Abstract
Background
To counteract the syndemics of HIV and alcohol in sub-Saharan Africa, international collaborations have developed interventions to reduce alcohol consumption. Reliable and accurate methods are needed to estimate alcohol use outcomes. A direct alcohol biomarker called phosphatidylethanol (PEth) has been shown to validate heavy, daily drinking, but the literature indicates mixed results for moderate and non-daily drinkers, including among HIV-infected populations. This study examined the associations of the PEth biomarker with self-report alcohol use at 2 time points in 127 HIV-infected outpatient drinkers in western Kenya.
Methods
Participants were consecutively enrolled in a randomized clinical trial to test the efficacy of a behavioral intervention to reduce alcohol use in Eldoret, Kenya. They endorsed current alcohol use, and a minimum score of 3 on the Alcohol Use Disorders Identification Test-Consumption or consuming ≥ 6 drinks per occasion at least monthly in the past year. Study interviews and blood draws were conducted at baseline and at 3 months post-treatment from July 2012 through September 2013. Alcohol use was assessed using the Timeline Followback questionnaire. Blood samples were analyzed for presence of the PEth biomarker and were compared to self-reported alcohol use. We also conducted semi-structured interviews with 14 study completers in February through March 2014.
Results
Baseline data indicated an average of moderate-heavy alcohol use: 50% drinking days and a median of 4.5 drinks per drinking day. At baseline, 46% of women (31 of 67) and 8% of men (5 of 60) tested negative for PEth (p<.001). At the 3-month follow-up, 93% of women (25 of 27) and 97% of men (30 of 31) who reported drinking tested positive, while 70% of women (28 of 40) and 35% of men (10 of 29) who denied drinking tested negative for PEth. Interviews were consistent with self-reported alcohol use among 13 individuals with negative baseline results.
Conclusions
These results add to the growing literature showing lack of agreement between self-report and PEth results among unhealthy and non-daily drinkers, particularly women. More research is needed to determine at what level of consumption over what period of time PEth becomes a reliable and accurate indicator of alcohol use.
Keywords: alcohol, PEth, biomarker, HIV, Kenya
Introduction
Approximately two-thirds of the world’s 33.2 million individuals infected with the HIV virus live in sub-Saharan Africa. Several Africa-based studies have provided evidence for the presence of a high rate of alcohol dependence (Othieno et al., 2000, Saunders et al., 1993a, World Health, 2004), often involving the consumption of inexpensive local brew with high ethanol content (Papas et al., 2010b). In Eldoret, Kenya, our work has also shown that an average chang’aa drink (locally made spirit) is equal to two U.S. standard drinks (Papas et al., 2010b), and prevalence of hazardous drinking was reported among HIV (53%) and general medicine (68%) outpatients (Shaffer et al., 2004). Alcohol use displays a dose–response association with imperfect adherence to antiretrovirals (ARVs) (Braithwaite et al., 2005), with comorbid medical diseases and acquired immune deficiency syndrome (AIDS)- defining conditions (Justice et al., 2006), and has been associated with increased risk of unprotected sex (Apostolopoulos et al., 2002, Seage et al., 2002). In Kenya, alcohol use correlates with HIV infection (Ayisi et al., 2000, Hargreves, 2002) and with risk of sexually transmitted infections (Feldblum et al., 2000, Lavreys, 2003). To counteract the syndemics of HIV and alcohol use in sub Saharan Africa, international collaborations have developed interventions to reduce risky behaviors such as alcohol consumption (e.g. Parry et al., 2014).
In order to estimate alcohol use outcomes in resource-limited settings, reliable and accurate methods are needed. Multimodal alcohol assessment is recommended, including both self-report and biological measures, because each method of measurement has strengths and weaknesses. Together they likely provide the basis for a more accurate estimate of participants’ past alcohol consumption than either does alone, given the absence of a gold standard of measurement. Our international research collaboration has engaged in a program of research to reduce alcohol use among HIV-infected outpatients in western Kenya across a range of drinking patterns (Papas et al., 2011, Papas et al., 2010a, Papas et al., 2010b, Papas et al., 2012). As part of our ongoing research, we used both culturally adapted self-report measures and biological measures of alcohol use. We have previously described our adaptation of the self-report primary outcome measure the Timeline Followback (TLFB) (Papas et al., 2011), as well as multimodal alcohol assessment (Mwaniki et al., 2015). To complement self-report measures, we incorporated two biological measures of alcohol use and a clinical measure of alcohol withdrawal. We administer at all visits the Alco Screen® saliva test, shown to be highly associated with blood ethanol concentration as a point-prevalence measure of use (McColl et al., 1979), and a validated assessment for alcohol withdrawal symptoms, the Clinical Institute Withdrawal Assessment for Alcohol (Sullivan et al., 1989). We also obtain blood samples for analysis of a direct biomarker of alcohol use called phosphatidylethanol (PEth).
PEth is the name of a group of phospholipids formed in the cell wall from phosphatidylcholine in the presence of alcohol by the enzyme phospholipase D. In blood, PEth resides primarily within the red blood cells and has a half-life of 4 days (Varga et al., 2000). When examining PEth in both HIV-infected and non-HIV-infected samples, PEth has been shown to be a valid indicator of heavy drinking, with reporting periods typically from several hours to several weeks (Aradottir et al., 2006, Viel et al., 2012, Wurst et al., 2010, Hartmann et al., 2007, Hahn et al., 2015, Hahn et al., 2012). Some studies in heavy drinking non-HIV infected samples suggested that PEth demonstrated 100% specificity, because PEth cannot be formed without the presence of alcohol (Hartmann et al., 2007).
