Abstract
Background
Axillary surgery is not considered therapeutic in patients with clinical T1-T2 N0 breast cancer. The importance of axillary staging is eroding in an era where tumor biology, as defined by biomarker and gene expression profile, is increasingly important in medical decision making. We hypothesize that axillary ultrasound (AUS) is a noninvasive alternative to sentinel lymph node biopsy (SLNB), and AUS could replace SLNB without compromising patient care.
Study Design
Patients with clinical T1-T2 N0 breast cancer and normal AUS were eligible for enrollment. Subjects were randomized to no further axillary staging (Arm 1) versus SLNB (Arm 2). Descriptive statistics were used to describe the results of the pilot phase of the randomized controlled trial.
Results
68 subjects were enrolled in the pilot phase of the trial (34 subjects in Arm 1, no further staging; 32 subjects in Arm 2, SLNB, and 2 subjects voluntarily withdrew from the trial). The median age was 61 years (range 40-80) in Arm 1 and 59 years (range 31-81) in Arm 2, and there were no significant clinical or pathologic differences between the arms. Median follow-up was 17 months (range 1-32). The negative predictive value (NPV) of AUS for identification of clinically significant axillary disease (> 2.0 mm) was 96.9%. No axillary recurrences have been observed in either arm.
Conclusions
Successful completion of the pilot phase of the randomized controlled trial confirms the feasibility of the study design, and provides prospective evidence supporting the ability of AUS to exclude clinically significant disease in the axilla. The results provide strong support for a phase 2 randomized controlled trial.
INTRODUCTION
The current standard of care for patients with a new diagnosis of clinical T1-T2 N0 breast cancer is to perform a sentinel lymph node biopsy (SLNB). SLNB is an invasive surgical procedure based on the hypothesis that the pathologic status of the “sentinel” lymph node(s) predict the pathologic status of the corresponding lymph node basin. In current practice, radiolabeled sulfur colloid and/or blue dye are injected into the breast near the primary breast cancer and travel via lymphatics to the sentinel lymph node(s) (SLN), which are surgically removed through an incision in the axilla. If the SLN are free of metastases, it is unlikely that any other lymph nodes will have metastases. Thus, SLNB provides important staging information that can be used to tailor adjuvant therapies to individual patients.
Despite this elegant hypothesis, we believe that SLNB is already an outdated procedure, and an invasive procedure may no longer be required for staging the axilla in early stage breast cancer patients. There are several important reasons to consider a noninvasive alternative to SLNB. First, SLNB is an invasive surgical procedure. Although SLNB has fewer and less severe complications compared to axillary lymph node dissection (ALND) [1-4], it is not a risk-free procedure. Large prospective trials such as ACOSOG Z0010, NSABP B-32, and the ALMANAC trial have documented SLNB complications including allergic reactions to isosulfan blue dye (0.1-1.0%), wound infection (1.0-10%), seroma (7.1%), paresthesias (8.6-11%), lymphedema (6.9%), and hematoma (1.4%) [2, 5-7]. For many breast cancer patients, SLNB is more morbid than partial mastectomy. Second, axillary surgery is not considered therapeutic in early stage breast cancer. Level 1 evidence supports the hypothesis that axillary surgery is not therapeutic in patients with early stage, clinically node-negative disease. The ACOSOG Z0011 trial randomized women with clinical T1-T2 N0 breast cancer and a positive SLNB to no further axillary surgery or to completion ALND. The Z0011 trial demonstrated no local control or survival advantage with completion ALND, even in the setting of known axillary disease [8, 9]. Similarly, the International Breast Cancer Study Group (IBSCG) 23-01 trial randomized women with micrometastatic disease in the SLN to no further axillary surgery or to completion ALND. This trial also demonstrated no local control or survival advantage associated with ALND [10]. Retrospective studies also support the concept that axillary surgery is not therapeutic in early stage breast cancer [11-17]. Third, the importance of axillary staging information provided by SLNB is decreasing in importance. Decisions regarding adjuvant systemic therapy are complex, and integrate a significant amount of clinical and pathologic information such as patient age, medical history, menopausal status, tumor size, biomarker profile, gene expression profile, and axillary lymph node status. Although axillary staging remains important, it is of decreasing importance as a driver of medical oncology decision making. For instance, chemotherapy is now recommended in most patients with triple-negative breast cancer (TNBC), including patients with node-negative disease. The importance of axillary staging may be further eroded in the future, as gene expression profiles, such as Oncotype DX, are increasingly used to not only better define a patient's risk of distant recurrence, but also to predict response to specific therapies, such as chemotherapy [18-26].
