Abstract
Liver fatty acid binding protein (FABP1, L-FABP) has high affinity for and enhances uptake of arachidonic acid (ARA, C20:4, n-6) which, when esterified to phospholipids, is the requisite precursor for synthesis of endocannabinoids (EC) such as arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG). The brain derives most of its ARA from plasma, taking up ARA and transporting it intracellularly via cytosolic fatty acid binding proteins (FABPs 3,5, and 7) localized within the brain. In contrast, the much more prevalent cytosolic FABP1 is not detectable in the brain but is instead highly expressed in the liver. Therefore, the possibility that FABP1 outside the central nervous system may regulate brain AEA and 2-AG was examined in wild-type (WT) and FABP1 null (LKO) male mice. LKO increased brain levels of AA-containing EC (AEA, 2-AG), correlating with increased free and total ARA in brain and serum. LKO also increased brain levels of non-ARA that contain potentiating endocannabinoids (EC*) such as OEA, PEA, 2-OG, and 2-PG. Concomitantly, LKO decreased serum total ARA-containing EC, but not non-ARA endocannabinoids. LKO did not elicit these changes in the brain EC and EC* due to compensatory upregulation of brain protein levels of enzymes in EC synthesis (NAPEPLD, DAGLα) or cytosolic EC chaperone proteins (FABPs 3, 5, 7, SCP-2, HSP70), or cannabinoid receptors (CB1, TRVP1). These data show for the first time that the non-CNS fatty acid binding protein FABP1 markedly affected brain levels of both ARA-containing endocannabinoids (AEA, 2-AG) as well as their non-ARA potentiating endocannabinoids.
Keywords: mouse, liver fatty acid binding protein, gene ablation, brain, endocannabinoid
INTRODUCTION
Cannabinoid (CB) receptors together with their endogenous endocannabinoid (EC) ligands, the arachidonic acid (ARA)-containing arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG), constitute a novel system for modulating pain and inflammation by both central and peripheral mechanisms (Sarchielli et al. 2007; Christie and Mallet 2009; Sagar et al. 2012; Fine and Rosenfeld 2013; Guasti et al. 2009; De Petrocellis et al. 2000; Luongo et al. 2015). Although some ARA can be synthesized de novo in brain, most ARA is taken up from plasma (Mitchell and Hatch 2011; Bazinet and Laye 2014). However, a high hepatic clearance rate significantly diminishes plasma availability (Mashek 2013; Havel et al. 1962; Kohout et al. 1971). Yet, little is known about hepatic factors contributing to high ARA clearance and their impact on the EC system. One potential candidate is liver fatty acid binding protein (FABP1, L-FABP).
The liver expresses very high amounts of FABP1, representing 2–10% of cytosolic protein or 0.1–1.0 mM (Atshaves et al. 2010; Huang et al. 2014; McArthur et al. 1999; Favretto et al. 2013). FABP1 accounts for >80% of cytosolic long chain fatty acid (LCFA) and LCFA-CoA thioester binding capacity (Martin et al. 2003a; Martin et al. 2003b). FABP1 has high affinity for n-6 PUFA such as ARA as well as γ-linolenic acid (γ-LNA, C18:2, n-6) which can be metabolized to ARA in liver, but much less so in brain (Frolov et al. 1997; Murphy et al. 1999). FABP1 overexpression stimulates cellular uptake of ARA and ARA analogues (McIntosh et al. 2010; Murphy et al. 1996b; Murphy et al. 1996a; Schroeder et al. 1993) more than other LCFA (Murphy et al. 1996b; Murphy et al. 1996a; Prows et al. 1995; Murphy 1998; McIntosh et al. 2010; Martin et al. 2003a; Storey et al. 2012a). FABP1 expression is highly elevated in obesity (Morrow et al. 1979), alcoholic fatty liver disease (AFLD) (Pignon et al. 1987; Gyamfi et al. 2008), non-alcoholic fatty liver disease (NAFLD) (Yang et al. 1987; Higuchi et al. 2011; Charlton et al. 2009; Baumgardner et al. 2007) and NAFLD in subjects expressing the highly prevalent FABP1 T94A substitution (Peng et al. 2012; McIntosh et al. 2014).
Since FABP1 is not detectable in brain (Owada et al. 2006; Myers-Payne et al. 1996a; Avdulov et al. 1998), these findings suggest that FABP1 expressed in tissues (especially liver) outside the central nervous system may nevertheless influence the brain endocannabinoid system. Therefore, this possibility was tested using male WT and FABP1 gene ablated (LKO) male mice to address the potential impact of LKO ablation on the brain endocannabinoid system. Our findings indicate that FABP1 ablation markedly increased the levels of endocannabinoids (AEA, 2-AG) along with brain and serum free ARA and total ARA. Concomitantly, LKO also increased brain levels of potentiating ethanolamides (OEA, PEA) and 2-monoacylglycerols (2-OG, 2-PG) in brains.
MATERIALS AND METHODS
Materials
Purified unlabeled N-acylethanolamides (NAEs) and 2-monoacylglycerols (2-MGs) as well as their deuterated analogues were from Cayman Chemical (Ann Arbor, MI) as follows: n-6 arachidonoylethanolamine (AEA), oleoylethanolamide (OEA), palmitoylethanolamide (PEA), n-3 docosahexaenoylethanolamide (DHEA), n-3 eicosapentaenoylethanolamide (EPEA), 2-arachidonoylglycerol (2-AG), 2-oleoylglycerol (2-OG), 2-palmitoylglycerol (2-PG), AEA-d4, OEA-d2, PEA-d4, DHEA-d4, EPEA-d4, 2-AG-d8, and arachidonic acid-d8 (ARA-d8, 20:4n-6-d8. Cis-parinaric acid was from Invitrogen (Grand Island, NY, USA). All reagents and solvents were the highest grade commercially available.
Proteins and Antibodies
Recombinant murine FABP1 (Martin et al. 2009a; Frolov et al. 1997), murine acyl-CoA binding protein (ACBP) (Chao et al. 2003; Frolov and Schroeder 1998) and human sterol carrier protein-2 (SCP-2) (Matsuura et al. 1993; Martin et al. 2008; Frolov et al. 1996) were isolated, delipidated, and purity determined as in cited papers. Rabbit polyclonal antibodies against SCP2 were as in (Atshaves et al. 1999). Antibodies to the following proteins were commercially obtained: caveolin-1 (CAV1; 610060) polyclonal anti-rabbit (BD Transduction Laboratories, Lexington, KY); fatty acid transport protein 1 (FATP-1; sc-25541) anti-rabbit and fatty acid binding protein-3 (FABP3; sc-58275) monoclonal anti-mouse, fatty acid binding protein-7 (FABP7; sc-30088) polyclonal anti-rabbit, FABP1 (sc-16064) polyclonal anti-mouse, N-acylethanolamide hydrolyzing acid amidase (NAAA; sc-100470) monoclonal anti-mouse, ß-Actin (sc-47778) monoclonal anti-mouse (Santa Cruz Biotech, Santa Cruz, CA); fatty acid binding protein-5 (FABP5; RD181060100) (BioVendor R&D, Asheville, NC); fatty acid translocase/cluster of differentiation 36/thrombospondin receptor (FAT/CD36; RDI-M1537db) monoclonal anti-mouse (Research Diagnostics (Flanders, NJ); anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; MAB374) monoclonal anti-mouse (Millipore, Billerica, MA); heat shock protein-70 (HSP70; MA3-028) monoclonal anti-mouse (Pierce Antibodies, Rockford, IL); diacylglycerol lipase α (DAGLα; 13626 Cell Signaling, Danvers, MA); 2-monoacylglycerol lipase (MAGL; 310212, Cayman Chemical Co, Ann Arbor, MI); Cytochrome C oxidase 4 (COX4, ab16056) polyclonal anti-rabbit, antibody to cannabinoid receptor-1 (CB1; AB172970), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, AB8245), fatty acid amide hydrolase (FAAH, AB54615), N-acylphosphatidylethanolamine phospholipase D (NAPEPLD; AB95397) (Abcam, Cambridge, MA); transient receptor potential cation channel subfamily V member 1 (TRVP-1: 75j-254) (Antibodies Inc., Davis, CA).
Animal Care
Animal experimental protocols were approved by the Institutional Animal Care and Use Committee at Texas A&M University. Male inbred C57BL/6NCr mice were from the National Cancer Institute (Frederick Cancer Research and Development Center, Frederick, MD). FABP1 null (LKO) mice on the same C57BL/6NCr background were generated by our laboratory as described previously (Martin et al. 2003a) and backcrossed to C57BL/6NCr to the N10 generation. For colony maintenance, mice were fed a standard rodent chow mix [5% calories from fat; D8604 Teklad Rodent Diet, Teklad Diets (Madison, WI)] and were maintained in barrier cages on ventilated racks at 12-hr light/dark cycle in a temperature controlled facility (25°C) with ad libitum food and water until study initiation. Mice were sentinel monitored quarterly and confirmed free of all known rodent pathogens.
Phytol-free, Phytoestrogen-free Diet
To avoid potential dietary complications on impact of FABP1 gene ablation, WT and FABP1 ablated mice (8 wk old) were fed a defined phytol-free (Ellinghaus et al. 1999; Hanhoff et al. 2005; Fuchs et al. 2001; Hanhoff et al. 2001; Wolfrum et al. 1999b; Seedorf et al. 1998), phytoestrogen-free (Thigpen et al. 1999a; Thigpen et al. 1999b) control chow for 4 weeks and, following an overnight fast, serum and brain were collected/flash frozen and stored at −80°C.
Extraction of Fatty Acyl Ethanolamides and 2-Monoacylglycerols
Lipids were extracted from mouse brain or serum essentially as described in (Wang et al. 2003) with the following modifications: Frozen mouse brain (100–200 mg tissue) was homogenized in 1.0 mL of ice-cold homogenization buffer containing 2000 pg each of the following deuterated standards (AEA-d4, OEA-d2, PEA-d4, DHEA-d4, EPEA-d4, and 2-AG-d8; Cayman Chemical, Ann Arbor, MI). Similar amounts of the respective deuterated standards were added to an aliquot (50 μL) of the serum prior to lipid extraction as described above. The final lipid residue was reconstituted in 100 μL of ice-cold methanol, purged with nitrogen, and stored at −80°C until analysis by liquid chromatography-mass spectrometry (LC-MS).
Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis to Quantify Brain and Serum N-Acyl Ethanolamides (NAEs)
NAEs (AEA, OEA, PEA, DHEA, EPEA) were identified and quantified in the Protein Chemistry Laboratory (directed by Dr. Larry Dangott at Texas A&M) essentially as in (Jian et al. 2010) with the following modifications: separation by liquid chromatography was achieved by Zorbax Eclipse XDB-C18 analytical column (4.6 mm × 150 mm × 5-micron, Agilent Technologies, Santa Clara, CA) and Accela 1250 LC pump (Thermo Fisher, Waltham, MA). Mass spectrometry analysis was performed by Exactive Orbitrap mass spectrometer (Thermo Fisher) equipped with an atmospheric-pressure heated electo-spray ionization source in positive polarity mode. Capillary temperature was 300°C, spray voltage 4.0 kV, capillary voltage 40.0 V, tube lens voltage 105.0 V, and skimmer voltage 26.0 V. Fatty acyl ethanolamides in the tissue samples were identified on the basis of column retention time, parent ion molecular weight (M+1), and comparison with an external standard bracket that contained a known amount (100, 200, 500, 1000100, 200, 500, 2000, or 5000 pg) of each non-deuterated NAE (AEA, OEA, PEA, DHEA, EPEA) as well as 400 pg of the respective deuterated NAE as above. Quantification of each fatty acyl ethanolamine in the tissue sample was achieved using Xcalibur software (Thermo Fisher) followed by comparison of peak area with appropriate standard peak area. Values are expressed as pmol of NAE/g wet tissue.
Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis of Brain and Serum 2-Monoacylglycerols (2-MGs)
2-MGs (2-AG, 2-OG, and 2-PG) were identified and quantified in the Protein Chemistry Laboratory (directed by Dr. Larry Dangott at Texas A&M) as in (Kaczocha et al. 2014) with the following modifications: Separation by liquid chromatography was achieved as above but solvent conditions were as in (Kaczocha et al. 2014). Mass spectrometry analysis was achieved as described above with the following changes: capillary voltage at 42.5 V, tube lens voltage at 70.0 V, skimmer voltage at 20.0 V. All other mass spectrometry settings were as described above. 2-MGs were identified and quantified with Xcalibur software (Thermo Fisher). For NAEs aside from the external standard bracket there was a known amount (100, 200, 500, 1000100, 200, 500, 2000, or 5000 pg) of each non-deuterated 2-AG, 2-OG, or 2-PG plus 400 pg of 2-AG-d8. Values are expressed as nmol of 2-MG/g wet tissue.
