Summary
Our prior study utilized both in vitro and in vivo multiple myeloma (MM) xenograft models to show that a novel alkylator melphalan-flufenamide (Melflufen) is a more potent anti-MM agent than melphalan and overcomes conventional drug resistance. Here we examined whether this potent anti-MM activity of melflufen versus melphalan is due to their differential effect on DNA damage and repair signalling pathways via γ-H2AX/ATR/CHK1/Ku80. Melflufen-induced apoptosis was associated with dose- and time-dependent rapid phosphorylation of γ-H2AX. Melflufen induces γ-H2AX, ATR, and CHK1 as early as after 2h exposure in both melphalan-sensitive and –resistant cells. However, melphalan induces γ-H2AX in melphalan-sensitive cells at 6h and 24h; no γ-H2AX induction was observed in melphalan-resistant cells even after 24h exposure. Similar kinetics was observed for ATR and CHK1 in meflufen- versus melphalan-treated cells. DNA repair is linked to melphalan-resistance; and importantly, we found that melphalan, but not melflufen, upregulates Ku80 that repairs DNA double-strand breaks. Washout experiments showed that a brief (2h) exposure of MM cells to melflufen is sufficient to initiate an irreversible DNA damage and cytotoxicity. Our data therefore suggest that melflufen triggers a rapid, robust, and an irreversible DNA damage which may account for its ability to overcome melphalan-resistance in MM cells.
Keywords: Myeloma, Apoptosis, Novel therapeutics, alkylating agents, melflufen, melphalan
Introduction
Multiple myeloma (MM) remains incurable despite novel therapies, highlighting the need for further identification of factors mediating disease progression and resistance (Anderson, 2011; Dimopoulos et al, 2011). The alkylating agent melphalan (mustard-L-phenylalanine) prolongs survival in multiple myeloma (MM) patients; however, it is associated with toxicities and development of drug-resistance. Nevertheless, the combination of melphalan and prednisone has proven effective MM therapy in non-transplant candidates. High-dose melphalan (HDM) together with autologous stem cell transplantation (ASCT) increased progression-free and overall survival in transplant candidates (Attal et al, 1996; Barlogie et al, 2006; Child et al, 2003; Falco et al, 2013). Furthermore, combination of melphalan plus steroids with bortezomib, thalidomide or lenalidomide has improved response extent and frequency, as well as prolonged progression-free and overall survival in elderly newly diagnosed patients (Falco et al, 2013; Berenson et al, 2006; Palumbo et al, 2007; 2013; Mateos et al, 2010a; 2010b; Facon et al, 2007). Integration of novel therapies into the transplant paradigm as induction, consolidation and maintenance has further improved outcome (Rajkumar 2010; Ludwig et al, 2012).
Based on the studies showing utility of melphalan as an anti-MM agent (Anderson 2012), recent research efforts have focused on the development of a novel alkylator to prevent drug-resistance, and increase tumour specificity. For example, pharmacological screening of alkylating oligopeptides led to the development of the novel compound melflufen (Melphalan flufenamide; previously called J1) (Gullbo et al, 2003; 2004; Wickstrom et al, 2007) which is a highly lipophilic alkylator. The lipophilicity characteristic of melflufen allows for rapid cellular uptake, followed by its hydrolysis via intracellular peptidases and the release of active metabolite melphalan. Melphalan is hydrophilic and its intracellular accumulation correlates with peptidase expression/activity. Aminopeptidase N, which hydrolyses melflufen into melphalan, is elevated in MM cells (Chauhan et al, 2013). Because of the differential rate of transport of melflufen into cells (rapid) and free melphalan out of cells (slow), a high intracellular concentration of melphalan is achieved as a result of the intracellular cleavage. Thus, treatment with melflufen efficiently results in the intracellular trapping of melphalan. Importantly, melflufen allows for a more rapid and higher intracellular accumulation of melphalan in tumour cells than is achievable by direct exposure to equimolar doses of melphalan (Wickstrom et al, 2011). Studies using solid tumour models showed that melflufen triggers at least a 10-fold higher loading of melphalan, associated with high tumour cell cytotoxicity (Anderson et al, 2012; Gullbo et al, 2003; 2004; Wickstrom et al, 2007;2011;2010). In the context of MM, we demonstrated that melflufen is a more potent anti-MM agent than melphalan, overcomes melphalan-resistance, and induce synergistic anti-MM activity in combination with bortezomib, lenalidomide or dexamethasone (Chauhan et al, 2013). Here we extended our prior study to examine 1) the induction kinetics of DNA damage and repair signalling pathways in melphalan versus melflufen-treated MM cells; and 2) whether melflufen-induced cytotoxicity is associated with induction of an irreversible DNA damage in MM cells.
Material and Methods
Cell culture and reagents
Melphalan-sensitive (MM.1S, RPMI-8226) and melphalan-resistant (LR-5: melphalan-resistant derivative of RPMI-8226) cell lines were cultured with RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 2mM L-glutamine, 100 units/ml Penicillin and 100 µg/ml streptomycin. Melflufen was obtained from Oncopeptides AB (Stockholm, Sweden). Melphalan was purchased from Sigma Chemical Company (St Louis, MO) and Apoteket AB, Sweden. Recombinant interleukin 6 (IL6) was purchased from Peprotech (Rocky Hill, New Jersey, USA).
Isolation of MM patient cells
Studies involving patient MM cells were performed following Institutional Review Board (IRB)-approved protocols at Dana-Farber Cancer Institute and Brigham and Women’s Hospital (Boston, USA). Informed consent was obtained, and patient samples were anonymized prior to experimental use. MM patient tumour cells were isolated from both bone marrow (BM) and peripheral blood mononuclear cells (PBMCs) by magnetically activated cell sorting (MACS) using CD138 microbeads kit (Miltenyi Biotec, Auburn, CA), as previously described (Chauhan et al, 2012;2013). Peripheral blood (PB) samples from normal healthy donors were obtained from the Kraft Family Blood Donor Center at Dana-Farber Cancer Institute and Brigham and Women’s Hospital (Boston, USA). All donor blood samples were used according to IRB-approved protocols (DFCI and BWH, Boston, USA).
Cell viability, proliferation, and apoptosis assays
Cell viability and proliferation was assessed using colorimetric assays (MTT[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]; Calbiochem; Billerica, MA, USA or WST: Clontech; Mountain View, CA, USA) (Chauhan et al, 2012). Apoptosis was measured with Annexin V- fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit, as per manufacturer’s instructions (BD Biosciences, San Jose, CA), followed by an analysis on a fluorescence-activated cell sorting (FACS) Canto II (BD Biosciences). All FACS Data were analysed using FACS DIVA (BD Biosciences) and FlowJO softwares (ver 7.6.5, Tree Star Inc, Ashland, OR, USA).
Studies were performed using equimolar concentrations of melflufen to melphalan, as in our prior study (Chauhan et al, 2013). For washout experiments, MM cells were treated with inhibitors for 1–2h; cells were washed and resuspended in complete medium without the drugs for further analysis. For viability study, cells were cultured for 48h, and viability was assessed from MTT assay. For cell cycle analysis, MM cells were cultured without the inhibitors, harvested at different time points, and subjected to PI staining.
Western blot analysis
Total cell lysates were prepared from MM cells treated with equimolar concentrations of melflufen and melphalan. Immunoblot analysis was performed as previously described (Chauhan et al., 2012; Chauhan et al, 2013) using antibodies specific against γ-H2AX, TTP53, ATR, CHK1, Ku80, GAPDH, Actin or α-Tubulin (Cell Signaling, Danvers, MA, USA). To determine the kinetics of induction of various DNA damage or repair signalling molecules, the MM cells were treated with equimolar concentrations of melflufen or melphalan for 2, 4, 6 and 24h; protein lysates were prepared, and subjected to immunoblot analysis. For flow cytometric analysis of γ-H2AX (pS139), MM cells were treated with equimolar concentration of melflufen or melphalan and harvested at indicated time points. The cells were fixed and stained with Alexa Fluor 647 conjugated anti-H2AX (pS139) antibody (Ab), and subjected to FACS analysis (Chauhan et al, 2013). Similar experiments were also performed using normal healthy donor-derived total mononuclear cells (MNCs). Melflufen- or melphalan-treated normal MNCs were additionally analysed for γ-H2AX on different subpopulations of MNCs; normal T or B cells, based on 2-colour staining, either CD3-FITC plus γ-H2AX-Alexa-647 (pan T cells), or CD20-FITC plus γ-H2AX-Alexa-647 (B cells), respectively.
