SUMMARY
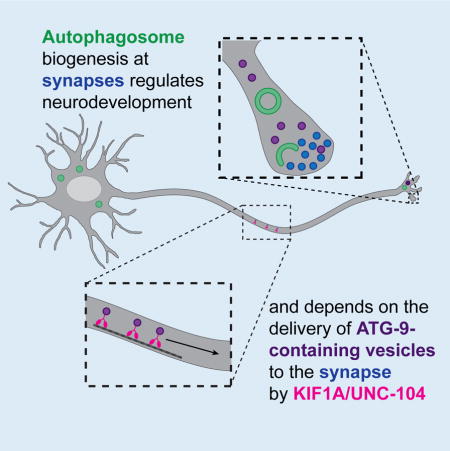
Autophagy is a cellular degradation process essential for neuronal development and survival. Neurons are highly polarized cells in which autophagosome biogenesis is spatially compartmentalized. The mechanisms and physiological importance of this spatial compartmentalization of autophagy in the neuronal development of living animals are not well understood. Here we determine that, in C. elegans neurons, autophagosomes form near synapses and are required for neurodevelopment. We first determine, through unbiased genetic screens and systematic genetic analyses, that autophagy is required cell-autonomously for presynaptic assembly and for axon outgrowth dynamics in specific neurons. We observe autophagosome biogenesis in the axon near synapses, and this localization depends on the synaptic vesicle kinesin, KIF1A/UNC-104. KIF1A/UNC-104 coordinates localized autophagosome formation by regulating the transport of the integral membrane autophagy protein, ATG-9. Our findings indicate that autophagy is spatially regulated in neurons through the transport of ATG-9 by KIF1A/UNC-104 to regulate neurodevelopment.
eTOC Blurb
Autophagy is a degradation process essential for neurodevelopment. Stavoe, Hill et al. uncover spatial regulation of autophagy in C. elegans neurons. They show that autophagosomes form near synapses and are required for presynaptic assembly and axon outgrowth dynamics. Local autophagosome biogenesis depends on kinesin KIF1A/UNC-104-mediated transport of autophagy protein ATG-9.
INTRODUCTION
Macroautophagy (hereafter called autophagy) is an evolutionarily conserved cellular degradation process best known for its role in cellular homeostasis (Feng et al., 2014; Marino et al., 2011; Son et al., 2012; Wu et al., 2013a; Zhang and Baehrecke, 2015). While autophagy is induced under stress conditions in yeast and many mammalian cells, in neurons, autophagosome formation is a constitutively active process (Lee, 2012; Wong and Holzbaur, 2015; Xilouri and Stefanis, 2010). Basal levels of autophagy are essential for neuronal survival, and neuron-specific inhibition of the autophagy pathway results in axonal degeneration and neuronal cell death (Hara et al., 2006; Komatsu et al., 2007; Yang et al., 2013; Yue et al., 2009).
Autophagy can regulate axon morphogenesis and synaptic physiology in neurons (Binotti et al., 2015; Hernandez et al., 2012; Rudolf et al., 2016; Shehata and Inokuchi, 2014; Torres and Sulzer, 2012; Yamamoto and Yue, 2014). For example, knockdown of the autophagic protein Atg7 in murine neurons results in longer axons, while activation of the autophagy pathway with rapamycin results in shorter neurites (Ban et al., 2013; Chen et al., 2013). In Drosophila, disruption of autophagy decreases the size of the neuromuscular junction, while induction of autophagy increases synaptic boutons and neuronal branches (Shen and Ganetzky, 2009). These changes in the axon are dependent, at least in part, on the degradation of cytoskeletal regulatory proteins and structures (Ban et al., 2013). Consistent with these observations, autophagosomes form at the tips of actively elongating axons in cultured neurons and contain membrane and cytoskeletal components (Bunge, 1973; Hollenbeck, 1993; Hollenbeck and Bray, 1987; Maday et al., 2012).
Most of our knowledge of autophagosome biogenesis comes from studies conducted either in yeast or mammalian cell culture (Abada and Elazar, 2014; Hale et al., 2013; Reggiori and Klionsky, 2013). Less is known about how autophagy is regulated in vivo in multicellular organisms during development and stress (Wu et al., 2013a; Zhang and Baehrecke, 2015). In developmental programs in metazoans, autophagy plays critical roles by degrading substrates at specific transitions (Hale et al., 2013; Tsukamoto et al., 2008; Wu et al., 2013a; Zhang et al., 2009). Autophagy also plays important roles during the development of the nervous system (Boland and Nixon, 2006; Cecconi et al., 2007; Lee et al., 2013; Yamamoto and Yue, 2014). However, the specific roles of autophagy in the coordination of neurodevelopmental events are less clear.
Neurons are highly polarized cells. In primary neurons, autophagosomes are observed to form at the distal end of the axon, indicating compartmentalization and spatial regulation of autophagosome biogenesis (Ariosa and Klionsky, 2015; Ashrafi et al., 2014; Hollenbeck, 1993; Hollenbeck and Bray, 1987; Maday and Holzbaur, 2014; Maday et al., 2012; Yue, 2007). Furthermore, autophagosome biogenesis requires the ordered recruitment of assembly factors to the distal axon (Maday and Holzbaur, 2014; Maday et al., 2012). How localized recruitment is regulated in neurons to specify autophagosome biogenesis is not well understood.
In this study, we conducted unbiased forward genetic screens to identify pathways involved in presynaptic assembly in C. elegans and identified an allele of atg-9. ATG-9 is known for its role in autophagosome biogenesis (Feng et al., 2016; Lang et al., 2000; Noda et al., 2000; Reggiori et al., 2004; Stanley et al., 2013; Wang et al., 2013; Yamamoto et al., 2012). Through genetic and cell biological approaches, we determined that the autophagy pathway is required cell-autonomously to promote presynaptic assembly in the interneuron AIY. We performed systematic analyses with cell biological markers for cytoskeletal organization, active zone position and synaptic vesicle clustering to establish that eighteen distinct autophagy genes promote proper neurodevelopment in C. elegans. We examined different neuron types to determine that autophagy regulates synaptic positions and axon outgrowth in specific neurons. We found that ATG-9 is transported to the tip of growing neurites or to synaptic regions by the synaptic vesicle kinesin UNC-104/KIF1A. Transport of ATG-9, in turn, regulates synaptic autophagosome biogenesis. We propose that transport of ATG-9 by UNC-104/KIF1A confers spatial regulation of autophagy in neurons.
RESULTS
Mutant allele wy56 displays defects in AIY synaptic vesicle clustering
During development, the AIY interneurons of C. elegans establish a stereotyped pattern of en passant (along the length of the axon) synaptic outputs. This pattern is reproducible across animals and displays specificity for both synaptic partners and positions (Figures 1A–1C, S1A) (Colón-Ramos et al., 2007; White et al., 1986). The positions of these en passant synapses in AIY are instructed by glia-derived Netrin, which directs local organization of the actin cytoskeleton, active zone localization and synaptic vesicle clustering (Colón-Ramos et al., 2007; Stavoe et al., 2012; Stavoe and Colón-Ramos, 2012). To identify the mechanisms underlying these early events that organize synaptogenesis, we performed visual forward genetic screens using the synaptic vesicle marker GFP::RAB-3 in AIY. From these screens, we identified the allele wy56, which displays a highly penetrant, abnormal distribution of synaptic vesicle proteins GFP::RAB-3 and SNB-1::YFP in the dorsal turn region of the AIY neurite (termed Zone 2; enclosed in dashed box; Figures 1E–1G, 2B–2C and S1B).
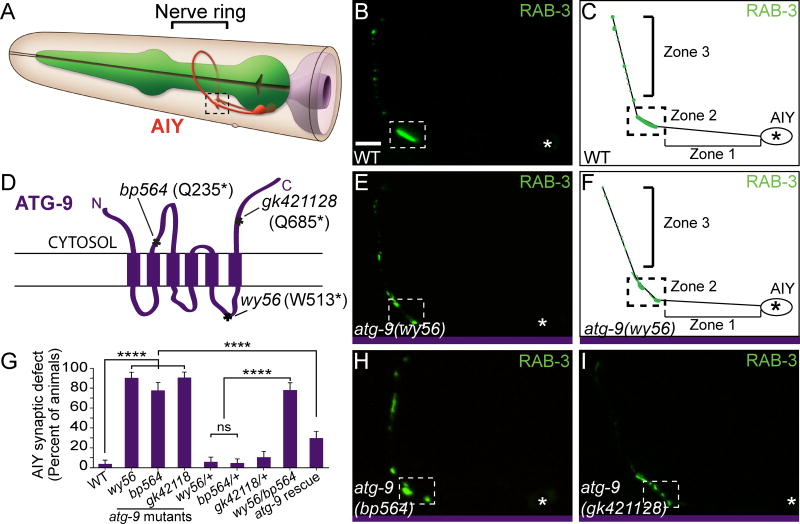
Figure 1. ATG-9 is required for AIY synaptic vesicle clustering.
(A) Schematic of AIY interneurons (red) in the nerve ring (indicated by bracket) in the head of the worm. Reprinted with permission from wormatlas.org (Z. Altun). (B) Distribution of synaptic vesicles (visualized with GFP::RAB-3) in a representative wild type animal and represented in a schematic diagram (C). Throughout the manuscript, Zone 1 corresponds to an asynaptic region of the AIY neurite, Zone 2 (enclosed by dashed box) corresponds to a synaptic rich region in the dorsal turn of the neurite and Zone 3 corresponds to a synaptic region with intermittent presynaptic clusters. We focus the characterization of our phenotypes to Zone 2, as described previously (Colón-Ramos et al., 2007; Stavoe et al., 2012; Stavoe and Colón-Ramos, 2012). (D) Schematic of ATG-9 transmembrane domains with bp564, wy56 and gk421128 lesions and corresponding protein effects indicated. (E) Distribution of synaptic vesicles in an atg-9(wy56) mutant animal and represented in a cartoon diagram (F). (G) Quantification of the AIY presynaptic phenotype in wild type, three atg-9 mutant backgrounds, heterozygous atg-9 animals, atg-9 (wy56/bp564) transheterozygotes, and atg-9(bp464) mutant animals that contain a pan-neuronal (Punc-14) atg-9 rescuing construct. Error bars represent 95% confidence interval. ****, P< 0.0001 between indicated groups by Fisher’s exact test. (H–I) Distribution of synaptic vesicles in atg-9(bp564) (H) and atg-9(gk421128) (I) mutant animals. Each image is a maximal projection of a confocal z-stack; the asterisk denotes the location of the cell body and the dashed box encloses AIY Zone 2. Scale bar (in B for B, E, H, and I), 5 μm.
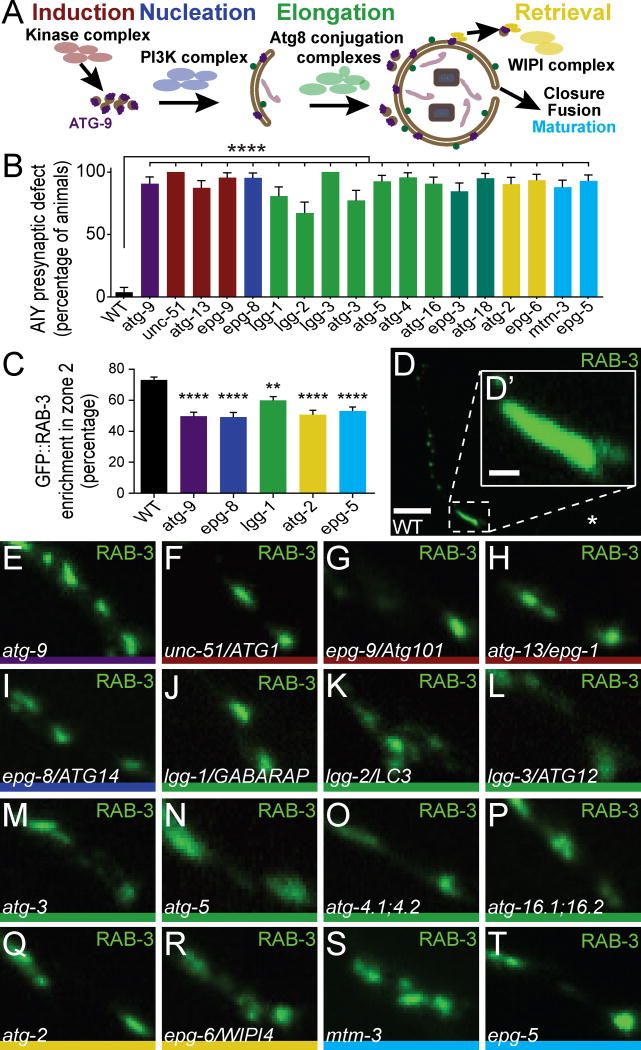
Figure 2. The autophagy pathway is required for AIY synaptic vesicle clustering.
