Abstract
Background
The duration of use and efficacy of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) is limited by distal ischemia. We developed a hybrid endovascular-extracorporeal circuit variable aortic control (VAC) device to extend REBOA duration in a lethal model of hemorrhagic shock to serve as an experimental surrogate to further development of endovascular variable aortic control (EVAC) technologies.
Methods
Nine Yorkshire-cross swine were anesthetized, instrumented, splenectomized, and subjected to 30% liver amputation. Following a short period of uncontrolled hemorrhage, REBOA was instituted for 20 minutes. Automated variable occlusion in response to changes in proximal mean arterial pressure was applied for the remaining 70 minutes of the intervention phase using the automated extracorporeal circuit. Damage control surgery and whole blood resuscitation then occurred and the animals were monitored for a total of 6 hours.
Results
Seven animals survived the initial surgical preparation. After 20 minutes of complete REBOA, regulated flow was initiated through the extracorporeal circuit to simulate VAC and provide perfusion to distal tissue beds during the 90-minute intervention phase. Two animals required circuit occlusion for salvage, while five animals tolerated sustained, escalating restoration of distal blood flow prior to surgical hemorrhage control. Animals tolerating distal flow had preserved renal function, maintained proximal blood pressure, and rapidly weaned from complete REBOA.
Conclusion
We combined a novel automated, extracorporeal circuit with complete REBOA to achieve endovascular variable aortic control in a swine model of uncontrolled hemorrhage. Our approach regulated proximal aortic pressure, alleviated supra-normal values above the balloon, and provided controlled distal aortic perfusion that reduced ischemia without inducing intolerable bleeding. This experimental model serves as a temporary surrogate to guide future EVAC catheter designs that may provide transformational approaches to hemorrhagic shock.
Keywords: REBOA, endovascular variable aortic control, hemorrhage control, aortic occlusion
Background
Endovascular techniques are a less invasive and more efficient way to manage vascular injury and shock(1-6), and resuscitative endovascular balloon occlusion of the aorta (REBOA) has emerged as an alternative to resuscitative thoracotomy with aortic clamping. However, despite promising reports and the development of smaller caliber REBOA devices, concerns exist including: distal ischemia, reperfusion injury, and rebound hypotension upon deflation of the balloon. (2,3,5) Additionally, the effects of prolonged supra-physiologic pressure proximal to the balloon may damage the heart, lungs, and brain. These limitations notwithstanding, the less invasive nature of REBOA makes it attractive for aortic control and the development of new approaches to this procedure provide great opportunity for therapeutic advances.
While REBOA is effective at restoring proximal perfusion, there is a time threshold beyond which the deleterious effects of aortic occlusion outweigh its initial benefit. However, for clinical scenarios in which the time interval between REBOA and definitive surgical management exceeds this threshold, a more advanced strategy is needed.(7) Variable or partial aortic occlusion that permits a controlled amount of distal perfusion while maintaining proximal aortic pressure is one strategy that may extend the window for endovascular therapies. Variable aortic control (VAC) may be particularly useful if used following an initial period of complete aortic occlusion that promotes distal hemostasis and maximizes proximal perfusion. VAC invokes the principle of permissive hypotension in an attempt to minimize hemorrhage and promote clot stabilization in the area of vascular disruption (e.g. liver, kidney, spleen, pelvis).(8) This strategy is effective not only because reduced blood pressure promotes clot stabilization, but also because regional blood flow is reduced in the face of hypovolemia. While the interaction of pressure, vascular tone, and blood flow varies with each injury scenario, it must be considered as new approaches to aortic control are explored. Regulated VAC is predicated on a new concept of “permissive regional hypoperfusion” that achieves equilibrium among: 1) reduced perfusion pressure to promote clot stabilization, 2) a low rate of of distal flow to mitigate compensatory vasodilation and provide tissue perfusion and 3) the avoidance of undue bleeding or exsanguination.
Although not yet widely described in the management of trauma and hemorrhagic shock, VAC-like procedures are used in cardiac failure, ischemic stroke, and the deployment of aortic stent grafts.(9) Recognizing that blood flow across any REBOA device involves the interplay of hemodynamics, ongoing blood loss, and changes in aortic diameter, automation will be required to continuously adjust the degree of aortic occlusion and account for other factors associated with the patient, the injury, and the clinical setting. When developed, automated endovascular variable aortic control (EVAC) combined with judicious use of resuscitative fluid has the potential to sustain an injured patient for a longer period of time prior to definitive hemostasis when compared to existing technology.
In the absence of such an automated endovascular device, a translational model is required to study of the feasibility and physiologic effects of variable aortic control in the setting of hemorrhagic shock. Our experimental approach was to develop an automated extracorporeal circuit that could offload proximal aortic pressure in the setting of REBOA and deliver distal perfusion, controlling for the variables of flow and pressure. In this context, the hybrid of REBOA and the extracorporeal circuit serve as a surrogate for EVAC. This configuration will allow us to investigate dynamic partial aortic occlusion and permissive regional hypoperfusion in an experimental model. The objective of this study is to describe the experimental setup and examine the feasibility of a combined endovascular-extracorporeal approach to automated variable aortic control in a lethal model of torso hemorrhage and shock.
Methods
Overview of Experimental Design
This study was approved by the Institutional Animal Care and Use Committee at David Grant USAF Medical Center, Travis Air Force Base, California. All animal care and use was in strict compliance with the Guide for the Care and Use of Laboratory Animals in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Healthy adult, castrate male and non-pregnant female Yorkshire-cross swine (Sus scrofa), obtained from the University of California, Davis, were acclimated for a minimum of 7 days. At the time of experimentation, animals weighed between 72 and 90 kg, with an age between 5 and 7 months.