Assessment of phosphorylated H2AX (γ-H2AX) foci formation
RPMI-8226 and LR-5 cells (1×106 cells/ml) were treated with melflufen or melphalan (3 µM) for 4h, and fixed in 4% formaldehyde for 20 mins; cells were washed and resuspended in blocking buffer for 45 min at room temperature, followed by incubation with primary anti-γH2AX Ab (Cell Signaling) overnight at 4°C. The cells were then incubated with Alexa fluor 488-conjugated secondary Ab for 1h, washed, and subjected to DAPI staining. The slides were mounted with anti-fading mounting medium, and images were taken using Nikon inverted wide-field fluorescence microscope, followed by analysis using Image J software (National Institute of Mental Health (NIMH), National Institute of Health (NIH), Bethesda, MD, USA).
Assessment of Irreversible DNA damage with Acridine Orange (AO) Staining
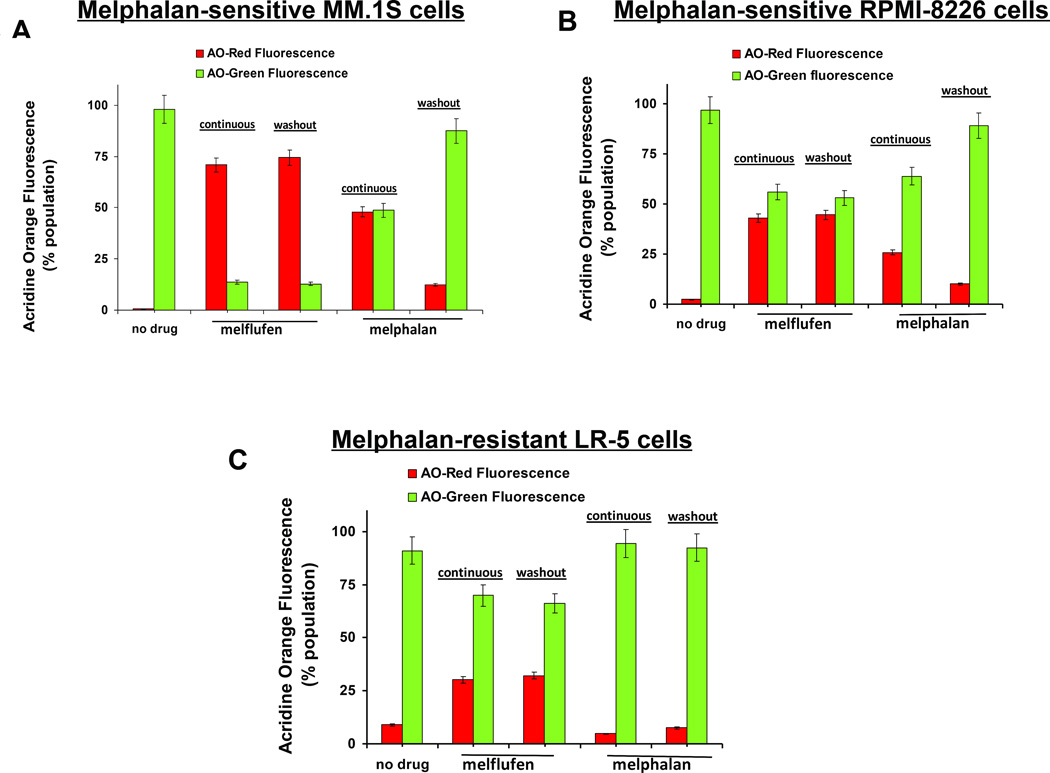
AO is a metachromatic dye that can differentially stain double (ds)- and single-stranded (ss) nucleic acids (ds: Green Fluorescence; ss: Red). Apoptotic condensed chromatin is much more sensitive to DNA denaturation than normal chromatin, as assessed by an intense red fluorescence and a reduced green emission that occurs upon mild acid denaturation (Traganos et al, 1977; Darzynkiewicz et al, 2011). MM cells were treated with equimolar melflufen or melphalan for 2h; cells were then washed with plain medium to remove drugs, and cultured in complete culture medium for 48h without drugs, followed by AO staining in the following 4-step process: 1) After 48h, the cells were washed and fixed in 70% (vol/vol) ethanol (4-h incubation on ice, or overnight at 4 deg C); 2) Cells were then washed thrice in ice cold phosphate-buffered saline (PBS); resuspended in 200 µl RNAse A solution (final concentration 250 µg/ml), and incubated for 1h at 37°C; 3) Cells were again washed, and resuspended in PBS followed by 30 s treatment with 50 mM HCl at room temperature; and 4) AO staining solution (Life Technologies Inc, Carlsbad, CA, USA) was added to each tube (12 µM final concentration), and cells were analysed by flow cytometry for AO green versus red fluorescence (excitation 488 nm; Green fluorescence in FITC channel, red fluorescence >600 nm, perdinin-chlorophyll – cyanin 5.5 [Per-CP Cy 5.5} channel). AO-Red Fluorescence and AO-2colors Green/Red Fluorescence were quantitative, as in previous study (Darzynkiewicz et al, 2011). As a comparator, the effect of continuous drug treatment for 48h was also examined using AO staining.
Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labelling (TUNEL) Assay
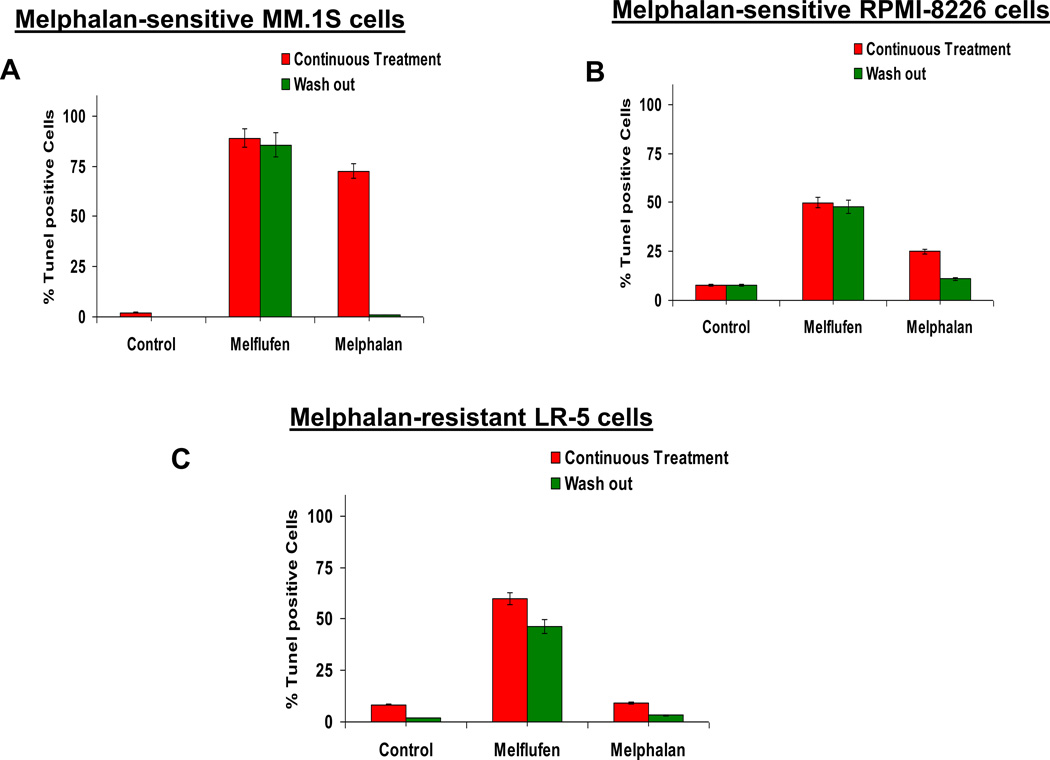
Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) assay detects apoptotic cells with extensive DNA damage (Loo, 2002; Karlsson et al, 2003). The method is based on the ability of TdT to identify blunt ends or nicks in double-stranded DNA breaks, independent of a template, and catalyse the addition of dUTPs to that end. APO-DIRECT™ assay kit (Cat. No: 6536KK, BD Biosciences) was utilized to label DNA breaks in a single-step method with FITC-dUTP. MM cells were treated with equimolar concentrations of melflufen or melphalan for 2h; cells were then washed with plain medium to remove drugs, and resuspended in complete culture medium for 48h, followed by end-labelling with FITC-conjugated dUTP and analysis/quantification of TUNEL positive cells by FACS. As a comparator, cells were also treated for 48h continuously and analysed for apoptosis as described above.
Statistical Analysis
Statistical significance of differences observed in drug-treated vs. control culture was determined using the Student’s t test. The minimal level of significance was p < 0.05 (Graph Pad PRISM, La Jolla, CA, USA).