AUS is a noninvasive alternative to SLNB for staging of the axilla in early stage breast cancer. AUS can identify disease in axillary lymph nodes (ALN) based on the size and morphologic features of the lymph nodes [27, 28]. Several reports have documented the accuracy of AUS with or without fine needle aspiration biopsy (FNA) [29-34], and a 2011 meta-analysis by Houssami et al. reported a 79.6% sensitivity, 98.3% specificity, and 97.1% positive predictive value for AUS with FNA or core-needle biopsy (CNB) [35]. However, following publication of the ACOSOG Z0011 trial results, the role of AUS in axillary staging has become less clear [36, 37]. We recently completed a retrospective study specifically focused on the ability of AUS to accurately exclude clinically significant disease in the axilla [38]. AUS had a sensitivity of 76% and a negative predictive value (NPV) of 89% for the detection of clinically significant disease (> 2.0 mm). On multivariate analysis, no patient or tumor characteristics significantly impacted the sensitivity, specificity, or NPV of AUS detection of metastatic breast cancer to the axilla, and the median size of a “missed” ALN metastasis was 2.0 mm (range 0.1-21 mm, mean 3.7 mm).
Taken together, the fact that axillary surgery is not therapeutic, the limited impact of axillary staging in the modern era of breast cancer treatment, and the excellent performance characteristics of AUS for the detection of clinically significant disease, strongly support prospective investigations of AUS for axillary staging in breast cancer. We recently completed the pilot phase of a randomized controlled trial focused on the role of AUS in axillary staging to assess the feasibility of such a trial, and obtain prospective information about the performance characteristics of AUS for detection of clinically significant axillary disease.
METHODS
Randomized controlled trial
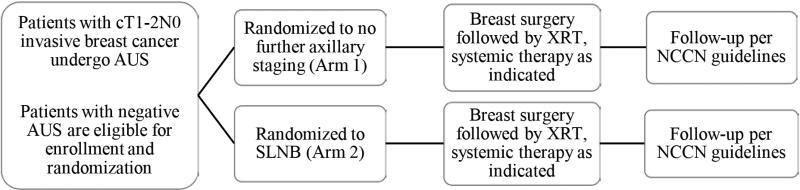
Institutional Review Board approval was obtained prior to initiation of the randomized controlled trial (NCT01821768). Subjects were recruited from the Siteman Cancer Center at Washington University School of Medicine (WUSM). Eligible participants included women aged 18 and over with newly diagnosed clinical T1-2 N0 M0 breast cancer who were candidates for breast conservation therapy and a normal AUS. AUS was considered abnormal based on consensus criteria (hypoechoic nodes, loss of the fatty hilum, and/or focal cortical thickening or bulge ≥ 4.0 mm). AUS was performed by radiologists specializing in breast imaging prior to initiation of any therapy. Patients were enrolled by Siteman Cancer Center research support staff. After enrollment, subjects were randomized in a 1:1 ratio to no further axillary staging (Arm 1), or SLNB (Arm 2) (Figure 1). Randomization was assigned using SAS PROC PLAN and was performed in small blocks of 4-6 patients using the WUSM RedCap website. In addition to definitive surgery, subjects received adjuvant systemic and/or radiation therapy as clinically indicated. Subjects in Arm 1 (no further axillary staging) were considered pathologically node-negative for the purposes of adjuvant systemic therapy decisions and radiation treatment planning. Axillary radiation was prohibited. Subjects were followed as per National Comprehensive Cancer Network (NCCN) guidelines with at least semi-annual clinical examination, annual mammography for patients with intact breasts, and additional imaging as indicated by symptoms or examination. Data recorded included patient age; tumor size, histology, grade, and receptor status; presence and type of postoperative complications; adjuvant therapy received; use and type of neoadjuvant systemic therapy; presence and location of disease recurrence; and patient death due to breast cancer or any cause. For patients in Arm 2 (SLNB), the number of sentinel nodes removed and pathologic status of the lymph nodes was recorded.
Figure 1.
Study schema. AUS, axillary ultrasound; SLNB, sentinel lymph node biopsy; XRT, radiation therapy; NCCN, National Comprehensive Cancer Center Network.
The primary objective of the pilot phase of the randomized controlled trial was to demonstrate feasibility of the trial design, and obtain prospective evidence supporting the ability of AUS to exclude clinically significant disease in the axilla. The primary objective of the phase 2 randomized controlled trial is to determine if axillary recurrence rates for patients randomized to Arm 1 (no SLNB) are equivalent to axillary recurrence rates for patients randomized to Arm 2 (SLNB).
Data analysis
The distribution of demographic and clinical characteristics in each arm was summarized using descriptive statistics and compared by two-sample t-test, Mann-Whitney rank-sum test, or Fisher's exact test as appropriate. All analyses were two-sided and significance was set at a p value of 0.05. For subjects in Arm 2, the negative predictive value (NPV) of AUS was calculated. Statistical analyses were performed using SAS (SAS Institutes, Cary, NC).
