SUMMARY
Lithium has been a mainstay for the treatment of bipolar disorder, yet the molecular mechanisms underlying its action remain enigmatic. Bisphosphate 3′-nucleotidase (BPNT-1) is a lithium-sensitive phosphatase that catalyzes the breakdown of cytosolic 3′phosphoadenosine 5′-phosphate (PAP), a byproduct of sulfation reactions utilizing the universal sulfate group donor 3′phosphoadenosine 5′-phosphosulfate (PAPS) (Figure 1A) [1–3]. Loss of BPNT-1 leads to the toxic accumulation of PAP in yeast and non-neuronal cell types in mice [4,5]. Intriguingly, BPNT-1 is expressed throughout the mammalian brain [4], and it has been hypothesized that inhibition of BPNT-1 could contribute to the effects of lithium on behavior [5]. Here, we show that loss of BPNT-1 in Caenorhabditis elegans results in the selective dysfunction two neurons, the bilaterally symmetric pair of ASJ chemosensory neurons. As a result, BPNT-1 mutants are defective in behaviors dependent on the ASJ neurons, such as dauer exit and pathogen avoidance. Acute treatment with lithium also causes dysfunction of the ASJ neurons, and we show that this effect is reversible, and mediated specifically through inhibition of BPNT-1. Finally, we show that the selective effect of lithium on the nervous system is due in part to the limited expression of the cytosolic sulfotransferase SSU-1 in the ASJ neuron pair. Our data suggest that lithium, through inhibition of BPNT-1 in the nervous system, can cause selective toxicity to specific neurons, resulting in corresponding effects on behavior of C. elegans.
Graphical abstract
RESULTS AND DISCUSSION
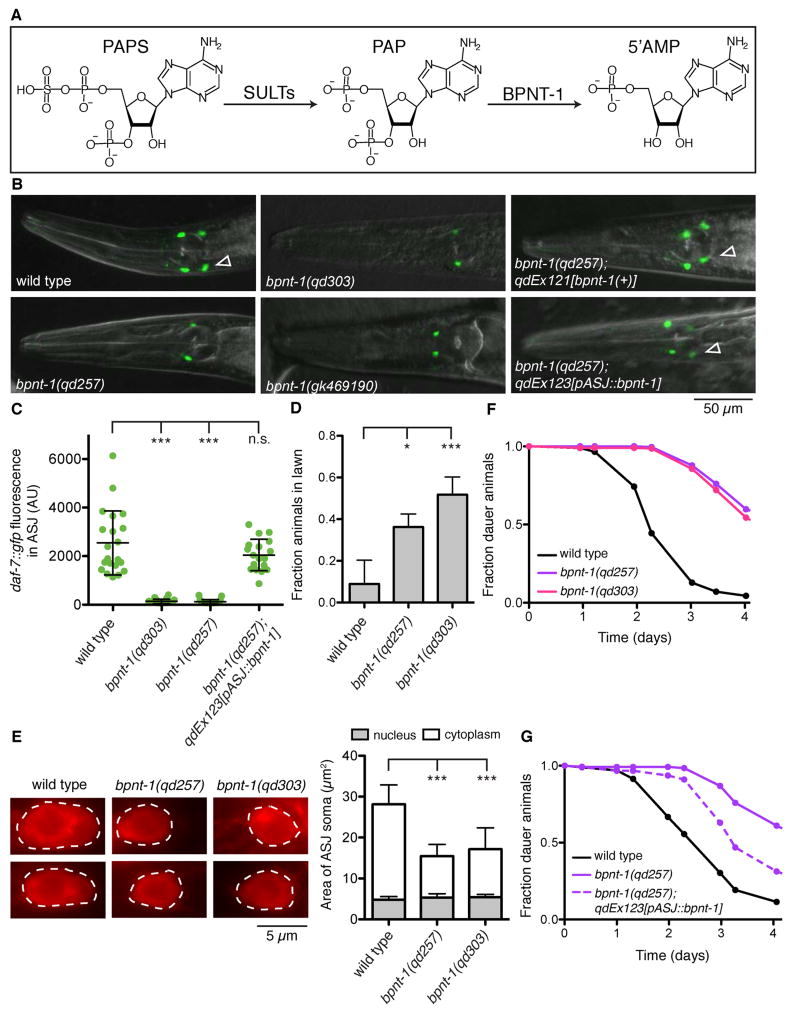
We have previously shown that the daf-7 gene, encoding a TGF-β ligand that regulates diverse behaviors in C. elegans [6,7], is transcriptionally activated in the ASJ chemosensory neuron pair upon exposure to the pathogenic bacteria Pseudomonas aeruginosa [8]. We conducted a forward genetic screen for animals defective in this transcriptional response, and identified one such mutant, qd257, which carries a D119N missense mutation in bpnt-1 (Figures 1B–C). This aspartic acid residue is conserved from yeast to humans, and is required for the catalytic activity of the superfamily of lithium-sensitive phosphatases [9,10]. Confirming the identity of qd257 as an allele of bpnt-1, we used CRISPR/Cas9-mediated genome editing to generate a loss-of-function bpnt-1 allele, qd303, in which a deletion/insertion leads to a frameshift at amino acid 20 followed by a premature stop codon. Animals carrying bpnt-1(qd303), or the W294X nonsense mutation bpnt-1(gk469190), also fail to express daf-7 in the ASJ neurons when exposed to P. aeruginosa (Figures 1B–C). Notably, the constitutive expression of daf-7 in the ASI neuron pair is unaffected by loss of bpnt-1 (Figure 1B). These data suggest that BPNT-1 is required for the expression of daf-7 specifically in the ASJ neurons.