Extraction of Serum and Brain Lipids for Determination of Free ARA, Total ARA, and protein
Homogenized brain tissue or serum was thawed on ice and added directly to a Tenbroeck homogenizer followed by addition of 2 mL of hexane:2-propanol (3:2 by volume) and 2 μg of ARA-d8 internal standard. Once tissue was homogenized, the extract was removed using a silanized Pasture pipette, saved in a silanized screw top test tube and then the homogenizer was washed two times with 2 mL of hexane:2-propanol (3:2 by volume) and the washes added to the initial extract. After homogenization the extract was dried under nitrogen (g) to ~1 mL to facilitate precipitation of organic soluble protein and the protein residue was pelleted by centrifugation at 3,250 × g for 15 min at 4 °C. The supernatant was removed and added to a new silanized screw top tube while the protein was saved for later quantification. The supernatant was dried under nitrogen (g) and 1 mL of hexane:2-propanol (3:2 by volume) was added to dissolve the extract. To quantify ARA in serum samples, 10 μL of serum was added to 1 mL of hexane:2-propanol (3:2 by volume) followed by addition of 1 μg of ARA-d8 internal standard. The extracts were then split in half to determine both free and total ARA.
To determine free (unesterified) ARA, half of the lipid extract was dried down under nitrogen (g) and 150 μL of hexane:2-propanol (3:2 by volume) was used to transfer extract into a silanized microinsert. The tube containing extract was washed with 150 μL of hexane:2-propanol (3:2 by volume) and added to the microinsert. The extract was dried down under nitrogen (g) followed by the addition of 20 μL of Acetonitrile and 20 μL of H2O (1:1 by volume) and proceeded to LC-MS analysis.
To determine total ARA, half of the lipid extract was saponified by transferring to a new screw top test tube, evaporated under nitrogen (g), and redissolved in 180 μL Methanol and 20 μL of 5 M KOH in water. Test tubes were tightly capped and placed in a water bath at 60 °C for 1 h. Following the water bath, the extracts were neutralized with the addition of 20 μL 5 M HCl. To extract the lipids 780 μL of 0.9% NaCl was added followed by three extractions with 2 mL hexane. The hexane extracts were combined in a new silanized tube. Extracts were dried under nitrogen (g) followed by the addition of 1 mL acetonitrile, with 10 μL of extract transferred to silanized microinserts followed by the addition of 10 μL of acetonitrile and 20 μL of H2O (1:1 by volume) and proceeded to LC-MS analysis. Protein content in the tissue residue was quantified using a modified dye-binding assay utilizing bovine serum albumin containing 0.2 M KOH as a standard (Bradford 1976; Murphy and Horrocks 1993).
Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis of Brain and Serum ARA
The above lipid extracts prepared for analysis of free ARA or saponified for determination of total ARA were analyzed by LC-MS. ARA was resolved using a Waters ACUITY UPLC HSS T3 column (1.8 μM, 100 Å pore diameter, 2.1×150mm, Waters, Milford, MA) with an ACUITY UPLC HSS T3 precolumn (1.8 μM, 100 Å pore diameter, 2.1×5mm, Waters) at a temperature of 55°C. The LC system consisted of a Waters ACUITY UPLC pump with a well plate autosampler set at 8 °C. Ten microliters of sample was injected on to the column. The separation was based on the separation described previously (Brose et al. 2013; Brose and Golovko 2013). Retention time for ARA was 14.1 min. The LC eluent was analyzed using a triple quadrupole mass spectrometer (Xevo TQ-S, Waters) with electrospray ionization operated in negative ion mode. The capillary voltage was 2.35 kV and the cone voltage was 30V. The desolvation temperature was 550 °C and the source temperature was 150 °C. The desolvation gas flow was 1000 L/hr, the cone gas flow was 150 L/hr, and the nebulizer gas was at 7.0 Bar. MassLynx V4.1 software (Waters) was used for instrument control, acquisition and sample analysis. The ARA was quantified using ARA-d8 as the internal standard. The analytes were monitored in MRM mode using 12 V for collision energy and 303.07/259.21 and 310.93/267.21 mass transitions for ARA and ARA-d8, respectively.
Brain Protein Levels of Enzymes and Other Proteins in the Endocannabinoid System
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis was performed on brain post-nuclear supernatants (PNS) analogous to those described earlier (Atshaves et al. 2007b) in order to determine relative protein levels of: caveolin-1 (CAV1), fatty acid binding protein-3 (FABP3), fatty acid binding protein-7 (FABP7), fatty acid transport protein 1 (FATP1), fatty acid transport protein 4 (FATP4), fatty acid translocase/thrombospondin receptor (FAT/CD36), heat shock protein-70 (HSP70), N-acylethanolamide hydrolyzing acid amidase (NAAA), sterol carrier protein 2 (recognizing 58 kDa SCP-x, 15 kDa pro-SCP-2, and 13.2 kDa SCP-2). GAPDH, ß-Actin or COX-4 was used as internal gel-loading control. HRP conjugated antibodies were exposed to the Super Signal West Pico chemiluminescent substrate (34077, Pierce, Rockford, IL) and images were taken with an Image Quant LAS 4000 mini (GE Healthcare Life Sciences, Marlborough, MA). Alkaline Phosphatase conjugated antibodies were exposed to BCIP/NBT solution (B6404, Sigma Aldrich); images were captured using an Epson Perfection V700 Photo scanner (Long Beach, CA). Proteins were quantified by densitometric analysis using ImageJ software (National Institutes of Health, Bethesda, MD).
Additional sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis was performed using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control analogous to those described earlier (Kaczocha et al. 2009; Kaczocha et al. 2015) in order to determine relative protein levels of cannabinoid receptor-1 (CB1), diacylglycerol lipase α (DAGLα), fatty acid amide hydrolase (FAAH), fatty acid binding protein-5 (FABP5), 2-monoacylglycerol lipase (MAGL), N-acylphosphatidylethanolamine phospholipase D (NAPEPLD), transient receptor potential cation channel subfamily V member 1 (TRVP-1, vanilloid receptor-1). Blots were developed using Immuno-star HRP substrate (Bio-Rad, Hercules, CA) and scanned using a C-DiGiT scanner (Li-COR, Lincoln, NE) for densitometric analysis. Protein band intensities were quantified and normalized to the respective GAPDH intensities.
For relative protein levels, representative cropped Western blot images were inserted into figure panels similarly as in earlier publications where individual blots were separated by a white line/space (Martin et al. 2014; Petrescu et al. 2013; Huang et al. 2013; Storey et al. 2012a; Storey et al. 2012b). For quantitative Western blotting of fatty acid binding protein-1 (FABP1, L-FABP), a recombinant murine FABP1 standard curve was used similarly as in (Atshaves et al. 2005; Atshaves et al. 2007a; Atshaves et al. 2009; Atshaves et al. 2004b). Images of the blots were obtained with an Epson Perfection V700 Photo scanner (Long Beach, CA) for protein quantification by densitometric analysis using ImageJ software (NIH, Bethesda, MD) as described earlier (Atshaves et al. 2007b).
Sterol Carrier Protein-2 Binding of Arachidonoylethanolamine (AEA) and 2-Arachidonoylglycerol (2-AG)
A cis-parinaric acid displacement assay (Frolov et al. 1996) and a cis-parinaroyl-CoA displacement assay (Huang et al. 2005) were used to determine AEA and 2-AG binding to SCP-2 and ACBP, respectively. SCP-2 binds cis-parinaric acid with Kd = 0.18 ± 0.01 μM, n = 1.13 ± 0.04—consistent with a single binding site (Frolov et al. 1996). In the displacement assay, SCP-2 (500 nM) was first equilibrated with cis-parinaric acid (500 nM) in 10 mM potassium phosphate buffer (pH = 7.4), followed by titration with increasing amounts of AEA (0–5 μM) or 2-AG (0–3 μM). At each ligand concentration, fluorescence emission of cis-parinaric acid was recorded using a Cary Eclipse fluorescence spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) with excitation wavelength 304 nm, emission scan 350–500 nm. The data were corrected with the following blanks/controls: protein only, cis-parinaric acid only, cis-parinaric acid with increasing amount of ligand, and corrected for photobleaching. Sample temperature was maintained at 24°C with a circulating water bath. Displacement curves were constructed by plotting the percentage of cis-parinaric acid fluorescence remaining (at emission maximum, 420 nm) versus ligand concentration. EC50 was obtained from the displacement curve. Ki values were calculated from the Kd = 0.18 ± 0.01 μM value for cis-parinaric acid and the experimentally determined EC50 according to the equation: EC50/[cis-parinaric acid] = Ki/Kd. It is important to note that AEA and 2-AG binding to SCP-2 was specific since neither AEA nor 2-AG was bound by the cytosolic acyl-CoA binding protein (ACBP) as determined with a cis-parinaroyl-CoA displacement assay (Huang et al. 2005) (data not shown).
Statistical Analysis
Values represent the mean ± standard error of the mean (SEM). Statistical analysis was performed by one-way analysis of variance (ANOVA), followed by the Newman-Keuls post-hoc analysis either with GraphPad software (La Jolla, CA) or Sigma Plot software (Systat, San Jose, CA). Statistical significance was assigned to values with p < 0.05.
RESULTS
FABP1 Ablation (LKO) Selectively Increased Brain N-acylethanolamides and 2-monoacylglycerols of the Brain Endocannabinoid System
Arachidonoylethanolamine (AEA) (Fig 1A) was detected by LC/MS in brains of wild-type (WT) male mice at levels in the range of those reported by others (Kaczocha et al. 2014; Wood et al. 2010; Bazinet and Laye 2014; Long et al. 2008; Schlosburg et al. 2010; Ignatowska-Jankowska et al. 2014; Nomura et al. 2011). LKO increased brain levels of AEA by 1.7-fold (Fig 1A) and even more so the non-ARA containing OEA and PEA by 2–3 fold (Fig 1B,C). In contrast, LKO did not alter the brain levels of DHEA (Fig 1D) and EPEA (Fig 1E), respectively. These antagonistic NAEs displace ARA from membrane phospholipids and decrease ARA containing phospholipid synthesis to thereby lower AEA and 2-AG production (Naughton et al. 2013).
Figure 1.
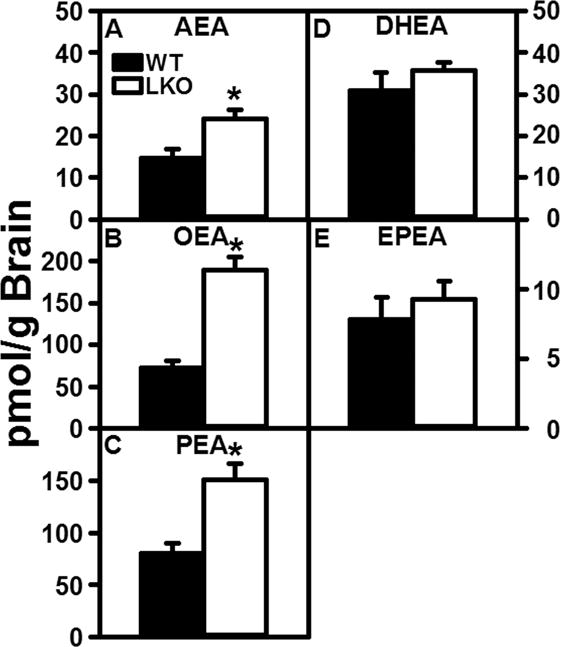
FABP1 gene ablation markedly increases brain N-acylethanolamide levels. C57BL/6N male WT and FABP1 gene ablated mice (8 wk old) were fed phytol-free, phytoestrogen-free control chow for 4 weeks, overnight fasted, and brains removed/flash frozen and stored at −80°C. LC-MS analysis to quantify N-acylethanolamines using deuterated internal standards (Cayman Chemical) was performed as described in Materials and Methods to quantify (A) arachidonoylethanolamide (AEA), (B) oleoylethanolamide (OEA), (C) palmitoylethanolamide (PEA), (D) docosahexaenoylethanolamide (DHEA), and (E) eicosapentaenoylethanolamide (EPEA). Results are presented as mean ± SEM (n = 8); *, p < 0.05 for LKO vs WT.
LC/MS also detected the important 2-monoacylglycerol endocannabinoid 2-AG (Fig 2A), the major endogenous ligand activator of cannabinoid (CB) receptors, in brains of WT mice at levels in the range of those reported by others (Kaczocha et al. 2014; Wood et al. 2010; Bazinet and Laye 2014; Long et al. 2008; Schlosburg et al. 2010; Ignatowska-Jankowska et al. 2014; Nomura et al. 2011). Again LKO increased brain 2-AG as well as the non-ARA containing 2-OG and 2-PG by 1.5 to 3.5-fold (Fig 2A–C).
Figure 2.