Results
Anti-MM activity of melflufen is associated with rapid phosphorylation of DNA damage marker protein H2AX
MM cell lines (MM.1S, INA-6, ANBL-6.WT, ANBL-6.BR, MM.1R, RPMI-8226, ARP1, or Dox-40) were treated with various concentrations of melflufen for 24h, and analysed for cell viability. A significant concentration-dependent decrease in the viability of all cell lines was noted in response to melflufen treatment (Fig 1B). A head-to-head analysis of melflufen and melphalan showed a significantly more potent cytotoxicity of melflufen (>10-fold) than melphalan against dexamethasone-sensitive MM.1S cells (Fig 1C). The different 50% inhibitory concentration (IC50) of melflufen noted against MM cell lines may be due to the distinct cytogenetics and/or intrinsic drug resistance characteristics (Davies et al, 2003) as well as slightly varying intrinsic aminopeptidase levels of MM cell lines, and are consistent with our prior study (Chauhan et al, 2013).
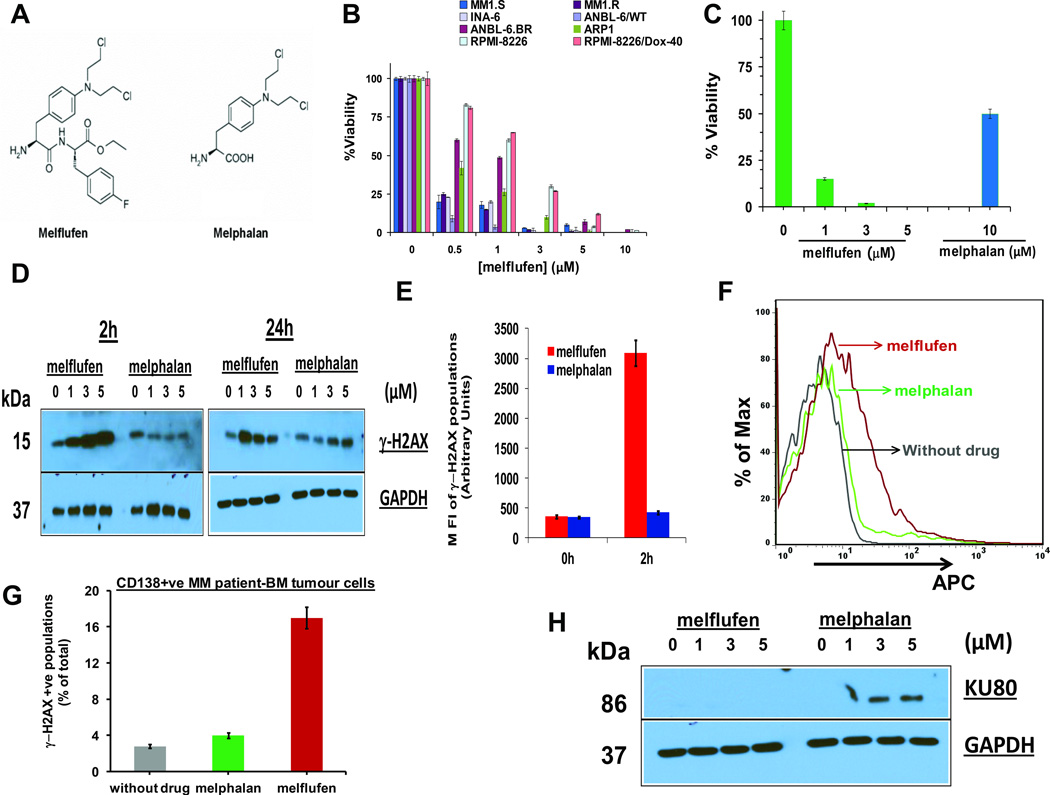
Figure 1. Anti-MM activity of melflufen is associated with dose- and time- dependent rapid phosphorylation of H2AX.
(A) Structure of Melphalan Flufenamide (left panel; Melflufen) and Melphalan (right panel). (B) MM cell lines were treated with indicated doses of melflufen for 24h, and cytotoxicity was assessed using MTT assay (n = 3, mean ± SD; p < 0.005 for all cell lines). (C) MM.1S cells were treated with melflufen or melphalan at the indicated concentrations for 48h, and cytotoxicity was measured by MTT assay (n = 3, mean ± SD; p < 0.005). (D) MM.1S cells were treated with equimolar concentrations of melflufen or melphalan for 2h and 24h; protein lysates were subjected to immunoblot analysis using antibodies specific against γ-H2AX and GAPDH. (E) MM.1S cells were treated with equimolar melflufen to melphalan (1 µM) concentrations for 2h. Cells were fixed and stained with Alexa Fluor 647 conjugated anti-H2AX (pS139) antibody, and then subjected to FACS analysis. Bar Graph: Quantification of γ-H2AX populations (mean ± SD; p< 0.05; n=3). (F) MM patient cells (CD138+, no of samples = 3) were first treated with melflufen (5 µM) or melphalan (10 µM) for 1h and then washed with PBS to remove drugs. Cells were fixed and stained with Alexa Fluor 647 conjugated anti-H2AX (pS139) antibody, and subjected to FACS analysis. (G) Bar Graph: Quantification of γ-H2AX-positive cell population shown in Figure 1F (mean ± SD; p< 0.05; n=3). (H) MM.1S cells were treated with indicated concentrations of melflufen or melphalan for 24h; protein lysates were subjected to immunoblot analysis using Ku80 and GAPDH Abs.
We next examined whether the enhanced anti-MM activity of melflufen vs. melphalan is linked to the differential kinetics and/or intensity of DNA damage-associated signalling. An early event in the response of mammalian cells to DNA double-strand breaks is the phosphorylation of histone H2AX (γ-H2AX) at the sites in proximity to DNA breaks (Burma et al, 2001). To determine the effect on γ-H2AX, MM.1S cells were treated with various equimolar concentrations of melflufen or melphalan for 2 and 24h, and protein lysates were analysed for γ-H2AX. Melflufen, but not melphalan, triggered a robust upregulation of γ-H2AX as early as after 2h exposure of cells (Fig 1D, left panel). Of note, low concentrations of melflufen (1 µM) induced γ-H2AX, whereas no such effect was noted even at higher concentrations of melphalan (5 µM) (Fig 1D, left panel). Similar results were observed for γ-H2AX at longer periods (24h) of melflufen treatment, whereas melphalan modestly induced γ-H2AX (Fig 1D, right panel). The alterations in γ-H2AX in response to melflufen or melphalan were further confirmed by flow cytometry using anti-H2AX (pS139) Ab (Fig 1E).
We next utilized MM patient tumour cells to confirm our findings in MM cell lines. Tumour cells from the BM samples of relapsed/refractory (to Bortezomib and Lenalidomide) MM patients (RRMM; n=3) were treated with equimolar concentrations of melflufen or melphalan for 1h, followed by analysis of γ-H2AX induction. Melflufen triggered a significant upregulation of γ-H2AX, whereas a modest induction of γ-H2AX was observed in melphalan-treated patient MM cells (Fig 1F and Fig 1G). As for MM cells lines, we noted an early onset of DNA damage in melflufen- versus melphalan-treated patient MM cells. Similar experiments were performed using normal healthy donor-derived total mononuclear cells (MNCs), B or T cells (No of healthy donors=3). Specifically, total MNCs, B or T cells were treated with melflufen or melphalan (5–10 µM) for 1h and then subjected to γ-H2AX analysis. A minimal γ-H2AX induction was noted in normal PBMCs, B or T cells in response to treatment with either melflufen or melphalan (Supplementary Fig 1). As a positive control, a significant γ-H2AX induction was observed in melflufen-treated patient MM cells (Supplementary Fig 1). Together, these data suggest a favourable therapeutic index for melflufen in MM.
Earlier studies showed that the mechanism(s) mediating melphalan-resistance include activation of DNA repair pathways (Spanswick et al, 2002; Sousa et al, 2013; Wang et al, 2001). We therefore examined the effect of melflufen vs. melphalan by analysing changes in a key DNA repair protein Ku80 (Featherstone & Jackson, 1999; Fell & Schild-Poulter, 2012). Specifically, Ku80 binds to double-strand break ends and mediates the non-homologous end joining DNA repair pathway. MM.1S cells were treated with various equimolar concentrations of melflufen or melphalan for 24h, and protein lysates were analysed for Ku80. A robust upregulation of Ku80 was observed in melphalan-treated cells, which was not observed even at high concentrations of melflufen (5 µM) (Fig 1H). The fact that Ku is essential for binding to broken DNA and recruiting other proteins to facilitate the processing and ligation of the DNA broken ends (Brown et al, 2015; Fell & Schild-Poulter, 2015), coupled with our data showing lack of Ku80 induction in melflufen-treated cells, suggests that melflufen-induced DNA damage is not associated with a functional DNA repair mechanism. Moreover, the finding that melphalan induces Ku80 is consistent with prior studies linking activation of DNA repair with melphalan-resistance (Spanswick et al, 2002; Sousa et al, 2013; Wang et al, 2001).
