Abstract
Background
The dual role of B cells as drivers and suppressors of the immune responses have underscored the need to trace the fate of B cells recognizing donor MHC Class I and Class II following allograft transplantation.
Methods
In this study we used donor Class II tetramers to trace the fate of I-Ed-specific B cells following primary immunization with BALB/c spleen cells, or cardiac transplantation, in naïve or sensitized C57BL/6 recipients. We combined this approach with genetic lineage tracing of memory B cells in activation-induced cytidine deaminase regulated Cre transgenic mice (AID-Cre) crossed to the ROSA26-EYFP reporter mice to track endogenous I-Ed-specific memory B cell generation.
Results
Immunization with BALB/c splenocytes or heart transplantation induced an expansion and differentiation of I-Ed-specific B cells into germinal center B cells, whereas BALB/c heart transplantation into sensitized recipients induced the preferential differentiation into antibody-secreting cells. A 10.8-fold increase in the frequency of I-Ed-specific memory B cells was observed by day 42 postimmunization. Treatment with CTLA4-Ig starting on day 0 or day 7 postimmunization abrogated I-Ed-specific memory B cell generation and sensitized humoral responses, but not if treatment commenced on day 14.
Conclusion
The majority of donor-specific memory B cells are generated between days 7–14 postimmunization, thus revealing a flexible timeframe whereby delayed CTLA4-Ig administration can inhibit sensitization and the generation of memory graft-reactive B cells.
Introduction
Improved diagnosis of donor-specific antibodies (DSA) has led to the current understanding that antibody-mediated rejection (ABMR) is the leading cause of kidney allograft failure in the clinic 1–5. Antibody-mediated rejection manifests as microcirculation lesions and specific transcript changes that signify antibody-mediated endothelial injury, interferon-γ effects and the recruitment of natural killer cells. Because the main cause of late kidney transplant failure is correlated with ABMR, and T cell-mediated rejection, which is common early but progressively disappears over time posttransplant, is not associated with graft failure 2,3, clinicans have concluded that current immunosuppression is relatively ineffective in preventing ABMR, especially once DSA is detected, and that new immunosuppressive agents are required for successfully preventing ABMR.
Donor-specific antibodies are produced by T-dependent alloreactive B cells that, upon encounter with alloantigen, differentiate into antibody-producing short-lived plasmablasts that are responsible for the acute production of antibodies, as well as long-lived plasma cells, which are responsible for serological memory 6. In addition, some activated alloreactive B cells differentiate into quiescent memory B cells that, upon antigen re-encounter, differentiate rapidly into plasmablasts capable of producing high affinity antibodies 6,7. B cells can also display antibody-independent functions; Zeng et al 8,9 reported that chronic allograft vasculopathy was dependent on T cells but B cells played critical roles in supporting splenic lymphoid architecture and serving as antigen-presenting cells to alloreactive T cells. In an elegant cell distance mapping study, Chang et al 9 reported that 80% of T cells with a T follicular helper phenotype (Tfh) were engaged in tight cognate interaction with B cells in biopsies diagnosed with mixed T cell and antibody-mediated rejection; in contrast only 15% of the T cells were similarly engaged in biopsies with T cell-mediated rejection. These data suggest that B cells may play an important role as antigen presenting cells within the allograft in distinct types of graft rejection.
There is also emerging evidence that B cells may play an immunomodulatory role and facilitate the development of transplantation tolerance 10–17. In those studies, IL-10 produced by B cells have been shown to play a critical role, but the phenotype and the antigen-specificity of the IL-10 producing B cells, and the micro-anatomical location of these IL-10-producing Bregs that allow them to modulate T cell responses, require further clarification. Additionally observations that operationally tolerant kidney transplant recipients have enriched subsets of B cells compared to stable recipients on immunosuppression have lead some investigators to hypothesize a role for B cells, and potentially regulatory B cells, in clinical transplant tolerance 18–24. Collectively these findings have intensified interest in understanding the fate of alloreactive B cells in rejection and tolerance.
Given the dual role of B cells as drivers and suppressors of the immune responses, there is a need to trace the fate of endogenous alloreactive B cells under different transplant scenarios. We have previously reported that MHC Class I tetramers can be used to identify donor Class I reactive B cells in mice 7,25. However clinical literature implicates a strong pathogenic role for anti-donor MHC Class II antibodies, and that their presence alone or in combination with anti-Class I antibodies predict worse outcome compared to anti-Class I antibodies alone 26,27,28. Because MHC Class II antigens are expressed in a limited abundance on only a subset of cells, whereas Class I is expressed in abundance on all nucleated cells, there is a possibility that the fate of B cells specific for donor Class I and Class II may differ.
In this study we report on the fate of donor MHC Class II-reactive B cells in C57BL/6 mice during primary immunization, or after cardiac transplantation in naïve or sensitized recipients. To specifically detect donor-MHC Class II-reactive B cells, we used a set of I-Ed tetramers conjugated to PE or APC to identify the B cells specific for I-Ed away from those that recognize the fluorochrome 7,25,29. We selected the I-Ed tetramer presenting the FIEWNKLRFRQGLEW peptide because I-Ed is not expressed by C57BL/6 mice and FIEWNKLRFRQGLEW is a peptide from the mouse immunoglobulin heavy chain variable region, reasoning that these features may result in the detection of a larger frequency of responding B cells compared to I-Ad tetramers. These I-Ed tetramers were then used to enumerate and track the differentiation of endogenous I-Ed-reactive B cells into GC cells by flow cytometry, while immobilized I-Ed monomers were used in ELISA and ELISPOT assays to quantify circulating antibodies and the frequency of plasmablasts, respectively. By combining the tetramer approach with lineage tracking of memory B cells in activation-induced cytidine deaminase regulated Cre transgenic mice (AID-Cre) crossed to the ROSA26-EYFP reporter mice 30–32, we are able to demonstrate the kinetics of memory B cell generation and the ability of delayed CTLA4-Ig to inhibit the generation of memory I-Ed-specific B cells.