FABP1 gene ablation increases brain 2-monoacylglycerol levels. C57BL/6N male WT and FABP1 gene ablated mice (8 wk old) were fed phytol-free, phytoestrogen-free control chow for 4 weeks, overnight fasted, brains removed/flash frozen and stored at −80°C. LC-MS analysis to quantify 2-monoacylglyerols using deuterated internal standards (Cayman Chemical) was performed as described in Materials and Methods to quantify (A) 2-arachidonoylglycerol (2-AG), (B) 2-oleoylglycerol (2-OG), and (C) 2-palmitoylglycerol (2-PG). Results are presented as mean ± SEM (n = 8); *, p < 0.05 for LKO vs WT.
Taken together, these data showed that loss of FABP1 increased not only the levels of ARA containing AEA and 2-AG, but also the levels of most of the potentiating NAEs (OEA, PEA) and 2-MGs (2-OG, 2-PG).
FABP1 Ablation (LKO) Increased Brain and/or Serum Levels of Free and Total ARA
Since brain free and total ARA are primarily derived from ARA taken up from serum, it was essential to determine if the elevated brain AEA and 2-AG levels in LKO mice were associated with increased levels of free and/or esterified ARA in brain and serum of LKO mice. LKO significantly increased brain free ARA levels by 26% and concomitantly increased brain total ARA by 31% (Fig. 3A). Since brain total ARA reflected primarily esterified ARA, this indicated that the LKO induced increase in brain AEA and 2-AG levels (Figs. 1A,2A) was not due to increased hydrolysis/degradation of esterified ARA from brain phospholipids, but reflected a net increase in total ARA mass. Finally, it is important to note that the LKO-induced 31% increase in brain total ARA was associated with a parallel 31% increase in serum total ARA (Fig. 3B). Although not statistically significant, LKO also trended to slightly increase serum free ARA levels (Fig. 3B).
FIGURE 3.
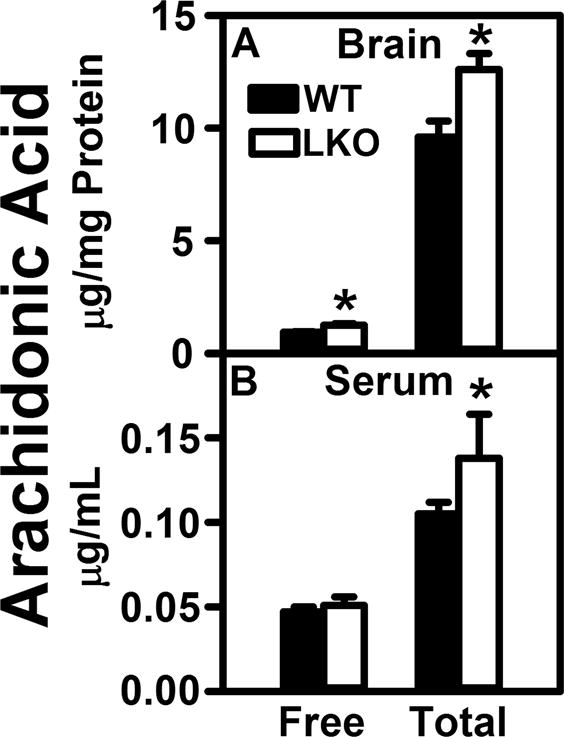
FABP1 gene ablation impacts serum and brain levels of unesterified and total arachidonic acid. WT and FABP1 gene ablated mice (8 wk old) were fed phytol-free, phytoestrogen-free control chow for 4 weeks, overnight fasted, serum and brain collected/flash frozen, and stored at −80°C. Lipid extraction and LC-MS analysis to quantify arachidonic acid using deuterated internal standard (Cayman Chemical) was performed as described in Materials and Methods to quantify (A) total arachidonic acid (total ARA) and (B) free arachidonic acid (free ARA). Results are presented as mean ± SEM (n =5–7); *, p < 0.05 for LKO vs WT
FABP1 Ablation (LKO) Differentially impacted Serum Levels of N-acylethanolamides and 2-monoacylglycerols of the Circulatory Endocannabinoid System
Many of the NAEs and 2-MGs present in brain of wild-type mice were also detected in serum (Fig 4A–G) at levels in the range of those reported in serum by others (Wood et al. 2010; Sailler et al. 2014). In contrast, while EPEA was present in brain (Fig. 1), it was not detectable in serum (not shown). This was consistent with results of others and correlated with nearly 20-fold lower serum level of EPA as compared with DHA precursors (Wood et al. 2010; Sailler et al. 2014). LKO differentially impacted serum levels of ARA-containing endocannabinoids. LKO slightly (15%) decreased the level of the ARA-containing NAE, i.e. AEA (Fig. 4A), while conversely increasing that of the ARA-containing 2-AG by 2.2-fold (Fig. 4E). With regards to the non-ARA containing potentiating NAEs and 2-MGs, LKO significantly decreased serum levels of OEA, PEA, and DHEA (Fig. 4B–D), but had no impact on the serum levels of 2-OG or 2-PG (Fig. 4F,G).
FIGURE 4.
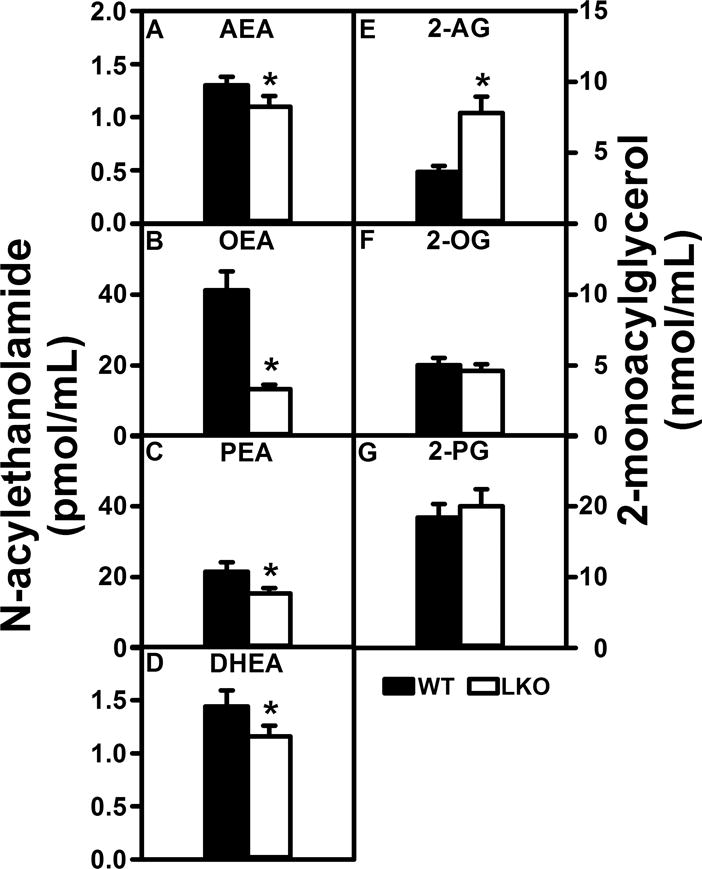
FABP1 gene ablation decreases serum N-acylethanolamide and 2-monoacylglycerol levels. All conditions were as described in legends to Figs. 1 and 2 except that NAEs and 2-MGs were determined in serum as described in Materials and Methods to quantify (A) arachidonoylethanolamide (AEA), (B) oleoylethanolamide (OEA), (C) palmitoylethanolamide (PEA), (D) docosahexaenoylethanolamide (DHEA), (E) 2-arachidonoylglycerol (2-AG), (F) 2-oleoylglycerol (2-OG), and (G) 2-palmitoylglycerol (2-PG). Results are presented as mean ± SEM (n = 8); *, p < 0.05 for LKO vs WT.
In summary, LKO increased the overall total level of ARA-containing endocannabinoids in serum by 1.8-fold. This was consistent with the LKO-induced increase in serum total ARA and trend toward increased serum free ARA noted in Fig. 3.
FABP1 ablation (LKO) did not Elicit Overall Concomitant Upregulation of Brain Membrane Transport/Translocase Proteins For Uptake of Fatty Acids such as ARA
Four membrane associated proteins are involved in translocation/uptake of long chain fatty acids such as arachidonic acid (ARA) including CD36/FAT, Caveolin-1, FATP1 and FATP4 (Mitchell and Hatch 2011). Western blotting showed that FABP1 ablation selectively decreased the protein level of only one plasma membrane fatty acid transporter, i.e. FAT/CD36 (Fig 5A), while concomitantly trending to increase the levels of other membrane fatty acid transporters such as caveolin-1 and FATP4 (Fig. 5B,D) and not altering that of FATP1 (Fig. 5C). Thus, the LKO-induced increases in brain AEA and 2-AG levels (Fig. 1A,2A) were attributable to a net increase in expression of membrane proteins involved in the uptake of LCFAs such as ARA into the brain.
Figure 5.
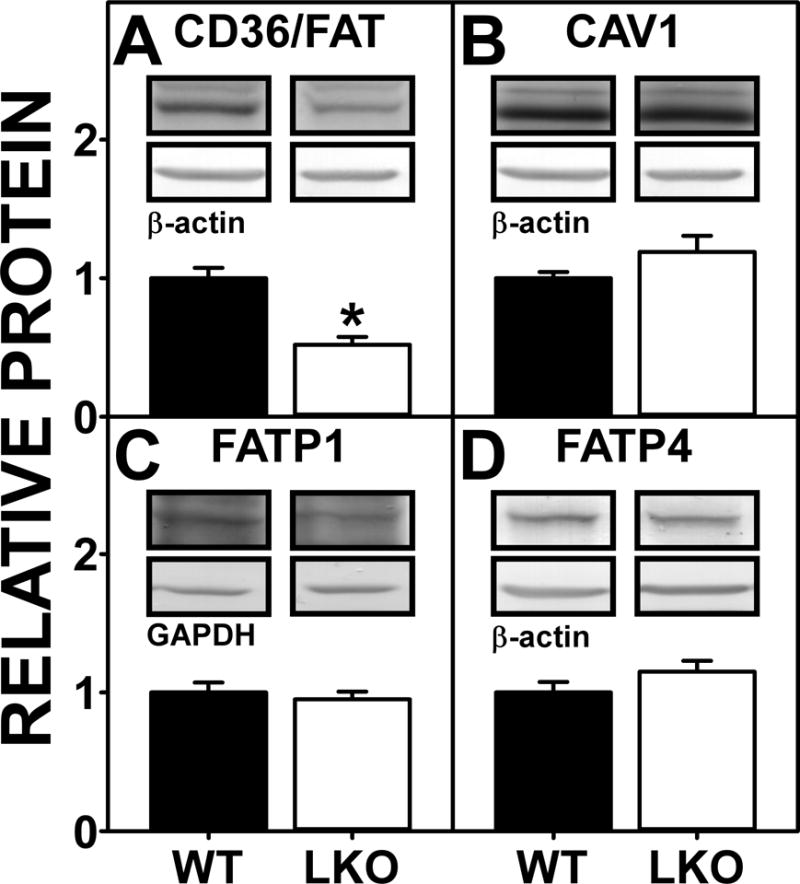
Effect of FABP1 gene ablation on protein levels of brain membrane proteins involved in ARA uptake. C57BL/6N male WT and FABP1 gene ablated mice (8 wk old) were fed phytol-free, phytoestrogen-free control chow for 4 weeks, overnight fasted, and brains removed/flash frozen and stored at −80°C. Aliquots of brain homogenate proteins were examined by SDS-PAGE and subsequent Western blot analysis as described previously (Martin et al. 2015) to determine levels of (A) CD36/FAT, (B) CAV1, (C) FATP1, and (D) FATP4. Insets show representative Western blot images of the respective protein (upper blot) and the gel-loading control protein (β-Actin or GAPDH, lower blot). Relative protein levels were normalized to gel-loading control protein; values were compared to WT set to 1. Results are mean ± SEM (n = 7); *, p < 0.05 for LKO vs WT.
Impact of FABP1 ablation (LKO) on Brain Cytosolic ‘Chaperone’ Proteins Involved in ARA Uptake and Intracellular Transport
Once translocated into brain cells by membrane associated transport/translocase proteins (Mitchell and Hatch 2011), ARA’s poor aqueous solubility also requires the presence of intracellular cytosolic ‘chaperone’ proteins that facilitate ARA transport/targeting to synthetic (endoplasmic reticulum) or degradative organelles (mitochondria) (Mitchell and Hatch 2011; Owada 2008; Moulle et al. 2012; Murphy et al. 2005). This function is served by two different families of cytosolic ARA binding proteins distributed in brain: i) FABPs 3, 5, and 7 (Pu et al. 1999b; Pu et al. 1999a; Myers-Payne et al. 1996b; Murphy et al. 2005; Moulle et al. 2012), and ii) sterol carrier protein-2 (SCP-2) (Frolov et al. 1996; Pu et al. 1998; Avdulov et al. 1998; Myers-Payne et al. 1996a). LKO decreased the protein levels of FABP3 and FABP5 (Fig 6A,B) without altering those of FABP7 or SCP2 (Fig 6B–D).
