Abstract
Ozone (O3)-related cardiorespiratory effects are a growing public health concern. Ground level O3 can exacerbate pre-existing respiratory conditions; however, research regarding therapeutic interventions to reduce O3-induced lung injury is limited. In patients with chronic obstructive pulmonary disease, hypoxia-associated pulmonary hypertension (HPH) is a frequent comorbidity that is difficult to treat clinically, yet associated with increased mortality and frequency of exacerbations. In this study, we hypothesized that established HPH would confer vulnerability to acute O3 pulmonary toxicity. Additionally, we tested whether improvement of pulmonary endothelial barrier integrity via rho-kinase inhibition could mitigate pulmonary inflammation and injury. To determine if O3 exacerbated HPH, male C57BL/6 mice were subject to either 3 wks continuous normoxia (20.9% O2) or hypoxia (10.0% O2), followed by a 4-h exposure to either 1 ppm O3 or filtered air (FA). As an additional experimental intervention fasudil (20mg/kg) was administered intraperitoneally prior to and after O3 exposures. As expected, hypoxia significantly increased right ventricular pressure and hypertrophy. O3 exposure in normoxic mice caused lung inflammation but not injury, as indicated by increased cellularity and edema in the lung. However, in hypoxic mice, O3 exposure led to increased inflammation and edema, along with a profound increase in airway hyperresponsiveness to methacholine. Fasudil administration resulted in reduced O3-induced lung injury via the enhancement of pulmonary endothelial barrier integrity. These results indicate that increased pulmonary vascular pressure may enhance lung injury, inflammation and edema when exposed to pollutants, and that enhancement of pulmonary endothelial barrier integrity may alleviate such vulnerability.
Keywords: Ozone, hypoxia, pulmonary hypertension, chronic obstructive pulmonary disease (COPD), fasudil, inflammation
INTRODUCTION
Chronic mild-to-moderate hypoxia-associated pulmonary arterial hypertension (HPH) occurs concurrently with pre-existing respiratory diseases, such as chronic obstructive pulmonary disease (COPD) (Christman et al., 1992). Clinical PAH is often defined as a sustained elevation of pulmonary arterial pressure ≥ 25 mmHg and prevalence can range dramatically depending on the severity of COPD (Chaouat et al., 2008). Chronic alveolar remodeling from cigarette smoking and environmental exposures leads to a decrease in gas exchange surface area and subsequent impairments in oxygenation (Tuder et al., 2007). Alveolar hypoxia, in turn, causes elevated pulmonary vascular resistance and pulmonary pressure (Jeffery, 2001). HPH is frequently a complication in COPD patients and is predictive of increased frequency and severity of exacerbations, poorer quality of life, and worse overall prognosis (Kessler et al., 1999; McGhan et al., 2007; Terzano et al., 2010; Wells et al., 2012).
Importantly, patients with COPD are at greater risk for hospital admission subsequent to exposure to airborne environmental insults, such as inhaled particulates or oxidant gases (Sunyer et al., 1993; Halonen et al., 2008). Long-term air pollution exposure and residential proximity to a busy roadway was associated with incidence of COPD in women (Schikowski et al., 2005). Additionally, multiple studies indicate that COPD-related emergency room visits increase in tandem with levels of air pollution (Sunyer et al., 1993; Anderson et al., 1997; Halonen et al., 2008). One study examining 94 patients in London found that outdoor air pollution was specifically associated with acute symptoms in COPD patients including dyspnea (Peacock et al., 2011). Unfortunately, few COPD patients report modifying their daily routine based on daily air pollution forecast, indicating alternative means to reduce environmental exacerbations are needed, aside from behavioral modifications (Dell et al., 2015).
Treatment of elevated pulmonary pressure with classical vasoactive drugs such as sildenafil, bosentan, or prostenoids is complicated by the deterioration of gas exchange in the deficient COPD lung (Stolz et al., 2008; Blanco et al., 2010; Seeger et al., 2013), however other aspects of elevated pulmonary vascular pressure, such as the risk of vascular leakage, might be valuable therapeutic targets. Sildenafil, in particular, inhibits phosphodiesterase-5, which in turn increases levels of cGMP thereby promoting smooth muscle relaxation and vasodilation. However, this potent vasodilator is ineffective in the COPD lung as its dilatory effects tend to decrease ventilation-perfusion matching and ultimately decrease gas exchange. Inhibition of the RhoA/Rho Kinase pathway has been shown to improve endothelial barrier integrity and is protective in models of vascular injury and inflammation (Gibson et al., 2014). Fasudil hydrochloride is a potent Rho-kinase inhibitor that has been clinically developed to treat pulmonary hypertension (Ishikura et al., 2006; Fujita et al., 2010) as well as improve blood brain barrier permeability by protection of tight junction proteins and ROCK inhibition (Fujii et al., 2012).
Therefore, this study was implemented to examine whether HPH contributes to exacerbated responses to ozone (O3), which is a ground-level airborne pollutant formed when substrates such as volatile organic compounds and nitrogen dioxide, mostly from anthropogenic sources, interact with ultraviolet light. O3 remains a major global concern with a demonstrated cardiopulmonary health impact, especially in COPD patients and has been associated specifically with exacerbations in COPD (Desqueyroux et al., 2002; Malig et al., 2015). Additionally, airway hyperreactivity has been implicated following O3 exposure, in part due to irritation of airway nerves (Fabbri et al., 1984; Kasahara et al., 2015; Williams et al., 2015). However, it remains unknown whether elevated pulmonary vascular pressure contributes to worsened outcomes related to environmental exposures.