Figure 1. Bisphosphate 3′-nucleotidase (BPNT-1) is required for the function of the ASJ neurons.
(A) BPNT-1 catalyzes the breakdown of cytosolic 3′phosphoadenosine 5′-phosphate (PAP), a byproduct of sulfation reactions utilizing the universal sulfur donor 3′phosphoadenosine 5′-phosphosulfate (PAPS). (B) daf-7 expression pattern in animals exposed to P. aeruginosa for 16 h. Empty triangles indicate the ASJ neurons (posterior) when visible. The ASI neuron pair (anterior) is unaffected by loss of BPNT-1. All genotypes contain ksIs2[daf-7p::gfp]. (C) Maximum fluorescence of daf-7p::gfp in the ASJ neurons after 16 h exposure to P. aeruginosa. All genotypes contain ksIs2[daf-7p::gfp]. (D) Lawn occupancy of animals on P. aeruginosa after 20 h. All genotypes contain npr-1(215F). (E) Fluorescence microscopy of the ASJ cell body (dashed line) visualized with a red-fluorescent lipophilic dye (left). Area of the ASJ cell body divided into nuclear and cytoplasmic compartments (right). (F–G) Fraction of animals that have exited the dauer developmental diapause state. All genotypes contain daf-2(e1368). For all panels: *** P < 0.001, * P < 0.05 as determined by one-way ANOVA followed by Dunnett’s Multiple Comparison Test. n.s. = not significant. Error bars indicate standard deviation. See also Figures S1 and S2.
BPNT-1 is conserved in all eukaryotes, and belongs to a family of lithium-sensitive phosphatases that includes inositol monophosphatase and inositol polyphosphate-1-phosphatase (Figure S1). Loss of BPNT-1 in mice leads to the toxic accumulation of PAP and death by liver failure at 45 days [4], and so we were surprised that bpnt-1 mutants were viable and appeared healthy. We used fluorescent in situ hybridization to analyze the bpnt-1 expression pattern and observed broad mRNA expression throughout the animal (Figure S2), confirming previous high-throughput expression analysis [11]. bpnt-1 mRNA was almost entirely absent in the bpnt-1(qd303) mutant, demonstrating that the bpnt-1 expression pattern was specific (Figure S2). Transgenic expression of genomic bpnt-1 DNA, or bpnt-1 cDNA under a promoter that drives expression exclusively in the ASJ neurons, each rescued the daf-7 expression defect in the ASJ neurons (Figures 1B–C). Consistent with this observation and our previous results that established a role for daf-7 induction in promoting pathogen avoidance [8], bpnt-1 mutant animals are defective in the protective behavioral avoidance of P. aeruginosa (Figure 1D). We therefore conclude that despite its widespread expression, BPNT-1 is not essential in C. elegans, and is acting cell-autonomously to promote the function of the ASJ neurons.