Sensitivity has been reported to range as high as 100% in heavy drinkers (Wurst et al 2004). The literature indicates mixed results for more moderate and non-daily drinkers among HIV-infected and non-HIV-infected samples (Hahn et al., 2015, Varga et al., 1998, Viel et al., 2012, Helander et al., 2012). More detailed research on PEth formation and elimination has revealed that the presence of PEth may be affected by many factors. For example, researchers have found that PEth in non-HIV infected samples can be formed in vitro after alcohol consumption has ended, and that the formation rate is individual, so it may not be possible to correlate PEth in blood exactly to the absolute amount of ingested ethanol (Aradottir et al., 2004). In an experiment in which ethanol was incubated with red blood cells collected from 12 PEth-negative (limit of quantification 0.2) individuals, PEth formed in-vitro at twice the rate in those cells collected from chronic heavy drinkers versus those from healthy volunteers who had abstained for the prior two weeks (Varga and Alling, 2002). Other researchers have suggested that PEth may have remained in the body for longer than 21 days, that is sometime during the 90-day period prior to alcohol being consumed (Hahn et al., 2012). Additionally, studies among healthy volunteers who drank limited quantities of alcohol resulted in variable or in some cases undetectable PEth presence (Gnann et al., 2012, Varga et al., 1998). For example, Gnann and colleagues provided alcohol to 11 healthy volunteers on 5 successive days between 21 prior and 16 subsequent days of alcohol abstinence. Alcohol was provided to achieve 1 g/kg of blood ethanol concentration (equivalent to .106%). Using newer LC-MS/MS methods, they found variable levels of PEth formation and elimination among the volunteers. Ten of 11 volunteers had detectable PEth values 1 hour after the start of drinking, ranging from 45 to 138 ng/ml. Concentrations of PEth increased continuously and reached maximum concentrations of 74 to 237 ng/ml between days 3 and 6. Four of 11 volunteers continued to test positive for PEth after 16 days of alcohol abstinence (Gnann et al., 2012).
Although early studies have focused on non-HIV infected samples, more recent literature has emerged examining relationships among HIV-infected samples, both in the U.S. and Africa. Hahn and colleagues conducted a validation study among 77 HIV-infected Ugandans using daily breathalyzer testing during visits by study staff and a collateral report to validate self-reported drinking. After 21 days, they then examined PEth levels (limit of detection 10 ng/ml). The authors concluded that PEth properties were best when measured for a duration of 21 days (versus 7 and 14 days) of alcohol use. They reported 88% sensitivity and 88.5% specificity for PEth’s property to detect any level of drinking in the past 21 days. PEth results were correlated with number of drinking days in the prior 21 days (r=0.74). Authors noted that the sample consisted of heavy drinkers, such that there was little difference between the “any drinking” and “heavy drinking” subgroups. In a second study with a small sample of drinkers, these researchers compared PEth levels against self-reported alcohol use for the past 30 days (limit of quantification 8 ng/nl) among 150 HIV-infected ARV-naïve Ugandans, 21 of whom reported drinking. This sample was characterized by low levels of reported drinking (median 10% drinking days in the past month). They observed positive PEth levels for 16% (13 of 82) of women and 43% (16 of 37) of men who denied drinking. They also found that 25% (4 of 16) of women (and no men) (0 of 15) who reported drinking demonstrated negative PEth results (Bajunirwe et al., 2014). Recent literature pooling PEth results across 3 observational studies and 4 clinical trials of HIV-infected individuals (6 in the U.S. and 1 in Uganda, total n=1159) reported the following ranges of PEth-positive results among these studies: reported abstainers, 8% to 17% positive; reported unhealthy drinkers, 46% to 100% positive; reported lower-risk drinkers, 15% to 70% positive. All studies employed a limit of quantification of 8 ng/nl. Unhealthy drinking was defined largely according to National Institute on Alcohol Abuse and Alcoholism guidelines, i.e. more than 7 drinks/week for women or 14 drinks/week for men, or ≥ 4 drinks/day women, ≥5 drinks/day men. Lower risk drinkers were defined as those who reported drinking that did not meet unhealthy drinking criteria (Hahn et al., 2015).
Given the lack of agreement between self-report and PEth when examining PEth as an indicator of alcohol use, particularly among non-daily drinkers, further exploration is needed. The purpose of this study was to explore the relationship between PEth and self-reported alcohol use among a larger sample of 127 HIV-infected moderate-heavy drinking outpatients, on average, in western Kenya across two comparative time points. We examined associations of PEth with different measures of alcohol consumption, and with several demographic and clinical characteristics.
Materials and Methods
Participants
Participants were 127 (53% women) HIV-infected outpatients in western Kenya. They were consecutively enrolled in a randomized clinical trial to test the efficacy of a Cognitive Behavioral Therapy intervention to reduce alcohol use among HIV-infected outpatients in Eldoret, Kenya. Inclusion criteria for the trial were: age 18 years or older, enrollment as an HIV outpatient in any of 4 AMPATH HIV clinics and living within one hour travel distance from the Eldoret AMPATH clinic. Participants were recruited if they reported any amount of alcohol use in the past 30 days and endorsed unhealthy drinking as indicated by either: 1) at risk drinking (minimum score of 3 on the Alcohol Use Disorders Identification Test-Consumption (AUDIT-C) (Gordon et al., 2001, Saunders et al., 1993b), or 2) consuming ≥ six drinks per occasion at least monthly in the past year. Participants also were required to have a verbal working knowledge of Kiswahili. Exclusion criteria included active psychosis or active suicidality, which were assessed through screening. Positive screens were followed with a referral to the psychiatry department.