MATERIALS AND METHODS
Animals
C57BL/6 mice (male, 6–8 weeks old at beginning of studies; Harlan Laboratories, Indianapolis, IN) were housed four per cage and allowed to acclimate over the course of two weeks after their arrival at UNM under AAALAC housing conditions. Mice were subject to a light/dark cycle of 12-h and had access to water and standard chow ad libitum (Harlan). Mice were euthanized via cardiac exsanguination while under isoflurane anesthesia. Sacrifice of animals was staggered due to space limitations of the hypoxia chamber. All procedures performed were approved by the UNM Institutional Animal Care and Use Committee (IACUC).
Exposures
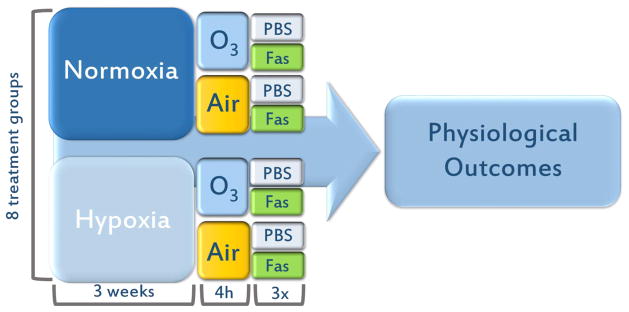
Mice were subject to acute hypoxia (10.0% O2) or normoxia (20.9% O2) 24 h a day for 3 wks, followed by a single exposure to O3. Hypoxia (10% O2) was monitored continuously in the exposure chambers and remained at 10% for 3 weeks. Food and water was changed on an as needed basis and cages, including bedding was changed once per week. An OREC silent arc discharge O3 generator (Osmonics, Phoenix, AZ) was used to expose rodents to O3, and the concentration was continuously monitored over the course of 4h; all terminal endpoints were obtained 18–20 h following the end of this O3 exposure. Water was available for mice during the exposure, but food was withheld. Exposures took place in a chamber without bedding to prevent O3 scavenging. The initial study involved four treatment groups: normoxia then filtered air (Nx,FA), normoxia then O3 (Nx,O3), hypoxia then filtered air (Hx,FA), hypoxia then O3 (Hx,O3). In a second study, fasudil or PBS were intraperitoneally injected at 20mg/kg at 3 timepoints: once before O3 exposure, once after O3 exposure, and once the following day before euthanasia (Fig. 1). A total of 8 treatment groups received either fasudil or PBS: Nx,FA,Fas; Nx,O3,Fas; Hx,FA,Fas; Hx,O3,Fas or Nx,FA,PBS; Nx,O3,PBS; Hx,FA,PBS; Hx,O3,PBS.
Figure 1. Study Design.
C57BL/6 mice (n=6–10 per treatment group) received either 3 wks of or FA. Mice were injected with either continuous normoxia or hypoxia followed by 4 h 1ppm O3 exposure. Data were subject Fasudil (20mg/kg) or PBS (vehicle) before, after and 24 h post-O3 to a two-way ANOVA statistical analysis and expressed as a mean +/− SEM.
Bronchoalveolar lavage fluid collection and cell counts
Immediately after euthanasia, a cannula was inserted into the trachea and lungs were lavaged with 1 mL phosphate buffered saline solution (PBS). Brochoalveolar lavage fluid (BAL) was centrifuged and pelleted cells were resuspended in PBS. Total cell counts, which included macrophages, neutrophils, lymphocytes, eosinophils, and basophils, were analyzed in triplicate using a hemocytometer and trypan blue staining.
Lung resistance and methacholine reactivity measurement
Twenty-four hours after O3 exposure, airway resistance was monitored using a methacholine (MCh) challenge using the FlexiVent system (SCIREQ, Montreal, Quebec, Canada). While under isofluorane anesthesia, a 20-gauge needle was inserted into tracheal incision. The cannula was then inserted on the Flexivent system and the mouse was artificially ventilated, as previously described (Mishra et al., 2008). Lung resistance was determined at baseline and after dose-response challenges with nebulized MCh (0–50 mg/ml) to assess airway hyperreactivity (AHR). Mice used for airway reactivity measurement were not used for measurements of lung weights, BAL, and RV pressure.
Right ventricular pressure
Right ventricular pressure measurements were obtained using saline-filled catheters connected to pressure transducers (Cobe, Lakewood, CO) in an artificially ventilated, open-chest model, as previously described, with n=4–8 per treatment group (Paffett et al., 2012a; Paffett et al., 2012b). Continuous hemodynamic traces were recorded (AD Instruments) and right ventricular systolic pressure (RVSP) and right ventricular mean pressure (RVMP) were calculated from a stable recording of 20–30 seconds for each subject.
Right ventricular hypertrophy and lung weights
The heart and lung block was dissected and removed from the chest cavity following cardiac exsanguination. Wet lung weight was recorded and lungs were dried for 48 h at room temperature according to a previously established protocol (Campen et al., 2005; Lund et al., 2009). After drying, lung weights were recorded again and total lung water, as an index of edema, was calculated as the difference between wet and dry weights. The right ventricle and the left ventricle + septum were dissected under a light microscope and weighed immediately to assess the presence of right ventricular hypertrophy. Fulton’s index was calculated (Fulton’s index = RV weight/LVS weight) to assess right ventricular cardiac remodeling subsequent to hypoxia.
