. Immunoglobulin G4-related sclerosing cholangitis in patients resected for presumed malignant bile duct strictures. Br J Surg. 2008 Jun;95(6):727–34. doi: 10.1002/bjs.6057. [DOI] [PubMed] [Google Scholar]
- 19.Farges O, Goutte N, Bendersky N, et al. Incidence and risks of liver resection: an all-inclusive French nationwide study. Ann Surg. 2012 Nov;256(5):697–704. doi: 10.1097/SLA.0b013e31827241d5. discussion -5. [DOI] [PubMed] [Google Scholar]
- 20.Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011 May;149(5):713–24. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 21.van den Esschert JW, de Graaf W, van Lienden KP, et al. Volumetric and functional recovery of the remnant liver after major liver resection with prior portal vein embolization : recovery after PVE and liver resection. J Gastrointest Surg. 2009 Aug;13(8):1464–9. doi: 10.1007/s11605-009-0929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson AL, Geller DA, Hemming AW, et al. Liver planning software accurately predicts postoperative liver volume and measures early regeneration. J Am Coll Surg. 2014 Aug;219(2):199–207. doi: 10.1016/j.jamcollsurg.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000 May;127(5):512–9. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 24.Martel G, Cieslak KP, Huang R, et al. Comparison of techniques for volumetric analysis of the future liver remnant: implications for major hepatic resections. HPB (Oxford) 2015 Dec;17(12):1051–7. doi: 10.1111/hpb.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Gaag NA, Rauws EAJ, van Eijck CHJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010 Jan 14;362(2):129–37. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 26.Wiggers JK, Coelen RJ, Rauws EA, et al. Preoperative endoscopic versus percutaneous transhepatic biliary drainage in potentially resectable perihilar cholangiocarcinoma (DRAINAGE trial): design and rationale of a randomized controlled trial. BMC gastroenterology. 2015;15:20. doi: 10.1186/s12876-015-0251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruys AT, van Beem BE, Engelbrecht MRW, et al. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol. 2012 Sep;85(1017):1255–62. doi: 10.1259/bjr/88405305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altman DG, Matthews JN. Statistics notes. Interaction 1: Heterogeneity of effects. BMJ. 1996 Aug 24;313(7055):486. doi: 10.1136/bmj.313.7055.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Klaveren D, Vergouwe Y, Farooq V, et al. Estimates of absolute treatment benefit for individual patients required careful modeling of statistical interactions. J Clin Epidemiol. 2015 Feb 27; doi: 10.1016/j.jclinepi.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssen KJM, Donders ART, Harrell FE, et al. Missing covariate data in medical research: to impute is better than to ignore. J Clin Epidemiol. 2010 Jul;63(7):721–7. doi: 10.1016/j.jclinepi.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan LM, Massaro JM, D’Agostino RB. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004 May 30;23(10):1631–60. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 32.Regimbeau JM, Fuks D, Le Treut Y-P, et al. Surgery for hilar cholangiocarcinoma: a multi-institutional update on practice and outcome by the AFC-HC study group. J Gastrointest Surg. 2011 Mar;15(3):480–8. doi: 10.1007/s11605-011-1414-0. [DOI] [PubMed] [Google Scholar]
- 33.Mansfield SD, Barakat O, Charnley RM, et al. Management of hilar cholangiocarcinoma in the North of England: pathology, treatment, and outcome. World J Gastroenterol. 2005 Dec 28;11(48):7625–30. doi: 10.3748/wjg.v11.i48.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker K, Neuburger J, Groene O, et al. Public reporting of surgeon outcomes: low numbers of procedures lead to false complacency. Lancet. 2013 Nov 16;382(9905):1674–7. doi: 10.1016/S0140-6736(13)61491-9. [DOI] [PubMed] [Google Scholar]
- 35.Abdalla EK, Barnett CC, Doherty D, et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002 Jun;137(6):675–80. doi: 10.1001/archsurg.137.6.675. discussion 80-1. [DOI] [PubMed] [Google Scholar]
- 36.Hemming AW, Reed AI, Howard RJ, et al. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003 May;237(5):686–91. doi: 10.1097/01.SLA.0000065265.16728.C0. discussion 91-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagino M, Kamiya J, Nishio H, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006 Mar;243(3):364–72. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoyama Y, Nagino M, Nimura Y. Mechanism of impaired hepatic regeneration in cholestatic liver. Journal of hepato-biliary-pancreatic surgery. 2007;14(2):159–66. doi: 10.1007/s00534-006-1125-1. [DOI] [PubMed] [Google Scholar]
- 39.Hemming AW, Reed AI, Fujita S, et al. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005 May;241(5):693–9. doi: 10.1097/01.sla.0000160701.38945.82. discussion 9-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seyama Y, Kubota K, Sano K, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003 Jul;238(1):73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kloek JJ, van der Gaag NA, Aziz Y, et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg. 2010 Jan;14(1):119–25. doi: 10.1007/s11605-009-1009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawakami H, Kuwatani M, Onodera M, et al. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J Gastroenterol. 2011 Feb;46(2):242–8. doi: 10.1007/s00535-010-0298-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