Effect of melflufen versus melphalan on DNA damage response proteins γ-H2AX/TP53/ATR/CHK1/Ku80 in melphalan-sensitive and -resistant MM cells
Our prior study (Chauhan et al, 2013) showed that melflufen triggers cytotoxicity against melphalan-resistant MM cells. We therefore next examined whether the higher potency and more rapid kinetics of action of melflufen than melphalan in tumour cells enables melflufen to overcome melphalan-resistance. To address this issue, we utilized a previously characterized (Hazelhurst et al., 2003) isogenic pair of melphalan-sensitive (RPMI-8226) and -resistant (RPMI-8226-derivative LR-5) MM cell lines, and examined the kinetics of DNA damage and repair-associated proteins (γ-H2AX/TP53/ATR/CHK1/Ku80). As in prior studies, melflufen induces cytotoxicity in melphalan-resistant LR-5 cells, whereas melphalan alone at tested concentrations does not significantly affect the viability of LR-5 cells (Fig 2A). Use of higher concentrations of melphalan (100 µM) confirmed resistance to melphalan in LR-5 cells (data not shown).
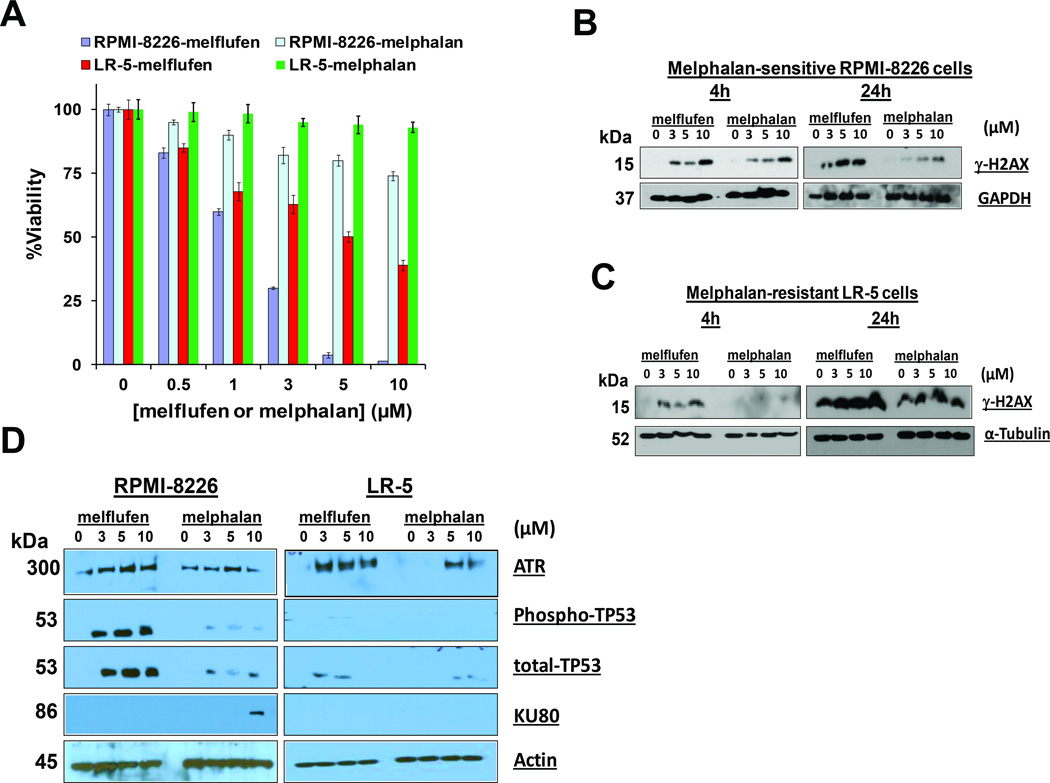
Figure 2. Melflufen induces dose- and time- dependent rapid phosphorylation of H2AX in melphalan-sensitive and resistant isogenic RPMI-8226 cell lines.
(A) Melphalan-sensitive RPMI-8226 (WT) and melphalan-resistant LR-5 cells were treated with indicated concentrations of melflufen for24h, and cytotoxicity was assessed using MTT assay (n = 3, mean ± SD; p < 0.005). (B) Melphalan-sensitive RPMI-8226 cells were treated with equimolar concentrations of melflufen or melphalan for 4h and 24h; protein lysates were subjected to immunoblot analysis using antibodies specific against γ-H2AX and GAPDH. (C) Melphalan-resistant LR-5 cells were treated with equimolar concentrations of melflufen or melphalan for 4h and 24h; protein lysates were subjected to immunoblot analysis using antibodies specific against γ-H2AX and α-Tubulin. (D) RPMI-8226 and LR-5 cells were treated with melflufen or melphalan for 24h; protein lysates were subjected to immunoblot analysis using antibodies specific against ATR, TP53, Ku80, and Actin.
We next treated RPMI-8226 and LR-5 cells with various equimolar concentrations of melflufen or melphalan for 4 and 24h, and protein lysates were analysed for γ-H2AX. Melflufen-treated RPMI-8226 and LR-5 cells showed a marked upregulation of γ-H2AX in a time and dose-dependent fashion (Fig 2B and 2C). An increase in γ-H2AX was observed in RPMI-8226 cells treated with melphalan, albeit to a lesser extent than if treated with melflufen (Fig 2B). Short term (4h) treatment of melphalan-resistant LR-5 cells with even higher concentrations of melphalan showed no induction of γ-H2AX, whereas longer time intervals of treatment (24h) led to a minimal increase in γ-H2AX (Fig 2C, right panel). Of note, baseline γ-H2AX levels (untreated control) increased in LR-5 cells after 24h culture, reflecting on-going DNA damage in MM cells as reported in our recent study (Cottini et al, 2015).
As for γ-H2AX, examination of DNA damage response proteins ATR and CHK1 also showed similar kinetics of induction in melflufen- and melphalan-treated cells (Fig 2D, and data not shown). Moreover, melflufen treatment upregulated TP53, a downstream target of ATR, in both RPMI-8226 and LR-5 cells (Fig 2D). On the other hand, although melphalan induced TP53 in melphalan-sensitive RPMI-8226 cells, but to a much lesser extent than melflufen, no significant TP53 induction was noted in melphalan-treated LR-5 cells. Analysis of Ku80 that repairs DNA double-strand breaks (DSBs) showed that melphalan, but not melflufen, triggered induction of Ku80 in RPMI-8226 cells. Consistent with melphalan-resistance of LR-5 cells, we observed no induction of Ku80 after treatment of these cells with melflufen (Fig 2D).
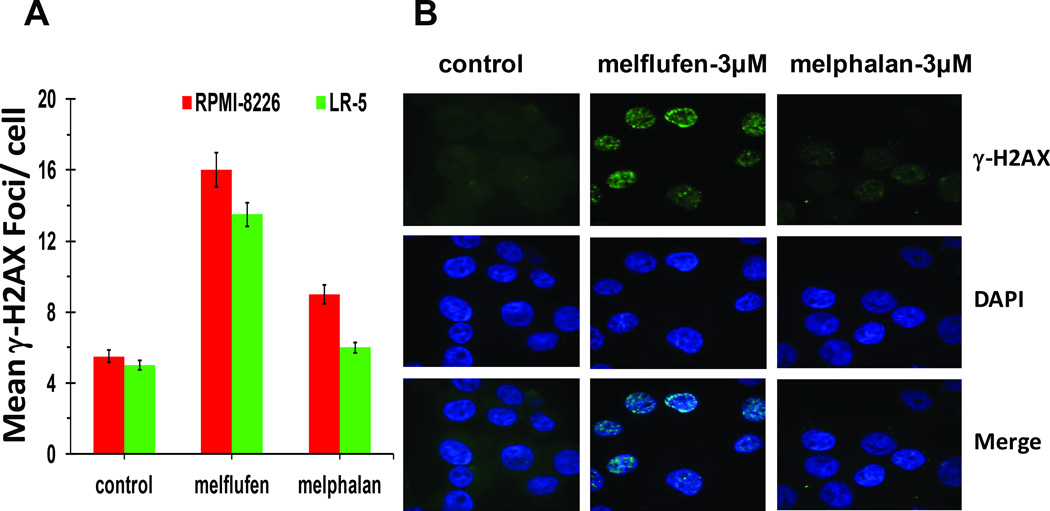
As noted above, phosphorylation of H2AX (γ-H2AX) is a critical step prior to the efficient recognition and repair of DNA damage, especially double strand breaks (DSBs). H2AX is rapidly phosphorylated at nascent double-strand break sites resulting in the formation of γ-H2AX foci, which serve as an important pharmacodynamic marker for DNA damage. We therefore next examined whether melflufen and melphalan trigger differential γ-H2AX foci formation in MM cells. RPMI-8226 and LR-5 cells were treated with equimolar concentrations of melflufen or melphalan for 4h, and were then subjected to dual immunofluorescence staining using DAPI and γ-H2AX Ab, followed by quantification of γ-H2AX foci. Melflufen triggered robust γ-H2AX foci formation in both melphalan-sensitive and resistant cells, whereas no significant γ-H2AX foci formation was observed in melphalan-treated LR-5 cells (Fig 3A; quantified γ-H2AX foci numbers/cell). Enhanced γ-H2AX foci formation in melflufen vs. melphalan-treated cells reflects a more pronounced induction of DNA DSBs in response to melflufen than melphalan. Consistent with its cytotoxic activity against melphalan-resistant LR-5 cells (Fig 2A), melflufen triggered robust γ-H2AX foci formation in these cells (Fig 3B). Taken together, our data suggests that melflufen triggers a more rapid DNA damage than melphalan.