Statistics
All statistics for terminal endpoints were computed using an ANOVA with a Holm-Sidak post-hoc test. Airway hyperreactivity responses were assessed with a repeated measures 2-factor (exposure condition, MCh concentration) with Tukey’s multiple comparison test. Resulting p-values ≤ 0.05 were considered significant. GraphPad Prism software (Montreal, Quebec, Canada) was utilized for all statistical analyses.
RESULTS
Chronic hypoxia induces RV hypertrophy, which is not altered by acute O3 exposure
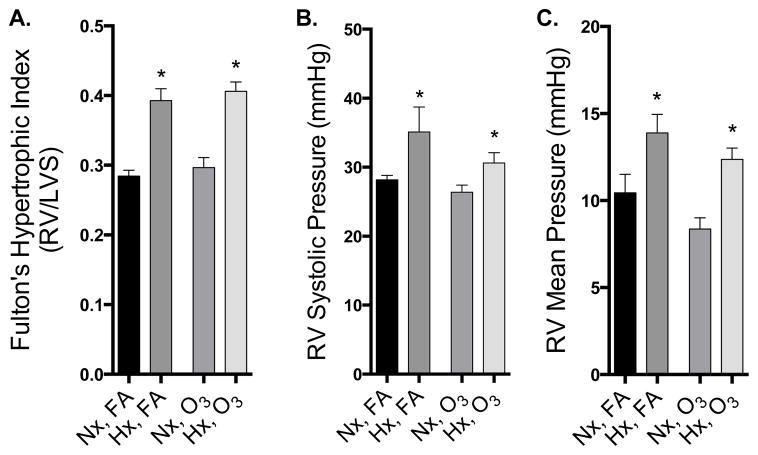
Mice were sacrificed 18–20 h following the end of O3 exposure, and 22–24 h following the end of the hypoxia exposure. At that time, Fulton’s index indicated substantial RV remodeling with a significantly higher RV/LVS ratio in the hypoxia-exposed groups (Fig. 2A). Additionally, significantly elevated RVSP and RVMP in hypoxia-treated mice compared to normoxic controls were noted, as indicated by a 2-factor ANOVA (Fig. 2B, C). Acute O3 exposure had no impact on RV remodeling or pressure in this model.
Figure 2. Right ventricular remodeling and RV pressure are elevated following 3 weeks of hypoxia, but not affected by O3.
A. Right ventricular remodeling in mice after 3 wks hypoxia (N=11–12/group). B. Right ventricular systolic pressure measured 24 h after cessation of hypoxia treatment (N=4–8/group). C. Right ventricular mean pressure measured 24 h after cessation of hypoxia treatment (N=4–8/group). Data were subject to a two-way ANOVA statistical analysis with Newman-Keuls posthoc test and expressed as a mean +/− SEM. Asterisks (*) indicate a significant effect of hypoxia treatment, regardless of O3 exposure.
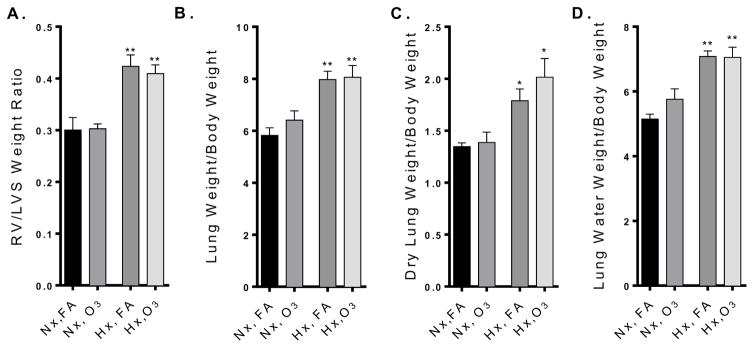
Ozone significantly exacerbates lung injury and inflammation
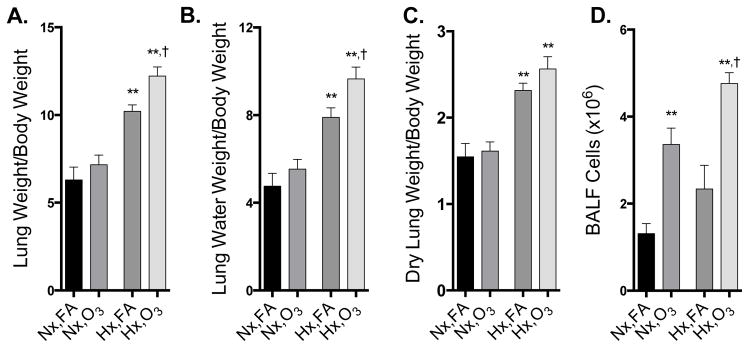
In normoxic mice, O3 did not lead to significant changes in lung weight or lung water weight, despite a clear increase in inflammatory cells in the BALF (Fig 3). Hypoxia alone significantly increased lung weight/body weight, lung water weight/body weight, and lung dry weight/body weight, but had no independent effect on BALF cell counts (Fig. 3). In hypoxic mice, O3 exposure resulted in significant elevation of lung weight/body weight, lung water/body weight and total BALF cells, although did not alter the dry lung weight/body weight ratio (Fig. 3).
Figure 3. Ozone-induced lung injury is exacerbated in mice with HPH.