(A) Schematic of the autophagosome biogenesis pathway, including the general stages: induction (red), nucleation (blue), elongation (green), retrieval (yellow), closure, fusion and maturation (cyan). (B) Quantification of the penetrance of the AIY presynaptic defect in wild type and autophagy pathway mutant animals. For all genotypes quantified, n>100 animals. Error bars represent 95% confidence interval. ****, P< 0.0001 between autophagy mutants and wild type by Fisher’s exact test. (C) Quantification of the relative distribution of GFP::RAB-3 in AIY Zone 2 in wild type and representative autophagy pathway mutants. For all genotypes quantified, n>28 neurons. Error bars represent SEM. **, P< 0.01; ****, P< 0.0001 between autophagy mutants and wild type by one-way ANOVA with Tukey’s post-hoc analysis. (D) Distribution of synaptic vesicles (visualized with GFP::RAB-3) in a representative wild type animal. (D′) Inset in (D) is enlarged Zone 2 region from (D). (E–T) Distribution of synaptic vesicles (visualized with GFP::RAB-3) in AIY Zone 2 of atg-9(gk421128) (E), unc-51(e369) (F), epg-9(bp320) (G), atg-13(bp414) (H), epg-8(bp251) (I), lgg-1(bp500) (J), lgg-2(tm5755) (K), lgg-3(tm1642) (L), atg-3(bp412) (M), atg-5(bp484) (N), atg-4.1(gk127286);atg-4.2(gk430078) double mutants (O), atg-16.1(gk668615);atg-16.2(gk145022) double mutants (P), atg-2(bp576) (Q), epg-6(bp242) (R), mtm-3(tm4475) (S), and epg-5(tm3425) (T) mutant animals. Each image is a maximal projection of a confocal z-stack; the asterisk denotes the location of the cell body and the dashed box encloses AIY Zone 2 in (D). Only the AIY Zone 2 region is depicted in (E–T), similar to the region depicted in (D′). Scale bar in D, 5 μm; scale bar in D′ for D′-T, 1 μm. See also Figures S1, S2 and Table S1.
We observed that both the penetrance and expressivity of wy56 mutant animals resembled those of other synaptogenic mutants in AIY, including mutants of actin-organizing molecules CED-5/DOCK-180, CED-10/RAC-1 and MIG-10/Lamellipodin (Stavoe et al., 2012; Stavoe and Colón-Ramos, 2012). Also similar to these actin-organizing mutants, the wy56 defective presynaptic pattern was observed early in development, at larval stage 1 (L1), just after hatching, suggesting that the phenotype emerges during embryogenesis (Figures S1I–S1O). Our findings indicate that wy56 corresponds to a lesion in a gene required early in development for correct formation of presynaptic sites in AIY.
wy56 is an allele of autophagy gene atg-9
To identify the genetic lesion of wy56, we performed single-nucleotide polymorphism (SNP) mapping, whole-genome sequencing and genetic rescue experiments. Our SNP mapping data indicate that wy56 is located between 0.05 Mb and 0.5 Mb on chromosome V. Whole genome sequencing of wy56 mutants revealed a point mutation in exon 8 of the atg-9 (AuTophaGy-9) gene, resulting in a G to A nucleotide transition that converts W513 to an opal/umber stop codon (Figure 1D). Two independent alleles of atg-9, atg-9(bp564) and atg-9(gk421128), with amber nonsense mutations at Q235 and Q685, respectively (Figure 1D) (Thompson et al., 2013; Tian et al., 2010), phenocopied the AIY presynaptic defect observed for wy56 mutants (Figures 1G–1I). Consistent with wy56 being an allele of atg-9, we also observed that wy56 fails to complement atg-9(bp564) and that expression of the ATG-9 cDNA under an early pan-neuronal promoter (punc-14) rescues the atg-9 presynaptic phenotype in AIY (Figure 1G). Together, our genetic data indicate that wy56 is a nonsense, loss-of-function mutation in the atg-9 gene.
The autophagy pathway is required for synaptic vesicle clustering in AIY
The atg-9 gene encodes a conserved, six-pass transmembrane protein that acts in the autophagy pathway (Lang et al., 2000; Noda et al., 2000; Young et al., 2006). Since ATG-9 is primarily known for regulating autophagosome biogenesis, we examined whether other components of the autophagy pathway are also required for synaptic vesicle clustering during synaptogenesis. Autophagosome biogenesis can be divided into four steps: initiation, nucleation, elongation, and retrieval. After biogenesis, autophagosomes mature prior to degradation of cargo. Distinct and specialized protein complexes mediate these steps, and homologs for these protein complexes have been identified in C. elegans (Figures 2A, S2I and Table S1) (Melendez and Levine, 2009; Tian et al., 2010). To evaluate the requirement of each of these distinct steps in synaptic vesicle clustering, we systematically examined existing alleles for each of these homologs using synaptic vesicle markers GFP::RAB-3 and SNB-1::YFP (Figures 2, S1, S2 and Table S1).
Autophagy is induced by a kinase complex composed of UNC-51/Atg1/ULK, EPG-9/Atg101 and ATG-13/EPG-1 (Feng et al., 2014; Kamada et al., 2000; Melendez and Levine, 2009; Reggiori et al., 2004). Examination of putative null alleles for unc-51/Atg1/ULK, epg-9/Atg101 and atg-13/epg-1 revealed highly penetrant AIY synaptic vesicle clustering defects which phenocopied those seen for atg-9 mutant animals, as visualized with synaptic vesicle-associated markers GFP::RAB-3 and SNB-1::YFP (>87% penetrance; n>100 animals for each genotype; Figures 2B, 2F–2H, S1C, and Table S1), suggesting that the initiation complex of autophagy is required for synaptic vesicle clustering in AIY.
Nucleation is mediated by a PI3K complex that promotes fusion of ATG-9-containing vesicles into a phagophore (Kihara et al., 2001; Obara et al., 2006). The nucleation complex consists of LET-512/Vps34, BEC-1/Atg6, EPG-8/Atg14, and VPS-15 (Melendez and Levine, 2009). Most of these genes also play important roles in other essential cellular pathways, and for this reason, null mutations in these genes are unviable (Kihara et al., 2001; Obara et al., 2006; Yang and Zhang, 2011). However, we were able to examine a putative null allele for the nucleation gene epg-8/Atg14, which is specific to the autophagy pathway (Table S1) (Yang and Zhang, 2011). epg-8 mutant animals exhibited highly penetrant AIY synaptic vesicle clustering defects as visualized with synaptic vesicle markers GFP::RAB-3 and SNB-1::YFP (95.4% of epg-8 mutant animals, n=108; Figures 2B–2C, 2I and S1D).
Once nucleated, the isolation membrane elongates via two ubiquitin-like conjugation complexes. In C. elegans, these complexes include: LGG-1/GABARAP and LGG-2/LC3 (the two C. elegans Atg8 homologs) (Alberti et al., 2010; Manil-Segalen et al., 2014; Wu et al., 2015), LGG-3/Atg12, ATG-5, ATG-7, ATG-10, ATG-4 (ATG-4.1 and ATG-4.2 in C. elegans), ATG-16 (ATG-16.1 and ATG-16.2 in C. elegans), and ATG-3 (Melendez and Levine, 2009). EPG-3/VMP1 and ATG-18/WIP1/2 are also implicated in downstream stages of elongation (Lu et al., 2011; Tian et al., 2010). We examined putative null alleles for atg-5, lgg-3/Atg12, lgg-1/GABARAP, lgg-2/LC3, epg-3, atg-18, and a hypomorphic allele of atg-3 (Table S1). Because atg-4 and atg-16 both have two homologs with redundant functions in C. elegans (Wu et al., 2012; Zhang et al., 2013), we built double mutant strains carrying putative null alleles for both homologs (atg-4.1;atg-4.2 double mutants and atg-16.1;atg-16.2 double mutants; Table S1). Consistent with elongation playing an important role in synaptic vesicle clustering in AIY, we observed that all elongation mutants display synaptic vesicle clustering defects in AIY that phenocopy the expressivity of other autophagy mutants (Figures 2B–2C, 2J–2P, S1E–S1F, S2G–S2H and Table S1). We note that alleles of the Atg8 homologs, lgg-1 and lgg-2, display 80.7% and 67.3% penetrance respectively. Since the lgg-2(tm5755) and lgg-1(bp500) alleles are putative nulls (Alberti et al., 2010; Manil-Segalen et al., 2014), our findings are consistent with these genes acting partially redundantly with each other or other autophagy machinery (Sato and Sato, 2011). atg-3 mutants with hypomorphic allele bp412 display 77.4% penetrance. The remaining elongation mutants, which are purported null alleles, display >85% penetrance (n>100 animals for all genotypes quantified; Figures 2B–2C, 2J–2P, S1E–S1F, S2G–S2H, and Table S1).
Next, ATG-9 is recovered from the isolation membrane by a retrieval complex, the double membrane closes, and the autophagosome matures into an autolysosome (Cullup et al., 2013; Lu et al., 2011; Reggiori et al., 2004; Tian et al., 2010; Wang et al., 2001; Wu et al., 2014; Zhao et al., 2013). We observed that putative null alleles for retrieval genes epg-6 and atg-2 and maturation genes mtm-3 and epg-5 (Table S1) display defects in AIY synaptic vesicle clustering, as visualized by synaptic vesicle markers GFP::RAB-3 and SNB-1::YFP (>88% penetrance; n>100 animals for all genotypes quantified; Figures 2B–2C, 2Q–2T and S1G–S1H).
Together, our findings indicate that components from all stages of the autophagy pathway are required for clustering of synaptic vesicle proteins in AIY during development. Our data further suggest that ATG-9 is acting within the autophagy pathway, not independently, to direct AIY synaptic vesicle clustering during development.
Selective autophagy genes atg-11, epg-2 and sqst-1 are not required for AIY synaptic vesicle clustering
Autophagy is best known for its nonselective role in degrading bulk cytoplasm (Mizushima and Klionsky, 2007). In neurons, nonselective autophagy locally degrades membrane and cytoskeletal components at the tips of actively elongating axons (Ban et al., 2013; Chen et al., 2013; Hollenbeck and Bray, 1987). However, the autophagy pathway can also selectively degrade specific organelles or target proteins in certain contexts. This process, known as selective autophagy, is dependent on the adaptor molecule Atg11, which interacts with cargo receptors to link specific protein targets to the autophagosome precursor membrane (Farre et al., 2013; He et al., 2006; Mao et al., 2013; Yorimitsu and Klionsky, 2005; Zaffagnini and Martens, 2016). Additional selective autophagy adaptors include the nematode-specific epg-2 (Tian et al., 2010) and the C. elegans homolog of p62, sqst-1 (Table S1) (Lamark et al., 2009; Lin et al., 2013). To determine whether selective autophagy acts in AIY synaptic vesicle clustering, we examined putative null alleles for atg-11, epg-2 and sqst-1 (Table S1). Unlike the other autophagy pathway mutants examined, we did not observe AIY presynaptic defects in these mutants (Figures S2B–S2D and data not shown). Therefore, our data suggest that selective autophagy adaptors ATG-11, EPG-2 and SQST-1 are not required for AIY synaptic vesicle clustering.
