Abstract
Background and Purpose
In heart failure (HF), left ventricular ejection fraction (LVEF) is inversely associated with mortality and cardiovascular (CV) outcomes. Its relationship with stroke is controversial, as is the effect of antithrombotic treatment. We studied the relationship of LVEF with stroke and CV events in HF patients, and the effect of different antithrombotic treatments.
Methods
In the Warfarin versus Aspirin in Reduced Ejection Fraction (WARCEF) trial, 2305 patients with systolic HF (LVEF ≤ 35%) and sinus rhythm were randomized to warfarin or aspirin and followed for 3.5±1.8 years. While no differences between treatments were observed on primary outcome (death, stroke or intracerebral hemorrhage), warfarin decreased the stroke risk. The present report compares the incidence of stroke and CV events across different LVEF and treatment subgroups.
Results
Baseline LVEF was inversely and linearly associated with primary outcome, mortality and its components (sudden and CV death) and HF hospitalization, but not myocardial infarction. A relationship with stroke was only observed for LVEF <15% (incidence rates: 2.04 vs. 0.95/100 pt. yrs; p=0.009), which more than doubled the adjusted stroke risk (adjusted HR: 2.125, 95% CI 1.182, 3.818; p=0.012). In warfarin-treated patients, each 5% LVEF decrement significantly increased the stroke risk (adjusted HR: 1.346, 95% CI 1.044, 1.737; p=0.022; p-value for interaction=0.04).
Conclusions
In patients with systolic HF and sinus rhythm, LVEF is inversely associated with death and its components, whereas an association with stroke exists for very low LVEF values. An interaction with warfarin treatment on stroke risk may exist.
Clinical Trial Registration - URL:http://www.clinicaltrials.gov. Unique identifier: NCT00041938
Keywords: Heart failure, echocardiography, transthoracic, heart ventricles, stroke, warfarin, aspirin, Mortality/Survival, Ischemic Stroke
In patients with heart failure (HF), a reduced left ventricular (LV) systolic function is associated with an increase in mortality and incidence of cardiovascular (CV) events. LV ejection fraction (LVEF) is the most widely accepted indicator of LV systolic function and is associated with CV outcomes. LVEF was shown to be inversely associated with CV mortality up to an LVEF of 45%, above which level the association is lost. In the Candesartan in Heart Failure Reduction in Mortality (CHARM) trial, all-cause mortality increased by 39% for every 10% reduction in LVEF below 45%.1 Similar results were reported by the Digitalis Investigation Group (DIG).2
Some aspects of the relationship between LVEF and CV events in HF remain controversial. An association between decreased LVEF and thromboembolic events (stroke or peripheral embolism) has been alternatively suggested3 or refuted.1, 2 While HF patients are often treated with antithrombotic agents (antiplatelet or systemic anticoagulation) to prevent thromboembolic complications and especially ischemic stroke, it is unclear whether lower LVEF may have a different impact on outcome depending on the antithrombotic treatment chosen.
In the Warfarin versus Aspirin in Reduced Ejection Fraction (WARCEF) trial, 2305 HF patients were randomized to aspirin or adjusted-dose warfarin and followed up for an average of 3.5 years. In the main results of the trial, patients on either treatment had similar rates of death and primary outcome (death, stroke or intracerebral hemorrhage), although patients on warfarin had significantly reduced incidence of stroke.4, 5 Here, we analyze the relationship of LVEF with mortality, stroke and CV outcomes in WARCEF, and possible interactions between LVEF and antithrombotic treatment.
MATERIALS AND METHODS
Study Patients
Details of the WARCEF trial enrollment were previously published.4 Briefly, from October 2002 through January 2010, a total of 2305 patients were enrolled in the trial (1119 in the United States and Canada and 1186 in Europe and Argentina) at 168 centers in 11 countries. Eligible patients were 18 years of age or older and had normal sinus rhythm, no contraindication to warfarin therapy, and an LVEF of 35% or less as assessed by quantitative echocardiography (or a wall-motion index of ≤1.2) or by radionuclide or contrast angiography within 3 months before randomization.
For details on eligibility criteria and study medications, please see online supplement at http://stroke.ahajournals.org. Patients were randomized to either adjusted dose-warfarin with target international normalized ratio (INR) of 2.75 (acceptable range: 2.0 to 3.5), or aspirin 325 mg daily in a double-blind, double-dummy design.
LVEF determination
LVEF assessment was performed by echocardiography at the individual sites. Mean time from echocardiogram performance to enrollment was 6.5 days. All echocardiograms were re-interpreted, blinded to treatment assignment, at a core echocardiography laboratory to confirm LVEF assessment. LVEF was determined by contrast angiography, radionuclide scanning, or MRI in 239 patients (10.4%).