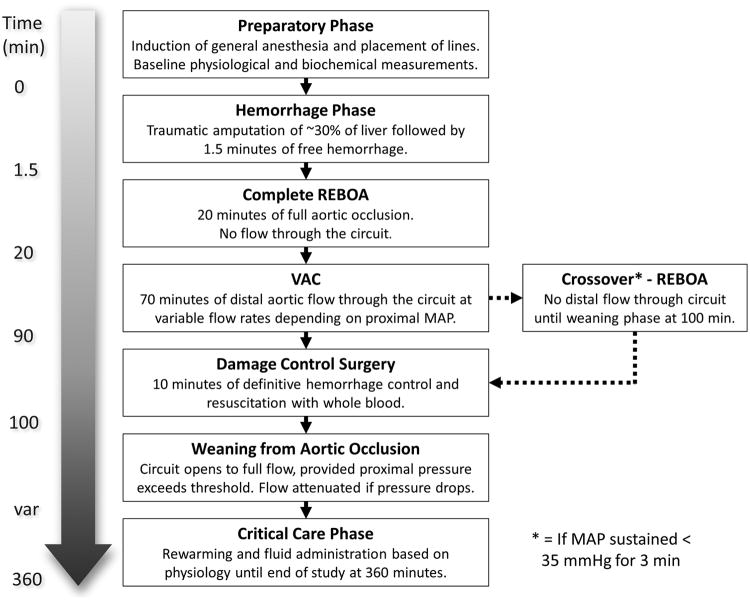
Following severe mixed venous and arterial liver injury and 1.5 minutes of free hemorrhage, animals underwent a 20-minute period of REBOA followed by restoration of variable, low-volume distal aortic flow using the extracorporeal circuit for the duration of the 90-minute period (Figure 1). Following this 90-minute period of REBOA with automated extra-corporeal perfusion (carotid to femoral), surgical hemostasis was performed via a laparotomy. The animal was then weaned from occlusion by gradually restoring flow through the extracorporeal circuit in response to proximal aortic pressure followed by REBOA catheter deflation. As would be the case in a real clinical scenario, these experiments were conducted in an “intention to treat” fashion. Animals unable to tolerate VAC reverted back to standard REBOA for the duration of the 70 minutes as a salvage maneuver.
Figure 1. Study Flow.
Animal Preparation
Animals were pre-medicated with 6.6 mg/kg tiletamine/zolazepam (TELAZOL, Fort Dodge Animal Health, Fort Dodge, IA) intramuscularly. Following isoflurane induction and endotracheal intubation, maintenance anesthesia consisted of 2% isoflurane in 100% oxygen. To overcome the vasodilatory effects of general anesthesia, an intravenous infusion of norepinephrine (0.01mcg/kg/hr) was initiated. Animals were mechanically ventilated with tidal volumes of 7-10 mL/kg and a respiratory rate of 10-15 breaths per minute sufficient to maintain end tidal CO2 at 40 ± 5 mm Hg. The pigs were placed on a warming blanked set at 39°C to minimize hypothermia.
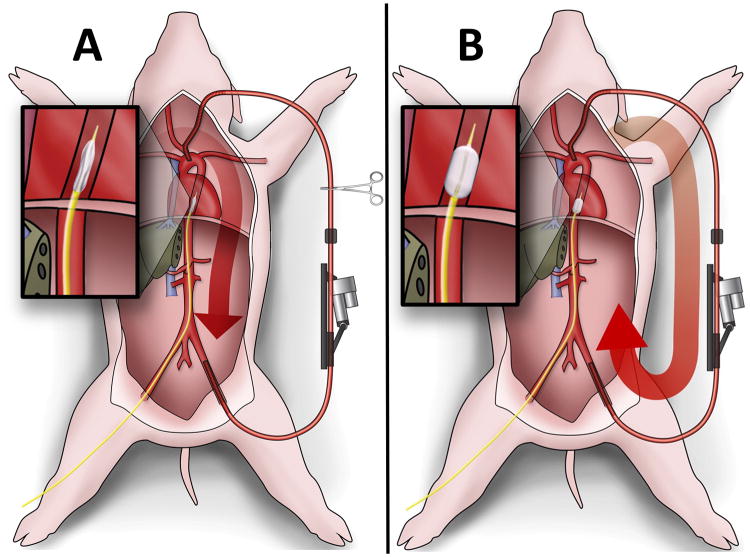
Both carotid arteries were exposed through a midline neck incision, the left brachial artery was exposed through an axillary incision, and the femoral arteries were accessed through separate oblique groin incisions. Arterial access was obtained for blood collection, proximal and distal hemodynamic monitoring, and to facilitate endovascular intervention. A 4mm perivascular Doppler flow probe (Transonic Corporation, Ithaca, NY) was secured around the right CCA to monitor cerebral blood flow. Additionally, a 12F 13cm introducer sheath (Cook Incorporated, Bloomington, IN) was inserted retrograde into the left FA, through which the occlusion balloon catheter was introduced (Figure 2A). Concurrently, a splenectomy was completed to minimize hemodynamic variation from auto-transfusion. Normal saline was administered at a maintenance rate of 5 mL/kg/hr.
Figure 2.
Schematic of Experimental Setup: A) Aortic cannulas placed in the carotid and femoral arteries are connected to the clamped circuit. B) Occlusion of the aorta with a CODA balloon and unclamping of the circuit diverted blood flow through the circuit. Data from the proximal pressure and inline flow monitors is relayed in real time back to the data acquisition system and on to the control box, which regulates flow in the circuit based on a prescribed algorithm.