A) Lung weight normalized to body weight B) Lung water weight normalized to body weight C) Dry lung weight normalized to body weight, and D) BALF inflammatory cell counts (n=5–6 per group). Group data were compared by an ANOVA with a Holm-Sidak post-hoc test and expressed as a mean +/− SEM. Asterisks (*) indicate difference from normoxia controls (*, p<0.05; **, p<0.01); crosses (†) indicates significant enhancement of effect by the hypoxia-O3 combination compared to hypoxia or ozone alone (p<0.05).
Lung resistance is increased with combined hypoxia and O3 exposure
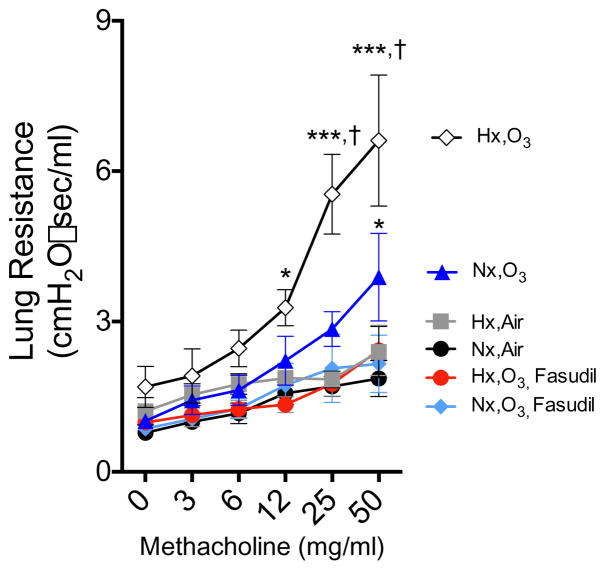
Hypoxia did not cause significant increases in airway hyperreactivity to MCh challenge (Fig. 4). O3 exposure in normoxic mice did induce a significant enhancement in resistance responses to MCh challenge. However, this effect was further potentiated in mice exposed to both hypoxia and O3 exposure, with the combined exposure resulting in a statistically significant increase in lung resistance compared to O3 alone. Treatment with fasudil was able to completely abrogate this hyperreactivity response in both HxO3 and NxO3 treatment groups.
Fig. 4. Airway hyperreactivity significantly increased with combined hypoxia and O3 after methacholine challenge.
Lung resistance after sequential methacholine administration (0–50 mg/mL). Data were subject to a two-way ANOVA statistical analysis with a Tukey’s multiple comparison test and expressed as a mean +/− SEM (n=6–8 per treatment group). Asterisks (*) indicate difference from normoxia-air controls (*, p<0.05; **, p<0.01; ***, p<0.001); crosses (†) indicates significant enhancement of effect by the hypoxia-O3 combination compared to O3 alone (p<0.05).
Fasudil intervention mitigates lung injury
In addition to blocking hyperreactivity changes due to O3 and hypoxia-O3 exposure, fasudil (20 mg/kg) i.p. administration resulted in mitigation of O3-induced lung injury (Fig. 5). Fasudil treatment followed the 3-week hypoxia regimen, and so did not alter the development of pulmonary hypertension in these mice, as indicated by RV/LVS ratios (Fig 5A). While hypoxia resulted in a significant increase in lung weight/ body weight, dry lung weight/ body weight and lung water/ body weight (Fig. 5B–D), there was no significant alteration of these parameters by O3 exposure.
Figure 5. Fasudil intervention significantly attenuates HPH-dependent ozone-induced lung injury.
A) RV/LVS weight ratio, B) lung weight normalized to body weight, C) dry lung weight normalized to body weight, and D) lung water weight normalized to body weight in mice administered a 20mg/mL i.p. Fasudil intervention after hypoxia, but before O3 exposure. Only the 3-week hypoxia treatment induced changes to any parameter, with O3 effects completely abrogated by the fasudil treatment. Group data were compared by an ANOVA with a Holm-Sidak post-hoc test and expressed as a mean +/− SEM. Asterisks (*) indicate difference from normoxia controls (*, p<0.05; **, p<0.01; N=4–6 per group).
DISCUSSION
O3-induced lung injury was exacerbated in the presence of a moderate pulmonary hypertension induced by 3 weeks of hypoxia in a mouse model. The presence of HPH led to enhanced O3 effects in terms of airway hyperresponsiveness and lung inflammation and edema. Additionally, i.p. injection with low-dose fasudil, administered after HPH had developed, mitigated lung injury by O3. While this study is a preliminary investigation into the relationship between HPH and environmental exposures, the results are consistent with a potential link to pollution-induced exacerbations in COPD patients with associated pulmonary hypertension.
Respiratory effects of O3 in mice are well-characterized, however, vascular pathology, including pulmonary hypertensive effects from O3 exposure has not been explored (Bauer and Kleeberger, 2010). Particulate matter air pollution has been studied in a limited manner in more severe models of pulmonary hypertension, but with similar findings of exacerbated outcomes. Gardner and colleagues explored interactions between monocrotaline-induced pulmonary hypertension and bolus intratracheal exposures to a residual oil fly ash particulate and specifically noted an interaction in terms of vascular inflammation in histological analysis (Gardner et al., 2004). In a similar study, rats treated with monocrotaline exhibited greater bradycardic, arrhythmic, and hypothermic responses to residual oil fly ash instillation compared to healthy rats (Campen et al., 2000), along with exacerbated lung injury outcomes (Kodavanti et al., 1999). However, in all f these studies, the time frame for monocrotaline-related treatment was likely insufficient to drive significant pulmonary hypertension (<14 days post injection) and still in the inflammatory phase of the pathology. Collectively, these studies did not focus on characterizing the pulmonary vascular hemodynamics and in only one study was RV remodeling reported (Campen et al., 2000).
