Abstract
Background and Purpose
Stroke is a potentially devastating complication of cardiac surgery. Identifying predictors of radiographic infarct may lead to improved stroke prevention for surgical patients.
Methods
We reviewed 129 post-operative brain MRIs from a prospective study of patients undergoing surgical aortic valve replacement (AVR). Acute infarcts were classified as watershed or embolic using pre-specified criteria.
Results
Acute infarct on MRI was seen in 79 of 129 patients (61%), interrater reliability for stroke etiology was high (κ =0.93). Embolic infarcts only were identified in 60 (46%), watershed only in 2 (2%), and both in 17 (13%). In multivariable logistic regression, embolic infarct was associated with aortic arch atheroma (OR=3.4, 95%CI 1.0-12.0, p=0.055), old subcortical infarcts (OR= 5.5, 95%CI 1.1-26.6, p=0.04), no history of PTCA or CABG (OR=4.0, 95%CI 1.2-13.7, p=0.03), and higher aortic valve gradient (OR=1.3 per 5mmHg, 95%CI 1.09-1.6, p=0.004). Watershed infarct was associated with internal carotid artery stenosis ≥70% (OR=11.7, 95%CI 1.8-76.8, p=0.01) and increased left ventricular ejection fraction (OR=1.6 per 5% increase, 95%CI 1.08-2.4, p=0.02).
Conclusions
The principal mechanism of acute cerebral infarction after AVR is embolism. There are distinct factors associated with watershed and embolic infarct, some of which may be modifiable.
Keywords: Ischemic Stroke, aortic valve replacement, aortic stenosis, magnetic resonance imaging (MRI), embolism
Introduction
Stroke complicating cardiac surgery is associated with prolonged length of stay, higher cost, and increased morbidity and mortality.1, 2 Peri-procedural radiographic infarcts are much more common than clinical events, and a greater understanding of the mechanisms of injury could lead to improved stroke prevention.
Prior attempts to determine the etiologies of infarcts after cardiac surgery have been limited by retrospective review of radiology reports, and imaging consisting of a mixture of head computed tomography (CT) or MRIs at non-standardized time-points. We investigated etiologic mechanisms of peri-procedural radiographic infarct from a prospective study of patients undergoing AVR for calcific AS with standardized MRI assessments.
Methods
Study design
Retrospective review of a prospective observational cohort study of subjects ≥ 65 years old undergoing open surgical AVR for calcific moderate-to-severe aortic stenosis. The study protocol was described in a prior publication.2 MRI with diffusion-weighted imaging (DWI) was obtained in 129 of 196 patients (66%) on median post-operative day 6 (IQR 5 – 8).
Infarct Classification System
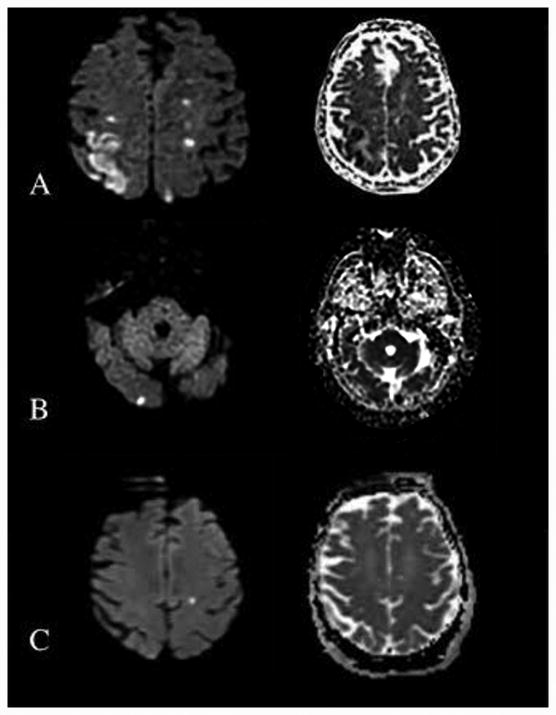
Two blinded neurologists independently reviewed MRIs for stroke location and type (embolic versus watershed). Acute infarct was defined as increased signal on DWI sequence with an appropriate apparent diffusion coefficient (ADC) correlate. Watershed infarcts were subcategorized into cortical border zone and internal border zone.3 Strokes were categorized as embolic if there were one or more DWI lesions in a non-watershed territory, or an isolated single small lesion (<1.5cm) in a possible watershed territory (Figure 1). Fluid attenuated inversion recovery (FLAIR) sequencing was reviewed for evidence of old infarction. Areas of disagreement were resolved independently by a third blinded neurologist.