Follow-up and outcome events
Follow-up was performed monthly by telephone or in person. An in person assessment was conducted quarterly for clinical evaluation.
Primary outcome of the trial was time to first event in a composite end point of ischemic stroke, intracerebral hemorrhage, or death. Individual outcomes were also recorded. For definitions of outcome events, please see online supplement.
Statistical analysis
Baseline demographics, clinical characteristics and various outcome events by LVEF categories were compared using ANOVA F-tests for continuous variables, Chi-squared tests for categorical variables, and log-rank tests for time-to-event outcomes. Univariable and multivariable Cox models were used to assess the effect of demographic and clinical variables on outcomes of interest.
To identify high-risk thresholds, we dichotomized LVEF at different cutoff points, and assessed the association between dichotomized LVEF and each outcome first with univariable Cox models, and then with adjustment for covariates. Incidence rates of outcome events stratified by optimal LVEF cutoff points were compared using Poisson regression.
Cox models were used to evaluate separately the association between LVEF and outcomes in patients treated with warfarin or aspirin, and assess any interaction between LVEF level and treatment type.
For warfarin treatment, time in therapeutic range (TTR) was compared in different stroke and LVEF subgroups using Wilcoxon’s rank sum test.
RESULTS
Mean LVEF in the study cohort was 24.7±7.5. Demographics and clinical characteristics of the cohort by LVEF category are illustrated in Table 1.
Table 1.
Demographics and clinical characteristics of the study cohort by LVEF categories *
| covariate | LVEF < 20% (n=603) |
LVEF 21%-25% (n=559) |
LVEF 26%-29% (n=533) |
LVEF ≥ 30% (n=610) |
p-value |
|---|---|---|---|---|---|
| Location | <0.001 | ||||
| Argentina | 18/603 (3.0 ) | 29/559 (5.2 ) | 15/533 (2.8 ) | 30/610 (4.9 ) | . |
| Europe | 284/603 (47.1 ) | 221/559 (39.5 ) | 249/533 (46.7 ) | 340/610 (55.7 ) | . |
| North America | 301/603 (49.9 ) | 309/559 (55.3 ) | 269/533 (50.5 ) | 240/610 (39.3 ) | . |
| Age - yr | 59.6 ± 11.6 | 60.6 ± 11.2 | 60.9 ± 11.4 | 62.0 ± 11.1 | 0.004 |
| Male sex | 486/603 (80.6 ) | 438/556 (78.8 ) | 419/531 (78.9 ) | 497/610 (81.5 ) | 0.598 |
| race or ethnic group | 0.018 | ||||
| Non-Hispanic white | 440/603 (73.0 ) | 407/555 (73.3 ) | 395/531 (74.4 ) | 491/610 (80.5 ) | . |
| Non-Hispanic black | 106/603 (17.6 ) | 88/555 (15.9 ) | 76/531 (14.3 ) | 62/610 (10.2 ) | . |
| Hispanic | 37/603 (6.1 ) | 47/555 (8.5 ) | 41/531 (7.7 ) | 41/610 (6.7 ) | . |
| Other | 20/603 (3.3 ) | 13/555 (2.3 ) | 19/531 (3.6 ) | 16/610 (2.6 ) | . |
| height - cm | 171.6 ± 9.2 | 171.8 ± 9.4 | 171.4 ± 9.1 | 171.8 ± 9.4 | 0.841 |
| weight - kg | 85.6 ± 20.0 | 86.0 ± 19.5 | 86.8 ± 19.7 | 86.2 ± 18.7 | 0.768 |
| Body-mass index, kg/m2 | 29.0 ± 6.3 | 29.0 ± 5.8 | 29.4 ± 5.9 | 29.1 ± 5.8 | 0.666 |
| Heart rate - beats/min | 74.5 ± 12.9 | 71.3 ± 11.9 | 71.4 ± 11.1 | 70.5 ± 11.5 | <0.001 |
| Educational level | 0.574 | ||||
| < High school | 248/603 (41.1 ) | 235/553 (42.5 ) | 238/530 (44.9 ) | 271/609 (44.5 ) | . |
| High-school graduate or some college |