Based on the findings of the present study, we propose that pulmonary hypertension in COPD patients may be a crucial factor conferring vulnerability to environmental pollutants. Pulmonary hypertension in COPD patients can range from mild to severe (Ppa<40mmHg) (Weitzenblum and Chaouat, 2005), and both prevalence and severity of pulmonary hypertension are associated with the severity of COPD. Most COPD patients exhibit mild-to-moderate increases in Ppa concomitant with decreases in lung function, but there are also a significant minority of COPD patients that exhibit more profound Ppa increases that may relate to arterial-alveolar oxygen gradients of simply other etiological causes of pulmonary hypertension (Thabut et al., 2005; Chaouat et al., 2008).
COPD exacerbations may be driven by a host of causes including viruses, bacteria, cigarette smoke, air pollution and pre-existing genetic and physiological factors (Sapey and Stockley, 2006). Air pollution exposure and exacerbation of COPD symptoms has been well documented in several studies (Sunyer et al., 1993; Anderson et al., 1997; Halonen et al., 2008), but pulmonary arterial hypertension has not specifically been addressed as a predisposing factor. Given that pulmonary hypertension is a relatively common comorbid condition in COPD and that several rat studies with a more severe pulmonary hypertension model (monocrotaline) suggest an interaction Campen et al., 2000; Kodavanti et al., 1999), our findings in a model of mild HPH offer strong support for pulmonary vascular pressure having an important role in determining the outcomes of environmental exposures in COPD patients.
Under hypoxic conditions (such as in COPD), blood flow to the upper respiratory zones increases due to vasoconstriction and elevated Ppa to improve the ventilation-perfusion ratio (V/Q) (Galvin et al., 2007). Therefore, clinically, PAH in COPD patients is often not treated because vasodilators may antagonize V/Q benefits and subsequently incur hypoxemia (Galiè et al., 2009). Furthermore, there are currently no pharmacological treatments indicated to oppose potential action of environmental pollutants in COPD patients. Because of these issues, we tested a pharmacological agent known to improve endothelial barrier integrity, fasudil, in terms of its ability to attenuate O3 inflammation and edema in the HPH model. Both Rac1 and RhoA of the Rho-kinase pathway are involved in endothelial cytoskeletal changes and are vital to endothelial barrier integrity in opposition to intracellular tethering forces (Wojciak-Stothard and Ridley, 2002). Activation of RhoA/Rho can cause changes in gene expression for over 300 cytoskeletal proteins involved in microfilament building, actomyosin structure, microfilament structure and endothelial cytoskeletal reorganization (Hall, 1998).
Attenuation of pulmonary lung injury has been demonstrated using the Rho kinase inhibitor fasudil as an intervention. Tasaka and colleagues found that intravenous injection of LPS from E.coli induced lung injury in mice and that fasudil pretreatment attenuated endothelial cytoskeletal rearrangements, resulting in decreased neutrophil infiltration (Tasaka et al., 2005). Still others that have used LPS to induce lung injury have found that fasudil decreases early stage sepsis and systemic inflammation by decreasing leukocyte transmigration and restoring endothelial barrier integrity (Ding et al., 2011). In an intestinal ischemia-reperfusion model, fasudil was found to mitigate lung injury, which is often a consequence of systemic shock (Li et al., 2011). Fasudil has also demonstrated improvement in stroke outcomes by improving cerebrovascular, endothelial cell barrier integrity (Satoh et al., 2001; Shibuya et al., 2005). Additionally, while we posit that fasudil benefits on reducing O3-induced airway hyperreactivity relate to the protection from lung edema and injury, fasudil may also relax airways through the rho-kinase pathway in airway smooth muscle (Gosens et al., 2004; Schaafsma et al., 2004). Regardless, considering that ambient air pollution exacerbates COPD symptoms, this study is the first PAH mouse model to establish that O3-induced lung injury may be prevented with prophylactic fasudil treatment.
However, inhibition of Rho kinase may also have limited the degree of pulmonary hypertension during O3 exposure in our model, which limits the strength of our conclusions related to endothelial barrier integrity. Because we were not able to continuously measure pulmonary arterial pressure throughout the 24h period between the onset of O3 exposure and the time of sacrifice, it is unclear if vascular pressure was reduced by fasudil in a meaningful way. Execution of such continuous pulmonary hemodynamic measurements is exceedingly challenging and infrequently reported due to technical issues (Campen et al., 2005; Schwenke et al., 2006); with the additional logistics of O3 exposure, continuous pulmonary hemodynamics monitoring was considered unworkable for the present study. In clinical studies, acute administration of fasudil has also been used to treat severe PAH patients (Fukumoto et al., 2005). Using intravenous administration of fasudil hydrochloride (30 mg for 30 min), it was found that mean pulmonary vascular resistance significantly decreased, while pulmonary artery pressure did not change in humans. High dose and/or long-term use of fasudil has been used to treat pulmonary hypertension in monocrotaline rodent models and, at similar concentrations to the present study, fasudil did reduce rat pulmonary arterial pressure; however, we estimate from their pharmacodynamics data that the effect in our model of 3 injections would amount to only a 10% mean reduction in pulmonary pressure over the 24h period (Jiang et al., 2007). Additionally, acute fasudil administration has clinically attenuated pulmonary pressure in PAH patients (Abe et al., 2006; Ishikura et al., 2006; Fujita et al., 2010), however in our study, we did not find a significant change in pressure after fasudil administration, despite confirmation of elevated right ventricular pressure in hypoxic mice. This may have been due to the timeframe that the pressure was obtained (24 h after the end of hypoxia). Future studies will focus on obtaining pressure immediately after the end of hypoxia.