Figure 1.
MR Diffusion weighted imaging (DWI) and corresponding apparent diffusion coefficient (ADC) sequences. (A) Left cortical borderzone watershed infarction and right internal borderzone watershed infarction. (B) Right occipital embolic infarct. (C) Left embolic infarct, although in an area of possible watershed distribution the lesion is singular and <1.5cm.
Statistical Analysis
Interrater reliability of the etiologic classifications was measured with κappa statistics. Demographic, clinical, and operative factors were tested for association with watershed and embolic stroke using t-test, Fisher exact, chi-squared, or Wilcoxon ranked sum, as appropriate. Factors in univariate analysis with p<0.1 were evaluated in a multivariable logistic regression model. Statistical analysis was performed using STATA 13.0 (College Station, TX).
Results
Acute infarct was seen in 79 of 129 patients (61%). Mean age 75 (±6) years, 66% male, and 7% nonwhite. Embolic strokes only were identified in 60 (46%), watershed only in 2 (2%), both in 17 (13%), and no infarct in 50 (39%). There was excellent interrater agreement on assessment of the presence of each of infarct type (κ =0.93). Table I(supplement) presents the demographic, clinical, and surgical characteristics, and univariable testing for each stroke type.
In multivariable logistic regression, embolic infarct was independently associated with moderate-to-severe ascending aortic atheroma (OR=3.4, 95%CI 1.0-12.0, p=0.055), the presence of old subcortical infarcts (OR= 5.5, 95%CI 1.1-26.6, p=0.04), no history of percutaneous transluminal coronary angioplasty (PTCA) or coronary artery bypass graft (CABG) (OR=4.0, 95%CI 1.2-13.7, p=0.03), and higher aortic valve gradient (OR=1.3 per 5mmHg, 95%CI 1.09-1.6, p=0.004). In a separate logistic regression model, watershed infarct was independently associated with internal carotid artery (ICA) stenosis ≥70% (OR=11.7, 95%CI 1.8-76.8, p=0.01) and increased left ventricular ejection fraction (OR=1.6 per 5% increase, 95%CI 1.08-2.4, p=0.02). Drop in blood pressure from baseline (obtained at pre-operative clinic visit) to intra-operative nadir was not associated with watershed or embolic infarct.
Discussion
Patients undergoing MRI after surgical AVR for calcific AS had a high rate of radiographic infarct (61%). The primary etiologic mechanism of infarct was embolism which is consistent with prior reports.4, 5 Aortic arch atheroma is a well-documented predictor of stroke in cardiac surgery and we identified a correlation of embolic stroke with moderate-to-severe aortic arch atheroma.6 Higher aortic valve gradient was also associated with embolic infarct likely reflecting greater calcification and increased atherosclerotic disease burden.7 Although coronary artery disease is a known risk factor for ischemic stroke,8 not having had prior PTCA or CABG was associated with embolic stroke in multivariate analysis possibly related to continuation of antiplatelets through the surgical period. Unfortunately, we did not have pre-operative medication data to confirm or refute this hypothesis. Finally, preoperative subcortical lesions on FLAIR was also associated with embolic infarct. Preexisting subcortical lesion burden is thought to occur in regions of hemodynamic impairment and reduced washout of small emboli.9, 10
Severe ICA stenosis was strongly associated with watershed infarction, consistent with the presumed mechanism for injury in these territories. More surprisingly, increased left ventricular ejection fraction was also associated with watershed infarct. Patients with higher ejection fraction may experience a larger drop in cerebral perfusion pressure when placed on bypass, however overall the drop in MAP was not associated with infarct, making this unlikely.
Strengths of our study include an aged, high-risk population, uniform imaging modality and timing, and serial clinical stroke ascertainment. Several limitations should be noted. Only 129 of 196 patients obtained MRI scans with DWI, mainly due to patient refusal and/or medical instability of the patient, which may have resulted in selection bias favoring milder brain injuries. Our cohort was selected from two tertiary hospitals, representing one academic institution, which may limit our generalizability. Lastly, there is evidence to suggest that hypoperfusion and embolism often coexist and their radiographic patterns are linked.10 However, the criteria used to determine infarct type appear to be reliable and valid, and there were distinct and plausible factors associated with each infarct mechanism.
Conclusion
Radiographic ischemic infarct after open surgical AVR for calcific aortic stenosis is common and the principal mechanism is embolism. Risk factors for radiographic infarction were generally distinct from clinical factors and some may be modifiable.