RESULTS
To evaluate the role of AUS as a noninvasive alternative to SLNB for staging of the axilla in breast cancer, we initiated a randomized controlled trial. Patients with clinical T1-T2 N0 breast cancer and normal AUS were eligible for enrollment. Subjects were randomized to no further axillary staging (Arm 1) versus SLNB (Arm 2) (Figure 1). Subject accrual began in April, 2013, and the pilot phase of the study was completed in December, 2015. 68 subjects were enrolled in the pilot phase of the trial. 34 subjects were randomized to Arm 1, 34 subjects were randomized to Arm 2, and 2 subjects randomized to Arm 2 withdrew from the trial. 34 patients from Arm 1, and 32 patients from Arm 2 completed the study interventions and are included in the analysis. The median age in Arm 1 was 61 (range 40-80 years) and the median age in Arm 2 was 59 (range 31-81). Median follow-up is 17 months (range 1-32 months). Subject demographic and tumor characteristics are summarized in Table 1. The two arms are well-matched in terms of patient age, tumor histology, tumor size, biomarker profile and length of follow-up.
Table 1.
Demographics, Tumor Characteristics, and Follow-Up for 66 Patients with Normal Axillary Ultrasound Randomized to No Surgical Axillary Staging (Arm 1) vs Sentinel Lymph Node Biopsy (Arm 2)
| Characteristic | Total n = 66 | Arm 1 n = 34 | Arm 2 n = 32 | p Value |
|---|---|---|---|---|
| Patient demographic | 0.106 | |||
| Median age, y, (range) | 60 (31-81) | 61 (40-80) | 59 (31-81) | |
| Median follow-up, months, (range) | 17 (1-32) | 17 (2-32) | 18 (1-32) | |
| Tumor histology, n (%) | 0.143 | |||
| Invasive ductal carcinoma | 58 (88) | 32 (94) | 26 (81) | |
| Invasive lobular carcinoma | 7 (11) | 1 (3) | 6 (19) | |
| Other histology | 1 (2) | 1 (3) | 0 (0) | |
| Tumor size, n (%) | 0.256 | |||
| T1a/mi | 5 (8) | 4 (12) | 1 (3) | |
| T1b | 22 (33) | 10 (29) | 12 (38) | |
| T1c | 27 (41) | 16 (47) | 11 (34) | |
| T2 | 12 (18) | 4 (12) | 8 (25) | |
| Tumor grade, n (%) | 0.595 | |||
| Tumor grade I | 25 (38) | 14 (41) | 11 (34) | |
| Tumor grade II | 28 (42) | 12 (35) | 16 (50) | |
| Tumor grade III | 12 (18) | 7 (21) | 5 (16) | |
| Tumor grade unknown | 1 (2) | 1 (3) | 0 (0) | |
| ER status, n (%) | 0.668 | |||
| ER+ | 61 (92) | 32 (94) | 29 (91) | |
| ER− | 5 (8) | 2 (6) | 3 (9) | |
| PR status, n (%) | 0.428 | |||
| PR+ | 59 (89) | 29 (85) | 30 (94) | |
| PR− | 7 (11) | 5 (15) | 2 (6) | |
| HER2 status, n (%) | 0.140 | |||
| HER2+ | 5 (8) | 3 (9) | 2 (6) | |
| HER2− | 57 (86) | 27 (80) | 30 (94) | |
| HER2 unknown or equivocal | 4 (6) | 4 (12) | 0 (0) |
ER, estrogen receptor; PR, progesterone receptor; HER2, Human epidermal receptor 2
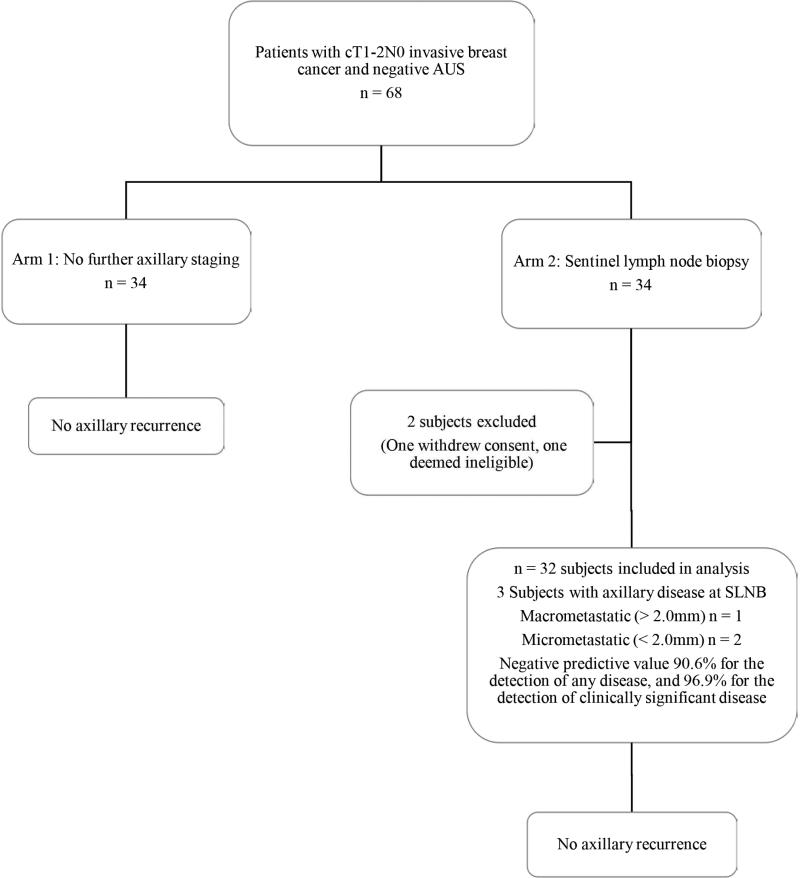
There were no unexpected adverse events in either arm. In Arm 2 (SLNB), there 2 cases of grade 1 lymphedema, one case of grade 1 infection, two cases of grade 2 seroma, one case of grade 1 paresthesia, and one wound hematoma requiring operative intervention. There have been no in-breast, axillary, or distant recurrences in either arm. Of the 32 subjects randomized to Arm 2 (SLNB), three were found to have axillary disease (Figure 2). One subject was a 47 year old with a T2, grade 2 invasive ductal carcinoma, ER+, PR+, and HER2−. There was a 1 mm micrometastasis in the SLN, and at ALND one of 15 nodes was found to have a focus of disease < 2.0 mm. The second subject was a 66 year old with a T1b, grade 2 invasive ductal carcinoma, ER+, PR+, and HER2−. One SLN was removed and had a single focus of disease < 2.0 mm. No axillary dissection was performed. The third subject was a 55 year old with a T2, grade 3 invasive ductal carcinoma, ER+, PR+, and HER2−. Her SLNB procedure failed to identify a SLN, and on ALND, two of 13 lymph nodes were positive, with the largest metastasis measuring 7 mm. The NPV of AUS for identification of any axillary disease was 90.6%, and the NPV for identification of clinically significant disease > 2.0 mm was 96.9%.