We determined that the gross morphology of the ASJ neurons was still intact in the bpnt-1 mutant background through labeling with a lipophilic dye. However, we also observed a reduction in the cytoplasmic volume of the ASJ neurons (Figure 1E). This led us to ask if the expression of other genes in the ASJ neurons and additional ASJ-dependent behaviors might be affected in the bpnt-1 mutant. Indeed, expression of the insulin-like peptide daf-28 [12], the G protein alpha subunit gpa-9 [13], and the thioredoxin trx-1 [14] were all decreased in the bpnt-1 mutant (Figures 2A–C). Expression of gpa-9 in tail neurons and expression of daf-28 in the ASI neurons was unaffected by loss of bpnt-1 (data not shown), further suggestive of ASJ-specific effects of the loss of BPNT-1 function. In addition, exit from the dauer reproductive diapause state, a behavior which requires the ASJ neurons [15,16], was severely impaired in animals lacking BPNT-1 (Figure 1F). The dauer exit defect was partially rescued by expressing bpnt-1 from an ASJ-specific promoter, demonstrating that BPNT-1 activity in the ASJ neurons is required for the dauer exit behavior (Figure 1G). From these observations we conclude that ASJ transcription and ASJ-dependent behaviors are defective in BPNT-1 mutants.
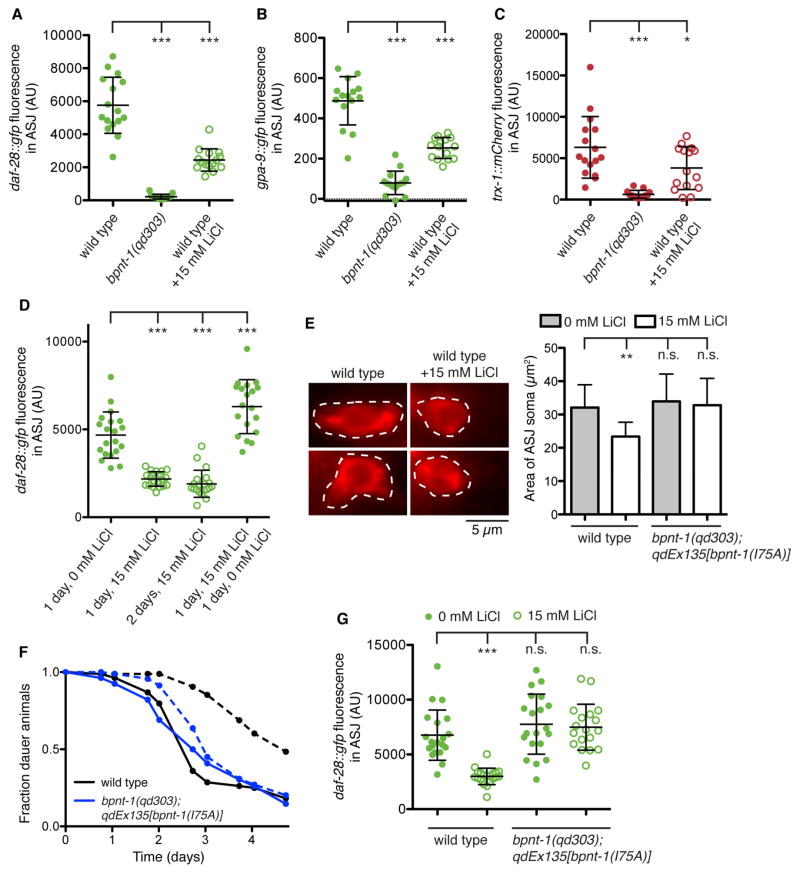
Figure 2. Lithium induces dysfunction of the ASJ neurons through inhibition of BPNT-1.
(A, D, G) Maximum fluorescence of daf-28::gfp in the ASJ neurons. All genotypes contain mgIs40[daf-28p::nls-GFP]. (B) Maximum fluorescence of gpa-9::gfp in the ASJ neurons. All genotypes contain pkIs586[gpa-9p::GFP]. (C) Maximum fluorescence of trx-1::mCherry in the ASJ neurons. All genotypes contain qdEx133[trx-1p::mCherry]. (E) Fluorescence microscopy of the ASJ cell body (dashed line) visualized with a red-fluorescent lipophilic dye (left). Area of the ASJ cell body (right). All genotypes contain mgIs40[daf-28p::nls-GFP]. (F) Fraction of animals that have exited the dauer developmental diapause state. All genotypes contain daf-2(e1368). Dashed lines indicate addition of 15 mM LiCl. For all panels: Open circles indicate addition of 15 mM LiCl. *** P < 0.001, ** P < 0.01, * P < 0.05 as determined by one-way ANOVA followed by Dunnett’s Multiple Comparison Test. n.s. = not significant. Error bars indicate standard deviation. See also Figure S3.