MATERIALS AND METHODS
Mice
C57BL/6 mice and BALB/c mice were purchased from Harlan Sprague Dawley, while BALB.B (C.B10-H2b/LilMcdJ), AID-Cre (B6;FVB-Tg(Aicda-cre)1Rcas/J) and ROSA26-EYFP mice (B6.129X1-Gt(ROSA)26Sor<tm1(EYFP)Cos>/J) were from Jackson Labs. All mouse procedures described in this study had been approved by the Institutional Animal Care and Use Committee (IACUC) of The University of Chicago.
Tetramers and Monomers
I-Ed- biotin monomers, I-Ed tetramers conjugated with phycoerythrin (PE) or allophycocyanin (APC) were from NIH Tetramer Core Facility (Atlanta, GA). The I-Ed was presenting the pCons-CDR1 (FIEWNKLRFRQGLEW) peptide. For the staining of 5 × 106 spleen and lymph node (LN) cells, saturating (0.1 μg) concentrations of I-Ed tetramer were used.
Antibodies and flow cytometry
Flow cytometry was performed with an LSRII or Fortessa (BD) and analyzed with Flowjo (Tree Star, Ashland, OR). All antibodies were purchased from Ebioscience, unless specified. For I-Ed-specific B cell staining, cells were preblocked at 4°C with 2.4G2, followed by incubation for 30 mins with lineage-specific antibodies (for dump channel: Anti-CD3 (17A2), anti-CD4 (GK1.5), anti-CD8α (53-6.7), anti-F4/80 (BM8, Biolegend), and anti-CD49b (DX5, Biolegend)), anti-B220 (RA3-6B.2), anti-IgD (11-26C.2a), anti-Fas (JO2, BD Pharmingen), and anti-GL7 (GL7), and followed by I-Ed tetramers.
Immunization
C57BL/6 mice were injected with 20 million BALB/c splenocytes in 4 sites on the flank. On day 10 after immunization, draining lymph nodes (LN) and nondraining LNs were collected (inguinal, axillary, brachial) respectively. In some experiments, separate groups of C57BL/6 mice were immunized with BALB/c or BALB.B splenocytes.
ELISpot Assay
For quantifying the frequency of anti-I-Ed IgG producing plasmablasts, plates were coated with streptavidin overnight at 37°C, washed, I-Ed-biotin monomers were added then washed and blocked with BSA or Superblock (Thermo, Cat. 37515) for 1 hour at room temperature. Splenocytes were seeded at 5 × 105 cells per well overnight at 37°C, then I-Ed-biotin monomers were added and incubated for 1 hour at room temperature. Plates were washed, Streptavidin-AP (Mabtech, Cat. 3310-10) and substrate added, and plates developed (Sigma, SIGMAFAST™ BCIP®/NBT Tablets). The plates were imaged on an ELISpot reader (ImmunoSpot Series Analyzer; Cellular Technology).
ELISA Assay
For quantifying the frequency of anti-I-Ed IgG producing ASCs, plate were coated with streptavidin overnight at 37°C, after several washes, I-Ed-biotin monomers were added and incubated for 1 hour at room temperature, plates were then blocked with superblock 1 hour at room temperature. To generate a standard curve, we used serum from acutely rejecting heart transplant recipients, arbitrarily assigning the titer of that serum to 10000 units. Sample sera were diluted (1:100) and incubated in I-Ed coated wells at room temperature for 2 hours, then plates were washed and anti-IgG conjugated to alkaline phosphatase (AP) (Jackson Immunoresearch, Cat. 115-055-071) was added. The substrate was used according to product information (Sigma, SIGMAFAST™ r-Nitrophenyl phosphate Tablets), and O.D values ere measured at 405 nm by a multiwall plate reader (BioRad).
Donor Specific Antibody (DSA) Assay
For DSA detection, serum were diluted (1:25) and incubated with 106 BALB/c splenocytes for 1 hour at 4°C, then the cells were washed and incubated with and B220 anti-IgG (Southern Biotech, Cat. 1030-02) for 30 minutes. Mean fluorescence intensity on the B220-negative cells were measured by flow cytometry.
Heart Transplantation
Hearts from BALB/c mice were transplanted into C57BL/6 recipients using techniques described previously 33.
CTLA4-Ig Immunosuppression
Immunized C57BL/6 mice were divided into 4 groups: (i) CTLA4-Ig D0+: CTLA4-Ig (ORENCIA [abatacept]; Bristol-Myers Squibb; 500 μg/mouse, intra-peritoneal injection) starting on Day 0–42; (ii) CTLA4-Ig D7+: CTLA4-Ig (500 μg/mouse) starting on Day 7–42; (iii) CTLA4-Ig D14+: CTLA4-Ig (500 μg/mouse) starting on Day 14–42; (iv) No Rx: Immunized but no treatment. All mice received a sub-cutaneous challenge of BALB/c splenocytes on day 56, and were bled at the indicated intervals. In some experiments, mice were sacrificed on day 42 post-immunization.
Statistical analysis
The statistical significance for differences in mean values, cell numbers, percentages was analyzed by student’s T test (unpaired) or ANOVA using Prism 5 (Graphpad, San Diego, CA). P values ≤ 0.05 were considered statistically significant.