The autophagy pathway acts cell-autonomously in AIY to promote synaptic vesicle clustering
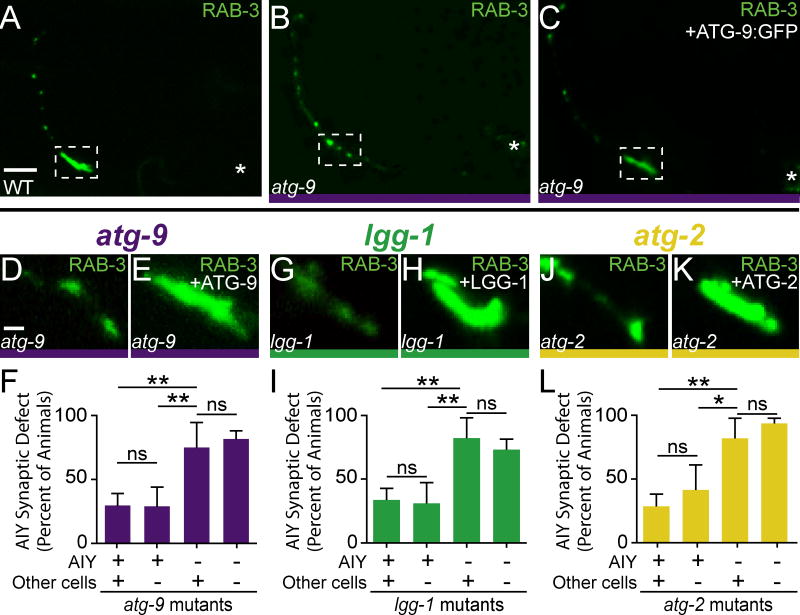
Next we examined whether autophagy acts cell-autonomously in AIY by performing mosaic analyses with representative mutants from distinct steps of the autophagy pathway. Briefly, mitotically-unstable rescuing arrays were used for each of the examined genes, and animals were scored for retention of the rescuing array in AIY and for the synaptic phenotype (Yochem and Herman, 2003). We observed that for atg-9(bp564), lgg-1(bp500) (elongation) and atg-2(bp576) (retrieval) mutants, retention of their rescuing arrays in AIY resulted in rescue of the AIY presynaptic defect, while retention of the array in other neurons (including postsynaptic partner RIA), but not in AIY, did not result in rescue of the AIY presynaptic defect (Figures 3D–3L). We also examined the endogenous expression pattern of atg-9 and atg-2 and observed that they are expressed in neurons, including AIY (Figures S3A–S3F). Together, our data suggest that the autophagy pathway acts cell-autonomously in AIY to promote synaptic vesicle clustering.
Figure 3. The autophagy pathway acts cell-autonomously to instruct AIY presynaptic assembly.
(A) Distribution of synaptic vesicles (visualized with GFP::RAB-3) in a representative wild type animal. (B–C) Distribution of synaptic vesicles (visualized with mCh::RAB-3, pseudocolored green) in an atg-9(bp564) mutant animal (B) and an atg-9(bp564) mutant expressing a panneuronal ATG-9::GFP rescuing construct (C). (D–L) Distribution of synaptic vesicles in AIY Zone 2 in atg-9(bp564) (D), lgg-1(bp500) (G), atg-2(bp576) (J) mutant animals and mutant animals expressing a corresponding rescuing array (E, H, and K). (F, I, L) Quantification of rescue of autophagy mutant animals expressing unstable rescuing transgenes. Error bars represent 95% confidence interval. **, P< 0.01; *, P< 0.05 between indicated groups by Fisher’s exact test. Each image is a maximal projection of a confocal z-stack; in A–C, the asterisk denotes the location of the cell body and the dashed box encloses AIY Zone 2. Scale bar (in A for A–C), 5 μm, (in D for D–E, G–H, J–K), 1 μm. See also Figure S3.
The autophagy pathway is required for F-actin accumulation and active zone protein localization at presynaptic sites
To understand how autophagy regulates synaptic vesicle clustering in AIY, we examined the requirement of autophagy for the different elements of presynaptic assembly. In previous studies, we determined that AIY presynaptic sites are specified through the local organization of the actin cytoskeleton and active zone proteins (Colón-Ramos et al., 2007; Stavoe et al., 2012; Stavoe and Colón-Ramos, 2012). Therefore, we first examined whether active zone proteins and the actin cytoskeleton were correctly organized at presynaptic sites in autophagy mutants.
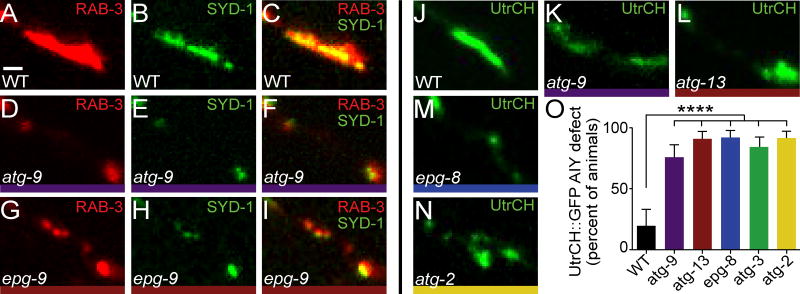
We observed that active zone protein SYD-1 is enriched and colocalizes with RAB-3 in AIY presynaptic regions in wild type animals (Figures 4A–4C); however, in autophagy mutants RAB-3 and SYD-1 are no longer enriched in the AIY Zone 2 presynaptic region (Figures 4D–4I and S3G–S3R). Furthermore, we observed that autophagy mutants also affected the presynaptic enrichment of the F-actin probe UtrCH::GFP (Figures 4J–4O). Together, our data indicate that defects in autophagy affect the localization of F-actin, synaptic vesicles and active zone proteins to presynaptic sites, and our data suggest that the autophagy pathway acts cell-autonomously in AIY to promote synaptogenesis.
Figure 4. The autophagy pathway is required for active zone assembly and F-actin organization.
(A–I) Distribution of synaptic vesicles in AIY Zone 2 (visualized with mCh::RAB-3), and localization of active zones in AIY (visualized with GFP::SYD-1) in wild type (A–C), atg-9(bp564) (D–F) and epg-9(bp320) (G–I) mutant animals. (J–N) F-actin organization in AIY Zone 2 (visualized with UtrCH::GFP) in wild type (J), atg-9(bp564) (K), atg-13(bp414) (L), epg-8(bp251) (M), and atg-2(bp576) (N) mutant animals. (O) Quantification of penetrance of F-actin Zone 2 enrichment defect in AIY. For all genotypes quantified, n>50 animals. Error bars represent 95% confidence interval. ****, P< 0.0001 between mutants and wild type by Fisher’s exact test. Each image is a maximal projection of a confocal z-stack; only AIY Zone 2 is depicted. Scale bar (in A for A–N), 1 μm. See also Figure S4.
In AIY, synaptogenesis is regulated by glia-derived Netrin signaling. Netrin is required for the local clustering of its receptor, UNC-40/DCC, in AIY presynaptic regions. UNC-40/DCC then activates a signal transduction pathway in AIY, which includes Rac GEF CED-5/DOCK180, MIG-10B/Lamellipodin and ABI-1/Abl Interactor 1. This pathway culminates in the local organization of F-actin and synaptic vesicle clustering at presynaptic sites (Colón-Ramos et al., 2007; Stavoe et al., 2012; Stavoe and Colón-Ramos, 2012). To examine if autophagy regulates these early signaling events, we visualized glia morphology and the subcellular localization of UNC-40::GFP and MIG-10B::GFP in AIY. We were unable to detect any defects in the development or morphology of the glia that specifies presynaptic assembly in AIY (Figure S4A–S4F). Furthermore, visualization of the areas of contact between the glia and AIY by GFP reconstitution across synaptic partners (GRASP) (Feinberg et al., 2008; Shao et al., 2013) did not reveal defects in the AIY-glia contacts in any of the examined autophagy mutants (data not shown). We also did not detect defects in the subcellular localization of UNC-40 or MIG-10B in the examined autophagy mutants (Figure S4G–S4J). Our findings indicate that autophagy is not required for the development of the glia that specifies the synaptic positions in AIY or for the subcellular localization of UNC-40 and MIG-10B during synaptogenesis. Our data are consistent with autophagy acting in parallel to known synaptogenic signaling pathways to regulate synapse formation in AIY. Importantly, our findings indicate that autophagy is required in vivo and during development for actin organization, active zone assembly and synaptic vesicle clustering.
Autophagosome biogenesis occurs at AIY presynaptic sites
In primary neuron culture, autophagosome biogenesis is spatially compartmentalized (Ariosa and Klionsky, 2015; Ashrafi et al., 2014; Bunge, 1973; Maday and Holzbaur, 2014; Maday et al., 2012; Yue, 2007). To determine when and where autophagosomes form in C. elegans neurons during development, we surveyed high magnification transmission electron micrographs (TEM) of neuronal somas and neurites in embryos. We observed autophagic vacuole (AV)-like organelles (identified by ultrastructural criteria as described (Bunge, 1973; Hernandez et al., 2012; Melendez et al., 2003; Yu et al., 2004), see also Experimental Procedures) in the cell bodies and neurites of developing embryonic neurons (Figures 5A–5C). Our findings are consistent with electron microscopy studies in primary neuron culture that reported the presence of autophagosomes in elongating axons (Bunge, 1973) and indicate that autophagosomes form in developing neurons in C. elegans embryos when axon outgrowth and synaptogenesis occur.
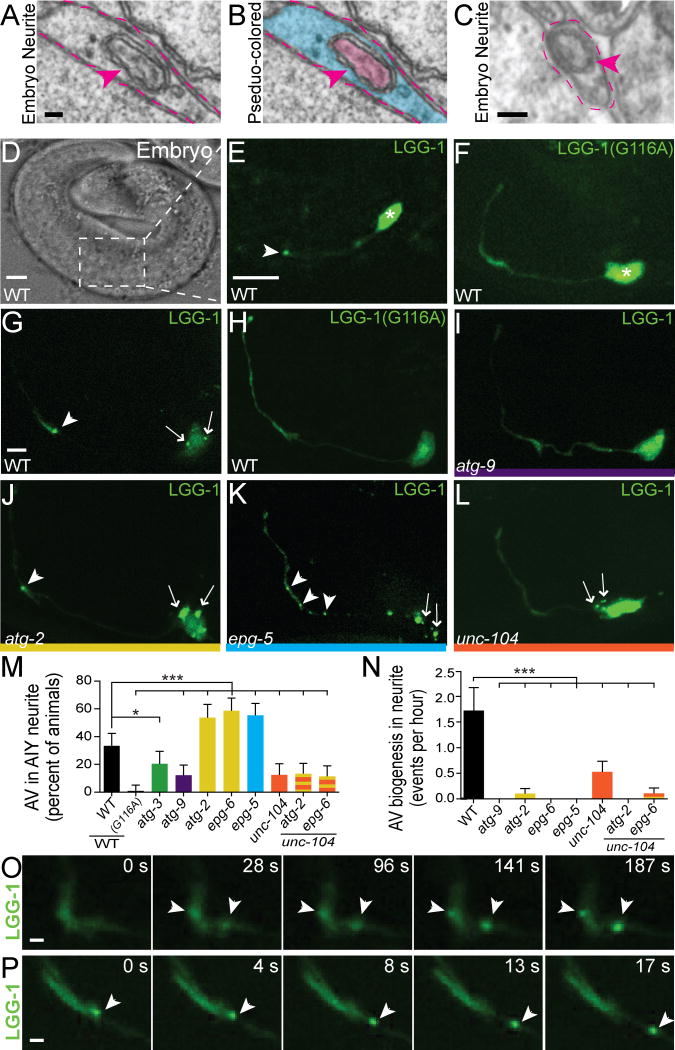
Figure 5. Autophagosome biogenesis occurs at AIY presynaptic sites during development.