Figure 3. Melflufen triggers robust γ-H2AX foci formation in melphalan-resistant LR-5 cells.
RPMI-8226 and LR-5 cells were treated with 3 µM of melflufen or melphalan for 4h, and then subjected to immunofluorescence (IF) staining (anti-γ-H2AX as primary and Alexa Fluor- 488 conjugated secondary antibodies; DAPI for nuclear staining). (A) Quantitative analysis of γ-H2AX foci formation in melflufen- or melphalan-treated (3 µM) RPMI-8226 and LR-5 cells.(mean±SD; P<0.05, n=3 fields per sample) (B) Representative IF showing γ-H2AX foci formation in melflufen- and melphalan-treated LR-5 cells.
Melflufen induces irreversible DNA damage in both melphalan-sensitive and -resistant MM cells
Our findings, showing that melflufen triggers early and robust DNA damage without activation of DNA repair protein Ku80, suggests that melflufen may trigger irreversible DNA damage and cytotoxicity. To validate this hypothesis, we next performed drug washout experiments, followed by correlative analyses of cell viability, γ-H2AX induction, and cell cycle. MM.1S, RPMI-8226, and LR-5 cells were treated with various concentrations of melflufen or melphalan for 1h; cells were then washed to remove drugs and cultured in complete medium without drugs for 48h, followed by analysis of cell viability. Results showed that short-term (1h) treatment with low concentrations of melflufen (range for IC90 to IC50: 0.5 µM- 3 µM) triggers significant cytotoxicity in both melphalan-sensitive (MM.1S, RPMI-8226) and melphalan-resistant (LR-5) cells (Fig 4A–4C). Similar short-term exposure of cell lines to equimolar concentrations of melphalan did not induce cytotoxicity in either RPMI-8226 or LR-5 cells (Fig 4A–4C). Higher concentrations of melphalan (IC50: 5 µM) decreased MM.1S cell viability, as reported previously (Chauhan et al, 2013).
Figure 4. Short term Melflufen exposure induces irreversible DNA damage in both melphalan-sensitive and resistant MM cells.
(A–C) MM.1S, RPMI-8226, and melphalan-resistant LR-5 cells were treated with melflufen or melphalan for 1h; cells were washed to remove drugs and then cultured in complete medium for 48h, followed by analysis of viability (mean ± SD; p < 0.05, n=3). (D) Melphalan-sensitive RPMI-8226 and melphalan-resistant LR-5 cells were treated with equimolar concentrations of melflufen or melphalan (3 µM) for 1h; cells were washed to remove drugs and then cultured in fresh complete medium for 24h or 48h, followed by immunoblot analysis of protein lysates using γ-H2AX and GAPDH antibodies.
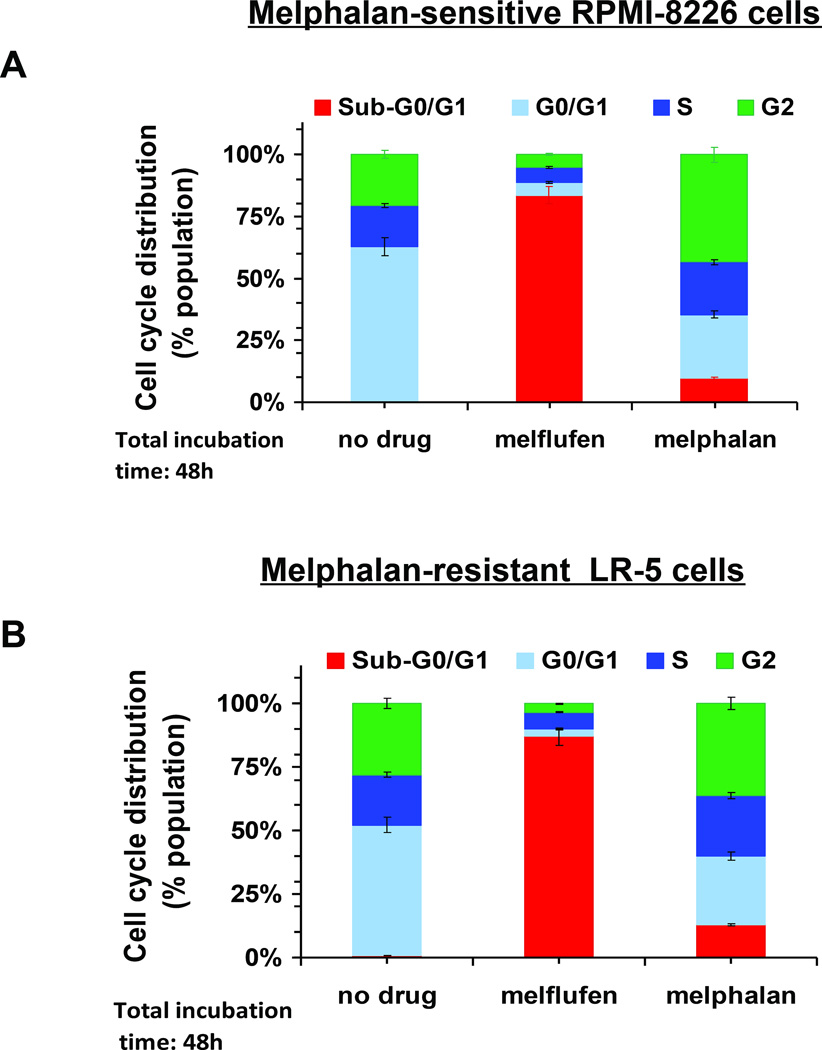
We next examined whether the cytotoxic activity profiles of melflufen and melphalan after short-term exposure correlate with γ-H2AX induction kinetics and cell cycle alterations in RPMI-8226 and LR-5 cells. Specifically, cells were treated with melflufen or melphalan (3 µM) for 1h; washed and cultured in complete medium without drugs for another 24 or 48h; and then analysed for γ-H2AX. As seen in Figure 4D, γ-H2AX induction is clearly evident in all melflufen-treated cells. Although melphalan-treated RPMI-8226 cells showed γ-H2AX signal after 24h, it disappeared after 48h (Figure 4D, right panel), consistent with on-going DNA repair in these cells maintaining their viability. Under identical experimental conditions as in Figure 4, examination of cell cycle changes showed that melflufen induces increased Sub G0/G1 phase in both melphalan-sensitive (~80%) and melphalan-resistant (77%) cells (Fig 5A and 5B). Of note, melphalan triggered only minimal (10%) accumulation of cells in Sub-G0/G1 phase (Fig 5A and 5B).
Figure 5. Melflufen induces Cell Cycle changes in MM cells.
(A and B) melphalan-sensitive (RPMI-8226) and -resistant (LR-5) cells were treated with melflufen (3 µM) or melphalan (10 µM) for 1h; cells were washed to remove drugs and then resuspended in complete medium for 48h, followed by cell cycle analysis using propidium iodide staining. Quantitative analysis of cells in different cell cycle phases is presented.
We next utilized MM patient tumour cells to assess whether melflufen induces irreversible DNA damage. Purified MM tumour cells were treated with melflufen (5 µM) or melphalan (10 µM) for 1h; washed and cultured in complete medium (containing recombinant IL6) without drugs for another 48h or 72h; and then analysed for viability using FACS. As expected, a significant increase in non-viable (dead) cells was observed in melflufen-treated cells due to an early and irreversible induction of DNA damage (Supplementary Fig 2). Interestingly, the number of dead cells in melphalan-treated cell populations slightly decreased from 48h to 72h (Supplementary Fig 2), indicating the presence of an active DNA repair pathway after melphalan washout.
