Abstract
Social play is a fundamental aspect of behavioral development in many species. Social play deprivation in rats alters dendritic morphology in the ventromedial prefrontal cortex (vmPFC) and we have shown that this brain region regulates responses to social defeat stress in Syrian hamsters. In this study, we tested whether play deprivation during the juvenile period disrupts dendritic morphology in the prefrontal cortex and potentiates the effects of social defeat stress. At weaning, male hamsters were either group-housed with peers or pair-housed with their mother, with whom they do not play. In adulthood, animals received acute social defeat stress or no-defeat control treatment. The hamsters were then tested for a conditioned defeat response in a social interaction test with a novel intruder, and were also tested for social avoidance of a familiar opponent. Brains were collected for Golgi-Cox staining and analysis of dendritic morphology in the infralimbic (IL), prelimbic (PL), and orbitofrontal cortex (OFC). Play-deprived animals showed an increased conditioned defeat response and elevated avoidance of a familiar opponent compared to play-exposed animals. Also, play-deprived animals showed increased total length and branch points in apical dendrites of pyramidal neurons in the IL and PL cortices, but not in the OFC. These findings suggest that social play deprivation in juvenile hamsters disrupts neuronal development in the vmPFC and increases vulnerability to the effects of social stress in adulthood. Overall, these results suggest that social play is necessary for the natural dendritic pruning process during adolescence and promotes coping with stress in adulthood.
Keywords: social play, social defeat, aggression, infralimbic cortex, prelimbic cortex
Social play is a common, though not universal, characteristic of the early life of most mammals and typically peaks during the juvenile period (Burghardt, 2005). Social play has long been considered beneficial to animals, including humans, although the specific benefits of play have been difficult to quantify (Martin & Caro, 1985; Pellis & Pellis, 2009). Play might serve to facilitate muscle growth and development of the motor system, strengthen social relationships, and enable practice of adult activities such as aggression, sex, and hunting (Graham & Burghardt, 2010). One intriguing possibility is that social play causes structural changes in the central nervous system that enable individuals to display appropriate, flexible, and adaptive behavior in adulthood (Pellis, Pellis, & Bell, 2010).
The primary brain regions responsible for the expression of play behavior and the motivation to play are subcortical (Siviy & Panksepp, 2011; Vanderschuren, Niesink, & Van Ree, 1997). It is noteworthy that decorticate rats are still able to perform the full array of play behavior exhibited by healthy control rats (Panksepp, Normansell, Cox, & Siviy, 1994; Pellis, Pellis, & Whishaw, 1992). The cortex, however, regulates those social aspects of play that allow animals to respond appropriately in specific contexts. Rats with lesions of the primary motor cortex do not exhibit the developmental changes in play behavior characteristic of healthy control animals (Kamitakahara, Monfils, Forgie, Kolb, & Pellis, 2007). Rats with lesions of the orbitofrontal cortex (OFC) fail to modify both their play and non-play behavior with respect to the identity of their social partner (Pellis et al., 2006). Also, ablation of the medial prefrontal cortex (mPFC) leads to a shift toward less complex defensive tactics, which functions to shorten individual bouts of play (Bell, McCaffrey, Forgie, Kolb, & Pellis, 2009). Likewise, animals that are deprived of social play during the juvenile period show impaired dendritic architecture in the prefrontal cortex, although subtle differences exist in the OFC and mPFC. Exposure to social play is necessary for pruning apical dendrites in the mPFC, whereas the experience of playing with multiple social partners promotes the proliferation of basal dendrites in the OFC (Bell, Pellis, & Kolb, 2010). The functional consequences of atypical dendritic morphology in the prefrontal cortex of play-deprived animals remain to be determined.
Early life social isolation leads to several long-term behavioral deficits that are likely mediated by social play deprivation. Isolated rats exhibit reduced affiliative behavior with conspecifics in adulthood (Hol, Van den Berg, Van Ree, & Spruijt, 1999), increased anxiety-like behavior in an elevated plus maze (Da Silva, Ferreira, Carobrez Ade, & Morato, 1996), and increased behavioral rigidity in a rule-learning task (Jones, Marsden, & Robbins, 1991). Also, when isolated rats are exposed to an aggressive animal in adulthood they are slower to submit than their non-isolated counterparts and they fail to show normal avoidant behavior after being socially defeated (van den Berg et al., 1999; Von Frijtag, Schot, van den Bos, & Spruijt, 2002). While rearing animals in isolation during the juvenile period results in the loss of many social experiences in addition to social play, several lines of evidence indicate that play deprivation is responsible for the deficits. For instance, the consequences of social isolation are attenuated when juvenile rats are given the opportunity to play with peers for one hour per day (Einon, Morgan, & Kibbler, 1978). In Syrian hamsters, at least two hours of social interaction per day may be necessary to compensate for the effects of social isolation (Vieira, Garcia, Rau, & Prado, 2005). Also, exposure to adults or drugged peers that do not play fails to reduce the motivation to play in juvenile rats, as well as the adverse effects of social isolation (Bean & Lee, 1991; Panksepp, Siviy, & Normansell, 1984). Because play deprivation is known to disrupt dendritic morphology in the prefrontal cortex, it is possible that many of the consequences of play deprivation are the result of improper development of the prefrontal cortex.
Broadly, the prefrontal cortex is known to regulate executive function, including the inhibition of inappropriate emotions, actions, and thoughts (Goldman-Rakic, 1996). The OFC contributes to impulse control and decision making in a delayed discounting procedure in which rats are given the choice of small immediate or large delayed rewards (Zeeb, Floresco, & Winstanley, 2010). Neural activity in the OFC also inhibits impulsive aggressive behavior in rats (de Bruin, van Oyen, & Van de Poll, 1983), monkeys (Izquierdo, Suda, & Murray, 2005), and humans (Mehta & Beer, 2010). Likewise, neural activity in the mPFC provides inhibitory control over conditioned fear (Do-Monte, Manzano-Nieves, Quinones-Laracuente, Ramos-Medina, & Quirk, 2015; Giustino & Maren, 2015), the neuroendocrine stress response (Radley, Gosselink, & Sawchenko, 2009), acquisition of anxiety and depression-like behavior (Vialou et al., 2014), and drug seeking behavior (Britt et al., 2012). Ventral portions of the mPFC (vmPFC) have also been implicated in behavioral and physiological responses to social defeat stress (Chen et al., 2015; Vialou, et al., 2014). In Syrian hamsters, acute social defeat stress leads to a dramatic shift from normal territorial aggression to submissive and defensive behavior during subsequent social interaction testing (Huhman et al., 2003). This defeat-induced change in agonistic behavior is called the conditioned defeat response. Pharmacological inactivation of the vmPFC prior to social defeat stress has been shown to increase the acquisition of the conditioned defeat response (Markham, Luckett, & Huhman, 2012). Similarly, we have shown that dominant hamsters exhibit both a reduced conditioned defeat response and increased c-Fos immunoreactivity in subregions of the vmPFC, the prelimbic (PL) cortex and infralimbic (IL) cortex, compared to subordinates and controls (Morrison et al., 2014; Morrison, Curry, & Cooper, 2012). Furthermore, pharmacological inactivation of the vmPFC reinstated the conditioned defeat response in dominant hamsters, but did not alter the conditioned defeat response in subordinates and controls (Morrison, Bader, McLaughlin, & Cooper, 2013). Overall, these findings are consistent with the view that neural activity in the PL and IL cortices promote coping and stress resilience (Christianson et al., 2014; Kumar et al., 2014; Lehmann & Herkenham, 2011).