Given that BPNT-1 enzymatic activity is potently inhibited by lithium in vitro [1–3], we asked whether the effects of lithium on C. elegans would mimic the loss of BPNT-1. Indeed, acute exposure to 15 mM LiCl significantly decreased the expression of all ASJ-specific genes, including daf-28, gpa-9, and trx-1 (Figures 2A–C). Similar to the effects of loss of BPNT-1, expression of these genes in other neurons was not affected by lithium treatment (data not shown). The inhibitory effects of lithium treatment were reversible – following 24 hours recovery, daf-28::gfp expression in the ASJ neurons had increased to levels slightly above untreated animals (Figure 2D). Acute lithium exposure also reduced the cytoplasmic volume of the ASJ neurons (Figure 2E). Additionally, dauer exit behavior was inhibited by lithium treatment to levels similar to bpnt-1 mutant animals (Figure 2F). These results indicate that the ASJ neurons are selectively and reversibly inhibited by lithium.
Lithium can inhibit the activity of phosphatases other than BPNT-1, such as inositol phosphatases, and we wondered if the effects of lithium were mediated by inhibition of BPNT-1. A mutation in the BPNT-1 yeast homolog MET22/HAL2 (V70A) has been characterized that does not affect its catalytic activity but confers 10-fold resistance to inhibition by lithium [10]. This hydrophobic residue is conserved not only in all BPNT-1 homologs, but in the homologous inositol phosphatases as well. In fact, introduction of the corresponding mutation into human inositol monophosphatase (I68A) or a bacterial inositol monophosphatase (L81A) renders these proteins lithium insensitive [10,17], suggesting that the homologous change would be effective on the much more closely related C. elegans BPNT-1. Introduction of this BPNT-1 variant (I75A in C. elegans) into the bpnt-1(qd303) mutant fully rescued the ASJ expression defect of daf-28 and in addition rendered the ASJ neuron resistant to the transcriptional and morphological effects of lithium treatment (Figures 2E and 2G). Animals expressing BPNT-1(I75A) were also resistant to the effects of lithium treatment on dauer exit, strongly suggestive that the effects of lithium on C. elegans dauer exit behavior are due to the inhibition of BPNT-1 (Figure 2F). Additionally, mutations in lithium-sensitive inositol monophosphatase TTX-7 or Golgi-resident PAP phosphatase GPAP-1 did not mimic the addition of lithium (Figure S3A). These data suggest that the inhibitory effects of lithium on the ASJ neurons are mediated through inhibition of the lithium-sensitive phosphatase BPNT-1.
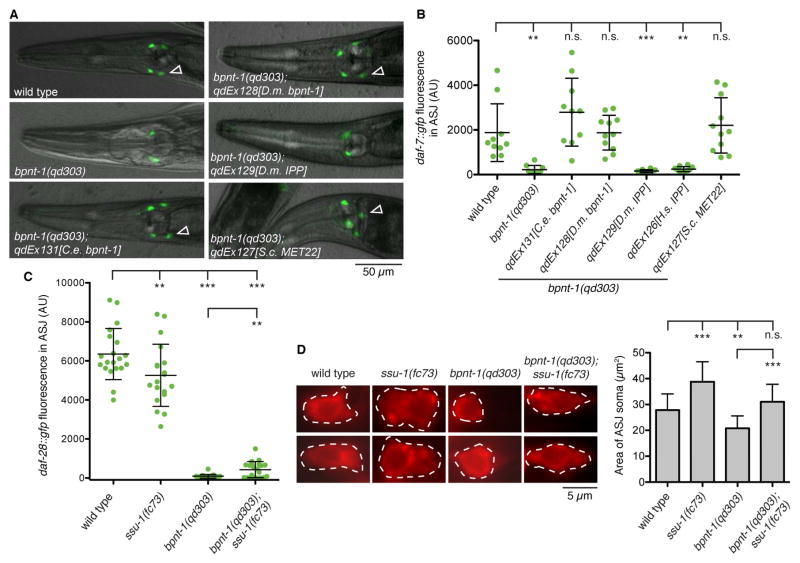
To identify the molecular mechanism by which loss of BPNT-1 leads to dysfunction of the ASJ neuron, we undertook cross-species rescue experiments with homologs of restricted substrate specificity. In addition to catalyzing the breakdown of PAP into 5′-AMP, BPNT-1 has been shown to have in vitro inositol polyphosphate-1-phosphatase (IPP) activity [2,3]. Intriguingly, although IPP was present in the common ancestor of all animals, as evidenced by its presence in cnidarians and sponges, IPP was lost in the nematode lineage (Figure S1). We therefore sought to determine if BPNT-1 might be functioning as an IPP in C. elegans neurons. Expression of bpnt-1 cDNA from C. elegans or D. melanogaster driven by the endogenous C. elegans bpnt-1 promoter rescued the daf-7 expression defect in the ASJ neurons (Figures 3A–B). However introduction of D. melanogaster or H. sapiens IPP cDNA was unable to rescue the daf-7 expression defect in bpnt-1 mutants, suggesting that the ASJ defect of bpnt-1 mutant animals is not due a loss of IPP activity (Figures 3A–B). Instead, introduction of the S. cerevisiae BPNT-1 homolog MET22/HAL2, which has been shown to have PAP phosphatase activity but weak IPP activity [1,2], fully rescued the bpnt-1(qd303) daf-7 expression phenotype (Figures 3A–B). These data support our hypothesis that loss of BPNT-1 causes ASJ neuronal dysfunction through the accumulation of PAP.