Development of the Variable Aortic Control Circuit
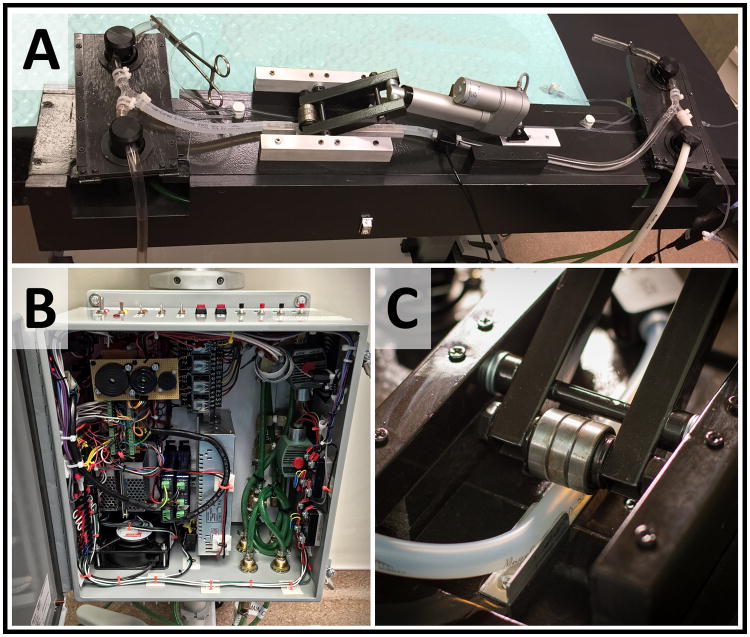
A custom extracorporeal, automated variable aortic control circuit capable of regulating distal aortic flow was constructed to experimentally replicate the desired functionality of EVAC when combined with REBOA (Figure 3A). The automation of the system was developed to facilitate a reliable and reproducible experimental design, minimizing potential for experimental bias. The hardware construct includes a commercially available flow monitor (Transonic, Ithaca, NY), a novel extracorporeal flow circuit (Figure 2A), and a custom designed control system (Figure 3B). Flow through the circuit was reliant on cardiac output and proximal blood pressure and regulated with a linear actuator that extrinsically compresses the circuit tubing (Figure 3c). In this manner, distal aortic flow was controlled across the spectrum from complete occlusion to unimpeded full flow.
Figure 3.
Components of the Variable Aortic Control Device: Transonic® TS410 Flowmeter receives input from an inline flow probe Custom control system utilizing an Arduino® microcontroller Flow circuit consists of a linear actuator, pneumatic, and electric pinch valves Close-up view of the linear actuator demonstrates the roller bearing/tubing interface
The configuration of the circuit was as follows. An 18Fr cannula was inserted into the left carotid artery and advanced to the level of the aortic arch. A second cannula was inserted retrograde into the left femoral artery to the level of the infrarenal aorta (Figure 2A). These cannulas are connected with 3/8 inch internal diameter tubing. To initiate flow through the circuit, REBOA was performed by positioning and inflating a CODA balloon (Cook Medical, Bloomington, IN) at the level of the diaphragm. To achieve distal flow, pneumatic pinch valves were unclamped to allow blood flow from the proximal carotid cannula through the VAC circuit and returning to the animal through the distal trans-femoral cannula. Circuit flow rates were assessed with an inline flow meter. The flow rate was precisely regulated to within +/- 5 mL/min of the prescribed flow rate utilizing the linear actuator. As the actuator extended and retracted, roller bearings on the opposing end of the lever arm variably compressed the tubing with a high degree of fidelity (approximately 0.01 mm step movement of the roller bearing) (Figure 2B). A complete technical description of the circuit control design is provided (Text, SDC 1).
Injury
The liver was marked along the planned transection plane, 2cm to the left of Cantlie's line, to provide amputation of approximately 80% of the left lateral lobe of the liver and 40% of the left medial lobe of the liver (approximately 30% of total liver volume) similar to previous descriptions.(10) At time 0, the liver was sharply transected and the abdomen rapidly closed. Complete occlusion of the aorta was achieved with REBOA 1.5 minutes following the initiation of injury (Figure 2B).
Intervention
REBOA and Restoration of Distal Aortic Flow with Automated Variable Aortic Control Circuit
Following 20 minutes of REBOA, automated variable aortic control was achieved by reintroducing oxygenated blood flow in a highly controlled fashion through the extracorporeal VAC circuit, around the inflated REBOA catheter, and into the distal aorta according to a pre-determined algorithm (Figure, SDC 2). Bypassing the inflated REBOA catheter with this circuit resulted in distal regional hypoperfusion for the remaining 70 minutes of the intervention period. During this period, the customized linear actuator on the VAC circuit continuously adjusted the degree of compression of the circuit tubing to maintain a pre-determined circuit flow rate provided MAP remained above a preset threshold of 50 mmHg (Figure, SDC 2). Below this threshold, the actuator restricted circuit blood flow to minimum flow rate of 100 mL/min to prevent death from complete cardiovascular collapse. Further, the VAC circuit was programmed to automatically clamp after 3 minutes of sustained pressure below 35 mmHg, reestablishing complete REBOA for the remainder of the 90-minute intervention period. Conversely, the VAC circuit increased the distal flow rate if a higher proximal MAP threshold was reached following 20 minutes of VAC. The flow rate was dynamic and based on proximal MAP, ranging from 100 mL/min to 300 mL/min (Figure, SDC 2). The initial flow rate of 150mL/min was determined during earlier iterations of model development, in which a flow rate of 150 mL/min after controlled hemorrhage of animals to class IV shock resulted in a proximal MAP of approximately 60 mmHg (data not shown). Two colloid boluses were allowed during VAC at T40 and T70, with administration predicated on a minimum MAP threshold and indicated by an audible alarm from the control system.
At the conclusion of the 90-minute intervention phase, the abdomen was reopened and liver hemorrhage was definitively controlled. Shed blood was quantified and fresh whole blood resuscitation was used to replenish the exact total volume of intra-abdominal shed blood.
Weaning from REBOA with Automated Variable Aortic Control Circuit and ICU Phase
Ten minutes following the start of damage control surgery, an automated weaning algorithm was initiated. When the proximal MAP exceeded the minimum threshold of 65 mmHg, the VAC circuit progressively opened to restore a full flow state. However, the circuit would also maintain or decrease flow to sustain the proximal MAP above 55 mmHg. Following a 5-minute period of full and unrestricted circuit flow, an alarm sounded to signal successful weaning from occlusion. The VAC circuit was clamped simultaneous to CODA balloon deflation to end REBOA and restore native inline aortic flow. The animal was then survived for 360 minutes from the start of the experiment. During this ICU phase, boluses were administered if the MAP dropped below 60 mmHg continuously for > 1 minute. The animals were euthanized after 360 minutes.