CONCLUSIONS
The hypoxia-induced pulmonary hypertension model led to a clear exacerbation of O3-related lung injury, consistent with the concept that elevated vascular pressures may promote edema and inflammation following oxidative lung injury. This study demonstrated that Rho kinase inhibition via fasudil may be a viable therapy to attenuate lung injury by enhancing endothelial barrier integrity and/or through the reduction in pulmonary pressure. Further studies are needed to determine if the results of this study may have clinical parallels in at-risk populations, such as COPD patients. Additionally, clinically-relevant therapies such as inhaled fasudil could be examined using this model of O3 and HPH (Nagaoka et al., 2005; Fujita et al., 2010).
Highlights.
Environmental exposures can exacerbate chronic obstructive pulmonary disease (COPD)
It is unknown if comorbid pulmonary hypertension may influence such effects in COPD patients
Pulmonary hypertension in a mouse model significantly exacerbated ozone-induced lung injury
Adverse ozone outcomes were largely attenuated by a rho kinase inhibitor, fasudil
Therapeutic benefit from rho kinase inhibition may be related to endothelial barrier integrity
Acknowledgments
This study was supported by the National Institutes of Health (ES014639), the Environmental Protection Agency (RD-83479601-0). Additionally, Katherine E. Zychowski received support from the Academic Science Education and Research Training (ASERT) program (K12GM088021).
Footnotes
Conflicts of Interest Statement
The authors declare no conflicts of interest with the contents of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Tawara S, Oi K, Hizume T, Uwatoku T, Fukumoto Y, Kaibuchi K, Shimokawa H. Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. Journal of cardiovascular pharmacology. 2006;48:280–285. doi: 10.1097/01.fjc.0000248244.64430.4a. [DOI] [PubMed] [Google Scholar]
- Anderson HR, Spix C, Medina S, Schouten JP, Castellsague J, Rossi G, Zmirou D, Touloumi G, Wojtyniak B, Ponka A. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. European respiratory journal. 1997;10:1064–1071. doi: 10.1183/09031936.97.10051064. [DOI] [PubMed] [Google Scholar]
- Bauer AK, Kleeberger SR. Genetic mechanisms of susceptibility to ozone-induced lung disease. Ann N Y Acad Sci. 2010;1203:113–119. doi: 10.1111/j.1749-6632.2010.05606.x. [DOI] [PubMed] [Google Scholar]
- Blanco I, Gimeno E, Munoz PA, Pizarro S, Gistau C, Rodriguez-Roisin R, Roca J, Barberà JA. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. American journal of respiratory and critical care medicine. 2010;181:270–278. doi: 10.1164/rccm.200907-0988OC. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Costa DL, Watkinson WP. Cardiac and Thermoregulatory Toxicity of Residual Oil Fly Ash in Cardiopulmonary-Compromised Rats. Inhal Toxicol. 2000;12(Suppl 2):7–22. doi: 10.1080/08958378.2000.11463196. [DOI] [PubMed] [Google Scholar]
- Campen MJ, Shimoda LA, O’Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol. 2005;99:2028–2035. doi: 10.1152/japplphysiol.00411.2005. [DOI] [PubMed] [Google Scholar]
- Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. European Respiratory Journal. 2008;32:1371–1385. doi: 10.1183/09031936.00015608. [DOI] [PubMed] [Google Scholar]
- Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. New England Journal of Medicine. 1992;327:70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- Dell SDM, Gershon AS, Chen H, To T, Foty RG. The Reported Perceptions And Behaviours Of Canadians With COPD Towards The Health Effects Of Air Pollution. Am J Respir Crit Care Med. 2015;191:A2296. [Google Scholar]
- Desqueyroux H, Pujet JC, Prosper M, Le Moullec Y, Momas I. Effects of air pollution on adults with chronic obstructive pulmonary disease. Archives of environmental health. 2002;57:554–560. doi: 10.1080/00039890209602088. [DOI] [PubMed] [Google Scholar]
- Ding RY, Zhao DM, Zhang ZD, Guo RX, Ma XC. Pretreatment of Rho kinase inhibitor inhibits systemic inflammation and prevents endotoxin-induced acute lung injury in mice. Journal of Surgical Research. 2011;171:e209–e214. doi: 10.1016/j.jss.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Fabbri LM, Aizawa H, Alpert SE, Walters EH, O’Byrne PM, Gold BD, Nadel JA, Holtzman MJ. Airway hyperresponsiveness and changes in cell counts in bronchoalveolar lavage after ozone exposure in dogs. The American review of respiratory disease. 1984;129:288–291. [PubMed] [Google Scholar]