The goal of this study was to determine whether social play exposure during the juvenile period in Syrian hamsters is necessary for normal neuronal development in the prefrontal cortex and buffers the effects of social defeat stress in adulthood. We hypothesized that play deprivation would increase the length and branch points of apical dendrites in the PL and IL cortices and decrease the length and branch points of basal dendrites in the OFC. In addition, we hypothesized that play deprivation would potentiate the conditioned defeat response. Female hamsters rarely play with their offspring and their presence does not compensate for the lack of social play with peers (Guerra, Takase, & de, 1999). Consequently, our method of social play deprivation involved pair-housing juvenile animals with their mother for several weeks after weaning other littermates. This treatment thus produced social play deprivation without disrupting other social interactions.
Methods
Subjects
Subjects were male Syrian hamsters (Mesocricetus auratus) obtained from our breeding colony that was derived from hamsters purchased from Charles River Laboratories (Wilmington, MA). All animals were housed in polycarbonate cages (12 cm × 27 cm × 16 cm) with corncob bedding, cotton nesting materials, and wire mesh tops. Animals were provided environmental enrichment, such as plastic shelters and paper cups, although enrichment objects were removed prior to social defeat stress. Food and water were available ad libitum. Subjects were individually housed one week prior to social defeat exposure and were handled repeatedly to habituate them to the stress of human contact. Also, cages were not changed during the week prior to social defeat to allow subjects to scent mark their territory. Animals were housed in a temperature-controlled colony room (20 ± 2 °C) and maintained on a 14:10 h light:dark cycle. All behavioral testing was performed during the first 3 h of the dark phase of their light cycle. All procedures were approved by the University of Tennessee Institutional Animal Care and Use Committee and are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Play Deprivation
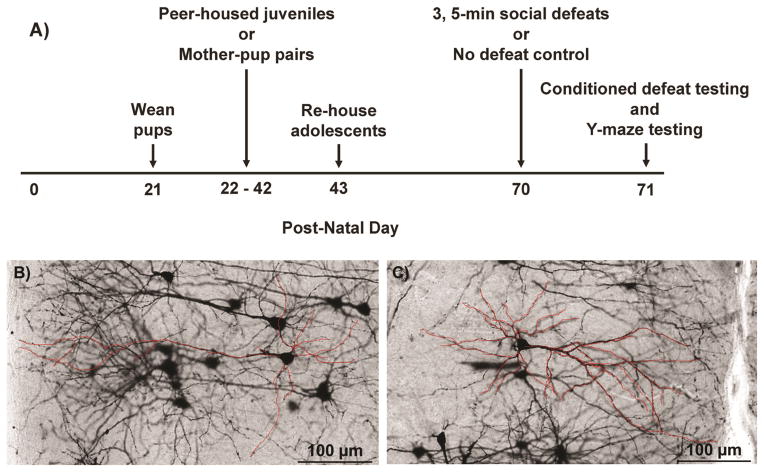
Male and female pups were housed with their mothers until weaning at postnatal day 21 (P21). At P21 male pups were separated into same-sex groups of three individuals (n = 21). One pup from each litter was not weaned and remained pair-housed with their mothers until P42 (n = 18; Figure 1a). We pair-housed juveniles with mothers to generate play deprivation and avoid potential confounding effects of complete social isolation. Mother-pup pairs were observed for 15 min three times per week and mothers were never observed playing with pups. These findings are consistent with previous studies showing that adult female hamsters rarely, if ever, play with juveniles (Guerra, et al., 1999). On P43, play-deprived animals were re-housed with their play-exposed littermates. We targeted P21–P42 for play deprivation because this is the period of early puberty when rates of social play peak in hamsters (Cervantes, Taravosh-Lahn, Wommack, & Delville, 2007; Pellis & Pellis, 1988).
Figure 1.
Representations of the experimental design and dendritic quantification. A) Schematic diagram of the experimental timeline. B) Representative photomicrograph of Golgi-Cox staining in the IL cortex for a play-exposed animal. C) Representative photomicrograph of Golgi-Cox staining in the IL cortex for a play-deprived animal. The 2D tracing of select neurons is shown in red (online version only) and was performed using MCID image analysis software while simultaneously viewing 3D neurons on a microscope at 40x magnification.
Social Stress and Behavioral Testing
Social defeat
In early adulthood (P63) all subjects were individually housed so that they could establish a territory prior to social defeat exposure. On P70, subjects (n = 21) were exposed to social defeat stress or served as no-defeat controls (n = 18). During social defeat stress, subjects were exposed to older, larger hamsters (> 6 months old, >190 g) that were individually housed. These larger animals were labeled resident aggressors and were prescreened to ensure that they reliably attacked and defeated intruders. Social defeat stress consisted of three, 5-min aggressive encounters in the home cage of a larger resident aggressor with a 5-min rest period in the subject’s home cage between each defeat episode. To correct for potential variation in the amount of aggression subjects received, the first defeat episode did not begin until the subject submitted to the first attack of the resident aggressor, which typically occurred within the first 60 sec of the encounter. Subjects submitted immediately in the second and third defeat episodes. No-defeat control animals were placed in the empty home cages of three separate resident aggressors for three 5-min exposures to control for the novel environment and olfactory cues associated with social defeat stress. Social defeats were digitally recorded for behavioral analysis. The duration of aggression displayed by the resident aggressor and the frequency of attacks were recorded. Whether or not subjects fought back against the resident aggressor during the first social defeat episode was also recorded. All aggressive encounters were carefully monitored for wounding and animals that received minor scratches were treated with antiseptic solution. No animal received a wound that resulted in pain or distress and none of the animals were removed from the study because of wounding.
Conditioned defeat testing
Hamsters were tested for a conditioned defeat response 24 hours after social defeat stress. social defeat stress. Testing consisted of a 5-min social interaction test during which a non-aggressive intruder was placed in the subject’s home cage. Nonaggressive intruders were younger, smaller hamsters (2 months old, < 120 g) that were group housed. These intruders displayed social and nonsocial behavior during conditioned defeat testing and did not direct agonistic behavior toward the subject. We digitally recorded all conditioned defeat testing sessions and quantified the behavior of subjects using Noldus Observer software (Noldus Information Technology). We quantified the total duration of the following categories of behavior: submissive/defensive (flee, avoid, upright and side defensive postures, tail-up, stretch-attend); aggressive (chase, attack, upright and side offensive postures); non-agonistic social (sniff, approach); and nonsocial (locomotion, grooming, nesting, feeding) (Huhman, et al., 2003). We also recorded the frequency of attacks and flees displayed by the subjects. All behavioral scoring was performed by a researcher blind to the experimental conditions of the subject. To established inter-rater reliability we achieved 90% agreement on the duration of submissive and defensive behavior in a subset of videos.