(A–C) Transmission electron micrographs of autophagic vacuole (AV)-like organelles in embryonic neuronal processes of C. elegans during the developmental time of axon outgrowth and synaptogenesis. Images were originally acquired by Richard Durbin (Durbin, 1987) and represent 500-minute-old embryo PR5_(PVPR) (A and B) and 550-minute-old embryo RDE (C). In all images, dashed lines outline cross-section of neurite; arrowheads indicate autophagosomes in neurite. Neurite and AV-like organelle in (A) is pseudocolored blue (neurite) and pink (AV) in (B). (D) Transmitted light image of a C. elegans embryo for reference. (E–F) Embryonic expression of GFP::LGG-1 (E) and GFP::LGG-1(G116A) (F) in AIY in a wild type animal. LGG-1(G116A) is a point mutant incapable of associating with autophagosomes (Mizushima et al., 2010; Zhang et al., 2015). (G–L) Distribution of autophagosomes visualized with GFP::LGG-1 (G, I–L) or GFP::LGG-1(G116A) (H) in AIY in wild type (G, H), atg-9(bp564) (I), atg-2(bp576) (J), epg-5(tm3425) (K) and unc-104(e1265) (L) mutant animals. (M) Quantification of the penetrance of animals with LGG-1 puncta in the neurite of AIY in wild type and mutant backgrounds. For all categories quantified, n>100 animals. Error bars represent 95% confidence interval. *, P< 0.05; ***< 0.001 between mutants and wild type by Fisher’s exact test. (N) Quantification of autophagosome (AV) biogenesis in the AIY neurite (as described in Experimental Procedures) in wild type and mutant backgrounds (n>10 videos per genotype). Error bars represent SEM. ***, P< 0.001 between mutants and wild type by one-way ANOVA with Tukey’s post-hoc analysis. (O–P) Time series depicting autophagosome biogenesis (O) (see also Supplemental Movie S1) and autophagosome retrograde movement (P) (see also Supplemental Movie S4). In E–L and O–P, arrowheads denote the location of LGG-1 puncta in the neurite and arrows denote the location of LGG-1 puncta in the cell body. Each image is a maximal projection of a confocal z-stack. Scale bars (in A for A–B, in C), 200 nm; (in D, in E for E–F, in G for G–L), 5 μm; (in O, in P), 1 μm. See also Figure S5 and Supplemental Movies S1–S4.
To understand the in vivo dynamics of autophagosomes in neurons, we examined the localization of autophagosomes in AIY using GFP::LGG-1. LGG-1 is the C. elegans homolog of Atg8/GABARAP, a molecule that is post-translationally modified to associate with autophagosomal membranes upon autophagy induction (Alberti et al., 2010; Kabeya et al., 2000; Kirisako et al., 1999; Lang et al., 1998; Mizushima et al., 2010; Tian et al., 2010; Zhang et al., 2015). Due to its dynamic but stable association with autophagosomes, GFP::LGG-1 and homologous markers are used as reliable cell biological probes for autophagosome dynamics (Manil-Segalen et al., 2014; Melendez et al., 2003; Mizushima et al., 2010; Tian et al., 2010; Zhang et al., 2015).
We observed GFP::LGG-1 was diffusely cytoplasmic throughout the AIY neurite and localized to small puncta in the AIY cell body. In 38.5% of wild type 3-fold embryos (n=26), we also observed LGG-1 puncta in the AIY neurite located in the synapse-rich region (Zone 2) (Figures 5D–5E). The localization pattern was similar post-embryonically in wild type animals (n=123) (Figures 5G and 5M). Additionally, this subcellular localization was not observed when we expressed GFP::LGG-1(G116A) (Figures 5F, 5H and 5M), which contains a point mutation that prevents LGG-1 lipidation and conjugation to autophagosomes (Mizushima et al., 2010; Zhang et al., 2015).
Next we examined GFP::LGG-1 in autophagy mutant backgrounds. LGG-1 fails to become conjugated to the autophagosome membrane in atg-3 mutants (Ichimura et al., 2000; Tanida et al., 2002; Tian et al., 2010). We observed fewer GFP::LGG-1 puncta in atg-3(bp412) mutants in the AIY cell body and neurites, consistent with a reduction of LGG-1 association with autophagosomes in these hypomorphic mutants (n=103; Figures 5M and S5B). We then examined GFP::LGG-1 in atg-2(bp576), epg-6(bp424) and epg-5(tm3425) mutants, all lesions in genes for late stages of autophagosome biogenesis and all known to result in the accumulation of defective autophagosomes (Figures 5J, 5K, 5M and S5C) (Lu et al., 2011; Mizushima et al., 2010; Shintani et al., 2001; Tian et al., 2010; Zhang et al., 2015). As expected, we observed a higher penetrance of animals displaying puncta in AIY neurites (54% of atg-2(bp576) mutants, 59% of epg-6(bp424) mutants and 55% of epg-5(tm3425) mutant animals; Figure 5M) when we blocked these late steps of the autophagy pathway. Together, our results indicate that autophagosomes are present in the AIY cell body and near presynaptic sites.
To elucidate the in vivo dynamics of autophagosome biogenesis in AIY, we performed time-lapse imaging. In wild type animals, we observed that a majority of the autophagosome biogenesis events in the neurite occurred in the synaptic regions, with 95% of them in the synaptic-rich Zone 2, at an average rate of 1.7 events/hour (n=20 events in 22 neurons; Figures 5N–5O; Supplemental Movies S1–S3). As expected, autophagosome biogenesis was reduced in autophagy mutants, with less than 0.1 events/hour observed for atg-9, atg-2, epg-6, and epg-5 mutant animals (Figure 5N). Autophagosome biogenesis was also reduced for late autophagy mutants (atg-2, epg-6, epg-5), in which partial or stalled autophagosomes accumulate, suggesting a negative feedback loop in which a block in autophagosome maturation halts new biogenesis in vivo. In wild type animals, the LGG-1 puncta that formed near presynaptic sites underwent retrograde trafficking, with 82% of motile autophagosomes (n=50) trafficking toward the cell body (Figures 5P and S5F; Supplemental Movie S4), an observation that is consistent with autophagosome trafficking studies in cultured mammalian neurons (Maday and Holzbaur, 2014; Maday et al., 2012).
Together, our results indicate that autophagosomes form in AIY near presynaptic sites and that autophagy is required for synaptogenesis. The observed spatial compartmentalization for autophagosome biogenesis in C. elegans neurons is consistent with findings from cultured vertebrate neurons, in which autophagosomes were observed to locally form in growth cones of actively elongating axons (Bunge, 1973; Hollenbeck, 1993; Hollenbeck and Bray, 1987; Maday et al., 2012). We extend those observations to show that local autophagosome biogenesis in neurons also occurs in vivo, near synapses and during development. Therefore, local autophagosome biogenesis in neurons is conserved, and compartmentalized autophagosome biogenesis may be important for function.
ATG-9 localizes to AIY presynaptic regions in an UNC-104/KIF1A-dependent manner
Local autophagy has been hypothesized to be critical for degrading substrates in axonal subcellular structures (Hollenbeck, 1993; Hollenbeck and Bray, 1987). How local autophagy is regulated to confer spatial selectivity during substrate degradation is not well understood. To determine how the location of autophagy is specified, we examined the subcellular localization of ATG-9, the only multipass transmembrane protein that is part of the core autophagy pathway (Feng et al., 2014; Noda et al., 2000; Young et al., 2006). In the nerve ring, we observed that the subcellular localization of a rescuing ATG-9::GFP transgene was reminiscent to that of a panneuronally-expressed synaptic vesicle-associated protein, RAB-3 (Figures 6A, 6D and 6E) (Mahoney et al., 2006). We then examined the endogenous localization of ATG-9 by creating transgenic animals in which the genomic ATG-9 locus was modified with a CRISPR-based knock-in of ATG-9::GFP (Figure 6B). C-terminal addition of GFP to ATG-9 does not disrupt its function (Figure 3A–3C and data not shown). Consistent with ATG-9 localizing to synaptic sites, we observed that the endogenous localization of ATG-9 was similar to panneuronally-expressed GFP::RAB-3 and ATG-9::GFP in both adults and 3-fold embryos (Figures 6C–6G, 6I–6J). To better understand the subcellular localization of ATG-9, we expressed ATG-9::GFP cell-specifically in AIY. Consistent with the endogenous expression pattern, we observed that ATG-9 was enriched in presynaptic regions and colocalized with RAB-3 in AIY (Figures 6L–6N). Our findings indicate that ATG-9 localizes to presynaptic sites in neurons.
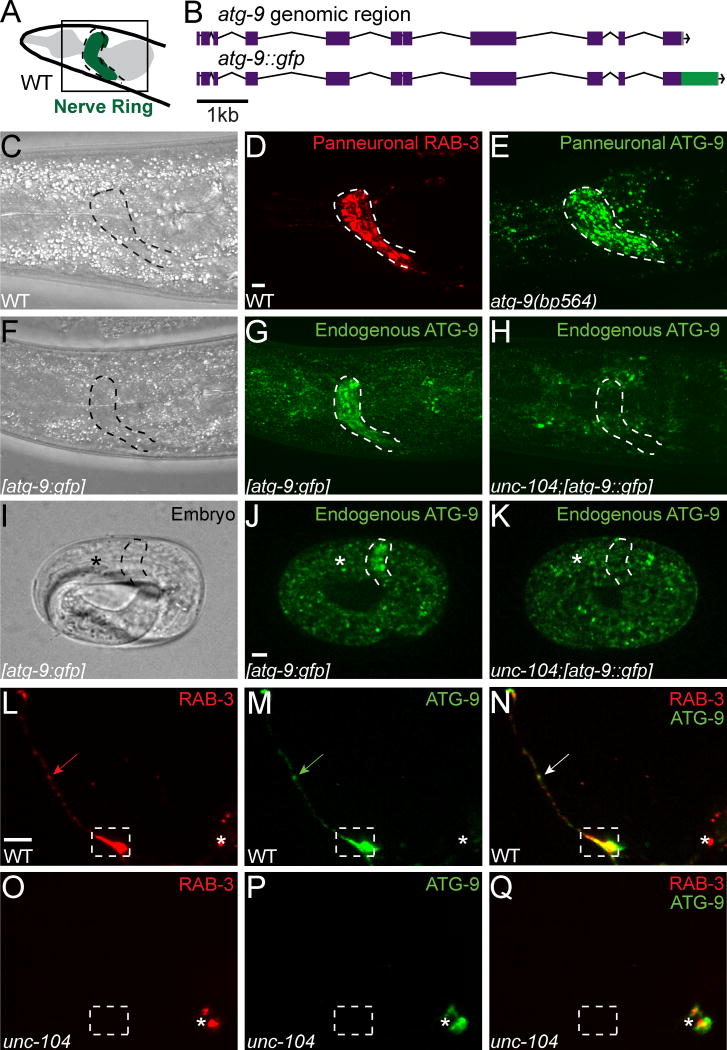
Figure 6. ATG-9 transport to AIY presynaptic zones requires KIF1A/UNC-104.
(A) Schematic of the nematode nerve ring (green, encircled by a dotted line) in C. elegans. (B) Schematic of the atg-9 genomic region in a wild type genome (top) and in the eGFP CRISPR knock-in (bottom). (C–D) Location of the nerve ring, referenced with a transmitted light image (C) and visualized with GFP::RAB-3 (pseudocolored red) expressed panneuronally with Prab-3 in a wild type animal (D). (E) Visualization of the subcellular localization of panneuronal ATG-9 (ATG-9::GFP rescuing array expressed with Punc-14). (F–K) Distribution of endogenous ATG-9 as visualized by CRISPR insertion of eGFP at the C-terminus of the genomic atg-9 (schematized in B) in adult (F–H) and embryo (I–K) in wild type (G and J) and unc-104(e1265) mutant (H and K) animals. Anterior end of the embryo (head) is indicated with an asterisk in I–K. (L–Q) Distribution of synaptic vesicles (visualized with mCh::RAB-3) (L and O) and ATG-9::GFP (M and P) in AIY (merge in N and Q) in wild type (L–N) and unc-104(e1265) mutant animals (O–Q). In L–Q, the asterisk denotes the location of the AIY cell body; the dashed box encloses AIY Zone 2. Each image is a maximal projection of a confocal z-stack. Scale bar (in D for C–H, in J for I–K and in L for L–Q), 5 μm.
ATG-9 is required for autophagosome biogenesis (Feng et al., 2016; He et al., 2009; Lang et al., 2000; Orsi et al., 2012; Reggiori and Tooze, 2012; Wang et al., 2013; Yamamoto et al., 2012; Young et al., 2006). In yeast, Atg9 localizes to small vesicles that nucleate to form the phagophore (Suzuki et al., 2015; Wang et al., 2013; Yamamoto et al., 2012). To understand how ATG-9 localizes to synaptic regions, we examined its subcellular localization in mutants for kinesins implicated in neuronal transport. UNC-14, a kinesin-1/KIF5 adaptor for neuronal transport of vesicles and synaptic precursors, interacts with UNC-51/ATG-1 to regulate axon outgrowth and neurodevelopment (Abe et al., 2009; Brown et al., 2009; Lai and Garriga, 2004; Ogura et al., 1997; Sakamoto et al., 2005). We did not observe any defects in synaptic vesicle clustering or ATG-9 localization in unc-14(e57) mutants (data not shown). Similarly, unc-116(e2310) mutants lacking the kinesin-1/KIF5 heavy chain and unc-16(ju146) mutants lacking kinesin-1/KIF5 regulator JIP3/Sunday Driver also did not display defects in ATG-9 localization (data not shown) (Byrd et al., 2001; Patel et al., 1993; Sakamoto et al., 2005; Yang et al., 2005).