Figure 2.
Pilot study results. The negative predictive value of axillary ultrasound was 90.6% for the detection of any disease in the axilla, and 96.9% for the detection of clinically significant (> 2.0 mm) disease in the axilla. AUS, axillary ultrasound.
DISCUSSION
Axillary staging impacts all the major stakeholders in breast cancer care, including patients, surgeons, medical oncologists, and radiation oncologists. Adoption of AUS for axillary staging represents a major paradigm shift, and AUS will not replace SLNB until a phase 3 randomized controlled trial documents the noninferiority of AUS. We have successfully completed the pilot phase of a randomized controlled trial comparing no further axillary staging to SLNB in patients with clinical T1-T2 N0 breast cancer and a negative AUS. Successful completion of the pilot phase of the trial confirms the feasibility of the study design. Feasibility is a critical issue as two recent randomized controlled trials evaluating the role of axillary surgery in clinical T1-T2 N0 breast cancer (ACOSOG Z0011 and IBCSG 23-01) were closed to enrollment without meeting accrual goals. These studies had poor accrual as patients and physicians had strong preconceptions about the potential therapeutic benefit of axillary surgery, making randomization problematic. Specifically, many patients and physicians were unwilling to consider less aggressive surgical management of the axilla. There is now strong evidence that axillary surgery is not therapeutic in patients with clinical T1-T2 N0 breast cancer, and the current goal of SLNB is limited to axillary staging. Demonstrating the feasibility of the randomized controlled trial design represents an important first step in evaluating AUS as a noninvasive alternative to SLNB.
The pilot phase of the randomized controlled trial also provides important prospective evidence confirming the performance characteristics of AUS for the exclusion of clinically significant disease. In Arm 2, the NPV of AUS for identification of any axillary disease was 90.6%, and the NPV for identification of clinically significant disease > 2.0 mm was 96.9%. These findings confirm the results of our retrospective study demonstrating the ability of AUS to accurately exclude clinically significant disease in the axilla [38]. In the pilot phase of the randomized controlled trial, the median size of a missed axillary metastasis was < 2.0 mm. This is consistent with our retrospective study and others demonstrating that patients with a false negative AUS typically have minimal disease in the axilla [38, 39]. Of note, micrometastatic disease in the axilla (disease < 2.0 mm) has almost no impact on prognosis and medical decision making [40-45]. Numerous studies have demonstrated that additional sections, immunohistochemistry (IHC), or molecular analysis of “negative” SLN will identify “occult” micrometastatic disease in a significant proportion of cases, and occult disease has minimal impact on clinical outcome. For example, a retrospective analysis of specimens obtained from NSABP B-32 identified occult metastases in 15.9% of cases when additional sections and IHC were performed [40]. Although the presence of occult metastases was associated with decreased overall survival, the difference was small (94.6% vs. 95.8%), and was not considered to be clinically significant. Given that micrometastatic disease in is not considered clinically relevant, current guidelines for the pathologic analysis of SLN are focused on identification of disease > 2.0 mm [46]. In this context, the relative inability of AUS to detect micrometastatic disease has questionable clinical significance.