Y-maze testing
To evaluate avoidance of familiar aggressors, we used a Y-maze test as previously described (Bader, Carboni, Burleson, & Cooper, 2014; Lai, Ramiro, Yu, & Johnston, 2005). Y-maze testing consisted of two, 3-min trials. The first trial was an empty trial to determine the animal’s preferred arm of the Y-maze apparatus. In the second trial, the resident aggressor from the initial defeat episode was placed in a stimulus box at the end of the subject’s preferred Y-maze arm. Stimulus animals were placed at the end of the subject’s preferred arm to avoid confounding side preferences with avoidance of the resident aggressor. For no-defeat control subjects, the resident aggressor whose cage was explored during the first empty cage exposure was placed in the stimulus box. The Y-maze was cleaned with 70% ethanol after each subject was tested to remove any residual odors. All trials were digitally recorded and behavior was quantified using Noldus Observer by a researcher blind to the experimental conditions. For each subject, we recorded the amount of time spent in six compartments: the start box, the stem of the ‘Y’, the basal part of each Y-maze arm, and the distal part of each Y-maze arm. The position of the subject within the Y-maze was determined by the location of its nose, and time spent in the distal compartment near the stimulus animal indicated proximity to the resident aggressor. Inter-rater reliability was established on a subset of videos by achieving 90% agreement on the location of the subject in each compartment.
Golgi-Cox Staining
Animals were euthanized and brains extracted within three days of conditioned defeat and Y-maze testing. Brain tissue was stained using a previously described Golgi-Cox protocol (Gibb & Kolb, 1998). Brains were briefly rinsed with deionized water to remove blood from the surface and immediately placed into a glass vial containing 5 mL of Golgi-Cox impregnation solution. The Golgi-Cox impregnation solution consisted of potassium chromate, potassium dichromate, and mercuric chloride, each at a concentration of 5% (g/mL), and was prepared at least five days before use. Vials were wrapped in aluminum foil to protect the tissue from light and were stored at 4°C. After 12–14 days, brains were transferred to 5 mL 30% (g/mL) sucrose and remained at 4°C until they were sectioned and mounted on slides. After at least 48 hrs in sucrose solution, 200 μm coronal sections were collected in a 6% (g/mL) sucrose solution using a vibrating microtome and immediately mounted onto 2% gelatin coated microscope slides. Slides were stored in a dark humidity chamber for no more than five days before the staining was visualized. Slides were submerged in 15% ammonium hydroxide, then Kodak Fixer (Adorama) solution for 30 minutes each, followed by progressive dehydration with ethanol and clearing in D-limonene. Finally, sections were cover-slipped using DPX mountant.
Dendrite Quantification
Dendritic morphology was quantified for pyramidal neurons located in the IL cortex, PL cortex, and OFC. Because IL and PL cortices are difficult to distinguish in Golgi-stained tissue, we created four templates based on the size and location of these cortical regions on four separate pages of the Syrian hamster stereotaxic atlas (Morin & Wood, 2001). The anterior-posterior position of each tissue section was determined and the appropriate template was overlaid onto each photomicrograph. The IL and PL cortices were identified within the borders of the template and Golgi-stained cells located in layers 2 and 3 of the IL and PL were selected for quantification. In order to be selected for quantification, neurons had to meet the following inclusion criteria: 1) somas were located in the middle one-third of the section; 2) apical and basal dendrites were fully impregnated with stain; and 3) the visibility of dendrites was not affected by stain precipitations, blood vessels, or heavy clusters of dendrites from other cells. Photomicrographs of stained neurons were captured at 20× magnification with an Olympus BX41 microscope. Images were loaded into MCID image analysis software, and dendrites were traced while simultaneously available for viewing at 40× magnification on the Olympus microscope. Tracing dendrites on two-dimensional photomicrographs in conjunction with viewing three-dimensional neurons resulted in a digital camera lucida-like system (Figure 1b,c). The image analysis software provided total dendritic length, and the number branch points was calculated by counting the number of bifurcations in the basal and apical dendritic field. Basal dendrites were branches originating from the cell body, and apical dendrites were branches originating from the main apical branch. The dendritic morphology was quantified from 4–6 cells per individual, per brain region. The average dendritic length and branch number was calculated for each individual in each brain region, and analyses were performed on these individual averages. Samples sizes for dendritic quantification differ for each brain region because of variability in tissue quality and Golgi-Cox staining. Dendrites were quantified blind to the experimental conditions, and inter-rater reliability was established by researchers achieving 90% agreement on total dendritic length of sample neurons.
Statistical Analysis
Two-way ANOVAs were used to test for main effects and an interaction between defeat (two levels: social defeat, no social defeat) and social play (two levels: play exposed, play deprived). Two-way ANOVAs were used to analyze behavior at conditioned defeat and Y-maze testing, as well as apical and basal dendritic morphology in individual brain regions. A Levene’s test for homogeneity of variance was used to test the assumption for parametric statistics. T-tests and chi-square tests were used to analyze aggressive behavior during social defeat stress. All statistical tests were two-tailed, and the α level was set at p ≤ 0.05.
Results
Conditioned Defeat Response
We quantified aggressive behavior of resident aggressors and subjects to document whether play-exposed and play-deprived animals had a similar social defeat experience. Play-exposed animals received an average of 224.4 sec (SE = 9.6) of aggression during social defeat stress, and play-deprived animals received an average of 200.5 sec (SE = 15.4) of aggression (t(19) = 1.40, p > .05). Similarly, play-exposed animals received an average of 13.2 attacks (SE = 1.0) and play-deprived animals received an average of 14.6 attacks (SE = 1.4) (t(19) = 0.84, p > .05). Thus, play-exposed and play-deprived animals did not significantly differ in the amount of aggression received during social defeat stress. Interestingly, play deprivation significantly increased the likelihood of animals fighting back against the resident aggressor during the first social defeat episode. Four of ten play-deprived animals fought back against the first resident aggressor, whereas zero of eleven play-exposed animals fought back (χ2 = 5.44, p = .020). These results indicate that play-deprived animals were more likely to initiate aggression prior to submitting to the resident aggressor compared to play-exposed animals.