Results
Tracking endogenous B cells with specificity for donor MHC Class II (I-Ed)
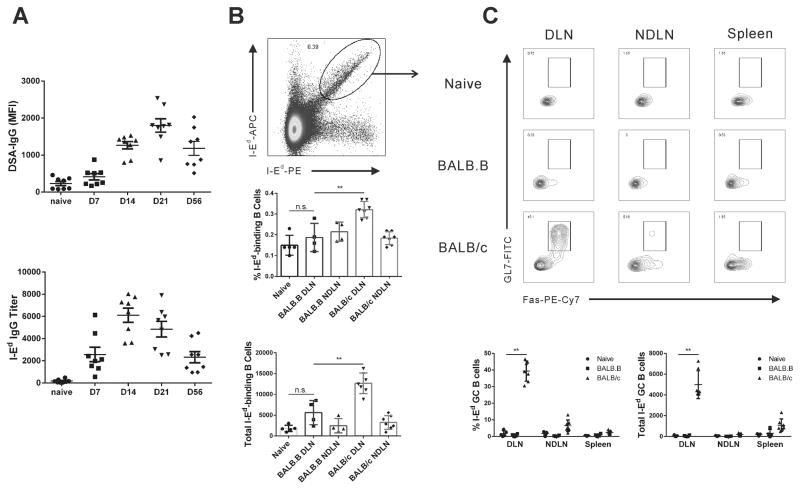
To identify I-Ed reactive B cells, we immunized C57BL/6 mice with 20×106 BALB/c splenocytes, injected subcutaneously in 4 sites on the flank, and confirmed the efficacy of immunization by testing for the presence of anti-BALB/c antibodies by flow cytometry and for anti-I-Ed antibody titers by ELISA (Fig 1A). Donor-specific IgG peaked on day 21 post-immunization whereas the anti-I-Ed specific antibodies peaked on day 14; both DSA and anti-I-Ed antibodies remained elevated at Day 56 post-immunization.
Figure 1. Kinetics of anti-BALB/c and anti-I-Ed antibody production and endogenous B cells in sensitized mice.
(A) C57BL/6 mice were sensitized with s.c. immunization with 20 million BALB/c splenocytes. Presence of anti-BALB/c antibodies was assessed by flow cytometry and anti-I-Ed antibody titers by ELISA. (B) C57BL/6 mice were injected with 20 million BALB/c or BALB.B splenocytes, and 10 days later, the total number of I-Ed-binding B cells was defined in the draining and non-draining lymph nodes (DLN, NDLN) of mice sensitized with BALB/c (I-Ed-positive) but not BALB.B (I-Ed-negative) splenocytes. (C) The percentage of I-Ed-binding B cells expressing a GC (Fas+GL7+) phenotype in the DLN, NDLN and spleen. Each dot represents an individual mouse, data are presented as mean ± SD, and statistically significant differences are indicated (*p<0.05; **p<0.01).
To trace the fate of anti-l-Ed B cells, C57BL/6 mice were immunized with 20×106 BALB/c (I-Ed positive) or BALB.B (I-Ed negative) splenocytes and were sacrificed 10 days later. The I-Ed-binding B cells were identified by staining with I-Ed-tetramers conjugated to APC and to PE (Fig 1B; Supplemental Figure 1), using a dual-fluorochrome, single tetramer method that we had previously described to detect anti-H-2Kd B cells 7,25. The percentage of I-Ed-binding B cells increased 2-fold and total number of I-Ed-binding B cells increased more than 7-fold in draining LN of mice sensitized with BALB/c (I-Ed-positive) compared to naïve controls, with no significant changes in the percentage and total numbers of I-Ed-binding B cells in the non-draining LNs or spleen. Within the draining LNs, a sizable percentage (44–48%) of I-Ed-binding B cells expressed a germinal center (GC; Fas+GL7+) phenotype, consistent with the primary response to alloantigen being a T cell- and GC-dependent response (Fig 1C). Importantly, the specificity of our method was demonstrated by not observing this pattern of expression in the few I-Ed-binding B cells of mice immunized with control BALB.B (I-Ed-negative) splenocytes (Fig 1B–C).
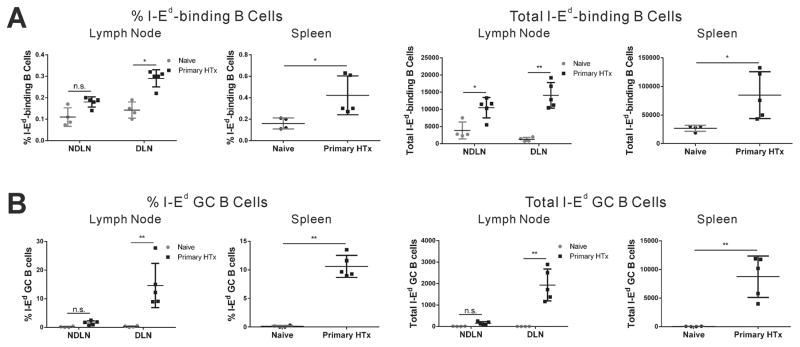
We next tracked I-Ed-binding B cells on day 10 after ectopic BALB/c heart transplantation into the abdominal cavity of C57BL/6 recipients. We observed significant 2–2.5 fold increases in the percentages of I-Ed-binding B cells in the draining mediastinal LNs and spleen. More impressive was when the total number of I-Ed-binding B cells were quantified; heart transplantation resulted in ~10–20 fold and ~3–4 fold increase in the total numbers I-Ed-binding B cells in the draining mediastinal LNs and spleen respectively (Fig 2A). Approximately 10–15% of the I-Ed-binding B cells in the spleen and draining LNs acquired a GC phenotype (Fig 2B). Thus compared to sub-cutaneous injection to the flank, the anti-I-Ed B cell response to the cardiac allograft was more systemic, but the differentiation into GC B cells was more modest (10–15% in transplant vs. 44–48% in immunization, of I-Ed-binding B cells with a GC phenotype in the draining LN; Fig 2B).
Figure 2. Tracking Class II-binding endogenous B cells in heart transplanted mice.
On day 10 after heterotopic BALB/c heart transplant into the abdomen of C57BL/6 recipients, a significant increase in the total number (A) and percentage of I-Ed-binding B cells with a GC phenotype (B) in the draining LN (mediastinal; DLN), non-draining peripheral LNs (NDLN) and spleen. Each dot represents an individual mouse, data are presented as mean ± SD, and statistically significant differences are indicated (*p<0.05; **p<0.01).