267/603 (44.3 ) | 231/553 (41.8 ) | 213/530 (40.2 ) | 236/609 (38.8 ) | . |
| College graduate or postgraduate |
88/603 (14.6 ) | 87/553 (15.7 ) | 79/530 (14.9 ) | 102/609 (16.7 ) | . |
| Systolic blood pressure - mmHg | 120.5 ± 18.2 | 122.3 ± 18.3 | 125.5 ± 18.5 | 127.6 ± 19.5 | <0.001 |
| Diastolic blood pressure - mmHg | 74.2 ± 11.3 | 73.5 ± 11.5 | 74.2 ± 11.6 | 74.9 ± 11.4 | 0.203 |
| NYHA classification | 0.135 | ||||
| I | 69/599 (11.5 ) | 75/553 (13.6 ) | 78/529 (14.7 ) | 93/609 (15.3 ) | . |
| II | 324/599 (54.1 ) | 314/553 (56.8 ) | 289/529 (54.6 ) | 340/609 (55.8 ) | . |
| III | 195/599 (32.6 ) | 154/553 (27.8 ) | 158/529 (29.9 ) | 173/609 (28.4 ) | . |
| IV | 11/599 (1.8 ) | 10/553 (1.8 ) | 4/529 (0.8 ) | 3/609 (0.5 ) | . |
| Distance covered on 6-minute walk - m |
353.5 ± 147.5 | 339.1 ± 146.2 | 340.4 ± 139.4 | 369.1 ± 151.5 | 0.002 |
| Pacemaker or defibrillator | 141/603 (23.4 ) | 142/551 (25.8 ) | 132/531 (24.9 ) | 109/610 (17.9 ) | 0.006 |
| Serum creatinine - mg/dL | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3 | 1.2 ± 0.3 | 0.677 |
| eGFR, mL/min | 69.0 ± 19.9 | 67.6 ± 20.0 | 69.5 ± 22.0 | 67.7 ± 20.6 | 0.310 |
| hemoglobin - g/dL | 14.1 ± 1.6 | 14.0 ± 1.6 | 14.0 ± 1.5 | 14.1 ± 1.6 | 0.731 |
| Serum sodium - mEq/L | 139.2 ± 6.4 | 139.4 ± 3.3 | 139.7 ± 3.3 | 140.0 ± 3.4 | 0.015 |
| Medical Comorbidities | |||||
| Diabetes Mellitus | 182/603 (30.2 ) | 183/551 (33.2 ) | 163/531 (30.7 ) | 194/609 (31.9 ) | 0.699 |
| Hypertension | 330/580 (56.9 ) | 300/530 (56.6 ) | 331/520 (63.7 ) | 406/602 (67.4 ) | <0.001 |
| Ischemic Cardiomyopathy | 254/603 (42.1 ) | 247/550 (44.9 ) | 239/531 (45.0 ) | 251/609 (41.2 ) | 0.453 |
| Myocardial Infarction | 263/602 (43.7 ) | 276/551 (50.1 ) | 273/531 (51.4 ) | 300/610 (49.2 ) | 0.045 |
| Atrial Fibrillation | 18/603 (3.0 ) | 25/551 (4.5 ) | 20/531 (3.8 ) | 23/610 (3.8 ) | 0.588 |
| Peripheral Vascular Disease | 55/603 (9.1 ) | 58/559 (10.4 ) | 70/533 (13.1 ) | 78/610 (12.8 ) | 0.092 |
| Prior stroke or TIA | 67/603 (11.1 ) | 68/551 (12.3 ) | 73/531 (13.7 ) | 86/610 (14.1 ) | 0.393 |
| Alcohol Consumption | <0.001 | ||||
| Current consumption, >2 oz/day |
141/603 (23.4 ) | 113/555 (20.4 ) | 130/531 (24.5 ) | 188/609 (30.9 ) | . |
| Previous consumption, >2 oz/day |
148/603 (24.5 ) | 141/555 (25.4 ) | 105/531 (19.8 ) | 112/609 (18.4 ) | . |
| Never consumed alcohol | 314/603 (52.1 ) | 301/555 (54.2 ) | 296/531 (55.7 ) | 309/609 (50.7 ) | . |
| Smoking status | 0.449 | ||||
| Current smoker | 122/602 (20.3 ) | 90/555 (16.2 ) | 93/531 (17.5 ) | 103/608 (16.9 ) | . |
| Former smoker | 307/602 (51.0 ) | 295/555 (53.2 ) | 275/531 (51.8 ) | 303/608 (49.8 ) | . |
| Never smoked | 173/602 (28.7 ) | 170/555 (30.6 ) | 163/531 (30.7 ) | 202/608 (33.2 ) | . |
| Medications | |||||
| ACE inhibitor or ARB | 593/603 (98.3 ) | 541/551 (98.2 ) | 522/529 (98.7 ) | 601/610 (98.5 ) | 0.922 |
| Beta-blocker | 538/603 (89.2 ) | 489/551 (88.7 ) | 490/530 (92.5 ) | 545/610 (89.3 ) | 0.163 |
| Aldosterone blocker | 231/355 (65.1 ) | 220/333 (66.1 ) | 175/296 (59.1 ) | 187/361 (51.8 ) | <0.001 |
| Nitrate | 143/603 (23.7 ) | 122/551 (22.1 ) | 141/530 (26.6 ) | 137/609 (22.5 ) | 0.296 |