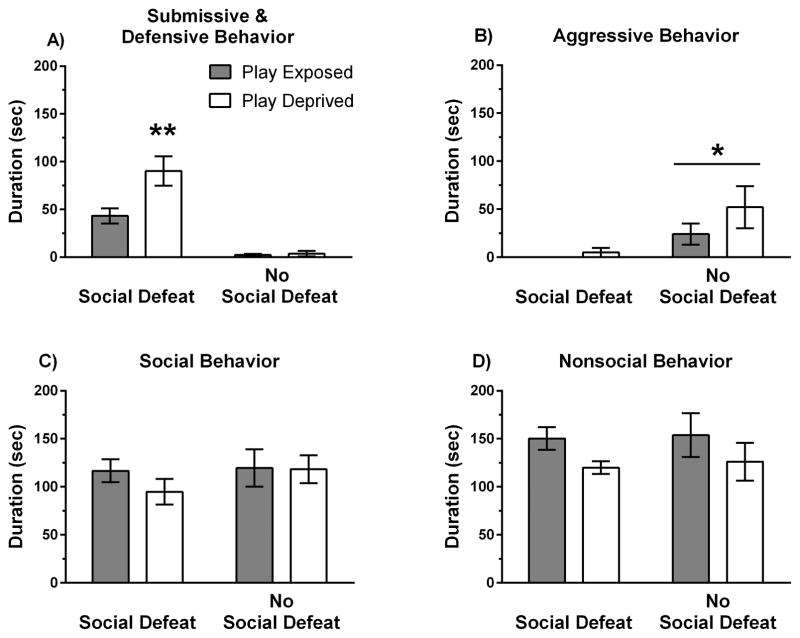
Twenty-four hours after social defeat stress animals received a 5-min social interaction test to measure the conditioned defeat response and a Y-maze test to measure avoidance of former opponents. We found that play-deprived animals exhibited increased submissive and defensive behavior during social interaction testing compared to play-exposed animals, which indicates an elevated conditioned defeat response. Specifically, we found a significant interaction between social defeat and play exposure on the duration of submissive and defensive behavior (F(1,35) = 6.16, p = .018; Figure 2a). In socially defeated subjects, play-deprived animals exhibited elevated submissive and defensive behavior compared to play-exposed animals, whereas in no-defeat control subjects there was no effect of play deprivation on submissive and defensive behavior. We also found a significant main effect of social defeat on aggressive behavior during social interaction testing (Figure 2b; F(1,35) = 10.70, p = .002). No-defeat control animals showed more aggression toward intruders compared to socially defeated animals. There was no significant effect of social defeat or play deprivation on social or nonsocial behavior during conditioned defeat testing (Figure 2c,d; p > .05). Altogether, these findings indicate that play deprivation resulted in an elevated conditioned defeat response in defeat animals, although it did not significantly alter agonistic behavior during social interaction testing in non-defeated animals.
Figure 2.
Play deprivation increased the conditioned defeat response. Mean durations (± SE) of A) submissive and defensive behavior, B) aggressive behavior, C) non-agonistic social behavior, and D) nonsocial behavior are shown during a 5-min test with a non-aggressive intruder. Data are shown for play-exposed (n = 11) and play-deprived (n = 10) animals that received social defeat stress and for play-exposed (n = 10) and play-deprived (n = 8) animals that did not receive social defeat stress. A significant interaction for submissive and defensive behavior indicates that social defeat stress produced a greater conditioned defeat response in play-deprived animals compared to play-exposed animals (** p < 0.05). A significant main effect for aggressive behavior indicates that social defeat stress reduced aggression during social interaction testing for both play-exposed and play deprived animals (* p < 0.05).
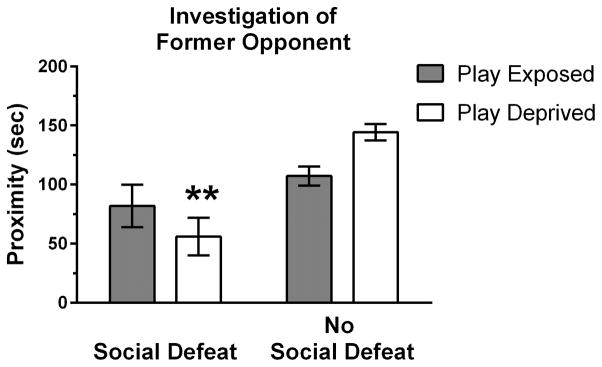
Y-maze testing was performed to investigate defeat-induced avoidance of former opponents. We found a significant interaction between social defeat and play deprivation on the amount of time animals maintained proximity with former opponents (F(1,35) = 4.92, p = .033; Figure 3). In socially defeated subjects, play-deprived animals spent less time in proximity to former opponents compared to play-exposed animals, whereas in non-defeated control subjects the opposite was found. These findings suggest that play deprivation leads to heightened attraction toward unfamiliar social partners and increased avoidance of former opponents following social defeat stress.
Figure 3.
Play deprivation increased avoidance of familiar opponents in a Y-maze test following social defeat stress. Mean duration (± SE) of proximity to a familiar opponent is shown for play-exposed (n = 11) and play-deprived (n = 10) animals that received social defeat stress and for play-exposed (n = 10) and play-deprived (n = 8) animals that did not receive social defeat stress. A significant interaction for the amount of time spent in proximity to a familiar opponent indicates that play-deprived animals exhibit greater avoidance following social defeat but greater approach following no-defeat treatment (i.e. empty cage exposure) compared to play-exposed animals (** p < 0.05).
Dendritic Morphology
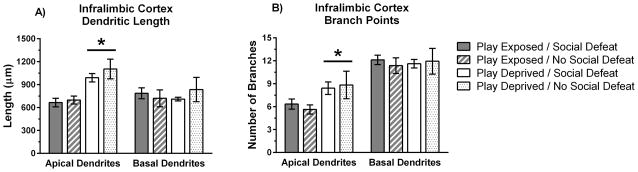
We found that play deprivation resulted in an increase in both total length of apical dendrites and the number of branch points in the IL cortex. There was a main effect of play deprivation such that play-deprived animals showed greater total length of apical dendrites in the IL cortex compared to play-exposed animals (F(1,23) = 28.00, p = .0001; Figure 4a). There was no significant effect of play deprivation on the length of basal dendrites in the IL cortex (F(1,23) = 0.03, p > .05). Also, there was no significant effect of social defeat on the length of apical or basal dendrites in the IL cortex (F(1,23) = 1.13, p > .05; F(1,23) = 0.07, p > .05, respectively). There was a main effect of play deprivation on dendritic branch points such that play-deprived animals showed an increased number of branch points on apical dendrites in the IL cortex compared to play-exposed animals (F(1,23) = 9.17, p = .006; Figure 4b). There was also no significant effect of play deprivation on branch points in basal dendrites (F(1,23) = 0.01, p > .05), and no significant effect of social defeat on branch points in apical or basal dendrites in the IL cortex (F(1,23) = 0.03, p > .05; F(1,23) = 0.04, p > .05, respectively).
Figure 4.
Play-deprivation increased total length and branch points for apical dendrites in the IL cortex. A) Mean dendritic length (± SE) for apical and basal dendrites in pyramidal neurons of IL layer 2/3. B) Mean branch points (± SE) for apical and basal dendrites in pyramidal neurons of IL layer 2/3. Data on dendritic morphology is shown for play-exposed (n = 9) and play-deprived (n = 6) animals that received social defeat stress and for play-exposed (n = 9) and play-deprived (n = 3) animals that did not receive social defeat stress. Significant main effects indicate that play-deprived animals showed increased apical dendritic length and branch points compared to play-exposed animals (* p < 0.05).