Tracking endogenous B cells with specificity for donor MHC Class II (I-Ed) in sensitized recipients
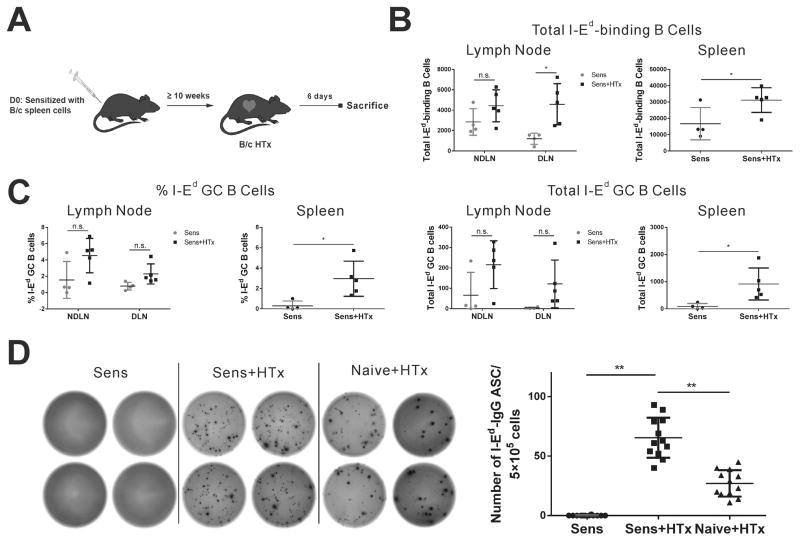
Clinical data suggest that the propensity to mount an alloantibody response posttransplantation is significantly higher in sensitized recipients compared to nonsensitized recipients maintained on standard immunosuppression 34–37. These data report on differences in susceptibility to immunosuppression in the primary vs. secondary alloantibody response, which could be explained by the increased frequency of memory T cells, memory B cells or plasma cells 6. To understand the fate of donor Class II-reactive B cells, C57BL/6 mice were sensitized with BALB/c spleen cells ≥10 weeks prior to challenge with a BALB/c heart transplantation (Fig 3A). The B cell response was measured at day 6 post-transplant, because sensitized mice display faster rejection kinetics that is associated with a transient anti-H-2Kd plasmablast response which peaks on day 6–7 and returns to baseline by day 14 7.
Figure 3. A plasmablast response predominates in sensitized mice receiving BALB/c heart transplantation.
(A) Experimental design of BALB/c heart transplantation into sensitized C57B/6 recipients. (B) Total number of I-Ed double positive B cells in LNs and spleen at day 6 after transplantation. (C) Only 1–7% of I-Ed double binding B cells in sensitized mice had a germinal center (Fas+GL7+) phenotype. (D) ELISPOT quantifying strong I-Ed-specific plasmablast response in sensitized HTx mice. Each dot represents an individual mouse, data are presented as mean ± SD, and statistically significant differences are indicated (*p<0.05; **p<0.01).
BALB/c heart transplantation into sensitized mice induced a significant increase in the total number of anti-I-Ed B cells in the draining LN and spleen but not in distal LNs (Fig 3B). In contrast, a GC response was not significantly detected in the draining LN and only ~2.5% of the total anti-I-Ed B cells in the spleen acquired a GC phenotype (Fig 3C). Consistent with the observations reported for anti-H-2Kd B cells 7. BALB/c heart transplant into sensitized recipients induced a rapid differentiation of B cells into plasmablasts, detected in an I-Ed IgG ELISPOT assay (Fig 3D). Plasmablast responses were also observed in naïve recipients of BALB/c hearts by day 10 post-transplantation, although they were markedly reduced compared to the response in sensitized recipients (Fig 3D). Taken collectively, our data demonstrate that the recall anti-I-Ed response in sensitized mice results in a rapid differentiation of pre-existing memory B cells into antibody-secreting cells. We note that the increase in total numbers of I-Ed B cells following heart transplantation into sensitized recipients was more modest compared to naïve recipients, however, this difference could be due to the different days posttransplant the analysis was performed, namely after 6 versus 10 days respectively, or to the B cells in sensitized mice rapidly differentiating into plasmablasts and down-regulating the membrane B cell receptor, thus precluding their detection by the I-Ed tetramers.
Kinetics of endogenous memory B cell generation during sensitization
Studies with model antigens have suggested that memory B cells are generated in 2 waves; early pre-GC wave that is associated with lower affinity memory B cells, and a later post-GC wave that is associated with higher affinity, class-switched memory B cells 29,30,38–41. Because the generation of the high-affinity memory B cells requires a successful GC response, delayed administration of reagents such as anti-CD154, administered on day 3–6 postimmunization, were shown to allow the generation of early pre-GC B cells but inhibit the output of post-GC memory B cells 41. We showed that CTLA4-Ig has a comparable ability as anti-CD154 to disrupt ongoing GC responses when administered at day 7 postimmunization with allogeneic BALB/c spleen cells in C57BL/6 mice 25, and here hypothesized that delayed CTLA4-Ig would also inhibit the generation of memory I-Ed-binding B cells.