In the pilot phase of the randomized controlled trial, there were 3 false negative AUS studies in Arm 2. Critical review of the adjuvant treatment recommendations in these patients suggests that the SLNB results did not alter medical decision making. Despite axillary disease identified by SLNB, these patients all had further evaluation with Oncotype DX testing at the request of the medical oncologist, and all were treated with endocrine therapy but not chemotherapy. These results highlight an important evolution in medical decision making. In the past, axillary staging was a key contributor to medical decision making. Presently, tumor biology as defined by biomarker profile (and gene expression profile in select cases) are increasingly used to not only better define a patient's risk of distant recurrence, but also to predict response to specific therapies, such as chemotherapy. Several large retrospective studies have used clinical specimens from randomized controlled trials to evaluate the utility of Oncotype DX in patients with node-negative and node-positive disease. Patients with ER+, node-negative disease and a low Oncotype DX recurrence score have a low risk of distant recurrence and receive little benefit from the addition of chemotherapy, whereas patients with a high recurrence score are significantly more likely to experience distant recurrence, and benefit from the addition of adjuvant chemotherapy [23, 24]. Similar results have been obtained in patients with ER+, node-positive disease [22]. These results have now been confirmed in prospective randomized controlled trials. The TAILORx trial enrolled ER+, node-negative breast cancer patients and randomized them to either endocrine therapy alone, or chemotherapy and endocrine therapy based on gene expression profile [21]. The results were recently published in the New England Journal of Medicine, dramatically confirming the value of Oncotype DX for medical decision making in node-negative patients [20]. Similar prospective studies are ongoing in node-positive disease, including the MINDACT and RxPONDER breast cancer trials [18, 19]. Although these prospective randomized controlled trials are ongoing, gene expression profiles are already being used to make adjuvant therapy recommendations, highlighting the decreasing importance of axillary staging. (Please note that current ASCO guidelines currently recommend against genomic testing in patients with node-positive breast cancer).
Radiation treatment planning may be influenced by the presence, absence, and/or extent of axillary disease. Specifically, the number of involved nodes may influence the selection of radiation fields. Patients who undergo BCT are typically treated with breast radiotherapy, which includes the lower portion of the axilla in many individuals. Axillary radiotherapy is typically directed at the axilla and the medial part of the supraclavicular fossa. Some radiation oncologists have proposed that radiation therapy may impact axillary recurrence rates in patients with low volume axillary disease [47]. In general, patients with four or more positive lymph nodes and selected patients with 1-3 positive nodes are treated with axillary and supraclavicular fields [48, 49]. However, in the post-ACOSOG Z0011 era, the exact extent of disease may not be available, as patients who have a positive SLNB may not undergo completion ALND. Further confusing this issue, the ACOSOG Z0011 study results suggest that axillary radiotherapy is not required for patients with low volume disease, although there is some concern about the validity of the ACOSOG Z0011 trial results, as the incidental coverage of level I and II axillary nodes seen with whole breast radiation may have contributed to the low axillary recurrence rate in the SLNB only arm (0.9%) [8, 9]. Jagsi et al. analyzed the Z0011 radiation fields to assess the extent of incidental axillary radiation received to determine if radiation treatment differed between the SLNB and completion ALND arms. Most patients did not receive axillary radiation, as outlined in the study protocol. Patients who had more lymph node disease were more likely to receive axillary radiation, but there was no significant difference in the use of prohibited nodal radiation fields between the two treatment groups [50]. Jagsi et al. concluded that patients with known SLN disease who do not undergo completion ALND should receive standard tangential radiation therapy but they were unable to make any definitive recommendations regarding axillary or supraclavicular-specific fields. Of note, the potential impact of a FN AUS on radiation oncology decision making has never been studied.
The performance characteristics of AUS vary widely in the literature [35, 51-56], and criteria for classifying ALN as positive or negative have not been clearly defined. Proposed criteria include cortical thickness, eccentric or focal cortical enlargement, lymph node shape, size, and echogenicity, and the presence or absence of a hyperechoic hilum. Bedi et al. demonstrated that focal cortical lobulation and/or hypoechoic appearance with absent hilum had the highest positive predictive value for the identification of metastatic disease [57]. We have adopted these criteria at our institution. Other studies have confirmed that criteria based on lymph node morphology improve the sensitivity and specificity of AUS compared to criteria based on size alone [34]. One limitation of the randomized controlled trial reported here is that radiologists at WUSM are specialized in breast imaging and have a particular interest in axillary staging; the sensitivity of AUS may be lower at other institutions or in the community setting.