Figure 6.

FABP1 gene ablation alters protein levels of brain cytosolic ‘chaperone’ fatty acid and endocannabinoid binding proteins. All conditions were as in Figure 5 except that Western blot analysis was performed as described (Martin et al. 2015;Kaczocha et al. 2009) to determine protein levels of (A) FABP3, (B) FABP5, (C) FABP7, (D) SCP-2, and (E) HSP70. Insets show representative Western blot images of the respective protein (upper blot) and the gel-loading control protein (GAPDH or β-Actin, lower blot). Relative protein levels were normalized to the gel-loading control protein; values were compared to WT set to 1. Results are presented as mean ± SEM (n = 7); *, p < 0.05 for LKO vs WT.
Impact of FABP1 Ablation (LKO) on Brain Protein Levels of Cytosolic ‘Chaperone’ Proteins Involved in AEA and 2-AG Re-uptake and Intracellular Transport
While translocation/transport of ARA across brain plasma membranes and intracellular membranes is mediated by membrane associated transport/translocase proteins (Mitchell and Hatch 2011), endocannabinoid reuptake across the plasma membrane is thought to be largely diffusional (Fowler 2013). Since endocannabinoids are very hydrophobic, however, intracellular transport targeting/degradation also requires cytosolic lipid transport proteins. Pioneering work by Kaczocha and others demonstrated for the first time that FABPs 3,5, and 7 serve as key cytosolic ‘chaperones’ for AEA and 2-AG reuptake/transport and targeting for degradation/hydrolysis within brain cells (Kaczocha et al. 2009; Kaczocha et al. 2012; Kaczocha 2009; Elmes et al. 2015; Yu et al. 2014; Owada et al. 2006; Owada 2008). Since HSP70 also binds endocannabinoids, it has been suggested to have a ‘chaperone’ function (Oddi et al. 2009). Furthermore, the finding that sterol carrier protein-2 (SCP-2) transfers endocannabinoid between membranes also suggests SCP-2 as a potential endocannabinoid binding/transfer ‘chaperone’ in the brain (Liedhegner et al. 2014). The possibility that SCP-2 bound AEA and/or 2-AG was examined by displacement of SCP-2 bound cis-parinaric acid. Indeed, AEA and 2-AG both displaced bound cis-parinaric acid from SCP-2 (Fig 7). Analysis of multiple displacement curves, together with known Kd = 0.18 ± 0.01 μM for SCP-2 binding cis-parinaric acid (Frolov et al. 1996), allowed calculation of SCP-2 affinities for AEA and 2-AG as described in Experimental Procedures. SCP-2 had high affinity for AEA, Ki = 0.29 ± 0.01 μM, and even slightly higher affinity for 2-AG, Ki = 0.20 ± 0.01 μM (Fig 5). Consequently, the impact of LKO on the levels of the brain FABPs, HSP70 and SCP-2 was determined.
Figure 7.
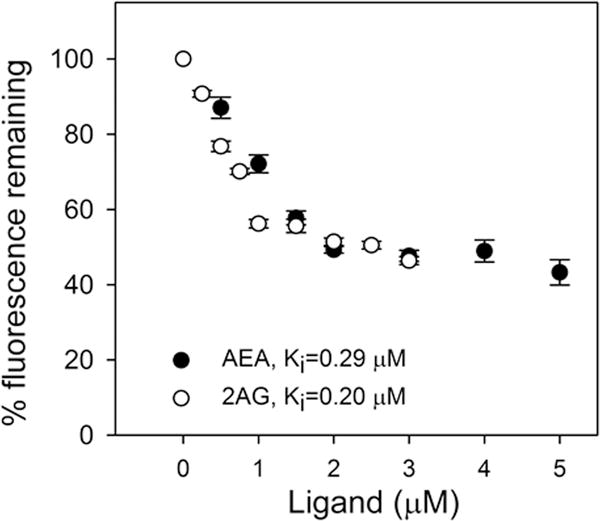
SCP-2 binds AEA and 2-AG: a fluorescent cis-parinaric acid displacement assay. SCP-2 (500 nM) was equilibrated with cis-parinaric acid (500 nM), and then titrated with increasing amounts of AEA (0–5 μM) or 2-AG (0–3 μM). As AEA (closed black circles) and 2-AG (open circles) concentration increased, cis-parinaric acid emission decreased (Ex = 304 nm, Em max = 420 nm). Kis were calculated from the known Kd for cis-parinaric acid binding and the EC50 for displacement of cis-parinaric acid by AEA and 2-AG as described in Materials and Methods. Results are presented as mean ± SEM (n = 6).
As indicated in the preceding section, LKO decreased brain protein levels of FABP3 and FABP5 (Fig. 6A,B), but not those of FABP7 (Fig. 6C). Concomitantly, LKO also decreased brain levels of HSP70 (Fig. 6E), but not those of SCP-2 (Fig. 6D). Thus, the observed higher brain levels of AEA and 2-AG in LKO mice were associated with an overall decrease in three out of five of the cytosolic endocannabinoid ‘chaperone’ proteins. In contrast to protein levels of the intracellular lipid transport proteins, RNA levels of FABP 3, 5 and 7 were increased in LKO males (Supplementary Fig 1A–C); however, SCP-2 RNA levels were decreased in LKO (Supplementary Fig 1D).
FABP1 Ablation (LKO) had Little Impact upon Brain Levels of Proteins Involved in AEA and 2-AG Synthesis and Degradation
Brain levels of AEA and 2-AG are regulated in part by the expression of several key enzymes in AEA and 2-AG synthesis (NAPEPLD, DAGLα) and/or degradation (FAAH, NAAA, MAGL) (Goparaju et al. 1989; Di Marzo et al. 1998; Blankman et al. 2007). LKO did not significantly alter the brain protein levels of any of these enzymes (Fig 8A–E). The lack of any difference in protein levels of these enzymes between WT and LKO mice did not correlate with levels of the respective RNAs (Supplementary Fig 2A–F).
Figure 8.
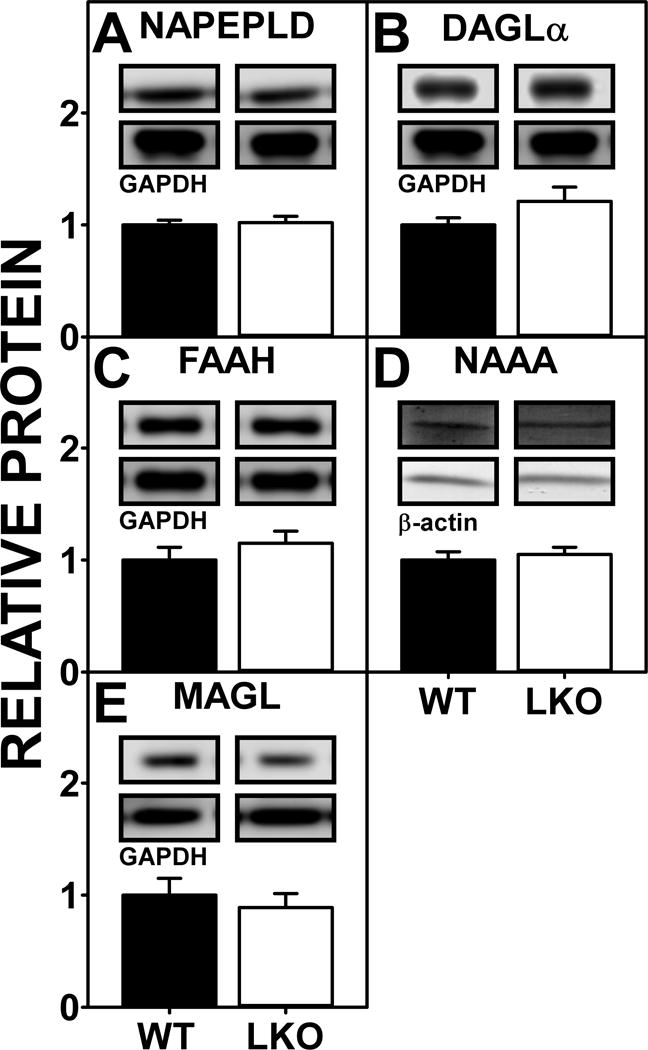
Impact of FABP1 gene ablation on protein levels of brain proteins involved in endocannabinoid synthesis and degradation. All conditions were as described in Figure 5 except that Western blot analysis was performed as described (Martin et al. 2015;Kaczocha et al. 2009) to determine protein levels of (A) NAPEPLD, (B) DAGLα, (C) FAAH, (D) NAAA, and (E) MAGL. Insets show representative Western blot images of the respective protein (upper blot) and the gel-loading control protein (GAPDH or β-Actin, lower blot). Relative protein levels were normalized to the gel-loading control protein; values were compared to WT set to 1. Results are presented as mean ± SEM (n = 7).
FABP1 Ablation (LKO) Has Little Impact on Brain Protein Levels of AEA and 2-AG Receptors of the Endocannabinoid System
The cannabinoid receptor most commonly found in brain is CB1, but TRPV1 and CB2 are also present at lower levels (Maccarrone et al. 2010). LKO did not significantly alter brain protein levels of CB1 and TRPV1 (Supplementary Fig 3E,F). The lack of change in the respective protein levels was not attributable to lack of change in RNA concentration since mRNA levels of these and other receptors/signaling proteins were actually elevated in response to FABP1 ablation (Supplementary Fig 3A–D). Thus, the FABP1 ablation-induced alterations in brain AEA and 2-AG levels were not associated with increased brain protein levels of endocannabinoid receptors of the endocannabinoid system.
Impact of FABP1 Ablation (LKO) on Brain Inflammatory Cytokine Levels
LKO not only reduced male brain concentrations of insulin (Supplementary Fig 4A), but also reduced brain levels of inflammatory cytokines MCP-1 (Supplementary Fig 4E), PAI-1 (Supplementary Fig 4F), and IL-6 (Supplementary Fig 4G). Thus, the LKO-induced increase in male mouse brain endocannabinoid levels correlated with decreased brain levels of the inflammation-related cytokines MCP-1, PAI-1 and IL-6.
DISCUSSION
Brain ARA, from which AEA and 2-AG are derived, is largely taken up from plasma (Mitchell and Hatch 2011; Bazinet and Laye 2014). Brain cytosolic fatty acid binding proteins, i.e. FABPs 3, 5, 7 (Myers-Payne et al. 1996b; Pu et al. 1999b; Pu et al. 1999a; Owada et al. 1996; Schnutgen et al. 1996; Bennett et al. 1994; Feng et al. 1994; Kurtz et al. 1994) act as ‘chaperones’ to facilitate poorly aqueous soluble ARA uptake, cytosolic transport, and intracellular targeting (Mitchell and Hatch 2011; Murphy 2015). Ablating or inhibiting brain FABPs, especially FABP3, reduces brain ARA uptake, and reduces AEA degradation (Yu et al. 2014; Murphy et al. 2005; Owada et al. 2006; Owada 2008; Moulle et al. 2012). Less appreciated is the fact that high first-pass hepatic clearance rate significantly diminishes plasma ARA availability for brain uptake by nearly 50% (Mashek 2013; Havel et al. 1962; Kohout et al. 1971; Zhou et al. 2002), while first-removal of oral cannabinoid was diminished even further by 90% (Huestis 2007; Mattes et al. 1993; Trevaskis et al. 2009; Grotenhermen 2003; Ashton 2001). Yet, little is known about hepatic factors contributing to brain AEA and 2-AG levels.