Melflufen Triggers irreversible DNA damage, as assessed by acridine orange (AO) staining and TUNEL assays
It is well established that the apoptotic DNA, in contrast to normal DNA, exhibits increased tendency to undergo DNA melting, with decreased resistance to acid or alkaline denaturation. We hypothesized that melflufen triggers irreversible DNA damage, and that the apoptotic DNA in melflufen-treated cells will exhibit decreased resistance to acid or alkaline denaturation compared to that after melphalan treatment. To validate this hypothesis, we utilized the metachromatic dye AO, which differentially stains double- and single-stranded DNA, to emit green and red fluorescence, respectively. The control/untreated cells emit mostly green fluorescence (non-apoptotic), with minimal baseline red fluorescence (apoptotic). As melflufen is more potent than melphalan, we expected that melflufen-treated cells stained with AO (after removal of RNA and HCL-induced denaturation), would emit more red fluorescence and less green fluorescence than melphalan-treated cells. Treatment of MM cells with melflufen, either for a short time (2h) followed by drug-free culture for 48h or for longer (48h) continuous treatment, led to a shift from green to red fluorescence compared to controls, indicating the presence of apoptotic DNA (Fig 6A–6C). Brief treatment with melflufen triggers DNA lesions that remain even after 48-h culture in drug-free medium. This provides evidence for irreversibility of DNA damage and/or a lack of active DNA repair in melflufen-treated cells. In contrast, short time (2h) exposure of cells to melphalan followed by 48h culture in the absence of drug showed predominance of green fluorescence representing non-apoptotic cells, reflecting an increased resistance to HCL-induced denaturation of DNA fragments, probably due to repair/reversal from apoptotic to non-apoptotic state after short term melphalan treatment. Furthermore, continuous exposure to melphalan resulted in a significant increase in AO red fluorescence (apoptotic ss-DNA) in melphalan-sensitive cells (Fig 6A–6B). As expected, the induction of AO red fluorescence after melphalan treatment of LR-5 cells was comparable to that of untreated cells (Fig 6C).
Figure 6. Analysis of melflufen or melphalan-induced DNA damage with Acridine Orange (AO).
(A–C) MM.1S (melflufen: 1 µM; melphalan: 3 µM), RPMI-8226 and LR-5 (melflufen:3 µM; melphalan :10 µM) cells were treated with drugs for 2h; cells were washed to remove drugs and then cultured in fresh complete medium for 48h, followed by AO staining of cells using FACS. In addition, cells were treated with drugs (as above) continuously for 48h, and then subjected to analysis with AO staining. Quantitative analysis of AO-Red Fluorescence and AO-Green Fluorescence is presented.
We further confirmed our data from AO staining using TUNEL assays. The TUNEL assay identifies apoptotic populations, as well as fractions that are undergoing DNA repair. MM.1S, RPMI-8226, and LR-5 cells were treated with equimolar concentrations of melflufen or melphalan for 2h, and then washed to remove drugs and cultured in complete medium for 48h. As a comparator, the effect of continuous drug treatment for 48h was also examined. A significantly increased TUNEL-positive cell population was seen in all cell lines after short term (2h) or prolonged continuous exposure of melflufen treatment (Fig 7A–7C). Conversely, short-term (2h) exposure of cells (MM.1S, RPMI-8226) to melphalan (followed by 48h of culture in drug-free complete medium) did not induce an increase in TUNEL-positive cells, consistent with data obtained using AO staining, associated with reversible DNA damage due to active DNA repair. Importantly, melflufen treatment triggered a marked increase in TUNEL-positive cells even in melphalan-resistant LR-5 cells (Fig 7C), which was not observed after melphalan treatment.
Figure 7. Analysis of melflufen or melphalan-induced DNA damage and apoptosis with TUNEL Assay.
(A–C) MM.1S (melflufen:1 µM; melphalan: 3 µM), RPMI-8226 and LR-5 (melflufen:3 µM; melphalan:10 µM) cells were treated with drugs for 2h; cells were washed to remove drugs and then cultured in complete medium for 48h, followed by FACS analysis of cells using TUNEL assay. In addition, cells were treated with drugs (as above) continuously for 48h, and then subjected to analysis using TUNEL assay. Bar graph shows the percentage of TUNEL positive cells under different treatment conditions.
Discussion
The lipophilicity characteristic of melflufen allows for rapid cellular uptake, followed by its hydrolysis via intracellular peptidases and the release of active metabolite melphalan. Due to the differential rate of transport of melflufen into cells (rapid) and free melphalan out of cells (slow), melflufen allows for a more rapid and higher intracellular accumulation of melphalan in tumour cells than is achievable by direct exposure to equimolar doses of melphalan (Wickstrom et al, 2011). Melflufen allows for a robust intracellular accumulation of melphalan in MM cells (Chauhan et al, 2013), and the accumulation with melflufen treatment correlates with cellular aminpeptidase expression/activity, which is quite high in tumour cells, including MM, and low/baseline in normal cells (Chauhan et al, 2013). Based on both in vitro and in vivo pre-clinical models, we demonstrated the potent anti-MM activity of melflufen at doses that are well tolerated in human MM xenograft mouse models (Chauhan et al, 2013). In the current study, we examined whether the potent anti-MM activity of melflufen versus melphalan is due to their differential effect on DNA damage and repair signalling pathways.
Melflufen triggered cytotoxicity in MM cell lines including, melphalan-resistant LR-5 cells and melflufen-induced DNA damage is significantly higher in MM tumour cells compared to normal cells (Chauhan et al, 2013). Results show that: 1) melflufen induces γ-H2AX in a dose- and time-dependent manner; and 2) melflufen-induced γ-H2AX is more rapid and robust than is triggered by equimolar concentrations of melphalan. The induction kinetics of γ-H2AX are consistent with our previous study (Chauhan et al, 2013) showing that melflufen allows for a more rapid and higher intracellular accumulation of melphalan in MM cells than is achievable by direct exposure to equimolar doses of melphalan.
The mechanism(s) mediating melphalan-resistance in MM cells include reduced cellular uptake of melphalan, and therefore increasing intracellular concentration of melphalan with melflufen overcame drug-resistance. Furthermore, we found that treatment of MM cells with melflufen induced a significantly higher levels of tumour suppressor protein TP53 than noted in melphalan-treated cells (Fig 2). ATR is an upstream regulator of TP53 in DNA-damaged cells (Cottini et al, 2015) and, consistent with our observation on TP53, we found higher levels of ATR in melflufen- versus melphalan-treated cells. It is likely that other upstream molecules, besides ATR, mediate TP53 induction in melflufen-treated cells.
Ku is essential for binding to broken DNA and recruiting other proteins to initiate the repair of broken DNA ends (Brown et al, 2015; Fell & Schild-Poulter, 2015), and a lack of Ku80 induction in melflufen-treated cells suggests that melflufen-induced DNA damage is not associated with a functional DNA repair mechanism. The rapid kinetics of melflufen processing result in higher intracellular concentrations of melphalan and induction of an irreversible DNA damage without DNA repair. Furthermore, the variation in Ku80 expression/changes among MM cell lines can be attributed to the different degree of constitutive on-going DNA damage levels as well as extent of molecular/cytogenetic heterogeneity observed in these cell lines. Conversely, melphalan-treatment induces Ku80, which is consistent with earlier studies linking activation of DNA repair with melphalan-resistance (Spanswick et al, 2002; Sousa et al, 2013; Wang et al, 2001). The immunofluorescence study assessing γ-H2AX foci formation shows that H2AX is recruited at the damaged DNA sites. The foci formation is an important pharmacodynamic marker for DNA damage, representing DSB in an almost 1:1 ratio (Loo, 2002). A robust γ-H2AX foci formation indicate a potent DSB formation in melfufen- versus melphalan-treated cells. Additionally, the drug washout experiments, together with analyses of cell viability, γ-H2AX induction and cell cycle, show that melflufen induces irreversible DNA damage in MM tumour cells, whereas washout experiments using melphalan indicate the presence of an active DNA repair process. Overall, our data suggest that melflufen triggers a more rapid DNA damage than melphalan in a dose- and time-dependent manner.