Study Procedures
Study interviews and blood draws were conducted at baseline and at 3 months post-treatment from July 2012 through September 2013. The sample consisted of consecutively enrolled participants who met study criteria and provided lab samples and interview data at both study time points. Twenty-five randomized participants were excluded: 8 due to their failure to provide 3-month follow-up data due to drop out; 2 were dismissed after admitting no alcohol use at baseline; 3 died from non-study-related causes; and 12 participants were not able to be located after study completion to consent to shipping dried blood spots outside of Kenya for analysis. Of the 36 participants whose lab results were negative for PEth at baseline, the first 14 (39%, 13 women) study completers of the 36 participants were enrolled in brief interviews to discuss report of alcohol use. These 10-minute semi-structured interviews were conducted from February to March 2014. During the interview, a gender-matched research assistant employed a nonjudgmental approach to confirm alcohol use at baseline by presenting possible explanations for misstating use. Participants were also informed of PEth lab results (negative vs. positive). The study protocol was reviewed and approved by institutional review boards at all affiliated universities. Participants provided written informed consent.
Alcohol assessment
Self-report alcohol use
Alcohol use was assessed by interview in a private setting using a computer survey interface with gender-matched research assistants. The 10-item AUDIT was administered at screening. Following enrollment, alcohol was assessed continuously from baseline through the end of the study, the 9-month post-treatment follow-up. If a participant missed a previous interview, the days since the last interview were assessed at the subsequent interview. For this study, we examined TLFB data for the past 30 days at baseline and for the past 30 days at the 3-month follow-up. We used the TLFB method, a well-established, reliable and valid retrospective calendar-based measure employing memory cues, to assess alcohol use (Maisto et al., 1979, Sobell et al., 1979, Sobell and Sobell, 1992, Sobell et al., 1988). Based on our previous work (Papas et al., 2010b), we adapted the TLFB to estimate use of local brew (chang’aa, spirit, and busaa, maize beer) by asking participants how much money they spent on personal consumption. Commercial drink was assessed by asking volume drunk for the respective time-periods. Reported cost and volume were then converted into grams of ethanol and divided by 14 g to achieve equivalence to a US standard drink. Seven-day re-test reliability using the adapted TLFB was 0.88 for percent drinking days and 0.92 for drinks per drinking day (Papas et al., 2011). Of the 127 participants in this study, 95% attended the 3-month follow-up interview within 2 weeks of target assessment date, and 98% reported within 30 days of the target date. Two participants reported for 3-month follow-up interviews at 36 and 42 days later, respectively.
Clinical and demographic variables
Clinical and demographic variables were obtained via self-report with the exception of BMI, CD4 count and ARV status, which were obtained from participant medical records. ARV status was also confirmed through self-report.
Other substance use
Besides alcohol, use of cigarettes, kuber (smokeless tobacco), marijuana and khat (stimulant leaf) were assessed. Use of other substances has not been found to be prevalent in this setting (Papas et al., 2011); however “other” substance use was also assessed.
Dried blood spot collection and PEth lab analysis
Whole blood was drawn and applied to dried blood spots (DBS). DBS were frozen immediately after collection at −80 °C. They were subsequently transported to United States Drug Testing Laboratory (USDTL), Des Plaines, IL, for testing and were stored at room temperature for approximately 2 days during transport. A validated liquid chromatography-tandem mass spectrometry method (LC-MS/MS) (Gnann et al., 2009, Helander and Zheng, 2009) was employed for the analysis of the most prevalent PEth isomer, palmitoyl (PEth 16:0)/oleoyl (PEth 18:1) on dried blood spots. PETH was detected in standard dried blood spot punches (3.1 mm) using an Agilent 6460 liquid chromatography-tandem mass spectrometry (LC-MS/MS) system following extraction into methanol (Jones, 2011). Studies have shown that PEth 16:0/18:1 is as sensitive as total PEth (Helander et al., 2012). The LC-MS/MS technique on DBS has been shown to produce results that highly correlate with PEth analyses conducted using fresh blood (Jones, 2011, Faller et al., 2011). In the current study, the limit of quantification was 8 ng/ml, and positive samples were confirmed using a second sampling of DBS.
Statistical analysis
Categorical variables were summarized as frequencies and the corresponding percentages. Continuous variables that assumed the Gaussian distribution were summarized as means with the corresponding standard deviation (SD), while those that violated the Gaussian assumptions were summarized as medians with corresponding 25th and 75th percentiles, here denoted as Interquartile range (IQR). Gaussian assumptions were assessed using empirical (Shapiro-Wilk test) as well as graphical approaches (box plots and normal probability plots).
Association between categorical variables was assessed using Pearson’s Chi Square test or test for proportions, and the association between continuous variables was assessed using Spearman rank correlation coefficient or independent t-tests. Continuous variables that assumed Gaussian distribution were compared using a two sample t-test. However, those that violated the assumptions were compared using two-sample Wilcoxon rank-sum (aka Mann Whitney U) test. Results were summarized in tables setting significance level (alpha) at 0.05. Data analysis was done using R software.
We measured common alcohol consumption variables using the TLFB: percent drinking days and average drinks per drinking day. In order to enable comparison to previous literature, we used a 30-day interval of the TLFB. Alcohol use was examined both dichotomously (no/yes) and continuously. PEth results were analyzed dichotomously (negative/positive). Boxplots were also developed by gender of the relationship between PEth levels and reported alcohol use.
Results
Baseline clinical, demographic and baseline substance use variables
Median age was 37.0 years (IQR 32.0-43.0). Average education was 8.5 years (SD 3.8). Participants had been diagnosed with HIV for a median of 5.6 years (IQR 2.9-8.6). Fifty-five percent of the sample was married. Eighty-two percent of participants were prescribed ARVs. Median CD4 count was 421.5 (IQR 317.5-559.0). Median BMI was 21.5 (IQR 19.6-24.6), which is in the average range. Women reported significantly lower education and more often being single, as well as longer duration since HIV diagnosis compared to men. Women also demonstrated significantly higher CD4 count and BMI compared to men.