- Fujii M, Duris K, Altay O, Soejima Y, Sherchan P, Zhang JH. Inhibition of Rho kinase by hydroxyfasudil attenuates brain edema after subarachnoid hemorrhage in rats. Neurochemistry international. 2012;60:327–333. doi: 10.1016/j.neuint.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Fukumoto Y, Saji K, Sugimura K, Demachi J, Nawata J, Shimokawa H. Acute vasodilator effects of inhaled fasudil, a specific Rho-kinase inhibitor, in patients with pulmonary arterial hypertension. Heart and vessels. 2010;25:144–149. doi: 10.1007/s00380-009-1176-8. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, Abe K, Takeshita A, Shimokawa H. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91:391–392. doi: 10.1136/hrt.2003.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS. Guidelines for the diagnosis and treatment of pulmonary hypertension. European heart journal. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- Galvin I, Drummond GB, Nirmalan M. Distribution of blood flow and ventilation in the lung: gravity is not the only factor. British journal of anaesthesia. 2007;98:420–428. doi: 10.1093/bja/aem036. [DOI] [PubMed] [Google Scholar]
- Gardner SY, McGee JK, Kodavanti UP, Ledbetter A, Everitt JI, Winsett DW, Doerfler DL, Costa DL. Emission-particle-induced ventilatory abnormalities in a rat model of pulmonary hypertension. Environ Health Perspect. 2004;112:872–878. doi: 10.1289/ehp.6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CL, Srivastava K, Sprigg N, Bath PM, Bayraktutan U. Inhibition of Rho-kinase protects cerebral barrier from ischaemia-evoked injury through modulations of endothelial cell oxidative stress and tight junctions. J Neurochem. 2014;129:816–826. doi: 10.1111/jnc.12681. [DOI] [PubMed] [Google Scholar]
- Gosens R, Schaafsma D, Bromhaar MMG, Vrugt B, Zaagsma J, Meurs H, Nelemans SA. Growth factor-induced contraction of human bronchial smooth muscle is Rho-kinase-dependent. European journal of pharmacology. 2004;494:73–76. doi: 10.1016/j.ejphar.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Halonen JI, Lanki T, Yli-Tuomi T, Kulmala M, Tiittanen P, Pekkanen J. Urban air pollution and asthma and COPD hospital emergency room visits. Thorax. 2008 doi: 10.1136/thx.2007.091371. [DOI] [PubMed] [Google Scholar]
- Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, Nakano T. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circulation Journal. 2006;70:174–178. doi: 10.1253/circj.70.174. [DOI] [PubMed] [Google Scholar]
- Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. American journal of respiratory and critical care medicine. 2001;164:S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Tawara S, Abe K, Takaki A, Fukumoto Y, Shimokawa H. Acute vasodilator effect of fasudil, a Rho-kinase inhibitor, in monocrotaline-induced pulmonary hypertension in rats. Journal of cardiovascular pharmacology. 2007;49:85–89. doi: 10.1097/FJC.0b013e31802df112. [DOI] [PubMed] [Google Scholar]
- Kasahara DI, Mathews JA, Park CY, Cho Y, Hunt G, Wurmbrand AP, Liao JK, Shore SA. ROCK insufficiency attenuates ozone-induced airway hyperresponsiveness in mice. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2015;309:L736–L746. doi: 10.1152/ajplung.00372.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 1999;159:158–164. doi: 10.1164/ajrccm.159.1.9803117. [DOI] [PubMed] [Google Scholar]
- Kodavanti UP, Jackson MC, Ledbetter AD, Richards JR, Gardner SY, Watkinson WP, Campen MJ, Costa DL. Lung injury from intratracheal and inhalation exposures to residual oil fly ash in a rat model of monocrotaline-induced pulmonary hypertension. J Toxicol Environ Health A. 1999;57:543–563. doi: 10.1080/009841099157502. [DOI] [PubMed] [Google Scholar]
- Li Y, Yao JH, Hu XW, Fan Z, Huang L, Jing HR, Liu KX, Tian XF. Inhibition of Rho kinase by fasudil hydrochloride attenuates lung injury induced by intestinal ischemia and reperfusion. Life sciences. 2011;88:104–109. doi: 10.1016/j.lfs.2010.10.028. [DOI] [PubMed] [Google Scholar]
- Lund AK, Lucero J, Lucas S, Madden MC, McDonald JD, Seagrave JC, Knuckles TL, Campen MJ. Vehicular Emissions Induce Vascular MMP-9 Expression and Activity Associated With Endothelin-1–Mediated Pathways. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:511–517. doi: 10.1161/ATVBAHA.108.176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malig BJ, Pearson DL, Chang YB, Broadwin R, Basu R, Green RS, Ostro B. A Time-Stratified Case-Crossover Study of Ambient Ozone Exposure and Emergency Department Visits for Specific Respiratory Diagnoses in California (2005–2008) Environ Health Perspect. 2015 doi: 10.1289/ehp.1409495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. CHEST Journal. 2007;132:1748–1755. doi: 10.1378/chest.06-3018. [DOI] [PubMed] [Google Scholar]
- Mishra NC, Langley RJ, Singh SP, Peña-Philippides JC, Koga T, Razani-Boroujerdi S, Hutt J, Campen M, Kim KC, Tesfaigzi Y. Nicotine primarily suppresses lung Th2 but not goblet cell and muscle cell responses to allergens. The Journal of Immunology. 2008;180:7655–7663. doi: 10.4049/jimmunol.180.11.7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, McMurtry IF, Oka M. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. American journal of respiratory and critical care medicine. 2005;171:494–499. doi: 10.1164/rccm.200405-637OC. [DOI] [PubMed] [Google Scholar]