One potential candidate serving in this capacity is FABP1 which represents 2–10% of cytosolic protein or 0.1–1.0 mM in liver cytosol depending on species (Atshaves et al. 2010; Huang et al. 2014; McArthur et al. 1999; Favretto et al. 2013). In fact, liver cytosolic FABP1 concentration is 20 to 100-fold higher than the sum of FABP 3, 5, and 7 present in brain cytosol (Myers-Payne et al. 1996b; Pu et al. 1999b; Pu et al. 1999a; Owada et al. 1996; Schnutgen et al. 1996; Bennett et al. 1994; Feng et al. 1994; Kurtz et al. 1994; Myers-Payne et al. 1996b; Pu et al. 1999b; Pu et al. 1999a; Owada et al. 1996; Schnutgen et al. 1996; Bennett et al. 1994; Feng et al. 1994; Kurtz et al. 1994). Further, FABP1 has high affinity for ARA as shown by: i) displacement of FABP1 bound fluorescent ANS (Ki=0.11+0.01μM) (Frolov et al. 1997; Schroeder et al. 1998); ii) FABP1 direct binding of fluorescent ARA analogue A5C (Kd=0.08+ 0.01μM) (McIntosh et al. 2010); and iii) preferential enrichment of native liver FABP1 with endogenously-bound ARA (Murphy et al. 1999). FABP1 even has high affinity for the ARA precursor linoleic acid (C18:2, n-6) which can be metabolized to ARA in liver, but much less so in brain (Frolov et al. 1997; Murphy et al. 1999). Significantly, FABP1 overexpression enhances cellular uptake of ARA and ARA analogues (McIntosh et al. 2010; Murphy et al. 1996b; Murphy et al. 1996a; Schroeder et al. 1993). These findings suggest that the liver may compete effectively with the brain for ARA uptake facilitated by the respective FABPs. On this basis, we hypothesized that FABP1 gene ablation would significantly impact brain levels of endocannabinoids, especially AEA and 2-AG. Our findings provided the following new insights:
First, brain levels of the ARA-containing endocannabinoid CB-receptor agonists (AEA, 2-AG) were inversely correlated with FABP1 level in liver. Complete loss of liver FABP1 (i.e. FABP1 ablation, LKO) increased levels of AEA and 2-AG in brain. These findings were associated with two major factors: i) LKO increased brain free and total ARA concomitant with increased availability of serum ARA (especially total ARA) for uptake by brain. Increased serum ARA availability upon FABP1 ablation was consistent with FABP1’s high affinity for ARA (Frolov et al. 1997; Schroeder et al. 1998; McIntosh et al. 2010) and with FABP1 overexpression increasing uptake of ARA and ARA analogues in transfected cells (McIntosh et al. 2010; Murphy et al. 1996b; Murphy et al. 1996a; Schroeder et al. 1993; McIntosh et al. 2010); ii) LKO decreased protein levels of brain cytosolic ARA and 2-AG ‘chaperone’ proteins such as FABP3, FABP5, and HSP70. Earlier studies showed that ablation (FABP3 null, FABP5 null) or chemical inhibition of brain FABPs increased brain AEA levels due to decreased AEA transport/targeting for intracellular degradation/hydrolysis by FAAH in the endoplasmic reticulum (Berger et al. 2012; Kaczocha 2009; Kaczocha et al. 2014; Leung et al. 2013; Yu et al. 2014). Conversely, overexpression of FABP5 or FABP7 in transformed cells (N18TG2 neuroblastoma or COS7) increased AEA uptake and hydrolysis (Kaczocha et al. 2009).
Second, brain levels of oleic acid or palmitic acid-containing potentiating NAEs and 2-MGs were also inversely correlated with liver FABP1. LKO increased brain levels of all these potentiating endocannabinoids in the order: oleic acid-containing (OEA, 2-OG) > palmitic acid-containing (PEA, 2-PG). The ‘entourage’ NAEs and/or 2-MGs are synthesized from oleic acid or palmitic acid containing phospholipids in brain, do not directly bind/activate CB receptors, but instead are thought to potentiate AEA (and/or 2-AG) activity by increasing AEA (and/or 2-AG) affinities for CB receptors or decrease AEA (and/or 2-AG) enzymatic degradation (Ho and Barrett 2008; Smart et al. 2002; Piomelli and Seaman 1993; Franklin et al. 2003; Ben-Shabat et al. 1998; Mechoulam et al. 1997). The mechanism(s) whereby by LKO elicits increased brain levels of the ‘entourage’ endocannabinoids are less clear but may exhibit significant overlap with those described above for ARA-containing endocannabinoids: i) LKO-induced decrease in brain levels of non-ARA endocannabinoids was also associated with reduced levels on cytosolic levels of ‘chaperone’ proteins (FABP3, FABP5, HSP70). Because of the close structural similarities the potentiating NAEs and 2-MGs to AEA and 2-AG, respectively, they are likely also bound by these brain cytosolic chaperone proteins—thereby similarly facilitating their transport/targeting to intracellular sites for hydrolysis/degradation; ii) LKO-induced increases in the oleic acid and palmitic acid-containing endocannabinoids may also, at least partially, be attributable to FABP1’s impact on hepatic clearance of palmitic and oleic acid clearance. Although saturated and monounsaturated fatty acids are mainly synthesized endogenously de novo in the brain (Mitchell and Hatch 2011; Bazinet and Laye 2014), nevertheless the brain will take up non-ARA fatty acids from plasma (Mitchell and Hatch 2011; Murphy et al. 2005; Chen et al. 2013). Palmitic acid and oleic acid represent about 10% and 30% of endogenously-bound fatty acids in native FABP1 isolated from liver (Murphy et al. 1999). FABP1 overexpression increases the uptake of non-ARA fatty acids or their analogues in transfected L-cells as well as being directly proportional to FABP1 level in a series of human HepG2 liver clones (Murphy et al. 1996b; Prows et al. 1995; Schroeder et al. 1993; Murphy 1998; McArthur et al. 1999; Wolfrum et al. 1999a). Conversely, LKO decreases uptake of saturated fatty acids (palmitic acid, NBD-stearic acid) and oleic acid in cultured primary mouse hepatocytes (Atshaves et al. 2004a; McIntosh et al. 2009; Storey et al. 2012a) and/or in vivo (Martin et al. 2003a; Erol et al. 2004; Newberry et al. 2003).
Third, changes in brain mRNA transcripts of genes encoding endocannabinoid system transporters, chaperones, enzymes, and receptors in general did not extend to respective proteins. LKO increased brain levels of several mRNA transcripts (Cnr1; Trvp1; Dagla; Faah; Mgll; Fabp 3, 5, 7), but the respective proteins were not increased. Kunos and colleagues first suggested that an analogous striking difference between the robust nearly 30-fold increase in Cnr1 mRNA, paralleled by only 3–4 fold increased CB1 protein, may result from the action of specific micro RNA (miRNA) that inhibit mRNA translation (Mukhopadyay et al. 2010). In support of this possibility, Target Scan identified a conserved miR-128 binding site in the 3’-untranslated region of the mouse Cnr1 gene (Mukhopadyay et al. 2010). CB1 receptor level is highly responsive to miR-26b and miR-494, while CB2 receptor level is responsive to miR-665 (Stringer et al. 2013; Mohnle et al. 2014). About 30%-90% of all genes are thought to be regulated by miRNA, with nearly 150 miRNA differentially expressed between male and female mouse brains (Dai and Ansar Ahmed 2014; D’Addario et al. 2013). Finally, it is important to note that endocannabinoids such as 2-AG itself upregulate expression of miR-188-3p while AEA differentially regulates up to 100 miRNA (Zhang et al. 2014; Jackson et al. 2014). Resolving the individual contributions of the potential multiple miRNAs will require further studies.
Fourth, the FABP1 gene ablation-induced increase in brain endocannabinoid levels was not associated with higher levels of inflammatory cytokines. ARA-containing phospholipids are the substrate not only for AEA and 2-AG synthesis, but also for formation of pro-inflammatory mediators (eicosanoids, prostamides)—thereby resulting in competition between these pathways for substrate ARA (Murphy 2015; Witkamp 2015). Although FABP1 enhances ARA uptake (McIntosh et al. 2010; Murphy et al. 1996b; Murphy et al. 1996a; Schroeder et al. 1993) and FABP1 ablation increases serum and brain ARA levels as shown herein, brain levels of ARA-derived inflammatory cytokines were not increased. This was consistent with earlier findings in livers of FABP1 gene ablated mice demonstrating the absence of steatosis, inflammation, or elevated levels of inflammation-related cytokines such as MCP-1, PAI-1, IL-6, or TNFα (Martin et al. 2009b). Taken together, these data suggested that brain production of ARA-containing endocannabinoids may be favored under conditions of high ARA availability.
In summary, the work described herein for the first time demonstrated that the non-CNS fatty acid binding protein FABP1 markedly affected brain levels of both ARA-containing as well as their non-ARA containing potentiating ‘entourage’ endocannabinoids. Hepatic FABP1 gene ablation significantly increased brain levels of ARA-containing endocannabinoids (AEA, 2-AG) as well as non-ARA containing ‘entourage’ NAE (OEA, PEA) and 2-MG (2-OG, 2-PG). This was associated with increased availability of ARA in serum and in brain of LKO mice. While the physiological significance of these findings remains to be explored, it should be noted that endogenous endocannabinoid (cannabis-like) CB agonists derived from arachidonic acid (AEA, 2-AG) constitute a novel modulatory system involved not only in nociception but also in appetite control/satiety and behavior (Sarchielli et al. 2007; Guasti et al. 2009; Fine and Rosenfeld 2013; Sagar et al. 2012; Luongo et al. 2015). For example, LKO may be on pain sensitivity. WT male mice exhibit high sensitivity to pain which is associated with low levels of brain AEA (Gonzalez et al. 2000; Craft et al. 2013; Rubino and Parolaro 2011; Moreno-Sanz et al. 2012). In contrast, increasing brain endocannabinoid levels by ablating or inhibiting brain FABPs (FABP3, 5, and 7) produces analgesia and suppresses inflammatory pain (Kaczocha et al. 2014; Kaczocha et al. 2015). Since LKO increases brain AEA level, this suggests that FABP1 loss or inhibition may increase analgesia in male mice analogous to ablation or inhibition of brain FABPs (FABP3, 5, and 7). Thus, it will be important in future studies to determine to what extent, if any, the loss or inhibition of FABP1 will have on behavior, pain and inflammatory pain. Another potential physiological impact of LKO-induced increases in brain AEA and OEA may be on food intake. Increased brain level of AEA correlates with increases food intake and de novo fatty acid synthesis by an SREBP1 mediated mechanism (Alswat 2015). In contrast, increased brain OEA decreases food intake and weight gain by inducing PPARα transcription of multiple genes involved in fatty acid oxidation (Fu et al. 2005; Maccarrone et al. 2010; Naughton et al. 2013). Since LKO similarly increased AEA and 2-OG levels in brain, the net effect of LKO was not to alter or only slightly increase food intake (Atshaves et al. 2005; Martin et al. 2005; Martin et al. 2009b; Martin et al. 2009a; Gajda and Storch 2015; Lagakos et al. 2011). Further studies with LKO mice, FABP1 overexpression, and FABP1 selective inhibitors are needed to elucidate the impact of FABP1 on pain sensitivity, behavior, and other aspects of brain endocannabinoid function.
Supplementary Material
Acknowledgments
This work was supported in part by the US Public Health Service/National Institutes of Health Grant R25 OD016574 (S.C., A.B.K.), Merial Veterinary Scholars Program, CVM (S.C., A.B.K.), DA035949 (M.K.), and NIH funded COBRE Mass Spec Core Facility Grant 5P30GM103329-04 (M.G.). We thank Ms. Svetlana Golovko for her excellent technical assistance with ARA LC-MS/MS analysis and Dr. Avery L. McIntosh for helpful assistance with schematic preparation.