| Calcium-channel blocker | 38/603 (6.3 ) | 51/550 (9.3 ) | 48/528 (9.1 ) | 66/610 (10.8 ) | 0.047 |
| Diuretic | 519/603 (86.1 ) | 446/551 (80.9 ) | 421/530 (79.4 ) | 469/610 (76.9 ) | 0.001 |
| Statin | 327/418 (78.2 ) | 340/408 (83.3 ) | 341/397 (85.9 ) | 386/455 (84.8 ) | 0.017 |
| Warfarin | 305/603 (50.6 ) | 287/559 (51.3 ) | 261/533 (49.0 ) | 289/610 (47.4 ) | 0.532 |
| Event | |||||
| Primary Outcome | 189 (41.7%) | 161 (39.3%) | 140 (35.1%) | 132 (30.3%) | 0.011 |
| Ischemic Stroke | 26 (30.3%) | 20 (5.0%) | 14 (3.7%) | 24 (6.0%) | 0.387 |
| Death | 162 (37.9%) | 139 (35.9%) | 124 (32.3%) | 106 (25.6%) | 0.008 |
| CV Death | 120 (28.8%) | 91 (24.5%) | 79 (20.8%) | 66 (15.9%) | 0.001 |
| Sudden Death | 69 (16.4%) | 43 (11.2%) | 46 (13.1%) | 37 (8.2%) | 0.010 |
| Myocardial Infarction | 21(4.3%) | 19 (7.1%) | 14 (3.5%) | 18 (3.8%) | 0.792 |
| Heart Failure Hospitalization | 150 (34.3%) | 112 (27.2%) | 104 (26.7%) | 85 (22.4%) | <0.001 |
For continuous variables, mean±SD were reported, and p-values were calculated using ANOVA F-test. For categorical variables, no/total no (%) were reported, and p-values were calculated using Chi-squared test. For time-to-event outcomes, no (Kaplan Meyer %) were reported, and p-values were calculated using log-rank test.
eGFR = estimated Glomerular Filtration Rate
The mean follow-up time was 3.5±1.8 years, and the total follow-up time was 8225 patient-years. Survival status was known for 97.0% of the patients. A total of 34 patients (1.5%) withdrew consent, and 35 (1.5%) were lost to follow-up.
Overall, 622 of the 2305 patients (27.0%) had a primary outcome (531 deaths [85.4%], 84 ischemic stroke [13.5%], and 7 intracerebral hemorrhage [1.1%]). 356 patients (15.4%) had CV death, 195 patients (8.5%) sudden death, 72 patients (3.1%) had an MI, and 451 patients (19.6%) experienced HF hospitalization.
LVEF and outcomes
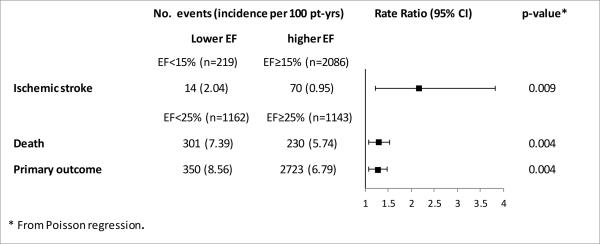
Table 1 also summarizes the frequency of outcome events by LVEF category. Incidence of primary outcome, death (all-cause, CV and sudden) and HF hospitalization increased progressively with decreasing LVEF; no such relationship was observed for stroke and MI.
Age, male sex, heart rate, diabetes, NYHA class, ischemic cardiomyopathy, prior stroke/TIA and serum creatinine level were significantly associated with the primary outcome. LVEF, body mass index, systolic blood pressure and presence of an internal defibrillator were inversely associated with this outcome (online supplement, Supplemental Table I).
While most of these variables were also associated with all-cause death (Supplemental Table II) and also with CV or sudden death and HF hospitalization, only LVEF <15% and prior stroke/TIA were associated with stroke (Supplemental Table III).
For the primary outcome and all-cause death, the LVEF cutoff point associated with the greatest increase in risk was 25%; for CV death, sudden death and HF hospitalization it was 20% (Supplemental Table IV). For all 5 outcomes, all LVEF cutoff points were associated with increased risk, confirming the linearity of the association. LVEF <15% was associated with a doubling of the risk of stroke; no other cutoff point was identified. No cutoff point of increased risk was identified for MI.