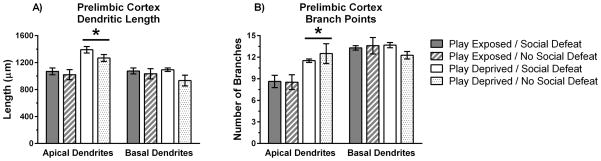
Play deprivation also increased total length and number of branch points in apical dendrites within the PL cortex. We found a main effect of play deprivation indicating that play-deprived animals showed greater total length of apical dendrites in the PL cortex compared to play-exposed animals (F(1,13) = 21.35, p = .0003; Figure 5a). Play deprivation did not significantly alter the length of basal dendrites in the PL cortex (F(1,13) = 0.47, p > .05). Also, there was no significant main effect of social defeat on the length of apical or basal dendrites in the PL cortex (F(1,13) = 1.96, p > .05; F(1,13) = 2.77, p > .05, respectively). We found that play-deprived animals also showed more branch points on apical dendrites in the PL cortex than did play-exposed animals (F(1,13) = 14.81, p = .002; Figure 5b). There was no significant main effect of play deprivation on branch points in basal dendrites (F(1,13) = 0.35, p > .05), and no significant main effects of social defeat on branch points in either apical or basal dendrites in the PL cortex (F(1,13) = 0.26, p > .05; F(1,13) = 0.56, p > .05, respectively).
Figure 5.
Play-deprivation increased total length and branch points for apical dendrites in the PL cortex. A) Mean dendritic length (± SE) for apical and basal dendrites in pyramidal neurons of PL layer 2/3. B) Mean branch points (± SE) for apical and basal dendrites in pyramidal neurons of PL layer 2/3. Data on dendritic morphology is shown for play-exposed (n = 4) and play-deprived (n = 5) animals that received social defeat stress and for play-exposed (n = 5) and play-deprived (n = 3) animals that did not receive social defeat stress. Significant main effects indicate that play-deprived animals showed increased apical dendritic length and branch points compared to play-exposed animals (* p < 0.05).
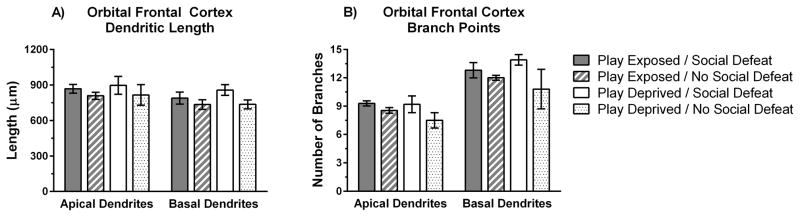
Play deprivation did not alter total dendritic length or the number of branch points within the OFC. We found no significant main effects of play deprivation or social defeat on total dendritic length of apical or basal dendrites in the OFC (p > .05, all tests; Figure 6a). Similarly, we found no significant main effects of play deprivation or social defeat on number of branch points in apical or basal dendrites in the OFC (p > .05, all tests; Figure 6b). Although sample sizes were reduced in the OFC because of fading of Golgi-Cox staining, there were no trends for an effect of play deprivation in well-stained OFC neurons.
Figure 6.
Play-deprivation did not significantly alter total dendritic length or branch points in the OFC. A) Mean dendritic length (± SE) for apical and basal dendrites in pyramidal neurons of OFC. B) Mean branch points (± SE) for apical and basal dendrites in pyramidal neurons of OFC. Data on dendritic morphology is shown for play-exposed (n = 4) and play-deprived (n = 5) animals that received social defeat stress and for play-exposed (n = 4) and play-deprived (n = 2) animals that did not receive social defeat stress.
Discussion
In this study we found that social play deprivation resulted in inappropriate aggressive behavior, an increased conditioned defeat response, and disrupted dendritic morphology in the IL and PL cortices. Play-deprived animals were more likely to attack the resident aggressor prior to losing the social defeat session. This finding suggests that play-deprived hamsters are more likely to show inappropriate aggression by attacking larger, aggressive animals who are protecting their home cage. However, appropriate forms of territorial aggression were not elevated when play-deprived hamsters were defending their own home cage. Among the non-defeated animals, play-deprived hamsters did not direct significantly more aggression toward smaller intruders during conditioned defeat testing. Importantly, play-deprived animals showed greater defeat-induced changes in behavior compared to play-exposed animals. Specifically, play-deprived animals showed increased submissive and defensive behavior during conditioned defeat testing and increased avoidance of familiar opponents in a Y-maze test. Finally, the behavioral changes found in play-deprived hamsters may be related to impaired development of the vmPFC. Play-deprived animals showed increased length and branch points within apical dendrites of pyramidal neurons in the IL and PL cortices, but not in the OFC.
Social isolation during the juvenile period in rats has been shown to impair social behavior, including aggression, in adulthood (Hol, et al., 1999; van den Berg, et al., 1999). Interestingly, social isolation leads to inappropriate reactions to aggressive individuals in adulthood as socially isolated rats fail to inhibit ongoing social behavior in the presence of an aggressor which results in more wounding (Von Frijtag, et al., 2002). The effects of social isolation in juvenile rats are likely related to play deprivation, because one hour of social interaction with peers is sufficient to minimize the effects of social isolation (Einon, et al., 1978). Compensating for the effects of social isolation in Syrian hamsters is less straightforward because even two hours of social interaction with peers fails to alleviate the effects of social isolation on play behavior (Vieira, et al., 2005). We avoided the potential confounds of social isolation by housing play-deprived hamsters with their mother after weaning the other littermates. Overall, it is likely that the behavioral and neural changes we identified in hamsters paired with their mother are the result of social play deprivation, because others have shown that contact with mother during the juvenile period does not compensate for lack of social play with peers (Guerra, et al., 1999).
The effects of play deprivation on future social behavior are related, but not identical, to the effects of chronic peripubertal stress. In male rats, chronic variable stress during puberty leads to increased aggression and anxiety-like behavior, and these behavioral changes are mediated, at least in part, by altered serotonin signaling in the OFC (Marquez et al., 2013) and glutamate and GABA signaling in central amygdala (Tzanoulinou, Riccio, de Boer, & Sandi, 2014). In male hamsters, chronic social defeat stress during early puberty leads to increased aggressive behavior and an earlier transition to adult forms of aggression (Delville, David, Taravosh-Lahn, & Wommack, 2003). In both rats and hamsters, these peripubertal stress-induced behavioral changes are regulated by elevated glucocorticoids during development (Veenit, Cordero, Tzanoulinou, & Sandi, 2013; Wommack, Salinas, & Delville, 2005). In contrast, play deprivation in rats does not alter anxiety-like behavior in the open field, elevated plus maze, or shock probe burying test (van den Berg, et al., 1999). Also, while play deprivation itself is not stressful, it impairs the extinction of a plasma corticosterone response following social defeat stress in adulthood (van den Berg, et al., 1999). These findings are consistent with a social buffering effect in which social housing during the juvenile period promotes an adaptive hormonal stress response in adulthood (Hennessy, Kaiser, & Sachser, 2009; Kaiser, Harderthauer, Sachser, & Hennessy, 2007). Also, chronic peripubertal stress and play deprivation have separate effects on dendritic morphology. In both male and female juvenile rats, chronic restraint stress decreases apical dendritic length in mPFC neurons and hippocampal CA3 neurons, while it increases total dendritic length in the basolateral amygdala (Eiland, Ramroop, Hill, Manley, & McEwen, 2012). On the other hand, play deprivation in juvenile rats increases apical dendritic length in mPFC neurons (Bell, et al., 2010; Himmler, Pellis, & Kolb, 2013).