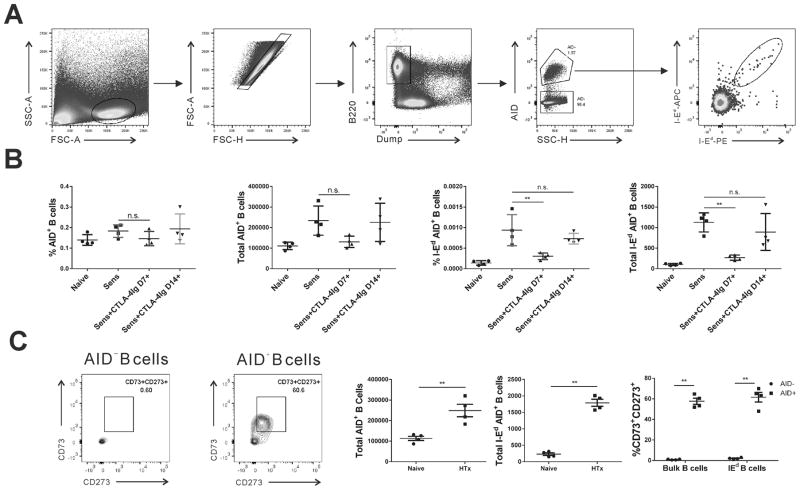
We quantified the frequency of anti-I-Ed memory B cells at day 30 postimmunization in 4 (with the naïve) experimental groups, immunized and treated with CTLA4-Ig from day 7 or from day 14, or immunized untreated and naïve controls. To identify memory B cells, we used bacterial artificial chromosome (BAC)-transgenic mice that express the Cre recombinase from the Aicda locus (AID protein; AID-Cre; Aicdatm1(cre)Mnz). These mice were bred with ROSA26-EYFP reporter mice such that EYFP is stably expressed in B cells upon Cre-mediated deletion of the floxed neomycin stop cassette 30,31. Thus, because all B cells responding to antigen and receiving T cell help will have expressed AID (and the Cre transgene), memory B cells will be unambiguously discriminated from naïve I-Ed-binding B cells. Total memory as well as the I-Ed-binding B cells were identified as resting B220+YFP+CD138−CD95low B cells and the gating strategy is illustrated in Fig 4A.
Figure 4. Tracking the changes in the percentage and total number of total and I-Ed memory B cells in AID-Cre-Rosa26-stop-EYFP mice.
(A) Detailed gating strategy of memory B cells. (B) The percentage and total number of AID+ splenic B cells was not significantly different between the experimental and control naïve groups (p>0.05). (C) Mice receiving CTLA4-Ig from day 7 demonstrated reduced percentage and total number of I-Ed-specific AID+ memory B cells at day 42 postimmunization, compared to untreated sensitized mice. In contrast, delayed treatment with CTLA4-Ig from day 14–42 failed to show the significance when compared with no treatment sensitized mice. (C) Naïve and C57BL/6 mice at >10 weeks post-BALB/c heart transplant, the majority of AID+ B cells, for both bulk and I-Ad-specific, were CD73+CD273+, whereas AID− B cells were CD73−CD273−. Each dot represents an individual mouse, data are presented as mean ± SD, and statistically significant differences are indicated (**p<0.01).
We observed a significant increase in both the percentage and total number of I-Ed-specific AID+ memory B cells at day 42 post-BALB/c immunization compared to naïve, accompanied by an increase in tddhe percentage and number of total AID+ memory B cells (Fig 4B); however the latter did not reach statistical significance. In the day 7–42 CTLA4-Ig treated group, we observed a significant reduction in the percentage and number of I-Ed-specific memory B cells, whereas there was no significant reduction when CTLA4-Ig was administered from day 14–42 postimmunization (Fig 4B). Thus, the majority of the I-Ed-specific memory B cells, marked by AID expression in this genetic lineage-tracing experiment, were mostly generated within the 7–14 day window after splenocyte immunization. Finally, we demonstrate that the total numbers of bulk as well as I-Ed-binding AID+ B cells were significantly increased at >10 weeks post-BALB/c heart transplantation, and were predominantly CD73+CD273+ (Fig 4C), consistent with their post-germinal center fate and with our previous report of memory IgG+ B cells 7.
Recall B cell responses is inhibited by delayed CTLA4-Ig administration
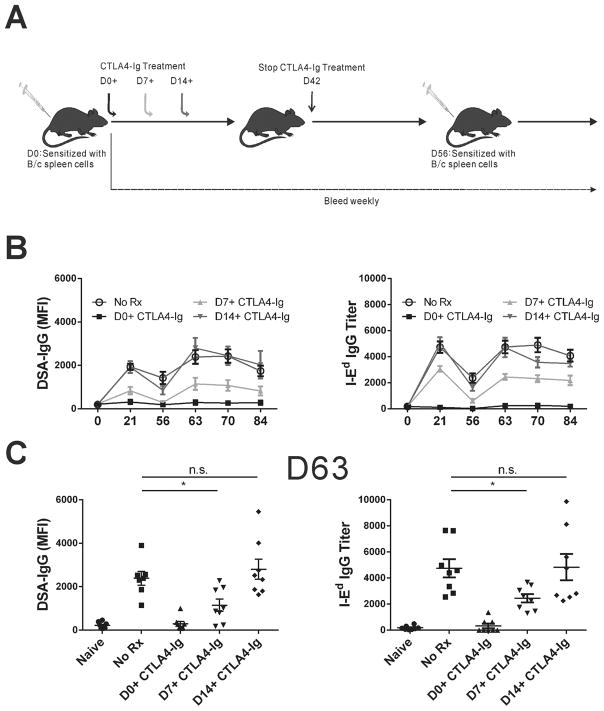
We next tested whether the quantification of the frequency of memory B cells early postsensitization would accurately predict the magnitude of the recall antibody response. In 3 groups of mice, we initiated CTLA4-Ig treatment on days 0, 7 or 14 postimmunization with BALB/c splenocytes and treatment was continued 2-times per week till day 42 (Fig 5A). Two weeks after cessation of CTLA4-Ig, the mice were challenged with BALB/c splenocytes and bled weekly to quantify DSA levels as well as anti-I-Ed titers (Fig 5B–C). Treatment with CTLA4-Ig from the day of immunization completely inhibited any DSA or anti-I-Ed production and prevented humoral responses when re-challenged with BALB/c splenocytes in the absence of CTLA4-Ig, consistent with the induction of immunological tolerance. In contrast, initiating CTLA4-Ig treatment on day 14 postimmunization had no significant effect, and the antibody responses were indistinguishable from untreated controls. These observations confirmed that the 2-week period following cessation of CTLA4-Ig treatment effectively cleared circulating CTLA4-Ig titers to a level that permitted normal recall antibody responses. Notably, the recall response in mice treated with CTLA4-Ig from day 7 was significantly reduced compared to both untreated controls and day 14+ CTLA-4 treated group. As illustrated for the day 63 time-point (14 days after challenge), this group of mice exhibited a 2.4-fold reduction in MFI for DSA and a 7.5-fold reduction in anti-I-Ed titers compared to untreated controls (Fig 5C). Thus delayed CTLA4-Ig significantly reduced the pool of memory anti-I-Ed–binding B cells that predicted the diminished recall anti-I-Ed antibody response.