Despite the success of our pilot trial we acknowledge that SLNB is currently considered an elegant, minimally invasive, and accurate procedure for axillary staging. In addition, the results of AUS are operator-dependent, and the excellent results that have been reported at select institutions with a specific interest in AUS may not be reproducible at all institutions. We believe that a phase 2 randomized controlled trial at select institutions with a special interest in the use of AUS for staging of the axilla in breast cancer is the most feasible and expeditious path forward at the present time. If the phase 2 trial confirms the performance characteristics of AUS and demonstrates the noninferiority of no further staging compared to SLNB, this will provide strong rationale for a larger, phase 3 trial in the context of an NCI cooperative group.
Conclusions
In summary, SLNB is an invasive surgical procedure, and represents an enormous investment in health care resources. Given that axillary surgery is not considered therapeutic in patients with clinical T1-T2 N0 breast cancer, and axillary staging information provided by SLNB is decreasing in importance, we believe that noninvasive alternatives to SLNB should be considered. AUS is relatively inexpensive, widely available, and demonstrates excellent performance characteristics for the exclusion of clinically significant disease. We have demonstrated the feasibility of a randomized controlled trial comparing no further axillary staging to SLNB in patients with clinical T1-T2 N0 breast cancer and negative AUS. Preliminary results provide prospective evidence confirming the performance characteristics of AUS. Taken together, the results provide strong rationale for a larger randomized controlled trial to rigorously evaluate the role of AUS in axillary staging.
ACKNOWLEDGEMENT
We thank the Longer Life Foundation for supporting this study. We thank Franco Basarabescu and Casey Rowe for their assistance with patient recruitment and study coordination, and Enid McIntosh for assistance with preparing the manuscript. We thank the breast imaging specialists at Washington University School of Medicine for their expertise performing axillary ultrasound. Specifically, Barbara Monsees, MD, Susan Holley, MD, PhD, Kimberly N Wiele, MD, Cheryl Herman, MD, Michelle Lee, MD, Valerie Reichert, MD, and Steven Poplack MD, PhD, all performed axillary ultrasound studies on patients included in this trial. Their dedication and expertise clearly contributed to the success of the trial.
Support: This study is funded by a grant from the Longer Life Foundation. Dr Tucker was supported by NCI grant T32 CA 009621. Dr Ademuyiwa was supported by an NCI grant 1K12 CA167540. Biostatistics services were provided by the Alvin J Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St Louis, MO. The Siteman Cancer Center is supported in part by NCI Cancer Center Support Grant #P30 CA91842.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the American Society of Breast Surgeons 16th Annual Meeting, Orlando, Florida, May 2015.
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Cyr is a paid consultant to Nanostring. Dr Margenthaler receives payment for lectures from Myriad Genetics and Genentech. Dr Appleton is a paid consultant to Hologic, Inc. and has provided expert trial testimony for Crivello Carlson law firm in Wisconsin, and Rensch and Rensch in Omaha, Nebraska.
REFERENCES
- 1.Fleissig A, Fallowfield LJ, Langridge CI, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat. 2006;95:279–293. doi: 10.1007/s10549-005-9025-7. [DOI] [PubMed] [Google Scholar]
- 2.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 3.Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102:111–118. doi: 10.1002/jso.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 5.Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13:491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 7.Land SR, Kopec JA, Julian TB, et al. Patient-reported outcomes in sentinel node-negative adjuvant breast cancer patients receiving sentinel-node biopsy or axillary dissection: National Surgical Adjuvant Breast and Bowel Project phase III protocol B-32. J Clin Oncol. 2010;28:3929–3936. doi: 10.1200/JCO.2010.28.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–432. doi: 10.1097/SLA.0b013e3181f08f32. discussion 432-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol. 2013;14:297–305. doi: 10.1016/S1470-2045(13)70035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cyr A, Gao F, Gillanders WE, et al. Disease recurrence in sentinel node-positive breast cancer patients forgoing axillary lymph node dissection. Ann Surg Oncol. 2012;19:3185–3191. doi: 10.1245/s10434-012-2547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fant JS, Grant MD, Knox SM, et al. Preliminary outcome analysis in patients with breast cancer and a positive sentinel lymph node who declined axillary dissection. Ann Surg Oncol. 2003;10:126–130. doi: 10.1245/aso.2003.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Hwang RF, Gonzalez-Angulo AM, Yi M, et al. Low locoregional failure rates in selected breast cancer patients with tumor-positive sentinel lymph nodes who do not undergo completion axillary dissection. Cancer. 2007;110:723–730. doi: 10.1002/cncr.22847. [DOI] [PubMed] [Google Scholar]