The apical and basal dendritic fields of pyramidal neurons receive afferent input from separate cortical and subcortical regions (Spratling, 2002). Local interneurons have been shown to project to the apical tree of pyramidal neurons in the mPFC where they promote feedforward inhibition (Gilabert-Juan, Castillo-Gomez, Guirado, Molto, & Nacher, 2013). Chronic restraint stress leads to retraction of apical dendrites of in layer 2/3 pyramidal neurons in the PL and IL (Radley et al., 2004; Shansky, Hamo, Hof, McEwen, & Morrison, 2009). These findings suggest that chronic stress in adulthood may impair the ability of local interneurons to control the activity of PL and IL pyramidal neurons. While it is interesting that play deprivation also targets apical dendrites, it remains unknown how increased apical dendritic length alters the activity of pyramidal neurons. Our study also addressed whether acute social defeat stress could produce dendritic remodeling in vmPFC neurons, but we failed to find an effect of acute social defeat on dendritic morphology. Interestingly, acute stress on a raised platform has recently been shown to reduce apical dendritic length in rat IL layer 2/3 neurons, although the stress-induced retraction is attenuated when rats experience fear conditioning and extinction training (Moench, Maroun, Kavushansky, & Wellman, 2016). One possibility for the lack of a social defeat effect is that defeat-induced changes in dendritic length did not persist following conditioned defeat and Y-maze testing. Also, play deprivation may have reduced the sensitivity of IL and PL neurons to the effects of acute social defeat. Likewise, play deprivation has been shown to prevent changes in dendritic length in mPFC neurons following nicotine exposure in adulthood (Himmler, et al., 2013).
Early studies of social play in Syrian hamsters indicated that play fighting in juveniles and aggression in adults constitute two separate types of behavior. During playful fights juveniles direct attacks toward the cheeks and face of peers, whereas adults direct attacks at the lower belly and rump (Pellis & Pellis, 1988). Delville and colleagues have proposed that play fighting in hamsters transitions into adult forms of aggression during mid-puberty and that the neural circuitry controlling play fighting is similar to that regulating aggression in adulthood. In adult hamsters flank marking is used by dominant animals to scent mark territory and maintain dominance relationships (Ferris, Axelson, Shinto, & Albers, 1987). Interestingly, juveniles flank mark after successful attacks during social play as they do during aggressive interactions in adulthood (Delville, et al., 2003). Also, similar brain regions exhibit elevated c-Fos expression following both play fighting and adult aggression, including vasopressin cells within the medial supraoptic nucleus and the nucleus circularis of the anterior hypothalamus (Cheng, Taravosh-Lahn, & Delville, 2008). While social play appears to be directly linked to the development of agonistic behavior in hamsters, our data on changes in mPFC dendritic morphology and the conditioned defeat response suggest that social play may also facilitate cortical regulation of social and emotional behavior in adulthood.
The prefrontal cortex is a late maturing brain structure and cortical grey matter tends to follow an inverted U-shaped curve during development (Giedd et al., 1999). The pruning of synaptic contacts during adolescence is thought to be critical for function of the prefrontal cortex in adulthood (Rakic, Bourgeois, Eckenhoff, Zecevic, & Goldman-Rakic, 1986; Selemon, 2013). Consequently, adolescence is a particularly sensitive period for cortical development and play deprivation may alter behavior in adulthood by disrupting the pruning of dendrites in the prefrontal cortex. In this study we found that play-deprived animals showed increased length and branch points in apical dendrites in the IL and PL cortices as well as an increased conditioned defeat response compared to play-exposed animals. These results suggest that pruning of dendrites in the IL and PL cortices during adolescence is important for responses to social stress in adulthood. Similarly, other data in Syrian hamsters indicate that pruning of dendrites and spines in the medial amygdala is associated with maturation of sexual behavior during adolescence (Zehr, Todd, Schulz, McCarthy, & Sisk, 2006). One possible consequence of changes in IL and PL dendritic morphology is an increase impulsivity. This possibility is consistent with the effects of social isolation. Social isolation in rats has been shown to increase impulsivity in a 5-choice serial reaction time task and reduce the sensitivity of vmPFC pyramidal neurons to dopaminergic input during whole-cell recordings (Baarendse, Counotte, O’Donnell, & Vanderschuren, 2013).
Because the vmPFC provides top-down inhibitory control on a variety of behavior and physiological processes in a wide range of species, play deprivation can have far reaching consequences. Neural activity in the vmPFC has been shown to regulate impulse control (Feja & Koch, 2014), reward sensitivity (Piantadosi, Khayambashi, Schluter, Kutarna, & Floresco, 2016), negative feedback on the hypothalamic-pituitary-adrenal axis (McKlveen et al., 2013), and the ability to cope with stressful events in adulthood (Maier & Watkins, 2010). We have shown that dominant hamsters exhibit a reduced conditioned defeat response compared to subordinates and controls and also exhibit elevated c-Fos expression in the PL and IL cortices (Morrison, et al., 2014; Morrison, et al., 2012). In addition, pharmacological inactivation of the vmPFC during social defeat stress prevents the reduced conditioned defeat response of dominant animals (Morrison, et al., 2013). Altogether, our findings suggest that play deprivation impairs dendritic morphology in the vmPFC and that dysregulated vmPFC neural activity in adulthood may potentiate the effects of social defeat stress.
The OFC also modulates the expression of social play in rats, and basal dendrites in the OFC are sensitive to the experience of social play with multiple partners (Bell, et al., 2010; Pellis, et al., 2006). The OFC and mPFC show opposite patterns of experience-dependent plasticity insofar as drug treatments and gonadal hormones that increase dendritic length and spine number in the mPFC tend to decrease it in the OFC (Kolb, Pellis, & Robinson, 2004). However, we failed to show that play deprivation alters dendritic morphology in the OFC of hamsters, and this discrepancy may be related to species and/or sex differences. While the effects of social play on dendritic architecture in the OFC have been described in female rats, corresponding studies in male rats have not yet been performed (Bell, et al., 2010). Although sex differences in rat social play are modest, males and females differ in the development of defensive tactics used during play bouts and how they adjust these tactics with different social partners (Pellis & Pellis, 1990; Pellis, et al., 2010). Our experimental design that compared juveniles raised in mother-pup pairs to those raised with multiple peers should have been sensitive to the effects of multiple play partners. However, rats exhibit more complex forms of social play compared to hamsters, and their OFC may be sensitive to social play with multiple partners whereas the hamster OFC may not (Pellis & Pellis, 1988, 1998).
Overall, social play in Syrian hamsters is necessary for the normal dendritic pruning that occurs in the vmPFC during development. While this experience-dependent structural reorganization of the vmPFC during puberty may constrain impulsivity and contribute to the emergence of appropriate forms of adult aggressive behavior, it also enables animals to cope with stressful life events in adulthood. Thus, social play trains animals to be resilient by fine-tuning the neural circuits that regulate emotional responses (Pellis, et al., 2010). Ultimately, one of the key functions of social play is promoting neuronal development within the vmPFC that is critical for flexible, adaptive behavior in adulthood.