Table 2 shows the hazard ratios for each outcome after adjustment for pertinent covariates, using the cutoff points of greatest increase in risk.
Table 2.
LVEF and outcome events (by optimal cutoff point of increased risk)
| Unadjusted HR (95% CI) |
p-value | Adjusted*
HR (95% CI) |
p-value | |
|---|---|---|---|---|
| LVEF < 15% vs. ≥15% | ||||
| Ischemic Stroke | 2.105 (1.186,3.738) | 0.011 | 2.125 (1.182, 3.818) | 0.012 |
| LVEF < 25% vs. ≥25% | ||||
| Primary outcome | 1.266 (1.081,1.484) | 0.004 | 1.250 (1.063, 1.469) | 0.007 |
| Death | 1.288 (1.085,1.529) | 0.004 | 1.252 (1.050, 1.492) | 0.012 |
| LVEF < 20% vs. ≥20% | ||||
| CV death | 1.481 (1.189,1.845) | <0.001 | 1.359 (1.085, 1.702) | 0.008 |
| Sudden death | 1.593 (1.187,2.136) | 0.002 | 1.481 (1.097, 1.999) | 0.010 |
| HF hospitalization | 1.520 (1.249,1.849) | <0.001 | 1.395 (1.142, 1.706) | 0.001 |
adjusted for age, gender, BMI, systolic BP, heart rate, smoking status, education, NYHA class (III, IV vs. I, II), diabetes, hypertension, ischemic cardiomyopathy, prior stroke or TIA, ICD presence, serum creatinine and hemoglobin for primary outcome, death, CV death and sudden death; adjusted for age, gender, BMI, systolic BP, smoking status, NYHA class (III, IV vs. I, II), diabetes, hypertension, ischemic cardiomyopathy, prior stroke or TIA, ICD presence, serum creatinine and hemoglobin for stroke; adjusted for age, gender, BMI, systolic BP, heart rate, smoking status, alcohol consumption, NYHA class (III, IV vs. I, II), diabetes, hypertension, ischemic cardiomyopathy, prior stroke or TIA, ICD presence, serum creatinine, hemoglobin and sodium for HF hospitalization.
The incidence of ischemic stroke, primary outcome and death, also stratified by optimal cutoff point for each outcome, is reported in Figure 1, which also reports the rate ratio for each event. Corresponding information for other outcomes is provided in Supplemental Figure I.
Figure 1.
Outcome incidence rates for ischemic stroke, primary outcome and death by LVEF (dichotomized at optimal cutoff points)
Effect of antithrombotic treatment
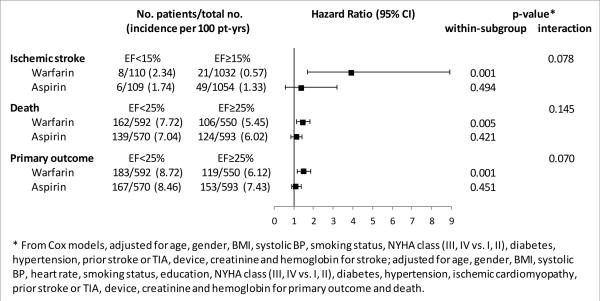
Figure 2 shows the outcome incidence rates for ischemic stroke, primary outcome and death by antithrombotic treatment (aspirin or warfarin), stratified by LVEF category (for other outcomes, please refer to Supplemental Figure II). Incidence rates were similar between aspirin- and warfarin-treated patients, as already known from the WARCEF main results4. The deleterious effect of a lower LVEF tended to be stronger in warfarin-treated than in aspirin-treated patients for all 3 outcomes. A trend towards a significant interaction between treatment type and LVEF was noted for ischemic stroke and primary outcome. An additional analysis by 5% LVEF decrements showed a significant interaction between LVEF and treatment for ischemic stroke only, with the warfarin-treated group showing a significantly greater stroke risk per each 5% LVEF decrement (adjusted HR: 1.346, 95% CI 1.044, 1.737; p=0.022) than the aspirin-treated group (adjusted HR: 0.971, 95% CI 0.805, 1.171; p=0.757; p-value for the interaction=0.04).
Figure 2.