- 14.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27:2946–2953. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 15.Yi M, Giordano SH, Meric-Bernstam F, et al. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol. 2010;17(Suppl 3):343–351. doi: 10.1245/s10434-010-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milgrom S, Cody H, Tan L, et al. Characteristics and outcomes of sentinel node-positive breast cancer patients after total mastectomy without axillary-specific treatment. Ann Surg Oncol. 2012;19:3762–3770. doi: 10.1245/s10434-012-2386-3. [DOI] [PubMed] [Google Scholar]
- 17.Rayhanabad J, Yegiyants S, Putchakayala K, et al. Axillary recurrence is low in patients with breast cancer who do not undergo completion axillary lymph node dissection for micrometastases in sentinel lymph nodes. Am Surg. 2010;76:1088–1091. [PubMed] [Google Scholar]
- 18.Ramsey SD, Barlow WE, Gonzalez-Angulo AM, et al. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemp Clin Trials. 2013;34:1–9. doi: 10.1016/j.cct.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutgers E, Piccart-Gebhart MJ, Bogaerts J, et al. The EORTC 10041/BIG 03-04 MINDACT trial is feasible: results of the pilot phase. Eur J Cancer. 2011;47:2742–2749. doi: 10.1016/j.ejca.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Sparano JA, Gray RJ, Makower DF, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zujewski JA, Kamin L. Trial assessing individualized options for treatment for breast cancer: the TAILORx trial. Future Oncol. 2008;4:603–610. doi: 10.2217/14796694.4.5.603. [DOI] [PubMed] [Google Scholar]
- 22.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8:R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 25.Prat A, Parker JS, Fan C, et al. Concordance among gene expression-based predictors for ER-positive breast cancer treated with adjuvant tamoxifen. Ann Oncol. 2012;23:2866–2873. doi: 10.1093/annonc/mds080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo SS, Mumby PB, Norton J, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol. 2010;28:1671–1676. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 27.Mustonen P, Farin P, Kosunen O. Ultrasonographic detection of metastatic axillary lymph nodes in breast cancer. Ann Chir Gynaecol. 1990;79:15–18. [PubMed] [Google Scholar]
- 28.Tate JJ, Lewis V, Archer T, et al. Ultrasound detection of axillary lymph node metastases in breast cancer. Eur J Surg Oncol. 1989;15:139–141. [PubMed] [Google Scholar]
- 29.Yamashita M, Hovanessian-Larsen L, Sener SF. The role of axillary ultrasound in the detection of metastases from primary breast cancers. Am J Surg. 2013;205:242–244. doi: 10.1016/j.amjsurg.2012.10.009. discussion 244-245. [DOI] [PubMed] [Google Scholar]
- 30.Diepstraten SC, Sever AR, Buckens CF, et al. Value of preoperative ultrasound-guided axillary lymph node biopsy for preventing completion axillary lymph node dissection in breast cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2014;21:51–59. doi: 10.1245/s10434-013-3229-6. [DOI] [PubMed] [Google Scholar]
- 31.Mills P, Sever A, Weeks J, et al. Axillary ultrasound assessment in primary breast cancer: an audit of 653 cases. Breast J. 2010;16:460–463. doi: 10.1111/j.1524-4741.2010.00952.x. [DOI] [PubMed] [Google Scholar]
- 32.Park SH, Kim MJ, Park BW, et al. Impact of preoperative ultrasonography and fine-needle aspiration of axillary lymph nodes on surgical management of primary breast cancer. Ann Surg Oncol. 2011;18:738–744. doi: 10.1245/s10434-010-1347-y. [DOI] [PubMed] [Google Scholar]
- 33.Solon JG, Power C, Al-Azawi D, et al. Ultrasound-guided core biopsy: an effective method of detecting axillary nodal metastases. J Am Coll Surg. 2012;214:12–17. doi: 10.1016/j.jamcollsurg.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Cools-Lartigue J, Meterissian S. Accuracy of axillary ultrasound in the diagnosis of nodal metastasis in invasive breast cancer: a review. World J Surg. 2012;36:46–54. doi: 10.1007/s00268-011-1319-9. [DOI] [PubMed] [Google Scholar]
- 35.Houssami N, Ciatto S, Turner RM, et al. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann Surg. 2011;254:243–251. doi: 10.1097/SLA.0b013e31821f1564. [DOI] [PubMed] [Google Scholar]
- 36.Hieken TJ. The promise of axillary imaging in individualized surgical management of breast cancer patients: another step forward. Ann Surg Oncol. 2014;21:3369–3371. doi: 10.1245/s10434-014-3853-9. [DOI] [PubMed] [Google Scholar]