Figure 5. Kinetics of anti-BALB/c and anti-I-Ed antibody production during the recall response.
A) Experimental design of recall response with CTLA4-Ig treatment. (B) Presence of anti-BALB/c antibodies was assessed by flow cytometry and anti-I-Ed antibody titers by ELISA. (C) On day 63, 1 week after recall, CTLA4-Ig D14+ mice showed similar I-Ed-specific antibody response compared to untreated mice, whereas CTLA4-Ig D7+ mice showed significantly reduced titer of I-Ed-specific antibodies. Each dot represents an individual mouse, data are presented as mean ± SD, and statistically significant differences are indicated (*p<0.05).
Discussion
Identification of B cells in peripheral blood with the potential to produce DSA may provide a complementary way of analyzing humoral responses at any given time in the posttransplant period. While solid-phase assays are now considered the gold standard for detecting donor-specific antibodies in the clinic, there remains significant limitations, including low or un-detectable DSA due to sequestration of antibodies in the graft, as well as high titers of DSA giving false negative results due to C1q interference 42. In addition, recent studies that mapped the repertoire of circulating antibodies and B cell repertoires suggest that only a small fraction (<5%) of memory B cells gave rise to bone marrow long-lived plasma cells responsible for serological memory 43. These observations raise the possibility that memory B cells may complement DSA measurements in assessing the sensitized allo-specific B cell response, and might even prove more sensitive.
It has become increasingly evident that memory alloreactive B cells play critical roles in the development of anti-donor HLA antibody responses upon transplantation of allografts in sensitized patients, and that such patients with circulating antibodies have much poorer outcomes compared to patients who do not develop antibodies, including those that had biopsy-confirmed acute rejection 1–5. Thus there is a need to understand the kinetics of memory B cell formation so that interventions that prevent memory B cell generation and their subsequent reactivation can be rationally designed. However, the interrogation of quiescent memory B cells in mice has been challenged by their low numbers, heterogeneity and the lack of a definitive cell surface marker. In humans, CD27 is used as a marker of memory B cells, but is present at very low levels on murine memory B cells 44. A number of approaches have been used to identify memory B cells in mice. A common approach has been to track class-switched IgG+ B cells in mice that had been immunized and rested 45,46, while others mark B cells during the early activation phase with a 5-bromo-2′-deoxyuridine (BrdU) and then identify the memory antigen-specific BrdU+ cells many weeks later 47,48. More recently, a genetic lineage-tracing approach has been used to identify B cells that had participated in the primary response by marking cells that have upregulated the Aicda gene; a feature of all antigen engaged and helped B cells 30,31. Using this approach, Dogan et al 30 reported that AID+ B cell memory appeared in several subsets comprising both IgM+ and IgG1 positive B cells that persisted for up to 8 months postimmunization. Subsequently, studies from the Jenkins laboratory demonstrated that IgM and IgG memory cells have distinct properties, and that unswitched memory B cells were generated early in the primary response whereas the isotype-switched memory B cells arose from the GC 29,39. Unswitched IgM+ memory B cells persisted for much longer times than IgG switched memory B cells, but in the presence of circulating antibodies, only switched memory B cells responded by differentiating primarily into plasmablasts. That memory B cells emerge as 2 distinct waves was confirmed by Kaji et al 41, using a different approach where the transcription factor bcl-6 is deleted in B cells and in T cells to prevent GC reaction. Post-GC memory B cells comprised cells capable of secreting somatically mutated high-affinity antibodies whereas the pre-GC memory B cells tended to have preserved unmutated variable genes that may be more relevant for responding to antigenic variants of invading pathogens. Those studies suggest that heterogeneity of memory B cells allows for flexibility in mounting the most robust type of protection.
We have previously identified memory donor-H-2Kd reactive B cells through their expression of IgG in long-term quiescent C57BL/6 mice following immunization with BALB/c spleen cells 7. However to be able to more broadly capture both switched and unswitched memory B cells, we used the approach of tracking quiescent B cells that had previously activated AID (and Cre) expression, and as a consequence became marked by EYFP expression. Coupling this approach with a delay in the start of CTLA4-Ig treatment to 7 days after sensitization, we showed that the generation of memory B cells occurred primarily between day 7–14 postimmunization. A reduction in frequency of I-Ed-binding memory B cells predicted markedly diminished recall donor- and I-Ed-specific antibody responses. Taken together, these data provide a timeframe whereby therapy with CTLA4-Ig may prevent humoral sensitization, and suggest that quantifying the frequency of donor-specific memory B cell may complement assessment of circulating DSA titers to predict the strength of the recall antibody response.
In summary, our study extends our previous studies with H-2Kd tetramers to report on the use of I-Ed tetramers to trace the fate of donor MHC Class II specific B cells. Despite profound difference in the magnitude of MHC Class I and Class II expression by the allograft, B cells recognizing donor Class I and Class II B cells respond comparably to cardiac allografts by differentiation into GC B cells in naïve recipients, and into plasmablasts in sensitized recipients. By combining this visualization approach with lineage tracing of memory B cells in AID-cre x Rosa26-StopfloxEYFP recipients, we observed that I-Ed-specific memory B cells were predominantly generated between days 7–14 postsensitization, thus providing insights into the timeframe whereby memory B cell generation can be prevented with CTLA4-Ig.