Data Collection
Physiologic data, including proximal and distal aortic pressures, visceral blood pressure (distal branch of the superior mesenteric artery), heart rate, core body temperature, right carotid artery blood flow, and ECG monitoring were continuously captured throughout the experiment using a multichannel data acquisition system (Biopac Systems Inc, Goleta, CA).
Results
Ex-vivo testing of the flow circuit revealed that the circuit and automated control mechanism were able to reliably achieve precise flow across all prescribed algorithms. (Figure, SDC 3A). During simulation of the weaning protocol, the circuit accurately reduced, increased or maintained the flow rate in response to the simulated proximal pressure (Figure, SDC 3B).
A total of 15 experiments were performed as part of this model validation study (6 model development and 9 model validation animals). Animal preparation, liver injury, open surgical technique, and post-hemorrhage resuscitation protocols were developed with the initial 6 model development animals. Two animals in the study group developed large air emboli from the liver injury and were excluded due to instant death after injury. The remaining 7 animals comprised the study cohort. All 7 sustained a large liver injury with intra-abdominal hemorrhage. The ACT for all animals was close to the desired target of 100 seconds. All 7 animals survived to the end of study and 2 reverted back to complete REBOA during the period of VAC due to sustained hypotension.
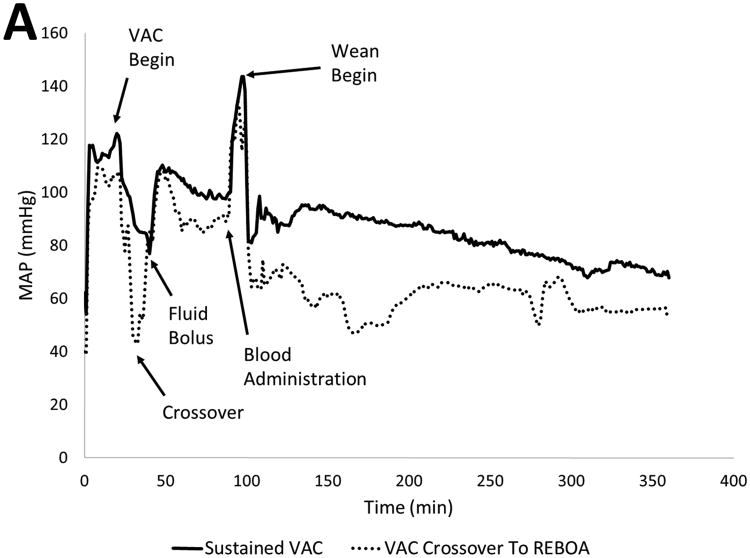
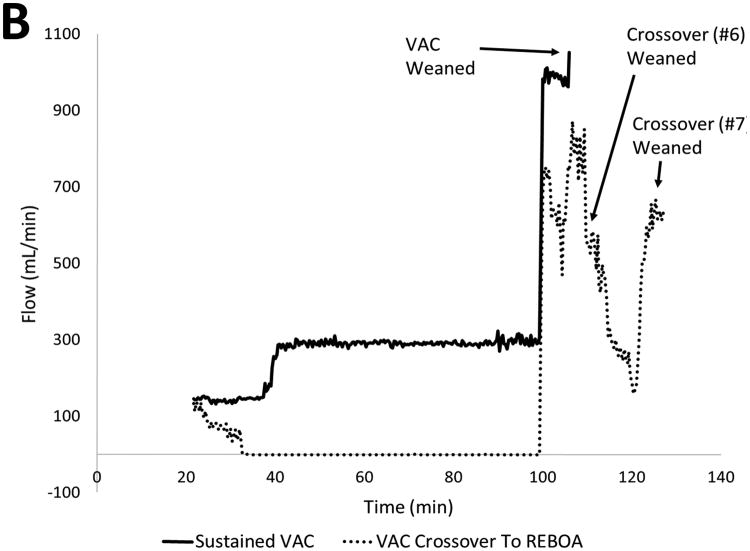
Baseline injury characteristics, resuscitation metrics, and outcomes are shown in Table 1. The 2 animals that did not tolerate distal flow required re-occlusion of the circuit after only 2 and 15 minutes, respectively. An averaged hemodynamic profile for all animals is shown in Figure 4A. The liver injury created a consistent and rapid decrease in the proximal MAP over the 1.5 minutes of free hemorrhage prior to intervention with a subsequent increase during the initial 20-minute period of complete REBOA. Animals that tolerated distal flow via the VAC circuit during the remaining 70 minutes consistently maintained a proximal MAP > 60mmHg, (Table 2). Furthermore, animals that tolerated sustained flow through the VAC circuit organ demonstrated continued distal organ function following the intervention with sustained urine production and lactate clearance. Animals that tolerated VAC were able to wean from partial flow to full native aortic flow without large fluctuations in proximal MAP (Figure 4B).
Table 1. Injury Characteristics, Resuscitation Metrics, and Outcomes.
| ID | Pre-Injury ACT | % Liver Resected | % EBL | Transfusion Volume | Crystalloids | Total UOP | Time to Wean (min) |
|---|---|---|---|---|---|---|---|
| 1 | 130 | 21 | 14.4 | 607 | 2800 | NA | 5.7 |
| 2 | 78 | 24 | 32.8 | 1358 | 2600 | 682 | 6.0 |
| 3 | 90 | 27 | 22.4 | 1354 | 4460 | 380 | 6.3 |
| 4 | 119 | 31 | 31.9 | 2004 | 4236 | 50 | 6.0 |
| 5 | 98 | 29 | 26.2 | 1618 | 4460 | 152 | 6.0 |
| 6* | 106 | 26 | 57.1 | 2709 | 4800 | 59 | 10.3 |
| 7* | 91 | 23 | 41.7 | 3104 | 5000 | 41 | 34.4 |
| Average | 101 | 25 | 32.5 | 1832 | 4050 | 227 | 10.7 |
| Std Dev | 16 | 3 | 12.8 | 794 | 886 | 234 | 9.8 |
Figure 4.