Acknowledgments
We are grateful to our team of graduate and undergraduate students for their technical assistance including Catie Clinard, Brooke Dulka, Steven Kyle, and Sam Adler. We also thank Sergio Pellis and Bryan Kolb for their advice on social play deprivation, Golgi-Cox staining, and dendritic quantification. This research was funded by NIH R21 MH098190 and a seed grant from the University of Tennessee NeuroNET Research Center.
References
- Baarendse PJ, Counotte DS, O’Donnell P, Vanderschuren LJ. Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacology. 2013;38(8):1485–1494. doi: 10.1038/npp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader LR, Carboni JD, Burleson CA, Cooper MA. 5-HT1A receptor activation reduces fear-related behavior following social defeat in Syrian hamsters. Pharmacol Biochem Behav. 2014;122:182–190. doi: 10.1016/j.pbb.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean G, Lee T. Social isolation and cohabitation with haloperidol-treated partners: effect on density of striatal dopamine D2 receptors in the developing rat brain. Psychiatry Research. 1991;36(3):307–317. doi: 10.1016/0165-1781(91)90029-o. [DOI] [PubMed] [Google Scholar]
- Bell HC, McCaffrey DR, Forgie ML, Kolb B, Pellis SM. The role of the medial prefrontal cortex in the play fighting of rats. Behav Neurosci. 2009;123(6):1158–1168. doi: 10.1037/a0017617. [DOI] [PubMed] [Google Scholar]
- Bell HC, Pellis SM, Kolb B. Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortices. Behav Brain Res. 2010;207(1):7–13. doi: 10.1016/j.bbr.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76(4):790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt GM. The Genesis of Animal Play: Testing the Limits. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Cervantes MC, Taravosh-Lahn K, Wommack JC, Delville Y. Characterization of offensive responses during the maturation of play-fighting into aggression in male golden hamsters. Developmental Psychobiology. 2007;49(1):87–97. doi: 10.1002/dev.20183. [DOI] [PubMed] [Google Scholar]
- Chen RJ, Kelly G, Sengupta A, Heydendael W, Nicholas B, Beltrami S, … Bhatnagar S. MicroRNAs as biomarkers of resilience or vulnerability to stress. Neuroscience. 2015;305:36–48. doi: 10.1016/j.neuroscience.2015.07.045. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Taravosh-Lahn K, Delville Y. Neural circuitry of play fighting in golden hamsters. Neuroscience. 2008;156(2):247–256. doi: 10.1016/j.neuroscience.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Flyer-Adams JG, Drugan RC, Amat J, Daut RA, Foilb AR, … Maier SF. Learned stressor resistance requires extracellular signal-regulated kinase in the prefrontal cortex. Front Behav Neurosci. 2014;8:348. doi: 10.3389/fnbeh.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva NL, Ferreira VM, de Carobrez PA, Morato GS. Individual housing from rearing modifies the performance of young rats on the elevated plus-maze apparatus. Physiol Behav. 1996;60(6):1391–1396. doi: 10.1016/s0031-9384(96)00254-5. [DOI] [PubMed] [Google Scholar]
- de Bruin JP, van Oyen HG, Van de Poll N. Behavioural changes following lesions of the orbital prefrontal cortex in male rats. Behav Brain Res. 1983;10(2–3):209–232. doi: 10.1016/0166-4328(83)90032-3. [DOI] [PubMed] [Google Scholar]
- Delville Y, David JT, Taravosh-Lahn K, Wommack JC. Stress and the development of agonistic behavior in golden hamsters. Horm Behav. 2003;44(3):263–270. doi: 10.1016/s0018-506x(03)00130-2. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Manzano-Nieves G, Quinones-Laracuente K, Ramos-Medina L, Quirk GJ. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J Neurosci. 2015;35(8):3607–3615. doi: 10.1523/jneurosci.3137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37(1):39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ, Kibbler CC. Brief periods of socialization and later behavior in the rat. Developmental Psychobiology. 1978;11(3):213–225. doi: 10.1002/dev.420110305. [DOI] [PubMed] [Google Scholar]
- Feja M, Koch M. Ventral medial prefrontal cortex inactivation impairs impulse control but does not affect delay-discounting in rats. Behav Brain Res. 2014;264:230–239. doi: 10.1016/j.bbr.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Axelson JF, Shinto LH, Albers HE. Scent marking and the maintenance of dominant/subordinate status in male golden hamsters. Physiology and Behavior. 1987;40:661–664. doi: 10.1016/0031-9384(87)90114-4. [DOI] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. Journal of Neuroscience Methods. 1998;79(1):1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, … Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gilabert-Juan J, Castillo-Gomez E, Guirado R, Molto MD, Nacher J. Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Struct Funct. 2013;218(6):1591–1605. doi: 10.1007/s00429-012-0479-1. [DOI] [PubMed] [Google Scholar]
- Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Front Behav Neurosci. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1996;351(1346):1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Graham KL, Burghardt GM. Current perspectives on the biological study of play: signs of progress. Quarterly Review of Biology. 2010;85(4):393–418. doi: 10.1086/656903. [DOI] [PubMed] [Google Scholar]
- Guerra RF, Takase E, de ONCR. Play fighting of juvenile golden hamsters (Mesocricetus auratus): effects of two types of social deprivation and days of testing. Behav Processes. 1999;47(3):139–151. doi: 10.1016/s0376-6357(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 2009;30(4):470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Himmler BT, Pellis SM, Kolb B. Juvenile play experience primes neurons in the medial prefrontal cortex to be more responsive to later experiences. Neurosci Lett. 2013;556:42–45. doi: 10.1016/j.neulet.2013.09.061. [DOI] [PubMed] [Google Scholar]
- Hol T, Van den Berg CL, Van Ree JM, Spruijt BM. Isolation during the play period in infancy decreases adult social interactions in rats. Behav Brain Res. 1999;100(1–2):91–97. doi: 10.1016/s0166-4328(98)00116-8. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44(3):293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25(37):8534–8542. doi: 10.1523/jneurosci.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GH, Marsden CA, Robbins TW. Behavioural rigidity and rule-learning deficits following isolation-rearing in the rat: neurochemical correlates. Behav Brain Res. 1991;43(1):35–50. doi: 10.1016/s0166-4328(05)80050-6. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Harderthauer S, Sachser N, Hennessy MB. Social housing conditions around puberty determine later changes in plasma cortisol levels and behavior. Physiol Behav. 2007;90(2–3):405–411. doi: 10.1016/j.physbeh.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Kamitakahara H, Monfils MH, Forgie ML, Kolb B, Pellis SM. The modulation of play fighting in rats: role of the motor cortex. Behav Neurosci. 2007;121(1):164–176. doi: 10.1037/0735-7044.121.1.164. [DOI] [PubMed] [Google Scholar]
- Kolb B, Pellis S, Robinson TE. Plasticity and functions of the orbital frontal cortex. Brain and Cognition. 2004;55(1):104–115. doi: 10.1016/S0278-2626(03)00278-1. [DOI] [PubMed] [Google Scholar]