- Paffett ML, Channell MM, Naik JS, Lucas SN, Campen MJ. Cardiac and vascular atrogin-1 mRNA expression is not associated with dexamethasone efficacy in the monocrotaline model of pulmonary hypertension. Cardiovascular toxicology. 2012a;12:226–234. doi: 10.1007/s12012-012-9158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffett ML, Hesterman J, Candelaria G, Lucas S, Anderson T, Irwin D, Hoppin J, Norenberg J, Campen MJ. Longitudinal in vivo SPECT/CT imaging reveals morphological changes and cardiopulmonary apoptosis in a rodent model of pulmonary arterial hypertension. PloS one. 2012b;7:e40910. doi: 10.1371/journal.pone.0040910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock JL, Anderson HR, Bremner SA, Marston L, Seemungal TA, Strachan DP, Wedzicha JA. Outdoor air pollution and respiratory health in patients with COPD. Thorax. 2011 doi: 10.1136/thx.2010.155358. thx-2010. [DOI] [PubMed] [Google Scholar]
- Sapey E, Stockley RA. COPD exacerbations· 2: Aetiology. Thorax. 2006;61:250–258. doi: 10.1136/thx.2005.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh SI, Utsunomiya T, Tsurui K, Kobayashi T, Ikegaki I, Sasaki Y, Asano T. Pharmacological profile of hydroxy fasudil as a selective rho kinase inhibitor on ischemic brain damage. Life sciences. 2001;69:1441–1453. doi: 10.1016/s0024-3205(01)01229-2. [DOI] [PubMed] [Google Scholar]
- Schaafsma D, Gosens R, Bos IST, Meurs H, Zaagsma J, Nelemans SA. Allergic sensitization enhances the contribution of Rho-kinase to airway smooth muscle contraction. British journal of pharmacology. 2004;143:477–484. doi: 10.1038/sj.bjp.0705903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikowski T, Sugiri D, Ranft U, Gehring U, Heinrich J, Wichmann HE, Kramer U. Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir Res. 2005;6:152. doi: 10.1186/1465-9921-6-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenke DO, Pearson JT, Mori H, Shirai M. Long-term monitoring of pulmonary arterial pressure in conscious, unrestrained mice. J Pharmacol Toxicol Methods. 2006;53:277–283. doi: 10.1016/j.vascn.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Seeger W, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galiè N, Ghio S, Gibbs S. Pulmonary hypertension in chronic lung diseases. Journal of the American College of Cardiology. 2013:62. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Hirai S, Seto M, Satoh S-i, Ohtomo E Fasudil Ischemic Stroke Study G. Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. Journal of the neurological sciences. 2005;238:31–39. doi: 10.1016/j.jns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Stolz D, Rasch H, Linka A, Di Valentino M, Meyer A, Brutsche M, Tamm M. A randomised, controlled trial of bosentan in severe COPD. European Respiratory Journal. 2008;32:619–628. doi: 10.1183/09031936.00011308. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Saez M, Murillo C, Castellsague J, Martinez F, Anto JM. Air pollution and emergency room admissions for chronic obstructive pulmonary disease: a 5-year study. American journal of epidemiology. 1993;137:701–705. doi: 10.1093/oxfordjournals.aje.a116730. [DOI] [PubMed] [Google Scholar]
- Tasaka S, Koh H, Yamada W, Shimizu M, Ogawa Y, Hasegawa N, Yamaguchi K, Ishii Y, Richer SE, Doerschuk CM. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. American journal of respiratory cell and molecular biology. 2005;32:504–510. doi: 10.1165/rcmb.2004-0009OC. [DOI] [PubMed] [Google Scholar]
- Terzano C, Conti V, Di Stefano F, Petroianni A, Ceccarelli D, Graziani E, Mariotta S, Ricci A, Vitarelli A, Puglisi G. Comorbidity, hospitalization, and mortality in COPD: results from a longitudinal study. Lung. 2010;188:321–329. doi: 10.1007/s00408-009-9222-y. [DOI] [PubMed] [Google Scholar]
- Thabut G, Dauriat G, Stern JB, Logeart D, Levy A, Marrash-Chahla R, Mal H. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127:1531–1536. doi: 10.1378/chest.127.5.1531. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Yun JH, Bhunia A, Fijalkowska I. Hypoxia and chronic lung disease. Journal of Molecular Medicine. 2007;85:1317–1324. doi: 10.1007/s00109-007-0280-4. [DOI] [PubMed] [Google Scholar]
- Weitzenblum E, Chaouat A. Severe pulmonary hypertension in COPD: is it a distinct disease? Chest. 2005;127:1480–1482. doi: 10.1378/chest.127.5.1480. [DOI] [PubMed] [Google Scholar]
- Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary AJ, Regan E, Bailey WC, Martinez FJ, Westfall E. Pulmonary arterial enlargement and acute exacerbations of COPD. New England Journal of Medicine. 2012;367:913–921. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AS, Mathews JA, Kasahara DI, Wurmbrand AP, Chen L, Shore SA. Innate and ozone-induced airway hyperresponsiveness in obese mice: role of TNF-α. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2015;308:L1168–L1177. doi: 10.1152/ajplung.00393.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascular pharmacology. 2002;39:187–199. doi: 10.1016/s1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]