Figure 3. Loss of BPNT-1 inhibits the ASJ neurons through accumulation of cytosolic PAP.
(A) daf-7 expression pattern in animals exposed to P. aeruginosa for 16 h. Empty triangles indicate the ASJ neurons (posterior) when visible. All genotypes contain ksIs2[daf-7p::gfp]. (B) Maximum fluorescence of daf-7p::gfp in the ASJ neurons after 16 h exposure to P. aeruginosa. All genotypes contain ksIs2[daf-7p::gfp]. (C) Maximum fluorescence of daf-28::gfp in the ASJ neurons. All genotypes contain mgIs40[daf-28p::nls-GFP]. (D) Fluorescence microscopy of the ASJ cell body (dashed line) visualized with a red-fluorescent lipophilic dye (left). Area of the ASJ cell body (right). All genotypes contain mgIs40[daf-28p::nls-GFP]. For all panels: *** P < 0.001, ** P < 0.01, * P < 0.05 as determined by one-way ANOVA followed by Dunnett’s Multiple Comparison Test. n.s. = not significant. Error bars indicate standard deviation. See also Figure S3.
How could loss of BPNT-1, which is widely expressed in C. elegans, selectively increase PAP levels and inhibit the two ASJ neurons? PAP is generated in the cytosol as a byproduct of sulfation reactions in which cytosolic sulfotransferases transfer a sulfate group from PAPS onto endogenous and xenobiotic targets such as hormones and catecholamines [18]. This is in contrast to Golgi-derived PAP which is generated by sulfotransferases that act on proteins and carbohydrates and is degraded by a Golgi-resident PAP 3′-phosphatase that is homologous to BPNT-1 [19]. Surprisingly, C. elegans has only one cytosolic sulfotransferase in its genome, SSU-1, and SSU-1 is exclusively expressed in the ASJ neurons [20,21]. We therefore asked if loss of SSU-1 could suppress the ASJ defect in BPNT-1 mutants. Indeed, loss of SSU-1 could partially suppress the daf-28 and daf-7 expression defects of BPNT-1 mutants, even though ssu-1 mutants themselves were slightly defective in these assays (Figures 3C and S3B). Loss of SSU-1 could also partially suppress the reduction of cytoplasmic volume of the ASJ cell body we observe in BPNT-1 mutant animals (Figure 3D). These data suggest that the specific expression of the cytosolic sulfotransferase SSU-1 in the ASJ neuron pair may contribute to the selective neuronal dysfunction caused by loss of BPNT-1.
The incomplete suppression of bpnt-1 phenotypes by mutation in ssu-1 could be due to residual cytosolic PAP produced by additional unidentified cytosolic sulfotransferases. Alternatively, Golgi-derived PAP may be enter the cytosol via PAPS/PAP antiporters in the Golgi membrane [22,23]. Golgi-derived PAP is normally degraded by a Golgi-resident PAP 3′-phosphatase homologous to BPNT-1 (GPAP-1 in C. elegans), and like bpnt-1 we find that animals carrying a loss-of-function mutation in gpap-1 are viable and appear healthy. However we constructed a balanced strain carrying mutations in both bpnt-1 and gpap-1 and found the double mutants to be inviable, displaying early larval lethality. This observation suggests that BPNT-1 and GPAP-1 act redundantly throughout the organism to degrade Golgi-produced PAP, which may explain the broad expression pattern of bpnt-1. However in the ASJ neuron pair, where there exists a unique source of cytosolic PAP due to expression of ssu-1, and perhaps an increased source of Golgi-derived PAP due to the secretory nature of the ASJ neurons, BPNT-1 is required for cellular function.