In contrast to normal DNA, apoptotic DNAs exhibit increased tendency to undergo DNA melting, with decreased resistance to acid or alkaline denaturation. The differential metachromatic AO-staining of MM cells revealed that apoptotic DNAs in melflufen-treated cells have decreased resistance to acid or alkaline denaturation compared to melphalan treatment after drug washout; this observation supports melflufen’s ability to induce irreversible DNA damage. The untreated MM cells exhibit minimal/baseline TUNEL +ve populations; indicative of an on-going spontaneous DNA damage in MM cells. Melflufen-treatment for a short period of time (2h) triggers DNA lesions that remain after 48h culture in drug-free medium, and thus provides evidence for irreversibility of DNA damage and/or a lack of active DNA repair in melflufen-treated cells. In contrast, based on both AO-staining and TUNEL assays, 2h of melphalan-treatment of MM cells followed by 48h drug-free culture shows reversal from an apoptotic to a non-apoptotic state, indicating an active repair process.
Collectively, our data provide mechanistic insights into the potent anti-MM activity of alkylating agent melflufen. The present study extends our prior report (Chauhan et al 2013) in which we utilized both in vitro and in vivo MM xenograft models to show that: 1) melflufen is a more potent anti-MM agent than melphalan and can overcome conventional drug resistance; and 2) the combination of melflufen with bortezomib, lenalidomide, or dexamethasone induces synergistic anti-MM activity. In the present study, we show that: 1) melflufen induces γ-H2AX in a dose- and time-dependent manner; 2) melflufen-induced γ-H2AX is more rapid and robust than that triggered by equimolar concentrations of melphalan; 3) Similar to γ-H2AX, melflufen also triggers induction of other DNA damage response proteins ATR/CHK1/TP53; 4) melflufen-induced DNA damage is not associated with Ku80-mediated DNA repair mechanism; 5) Melflufen triggers irreversible DNA damage and cytoxicity, evidenced by short term treatment and washout experiments; and 6) melflufen-induces irreversible DNA damage in melphalan-resistant MM cells and can overcome melphalan-resistance. Our preclinical data provide the framework for clinical evaluation of melflufen in MM. Importantly, an ongoing Phase-I/II clinical trial of melflufen shows promising activity in patients with relapsed and relapsed-refractory MM (median 4 prior lines of therapy) and is well tolerated (Magarotto et al, 2015). Moreover, melflufen is currently undergoing a multi-centre phase 2 clinical trial in MM and is on track for the next phase of clinical studies. Recent data from the clinical trials in MM patients were presented at the Annual meeting of the American Society of Hematology. Specifically, melflufen plus low dose dexamethasone appeared in early data to be well tolerated and has activity in this advanced RRMM population (Paba-Prada et al, 2014). Furthermore, melflufen showed promising activity in heavily pretreated RRMM patients where conventional therapies have failed. Haematological toxicity was common, but non-haematological AEs were infrequent (Voorhees et al, 2015).
Supplementary Material
Acknowledgments
Grant Support: This investigation was supported by National Institutes of Health Specialized Programs of Research Excellence (SPORE) grant P50100707, PO1-CA078378, and RO1 CA050947 (DC, and KCA); KCA is an American Cancer Society Clinical Research Professor.
Footnotes
Conflicts of Interest disclosure: EN is an employee of Oncopeptides, AB. JG is equity owner in Oncopeptides AB. DC is a consultant to Oncopeptides AB. Other co-authors have no competing financial interests.
Authors’ contributions: AR designed research, performed the experiments, analysed data and wrote the manuscript; DSD and YS contributed in to blot analysis and flow cytometry; JC synthesized melflufen; PGR provided clinical samples; DC designed research, analysed data and wrote the manuscript; and KCA analysed data and wrote the manuscript.
References
- Anderson KC. Oncogenomics to target myeloma in the bone marrow microenvironment. Clin Cancer Res. 2011;17:1225–1233. doi: 10.1158/1078-0432.CCR-10-3366. [DOI] [PubMed] [Google Scholar]
- Anderson KC. The 39th David A. Karnofsky Lecture: bench-to-bedside translation of targeted therapies in multiple myeloma. J Clin Oncol. 2012;30:445–452. doi: 10.1200/JCO.2011.37.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, McCoy J, Moore DF, Jr, Dakhil SR, Lanier KS, Chapman RA, Cromer JN, Salmon SE, Durie B, Crowley JC. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III USIntergroup Trial S9321. J Clin Oncol. 2006;24:929–936. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- Berenson JR, Yang HH, Sadler K, Jarutirasarn SG, Vescio RA, Mapes R, Purner M, Lee SP, Wilson J, Morrison B, Adams J, Schenkein D, Swift R. Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2006;24:937–944. doi: 10.1200/JCO.2005.03.2383. [DOI] [PubMed] [Google Scholar]
- Brown JS, Lukashchuk N, Sczaniecka-Clift M, Britton S, le Sage C, Calsou P, Beli P, Galanty Y, Jackson SP. Neddylation promotes ubiquitylation and release of Ku from DNA-damage sites. Cell Rep. 2015;11:704–714. doi: 10.1016/j.celrep.2015.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Tian Z, Nicholson B, Kumar KG, Zhou B, Carrasco R, McDermott JL, Leach CA, Fulcinniti M, Kodrasov MP, Weinstock J, Kingsbury WD, Hideshima T, Shah PK, Minvielle S, Altun M, Kessler BM, Orlowski R, Richardson P, Munshi N, Anderson KC. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell. 2012;22:345–358. doi: 10.1016/j.ccr.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Ray A, Viktorsson K, Spira J, Paba-Prada C, Munshi N, Richardson P, Lewensohn R, Anderson KC. In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan-flufenamide, against multiple myeloma cells. Clin Cancer Res. 2013;19:3019–3031. doi: 10.1158/1078-0432.CCR-12-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ. Medical Research Council Adult Leukaemia Working Party. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- Cottini F, Hideshima T, Suzuki R, Tai YT, Bianchini G, Richardson PG, Anderson KC, Tonon G. Synthetic Lethal Approaches Exploiting DNA Damage in Aggressive Myeloma. Cancer Discov. 2015;5:972–987. doi: 10.1158/2159-8290.CD-14-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Traganos F, Zhao H, Halicka HD, Skommer J, Wlodkowic D. Analysis of Individual Molecular Events of DNA Damage Response by Flow and Image Assisted Cytometry. Methods Cell Biol. 2011;103:115–147. doi: 10.1016/B978-0-12-385493-3.00006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies FE, Dring AM, Li C, Rawstron AC, Shammas MA, O'Connor SM, Fenton JA, Hideshima T, Chauhan D, Tai IT, Robinson E, Auclair D, Rees K, Gonzalez D, Ashcroft AJ, Dasgupta R, Mitsiades C, Mitsiades N, Chen LB, Wong WH, Munshi NC, Morgan GJ, Anderson KC. Insights into the multistep transformation of MGUS to myeloma using microarray expression analysis. Blood. 2003;102:4504–4511. doi: 10.1182/blood-2003-01-0016. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, San-Miguel JF, Anderson KC. Emerging therapies for the treatment of relapsed or refractory multiple myeloma. Eur J Haematol. 2011;86:1–15. doi: 10.1111/j.1600-0609.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, Renaud M, Harousseau JL, Guillerm G, Chaleteix C, Dib M, Voillat L, Maisonneuve H, Troncy J, Dorvaux V, Monconduit M, Martin C, Casassus P, Jaubert J, Jardel H, Doyen C, Kolb B, Anglaret B, Grosbois B, Yakoub-Agha I, Mathiot C, Avet-Loiseau H Intergroupe Francophone du Myélome. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- Falco P, Cavallo F, Larocca A, Rossi D, Guglielmelli T, Rocci A, Grasso M, Siez ML, De Paoli L, Oliva S, Molica S, Mina R, Gay F, Benevolo G, Musto P, Omedè P, Freilone R, Bringhen S, Carella AM, Gaidano G, Boccadoro M, Palumbo A. Lenalidomide-prednisone induction followed by lenalidomide-melphalan-prednisone consolidation and lenalidomide-prednisone maintenance in newly diagnosed elderly unfit myeloma patients. Leukemia. 2013;27:695–701. doi: 10.1038/leu.2012.271. [DOI] [PubMed] [Google Scholar]