- Kumar S, Hultman R, Hughes D, Michel N, Katz BM, Dzirasa K. Prefrontal cortex reactivity underlies trait vulnerability to chronic social defeat stress. Nat Commun. 2014;5:4537. doi: 10.1038/ncomms5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WS, Ramiro LL, Yu HA, Johnston RE. Recognition of familiar individuals in golden hamsters: a new method and functional neuroanatomy. J Neurosci. 2005;25(49):11239–11247. doi: 10.1523/JNEUROSCI.2124-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Herkenham M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci. 2011;31(16):6159–6173. doi: 10.1523/JNEUROSCI.0577-11.2011. 31/16/6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Luckett CA, Huhman KL. The medial prefrontal cortex is both necessary and sufficient for the acquisition of conditioned defeat. Neuropharmacology. 2012;62(2):933–939. doi: 10.1016/j.neuropharm.2011.09.026. S0028-3908(11)00450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez C, Poirier GL, Cordero MI, Larsen MH, Groner A, Marquis J, … Sandi C. Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl Psychiatry. 2013;3:e216. doi: 10.1038/tp.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Caro TM. On the functions of play and its role in behavioral development. Advances in the Study of Behavior. 1985;15:59–103. [Google Scholar]
- McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, Herman JP. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74(9):672–679. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PH, Beer J. Neural mechanisms of the testosterone-aggression relation: the role of orbitofrontal cortex. Journal of Cognitive Neuroscience. 2010;22(10):2357–2368. doi: 10.1162/jocn.2009.21389. [DOI] [PubMed] [Google Scholar]
- Moench KM, Maroun M, Kavushansky A, Wellman C. Alterations in neuronal morphology in infralimbic cortex predict resistance to fear extinction following acute stress. Neurobiology of Stress. 2016;3:23–33. doi: 10.1016/j.ynstr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A stereotaxic atlas of the golden hamster brain. New York: Academic Press; 2001. [Google Scholar]
- Morrison KE, Bader LR, Clinard CT, Gerhard DM, Gross SE, Cooper MA. Maintenance of dominance status is necessary for resistance to social defeat stress in Syrian hamsters. Behav Brain Res. 2014;270:277–286. doi: 10.1016/j.bbr.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, Bader LR, McLaughlin CN, Cooper MA. Defeat-induced activation of the ventral medial prefrontal cortex is necessary for resistance to conditioned defeat. Behav Brain Res. 2013;243:158–164. doi: 10.1016/j.bbr.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, Curry DW, Cooper MA. Social status alters defeat-induced neural activation in Syrian hamsters. Neuroscience. 2012;210:168–178. doi: 10.1016/j.neuroscience.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Normansell L, Cox JF, Siviy SM. Effects of neonatal decortication on the social play of juvenile rats. Physiol Behav. 1994;56(3):429–443. doi: 10.1016/0031-9384(94)90285-2. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8(4):465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Hastings E, Shimizu T, Kamitakahara H, Komorowska J, Forgie ML, Kolb B. The effects of orbital frontal cortex damage on the modulation of defensive responses by rats in playful and nonplayful social contexts. Behav Neurosci. 2006;120(1):72–84. doi: 10.1037/0735-7044.120.1.72. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play-fighting in the Syrian golden hamster Mesocricetus auratus Waterhouse, and its relationship to serious fighting during postweaning development. Developmental Psychobiology. 1988;21(4):323–337. doi: 10.1002/dev.420210404. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Developmental Psychobiology. 1990;23(3):215–231. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play fighting of rats in comparative perspective: a schema for neurobehavioral analyses. Neuroscience and Biobehavioral Reviews. 1998;23(1):87–101. doi: 10.1016/s0149-7634(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. The Playful Brain: Venturing to the Limits of Neuroscience. Oxford: Oneworld Publications; 2009. [Google Scholar]
- Pellis SM, Pellis VC, Bell HC. The function of play in the development of the social brain. American Journal of Play. 2010;2(3):278–298. [Google Scholar]
- Pellis SM, Pellis VC, Whishaw IQ. The role of the cortex in play fighting by rats: developmental and evolutionary implications. Brain Behav Evol. 1992;39(5):270–284. doi: 10.1159/000114124. [DOI] [PubMed] [Google Scholar]
- Piantadosi PT, Khayambashi S, Schluter MG, Kutarna A, Floresco SB. Perturbations in reward-related decision-making induced by reduced prefrontal cortical GABA transmission: Relevance for psychiatric disorders. Neuropharmacology. 2016;101:279–290. doi: 10.1016/j.neuropharm.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29(22):7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, … Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232(4747):232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry. 2013;3:e238. doi: 10.1038/tp.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cerebral Cortex. 2009;19(10):2479–2484. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviy SM, Panksepp J. In search of the neurobiological substrates for social playfulness in mammalian brains. Neurosci Biobehav Rev. 2011;35(9):1821–1830. doi: 10.1016/j.neubiorev.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Spratling MW. Cortical region interactions and the functional role of apical dendrites. Behav Cogn Neurosci Rev. 2002;1(3):219–228. doi: 10.1177/1534582302001003003. [DOI] [PubMed] [Google Scholar]
- Tzanoulinou S, Riccio O, de Boer MW, Sandi C. Peripubertal stress-induced behavioral changes are associated with altered expression of genes involved in excitation and inhibition in the amygdala. Transl Psychiatry. 2014;4:e410. doi: 10.1038/tp.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Developmental Psychobiology. 1999;34(2):129–138. doi: 10.1002/(SICI)1098-2302(199903). [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21(3):309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Veenit V, Cordero MI, Tzanoulinou S, Sandi C. Increased corticosterone in peripubertal rats leads to long-lasting alterations in social exploration and aggression. Front Behav Neurosci. 2013;7:26. doi: 10.3389/fnbeh.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, … Nestler EJ. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of DeltaFosB. J Neurosci. 2014;34(11):3878–3887. doi: 10.1523/jneurosci.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira ML, Garcia MP, Rau DDW, Prado AB. Effects of different opportunities for social interaction on the play fighting behavior in male and female golden hamsters (Mesocricetus auratus) Developmental Psychobiology. 2005;47(4):345–353. doi: 10.1002/dev.20101. [DOI] [PubMed] [Google Scholar]
- Von Frijtag JC, Schot M, van den Bos R, Spruijt BM. Individual housing during the play period results in changed responses to and consequences of a psychosocial stress situation in rats. Developmental Psychobiology. 2002;41(1):58–69. doi: 10.1002/dev.10057. [DOI] [PubMed] [Google Scholar]
- Wommack JC, Salinas A, Delville Y. Glucocorticoids and the development of agonistic behaviour during puberty in male golden hamsters. J Neuroendocrinol. 2005;17(12):781–787. doi: 10.1111/j.1365-2826.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Floresco SB, Winstanley CA. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology (Berl) 2010;211(1):87–98. doi: 10.1007/s00213-010-1871-2. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. Journal of Neurobiology. 2006;66(6):578–590. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]