Median total AUDIT scores (21.0, IQR 13.0-28.0) suggested high-risk drinking. There were no gender differences in the AUDIT-C quantity-frequency items; however, women reported a significantly higher median total AUDIT score than men. Fifty-one percent of the sample reported consuming ≥ 6 drinks per occasion at least monthly in the past year. Use of other substances besides alcohol was minimal. More men than women reported smoking tobacco. At baseline, 79% of participants reported drinking locally made spirits, and 56% drank locally made beer. Baseline TLFB data indicated moderate to heavy levels of alcohol use on average: 50% drinking days and a median of 4.5 drinks per drinking day (IQR 3.4-7.8). There were no significant gender differences at baseline in self-reported drinking using the TLFB (Table 1). Baseline level of drinking (percent drinking days and drinks per drinking day) was not significantly different between the 127 study participants and the 23 participants who did not provide PEth data.
Table 1.
Baseline demographic, clinical and substance use variables
| Variable | Total (n=127) |
Female (n=67, 52.8%) |
Male (n=60, 48.2%) |
P-value |
|---|---|---|---|---|
| n (%) or Mean ± SD or Median (IQR) | ||||
| Demographics | ||||
| Age (years) | 37.0 (32.0 – 43.0) | 35.0 (32.0 - 42.5) | 39.0 (34.0 – 43.3) | 0.082 |
| Education, highest year completed | 8.5 ± 3.8 | 7.3 ± 3.7 | 9.8 ± 3.4 | <.001 |
| Years since HIV diagnosisa | 5.6 (2.9 – 8.6) | 6.4 (4.1 – 9.2) | 4.9 (2.2 – 7.3) | 0.012 |
| Married | 70 (55.1%) | 23 (34.3%) | 47 (78.3%) | <.001 |
| Currently taking ARVs | 104 (81.9%) | 54 (80.6%) | 50 (83.3%) | 0.689 |
| CD4 cells (per cubic mm)b | 421.5 (317.5 – 559.0) |
485 (356.0 – 577.5) |
363.5 (279.0 – 480.8) |
0.003 |
| BMI (Kgs/m2)c | 21.5 (19.6 – 24.6) | 22.9 (20.1 – 26.6) | 20.2 (18.6 – 22.4) | 0.003 |
| AUDIT in the past year | ||||
| Audit-C (First 3 items’ score) | 6.0 (5.0 – 8.5) | 6.0 (5.0 – 8.0) | 6.5 (5.0 – 9.3) | 0.398 |
| Total score | 21.0 (13.0 – 28.0) | 24.0 (14.0 – 28.0) | 18.0 (11.8 25.0) | 0.033 |
| ≥6 drinks per occasion at least monthly in the past year |
65 (51.2%) | 34 (50.7%) | 31 (51.7%) | 1.000 |
| Substance use in the past 30 days | ||||
| Tobacco use | 32 (25.2%) | 8 (11.9%) | 24 (40.0%) | <.001 |
| Number of days using tobaccod | 30 (11.5 – 30.0) |
6.5 (2.8 – 14.0) |
30 (24.3 – 30.0) |
<.001 |
| Number of cigarettes per smoking dayd |
4.0 (2.0 – 5.0) | 2.5 (2.0 – 4.5) | 4.0 (2.8 – 5.0) | 0.260 |
| Marijuana use | 5 (3.9%) | 1 (1.5%) | 4 (6.7%) | 0.188e |
| Miraa use | 11 (8.7%) | 5 (7.5%) | 6 (10.0%) | 0.848 |
| Kuber use | 13 (10.2%) | 6 (9.0%) | 7 (11.7%) | 0.834 |
| Alcohol consumption in the past 30 days | ||||
| Drank chang'aa spirit | 100 (78.7%) | 53 (79.1%) | 47 (78.3%) | 0.916 |
| Drank busaa beer | 71 (55.9%) | 41 (61.2%) | 30 (50.0%) | 0.205 |
| Drinks per drinking day | 4.5 (3.4 - 7.8) | 4.3 (2.9 – 6.9) | 5.0 (3.6 – 7.9) | 0.200 |
| Percent drinking days | 50.0 (26.7 – 76.7) | 50.0 (26.7 – 75.0) | 48.3 (29.2 – 80.8) | 0.917 |
| PEth-positive | 91 (71.7%) | 36 (53.7%) | 55 (91.7%) | <.001 |
n = 122;
n = 124;
n = 115;
n=32, among those who used tobacco for the past 30 days
Result from Fisher’s exact test due to the small cell count
Associations between alcohol consumption and PEth
Baseline data
At baseline, we found that PEth results were negative for 28% of the 127 participants (n=36). PEth results were negative at baseline for 46% of women (31 of 67) and 8% of men (5 of 60) (p<.001) (Table 2). There were no significant differences at baseline in percent of participants on ARVs, BMI scores or CD4 count by PEth status (data not shown).
Table 2.
Associations between self-reported drinking and PEth status by gender and in total sample at baseline
| PEth Negative | PEth Positive | P-value | ||
|---|---|---|---|---|
| n (%) or Median (IQR) | ||||
| Women | ||||
| Any use no | – | – | – | |
| Any use yes | 31 (46.3%) | 36 (53.7%) | ||
| Men | ||||
| Any use no | – | – | – | |
| Any use yes | 5 (8.3%) | 55 (91.7%) | ||
| Total | ||||
| Any use no | – | – | – | |
| Any use yes | 36 (28.3%) | 91 (72.7%) | ||
| Drinks per drinking day | 4.3 (2.8 - 7.1) | 4.5 (3.5 - 8.0) | 0.406 | |
| Percent drinking days | 40.0 (25.8 - 74.2) | 50.0 (28.3 - 76.7) | 0.419 | |
Three-month follow-up data
At the 3-month follow-up, PEth status was highly associated with whether an individual reported any alcohol use in the past 30 days (p<.001). Forty-six percent of participants (58 of 127) reported drinking any alcohol, while 68% (86 of 127) of the sample demonstrated positive PEth results. Five percent of those who reported drinking (3 of 58) tested negative for PEth. Ninety-three percent of women (25 of 27) and 97% of men (30 of 31) who reported drinking tested positive, while 70% of women (28 of 40) and 34% of men (10 of 29) who denied drinking tested negative for PEth (Table 3). With regard to self-reported drinking, women reported significantly fewer drinks per drinking day than men (2.3 vs 3.9, p=.008). There were no other significant gender differences in self-reported drinking at the 3-month follow-up (data not shown).