However, examination of the synaptic vesicle kinesin UNC-104/KIF1A revealed a requirement for this kinesin in ATG-9 transport. In unc-104(e1265) mutant animals, synaptic vesicles fail to be transported to synapses and are instead restricted to the cell body (Hall and Hedgecock, 1991; Otsuka et al., 1991). Interestingly, we observed that in unc-104(e1265) mutant animals, ATG-9 does not localize to the nerve ring in embryos and adults (Figures 6H and 6K). Similarly, ATG-9 does not localize to AIY synaptic regions, and its localization is restricted to the cell body (Figures 6O–6Q). Our findings indicate that ATG-9 localizes to presynaptic sites in an UNC-104/KIF1A-dependent manner.
Autophagosome biogenesis in synaptic regions is dependent on UNC-104/KIF1A
UNC-104/KIF1A is a neuron-specific motor protein that regulates anterograde axonal transport of synaptic vesicle precursors (Otsuka et al., 1991). UNC-104/KIF1A-regulated distribution of synaptic vesicles and active zone proteins controls the spatial distribution of synapses during development (Wu et al., 2013b). Therefore, by regulating the transport of synaptic cargo to precise sites during development, the kinesin-3 UNC-104/KIF1A provides spatial specificity for synaptogenesis.
Given the observed role of UNC-104/KIF1A in transporting ATG-9 to presynaptic sites and the known role of ATG-9 in autophagosome biogenesis, we hypothesized that localization of ATG-9 by UNC-104/KIF1A would regulate the spatial distribution of autophagosomes to presynaptic sites. To test this hypothesis, we first observed autophagosomes in atg-9 mutant animals. Consistent with our hypothesis, in atg-9 mutant animals, we detected significant reductions in the number of animals with autophagosomes in the AIY neurite (12% of atg-9 mutant animals, compared with 33% wild type animals) and in the rate of autophagosome biogenesis in the neurite (we did not observe any biogenesis events in atg-9 mutants, n=20 neurons, compared to 1.7 events/hour in wild type, n=22 neurons) (Figures 5I and 5M–5N). We next examined the requirement of UNC-104/KIF1A for autophagosome enrichment in synaptic regions by visualizing GFP::LGG-1 in unc-104(e1265) mutants. While autophagosomes are still present in AIY cell bodies in unc-104(e1265) hypomorphic mutant animals (Figure 5L), we observed significant reductions in the number of animals with GFP::LGG-1 puncta in the AIY neurite (13% of unc-104 mutant animals; Figure 5M) and the rate of autophagosome biogenesis in the neurite (0.5 events/hour) (Figure 5N).
If UNC-104/KIF1A is important for local formation of autophagosomes, then it should act upstream of late autophagy genes atg-2 and epg-6 to suppress the accumulation of autophagosomes seen in these mutants. Indeed, we observed that atg-2(bp576);unc-104(e1265) and epg-6(bp424);unc-104(e1265) double mutants exhibited a significant reduction in the accumulation of autophagosomes in AIY presynaptic regions compared to atg-2(bp576) and epg-6(bp424) single mutants, indicating that unc-104 is epistatic to epg-6 and atg-2 (Figures 5M, S5D and S5E). Together our findings indicate that the presence of autophagosomes in synaptic regions is dependent on UNC-104/KIF1A and suggest that UNC-104/KIF1A regulates the spatial distribution of autophagosomes to presynaptic sites by mediating transport of ATG-9.
Autophagy is required in PVD for axon outgrowth
To better understand the role of autophagy in neurodevelopment, we examined multiple neuron classes in autophagy mutants for defects in neurodevelopmental events, including axon outgrowth, axon guidance and synaptic positioning. Interestingly, most of the neurons examined (HSN, RIA, DA9, RIB, and NSM) did not display phenotypes in the examined categories (Figures S6A–S6F and data not shown). In the nociceptive sensory neuron PVD (Figure 7A), we observed that autophagy was required for the length of the PVD axon at larval stage 4 (L4), but not for the morphology or timing of dendritic branching (Figures 7A–7G and data not shown).
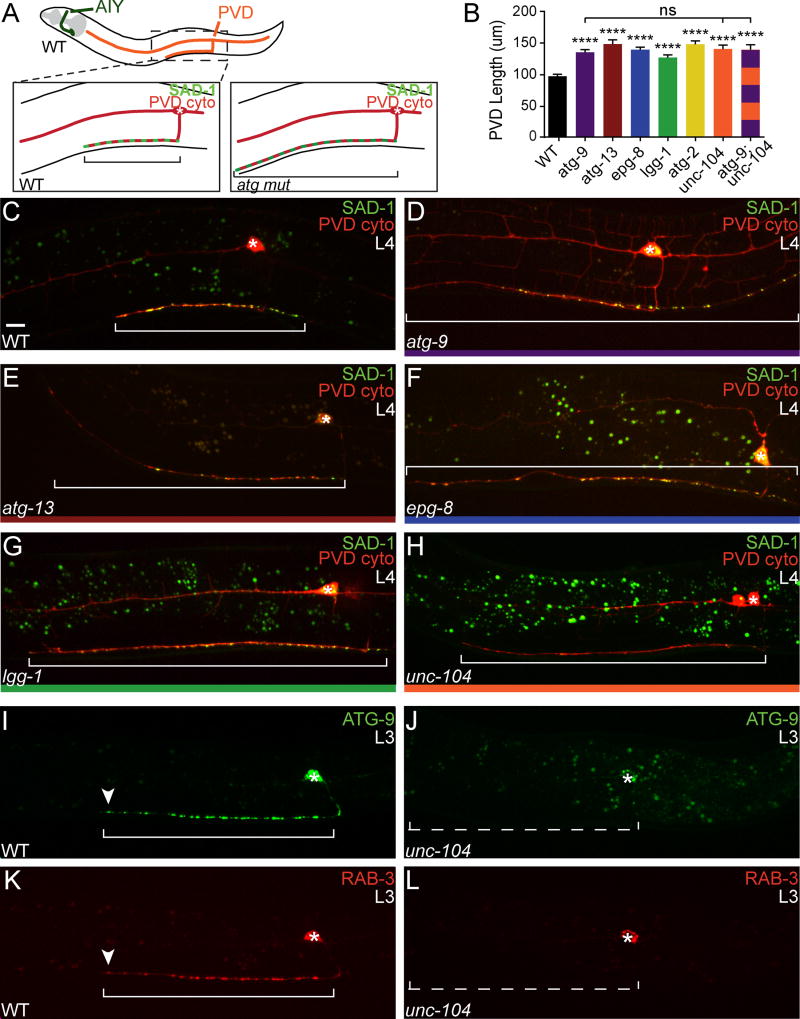
Figure 7. The autophagy pathway regulates the rate of PVD axon outgrowth.
(A) Diagram of AIY (dark green) and PVD (orange) neurons in C. elegans. Schematics of PVD axon morphology (red) and presynaptic sites (light green) in wild type (left box) and autophagy mutant animals (right box). (B) Quantification of PVD axon length in wild type and autophagy mutant L4 animals. Error bars represent SEM. ****, P< 0.0001 between mutants and wild type by one-way ANOVA with Tukey’s post-hoc analysis. (C–H) PVD morphology (visualized with cytoplasmic mCh) and presynapses (visualized with SAD-1::GFP) in the axon of PVD in wild type (C), atg-9(bp564) (D), atg-13(bp414) (E), epg-8(bp251) (F), lgg-1(bp500) (G), and unc-104(e1265) (H) mutant L4 animals. (I–L) Distribution of ATG-9::GFP (I and J) and synaptic vesicles (visualized with mCh::RAB-3) (K and L) in PVD in wild type (I and K) and unc-104(e1265) mutant animals (J and L). Arrowheads denote the tip of the axon in wild type animals (I and K). Each image is a maximal projection of a confocal z-stack; the asterisk denotes the location of the cell body; the bracket denotes length of the PVD axon; and the dotted bracket (J and L) denotes the PVD axon (not visible due to absence of presynaptic protein localization). Scale bar (in C for C–L), 5 μm. See also Figure S6.
The PVD axon begins to grow during larval stage 2 (L2) and continues to grow along the ventral nerve cord into adulthood (Maniar et al., 2012; Smith et al., 2010). To understand the requirement of autophagy in PVD axon outgrowth, we measured PVD axon length in L3, L4 and adult stages in wild type and autophagy pathway mutant animals. We observed that the rate of PVD axon outgrowth in autophagy mutants was significantly higher than the rate observed in wild type animals; this phenotype could be rescued upon neuronal expression of wild type copies of the respective autophagy genes, each representing a distinct step of autophagy (Figures 7B–7G and S6G–S6H). These data demonstrate that autophagy is required to regulate the rate of PVD axon outgrowth during development and are consistent with previous studies that demonstrated that knockdown of autophagy proteins results in longer axons (Ban et al., 2013). Our findings also indicate that autophagy is required in individual neurons to regulate distinct and specific neurodevelopmental events in vivo.
UNC-104/KIF1A is required for ATG-9 localization and axon outgrowth in PVD
We next examined the localization of ATG-9 in PVD. Similar to AIY, we observed that ATG-9::GFP localizes in a punctate pattern in the PVD axon (Figure 7I) and colocalizes with the synaptic vesicle marker mCh::RAB-3 (Figure 7K). Interestingly, we observed that in actively elongating neurons, both ATG-9::GFP and mCh::RAB-3 localize to the growing tip of the PVD axon (Figure 7I and 7K; arrowheads). In unc-104(e1265) mutant animals, both ATG-9 and RAB-3 did not localize to presynaptic sites or to the tip of the PVD axon and were observed instead in the PVD cell body (Figure 7J and 7L). These data suggest that, in PVD, as in AIY, UNC-104/KIF1A is important for the transport of ATG-9 in the axon.
In PVD, atg-9 mutants, like other autophagy mutants, display longer axons than wild type animals (Figure 7B and 7D). We hypothesized that if UNC-104/KIF1A was required in PVD for ATG-9 localization and autophagy, unc-104 mutants would phenocopy atg-9 mutant animals, with longer PVD axons. Consistent with our hypothesis, we observed that unc-104(e1265) mutants phenocopied the atg-9 mutant PVD axon length phenotype (Figures 7B and 7H). To our knowledge, this is the first time an axon outgrowth phenotype has been noted for the synaptic vesicle kinesin UNC-104/KIF1A. We hypothesized that if this newfound phenotype emerged from defects in ATG-9 transport, then unc-104(e1265);atg-9(bp564) double mutants would not enhance the length of either single mutant. Indeed, genetic analyses of unc-104(e1265);atg-9(bp564) double mutant animals revealed no enhancement of the PVD axon length of either single mutant, consistent with our model that unc-104 and atg-9 act in the same pathway to regulate PVD axon outgrowth (Figure 7B). Together our findings suggest that UNC-104/KIF1A is important for ATG-9 transport, which in turn regulates localized autophagy and neurodevelopmental events.
DISCUSSION
Autophagy regulates specific neurodevelopmental events in vivo. Less is known about the roles of autophagy in the execution of neurodevelopmental programs, especially in the context of intact, living animals. Our systematic, in vivo and single-cell analyses of the roles of autophagy during neurodevelopment indicate that autophagy is required for distinct and specific stages of neurodevelopment in different neuron types of C. elegans. We observe that in the interneuron AIY, autophagy is required to regulate presynaptic assembly, and 18 distinct autophagy mutants display consistent and specific defects in synaptogenesis. In a different neuron (sensory neuron PVD), autophagy is specifically required to regulate the rate of axon outgrowth. However, autophagy is not required for the neurodevelopment of all neurons. Therefore, in vivo, autophagy plays precise and cell-specific roles during development to contribute to the formation of the nervous system.