Supplementary Material
Acknowledgments
This work was supported in part by grants (1R01AI110513, P01AI097113) from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health. JSY received an American Heart Association post-doctoral fellowship award (15POST25700452) and was funded by a Respiratory Biology Training Grant (T32 HL07605).
MHC Class II tetramers were provided by the NIH Tetramer Core Facility (contract HHSN272201300006C).
Abbreviations
- ABMR
antibody-mediated rejection
- AID
activation-induced cytidine deaminase
- AP
alkaline phosphatase
- APC
allophycocyanin
- BrdU
5-bromo-2′-deoxyuridine
- DSA
donor-specific antibodies
- EYFP
enhanced yellow fluorescent protein
- FCS
fetal calf serum
- GC
germinal center
- HLA
human leukocyte antigen
- LN
lymph node
- PE
phycoerythrin
- MHC
Major histocompatibility complex
- Tfh
T follicular helper cell
Footnotes
Author contribution
Jinghui Yang: Conducted the experiments and performed the data analysis, and assisted in the writing of the manuscript.
Jianjun Chen: Developed the tetramer-staining protocol and supervised Jinghui Yang in all aspects of the B cell and data analysis.
James Young: Performed some of the memory B cell experiments and DSA quantification.
Qiang Wang: Maintained the mouse colony and genotyped the donor and recipient mice used in the experiments.
Dengping Yin: Performed all the transplants described in the study.
Roger Sciammas: Participated in the design of the study and edited the manuscript.
Anita S Chong: Conceived of the project, supervised the studies and data analysis, wrote and edited the manuscript.
The authors declare no conflicts of interest.
References
- 1.Gosset C, Lefaucheur C, Glotz D. New insights in antibody-mediated rejection. Curr Opin Nephrol Hypertens. 2014;23(6):597–604. doi: 10.1097/MNH.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 2.Halloran PF, Chang J, Famulski K, et al. Disappearance of T Cell-Mediated Rejection Despite Continued Antibody-Mediated Rejection in Late Kidney Transplant Recipients. J Am Soc Nephrol. 2015;26(7):1711–1720. doi: 10.1681/ASN.2014060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halloran PF, Reeve JP, Pereira AB, Hidalgo LG, Famulski KS. Antibody-mediated rejection, T cell-mediated rejection, and the injury-repair response: new insights from the Genome Canada studies of kidney transplant biopsies. Kidney Int. 2014;85(2):258–264. doi: 10.1038/ki.2013.300. [DOI] [PubMed] [Google Scholar]
- 4.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Rates and Determinants of Progression to Graft Failure in Kidney Allograft Recipients With De Novo Donor-Specific Antibody. Am J Transplant. 2015;15(11):2921–2930. doi: 10.1111/ajt.13347. [DOI] [PubMed] [Google Scholar]
- 5.Arias M, Rush DN, Wiebe C, et al. Antibody-mediated rejection: analyzing the risk, proposing solutions. Transplantation. 2014;98(Suppl 3):S3–21. doi: 10.1097/TP.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 6.Chong AS, Sciammas R. Memory B cells in transplantation. Transplantation. 2015;99(1):21–28. doi: 10.1097/TP.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Wang Q, Yin D, Vu V, Sciammas R, Chong AS. Cutting Edge: CTLA-4Ig Inhibits Memory B Cell Responses and Promotes Allograft Survival in Sensitized Recipients. J Immunol. 2015;195(9):4069–4073. doi: 10.4049/jimmunol.1500940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng Q, Ng YH, Singh T, et al. B cells mediate chronic allograft rejection independently of antibody production. J Clin Invest. 2014;124(3):1052–1056. doi: 10.1172/JCI70084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liarski VM, Kaverina N, Chang A, et al. Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci Transl Med. 2014;6(230):230ra246. doi: 10.1126/scitranslmed.3008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marino J, Paster JT, Trowell A, et al. B Cell Depletion With an Anti-CD20 Antibody Enhances Alloreactive Memory T Cell Responses After Transplantation. Am J Transplant. 2015 doi: 10.1111/ajt.13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeung MY, Ding Q, Brooks CR, et al. TIM-1 signaling is required for maintenance and induction of regulatory B cells. Am J Transplant. 2015;15(4):942–953. doi: 10.1111/ajt.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lal G, Nakayama Y, Sethi A, et al. Interleukin-10 From Marginal Zone Precursor B-Cell Subset Is Required for Costimulatory Blockade-Induced Transplantation Tolerance. Transplantation. 2015;99(9):1817–1828. doi: 10.1097/TP.0000000000000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JI, Rothstein DM, Markmann JF. Role of B cells in tolerance induction. Curr Opin Organ Transplant. 2015;20(4):369–375. doi: 10.1097/MOT.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherukuri A, Rothstein DM, Clark B, et al. Immunologic human renal allograft injury associates with an altered IL-10/TNF-alpha expression ratio in regulatory B cells. J Am Soc Nephrol. 2014;25(7):1575–1585. doi: 10.1681/ASN.2013080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Q, Yeung M, Camirand G, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121(9):3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KM, Stott RT, Zhao G, et al. TGF-beta-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J Immunol. 2014;44(6):1728–1736. doi: 10.1002/eji.201344062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KM, Kim JI, Stott R, et al. Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B cells. Am J Transplant. 2012;12(8):2072–2078. doi: 10.1111/j.1600-6143.2012.04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durand J, Huchet V, Merieau E, et al. Regulatory B Cells with a Partial Defect in CD40 Signaling and Overexpressing Granzyme B Transfer Allograft Tolerance in Rodents. J Immunol. 2015;195(10):5035–5044. doi: 10.4049/jimmunol.1500429. [DOI] [PubMed] [Google Scholar]