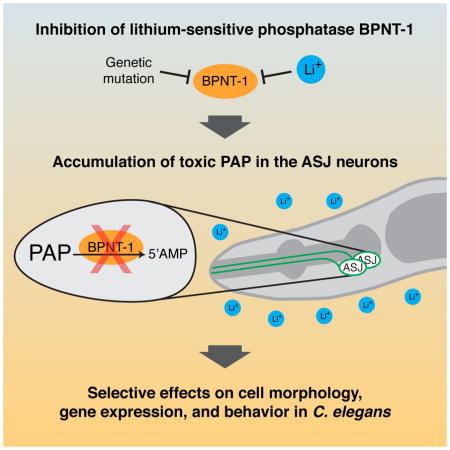
Inositol monophosphatase and inositol polyphosphate-1-phosphatase belong to a family of evolutionarily conserved phosphatases that are inhibited by lithium at therapeutic concentrations [24,25], which has led to the “inositol depletion” hypothesis for explaining lithium’s effects on the mammalian brain [26]. BPNT-1 has also been proposed to be a potential therapeutic target of lithium [5]. Here we provide evidence that inhibition of BPNT-1 can cause cell-selective dysfunction resulting in neuron-specific effects on behavior in C. elegans. The ASJ neurons of C. elegans are acutely sensitive to both the loss of BPNT-1 and the addition of lithium, perhaps due to their expression of the cytosolic sulfotransferase SSU-1 that generates cytosolic PAP (Figure 4). Accumulation of PAP can interfere with numerous cellular processes including sulfotransferase activity, RNA processing, nucleotide phosphorylation, and PARP activity [23,27–30]. Our data suggest that the differential expression of cytosolic sulfotransferases may result in neuron-selective effects of lithium through inhibition of BPNT-1 and buildup of cytosolic PAP. Intriguingly, cytosolic sulfotransferases are expressed selectively in the mammalian brain, particularly in neurons that synthesize catecholamine neurotransmitters [31,32], and we hypothesize that these populations of neurons may display enhanced susceptibility to inhibition of BPNT-1. We anticipate that our data in C. elegans on the neuron-selective effects of lithium, which acts through inhibition of the evolutionarily conserved phosphatase BPNT-1, may have implications for understanding the remarkable, and yet mechanistically poorly understood, effects of lithium therapy and toxicity in humans.
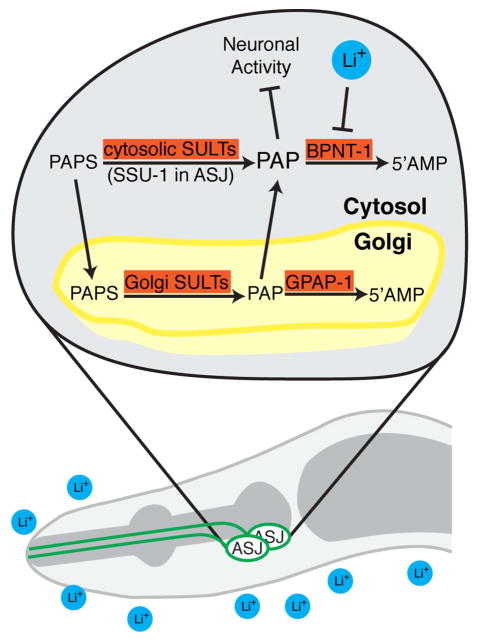
Figure 4. Lithium selectively inhibits the ASJ neurons of C. elegans through inhibition of BPNT-1.
We hypothesize that inhibition of the cytosolic PAP phosphatase BPNT-1 by lithium or genetic mutation leads to a buildup of toxic PAP, due to ASJ-specific expression of the cytosolic sulfotransferase SSU-1. PAP, in turn, causes alterations in cell morphology, transcription, and behavioral outputs of the ASJ neurons. BPNT-1 can also degrade PAP transported into the cytosol from the Golgi, which may explain the synthetic lethality between BPNT-1 and the Golgi-resident PAP phosphatase GPAP-1.
Supplementary Material
Highlights.
Lithium-sensitive BPNT-1 is selectively required for neuron function in C. elegans
Lithium treatment affects gene expression and behavior through inhibition of BPNT-1
Cytosolic sulfotransferase SSU-1 renders neurons sensitive to loss of BPNT-1
eTOC Blurb.
The molecular mechanisms underlying the action of lithium on the nervous system remainenigmatic. Meisel and Kim show that loss of the lithium-sensitive phosphatase BPNT-1, orinhibition of BPNT-1 by lithium, can cause selective toxicity to specific neurons, resulting incorresponding effects on behavior in the simple animal Caenorhabditis elegans.