Outcome incidence rates for ischemic stroke, primary outcome and death by antithrombotic treatment type, stratified by LVEF category
Since the interaction between LVEF and warfarin on stroke risk might be mediated by differences in TTR, this variable was examined in different stroke and LVEF subgroups. TTR was similar in patients with LVEF <15% or ≥15% (56.5±28.6% vs. 57.1±28.5%; p=0.793). TTR tended to be lower in patients who experienced a stroke during follow-up than in those who did not (45.9±27.9% vs. 57.2±28.4%; p=0.064); this trend was stronger in patients with LVEF <15% (36.1±24.1% vs. 57.6±28.5%; p=0.074) than in patients with LVEF ≥15% (49.0±29.0% vs. 57.2±28.4%; p=0.249).
DISCUSSION
The present study evaluates the effect of LVEF on ischemic stroke and other outcome events in HF patients with sinus rhythm and reduced LVEF treated with currently recommended HF medications and randomized to different antithrombotic treatments.
In patients with systolic HF, several hemodynamic variables have been shown to be associated with outcome, some of which easily obtainable such as systolic blood pressure6-8, pulse pressure9 and resting heart rate.8, 10 However, LVEF is the most widely used clinical indicator of LV systolic function and related risk for CV events in HF patients. The results of our study support the presence of a linear, inverse relationship between LVEF and the considered outcome events with the exception of stroke, for which a relationship was observed only for very low LVEF (<15%), and of MI, for which no relationship was observed. An LVEF <15% more than doubled the risk of stroke.
Comparison with previous studies
The observation of an inverse relationship between LVEF and death is in agreement with those from previous observational11, 12 and more recent, large-scale studies.1, 2, 13 Among the latter, results from the CHARM trial showed LVEF to be inversely and linearly associated with all-cause mortality and with all components of CV death for LVEF values below 45% over a median follow up of 38 months.1 The DIG trial also reported similar results. 2 Our study provides similar results in a more recently enrolled cohort, but notable differences exist. Our study only included patients with systolic HF, and an LVEF <35% was an inclusion criterion. However, an LVEF cutoff of 35% was indeed the one associated with increased mortality at 180 days in the recent Acute Studies of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial, and a linear relationship between decreasing LVEF and risk of death was only observed below that level.13 Compared with the CHARM cohort, the WARCEF cohort had differences in medical treatment that reflect the more recent conduct of the study. Patients in WARCEF were more often on beta-blockers (over 90% of patients vs. approximately 55%), aldosterone-blockers (60% vs. approximately 20%) and statins or other lipid lowering medications (over 80% vs. approximately 40%). The most important difference, however, is that all patients in WARCEF received an antithrombotic medication per-protocol, which may have affected the relationship between LVEF and thromboembolic events.
Unlike CHARM, which found no association between LVEF and incidence of stroke, we observed an increased stroke risk for extremely low LVEF values (<15%). This difference may reflect the very low incidence of stroke in CHARM (slightly over 1% over a mean follow up of approximately 3 years). The stroke incidence in WARCEF (slightly over 1% per year) is at the lower end of what traditionally reported in the HF literature (1.3% to 3.5% per year),14-17 probably reflecting the updated background medical treatment as well as the per-protocol antithrombotic agents. In the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), the rate of stroke and other thromboembolic events was 1.7% per year, and lower LVEF was associated with an increased risk of thromboembolic events, with the highest risk observed in patients with LVEF <20%.3 From this cumulative experience, it appears that LVEF may indeed be associated with stroke risk in systolic HF patients, but predominantly at very low LVEF levels. The possibility exists that very low LVEF may be associated with more frequent occurrence of undetected episodes of paroxysmal atrial fibrillation, an established stroke risk factor that might be involved in explaining this finding.
While CHARM documented a slight but significant increase in risk of MI (adjusted HR 1.14, 95% CI 1.01 to 1.28 per 10% LVEF reduction below 45%), no such increase was observed in WARCEF. Although the low frequency of MI (approximately 2% in CHARM and 3% in WARCEF in corresponding LVEF categories) prevents definitive conclusions, the antithrombotic treatments prescribed in WARCEF may have affected the relationship between LVEF and a predominantly thrombotic outcome such as MI.