Table 3.
Associations between self-reported drinking and PEth status by gender and in total sample at 3-month follow-up
| PEth Negative | PEth Positive | P-value | ||
|---|---|---|---|---|
| n (%) or Median (IQR) | ||||
| Women | ||||
| Any use no | 28 (70.0%) | 12 (30.0%) | <.001 | |
| Any use yes | 2 (7.4%) | 25 (92.6%) | ||
| Men | ||||
| Any use no | 10 (34.5%) | 19 (65.5%) | 0.005 | |
| Any use yes | 1 (3.2%) | 30 (96.8%) | ||
| Total | ||||
| Any use no | 38 (55.1%) | 31 (44.9%) | <.001 | |
| Any use yes | 3 (5.2%) | 55 (94.8%) | ||
| Drinks per drinking day | 4.0 (4.0 – 4.8) | 3.2 (1.9 – 5.6) | 0.380 | |
| Percent drinking days | 23.3 (16.7 – 26.7) | 10.0 (6.7 – 30.0) | 0.479 | |
We also examined the relationship between PEth status and self-reported drinking at the 3-month follow-up among those 31 women who were PEth-negative at baseline. Nineteen percent (6 of 31) of these women reported drinking also at the 3-month follow-up, and 2 of these women 6 (33%) again tested negative for PEth. At the 3-month follow-up, there were positive PEth results among 20% of women who denied drinking (5 of 25) (Table 4).
Table 4.
Associations between self-reported drinking and PEth status at 3-month follow-up among women who were PEth-negative at baseline
| PEth Negative n (%) |
PEth Positive n (%) |
Total n (%) |
Fisher’s exact P |
||
|---|---|---|---|---|---|
| Alcohol Use | No | 20 (80.0%) | 5 (20.0%) | 25 (80.6%) | 0.043 |
| Yes | 2 (33.3%) | 4 (66.7%) | 6 (19.4%) | ||
| Total | 22 (71.0%) | 9 (29.0%) | 31 (100.0%) |
Comparison between values of self-report and PEth indicators of alcohol consumption at baseline and 3-month follow-up
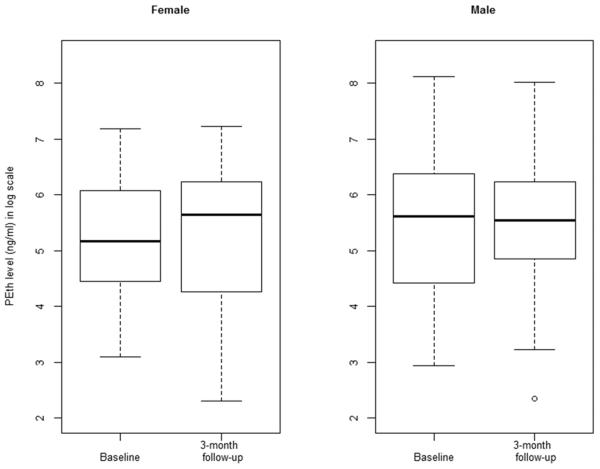
We reviewed the relationships between absolute values of PEth and self-report alcohol use in two ways. First, we examined distributions of PEth levels and associated medians at baseline and the 3-month follow-up in boxplots stratified by gender. Results showed lower median PEth levels at baseline when compared to the 3-month follow-up, and among women when compared to men (Figure 1). Then we examined Spearman correlations between detectable PEth levels and self-reported alcohol use in the total sample. Drinks per drinking day were significantly correlated with PEth levels at baseline (r=0.39, p<.001) and at 3-month follow-up (r=0.44, p<.001). Percent drinking days was not significantly correlated with PEth levels at either baseline (r=−0.07, p=0.509) or 3-month follow-up (r=−0.09, p=0.497).
Figure 1.
Box plot of logarithmic PEth levels at baseline and 3-month follow-up stratified by gender
Post-trial interviews
During brief interviews among study completers with PEth-negative results at baseline, one of the 14 participants admitted that at the baseline assessment she falsely reported drinking in order to gain the $6 per visit transport reimbursement. Her data were dropped from these analyses. The remaining 13 participants confirmed drinking at baseline. Seven individuals self-identified as occasional or not heavy drinkers, and 6 individuals self-identified as heavy drinkers. One woman noted that she drank regularly throughout the study from baseline to final follow-up. One man recalled during the interview the days he had drunk during the baseline period, including 3 days before baseline, and this was consistent with data recorded at baseline.
Discussion
This study examined associations of self-reported alcohol use with lab results of a direct biomarker of alcohol use called PEth among a sample of HIV-infected outpatients in western Kenya who reported, on average, moderate to heavy non-daily drinking. The results of this study showing lack of agreement between self-report and PEth indicators of alcohol use are consistent with recently published PEth literature in HIV-infected samples. This pertains both to discrepancies overall between PEth and self-reports of any drinking, and gender differences in the frequency of that discrepancy. For example, as part of a multi-study analysis, Cook and colleagues reported finding that 46% of 125 HIV-infected women participants enrolled in a U.S. trial for unhealthy drinkers tested negative for PEth. Authors had previously found 100% PEth positive results in a pilot study of 17 women (Hahn et al., 2015).