Acknowledgments
We thank W. Gilbert, H. R. Horvitz, the C. elegans Million Mutation Project and Knockout Consortium, and the Caenorhabditis Genetics Center (which is supported by the NIH, Office of Research Infrastructure Programs) for providing strains and reagents. We thank R. Saunders, F. Meisel, and the Kim and Horvitz lab members for helpful discussions. This study was supported by the NIH (GM084477 to D.H.K.).
Footnotes
AUTHOR CONTRIBUTIONS:
J.D.M. performed experiments. J.D.M. and D.H.K analyzed the data, interpreted results, and wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murguía JR, Bellés JM, Serrano R. A salt-sensitive 3′ (2′),5′-bisphosphate nucleotidase involved in sulfate activation. Science. 1995;267:232–234. doi: 10.1126/science.7809627. [DOI] [PubMed] [Google Scholar]
- 2.Spiegelberg BD, Xiong JP, Smith JJ, Gu RF, York JD. Cloning and characterization of a mammalian lithium-sensitive bisphosphate 3′-nucleotidase inhibited by inositol 1,4-bisphosphate. J Biol Chem. 1999;274:13619–13628. doi: 10.1074/jbc.274.19.13619. [DOI] [PubMed] [Google Scholar]
- 3.López-Coronado JM, Bellés JM, Lesage F, Serrano R, Rodriguez PL. A novel mammalian lithium-sensitive enzyme with a dual enzymatic activity, 3′-phosphoadenosine 5′-phosphate phosphatase and inositol-polyphosphate 1-phosphatase. J Biol Chem. 1999;274:16034–16039. doi: 10.1074/jbc.274.23.16034. [DOI] [PubMed] [Google Scholar]
- 4.Hudson BH, Frederick JP, Drake LY, Megosh LC, Irving RP, York JD. Role for cytoplasmic nucleotide hydrolysis in hepatic function and protein synthesis. Proceedings of the National Academy of Sciences. 2013;110:5040–5045. doi: 10.1073/pnas.1205001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegelberg BD, Cruz Dela J, Law T-H, York JD. Alteration of lithium pharmacology through manipulation of phosphoadenosine phosphate metabolism. J Biol Chem. 2005;280:5400–5405. doi: 10.1074/jbc.M407890200. [DOI] [PubMed] [Google Scholar]
- 6.Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- 7.Schackwitz WS, Inoue T, Thomas JH. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 8.Meisel JD, Panda O, Mahanti P, Schroeder FC, Kim DH. Chemosensation of bacterial secondary metabolites modulates neuroendocrine signaling and behavior of C. elegans. Cell. 2014;159:267–280. doi: 10.1016/j.cell.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollack SJ, Knowles MR, Atack JR, Broughton HB, Ragan CI, Osborne S, McAllister G. Probing the role of metal ions in the mechanism of inositol monophosphatase by site-directed mutagenesis. Eur J Biochem. 1993;217:281–287. doi: 10.1111/j.1432-1033.1993.tb18244.x. [DOI] [PubMed] [Google Scholar]
- 10.Albert A, Yenush L, Gil-Mascarell MR, Rodriguez PL, Patel S, Martínez-Ripoll M, Blundell TL, Serrano R. X-ray structure of yeast Hal2p, a major target of lithium and sodium toxicity, and identification of framework interactions determining cation sensitivity. J Mol Biol. 2000;295:927–938. doi: 10.1006/jmbi.1999.3408. [DOI] [PubMed] [Google Scholar]
- 11.Dupuy D, Bertin N, Hidalgo CA, Venkatesan K, Tu D, Lee D, Rosenberg J, Svrzikapa N, Blanc A, Carnec A, et al. Genome-scale analysis of in vivo spatiotemporal promoter activity in Caenorhabditis elegans. Nat Biotechnol. 2007;25:663–668. doi: 10.1038/nbt1305. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Kennedy SG, Ruvkun G. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes & Development. 2003;17:844–858. doi: 10.1101/gad.1066503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansen G, Thijssen KL, Werner P, van der Horst M, Hazendonk E, Plasterk RH. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet. 1999;21:414–419. doi: 10.1038/7753. [DOI] [PubMed] [Google Scholar]