Hemodynamic Data: A) Proximal MAP throughout the experiment. Note animals requiring crossover sustained precipitous drop upon EVAC, however rebounded upon re-occlusion. Following damage control, animals that tolerating sustained EVAC maintained a MAP near baseline for the remainder of the study. B) Circuit flow during EVAC phase. Averaged continuous MAP for five animals that tolerated EVAC showed sustained initial flow at 150 mL/min, with flow rate of 300 mL/min beyond T40 and immediately weaned from REBOA.
Table 2. Hemodynamic and Laboratory Analysis.
| ID | Init MAP | Min Free-Bleed MAP | Max Occlusion MAP | Min VAC MAP | Δ Hgb | Min pH | Max BE | Max K+ | Max Lactate | Lactate T360 | Max Creat |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | 57 | 171 | 104 | 1.3 | 7.36 | 0.1 | 4.8 | 5.4 | 5.1 | 2.7 |
| 2 | 83 | 52 | 154 | 33 | 5.3 | 7.14 | -10.3 | 6.7 | 14.5 | 5.0 | 2.9 |
| 3 | 82 | 45 | 116 | 63 | 3.7 | 7.21 | -7 | 6.2 | 12.1 | 7.2 | 3.1 |
| 4 | 77 | 39 | 116 | 72 | 5.8 | 7.25 | -7.3 | 6.3 | 9.2 | 7.5 | 2.9 |
| 5 | 67 | 41 | 117 | 58 | 4.3 | 7.30 | -5.1 | 6.4 | 9.2 | 4.2 | 3.8 |
| 6* | 76 | 32 | 67 | 17 | 5.7 | 7.01 | -12.2 | 6.8 | 13.3 | 6.7 | 2.6 |
| 7* | 71 | 47 | 162 | 29 | 6 | 7.03 | -14.7 | 6.8 | 13.4 | 10.1 | 2.9 |
| Average | 76 | 44 | 129 | 53 | 4.6 | 7.18 | -8.1 | 6.3 | 11.1 | 6.5 | 2.98 |
| Std Dev | 5 | 7 | 33 | 27 | 1.5 | 0.12 | 4.9 | 0.6 | 2.9 | 1.8 | 0.36 |
Discussion
The objective of this study was to demonstrate the feasibility of REBOA coupled with a novel automated extracorporeal circuit in order to establish a predicate model with which to study the emerging concept of automated EVAC. Findings demonstrate that initiation of this circuit in conjunction with complete REBOA effectively regulates proximal aortic pressure within desirable and pre-determined parameters while providing a variable degree of distal aortic perfusion that mitigates ischemia without leading to exsanguination. Observations from this study show complete survival from what is an otherwise uniformly fatal injury with a preserved distal organ function after 90 minutes of intervention and before definitive surgical control of hemorrhage. This study supports the principle of “permissive regional hypoperfusion” in the setting of non-compressible torso hemorrhage, provides a model to study it, and suggests a transforming potential for automated, endovascular variable aortic control (EVAC) in the setting of shock.
The advancement of endovascular technologies in response to ongoing clinical and translational research has resulted in the development of REBOA as a viable alternative to traditional thoracotomy and aortic cross clamping. However, REBOA still results in a significant ischemic insult to distal tissue beds and is only tolerated in short intervals.(3, 11) Extended periods of complete aortic occlusion can result in irreversible ischemic injury, permanent organ failure, limb ischemia, and death.(3, 4) Unfortunately, these consequences restrict the application of REBOA in many clinical scenarios to a technique of last resort. However, EVAC may offer new therapeutic options for non-compressible truncal injury. Similar to REBOA, EVAC would lessen the physiologic impact of aortic occlusion compared to traditional thoracotomy and aortic cross clamping. Additionally, EVAC maneuvers would likely require an abbreviated period of complete aortic occlusion to provide for early hemodynamic recovery and clot stabilization. What distinguishes EVAC from current resuscitative strategies is the early restoration of low volume distal flow to minimize the ischemic burden of sustained complete aortic occlusion. Furthermore, control of an EVAC device with smart technology is conceivable to regulate distal flow based on the patient's physiology. Moreover, the expanded capability of future low-profile endovascular devices would allow EVAC to be applied more liberally and earlier in the patient's care. The results of our current study highlight that this approach is rational and feasible.
The model in this series of experiments was constructed to be a temporary surrogate of automated variable aortic control and not the end solution. While examples exist in cardiovascular surgery of the use of extra-corporeal circuits to offload the left ventricle during aortic cross clamp and to deliver distal aortic perfusion during an aortic repair, none are automated to regulate a desired range of proximal aortic pressure or distal flow and are too cumbersome to be used in settings of trauma.(12, 13) This model confirms the effectiveness of the general approach to left ventricular off-loading and extends the concept to the setting of trauma and shock. Additionally, the model in this study, including the pressure-responsive computer algorithm of the circuit, provides a means by which to study the principles of automated, variable aortic control and permissive regional hypoperfusion in the setting of torso hemorrhage. The design of this model should also allow for the more efficient development of catheter-based devices including ones to accomplish regulated endovascular variable aortic occlusion (EVAC).
Our previous attempts to mitigate the deleterious effects of REBOA with existing endovascular technology utilized a similar porcine liver injury model.(10) A significant limitation of this prior experimental model of partial REBOA with balloon catheters was an inability to quantify or control the amount of blood flow being delivered to the distal aorta. Instead of measuring direct flow, the degree of occlusion was based on the pressure gradient proximal and distal to the balloon. Our current experience suggests an uncoupling of the relationship between pressure gradient and flow particularly during periods when circulating blood volume and/or cardiac output are changing (Figure, SDC 4). This finding led us to surmise that flow rates in prior studies may have exceeded what was physiologically tolerable in the face of an uncontrolled vascular injury. Thus, the lack of current balloon catheter technology to measure and precisely regulate distal aortic flow was the impetus for redesigning the experimental setup and creating an automated computer-controlled model of variable aortic control.