Level of drinking has been commonly cited as an explanation for lack of agreement between PEth and self-report (Helander et al., 2012, Varga et al., 1998). In a meta-analysis of PEth-related studies published through 2012, Viel et al. concluded that PEth is an efficient marker for chronic heavy drinking, and that the utility of PEth as a measurement of less frequent or non-heavy drinking levels remains inconclusive. The level of consumption over what period of time PEth becomes a reliable and accurate indicator of alcohol use is unknown. Hartmann and colleagues concluded that a threshold of total ethanol intake yielding detectable PEth seems to be around 1000 g (71.4 standard drinks), with a mean daily intake of about 50 g (3.6 standard drinks) (Hartmann et al., 2007). Aradottir (2004) also concluded that 50 g per day may be a useful threshold but noted that there are individual differences in the PEth formation rate. It should be noted that both studies used less sensitive HPLC methods. Using LC-MS/MS methods, Gnann and colleagues administered from 57 to 109 g alcohol daily to achieve 1 g/kg of blood ethanol concentration, resulting in maximum PEth concentrations of 74 to 237 ng/ml between days 3 and 6. Hahn’s 2012 PEth validation paper suggested that there was little difference in the sample between “any” drinking and “heavy” drinking because of pervasive heavy drinking. Participants reported consuming 2.9 drinks on 75% of the 21 days. Thus, the authors questioned whether PEth is measuring heavy drinking versus any drinking (Hahn et al., 2012). In our sample of moderate to heavy reported drinkers, percent drinking days at baseline was reported to be 50% and thus did not match the frequency of drinking criteria identified; however median drinks per drinking day (4.5) met heavy drinking criteria.
We also found that 45% of those who denied drinking (31 of 69) – this includes 19 of 29 men – at the 3-month follow-up tested positive for PEth. This may be attributed to several factors besides possible under-reporting: the limited properties of PEth biomarker, as yet unidentified factors associated with the HIV infection, or the sustained formation of PEth after cessation of drinking (Aradottir et al., 2004). For example, Hahn et al. (2012) conducted daily visits with breathalyzer testing and found that 3 of 26 individuals tested positive for PEth after 21 days, although self-report, collateral report, and breathalyzers were all negative. Authors questioned whether residual PEth continued to be found during the 90-day period prior to baseline, when participants last reported drinking (Hahn et al., 2012).
It should be noted that, in our sample, there may have been motivations to both under-report drinking, e.g., due to perceived stigma and fear of not accessing ARVS, social desirability after treatment engagement; and to over-report drinking, e.g., for secondary gain of transport reimbursement. Although our transport reimbursement of $6 per visit is considered standard compensation by the Moi University Institutional Research and Ethics board, some participants in the trial who live close to the study site have reported investing the money gained from study participation in small businesses. Importantly, our interviews after study completion with 39% of those who tested negative for PEth at baseline, mostly women, suggested veracity of self-report by nearly all participants. It should be noted that women in Kenya experience an impoverished and disempowered status when compared to men. One possible explanation for the increase in percent of PEth-positive results among women at the 3-month follow-up is the possibility that there was increased motivation among women to fabricate or exaggerate drinking patterns at baseline in order to gain access to study transport payments, then to report more honest levels during subsequent follow-ups. However, when we examined self-report at the 3-month follow-up of those 31 women who were PEth-negative at baseline, we noted mixed results. We found that 19% (6 of 31) continued to report drinking at the 3-month follow-up, and 2 of those 6 (33%) continued to test negative for PEth. Additionally, we noted at the 3-month follow-up positive PEth results among 20% of these women who denied drinking (5 of 25). Furthermore, when visually examining associations between PEth and self-report at the two time points, substantial heterogeneity with no dominant pattern are noted. Hence, it is difficult to identify any specific patterns of drinking that may account for change in percent of PEth-positive results and overall lack of agreement between PEth and self-report. Given that other studies have noted significant lack of agreement between self-report and PEth among women, there also may be some as yet unidentified biological gender difference in PEth formation. There were a significant number of individuals who denied drinking who tested positive for PEth and the reasons are likely multifactorial as previously discussed. In sum, in the absence of a gold standard biological measure, it is difficult to quantify and make attributions about the noted discrepancies between self-report and PEth results found in our study.
This study has limitations. These include 1) primary reliance on self-reported alcohol use given the infeasibility of collecting daily point-prevalence measures or collateral confirmation, and 2) limited generalizability of the focus on only a sample of HIV-infected, western Kenyan outpatients. Nonetheless, our results suggest that more research needs to be performed on the PEth biomarker to improve its utility as a biological measure of alcohol use. Questions that would be helpful to answer include the quantity of ethanol that must be consumed and over which time period in order to result in a positive PEth blood assay, and exploration of confounding factors associated with negative PEth results among confirmed drinkers. Also, exploring other cutoffs of PEth beyond the commonly employed range of 8 ng/nl to 20 ng/nl may be useful to improve PEth’s utility among non-daily drinkers.
Acknowledgments
Sources of support: This research was funded by grant R01AA020805 from the U.S. National Institute on Alcohol Abuse and Alcoholism (NIAAA). It was also supported in part by a grant to the USAID-AMPATH Partnership from the United States Agency for International Development as part of the President’s Emergency Plan for AIDS Relief. Preparation of this manuscript was supported in part by NIAAA grant 2K05 16928. We thank the participants for their role in this project.