Effect of antithrombotic treatment
In the main results of WARCEF, patients on warfarin or aspirin treatment had similar rates of death and primary outcome, although patients on warfarin treatment had significantly reduced incidence of ischemic stroke.4, 5 In the present analysis, lower LVEF tended to have a more deleterious effect in patients treated with warfarin than in patients receiving aspirin with respect to primary outcome, death and ischemic stroke (Figure 2). The only significant interaction between LVEF and treatment was observed for stroke, where each 5% decrease in LVEF was associated with a 35.6% increase in the adjusted risk of stroke in patients treated with warfarin (p=0.02). Warfarin-treated patients with LVEF <15% showed an increased stroke rate, whereas those with higher LVEF had very low stroke incidence, actually lower than that of aspirin-treated patients. The hypothesis that patients with extremely low LVEF might have greater difficulty in maintaining an adequate TTR was not confirmed in our analysis, as mean TTR was nearly identical in patients with LVEF <15% or ≥15%; however, the mean TTR tended to be lower in patients with LVEF <15% who experienced a stroke than in patients with LVEF <15% who did not (p=0.074). Taken together, these results suggest that, although maintaining an adequate TTR may not necessarily be more difficult in patients with extremely low LVEF, the stroke risk may increase in them when an adequate TTR is for any reason not achieved. Given the low number of incident strokes, these results should be regarded with a degree of caution; however, particular emphasis should be placed on INR control in patients with very low LVEF, and switching to another antithrombotic agent considered when INR management proves difficult. The use of newer oral anticoagulants in patients with severely reduced LVEF and sinus rhythm, while an appealing possibility, requires appositely designed and powered clinical trials to assess safety and efficacy of these drugs in this very specific clinical setting.
Our study has strengths and limitations. Strengths are the relatively large cohort of HF patients in sinus rhythm, the central interpretation of echocardiograms and consequent standardization of LVEF measurement, and the ability to investigate the effect of two antithrombotic treatments. Among the limitations, the study only included patients with systolic HF, therefore the effect of LVEF on outcomes within a normal or mildly decreased LVEF could not be evaluated. The incidence of some outcomes was rather low, therefore the related results for subgroup analyses should be considered exploratory.
SUMMARY
In patients with systolic HF in sinus rhythm treated with currently recommended HF regimen and antithrombotic medications, LVEF is inversely associated with death and its various components, ischemic stroke and HF hospitalization, but not MI; the association is linear for most outcomes, but an increased stroke risk is observed only for very low LVEF (<15%); an interaction between LVEF and warfarin treatment may exist on stroke risk, which deserves further investigation.
Supplementary Material
Acknowledgments
The authors thank Michelle Bierig, RDCS, and Rui Liu, MD, for their help with the echocardiographic measurements.
Sources of Funding
The study was funded by U01-NS-043975 (S. Homma) and U01-NS-039143 (J.L.P. Thompson) from the National Institute of Neurological Diseases and Stroke NINDS.
Footnotes
Disclosures
Dr. Homma reports receiving payment from AGA Medical (now St. Jude Medical) for his work as a member of a data and safety monitoring board and consulting fees from Boehringer Ingelheim; Dr. Levin, receiving consulting fees from United Healthcare; Dr. Teerlink, receiving research grants and/ or consulting fees from Amgen, Bayer, Cardio3 Bioscience, Cytokinetics, Mast Therapeutics, Medtronic, Novartis, St. Jude and Trevena; Dr. Labovitz, receiving grant support from Boehringer Ingelheim and BMS Pfizer, lecture fees from Boehringer Ingelheim, and fees for the development of educational presentations from the American College of Cardiology; Dr. Anker, receiving consulting fees from Vifor, Bayer, Janssen, Novartis, Relypsa, ZS-Pharma, and Thermo Fisher, grant support from Vifor Pharma, and Abbott Vascular, and lecture fees from Vifor, Novartis, and Stealth Peptides; Dr. Ponikowski, receiving consulting fees from Bayer, Boehringer Ingelheim, Coridea, Corthera, Johnson & Johnson, Pfizer, Respicardia, and Vifor Pharma, grant support from Vifor Pharma on behalf of himself and his institution, and lecture fees from Abbott, Boehringer Ingelheim, Merck Serono, Pfizer, Respicardia, Sanofi-Aventis, Servier, and Vifor Pharma; and Dr. Lip, receiving consulting fees from Bayer/Janssen, Astellas, Merck, Sanofi, BMS/Pfizer, Biotronik, Medtronic, Portola, Boehringer Ingelheim, Microlife and Daiichi-Sanky, speakers bureau fees from Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Microlife, Roche and Daiichi-Sankyo, and payment for the development of educational presentations from Bayer, Boehringer Ingelheim, and Merck. No other potential conflict of interest relevant to this article was reported.