Our bench-top testing has validated the feasibility of tightly regulating flow with computer-controlled algorithms in an ex-vivo flow circuit (Figures, SDC 3 and SDC 4). Additionally, we have been able to replicate this precise control in our large animal model. Utilizing the described EVAC resuscitation paradigm, we have noted significant improvement in immediate survival for animals undergoing EVAC as compared to previous work with a similar liver injury model.(10) In this present study, all animals survived to the end of the experiment. This suggests that even in critically injured animals, attempts to reinstate partial distal flow did not ultimately compromise short-term salvage of the animal.
Current technologies in endovascular aortic occlusion are dependent on manual inflation of a REBOA balloon.(6, 14, 15) Small changes in the diameter of an occlusion balloon result in large changes in flow (based on the Poiseuille's Law), making control of distal flow by manual adjustment of balloon diameter extremely difficult. While waiting for the technological advances that will make endovascular graded occlusion of the aorta possible, we have utilized our extracorporeal circuit as a stepping stone to demonstrate the utility of automated computer control of variable aortic occlusion. The development of catheters capable of EVAC with computer-controlled automation promises a more efficient and responsive system during resuscitation. Furthermore, the creation of an automated endovascular aortic occlusive device will help “cognitively offload” healthcare providers and free them to focus efforts on key manual resuscitative interventions, from the preparation of a patient for transport to providing definitive surgical hemorrhage control, while automated aortic control maximizes a patient's physiology.
Automated computer-controlled variable aortic control offers new advances in weaning patients from aortic occlusion. Current methods of weaning a patient from complete aortic occlusion are imprecise and poorly studied. Upon reintroduction of aortic flow, the redistribution of blood with accumulated ischemic metabolites results in ischemia reperfusion injury, systemic inflammatory response, and often hemodynamic collapse.(16, 17) As many as 50% of patients treated with traditional REBOA require re-occlusion of the aorta, adding to the existing ischemic burden.(3, 4) Besides minimizing distal ischemia through partial aortic flow, an automated-computer controlled model of EVAC provides an alternative to the dichotomous open or occluded paradigm provided by REBOA. These initial experiments have demonstrated that our weaning protocol is able to rapidly transition from complete occlusion to open flow automatically in under 20 minutes. The weaning in both animals that required re-occlusion of the aorta occurred successfully without hemodynamic collapse utilizing this algorithm despite significant ischemic burden at the time of initiation of weaning. Moreover, the sustained distal aortic flow for the 5 other animals facilitated immediate weaning from occlusion, presumably due to decreased distal ischemic burden. This rapid return of full aortic flow further underscores how EVAC might minimize the physiologic impact of aortic occlusion.
Beyond translational experiments for hemorrhage, the variable aortic control afforded by this experimental model could be utilized in polytrauma models to evaluate effects of supraphysiologic blood flow on proximal vascular beds. In particular, this model is ideally suited to evaluate effects of partial and complete aortic occlusion on patients with concomitant traumatic brain injury (TBI), a patient group that has faired poorly with traditional REBOA techniques.(3, 4) This expanded capability may even limit the inherent physiologic variation in large animal experiments by providing precise regulation of proximal or distal arterial blood pressure or flow for extended periods of time. Such applications could accelerate the transition toward more clinically relevant models of traumatic brain injury and ischemia-reperfusion injury.
The present proof-of-concept study is limited by a lack of a comparison group receiving only REBOA for the entire 90-minute intervention phase and prevents broader interpretation of the results. Likewise, these experiments did not include a group in which no intervention was performed after injury. However, the author's previous experience utilizing a similar liver injury model suggests a uniformly, rapidly fatal injury in the absence of expeditious application of complete REBOA.(10) Additionally, the use of large diameter aortic cannulas in the femoral artery likely resulted in flow restriction to the hindlimb of the animal and may have artificially increased the ischemic burden. Limitations notwithstanding, this study provides an important first step toward novel resuscitation paradigms and experimental methodology.
Conclusion
This study demonstrates the life-saving potential of a novel, automated, extracorporeal circuit used in conjunction with complete resuscitative endovascular balloon occlusion of the aorta. Initiation of the extracorporeal circuit in this study regulated proximal aortic pressure within a pre-determined range, alleviating supra-normal values above the balloon occlusion. This approach also provided controlled distal aortic perfusion that reduced end-organ ischemia without inducing intolerable bleeding, confirming the principle of permissive regional hypoperfusion. The endovascular-extracorporeal approach in this study serves as a temporary surrogate for future automated variable aortic control designs – including the development of low profile, endovascular devices – that should be pursued as potentially transformational approaches to non-compressible torso hemorrhage and shock.
Supplementary Material
Text, Supplemental Digital Content 1. Control of the VAC circuit was accomplished using a custom control panel (Figure 3B). This device housed the hardware and components necessary to provide automation of the experimental process, with electrical and pneumatic input and output connections for all associated equipment. The Arduino Mega 2560 programmable microcontroller (Arduino, Sommerville, MA) provided the computational control for the circuit. The control panel received flow and pressure data from the Transonic TS410 flowmeter and the Biopac MP150 data acquisition system (Biopac Systems Incorporated, Goleta, California), respectively. The microcontroller communicated via a serial connection to a PC, which served as an interface terminal for calibration, initialization of and data acquisition from the system. The software for control of the system was developed within the Arduino software integrated development environment (IDE), utilizing custom algorithms to precisely regulate flow through the circuit based on closed loop feedback between the flowmeter and the actuator position. The actuator was controlled by a motor controller (Pololu Jrk 21v3 USB motor controller, Las Vegas, Nevada) housed within the flow circuit assembly and received input via a serial connection from the main control panel.