Abbreviations
- ACBP
acyl CoA binding protein
- Adrbk2
G protein coupled receptor kinase-2 (GRK-2)
- AEA
n-6 arachidonoyl-ethanolamide (anandamide)
- 2-AG
2-arachidonoyl glycerol
- ARA
arachidonic acid (C20:4, n-6)
- Cnr1
cannabinoid receptor-1 (CB1)
- Cnr2
cannabinoid receptor-2 (CB2)
- Dagla
diacylglycerol lipase A (DAGL-A)
- Daglb
diacylglycerol lipase B (DAGL-B)
- DHEA
n-3 docosahexaenoyl ethanolamide
- EC
endocannabinoid
- EC*
non-arachidonic acid containing endocannabinoid
- EPEA
n-3 eicosapentaenoyl ethanolamide
- Faah
fatty acid amide hydrolase (FAAH)
- Fabp1
liver fatty acid binding protein-1 (FABP1, L-FABP)
- Fabp3
fatty acid binding protein-3 (FABP-3)
- Fabp5
fatty acid binding protein-5 (FABP-5)
- Fabp7
fatty acid binding protein-7 (FABP-7)
- FAT/CD36
fatty acid translocase/thrombospondin receptor
- FATP-1
fatty acid transport protein-1
- FATP-4
fatty acid transport protein-4
- GPCR
G-protein coupled receptor
- HSP70
heat shock protein-70
- LKO
FABP1 gene ablated mouse on C57BL/6NCr background
- LCFA
long chain fatty acid
- LCFA-CoA
long chain fatty acyl CoA
- γ-LNA
γ-linolenic acid (C18:2, n-6)
- 2-MG
2-monoacylglycerol
- Mgll
2-monoacylglycerol lipase (MGL)
- Naaa
N-acylethanolamide-hydrolyzing acid amidase (NAAA)
- NAE
N-acyl ethanolamides
- NAPE
N-acylphosphatidylethanolamine
- Nape-pld
N-acylphosphatidylethanolamine phospholipase-D (NAPE-PLD)
- OEA
oleoyl ethanolamide
- 2-OG
2-oleoyl glycerol
- PEA
palmitoyl ethanolamide
- 2-PG
2-palmitoyl glycerol
- Scp2
sterol carrier protein-2 (SCP-2)
- Trvp-1
transient receptor potential cation channel subfamily V member 1 (TRVP-1, vanilloid receptor-1)
- WT
wild-type C57BL/6NCr mouse
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions: Experiments were designed, performed, and analyzed by the following: GGM and LJD for Figures 1, 2, 4 and GGM for Supplemental Figure 4; DRS, EJM, and MYG for Figure 3; SC, DL, and KKL for Figures 5, 6A, C–E, 8D and Supplemental Figures 1, 2, 3A–D; HH for Figure 7; XP and MK for Figure 6B, 8A–C, E and Supplemental Figure 3E, F. FS, ABK, MK, and EJM conceived and coordinated the study and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
References
- Alswat KA. The role of endocannabinoid system in fatty liver disease and therapeutic potential. Saudi Journal of Gastroenterology. 2015;19:144–151. doi: 10.4103/1319-3767.114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101–106. doi: 10.1192/bjp.178.2.101. [DOI] [PubMed] [Google Scholar]
- Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) and Dietary Obesity. Journal of Nutritional Biochemisty. 2010;21:1015–1032. doi: 10.1016/j.jnutbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atshaves BP, McIntosh AL, Martin GG, Landrock D, Payne HR, Bhuvanendran S, Landrock K, Lyuksyutova OI, Johnson JD, Macfarlane RD, Kier AB, Schroeder F. Overexpression of sterol carrier protein-2 differentially alters hepatic cholesterol accumulation in cholesterol-fed mice. J Lipid Res. 2009;50:1429–1447. doi: 10.1194/jlr.M900020-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atshaves BP, McIntosh AL, Landrock D, Payne HR, Mackie J, Maeda N, Ball JM, Schroeder F, Kier AB. Effect of SCP-x gene ablation on branched-chain fatty acid metabolism. Am J Physiol. 2007a;292:939–951. doi: 10.1152/ajpgi.00308.2006. [DOI] [PubMed] [Google Scholar]
- Atshaves BP, McIntosh AL, Lyuksyutova OI, Zipfel WR, Webb WW, Schroeder F. Liver fatty acid binding protein gene ablation inhibits branched-chain fatty acid metabolism in cultured primary hepatocytes. J Biol Chem. 2004a;279:30954–30965. doi: 10.1074/jbc.M313571200. [DOI] [PubMed] [Google Scholar]
- Atshaves BP, McIntosh AL, Payne HR, Gallegos AM, Landrock K, Maeda N, Kier AB, Schroeder F. Sterol carrier protein-2/sterol carrier protein-x gene ablation alters lipid raft domains in primary cultured mouse hepatocytes. J Lipid Res. 2007b;48:2193–2211. doi: 10.1194/jlr.M700102-JLR200. [DOI] [PubMed] [Google Scholar]
- Atshaves BP, McIntosh AL, Payne HR, Mackie J, Kier AB, Schroeder F. Effect of branched-chain fatty acid on lipid dynamics in mice lacking liver fatty acid binding protein gene. Am J Physiol. 2005;288:C543–C558. doi: 10.1152/ajpcell.00359.2004. [DOI] [PubMed] [Google Scholar]
- Atshaves BP, Payne HR, McIntosh AL, Tichy SE, Russell D, Kier AB, Schroeder F. Sexually dimorphic metabolism of branched chain lipids in C57BL/6J mice. J Lipid Res. 2004b;45:812–830. doi: 10.1194/jlr.M300408-JLR200. [DOI] [PubMed] [Google Scholar]
- Atshaves BP, Petrescu A, Starodub O, Roths J, Kier AB, Schroeder F. Expression and Intracellular Processing of the 58 kDa Sterol Carrier Protein 2/3-Oxoacyl-CoA Thiolase in Transfected Mouse L-cell Fibroblasts. J Lipid Res. 1999;40:610–622. [PubMed] [Google Scholar]
- Avdulov NA, Chochina SV, Myers-Payne S, Hubbell T, Igbavboa U, Schroeder F, Wood WG. Expression and lipid binding of sterol carrier protein-2 and liver fatty acid binding proteins: differential effects of ethanol in vivo and in vitro. In: Riemersma R. A. A. R. K. R. W. a. W. R, editor. Essential Fatty Acids and Eicosanoids: Invited Papers from the Fourth International Congress. American Oil Chemists Society Press; Champaign, IL: 1998. pp. 324–327. [Google Scholar]
- Baumgardner JN, Shankar K, Hennings L, Badger TM, Ronis MJJ. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am J Physiol Gastrointest and Liver Phys. 2007;294:G27–G38. doi: 10.1152/ajpgi.00296.2007. [DOI] [PubMed] [Google Scholar]
- Bazinet RP, Laye S. PUFA and their metabolites in brain function and disease. Nature Reviews Neuroscience. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- Ben-Shabat S, Fride E, Sheskin T, et al. An entoruage effects: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharm. 1998;353:23–31. doi: 10.1016/s0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- Bennett E, Stenvers KL, Lund PK, Popko B. Cloning and characterization of a cDNA encoding a novel fatty acid binding protein from rat brain. J Neurochem. 1994;63:1616–1624. doi: 10.1046/j.1471-4159.1994.63051616.x. [DOI] [PubMed] [Google Scholar]
- Berger WT, Ralph BP, Kaczocha M, Sun J, Balius TE, Rizzo RC, Haj-Dahmane S, Ojima I, Deutsch DG. Targeting FABP anandamide transporters–a novel strategy for development of anti-inflammatory and anti-nociceptive drugs. PLoS ONE. 2012;7:e50968. doi: 10.1371/journal.pone.0050968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. Comprehensive profile of brain enzymes that hydrolyze endocannabinoid 2-arachidonoylglycerol. Chemistry and Biology. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brose S, Baker A, Golovko MY. A fast one-step extraction and UPLC-MS/MS Analysis for E2/D2 series prostaglandins and isoprostanes. Lipids. 2013;48:411–419. doi: 10.1007/s11745-013-3767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose SA, Golovko MY. Eicosanoid post-mortem induction in kidney tissue is prevented by microwave irradiation. Prost Leukot Essen Fatty Acids. 2013;89:313–318. doi: 10.1016/j.plefa.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamouton J, Latruffe N. PPARa/HNF4a interplay on diversified response elements. Relevance in the regulation of liver peroxisomal fatty acid catabolism. Curr Drug Metabolism. 2012;13:1436–1453. doi: 10.2174/138920012803762738. [DOI] [PubMed] [Google Scholar]
- Chao H, Zhou M, McIntosh A, Schroeder F, Kier AB. Acyl CoA binding protein and cholesterol differentially alter fatty acyl CoA utilization by microsomal acyl CoA: cholesterol transferase. J Lipid Res. 2003;44:72–83. doi: 10.1194/jlr.m200191-jlr200. [DOI] [PubMed] [Google Scholar]
- Charlton M, Viker K, Krishnan A, Sanderson S, Veldt B, Kaalsbeek AJ, Kendrick M, Thompson G, Que F, Swain J, Sarr M. Differential expression of lumican and fatty acid binding protein-1: new insights into the histologic spectrum of nonalcoholic fatty liver disease. Hepatology. 2009;49:1375–1384. doi: 10.1002/hep.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CT, Domenichiello AF, Trepanier M-O, Liu Z, Masoodi M, Bazinet RP. Low levels of EPA in rat brain phospholipids are maintained via multiple redundant mechanisms. J Lipid Res. 2013;54:2422. doi: 10.1194/jlr.M038505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ, Mallet C. Endocannabinoids can open the pain gate. Sciencesignaling. 2009;2:pe57. doi: 10.1126/scisignal.288pe57. [DOI] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: a reflection of differences in endocannabinoid system? Life Sci. 2013;92:476–481. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Addario C, Di Francesco A, Pucci M, Agro AF, Maccarrone M. Epigenetic mechanisms and endocannabinoid signaling. FEBS J. 2013;280:1905–1917. doi: 10.1111/febs.12125. [DOI] [PubMed] [Google Scholar]
- Dai R, Ansar Ahmed S. Sexual dimorphism of miRNA expression: a new perspective in understanding the sex bias of autoimmune disease. Therapeutics and Clinical Risk Management. 2014;10:151–163. doi: 10.2147/TCRM.S33517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Melck D, Bisogno T, Di Marzo V. Endocannabinoids and fatty acid amides in cancer, inflammation, and related disorders. Chem Phys Lip. 2000;108:191–209. doi: 10.1016/s0009-3084(00)00196-1. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, Sugiura T, et al. The novel endogenous cannabinoid 2-AG is inactivated by neuronal- and basophil-like cells: connections with anandamide. Biochem J. 1998;331:15–19. doi: 10.1042/bj3310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus P, Wolfrum C, Assmann G, Spener F, Seedorf U. Phytanic acid activates the peroxisome proliferator-activated receptor alpha (PPARalpha) in sterol carrier protein-2-/sterol carrier protein x-deficient mice. J Biol Chem. 1999;274:2766–2772. doi: 10.1074/jbc.274.5.2766. [DOI] [PubMed] [Google Scholar]
- Elmes MW, Kaczocha M, Berger WT, Leung KN, Ralph BP, Wang L, Sweeney JM, Miyauchi JT, Tsirka SE, Ojima I, Deutsch DG. Fatty acid binding proteins are intracellular carriers for delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) J Biol Chem. 2015;290:8711–8721. doi: 10.1074/jbc.M114.618447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol E, Kumar LS, Cline GW, Shulman GI, Kelly DP, Binas B. Liver fatty acid-binding protein is required for high rates of hepatic fatty acid oxidation but not for the action of PPAR-a in fasting mice. FASEB J. 2004;18:347–349. doi: 10.1096/fj.03-0330fje. [DOI] [PubMed] [Google Scholar]
- Favretto F, Assfalg M, Gallo M, Cicero DO, D’Onofrio M, Molinari H. Ligand binding promiscuity and human liver fatty acid binding protein: structural and dynamic insights from an interaction study with glycocholate and oleate. ChemBioChem. 2013;14:1807–1819. doi: 10.1002/cbic.201300156. [DOI] [PubMed] [Google Scholar]
- Feng L, Hatten ME, Heintz N. Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron. 1994;12:895–908. doi: 10.1016/0896-6273(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Fine PG, Rosenfeld MJ. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Medical Journal. 2013;4:e0022. doi: 10.5041/RMMJ.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ. Transport of endocannabinoids across plasma membrane and within the cell. FEBS J. 2013;280:1895–1904. doi: 10.1111/febs.12212. [DOI] [PubMed] [Google Scholar]
- Franklin A, Parmentier-Batteur S, Walter L, Greenbert DA, Stella N. Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility. J Neuroscience. 2003;23:7767–7775. doi: 10.1523/JNEUROSCI.23-21-07767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov A, Cho TH, Billheimer JT, Schroeder F. Sterol carrier protein-2, a new fatty acyl coenzyme A-binding protein. J Biol Chem. 1996;271:31878–31884. doi: 10.1074/jbc.271.50.31878. [DOI] [PubMed] [Google Scholar]
- Frolov A, Cho TH, Murphy EJ, Schroeder F. Isoforms of rat liver fatty acid binding protein differ in structure and affinity for fatty acids and fatty acyl CoAs. Biochemistry. 1997;36:6545–6555. doi: 10.1021/bi970205t. [DOI] [PubMed] [Google Scholar]
- Frolov AA, Schroeder F. Acyl coenzyme A binding protein: conformational sensitivity to long chain fatty acyl-CoA. J Biol Chem. 1998;273:11049–11055. doi: 10.1074/jbc.273.18.11049. [DOI] [PubMed] [Google Scholar]
- Fu J, Oveisi F, Gaetani S, Lin E, Piomelli D. Oleoylethanolamine, an endogenous PPAR-a agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology. 2005;48:1153. doi: 10.1016/j.neuropharm.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Hafer A, Muench C, Kannenberg F, Teichmann S, Scheibner J, Stange EF, Seedorf U. Disruption of the sterol carrier protein 2 gene in mice impairs biliary lipid and hepatic cholesterol metabolism. J Biol Chem. 2001;276:48058–48065. doi: 10.1074/jbc.M106732200. [DOI] [PubMed] [Google Scholar]