- 14.Miranda-Vizuete A, Fierro González JC, Gahmon G, Burghoorn J, Navas P, Swoboda P. Lifespan decrease in a Caenorhabditis elegans mutant lacking TRX-1, a thioredoxin expressed in ASJ sensory neurons. FEBS Letters. 2006;580:484–490. doi: 10.1016/j.febslet.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 15.Cornils A, Gloeck M, Chen Z, Zhang Y, Alcedo J. Specific insulin-like peptides encode sensory information to regulate distinct developmental processes. Development. 2011;138:1183–1193. doi: 10.1242/dev.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bargmann CI, Horvitz HR. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 1991;251:1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- 17.Nigou J, Dover LG, Besra GS. Purification and biochemical characterization of Mycobacterium tuberculosis SuhB, an inositol monophosphatase involved in inositol biosynthesis. Biochemistry. 2002;41:4392–4398. doi: 10.1021/bi0160056. [DOI] [PubMed] [Google Scholar]
- 18.Strott CA. Sulfonation and molecular action. Endocr Rev. 2002;23:703–732. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- 19.Frederick JP, Tafari AT, Wu S-M, Megosh LC, Chiou S-T, Irving RP, York JD. A role for a lithium-inhibited Golgi nucleotidase in skeletal development and sulfation. Proceedings of the National Academy of Sciences. 2008;105:11605–11612. doi: 10.1073/pnas.0801182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hattori K, Inoue M, Inoue T, Arai H, Tamura H-O. A novel sulfotransferase abundantly expressed in the dauer larvae of Caenorhabditis elegans. J Biochem. 2006;139:355–362. doi: 10.1093/jb/mvj041. [DOI] [PubMed] [Google Scholar]
- 21.Carroll BT, Dubyak GR, Sedensky MM, Morgan PG. Sulfated signal from ASJ sensory neurons modulates stomatin-dependent coordination in Caenorhabditis elegans. J Biol Chem. 2006;281:35989–35996. doi: 10.1074/jbc.M606086200. [DOI] [PubMed] [Google Scholar]
- 22.Dejima K, Murata D, Mizuguchi S, Nomura KH, Izumikawa T, Kitagawa H, Gengyo-Ando K, Yoshina S, Ichimiya T, Nishihara S, et al. Two Golgi-resident 3′-Phosphoadenosine 5′-phosphosulfate transporters play distinct roles in heparan sulfate modifications and embryonic and larval development in Caenorhabditis elegans. Journal of Biological Chemistry. 2010;285:24717–24728. doi: 10.1074/jbc.M109.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozeran JD, Westley J, Schwartz NB. Kinetics of PAPS translocase: evidence for an antiport mechanism. Biochemistry. 1996;35:3685–3694. doi: 10.1021/bi951302u. [DOI] [PubMed] [Google Scholar]
- 24.York JD, Majerus PW. Isolation and heterologous expression of a cDNA encoding bovine inositol polyphosphate 1-phosphatase. Proc Natl Acad Sci USA. 1990;87:9548–9552. doi: 10.1073/pnas.87.24.9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diehl RE, Whiting P, Potter J, Gee N, Ragan CI, Linemeyer D, Schoepfer R, Bennett C, Dixon RAF. Cloning and expression of bovine brain inositol monophosphatase. J Biol Chem. 1990;265:5946–5949. [PubMed] [Google Scholar]
- 26.Berridge MJ, Downes CP, Hanley MR. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982;206:587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramaswamy SG, Jakoby WB. (2′)3′,5′-Bisphosphate nucleotidase. J Biol Chem. 1987;262:10044–10047. [PubMed] [Google Scholar]
- 28.Dichtl B, Stevens A, Tollervey D. Lithium toxicity in yeast is due to the inhibition of RNA processing enzymes. Embo J. 1997;16:7184–7195. doi: 10.1093/emboj/16.23.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider B, Xu YW, Janin J, Véron M, Deville-Bonne D. 3′-Phosphorylated nucleotides are tight binding inhibitors of nucleoside diphosphate kinase activity. J Biol Chem. 1998;273:28773–28778. doi: 10.1074/jbc.273.44.28773. [DOI] [PubMed] [Google Scholar]
- 30.Toledano E, Ogryzko V, Danchin A, Ladant D, Mechold U. 3′-5′ phosphoadenosine phosphate is an inhibitor of PARP-1 and a potential mediator of the lithium-dependent inhibition of PARP-1 in vivo. Biochem J. 2012;443:485–490. doi: 10.1042/BJ20111057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salman ED, Kadlubar SA, Falany CN. Expression and localization of cytosolic sulfotransferase (SULT) 1A1 and SULT1A3 in normal human brain. Drug Metab Dispos. 2009;37:706–709. doi: 10.1124/dmd.108.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sidharthan NP, Minchin RF, Butcher NJ. Cytosolic sulfotransferase 1A3 is induced by dopamine and protects neuronal cells from dopamine toxicity: role of D1 receptor-N-methyl-D-aspartate receptor coupling. Journal of Biological Chemistry. 2013;288:34364–34374. doi: 10.1074/jbc.M113.493239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.