Our cell biological and genetic evidence suggest that autophagy controls neurodevelopment by directly or indirectly regulating cytoskeletal structures in the axon. During development, neuronal structures such as synapses and growth cones are dynamically formed, altered or eliminated in response to developmental cues (Kolodkin and Tessier-Lavigne, 2011). Underlying the transitions during neurodevelopment are mechanisms that regulate cytoskeletal dynamics (Nelson et al., 2013; Shen and Cowan, 2010). In our study, we found that autophagy mutants phenocopied previously identified mutations in genes that regulate the cytoskeleton during presynaptic assembly (Colón-Ramos et al., 2007; Stavoe et al., 2012; Stavoe and Colón-Ramos, 2012). We also found that disruption of the autophagy pathway in AIY results in disordered cytoskeletal structures, abnormal active zones and mislocalized synaptic vesicles. Our findings are consistent with studies in Drosophila that demonstrate that autophagy regulates the development of neuromuscular junctions and with studies in vertebrates that demonstrate that autophagy-dependent changes in axon length rely on the degradation of cytoskeletal regulatory proteins (Ban et al., 2013; Shen and Ganetzky, 2009). Furthermore, our data complement studies that establish that, in actively elongating axons, autophagosomes at the tip of axons contain cytoskeletal components (Hollenbeck and Bray, 1987) and with studies that show that induction of autophagy results in degradation of cytoskeletal components and inhibition of neurite outgrowth (Chen et al., 2013). We note that loss of autophagy in neurons does not result in the pleiotropic defects one would expect from general loss of cytoskeletal regulation or cellular homeostasis. Instead, autophagy mutants display precise neurodevelopmental phenotypes in specific neurons, suggesting that regulation of autophagy is required during development. In yeast and mammalian cells, autophagosome size and number are tightly controlled through transcriptional and post-translational mechanisms (Jin and Klionsky, 2014a, b). We hypothesize that similar mechanisms might regulate the precise deployment of autophagosomes in metazoan neurons, thereby modulating controlled degradation of cytoskeletal structures during neurodevelopmental transitions.
Our study demonstrates that autophagosome biogenesis is compartmentalized in the axons of living animals. Previous studies have demonstrated that autophagosome biogenesis occurs in the distal axons of cultured neurons, suggesting a regulated segregation of autophagosome biogenesis in neurons (Bunge, 1973; Hollenbeck, 1993; Hollenbeck and Bray, 1987; Maday and Holzbaur, 2014; Maday et al., 2012). We now show that compartmentalization of autophagosome biogenesis in neurons is also observed in vivo and that autophagosomes are enriched in synaptic regions in both adult and embryonic animals. This synaptic enrichment might confer a regulatory step by localizing the spatial activity of this cellular degradation pathway.
We find that local transport of ATG-9-containing vesicles acts as a permissive cue to compartmentalize autophagosome biogenesis. Most autophagy proteins are cytosolic, and their association with the autophagosome can be induced through post-translational modifications (Xie et al., 2015). However, ATG-9 is a six-pass transmembrane protein and the only integral membrane protein that is part of the core machinery of the autophagy pathway (Lang et al., 2000; Noda et al., 2000; Young et al., 2006). In yeast, Atg9 localizes to small (30–60nm) vesicles and promotes the formation of the autophagosome precursor (or isolation) membrane (Yamamoto et al., 2012). Little is known regarding the regulated transport of these Atg9-containing vesicles or if their transport limits the sites of autophagosome biogenesis. In our study, we observed that ATG-9 localizes to presynaptic regions and to the tip of the growing axon. Our findings in C. elegans neurons are consistent with studies in mammalian neurons, which demonstrate that Atg9 is enriched in varicosities in axons and colocalizes with synaptic proteins (Tamura et al., 2010). We determined that the localization of ATG-9 to the axon is regulated by the synaptic vesicle kinesin UNC-104/KIF1A, likely through the transport of ATG-9-containing vesicles. Disruption of ATG-9 transport in unc-104 mutants resulted in reduced rates of autophagosome biogenesis and a reduced number of animals with autophagosomes in AIY neurites. Our findings provide mechanistic insights on how transport of the integral membrane protein ATG-9 provides spatial specificity of autophagosome biogenesis to presynaptic compartments and the distal axon.
Taken together, we propose the following model to explain how local regulation of autophagy during development could both restrain axon outgrowth in PVD and promote presynaptic assembly in AIY. We hypothesize that the UNC-104/KIF1A-dependent delivery of ATG-9 to the PVD growth cone is required for autophagy to remodel the growth cone, potentially through degradation of growth cone components. This results in slower growth cone velocity, while loss of autophagy results in unrestricted axon outgrowth in PVD. This model is consistent with findings from cell culture and mammalian neurons, which reveal that disruption of autophagy results in longer neurites, while promotion of autophagy results in shorter neurites (Ban et al., 2013; Chen et al., 2013). In AIY, formation of the synaptic-rich region Zone 2 is partially instructed by the extracellular cue Netrin (Colón-Ramos et al., 2007). We hypothesize that, after AIY axon outgrowth, autophagy may be important for locally remodeling subcellular structures, such as the cytoskeleton, to facilitate presynaptic assembly in the synaptic-rich AIY Zone 2. Thus, in both AIY and PVD, autophagy may be required during neurodevelopment to locally remove cytoskeletal structures remaining from axon outgrowth to facilitate the creation of new functional domains in the subsequent stages of neurodevelopment.
In summary, our findings suggest that in neurons of living animals, regulated transport of autophagy components, such as ATG-9, permits compartmentalized autophagosome biogenesis and progression of neurodevelopmental events. Autophagy has also been implicated in postdevelopmental events in neurons, such as synaptic transmission and vesicle recycling (Binotti et al., 2015; Hernandez et al., 2012; Wang et al., 2015). Although we focused on the characterization of developmental phenotypes, we note that autophagy protein expression persists in adults and hypothesize that the mechanisms reported here could influence synaptic physiology and function postdevelopmentally.
EXPERIMENTAL PROCEDURES
For extended experimental procedures, please see Supplemental Information.
CRISPR Transgenics
We used a CRISPR protocol (Dickinson et al., 2015) to create atg-9(ola270[atg-9::gfp::SEC]), in which the eGFP coding sequence and the self-excision cassette are inserted in place of the atg-9 stop codon, and atg-9(ola274[atg-9::gfp]), in which the self-excision cassette is excised.
Electron Micrographs
We surveyed hundreds of high magnification electron micrographs from the archives of the Center for C. elegans Anatomy (Hall lab) to look for evidence of autophagy in embryonic neurons. The archive includes images contributed by Richard Durbin, John White (MRC/LMB, Cambridge) and Carolyn Norris (Hedgecock lab), many of which are publically available on www.WormImage.org. Neuronal autophagic vacuole-like organelles were identified as previously described (Bunge, 1973; Hernandez et al., 2012; Melendez et al., 2003; Yu et al., 2004). Autophagosomes in C. elegans are relatively small, owing to the small size of nematode cells (Melendez et al., 2003). We include the provenance of selected micrographs in the Figure legends.
AIY and PVD Quantifications
AIY presynaptic defect
To quantify the penetrance of the AIY presynaptic defect, we used the integrated transgenic line wyIs45 in the specified mutant backgrounds and quantified penetrance as previously described (Colón-Ramos et al., 2007; Stavoe et al., 2012; Stavoe and Colón-Ramos, 2012). Briefly, Zone 2 was defined morphologically as the region of the AIY process, which turns dorsally from the anterior ventral nerve cord into the nerve ring in adult animals We scored the number of animals displaying normal or abnormal Zone 2 synaptic patterns relative to wild type animals.
GFP::Rab-3 Enrichment in AIY
To quantify the enrichment of GFP::RAB-3 in AIY Zone 2, we measured the total fluorescence intensity across the AIY Zone 2 and Zone 3 regions in confocal maximal projection micrographs. Enrichment was defined as the total fluorescence intensity of Zone 2 divided by the total fluorescence intensity of both Zones 2 and 3. Fluorescence intensity (after background subtraction) was determined by tracing the AIY neurite using the line scan function in FIJI (Schindelin et al., 2012). For this quantification, Zone 2 was defined as 20% of the length of the entire AIY synaptic region (Zones 2 and 3).
GFP::LGG-1 in AIY
The GFP::LGG-1 probe used in these studies is the same one that has been validated in C. elegans by immuno-labeling electron microscopy studies with both anti-GFP and anti-LGG-1 and used to examine the in vivo dynamics of autophagosomes in hypodermal seam cells and early embryos (Manil-Segalen et al., 2014; Melendez et al., 2003; Tian et al., 2010; Zhang et al., 2015). To quantify the penetrance of autophagosome (GFP::LGG-1) puncta in the AIY neurite, we scored for presence or absence of LGG-1 puncta in the AIY neurite. For the rates of autophagosome biogenesis, we captured confocal micrographs (z-stacks) once every minute for 30 minutes. We counted the number of GFP::LGG-1 puncta that appeared within the movie and report these as biogenesis events in Figure 5N.
Statistical Analyses
To determine statistical significance for categorical data, we used Fisher’s exact test. Error bars represent 95% confidence intervals.
Statistical significance for continuous data was determined using one-way ANOVA with post hoc analysis by Tukey’s multiple comparisons test or Student’s t-test using PRISM software. Error bars for continuous data were calculated using standard error of the mean (SEM).
Supplementary Material
HIGHLIGHTS.
The autophagy pathway is required for presynaptic assembly in vivo
The autophagy pathway acts cell-autonomously and in specific neurons in development
Autophagosome biogenesis occurs in compartmentalized axonal regions near synapses
The synaptic vesicle kinesin UNC-104/KIF1A transports ATG-9 to presynaptic sites
Acknowledgments
We thank members of the Colón-Ramos lab, Erika Holzbaur (University of Pennsylvania), Hannes Buelow (Albert Einstein College of Medicine) and Alicia Meléndez (Queens College, CUNY) for their thoughtful comments on the manuscript and the project. We are particularly indebted to Kang Shen (Stanford University) for reagents, thoughtful comments and advice during the course of this project. We thank summer interns Jihane Jadi (University of North Carolina, Chapel Hill) and Ninoshka Caballero-Colón (University of Puerto Rico, Humacao) for experimental assistance. We thank the Caenorhabditis Genetics Center for mutant strains, Cori Bargmann (Rockefeller University) for CX9797 (kyIs445) strain, David Miller (Vanderbilt University) for NC1686 (wdIs51) strain, the Mitani laboratory of the Tokyo Women’s Medical University School of Medicine for autophagy mutant strains, and the Hong Zhang laboratory at the Institute of Biophysics, Chinese Academy of Sciences for autophagy mutant alleles. We thank Z. Altun (www.wormatlas.org) for diagrams used in figures. We thank Bob Goldstein (University of North Carolina at Chapel Hill) for CRISPR Self-excision cassette (SEC) constructs and advice.
We are grateful to Steven Cook for his help in choosing and copying high-resolution electron micrographs from the Hall lab archive. We thank John White and Jonathan Hodgkin for their help in contributing thousands of electron micrographs from the MRC/LMB collection to the Hall lab.
We thank the Research Center for Minority Institutions program and the Instituto de Neurobiología de la Universidad de Puerto Rico for providing a meeting and brainstorming platform. This work was partially conducted at the Marine Biological Laboratories at Woods Hole, under a Whitman research award to DACR. This work was funded by the U01HD075602, R01NS076558 and R24OD016474 NIH grants to DACR. DHH, the Center of C. elegans Anatomy and www.WormImage.org were supported by NIH OD 010943. AKHS and SEH were supported by Cellular and Molecular Biology Training grant T32-GM007223 from the NIH. SEH was also supported by NSF Graduate Research Fellowship DGE-1122492.