References
- Apostolopoulos Y, Sonmez S, Yu CH. HIV-risk behaviors of American spring break vacationers: A case of situational disinhibition? International Journal of STD & AIDS. 2002;13:733–743. doi: 10.1258/095646202320753673. [DOI] [PubMed] [Google Scholar]
- Aradottir S, Asanovska G, Gjerss S, Hansson P, Alling C. Phosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol. 2006;41:431–7. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- Aradottir S, Moller K, Alling C. Phosphatidylethanol formation and degradation in human and rat blood. Alcohol Alcohol. 2004;39:8–13. doi: 10.1093/alcalc/agh003. [DOI] [PubMed] [Google Scholar]
- Ayisi JG, Van Eijk AM, Ter Kuil OF, Kolczak MS, Otieno JA, Misore AO, Kager PA, Steketee RW, Nahlen BL. Risk factors for HIV infection among asymptomatic pregnant women attending an antenatal clinic in western Kenya. International Journal of STD & AIDS. 2000;11:393–401. doi: 10.1258/0956462001916119. [DOI] [PubMed] [Google Scholar]
- Bajunirwe F, Haberer JE, Boum Y, 2nd, Hunt P, Mocello R, Martin JN, Bangsberg DR, Hahn JA. Comparison of self-reported alcohol consumption to phosphatidylethanol measurement among HIV-infected patients initiating antiretroviral treatment in southwestern Uganda. PLoS One. 2014;9:e113152. doi: 10.1371/journal.pone.0113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, Mcginnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, Cook RL, Gordon A, Bridges MW, Seiler JF, Justice AC. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- Faller A, Richter B, Kluge M, Koenig P, Seitz HK, Thierauf A, Gnann H, Winkler M, Mattern R, Skopp G. LC-MS/MS analysis of phosphatidylethanol in dried blood spots versus conventional blood specimens. Anal Bioanal Chem. 2011;401:1163–6. doi: 10.1007/s00216-011-5221-y. [DOI] [PubMed] [Google Scholar]
- Feldblum PJ, Kuyoh M, Omari M, Ryan KA, Bwayo JJ, Welsh M. Baseline STD prevalence in a community intervention trial of the female condom in Kenya. Sexually Transmitted Infections. 2000;76:454–456. doi: 10.1136/sti.76.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnann H, Weinmann W, Engelmann C, Wurst FM, Skopp G, Winkler M, Thierauf A, Auwarter V, Dresen S, Ferreiros Bouzas N. Selective detection of phosphatidylethanol homologues in blood as biomarkers for alcohol consumption by LC-ESI-MS/MS. J Mass Spectrom. 2009;44:1293–9. doi: 10.1002/jms.1608. [DOI] [PubMed] [Google Scholar]
- Gnann H, Weinmann W, Thierauf A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res. 2012;36:1507. doi: 10.1111/j.1530-0277.2012.01768.x. [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Maisto SA, Mcneil M, Kraemer KL, Conigliaro RL, Kelley ME, Conigliaro J. Three questions can detect hazardous drinkers. The Journal of Family Practice. 2001;50:313–320. [PubMed] [Google Scholar]
- Hahn JA, Cook RL, Saitz R, Cheng DM, Muyindike WR, Hu X, Brunback B, Ventura A, Lloyd-Travaglini C, Winter M, Emenyonu NI, Mcginnis KA, Holt S, Danton C, Edelman EJ, Justice AJ, Samet JH, Fiellin DA. PEth (Phosphatidylethanol) versus Self-Report: A Comparison for Assessing Alcohol Use in Research Studies of Heterogeneous HIV-Infected Populations. Alcohol Clin Exp Res. 2015;39 (poster presentation) [Google Scholar]
- Hahn JA, Dobkin LM, Mayanja B, Emenyonu NI, Kigozi IM, Shiboski S, Bangsberg DR, Gnann H, Weinmann W, Wurst FM. Phosphatidylethanol (PEth) as a biomarker of alcohol consumption in HIV-positive patients in sub-Saharan Africa. Alcohol Clin Exp Res. 2012;36:854–62. doi: 10.1111/j.1530-0277.2011.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreves JR. Socioeconomic status and risk of HIV infection in an urban population in Kenya. Tropical Medicine and International Health. 2002;7:793–802. doi: 10.1046/j.1365-3156.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- Hartmann S, Aradottir S, Graf M, Wiesbeck G, Lesch O, Ramskogler K, Wolfersdorf M, Alling C, Wurst FM. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12:81–4. doi: 10.1111/j.1369-1600.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- Helander A, Peter O, Zeng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol and alcoholism (Oxford) 2012;47:552. doi: 10.1093/alcalc/ags065. [DOI] [PubMed] [Google Scholar]
- Helander A, Zheng Y. Molecular species of the alcohol biomarker phosphatidylethanol in human blood measured by LC-MS. Clin Chem. 2009;55:1395–405. doi: 10.1373/clinchem.2008.120923. [DOI] [PubMed] [Google Scholar]
- Jones J, Jones M, Plate C, Lewis D. The Detection of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol in Human Dried Blood Spots. Analytical Methods. 2011;3:1101–1106. [Google Scholar]
- Justice AC, Lasky E, Mcginnis KA, Griffith T, Skanderson M, Conigliaro J, Fultz S, Crothers K, Rabeneck L, Rodriguez-Barradas M, Weissman S, Bryant K. Comorbid disease and alcohol use among veterans with HIV infection: a comparison of measurement strategies. Medical Care. 2006;44:S52–60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- Lavreys L. Human herpesvirus 8: seroprevalence and correlates in prostitutes in Mombasa, Kenya. Journal of Infectious Disease. 2003;187:359–363. doi: 10.1086/367703. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Sobell LC, Sobell MB. Comparison of alcoholics' self-reports of drinking behavior with reports of collateral informants. Journal of Consulting and Clinical Psychology. 1979;47:106–112. [PubMed] [Google Scholar]
- Mccoll KE, Whiting B, Moore MR, Goldberg A. Correlation of ethanol concentrations in blood and saliva. Clin Sci (Lond) 1979;56:283–6. doi: 10.1042/cs0560283. [DOI] [PubMed] [Google Scholar]