- Gajda AM, Storch J. Enterocyte fatty acid binding proteins (FABPs): different functions of liver and intestinal FABPs in the intestine. Prost Leukot Essen Fatty Acids. 2015;93:9–15. doi: 10.1016/j.plefa.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Bisogno T, Wenger T, et al. Sex steroid influence on CB1 receptor mRNA and endocannabinoid levels in anterior pituitary gland. Biochem Biophy Res Comm. 2000;270:260–266. doi: 10.1006/bbrc.2000.2406. [DOI] [PubMed] [Google Scholar]
- Goparaju SK, Ueda N, Yamaguchi H, Yamamoto S. Anandamide amidohydrolase reacting with 2-AG, another cannabinoid receptor ligand. FEBS Lett. 1989;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F. Clin Pharmacokinet. 2003;42:327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Guasti L, Richardson D, Jhaveri M, et al. Minocycline treatment inhibits microglial activation and alters spinal levels of endocannabinoids in a rat model of neuropathic pain. Molecular Pain. 2009;5 doi: 10.1186/1744-8069-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamfi MA, He L, French SW, Damjanov I, Wan YJY. Hepatocyte retinoid X receptor alpha-dependent regulation of lipid homeostasis and inflammatory cytokine expression contributes to alcohol-induced liver injury. J Pharm Exp Ther. 2008;324:443–453. doi: 10.1124/jpet.107.132258. [DOI] [PubMed] [Google Scholar]
- Hanhoff T, Benjamin S, Borchers T, Spener F. Branched-chain fatty acids as activators of peroxisome proliferators. Eur J Lip Sci Technol. 2005;107:716–729. [Google Scholar]
- Hanhoff T, Wolfrum C, Ellinghaus P, Seedorf U, Spener F. Pristanic acid is activator of PPARalpha. Eur J Lipid Sci. 2001;103:75–80. [Google Scholar]
- Havel RJ, Felts JM, Van Duyne CM. Formation and fate of endogenous triglycerides in blood plasma of rabbits. J Lipid Res. 1962;3:297–308. [Google Scholar]
- Higuchi N, Kato M, Tanaka M, et al. Effects of insulin resistance and hepatic lipid accumulation on hepatic mRNA expression levels of apoB, MTP, and L-FABP in non-alcoholic fatty liver disease. Exp and Ther Med. 2011;2:1077–1081. doi: 10.3892/etm.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WSV, Barrett DAR. Entourage effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxaton to anandamide occur through TRPV1 receptors. Br J Pharmacol. 2008;155:837–846. doi: 10.1038/bjp.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Atshaves BP, Frolov A, Kier AB, Schroeder F. Acyl-coenzyme A binding protein expression alters liver fatty acyl coenzyme A metabolism. Biochemistry. 2005;44:10282–10297. doi: 10.1021/bi0477891. [DOI] [PubMed] [Google Scholar]
- Huang H, McIntosh AL, Martin GG, Landrock K, Landrock D, Gupta S, Atshaves BP, Kier AB, Schroeder F. Structural and functional interaction of fatty acids with human liver fatty acid binding protein (L-FABP) T94A variant. FEBS J. 2014;281:2266–2283. doi: 10.1111/febs.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, McIntosh AL, Martin GG, Petrescu AD, Landrock K, Landrock D, Kier AB, Schroeder F. Inhibitors of fatty acid synthesis induce PPARa-regulated fatty acid b-oxidative enzymes: synergistic roles of L-FABP and glucose. PPAR Research. 2013;2013:1–22. doi: 10.1155/2013/865604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4:1770–1804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Ghosh S, Crowe MS, et al. In vivo characterization of the highly selective inhibitor KML29: antinociceptive activity without cannabimimetic side effects. Br J Pharmacol. 2014;171:1392–1407. doi: 10.1111/bph.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AR, Nagarkatti P, Nagarkatti M. Anandamide attenuates Th-17 cell-mediated delayed type hypersensitivity response by triggering IL-10 production and consequent miRNA induction. PLoS ONE. 2014;9:e93954. doi: 10.1371/journal.pone.0093954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian W, Edom R, Weng N, Zannikos P, Zhang Z, Wang H. Validation and application of an LC-MS/MS method for quantitation of three fatty acid ethanolamides as biomarkers for fatty acid hydrolase inhibitionin human placenta. J Chrom B. 2010;878:1687–1699. doi: 10.1016/j.jchromb.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Kaczocha LM, Glaser ST, Deutsch DG. Identification of intracellular carriers for the endocannabinoid anandamide. Proc Natl Acad Sci U S A. 2009;106:6375–6380. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M. Ph D Thesis. Stony Brook University; 2009. Role of fatty acid binding proteins and FAAH-2 in endocannabinoid uptake and inactivation. [Google Scholar]
- Kaczocha M, Glaser ST, Maher T, Clavin B, Hamilton J, O’Rourke J, Rebecchi M, Puopolo M, Owada Y, Thanos PK. Fatty acid binding protein deletion suppresses inflammatory pain through endocannabinoid/N-acylethanolamine-dependent mechanisms. Molecular Pain. 2015;11:52. doi: 10.1186/s12990-015-0056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Rebecchi MJ, Ralph BP, Teng Y-HG, Berger WT, Galbavy W, Elmes MW, Glaser ST, Wang L, Rizzo RC, Deutsch DG, Ojima I. Inhibition of fatty acid binding protein elevates brain anandamide levels and produces analgesia. PLoS ONE. 2014;9:e94200. doi: 10.1371/journal.pone.0094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczocha M, Vivieca S, Sun J, Glaser ST, Deutsch DG. Fatty acid binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J Biol Chem. 2012;287:3415–3424. doi: 10.1074/jbc.M111.304907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout M, Kohoutova B, Heimberg M. The regulaton of hepatic triglyceride metabolism by free fatty acids. J Biol Chem. 1971;246:5067–5074. [PubMed] [Google Scholar]
- Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Muller T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- Lagakos WS, Gajda AM, Agellon LB, Binas B, Choi V, Mandap B, Russnak T, Zhou YX, Storch J. Different functions of intestinal and liver-type fatty acid binding proteins in intestine and in whole body energy homeostasis. Am J Physiol Gastrointest and Liver Phys. 2011;300:G803–G814. doi: 10.1152/ajpgi.00229.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K, Elmes MW, Glaser ST, Deutsch DG, Kaczocha M. Role of FAAH-like anandamide transporter in anandamide inactivation. PLoS ONE. 2013;8:e79355. doi: 10.1371/journal.pone.0079355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedhegner ES, Vogt CD, Sem DS, Cunninham CW, Hillard CJ. Sterol carrier protein-2: binding protein for endocannabinoids. Mol Neurobiol. 2014;50:149–158. doi: 10.1007/s12035-014-8651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, et al. Selective blockade of 2-arachidonoyl hydrolysis produces cannabinoid behavioral effects. Nature Chemical Biology. 2008 Nov 23; doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo L, Malcangio M, Salvemini D, Starowicz K. Chronic pain: New insights in molecular and cellular mechanisms. BioMed Res Int. 2015:1–2. doi: 10.1155/2015/676725. doi.org/10.1155/2015/676725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Gasperi V, Catani MV, Diep TI, Dainese E, Hansen HS, Avigliano L. The endocannabinoid system and its relevance to nutrition. Annu Rev Nutr. 2010;30:423–440. doi: 10.1146/annurev.nutr.012809.104701. [DOI] [PubMed] [Google Scholar]
- Martin GG, Atshaves BP, Landrock KK, Landrock D, Storey SM, Howles PN, Kier AB, Schroeder F. Ablating L-FABP in SCP-2/SCP-x null mice impairs bile acid metabolism and biliary HDL-cholesterol secretion. Am J Physiol Gastrointest and Liver Phys. 2014;307:G1130–G1143. doi: 10.1152/ajpgi.00209.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GG, Atshaves BP, Huang H, McIntosh AL, Williams BW, Pai P-J, Russell DH, Kier AB, Schroeder F. Hepatic phenotype of liver fatty acid binding protein (L-FABP) gene ablated mice. Am J Physiol. 2009a;297:G1053–G1065. doi: 10.1152/ajpgi.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GG, Atshaves BP, Landrock KK, Landrock D, Schroeder F, Kier AB. Loss of L-FABP, SCP-2/SCP-x, or both Induces Hepatic Lipid Accumulation in Female Mice. Arch Biochem Biophys. 2015;580:41–49. doi: 10.1016/j.abb.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) gene ablation alters liver bile acid metabolism in male mice. Biochem J. 2005;391:549–560. doi: 10.1042/BJ20050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein gene ablation enhances age-dependent weight gain in male mice. Mol Cell Biochem. 2009b;324:101–115. doi: 10.1007/s11010-008-9989-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid binding protein (L-FABP) gene. J Biol Chem. 2003a;278:21429–21438. doi: 10.1074/jbc.M300287200. [DOI] [PubMed] [Google Scholar]
- Martin GG, Hostetler HA, McIntosh AL, Tichy SE, Williams BJ, Russell DH, Berg JM, Spencer TA, Ball JA, Kier AB, Schroeder F. Structure and function of the sterol carrier protein-2 (SCP-2) N-terminal pre-sequence. Biochem. 2008;47:5915–5934. doi: 10.1021/bi800251e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GG, Huang H, Atshaves BP, Binas B, Schroeder F. Ablation of the liver fatty acid binding protein gene decreases fatty acyl CoA binding capacity and alters fatty acyl CoA pool distribution in mouse liver. Biochem. 2003b;42:11520–11532. doi: 10.1021/bi0346749. [DOI] [PubMed] [Google Scholar]
- Mashek DG. Hepatic fatty acid trafficking: multiple forks in the road. Adv Nutr. 2013;4:697–710. doi: 10.3945/an.113.004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura JE, George HJ, Ramachandran N, Alvarez JG, Strauss JFI, Billheimer JT. Expression of the mature and the pro-form of human sterol carrier protein 2 in Escherichia coli alters bacterial lipids. Biochemistry. 1993;32:567–572. doi: 10.1021/bi00053a023. [DOI] [PubMed] [Google Scholar]
- Mattes RD, Shaw LM, Edling-Owens J, Engelman K, Elsohly MA. Bypassing the first-pass effect for the therapeutic use of cannabinoids. Pharm Biochem and Behavior. 1993;44:745–747. doi: 10.1016/0091-3057(93)90194-x. [DOI] [PubMed] [Google Scholar]
- McArthur MJ, Atshaves BP, Frolov A, Foxworth WD, Kier AB, Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J Lipid Res. 1999;40:1371–1383. [PubMed] [Google Scholar]
- McIntosh AL, Huang H, Atshaves BP, Wellburg E, Kuklev DV, Smith WL, Kier AB, Schroeder F. Fluorescent n-3 and n-6 very long chain polyunsaturated fatty acids: three photon imaging and metabolism in living cells overexpressing liver fatty acid binding protein. J Biol Chem. 2010;285:18693–18708. doi: 10.1074/jbc.M109.079897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AL, Atshaves BP, Hostetler HA, Huang H, Davis J, Lyuksyutova OI, Landrock D, Kier AB, Schroeder F. Liver type fatty acid binding protein (L-FABP) gene ablation reduces nuclear ligand distribution and peroxisome proliferator activated receptor-alpha activity in cultured primary hepatocytes. Arch Biochem Biophys. 2009;485:160–173. doi: 10.1016/j.abb.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AL, Huang H, Storey SM, Landrock K, Landrock D, Petrescu AD, Gupta S, Atshaves BP, Kier AB, Schroeder F. Human FABP1 T94A variant impacts fatty acid metabolism and PPARa activation in cultured human female hepatocytes. Am J Physiol Gastrointest and Liver Phys. 2014;307:G164–G176. doi: 10.1152/ajpgi.00369.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Hanus L, et al. Anandamide may mediate sleep induction. Nature. 1997;389:25–26. doi: 10.1038/37891. [DOI] [PubMed] [Google Scholar]
- Mitchell RW, Hatch GM. Fatty acid transport into the brain: of fatty acid fables and lipid tails. Prost Leukot Essen Fatty Acids. 2011;85:293–302. doi: 10.1016/j.plefa.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Mohnle P, Schutz SV, Schmidt M, et al. miR-665 is invovled inteh regulation of the expression of the cardioprotective CB2 in patients with severe heart failure. Biochem Biophy Res Comm. 2014;451:516–521. doi: 10.1016/j.bbrc.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Moreno-Sanz G, Sasso O, Guijarro A, Piomelli D. Pharmacological characterization of the peripheral FAAH inhibitor URB937 in female rodents: interaction with the Abcg2 transporter in the blood-placenta barrier. Br J Pharmacol. 2012;167:1620–1628. doi: 10.1111/j.1476-5381.2012.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow FD, Allen CE, Martin RJ. Intracellular fatty acid-binding protein: Hepatic levels in lean and obese rats. Fed Proc. 1979;38:280. [Google Scholar]