- 37.Verheuvel NC, van den Hoven I, Ooms HW, et al. The Role of Ultrasound-Guided Lymph Node Biopsy in Axillary Staging of Invasive Breast Cancer in the Post-ACOSOG Z0011 Trial Era. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-4071-1. [DOI] [PubMed] [Google Scholar]
- 38.Tucker NS, Cyr AE, Ademuyiwa FO, et al. Axillary ultrasound accurately excludes clinically significant lymph node disease in patients with early stage breast cancer. Ann Surg. 2015 doi: 10.1097/SLA.0000000000001549. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stachs A, Gode K, Hartmann S, et al. Accuracy of axillary ultrasound in preoperative nodal staging of breast cancer - size of metastases as limiting factor. Springerplus. 2013;2:350. doi: 10.1186/2193-1801-2-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weaver DL, Ashikaga T, Krag DN, et al. Effect of occult metastases on survival in node-negative breast cancer. N Engl J Med. 2011;364:412–421. doi: 10.1056/NEJMoa1008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giuliano AE, Hawes D, Ballman KV, et al. Association of occult metastases in sentinel lymph nodes and bone marrow with survival among women with early-stage invasive breast cancer. JAMA. 2011;306:385–393. doi: 10.1001/jama.2011.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cote RJ, Peterson HF, Chaiwun B, et al. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer. International Breast Cancer Study Group. Lancet. 1999;354:896–900. doi: 10.1016/s0140-6736(98)11104-2. [DOI] [PubMed] [Google Scholar]
- 43.Chen SL, Hoehne FM, Giuliano AE. The prognostic significance of micrometastases in breast cancer: a SEER population-based analysis. Ann Surg Oncol. 2007;14:3378–3384. doi: 10.1245/s10434-007-9513-6. [DOI] [PubMed] [Google Scholar]
- 44.de Boer M, van Deurzen CH, van Dijck JA, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med. 2009;361:653–663. doi: 10.1056/NEJMoa0904832. [DOI] [PubMed] [Google Scholar]
- 45.Tvedskov TF, Jensen MB, Balslev E, et al. Stage migration after introduction of sentinel lymph node dissection in breast cancer treatment in Denmark: a nationwide study. Eur J Cancer. 2011;47:872–878. doi: 10.1016/j.ejca.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 46.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haffty BG, Hunt KK, Harris JR, Buchholz TA. Positive sentinel nodes without axillary dissection: implications for the radiation oncologist. J Clin Oncol. 2011;29:4479–4481. doi: 10.1200/JCO.2011.36.1667. [DOI] [PubMed] [Google Scholar]
- 49.Caudle AS, Hunt KK, Kuerer HM, et al. Multidisciplinary considerations in the implementation of the findings from the American College of Surgeons Oncology Group (ACOSOG) Z0011 study: a practice-changing trial. Ann Surg Oncol. 2011;18:2407–2412. doi: 10.1245/s10434-011-1593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jagsi R, Chadha M, Moni J, et al. Radiation Field Design in the ACOSOG Z0011 (Alliance) Trial. J Clin Oncol. 2014;32:3600–3606. doi: 10.1200/JCO.2014.56.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bedrosian I, Bedi D, Kuerer HM, et al. Impact of clinicopathological factors on sensitivity of axillary ultrasonography in the detection of axillary nodal metastases in patients with breast cancer. Ann Surg Oncol. 2003;10:1025–1030. doi: 10.1245/aso.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 52.Deurloo EE, Tanis PJ, Gilhuijs KG, et al. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur J Cancer. 2003;39:1068–1073. doi: 10.1016/s0959-8049(02)00748-7. [DOI] [PubMed] [Google Scholar]
- 53.van Rijk MC, Deurloo EE, Nieweg OE, et al. Ultrasonography and fine-needle aspiration cytology can spare breast cancer patients unnecessary sentinel lymph node biopsy. Ann Surg Oncol. 2006;13:31–35. doi: 10.1245/ASO.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 54.Bonnema J, van Geel AN, van Ooijen B, et al. Ultrasound-guided aspiration biopsy for detection of nonpalpable axillary node metastases in breast cancer patients: new diagnostic method. World J Surg. 1997;21:270–274. doi: 10.1007/s002689900227. [DOI] [PubMed] [Google Scholar]
- 55.Yang WT, Ahuja A, Tang A, et al. High resolution sonographic detection of axillary lymph node metastases in breast cancer. J Ultrasound Med. 1996;15:241–246. doi: 10.7863/jum.1996.15.3.241. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez S, Anorbe E, Alcorta P, et al. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006;186:1342–1348. doi: 10.2214/AJR.05.0936. [DOI] [PubMed] [Google Scholar]
- 57.Bedi DG, Krishnamurthy R, Krishnamurthy S, et al. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: in vitro sonographic study. AJR Am J Roentgenol. 2008;191:646–652. doi: 10.2214/AJR.07.2460. [DOI] [PubMed] [Google Scholar]