- Mwaniki MM, Gakinya BN, Maisto SA, Sidle JE, Klein DE, Baliddawa JB, Martino S, Liu T, Schlaudt KL, Papas RK. Culturally Adapted Multimodal Alcohol Assessment in Western Kenya. Alcohol Clin Exp Res. 2015;39 [Google Scholar]
- Othieno CJ, Kathuku DM, Ndetei DM. Substance abuse in outpatients attending rural and urban health centres in Kenya. East African Medical Journal. 2000;77:592–595. doi: 10.4314/eamj.v77i11.46728. [DOI] [PubMed] [Google Scholar]
- Papas RK, Gakinya BN, Baliddawa JB, Martino S, Bryant KJ, Meslin EM, Sidle JE. Ethical issues in a stage 1 cognitive-behavioral therapy feasibility study and trial to reduce alcohol use among HIV-infected outpatients in western Kenya. J Empir Res Hum Res Ethics. 2012;7:29–37. doi: 10.1525/jer.2012.7.3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas RK, Sidle JE, Gakinya BN, Baliddawa JB, Martino S, Mwaniki MM, Songole R, Omolo OE, Kamanda AM, Ayuku DO, Ojwang C, Owino-Ong'or WD, Harrington M, Bryant KJ, Carroll KM, Justice AC, Hogan JW, Maisto SA. Treatment outcomes of a stage 1 cognitive-behavioral trial to reduce alcohol use among human immunodeficiency virus-infected out-patients in western Kenya. Addiction. 2011;106:2156–66. doi: 10.1111/j.1360-0443.2011.03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas RK, Sidle JE, Martino S, Baliddawa JB, Songole R, Omolo OE, Gakinya BN, Mwaniki MM, Adina JO, Nafula T, Owino-Ong'or WD, Bryant KJ, Carroll KM, Goulet JL, Justice AC, Maisto SA. Systematic cultural adaptation of cognitive-behavioral therapy to reduce alcohol use among HIV-infected outpatients in western Kenya. AIDS Behav. 2010a;14:669–78. doi: 10.1007/s10461-009-9647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas RK, Sidle JE, Wamalwa ES, Okumu TO, Bryant KL, Goulet JL, Maisto SA, Braithwaite RS, Justice AC. Estimating alcohol content of traditional brew in Western Kenya using culturally relevant methods: the case for cost over volume. AIDS Behav. 2010b;14:836–44. doi: 10.1007/s10461-008-9492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry CD, Morojele NK, Myers BJ, Kekwaletswe CT, Manda SO, Sorsdahl K, Ramjee G, Hahn JA, Rehm J, Shuper PA. Efficacy of an alcohol-focused intervention for improving adherence to antiretroviral therapy (ART) and HIV treatment outcomes - a randomised controlled trial protocol. BMC Infect Dis. 2014;14:500. doi: 10.1186/1471-2334-14-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Amundsen A, Grant M. Alcohol consumption and related problems among primary health care patients: WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-I. Addiction. 1993a;88:349–362. doi: 10.1111/j.1360-0443.1993.tb00822.x. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, Delafuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993b;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Seage GR, 3rd, Holte S, Gross M, Koblin B, Marmor M, Mayer KH, Lenderking WR. Case-crossover study of partner and situational factors for unprotected sex. Journal of Acquired Immune Deficiency Syndrome. 2002;31:432–439. doi: 10.1097/00126334-200212010-00010. [DOI] [PubMed] [Google Scholar]
- Shaffer DN, Njeri R, Justice AC, Odero WW, Tierney WM. Alcohol abuse among patients with and without HIV infection attending public clinics in western Kenya. East African Medical Journal. 2004;81:594–598. [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behavior Research & Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback: A Technique for Assessing Self-Reported Alcohol Consumption. In: LITTEN RZ, ALLEN J, editors. Measuring Alcohol Consumption. Humana Press; Totowa, New Jersey: 1992. [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. British Journal of Addiction. 1988;83(4):393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CJ, Sellers EM. Assessment of alcohol withdrawal: The revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) British Journal of Addiction. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Varga A, Alling C. Formation of phosphatidylethanol in vitro in red blood cells from healthy volunteers and chronic alcoholics. J Lab Clin Med. 2002;140:79–83. doi: 10.1067/mlc.2002.125292. [DOI] [PubMed] [Google Scholar]
- Varga A, Hansson P, Johnson G, Alling C. Normalization rate and cellular localization of phosphatidylethanol in whole blood from chronic alcoholics. Clin Chim Acta. 2000;299:141–50. doi: 10.1016/s0009-8981(00)00291-6. [DOI] [PubMed] [Google Scholar]
- Varga A, Hansson P, Lundqvist C, Alling C. Phosphatidylethanol in blood as a marker of ethanol consumption in healthy volunteers: comparison with other markers. Alcohol Clin Exp Res. 1998;22:1832–7. [PubMed] [Google Scholar]
- Viel G, Boscolo-Berto R, Cecchetto G, Fais P, Nalesso A, Ferrara SD. Phosphatidylethanol in blood as a marker of chronic alcohol use: a systematic review and meta-analysis. Int J Mol Sci. 2012;13:14788–812. doi: 10.3390/ijms131114788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health, O . Global Status Report on Alcohol 2004 [Online] World Health Organization; Geneva: 2004. [Google Scholar]
- Wurst FM, Thon N, Aradottir S, Hartmann S, Wiesbeck GA, Lesch O, Skala K, Wolfersdorf M, Weinmann W, Alling C. Phosphatidylethanol: normalization during detoxification, gender aspects and correlation with other biomarkers and self-reports. Addict Biol. 2010;15:88–95. doi: 10.1111/j.1369-1600.2009.00185.x. [DOI] [PubMed] [Google Scholar]