- Moulle VSF, Cansell C, Luquet S, Cruciani-Guglielmacci C. Multiple roles of fatty acid handling proteins in brain. Frontiers in Physiology. 2012;3:1–6. doi: 10.3389/fphys.2012.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadyay B, Liu J, Osei-Hylaman D, Kunos G. Transcriptional regulation of the cannabinoid receptor-1 expression in the liver by retinoid acid via retinoid acid receptor-g. J Biol Chem. 2010;285:19002–19011. doi: 10.1074/jbc.M109.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EJ. L-FABP and I-FABP expression increase NBD-stearate uptake and cytoplasmic diffusion in L-cells. Am J Physiol. 1998;275:G244–G249. doi: 10.1152/ajpgi.1998.275.2.G244. [DOI] [PubMed] [Google Scholar]
- Murphy EJ. Blood-brain barrier and brain fatty acid uptake: role of arachidonic acid and PGE2. J Neurochem. 2015 doi: 10.1111/jnc.13289. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Edmondson RD, Russell DH, Colles SM, Schroeder F. Isolation and characterization of two distinct forms of liver fatty acid binding protein from the rat. Biochim Biophys Acta. 1999;1436:413–425. doi: 10.1016/s0005-2760(98)00150-7. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Horrocks LA. A model for compression trauma: pressure-induced injury in cell cultures. J Neurotrauma. 1993;10:431–426. doi: 10.1089/neu.1993.10.431. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Owada Y, Kitanaka N, Kondo H, Glatz JFC. Brain arachidonic acid incorportion is decreased in heart FABP gene ablated mice. Biochem. 2005;44:6350–6360. doi: 10.1021/bi047292r. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Prows DR, Jefferson JR, Incerpi S, Hertelendy ZI, Heiliger CE, Schroeder F. Cis-parinaric acid uptake in L-cells. Arch Biochem Biophys. 1996a;335:267–272. doi: 10.1006/abbi.1996.0507. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Prows DR, Jefferson JR, Schroeder F. Liver fatty acid binding protein expression in transfected fibroblasts stimulates fatty acid uptake and metabolism. Biochim Biophys Acta. 1996b;1301:191–198. doi: 10.1016/0005-2760(96)00024-0. [DOI] [PubMed] [Google Scholar]
- Myers-Payne S, Fontaine RN, Loeffler AL, Pu L, Rao AM, Kier AB, Wood WG, Schroeder F. Effects of chronic ethanol consumption on sterol transfer protein in mouse brain. J Neurochem. 1996a;66:313–320. doi: 10.1046/j.1471-4159.1996.66010313.x. [DOI] [PubMed] [Google Scholar]
- Myers-Payne SC, Hubbell T, Pu L, Schnutgen F, Borchers T, Wood WG, Spener F, Schroeder F. Isolation and characterization of two fatty acid binding proteins from mouse brain. J Neurochem. 1996b;66:1648–1656. doi: 10.1046/j.1471-4159.1996.66041648.x. [DOI] [PubMed] [Google Scholar]
- Naughton SS, Mathai ML, Hryciw DH, McAinch AJ. Fatty acid modulation of the endocannabinoid system and the effect on food intake and metabolism. Int J Endocrinol. 2013:1–11. doi: 10.1155/2013/361895. ID 361895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry EP, Xie Y, Kennedy S, Buhman KK, Luo J, Gross RW, Davidson NO. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid binding protein gene. J Biol Chem. 2003;278:51664–51672. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- Nomura DK, Morrison BAE, Blankman JI, et al. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddi S, Fezza F, Pasquoriello N, et al. Molecular identification of albumin and Hsp70 as cytosolic anandamide binding proteins. Chemistry and Biology. 2009;16:624–632. doi: 10.1016/j.chembiol.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Owada Y. Fatty acid binding protein: localization and functional significance in brain. Tohoku J Exp Med. 2008;214:213–220. doi: 10.1620/tjem.214.213. [DOI] [PubMed] [Google Scholar]
- Owada Y, Abdelwahab SA, et al. Altered emotional behavioral responses in mice lacking brain type FABP gene. Eur J Neurosci. 2006;24:175–187. doi: 10.1111/j.1460-9568.2006.04855.x. [DOI] [PubMed] [Google Scholar]
- Owada Y, Yoshimoto T, Kondo H. Spatio-temporally differential expression of genes for three members of fatty acid binding proteins in developing and mature rat brains. Journal of Chemical Neuroanatomy. 1996;12:113–122. doi: 10.1016/s0891-0618(96)00192-5. [DOI] [PubMed] [Google Scholar]
- Peng X-E, Wu YL, Lu Q-Q, Ju Z-J, Lin X. Two genetic variants in FABP1 and susceptibility to non-alcoholic fatty liver disease in a Chinese population. Gene. 2012;500:54–58. doi: 10.1016/j.gene.2012.03.050. [DOI] [PubMed] [Google Scholar]
- Petrescu AD, Huang H, Martin GG, McIntosh AL, Storey SM, Landrock D, Kier AB, Schroeder F. Impact of L-FABP and glucose on polyunsaturated fatty acid induction of PPARa regulated b-oxidative enzymes. Am J Physiol Gastrointest and Liver Phys. 2013;304:G241–G256. doi: 10.1152/ajpgi.00334.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignon J-P, Bailey NC, Baraona E, Lieber CS. Fatty acid-binding protein: a major contributor to the ethanol-induced increase in liver cytosolic proteins in the rat. Hepatology. 1987;7:865–871. doi: 10.1002/hep.1840070512. [DOI] [PubMed] [Google Scholar]
- Piomelli S, Seaman C. Mechanism of red blood cell aging: relationship of cell density and cell age. [Review] Am J Hematology. 1993;42:46–52. doi: 10.1002/ajh.2830420110. [DOI] [PubMed] [Google Scholar]
- Prows DR, Murphy EJ, Schroeder F. Intestinal and liver fatty acid binding proteins differentially affect fatty acid uptake and esterification in L-Cells. Lipids. 1995;30:907–910. doi: 10.1007/BF02537481. [DOI] [PubMed] [Google Scholar]
- Pu L, Annan RS, Carr SA, Frolov A, Wood WG, Spener F, Schroeder F. Isolation and identification of a native fatty acid binding protein form mouse brain. Lipids. 1999a;34:363–373. doi: 10.1007/s11745-999-0374-8. [DOI] [PubMed] [Google Scholar]
- Pu L, Foxworth WB, Kier AB, Annan RS, Carr SA, Edmondson R, Russell D, Wood WG, Schroeder F. Isolation and Characterization of 26- and 30-kDa Rat Liver Proteins Immunoreactive to Anti-Sterol Carrier Protein-2 Antibodies. Protein Expression And Purification. 1998;13:337–348. doi: 10.1006/prep.1998.0908. [DOI] [PubMed] [Google Scholar]
- Pu L, Igbavboa U, Wood WG, Roths JB, Kier AB, Spener F, Schroeder F. Expression of Fatty Acid Binding Proteins Is Altered in Aged Mouse Brain. Molecular and Cellular Biochemistry. 1999b;198:69–78. doi: 10.1023/a:1006946027619. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front Behavioral Neurosci. 2011;5 doi: 10.3389/fnbeh.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar DR, Burston JJ, Woodhams SG, Chapman V. Dynamic changes to the endocannabinoid system in models of chronic pain. Phil Trans R Soc B. 2012;367:3300–3311. doi: 10.1098/rstb.2011.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailler S, Schmitz K, Jager E, Ferreiros N, et al. Regulation of circulating endocannabinoids associated with cancer and metastases in mice and humans. Oncoscience. 2014;1:272–282. doi: 10.18632/oncoscience.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarchielli P, Pini LA, Coppola F, et al. Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology. 2007;32:1384–1390. doi: 10.1038/sj.npp.1301246. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JI, Long JZ, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nature Neuroscience. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnutgen F, Borchers T, Muller T, Spener F. Heterologous expression and characterization of mouse brain fatty acid binding protein. Biol Chem Hoppe-Seyler. 1996;377:211–215. doi: 10.1515/bchm3.1996.377.3.211. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Jefferson JR, Powell D, Incerpi S, Woodford JK, Colles SM, Myers-Payne S, Emge T, Hubbell T, Moncecchi D, Prows DR, Heyliger CE. Expression of rat L-FABP in mouse fibroblasts: role in fat absorption. Mol Cell Biochem. 1993;123:73–83. doi: 10.1007/BF01076477. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Jolly CA, Cho TH, Frolov AA. Fatty acid binding protein isoforms: structure and function. Chem Phys Lipids. 1998;92:1–25. doi: 10.1016/s0009-3084(98)00003-6. [DOI] [PubMed] [Google Scholar]
- Scorletti E, Byrne CD. Omega-3 fatty acids, hepatic lipid metabolism, and NAFLD. Annu Rev Nutr. 2013;33:231–248. doi: 10.1146/annurev-nutr-071812-161230. [DOI] [PubMed] [Google Scholar]
- Seedorf U, Raabe M, Ellinghaus P, Kannenberg F, Fobker M, Engel T, Denis S, Wouters F, Wirtz KWA, Wanders RJA, Maeda N, Assmann G. Defective peroxisomal catabolism of branched fatty acyl coenzyme A in mice lacking the sterol carrier protein-2/sterol carrier protein-x gene function. Genes and Development. 1998;12:1189–1201. doi: 10.1101/gad.12.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer GC, Savinova OV, Harris WS. Fish oil–How does it reduce plasma triglycerides. Biochim Biophys Acta. 2012;1821:843–851. doi: 10.1016/j.bbalip.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Jonsson K-O, Vanvoorde S, Lambert DM, Fowler CJ. Entourage effects of N-acyl ethanolamines at human vanilloid receptors. Comparison of effects upon anandamide-induced vanilloid receptor activation and upon anandamide metabolism. Br J Pharmacol. 2002;136:452–458. doi: 10.1038/sj.bjp.0704732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey SM, McIntosh AL, Huang H, Martin GG, Landrock KK, Landrock D, Payne HR, Kier AB, Schroeder F. Loss of intracellular lipid binding proteins differentially impacts saturated fatty acid uptake and nuclear targeting in mouse hepatocytes. Am J Physiol Gastrointest and Liver Phys. 2012a;303:G837–G850. doi: 10.1152/ajpgi.00489.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey SM, McIntosh AL, Huang H, Martin GG, Landrock KK, Landrock D, Payne HR, Kier AB, Schroeder F. Intracellular cholesterol binding proteins enhance HDL-mediated cholesterol uptake in cultured primary mouse hepatocytes. Am J Physiol Gastrointest and Liver Phys. 2012b;302:G824–G839. doi: 10.1152/ajpgi.00195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer RL, Laufer BI, Klieber ML, Singh SM. Reduced expression of brain Cnr1 is coupled with an increased complementary miR-26b in a mouse model of fetal alcohol spectrum disorders. Clinical Epigenetics. 2013;5:14. doi: 10.1186/1868-7083-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Ahlmark KB, Kocklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab An Science. 1999a;49:530–536. [PubMed] [Google Scholar]
- Thigpen JE, Setchell KD, Goelz MF, Forsythe DB. The phytoestrogen content of rodent diets. Envron Health Persp. 1999b;107:A182–A183. doi: 10.1289/ehp.107-1566530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis NL, Shackleford DM, Charman WN, Edwards GA, et al. Intestinal lymphatic transport enhances the post-prandial oral bioavailability of a novel cannabinoid receptor agonist via avoidance of first-pass metabolism. Pharm Res. 2009;26:1486–1495. doi: 10.1007/s11095-009-9860-z. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is invovled in ethanol prference and its age-dpendent decline in mice. Proc Natl Acad Sci U S A. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkamp R. Fatty acids, endocannabinoids, and inflammation. Eur J Pharmacol. 2015 doi: 10.1016/j.ejphar.2015.08.051. http://dx.doi.org/10.1016;j.ejphar.2015.08.051. [DOI] [PubMed]
- Wolfrum C, Buhlman C, Rolf B, Borchers T, Spener F. Variation of liver fatty acid binding protein content in the human hepatoma cell line HepG2 by peroxisome proliferators and antisense RNA affects the rate of fatty acid uptake. Biochim Biophys Acta. 1999a;1437:194–201. doi: 10.1016/s1388-1981(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Ellinghaus P, Fobker M, Seedorf U, Assmann G, Borchers T, Spener F. Phytanic acid is ligand and transcriptional activator of murine liver fatty acid binding protein. J Lipid Res. 1999b;40:708–714. [PubMed] [Google Scholar]
- Wood JT, Williams JS, Makriyannis A. Dietary DHA supplementation alters select physiological endocannabinoid system metabolites in brain and plasma. J Lipid Res. 2010;51:1416–1423. doi: 10.1194/jlr.M002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, He XY, Schulz H. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J Biol Chem. 1987;262:13027–13032. [PubMed] [Google Scholar]
- Yu S, Levi L, Casadesus G, Kunos G, Noy N. Fatty acid binding protein 5 (FABP5) regulates cognitive function both by decreasing anandamide levels and by activating the nuclear receptor peroxisome proliferator activated receptor b/d (PPARb/d) in the brain. J Biol Chem. 2014;289:12748–12758. doi: 10.1074/jbc.M114.559062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Teng Z, Tang YP, Chen C. Synaptic and cognitive improvements by inhibition of 2-AG metabolism are through upregualtion of miR-188-3p in a mouse model of Alzheimer’s disease. J of Neuroscience. 2014;34:1419–1433. doi: 10.1523/JNEUROSCI.1165-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Vessby B, Nilsson A. Quantitative role of plasma free fatty acids in the supply of arachidonic acid to extrahepatic tissues in rats. J Nutr. 2002;132:2626–2631. doi: 10.1093/jn/132.9.2626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