- 19.Chesneau M, Michel L, Degauque N, Brouard S. Regulatory B cells and tolerance in transplantation: from animal models to human. Front Immunol. 2013;4:497. doi: 10.3389/fimmu.2013.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Texier L, Thebault P, Lavault A, et al. Long-term allograft tolerance is characterized by the accumulation of B cells exhibiting an inhibited profile. Am J Transplant. 2011;11(3):429–438. doi: 10.1111/j.1600-6143.2010.03336.x. [DOI] [PubMed] [Google Scholar]
- 21.Sagoo P, Perucha E, Sawitzki B, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1848–1861. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newell KA, Asare A, Kirk AD, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newell KA, Asare A, Sanz I, et al. Longitudinal Studies of a B Cell-Derived Signature of Tolerance in Renal Transplant Recipients. Am J Transplant. 2015;15(11):2908–2920. doi: 10.1111/ajt.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newell KA, Chong AS. Making a B-line for transplantation tolerance. Am J Transplant. 2011;11(3):420–421. doi: 10.1111/j.1600-6143.2010.03429.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Yin H, Xu J, et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. Am J Transplant. 2013;13(9):2280–2292. doi: 10.1111/ajt.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willicombe M, Moss J, Moran L, et al. Tubuloreticular Inclusions in Renal Allografts Associate with Viral Infections and Donor-Specific Antibodies. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015050478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiebe C, Nevins TE, Robiner WN, Thomas W, Matas AJ, Nickerson PW. The Synergistic Effect of Class II HLA Epitope-Mismatch and Nonadherence on Acute Rejection and Graft Survival. Am J Transplant. 2015;15(8):2197–2202. doi: 10.1111/ajt.13341. [DOI] [PubMed] [Google Scholar]
- 28.DeVos JM, Gaber AO, Knight RJ, et al. Donor-specific HLA-DQ antibodies may contribute to poor graft outcome after renal transplantation. Kidney Int. 2012;82(5):598–604. doi: 10.1038/ki.2012.190. [DOI] [PubMed] [Google Scholar]
- 29.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331(6021):1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dogan I, Bertocci B, Vilmont V, et al. Multiple layers of B cell memory with different effector functions. Nature Immunol. 2009;10(12):1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 31.Crouch EE, Li Z, Takizawa M, et al. Regulation of AID expression in the immune response. J Exp Med. 2007;204(5):1145–1156. doi: 10.1084/jem.20061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aiba Y, Kometani K, Hamadate M, et al. Preferential localization of IgG memory B cells adjacent to contracted germinal centers. Proc Natl Acad Sci U S A. 2010;107(27):12192–12197. doi: 10.1073/pnas.1005443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DY, ND, LM, et al. IFN-gamma production is specifically regulated by IL-10 in mice made tolerant. J Immunol. 2003;170(2):853–860. doi: 10.4049/jimmunol.170.2.853. [DOI] [PubMed] [Google Scholar]
- 34.Lucia M, Luque S, Crespo E, et al. Preformed circulating HLA-specific memory B cells predict high risk of humoral rejection in kidney transplantation. Kidney Int. 2015;88(4):874–887. doi: 10.1038/ki.2015.205. [DOI] [PubMed] [Google Scholar]
- 35.Mattiazzi AD, Centeno A, Amador A, et al. Highly Sensitized Patients: Miami Transplant Institute Experience. Clin Transpl. 2014:171–178. [PubMed] [Google Scholar]
- 36.Vo AA, Sinha A, Haas M, et al. Factors Predicting Risk for Antibody-mediated Rejection and Graft Loss in Highly Human Leukocyte Antigen Sensitized Patients Transplanted After Desensitization. Transplantation. 2015;99(7):1423–1430. doi: 10.1097/TP.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 37.Jordan SC, Choi J, Vo A. Kidney transplantation in highly sensitized patients. Br Med Bull. 2015;114(1):113–125. doi: 10.1093/bmb/ldv013. [DOI] [PubMed] [Google Scholar]
- 38.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med. 2006;203(2):305–310. doi: 10.1084/jem.20052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med. 2012;209(3):597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kometani K, Nakagawa R, Shinnakasu R, et al. Repression of the transcription factor Bach2 contributes to predisposition of IgG1 memory B cells toward plasma cell differentiation. Immunity. 2013;39(1):136–147. doi: 10.1016/j.immuni.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Kaji T, Ishige A, Hikida M, et al. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J Exp Med. 2012;209(11):2079–2097. doi: 10.1084/jem.20120127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tambur AR, Herrera ND, Haarberg KM, et al. Assessing Antibody Strength: Comparison of MFI, C1q, and Titer Information. Am J Transplant. 2015;15(9):2421–2430. doi: 10.1111/ajt.13295. [DOI] [PubMed] [Google Scholar]
- 43.Lavinder JJ, Horton AP, Georgiou G, Ippolito GC. Next-generation sequencing and protein mass spectrometry for the comprehensive analysis of human cellular and serum antibody repertoires. Curr Opin Chem Biol. 2015;24:112–120. doi: 10.1016/j.cbpa.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Xiao Y, Hendriks J, Langerak P, Jacobs H, Borst J. CD27 is acquired by primed B cells at the centroblast stage and promotes germinal center formation. J Immunol. 2004;172(12):7432–7441. doi: 10.4049/jimmunol.172.12.7432. [DOI] [PubMed] [Google Scholar]
- 45.Ridderstad A, Tarlinton DM. Kinetics of establishing the memory B cell population as revealed by CD38 expression. J Immunol. 1998;160(10):4688–4695. [PubMed] [Google Scholar]
- 46.Lalor PA, Nossal GJ, Sanderson RD, McHeyzer-Williams MG. Functional and molecular characterization of single, (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific, IgG1+ B cells from antibody-secreting and memory B cell pathways in the C57BL/6 immune response to NP. Eur J Immunol. 1992;22(11):3001–3011. doi: 10.1002/eji.1830221136. [DOI] [PubMed] [Google Scholar]
- 47.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J Immunol. 2010;185(12):7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204(9):2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.