Footnotes
AUTHOR CONTRIBUTIONS
AKHS, SEH and DACR designed the experiments; AKHS and SEH performed the experiments and data analyses. SEH, DHH and DACR examined the EM micrographs for autophagosomes. AKHS, SEH and DACR prepared the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abada A, Elazar Z. Getting ready for building: signaling and autophagosome biogenesis. EMBO reports. 2014;15:839–852. doi: 10.15252/embr.201439076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe N, Almenar-Queralt A, Lillo C, Shen Z, Lozach J, Briggs SP, Williams DS, Goldstein LS, Cavalli V. Sunday driver interacts with two distinct classes of axonal organelles. The Journal of biological chemistry. 2009;284:34628–34639. doi: 10.1074/jbc.M109.035022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti A, Michelet X, Djeddi A, Legouis R. The autophagosomal protein LGG-2 acts synergistically with LGG-1 in dauer formation and longevity in C. elegans. Autophagy. 2010;6:622–633. doi: 10.4161/auto.6.5.12252. [DOI] [PubMed] [Google Scholar]
- Ariosa AR, Klionsky DJ. Long-distance autophagy. Autophagy. 2015;11:193–194. doi: 10.1080/15548627.2015.1009790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. The Journal of cell biology. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban BK, Jun MH, Ryu HH, Jang DJ, Ahmad ST, Lee JA. Autophagy negatively regulates early axon growth in cortical neurons. Molecular and cellular biology. 2013;33:3907–3919. doi: 10.1128/MCB.00627-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binotti B, Pavlos NJ, Riedel D, Wenzel D, Vorbruggen G, Schalk AM, Kuhnel K, Boyken J, Erck C, Martens H, et al. The GTPase Rab26 links synaptic vesicles to the autophagy pathway. eLife. 2015;4:e05597. doi: 10.7554/eLife.05597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland B, Nixon RA. Neuronal macroautophagy: from development to degeneration. Molecular aspects of medicine. 2006;27:503–519. doi: 10.1016/j.mam.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Brown HM, Van Epps HA, Goncharov A, Grant BD, Jin Y. The JIP3 scaffold protein UNC-16 regulates RAB-5 dependent membrane trafficking at C. elegans synapses. Developmental neurobiology. 2009;69:174–190. doi: 10.1002/dneu.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge MB. Fine structure of nerve fibers and growth cones of isolated sympathetic neurons in culture. The Journal of cell biology. 1973;56:713–735. doi: 10.1083/jcb.56.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DT, Kawasaki M, Walcoff M, Hisamoto N, Matsumoto K, Jin Y. UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron. 2001;32:787–800. doi: 10.1016/s0896-6273(01)00532-3. [DOI] [PubMed] [Google Scholar]
- Cecconi F, Di Bartolomeo S, Nardacci R, Fuoco C, Corazzari M, Giunta L, Romagnoli A, Stoykova A, Chowdhury K, Fimia GM, et al. A novel role for autophagy in neurodevelopment. Autophagy. 2007;3:506–508. doi: 10.4161/auto.4616. [DOI] [PubMed] [Google Scholar]
- Chen JX, Sun YJ, Wang P, Long DX, Li W, Li L, Wu YJ. Induction of autophagy by TOCP in differentiated human neuroblastoma cells lead to degradation of cytoskeletal components and inhibition of neurite outgrowth. Toxicology. 2013;310:92–97. doi: 10.1016/j.tox.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Colón-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullup T, Kho AL, Dionisi-Vici C, Brandmeier B, Smith F, Urry Z, Simpson MA, Yau S, Bertini E, McClelland V, et al. Recessive mutations in EPG5 cause Vici syndrome, a multisystem disorder with defective autophagy. Nature genetics. 2013;45:83–87. doi: 10.1038/ng.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DJ, Pani AM, Heppert JK, Higgins CD, Goldstein B. Streamlined Genome Engineering with a Self-Excising Drug Selection Cassette. Genetics. 2015;200:1035–1049. doi: 10.1534/genetics.115.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin RM. Studies on the Development and Organization of the Nervous System of Caenorhabditis elegans. University of Cambridge; 1987. http://www.wormatlas.org. [Google Scholar]
- Farre JC, Burkenroad A, Burnett SF, Subramani S. Phosphorylation of mitophagy and pexophagy receptors coordinates their interaction with Atg8 and Atg11. EMBO reports. 2013;14:441–449. doi: 10.1038/embor.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg EH, Vanhoven MK, Bendesky A, Wang G, Fetter RD, Shen K, Bargmann CI. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Feng Y, Backues SK, Baba M, Heo JM, Harper JW, Klionsky DJ. Phosphorylation of Atg9 regulates movement to the phagophore assembly site and the rate of autophagosome formation. Autophagy. 2016;12:648–658. doi: 10.1080/15548627.2016.1157237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell research. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale AN, Ledbetter DJ, Gawriluk TR, Rucker EB., 3rd Autophagy: regulation and role in development. Autophagy. 2013;9:951–972. doi: 10.4161/auto.24273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DH, Hedgecock EM. Kinesin-related gene unc-is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- He C, Baba M, Klionsky DJ. Double duty of Atg9 self-association in autophagosome biogenesis. Autophagy. 2009;5:385–387. doi: 10.4161/auto.5.3.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Song H, Yorimitsu T, Monastyrska I, Yen WL, Legakis JE, Klionsky DJ. Recruitment of Atg9 to the preautophagosomal structure by Atg11 is essential for selective autophagy in budding yeast. The Journal of cell biology. 2006;175:925–935. doi: 10.1083/jcb.200606084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez D, Torres CA, Setlik W, Cebrian C, Mosharov EV, Tang G, Cheng HC, Kholodilov N, Yarygina O, Burke RE, et al. Regulation of presynaptic neurotransmission by macroautophagy. Neuron. 2012;74:277–284. doi: 10.1016/j.neuron.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ. Products of endocytosis and autophagy are retrieved from axons by regulated retrograde organelle transport. The Journal of cell biology. 1993;121:305–315. doi: 10.1083/jcb.121.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ, Bray D. Rapidly transported organelles containing membrane and cytoskeletal components: their relation to axonal growth. The Journal of cell biology. 1987;105:2827–2835. doi: 10.1083/jcb.105.6.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- Jin M, Klionsky DJ. Regulation of autophagy: modulation of the size and number of autophagosomes. FEBS letters. 2014a;588:2457–2463. doi: 10.1016/j.febslet.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Klionsky DJ. Transcriptional regulation of ATG9 by the Pho23-Rpd3 complex modulates the frequency of autophagosome formation. Autophagy. 2014b;10:1681–1682. doi: 10.4161/auto.29641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. The EMBO journal. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. The Journal of cell biology. 2000;150:1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. The Journal of cell biology. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, Noda T, Ohsumi Y. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. The Journal of cell biology. 1999;147:435–446. doi: 10.1083/jcb.147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Wang QJ, Holstein GR, Friedrich VL, Jr, Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T, Garriga G. The conserved kinase UNC-51 acts with VAB-8 and UNC-14 to regulate axon outgrowth in C. elegans. Development. 2004;131:5991–6000. doi: 10.1242/dev.01457. [DOI] [PubMed] [Google Scholar]
- Lamark T, Kirkin V, Dikic I, Johansen T. NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell cycle. 2009;8:1986–1990. doi: 10.4161/cc.8.13.8892. [DOI] [PubMed] [Google Scholar]
- Lang T, Reiche S, Straub M, Bredschneider M, Thumm M. Autophagy and the cvt pathway both depend on AUT9. Journal of bacteriology. 2000;182:2125–2133. doi: 10.1128/jb.182.8.2125-2133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Schaeffeler E, Bernreuther D, Bredschneider M, Wolf DH, Thumm M. Aut2p and Aut7p, two novel microtubule-associated proteins are essential for delivery of autophagic vesicles to the vacuole. The EMBO journal. 1998;17:3597–3607. doi: 10.1093/emboj/17.13.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA. Neuronal autophagy: a housekeeper or a fighter in neuronal cell survival? Experimental neurobiology. 2012;21:1–8. doi: 10.5607/en.2012.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Hwang SK, Lee JA. Neuronal autophagy and neurodevelopmental disorders. Experimental neurobiology. 2013;22:133–142. doi: 10.5607/en.2013.22.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Yang P, Huang X, Zhang H, Lu Q, Zhang H. The scaffold protein EPG-7 links cargo-receptor complexes with the autophagic assembly machinery. The Journal of cell biology. 2013;201:113–129. doi: 10.1083/jcb.201209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Yang P, Huang X, Hu W, Guo B, Wu F, Lin L, Kovacs AL, Yu L, Zhang H. The WD40 repeat PtdIns(3)P-binding protein EPG-6 regulates progression of omegasomes to autophagosomes. Developmental cell. 2011;21:343–357. doi: 10.1016/j.devcel.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Maday S, Holzbaur EL. Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Developmental cell. 2014;30:71–85. doi: 10.1016/j.devcel.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. The Journal of cell biology. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney TR, Liu Q, Itoh T, Luo S, Hadwiger G, Vincent R, Wang ZW, Fukuda M, Nonet ML. Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegans. Molecular biology of the cell. 2006;17:2617–2625. doi: 10.1091/mbc.E05-12-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniar TA, Kaplan M, Wang GJ, Shen K, Wei L, Shaw JE, Koushika SP, Bargmann CI. UNC-33 (CRMP) and ankyrin organize microtubules and localize kinesin to polarize axon-dendrite sorting. Nature neuroscience. 2012;15:48–56. doi: 10.1038/nn.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manil-Segalen M, Lefebvre C, Jenzer C, Trichet M, Boulogne C, Satiat-Jeunemaitre B, Legouis R. The C. elegans LC3 acts downstream of GABARAP to degrade autophagosomes by interacting with the HOPS subunit VPS39. Developmental cell. 2014;28:43–55. doi: 10.1016/j.devcel.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Mao K, Wang K, Liu X, Klionsky DJ. The scaffold protein Atg11 recruits fission machinery to drive selective mitochondria degradation by autophagy. Developmental cell. 2013;26:9–18. doi: 10.1016/j.devcel.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino G, Madeo F, Kroemer G. Autophagy for tissue homeostasis and neuroprotection. Current opinion in cell biology. 2011;23:198–206. doi: 10.1016/j.ceb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Melendez A, Levine B. Autophagy in C. elegans. WormBook: the online review of C elegans biology. 2009:1–26. doi: 10.1895/wormbook.1.147.1. [DOI] [PubMed] [Google Scholar]
- Melendez A, Talloczy Z, Seaman M, Eskelinen EL, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annual review of nutrition. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JC, Stavoe AK, Colón-Ramos DA. The actin cytoskeleton in presynaptic assembly. Cell adhesion & migration. 2013;7:379–387. doi: 10.4161/cam.24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T, Kim J, Huang WP, Baba M, Tokunaga C, Ohsumi Y, Klionsky DJ. Apg9p/Cvt7p is an integral membrane protein required for transport vesicle formation in the Cvt and autophagy pathways. The Journal of cell biology. 2000;148:465–480. doi: 10.1083/jcb.148.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara K, Sekito T, Ohsumi Y. Assortment of phosphatidylinositol 3-kinase complexes--Atg14p directs association of complex I to the pre-autophagosomal structure in Saccharomyces cerevisiae. Molecular biology of the cell. 2006;17:1527–1539. doi: 10.1091/mbc.E05-09-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K, Shirakawa M, Barnes TM, Hekimi S, Ohshima Y. The UNC-14 protein required for axonal elongation and guidance in Caenorhabditis elegans interacts with the serine/threonine kinase UNC-51. Genes & development. 1997;11:1801–1811. doi: 10.1101/gad.11.14.1801. [DOI] [PubMed] [Google Scholar]
- Orsi A, Razi M, Dooley HC, Robinson D, Weston AE, Collinson LM, Tooze SA. Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy. Molecular biology of the cell. 2012;23:1860–1873. doi: 10.1091/mbc.E11-09-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka AJ, Jeyaprakash A, Garcia-Anoveros J, Tang LZ, Fisk G, Hartshorne T, Franco R, Born T. The C. elegans unc-gene encodes a putative kinesin heavy chain-like protein. Neuron. 1991;6:113–122. doi: 10.1016/0896-6273(91)90126-k. [DOI] [PubMed] [Google Scholar]