- Featherstone C, Jackson SP. Ku, a DNA repair protein with multiple cellular functions? Mutat Res. 1999;434:3–15. doi: 10.1016/s0921-8777(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Fell VL, Schild-Poulter C. Ku regulates signaling to DNA damage response pathways through the Ku70 von Willebrand A domain. Mol Cell Biol. 2012;32:76–87. doi: 10.1128/MCB.05661-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell VL, Schild-Poulter C. The Ku heterodimer: function in DNA repair and beyond. Mutat Res Rev Mutat Res. 2015;763:15–29. doi: 10.1016/j.mrrev.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Gullbo J, Wallinder C, Tullberg M, Lovborg H, Ehrsson H, Lewensohn R, Nygren P, Luthman K, Larsson R. Antitumor activity of the novel melphalan containing tripeptide J3 (L-prolyl-L-melphalanyl-p-L-fluorophenylalanine ethyl ester): comparison with its m-L-sarcolysin analogue P2. Mol Cancer Ther. 2003;2:1331–1339. [PubMed] [Google Scholar]
- Gullbo J, Lindhagen E, Bashir-Hassan S, Tullberg M, Ehrsson H, Lewensohn R, Nygren P, De La Torre M, Luthman K, Larsson R. Antitumor efficacy and acute toxicity of the novel dipeptide melphalanyl-p-L-fluorophenylalanine ethyl ester (J1) in vivo. Invest New Drugs. 2004;22:411–420. doi: 10.1023/B:DRUG.0000036683.10945.bb. [DOI] [PubMed] [Google Scholar]
- Hazlehurst LA, Enkemann SA, Beam CA, Argilagos RF, Painter J, Shain KH, Saporta S, Boulware D, Moscinski L, Alsina M, Dalton WS. Genotypic and phenotypic comparisons of de novo and acquired melphalan resistance in an isogenic multiple myeloma cell line model. Cancer Res. 2003;63:7900–7906. [PubMed] [Google Scholar]
- Karlsson A, Deb-Basu D, Cherry A, Turner S, Ford J, Felsher DW. Defective double-strand DNA break repair and chromosomal translocations by MYC overexpression. PNAS. 2003;100:9974–9979. doi: 10.1073/pnas.1732638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo DT. TUNEL Assay: An Overview of Techniques. In: Didenko VV, editor. Methods in Molecular Biology: In Situ Detection of DNA Damage: Methods and Protocols. Vol. 203. Totowa, NJ: Humana Press Inc.; 2002. pp. 21–30. 2002. [DOI] [PubMed] [Google Scholar]
- Ludwig H, Durie BG, McCarthy P, Palumbo A, San Miguel J, Barlogie B, Morgan G, Sonneveld P, Spencer A, Andersen KC, Facon T, Stewart KA, Einsele H, Mateos MV, Wijermans P, Waage A, Beksac M, Richardson PG, Hulin C, Niesvizky R, Lokhorst H, Landgren O, Bergsagel PL, Orlowski R, Hinke A, Cavo M, Attal M International Myeloma Working Group. IMWG consensus on maintenance therapy in multiple myeloma. Blood. 2012;119:3003–3015. doi: 10.1182/blood-2011-11-374249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarotto V, Sonneveld P, Voorhees P, Paba-Prada C, Plesner T, Mellqvist U-h, Byrne C, Harmenberg J, Nordström E, Palumbo A, Richardson P. Encouraging Preliminary Data In Ongoing Open-label Phase 1/2 Study Of Safety And Efficacy Of Melflufen And Dexamethasone For Patients With Relapsed And Relapsed-Refractory Multiple Myeloma. Annual Meeting of the European Hematology Association (EHA) meeting. Haematologica. 2015 Jun;100 Issue Supplement 1, 1-800; Abstract No: poster#285. [Google Scholar]
- Mateos MV, Oriol A, Martinez-Lopez J, Gutierrez N, Teruel AI, de Paz R, García-Laraña J, Bengoechea E, Martín A, Mediavilla JD, Palomera L, de Arriba F, González Y, Hernández JM, Sureda A, Bello JL, Bargay J, Peñalver FJ, Ribera JM, Martín-Mateos ML, García-Sanz R, Cibeira MT, Ramos ML, Vidriales MB, Paiva B, Montalbán MA, Lahuerta JJ, Bladé J, Miguel JF. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010a;11:934–941. doi: 10.1016/S1470-2045(10)70187-X. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Esseltine DL, Liu K, Cakana A, van de Velde H, San Miguel JF. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010b;28:2259–66. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- Paba-Prada C, Palumbo A, Mellqvist U-H, Voorhees PM, Plesner T, Sonneveld P, Byrne C, Harmenberg J, Nordstrom E, Richardson PG. Determination of the MTD and Encouraging Results in an Ongoing Open-Label Phase 1/2a Study of the Safety and Efficacy of Melflufen and Dexamethasone in Combination for Patients with Relapsed and Relapsed-Refractory Multiple Myeloma (RRMM) Blood (ASH Annual Meeting Abstracts) 2014;124:2123. [Google Scholar]
- Palumbo A, Ambrosini MT, Benevolo G, Pregno P, Pescosta N, Callea V, Cangialosi C, Caravita T, Morabito F, Musto P, Bringhen S, Falco P, Avonto I, Cavallo F, Boccadoro M Italian Multiple Myeloma Network; Gruppo Italiano Malattie Ematologicche dell'Adulto. Bortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma. Blood. 2007;109:2767–2772. doi: 10.1182/blood-2006-08-042275. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Waage A, Hulin C, Beksac M, Zweegman S, Gay F, Gimsing P, Leleu X, Wijermans P, Sucak G, Pezzatti S, Juliusson G, Pégourié B, Schaafsma M, Galli M, Turesson I, Kolb B, van der Holt B, Baldi I, Rolke J, Ciccone G, Wetterwald M, Lokhorst H, Boccadoro M, Rodon P, Sonneveld P. Safety of thalidomide in newly diagnosed elderly myeloma patients: an individual patient data meta-analysis of six randomized trials. Haematologica. 2013;98:87–94. doi: 10.3324/haematol.2012.067058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV. Optimising bortezomib in newly diagnosed multiple myeloma. Lancet Oncol. 2010;11:909–910. doi: 10.1016/S1470-2045(10)70199-6. [DOI] [PubMed] [Google Scholar]
- Sousa MML, Zub KA, Aas PA, Hanssen-Bauer A, Demirovic A, Sarno A, Tian E, Liabakk NB, Slupphaug G. An Inverse Switch in DNA Base Excision and Strand Break Repair Contributes to Melphalan Resistance in Multiple Myeloma Cells. PLOS ONE. 2013;8:e55493. doi: 10.1371/journal.pone.0055493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick VJ, Craddock C, Sekhar M, Mahendra P, Shankaranarayana P, Hughes RG, Hochhauser D, Hartley JA. Repair of DNA interstrand crosslinks as a mechanism of clinical resistance to melphalan in multiple myeloma. Blood. 2001;100:224–229. doi: 10.1182/blood.v100.1.224. [DOI] [PubMed] [Google Scholar]
- Traganos F, Darzynkiewicz Z, Sharpless T, Melamed MR. Simultaneous staining of ribonucleic and deoxyribonucleic acids in unfixed cells using acridine orange in a flow cytofluorometric system. J Histochem Cytochem. 1977;25:46–56. doi: 10.1177/25.1.64567. [DOI] [PubMed] [Google Scholar]
- Voorhees PM, Magarotto V, Sonneveld P, Plesner T, Mellqvist U-H, Paba-Prada C, Byrne C, Harmenberg J, Nordstrom E, Palumbo A, Richardson PG. Efficacy of Melflufen, a Peptidase Targeted Therapy, and Dexamethasone in an Ongoing Open-Label Phase 2a Study in Patients with Relapsed and Relapsed-Refractory Multiple Myeloma (RRMM) Including an Initial Report on Progression Free Survival. Blood (ASH Annual Meeting Abstracts) 2015;126:3029. [Google Scholar]
- Wang Z-M, Chen Z-P, Xu Z-Y, Christodoulopoulos G, Bello V, Mohr G, Aloyz R, Panasci LC. In Vitro Evidence for Homologous Recombinational Repair in Resistance to Melphalan. J Natl Cancer Inst. 2001;93:1473–1478. doi: 10.1093/jnci/93.19.1473. [DOI] [PubMed] [Google Scholar]
- Wickstrom M, Johnsen JI, Ponthan F, Segerstrom L, Sveinbjornsson B, Lindskog M, Lövborg H, Viktorsson K, Lewensohn R, Kogner P, Larsson R, Gullbo J. The novel melphalan prodrug J1 inhibits neuroblastoma growth in vitro and in vivo. Mol Cancer Ther. 2007;6:2409–2417. doi: 10.1158/1535-7163.MCT-07-0156. [DOI] [PubMed] [Google Scholar]
- Wickstrom M, Larsson R, Nygren P, Gullbo J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011;102:501–508. doi: 10.1111/j.1349-7006.2010.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom M, Viktorsson K, Lundholm L, Aesoy R, Nygren H, Sooman L, Fryknäs M, Vogel LK, Lewensohn R, Larsson R, Gullbo J. The alkylating prodrug J1 can be activated by aminopeptidase N, leading to a possible target directed release of melphalan. Biochem Pharmacol. 2010;79:1281–1290. doi: 10.1016/j.bcp.2009.12.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.