Reference List
- (1).Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–44. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- (2).Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–42. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- (3).Freudenberger RS, Hellkamp AS, Halperin JL, Poole J, Anderson J, Johnson G, et al. Risk of thromboembolism in heart failure: an analysis from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Circulation. 2007;115:2637–41. doi: 10.1161/CIRCULATIONAHA.106.661397. [DOI] [PubMed] [Google Scholar]
- (4).Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–69. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Homma S, Thompson JL, Sanford AR, Mann DL, Sacco RL, Levin B, et al. Benefit of warfarin compared with aspirin in patients with heart failure in sinus rhythm: a subgroup analysis of WARCEF, a randomized controlled trial. Circ Heart Fail. 2013;6:988–97. doi: 10.1161/CIRCHEARTFAILURE.113.000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Meredith PA, Ostergren J, Anand I, Puu M, Solomon SD, Michelson EL, et al. Clinical outcomes according to baseline blood pressure in patients with a low ejection fraction in the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity) Program. J Am Coll Cardiol. 2008;52:2000–7. doi: 10.1016/j.jacc.2008.09.011. [DOI] [PubMed] [Google Scholar]
- (7).Ambrosy AP, Vaduganathan M, Mentz RJ, Greene SJ, Subacius H, Konstam MA, et al. Clinical profile and prognostic value of low systolic blood pressure in patients hospitalized for heart failure with reduced ejection fraction: insights from the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) trial. Am Heart J. 2013;165:216–25. doi: 10.1016/j.ahj.2012.11.004. [DOI] [PubMed] [Google Scholar]
- (8).Maeder MT, Kaye DM. Differential impact of heart rate and blood pressure on outcome in patients with heart failure with reduced versus preserved left ventricular ejection fraction. Int J Cardiol. 2012;155:249–56. doi: 10.1016/j.ijcard.2010.10.007. [DOI] [PubMed] [Google Scholar]
- (9).Regnault V, Lagrange J, Pizard A, Safar ME, Fay R, Pitt B, et al. Opposite predictive value of pulse pressure and aortic pulse wave velocity on heart failure with reduced left ventricular ejection fraction: insights from an Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) substudy. Hypertension. 2014;63:105–11. doi: 10.1161/HYPERTENSIONAHA.113.02046. [DOI] [PubMed] [Google Scholar]
- (10).Castagno D, Skali H, Takeuchi M, Swedberg K, Yusuf S, Granger CB, et al. Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure: results from the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity) program. J Am Coll Cardiol. 2012;59:1785–95. doi: 10.1016/j.jacc.2011.12.044. [DOI] [PubMed] [Google Scholar]
- (11).St John SM, Pfeffer MA, Moye L, Plappert T, Rouleau JL, Lamas G, et al. Cardiovascular death and left ventricular remodeling two years after myocardial infarction: baseline predictors and impact of long-term use of captopril: information from the Survival and Ventricular Enlargement (SAVE) trial. Circulation. 1997;96:3294–9. doi: 10.1161/01.cir.96.10.3294. [DOI] [PubMed] [Google Scholar]
- (12).McDermott MM, Feinglass J, Lee PI, Mehta S, Schmitt B, Lefevre F, et al. Systolic function, readmission rates, and survival among consecutively hospitalized patients with congestive heart failure. Am Heart J. 1997;134:728–36. doi: 10.1016/s0002-8703(97)70057-7. [DOI] [PubMed] [Google Scholar]
- (13).Toma M, Ezekowitz JA, Bakal JA, O'Connor CM, Hernandez AF, et al. The relationship between left ventricular ejection fraction and mortality in patients with acute heart failure: insights from the ASCEND-HF Trial. Eur J Heart Fail. 2014;16:334–41. doi: 10.1002/ejhf.19. [DOI] [PubMed] [Google Scholar]
- (14).Fuster V, Gersh BJ, Giuliani ER, Tajik AJ, Brandenburg RO, Frye RL. The natural history of idiopathic dilated cardiomyopathy. Am J Cardiol. 1981;47:525–31. doi: 10.1016/0002-9149(81)90534-8. [DOI] [PubMed] [Google Scholar]
- (15).Dunkman WB, Johnson GR, Carson PE, Bhat G, Farrell L, Cohn JN. Incidence of thromboembolic events in congestive heart failure. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI94–101. [PubMed] [Google Scholar]
- (16).Katz SD, Marantz PR, Biasucci L, Jondeau G, Lee K, Brennan C, et al. Low incidence of stroke in ambulatory patients with heart failure: a prospective study. Am Heart J. 1993;126:141–6. doi: 10.1016/s0002-8703(07)80021-4. [DOI] [PubMed] [Google Scholar]
- (17).Dries DL, Rosenberg YD, Waclawiw MA, Domanski MJ. Ejection fraction and risk of thromboembolic events in patients with systolic dysfunction and sinus rhythm: evidence for gender differences in the studies of left ventricular dysfunction trials. J Am Coll Cardiol. 1997;29:1074–80. doi: 10.1016/s0735-1097(97)00019-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.