- Patel N, Thierry-Mieg D, Mancillas JR. Cloning by insertional mutagenesis of a cDNA encoding Caenorhabditis elegans kinesin heavy chain. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:9181–9185. doi: 10.1073/pnas.90.19.9181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F, Klionsky DJ. Autophagic processes in yeast: mechanism, machinery and regulation. Genetics. 2013;194:341–361. doi: 10.1534/genetics.112.149013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F, Tooze SA. Autophagy regulation through Atg9 traffic. The Journal of cell biology. 2012;198:151–153. doi: 10.1083/jcb.201206119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F, Tucker KA, Stromhaug PE, Klionsky DJ. The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Developmental cell. 2004;6:79–90. doi: 10.1016/s1534-5807(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Rudolf R, Khan MM, Wild F, Hashemolhosseini S. The impact of autophagy on peripheral synapses in health and disease. Frontiers in bioscience. 2016;21:1474–1487. doi: 10.2741/4467. [DOI] [PubMed] [Google Scholar]
- Sakamoto R, Byrd DT, Brown HM, Hisamoto N, Matsumoto K, Jin Y. The Caenorhabditis elegans UNC-14 RUN domain protein binds to the kinesin-1 and UNC-16 complex and regulates synaptic vesicle localization. Molecular biology of the cell. 2005;16:483–496. doi: 10.1091/mbc.E04-07-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Sato K. Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science. 2011;334:1141–1144. doi: 10.1126/science.1210333. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Watanabe S, Christensen R, Jorgensen EM, Colon-Ramos DA. Synapse location during growth depends on glia location. Cell. 2013;154:337–350. doi: 10.1016/j.cell.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata M, Inokuchi K. Does autophagy work in synaptic plasticity and memory? Reviews in the neurosciences. 2014;25:543–557. doi: 10.1515/revneuro-2014-0002. [DOI] [PubMed] [Google Scholar]
- Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harbor perspectives in biology. 2010;2:a001842. doi: 10.1101/cshperspect.a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Ganetzky B. Autophagy promotes synapse development in Drosophila. The Journal of cell biology. 2009;187:71–79. doi: 10.1083/jcb.200907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T, Suzuki K, Kamada Y, Noda T, Ohsumi Y. Apg2p functions in autophagosome formation on the perivacuolar structure. The Journal of biological chemistry. 2001;276:30452–30460. doi: 10.1074/jbc.M102346200. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Watson JD, Spencer WC, O’Brien T, Cha B, Albeg A, Treinin M, Miller DM., 3rd Time-lapse imaging and cell-specific expression profiling reveal dynamic branching and molecular determinants of a multi-dendritic nociceptor in C. elegans. Developmental biology. 2010;345:18–33. doi: 10.1016/j.ydbio.2010.05.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son JH, Shim JH, Kim KH, Ha JY, Han JY. Neuronal autophagy and neurodegenerative diseases. Experimental & molecular medicine. 2012;44:89–98. doi: 10.3858/emm.2012.44.2.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley RE, Ragusa MJ, Hurley JH. The beginning of the end: how scaffolds nucleate autophagosome biogenesis. Trends in cell biology. 2013 doi: 10.1016/j.tcb.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavoe AK, Nelson JC, Martinez-Velazquez LA, Klein M, Samuel AD, Colón-Ramos DA. Synaptic vesicle clustering requires a distinct MIG-10/Lamellipodin isoform and ABI-1 downstream from Netrin. Genes & development. 2012;26:2206–2221. doi: 10.1101/gad.193409.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavoe AKH, Colón-Ramos DA. Netrin instructs synaptic vesicle clustering through Rac GTPase, MIG-10, and the actin cytoskeleton. Journal of Cell Biology. 2012;197:75–88. doi: 10.1083/jcb.201110127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki SW, Yamamoto H, Oikawa Y, Kondo-Kakuta C, Kimura Y, Hirano H, Ohsumi Y. Atg13 HORMA domain recruits Atg9 vesicles during autophagosome formation. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:3350–3355. doi: 10.1073/pnas.1421092112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Shibata M, Koike M, Sasaki M, Uchiyama Y. Atg9A protein, an autophagy-related membrane protein, is localized in the neurons of mouse brains. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2010;58:443–453. doi: 10.1369/jhc.2010.955690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Tanida-Miyake E, Komatsu M, Ueno T, Kominami E. Human Apg3p/Aut1p homologue is an authentic E2 enzyme for multiple substrates, GATE-16, GABARAP, and MAP-LC3, and facilitates the conjugation of hApg12p to hApg5p. The Journal of biological chemistry. 2002;277:13739–13744. doi: 10.1074/jbc.M200385200. [DOI] [PubMed] [Google Scholar]
- Thompson O, Edgley M, Strasbourger P, Flibotte S, Ewing B, Adair R, Au V, Chaudhry I, Fernando L, Hutter H, et al. The million mutation project: a new approach to genetics in Caenorhabditis elegans. Genome research. 2013;23:1749–1762. doi: 10.1101/gr.157651.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Li Z, Hu W, Ren H, Tian E, Zhao Y, Lu Q, Huang X, Yang P, Li X, et al. C. elegans screen identifies autophagy genes specific to multicellular organisms. Cell. 2010;141:1042–1055. doi: 10.1016/j.cell.2010.04.034. [DOI] [PubMed] [Google Scholar]
- Torres CA, Sulzer D. Macroautophagy can press a brake on presynaptic neurotransmission. Autophagy. 2012;8:1540–1541. doi: 10.4161/auto.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto S, Kuma A, Murakami M, Kishi C, Yamamoto A, Mizushima N. Autophagy is essential for preimplantation development of mouse embryos. Science. 2008;321:117–120. doi: 10.1126/science.1154822. [DOI] [PubMed] [Google Scholar]
- Wang CW, Kim J, Huang WP, Abeliovich H, Stromhaug PE, Dunn WA, Jr, Klionsky DJ. Apg2 is a novel protein required for the cytoplasm to vacuole targeting, autophagy, and pexophagy pathways. The Journal of biological chemistry. 2001;276:30442–30451. doi: 10.1074/jbc.M102342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Menon S, Yamasaki A, Chou HT, Walz T, Jiang Y, Ferro-Novick S. Ypt1 recruits the Atg1 kinase to the preautophagosomal structure. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9800–9805. doi: 10.1073/pnas.1302337110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Martin S, Papadopulos A, Harper CB, Mavlyutov TA, Niranjan D, Glass NR, Cooper-White JJ, Sibarita JB, Choquet D, et al. Control of autophagosome axonal retrograde flux by presynaptic activity unveiled using botulinum neurotoxin type a. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:6179–6194. doi: 10.1523/JNEUROSCI.3757-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhanditis elegans. Philosophical Transactions of the Royal Society of London. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wong YC, Holzbaur EL. Autophagosome dynamics in neurodegeneration at a glance. Journal of cell science. 2015;128:1259–1267. doi: 10.1242/jcs.161216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Li Y, Wang F, Noda NN, Zhang H. Differential function of the two Atg4 homologues in the aggrephagy pathway in Caenorhabditis elegans. The Journal of biological chemistry. 2012;287:29457–29467. doi: 10.1074/jbc.M112.365676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Watanabe Y, Guo XY, Qi X, Wang P, Zhao HY, Wang Z, Fujioka Y, Zhang H, Ren JQ, et al. Structural Basis of the Differential Function of the Two C. elegans Atg8 Homologs, LGG-1 and LGG-2, in Autophagy. Molecular cell. 2015;60:914–929. doi: 10.1016/j.molcel.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Wu X, Won H, Rubinsztein DC. Autophagy and mammalian development. Biochemical Society transactions. 2013a;41:1489–1494. doi: 10.1042/BST20130185. [DOI] [PubMed] [Google Scholar]
- Wu Y, Cheng S, Zhao H, Zou W, Yoshina S, Mitani S, Zhang H, Wang X. PI3P phosphatase activity is required for autophagosome maturation and autolysosome formation. EMBO reports. 2014;15:973–981. doi: 10.15252/embr.201438618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YE, Huo L, Maeder CI, Feng W, Shen K. The balance between capture and dissociation of presynaptic proteins controls the spatial distribution of synapses. Neuron. 2013b;78:994–1011. doi: 10.1016/j.neuron.2013.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Kang R, Sun X, Zhong M, Huang J, Klionsky DJ, Tang D. Posttranslational modification of autophagy-related proteins in macroautophagy. Autophagy. 2015;11:28–45. doi: 10.4161/15548627.2014.984267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M, Stefanis L. Autophagy in the central nervous system: implications for neurodegenerative disorders. CNS & neurological disorders drug targets. 2010;9:701–719. doi: 10.2174/187152710793237421. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Yue Z. Autophagy and its normal and pathogenic states in the brain. Annual review of neuroscience. 2014;37:55–78. doi: 10.1146/annurev-neuro-071013-014149. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kakuta S, Watanabe TM, Kitamura A, Sekito T, Kondo-Kakuta C, Ichikawa R, Kinjo M, Ohsumi Y. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. The Journal of cell biology. 2012;198:219–233. doi: 10.1083/jcb.201202061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HY, Mains PE, McNally FJ. Kinesin-1 mediates translocation of the meiotic spindle to the oocyte cortex through KCA-1, a novel cargo adapter. The Journal of cell biology. 2005;169:447–457. doi: 10.1083/jcb.200411132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Zhang H. The coiled-coil domain protein EPG-8 plays an essential role in the autophagy pathway in C. elegans. Autophagy. 2011;7:159–165. doi: 10.4161/auto.7.2.14223. [DOI] [PubMed] [Google Scholar]
- Yang Y, Coleman M, Zhang L, Zheng X, Yue Z. Autophagy in axonal and dendritic degeneration. Trends in neurosciences. 2013;36:418–428. doi: 10.1016/j.tins.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem J, Herman RK. Investigating C. elegans development through mosaic analysis. Development. 2003;130:4761–4768. doi: 10.1242/dev.00701. [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Klionsky DJ. Atg11 links cargo to the vesicle-forming machinery in the cytoplasm to vacuole targeting pathway. Molecular biology of the cell. 2005;16:1593–1605. doi: 10.1091/mbc.E04-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AR, Chan EY, Hu XW, Kochl R, Crawshaw SG, High S, Hailey DW, Lippincott-Schwartz J, Tooze SA. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. Journal of cell science. 2006;119:3888–3900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
- Yu WH, Kumar A, Peterhoff C, Shapiro Kulnane L, Uchiyama Y, Lamb BT, Cuervo AM, Nixon RA. Autophagic vacuoles are enriched in amyloid precursor protein-secretase activities: implications for beta-amyloid peptide over-production and localization in Alzheimer’s disease. The international journal of biochemistry & cell biology. 2004;36:2531–2540. doi: 10.1016/j.biocel.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Yue Z. Regulation of neuronal autophagy in axon: implication of autophagy in axonal function and dysfunction/degeneration. Autophagy. 2007;3:139–141. doi: 10.4161/auto.3602. [DOI] [PubMed] [Google Scholar]
- Yue Z, Friedman L, Komatsu M, Tanaka K. The cellular pathways of neuronal autophagy and their implication in neurodegenerative diseases. Biochimica et biophysica acta. 2009;1793:1496–1507. doi: 10.1016/j.bbamcr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffagnini G, Martens S. Mechanisms of Selective Autophagy. Journal of molecular biology. 2016;428:1714–1724. doi: 10.1016/j.jmb.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Baehrecke EH. Eaten alive: novel insights into autophagy from multicellular model systems. Trends in cell biology. 2015;25:376–387. doi: 10.1016/j.tcb.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Chang JT, Guo B, Hansen M, Jia K, Kovacs AL, Kumsta C, Lapierre LR, Legouis R, Lin L, et al. Guidelines for monitoring autophagy in Caenorhabditis elegans. Autophagy. 2015;11:9–27. doi: 10.1080/15548627.2014.1003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu F, Wang X, Du H, Wang X, Zhang H. The two C. elegans ATG-16 homologs have partially redundant functions in the basal autophagy pathway. Autophagy. 2013;9:1965–1974. doi: 10.4161/auto.26095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yan L, Zhou Z, Yang P, Tian E, Zhang K, Zhao Y, Li Z, Song B, Han J, et al. SEPA-1 mediates the specific recognition and degradation of P granule components by autophagy in C. elegans. Cell. 2009;136:308–321. doi: 10.1016/j.cell.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhao YG, Wang X, Xu L, Miao L, Feng D, Chen Q, Kovacs AL, Fan D, Zhang H. Mice deficient in Epg5 exhibit selective neuronal vulnerability to degeneration. J Cell Biol. 2013;200:731–741. doi: 10.1083/jcb.201211014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.