Supplementary Material
Acknowledgments
This study was supported by a National Institutes of Health/National Heart Lung and Blood Institute Grant R01HL084375
Dr. Messé has received significant research funding from the National Institutes of Health for this study and for participation in the CT Surgery Network which is evaluating embolic protection devices in surgical aortic valve replacement. He has also received significant research funding from Glaxo Smith Kline for his role as co-PI of a neuroprotectant study for high risk surgical aortic repair.
Dr. Acker has received significant research funding from the National Institutes of Health for this study and for participation in the CT Surgery Network which is evaluating embolic protection devices in surgical aortic valve replacement.
Dr. Kasner has received significant research funding from the National Institutes of Health for this study
Ms. Fanning has received significant research funding from the National Institutes of Health for this study
Dr. Giovannetti has received significant research funding from the National Institutes of Health for this study
Dr. Ratcliffe has received significant research funding from the National Institutes of Health for this study
Dr. Bilello has received significant research funding from the National Institutes of Health for this study and for participation in the CT Surgery Network, which is evaluating embolic protection devices in surgical aortic valve replacement.
Dr. Bavaria has received compensation from Glaxo Smith Kline for his work as co-PI of a neuroprotectant study for high risk surgical aortic repair.
Dr. Floyd has received significant research funding from the National Institutes of Health for this study
Footnotes
Disclosures: Dr. Massaro, Dr. Torres, Dr. Szeto, Dr. Mohler have nothing to disclose
References
- 1.Puskas JD, Winston AD, Wright CE, Gott JP, Brown WM, 3rd, Craver JM, et al. Stroke after coronary artery operation: incidence, correlates, outcome, and cost. The Annals of thoracic surgery. 2000;69:1053–6. doi: 10.1016/s0003-4975(99)01569-6. [DOI] [PubMed] [Google Scholar]
- 2.Messe SR, Acker MA, Kasner SE, Fanning M, Giovannetti T, Ratcliffe SJ, et al. Stroke after aortic valve surgery: results from a prospective cohort. Circulation. 2014;129:2253–61. doi: 10.1161/CIRCULATIONAHA.113.005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mangla R, Kolar B, Almast J, Ekholm SE. Border zone infarcts: pathophysiologic and imaging characteristics. Radiographics : a review publication of the Radiological Society of North America, Inc. 2011;31:1201–14. doi: 10.1148/rg.315105014. [DOI] [PubMed] [Google Scholar]
- 4.Blossom GB, Fietsam R, Jr, Bassett JS, Glover JL, Bendick PJ. Characteristics of cerebrovascular accidents after coronary artery bypass grafting. The American surgeon. 1992;58:584–9. [PubMed] [Google Scholar]
- 5.Likosky DS, Marrin CA, Caplan LR, Baribeau YR, Morton JR, Weintraub RM, et al. Determination of etiologic mechanisms of strokes secondary to coronary artery bypass graft surgery. Stroke; a journal of cerebral circulation. 2003;34:2830–4. doi: 10.1161/01.STR.0000098650.12386.B3. [DOI] [PubMed] [Google Scholar]
- 6.Djaiani G, Fedorko L, Borger M, Mikulis D, Carroll J, Cheng D, et al. Mild to moderate atheromatous disease of the thoracic aorta and new ischemic brain lesions after conventional coronary artery bypass graft surgery. Stroke; a journal of cerebral circulation. 2004;35:e356–8. doi: 10.1161/01.STR.0000138783.63858.62. [DOI] [PubMed] [Google Scholar]
- 7.Adler Y, Vaturi M, Wiser I, Shapira Y, Herz I, Weisenberg D, et al. Nonobstructive aortic valve calcium as a window to atherosclerosis of the aorta. The American journal of cardiology. 2000;86:68–71. doi: 10.1016/s0002-9149(00)00830-4. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, Wolf PA, Verter J. Manifestations of coronary disease predisposing to stroke. The Framingham study. Jama. 1983;250:2942–6. [PubMed] [Google Scholar]
- 9.Derdeyn CP, Khosla A, Videen TO, Fritsch SM, Carpenter DL, Grubb RL, Jr, et al. Severe hemodynamic impairment and border zone--region infarction. Radiology. 2001;220:195–201. doi: 10.1148/radiology.220.1.r01jl09195. [DOI] [PubMed] [Google Scholar]
- 10.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Archives of neurology. 1998;55:1475–82. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.