Figure, Supplemental Digital Content 2. Experimental Design and Flow Algorithm: Sequence of events from initial cannulation through the end of experiment is outlined. The current study design includes four experimental arms, partial flow with wean (a) no flow with wean (b), partial flow without wean (c) and no flow without wean (d). Animals within the partial flow arm can crossover to complete circuit occlusion if the proximal pressure is sustained below 35 mmHg for 3 minutes. (MAP, mean arterial pressure)
Figure, Supplemental Digital Content 3. Impact of Simulated Pressure on Circuit Flow: A) Flow is initiated at 21.5 minutes, with a maximum flow rate of 150 mL/min until T40, as determined by the proximal pressure. When pressure is greater than 50 mmHg (graph regions 1,2,3), flow is at 150 mL/min. When pressure is less than 50 mmHg (graph region 4), flow is 100 mL/min. Beyond T40 through T100, higher flow rates are permitted (> 70 mmHg (1) = 300 mL/min (a), 60-70 mmHg (2) = 200 mL/min (b), 50-60 mmHg (3) = 150 mL/min (c), < 50 mmHg (4) = 100 mL/min). B) At T100, weaning occurs based on proximal pressure. When pressure is greater than 65 mmHg, the circuit flow will progressively increase to full open. Flow remains unchanged when pressure is between 55 mmHg and 65 mmHg. Flow decreases toward a minimal rate of 100 mL/min when pressure drops below 55 mmHg. The rate of opening or closing is determined by the difference of the pressure from the set point (i.e. a pressure of 100 mmHg results in faster weaning than a pressure of 70 mmHg). (MAP, mean arterial pressure)
Figure, Supplemental Digital Content 4. VAC Flow and Pressure Gradient Over Time: While pressure and flow at steady state may correlate, changes is other physiologic parameters may influence this relationship. A) During initiation of VAC following 20 mins of REBOA, the pressure gradient precipitously declines, while flow rate remains constant due to automated circuit control. This may be due to changes in volume status from ongoing blood loss, decreased cardiac output or other factors. However, after equilibration to VAC, pressure gradient and flow remain stable. B) Upon whole blood resuscitation, note that pressure gradient increases sharply while flow remains stable. This suggests that a converse strategy, where pressure gradient is tightly regulated, would result in variable and unpredictable flow rates.
Acknowledgments
The authors would like to acknowledge the tremendous support of Dr. J. Kevin Grayson, DVM, LtCol Robin Mitchell, Mr. Oren Gotlib, SSgt Elaine Spotts, SSgt Kelly Caneen, SrA Geoffrey O'Hair, Ms. Sally Knode, Mr. Robert Gibbons, SSgt Vanessa Lang, Mrs. Diane Gonzalez, Mr. Aric Sawyer, SrA Anna Ferro, Mrs. Eileen Foster and the entire staff of the 60th Clinical Investigation Facility, Travis AFB, CA.
Disclaimer: The animals involved in this study were procured, maintained, and used in accordance with the Laboratory Animal Welfare Act of 1966, as amended, and NIH 80-23, Guide for the Care and Use of Laboratory Animals, National Research Council.
The views expressed in this material are those of the authors, and do not reflect the official policy or position of the U.S. Government, the Department of Defense, the Department of the Air Force, or the University of California Davis. The work reported herein was performed under United States Air Force Surgeon General approved Clinical Investigation No. FDG20150002A.
There was no funding from the National Institutes of Health (NIH), Wellcome Trust, or the Howard Hughes Medical Institute (HHMI) for this work. No conflicts of interest were declared by any of the authors. The Clinical Investigation Facility, David Grant USAF Medical Center, Travis Air Force Base, California provided funding for this study.
Footnotes
Author Contributions: All authors contributed to the literature search and study design. T.K.W., L.P.N., M.A.J., R.M.R., S-A.F., and A.J.D. collected data. T.K.W., L.P.N., M.A.J., and T.E.R. performed data analysis and interpretation. T.K.W., L.P.N., M.A.J., and T.E.R. wrote the manuscript, which all authors critically revised.
Contributor Information
Timothy K. Williams, Email: timothy.williams.72@us.af.mil.
Lucas P. Neff, Email: lucas.neff.2@us.af.mil.
Michael A. Johnson, Email: ausjohnson@ucdavis.edu.
Rachel M. Russo, Email: rmrusso@ucdavis.edu.
Sarah-Ashley Ferencz, Email: saferencz@ucdavis.edu.
Anders J. Davidson, Email: ajdavidson@ucdavis.edu.
Todd E. Rasmussen, Email: todd.e.rasmussen.mail@mail.mil.
References
- 1.Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma Acute Care Surg. 2011;71(6):1869–72. doi: 10.1097/TA.0b013e31823fe90c. [DOI] [PubMed] [Google Scholar]
- 2.Brenner M, Moore L, Dubose J, Tyson G, McNutt M, Albarado R, Holcomb J, Scalea T, Rasmussen T. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma. 2013;75(3):506–11. doi: 10.1097/TA.0b013e31829e5416. [DOI] [PubMed] [Google Scholar]
- 3.Saito N, Matsumoto H, Yagi T, Hara Y, Hayashida K, Motomura T, Mashiko K, Iida H, Yokota H, Wagatsuma Y. Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2015;78(5):897–904. doi: 10.1097/TA.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 4.Matsuoka S, Uchiyama K, Shima H, Ohishi S, Nojiri Y, Ogata H. Temporary percutaneous aortic balloon occlusion to enhance fluid resuscitation prior to definitive embolization of post-traumatic liver hemorrhage. Cardiovasc Intervent Radiol. 2001;24(4):274–6. doi: 10.1007/s00270-001-0003-0. [DOI] [PubMed] [Google Scholar]
- 5.Biffl WL, Fox CJ, Moore EE. The role of REBOA in the control of exsanguinating torso hemorrhage. J Trauma Acute Care Surg. 2015;78(5):1054–8. doi: 10.1097/TA.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 6.Morrison JJ, Ross JD, Rasmussen TE, Midwinter MJ, Jansen JO. Resuscitative endovascular balloon occlusion of the aorta: a gap analysis of severely injured UK combat casualties. Shock. 2014;41(5):388–93. doi: 10.1097/SHK.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen T, Baer D, Doll B, Caravalho J. In the ‘golden hour’: combat casualty care research drives innovation to improve survivability and reimagine future combat care. Army AL&T Magazine. 2015:80–5. [Google Scholar]
- 8.Bickell WH, Wall MJ, Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331(17):1105–9. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 9.Hammer M, Jovin T, Wahr JA, Heiss WD. Partial occlusion of the descending aorta increases cerebral blood flow in a nonstroke porcine model. Cerebrovascular Diseases. 2009;28(4):406–10. doi: 10.1159/000235628. [DOI] [PubMed] [Google Scholar]
- 10.Russo RM, Williams TK, Grayson JK, Lamb CM, Cannon JW, Clement NF, Galante JM, Neff LP. Extending The Golden Hour: Partial Resuscitative Endovascular Balloon Occlusion Of The Aorta (P-REBOA) In A Highly Lethal Swine Liver Injury Model. J Trauma Acute Care Surg. 2016 doi: 10.1097/TA.0000000000000940. In Press. [DOI] [PubMed] [Google Scholar]
- 11.Avaro JP, Mardelle V, Roch A, Gil C, de Biasi C, Oliver M, Fusai T, Thomas P. Forty-minute endovascular aortic occlusion increases survival in an experimental model of uncontrolled hemorrhagic shock caused by abdominal trauma. J Trauma. 2011;71(3):720–6. doi: 10.1097/TA.0b013e318221a94a. [DOI] [PubMed] [Google Scholar]
- 12.Comerota AJ, White JV. Reducing morbidity of thoracoabdominal aneurysm repair by preliminary axillofemoral bypass. The American journal of surgery. 1995;170(2):218–22. doi: 10.1016/s0002-9610(99)80290-6. [DOI] [PubMed] [Google Scholar]
- 13.Coselli JS, editor. The use of left heart bypass in the repair of thoracoabdominal aortic aneurysms: current techniques and results. Semin Thorac Cardiovasc Surg. 2003 Oct;15(4):326–32. doi: 10.1053/s1043-0679(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 14.White JM, Cannon JW, Stannard A, Markov NP, Spencer JR, Rasmussen TE. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery. 2011;150(3):400–9. doi: 10.1016/j.surg.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Markov NP, Percival TJ, Morrison JJ, Ross JD, Scott DJ, Spencer JR, Rasmussen TE. Physiologic tolerance of descending thoracic aortic balloon occlusion in a swine model of hemorrhagic shock. Surgery. 2013;153(6):848–56. doi: 10.1016/j.surg.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Gelman S, Khazaeli M, Orr R, Henderson T, Reves J. Blood volume redistribution during cross-clamping of the descending aorta. Anesth Analg. 1994;78(2):219–24. doi: 10.1213/00000539-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Gelman S, McDowell H, Varner PD, Pearson J, Ebert J, Graybar G, Proctor J. The reason for cardiac output reduction after aortic cross-clamping. Am J Surg. 1988;155(4):578–86. doi: 10.1016/s0002-9610(88)80413-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text, Supplemental Digital Content 1. Control of the VAC circuit was accomplished using a custom control panel (Figure 3B). This device housed the hardware and components necessary to provide automation of the experimental process, with electrical and pneumatic input and output connections for all associated equipment. The Arduino Mega 2560 programmable microcontroller (Arduino, Sommerville, MA) provided the computational control for the circuit. The control panel received flow and pressure data from the Transonic TS410 flowmeter and the Biopac MP150 data acquisition system (Biopac Systems Incorporated, Goleta, California), respectively. The microcontroller communicated via a serial connection to a PC, which served as an interface terminal for calibration, initialization of and data acquisition from the system. The software for control of the system was developed within the Arduino software integrated development environment (IDE), utilizing custom algorithms to precisely regulate flow through the circuit based on closed loop feedback between the flowmeter and the actuator position. The actuator was controlled by a motor controller (Pololu Jrk 21v3 USB motor controller, Las Vegas, Nevada) housed within the flow circuit assembly and received input via a serial connection from the main control panel.
Figure, Supplemental Digital Content 2. Experimental Design and Flow Algorithm: Sequence of events from initial cannulation through the end of experiment is outlined. The current study design includes four experimental arms, partial flow with wean (a) no flow with wean (b), partial flow without wean (c) and no flow without wean (d). Animals within the partial flow arm can crossover to complete circuit occlusion if the proximal pressure is sustained below 35 mmHg for 3 minutes. (MAP, mean arterial pressure)
Figure, Supplemental Digital Content 3. Impact of Simulated Pressure on Circuit Flow: A) Flow is initiated at 21.5 minutes, with a maximum flow rate of 150 mL/min until T40, as determined by the proximal pressure. When pressure is greater than 50 mmHg (graph regions 1,2,3), flow is at 150 mL/min. When pressure is less than 50 mmHg (graph region 4), flow is 100 mL/min. Beyond T40 through T100, higher flow rates are permitted (> 70 mmHg (1) = 300 mL/min (a), 60-70 mmHg (2) = 200 mL/min (b), 50-60 mmHg (3) = 150 mL/min (c), < 50 mmHg (4) = 100 mL/min). B) At T100, weaning occurs based on proximal pressure. When pressure is greater than 65 mmHg, the circuit flow will progressively increase to full open. Flow remains unchanged when pressure is between 55 mmHg and 65 mmHg. Flow decreases toward a minimal rate of 100 mL/min when pressure drops below 55 mmHg. The rate of opening or closing is determined by the difference of the pressure from the set point (i.e. a pressure of 100 mmHg results in faster weaning than a pressure of 70 mmHg). (MAP, mean arterial pressure)
Figure, Supplemental Digital Content 4. VAC Flow and Pressure Gradient Over Time: While pressure and flow at steady state may correlate, changes is other physiologic parameters may influence this relationship. A) During initiation of VAC following 20 mins of REBOA, the pressure gradient precipitously declines, while flow rate remains constant due to automated circuit control. This may be due to changes in volume status from ongoing blood loss, decreased cardiac output or other factors. However, after equilibration to VAC, pressure gradient and flow remain stable. B) Upon whole blood resuscitation, note that pressure gradient increases sharply while flow remains stable. This suggests that a converse strategy, where pressure gradient is tightly regulated, would result in variable and unpredictable flow rates.