Abstract
Background and Aims: A national survey of photodynamic therapy (PDT) was carried out in order to understand the present situation in Japan and the efficacy of PDT for gastric cancer.
Materials and Methods: A questionnaire concerning with PDT was sent to all hospitals performing or previously performed PDT. The answers were collected and analyzed. An additional investigation about efficacy of PDT for gastric cancer was conducted for the main 3 hospitals.
Results: In 18 of 19 responded hospitals, PDT was performed for 386 cases of superficial early gastric cancer and for 27 cases of advanced gastric cancer. In the 3 main hospitals, a complete response was achieved in 42 of 57 patients (73.7%) of superficial early gastric cancer not indicated for surgery or other endoscopic treatments such as endoscopic submucosal dissection. No serious complication occurred.
Conclusions: PDT has been shown to be a safe and effective treatment for early gastric cancer, not only for the intramucosal type, but also for the submucosal invasion. PDT will be one of the important endoscopic treatments for gastric cancer especially in a super ageing society like Japan.
Keywords: gastric cancer, photodynamic therapy (PDT), national survey in Japan
1. Background
Photodynamic therapy (PDT) is a safe treatment for tumors and neovascularity; the representative of them is cancers, where selective necrosis of them is achieved through photochemical reactions. This treatment relies on the use of porphyrin derivatives such as porfimer sodium (Photofrin®, Pfizer) which are photosensitizers with high accumulation of tumor tissue and neovascularity, and the use of low power red light. 1)
As a result of a multi-institutional study of PDT using the excimer-dye laser (EDL: Hamamatsu Photonics K.K, Hamamatsu, Japan) and Photofrin® for the treatment of early gastric cancer 2), PDT for superficial early gastric cancer was approved by the Ministry of Health and Welfare and covered by the Japanese Universal Health Insurance Coverage System (JUHICS) (Figure 1) 3) in April 1996. The gastric cancerous lesions indicated for PDT were those that showed no sign of lymph node metastasis through diagnostic imaging and where endoscopic mucosal resection (EMR) was not indicated. The lesions had also to be a) limited to the submucosal layer (SM), with no ulceration and measuring 1–3 cm in diameter or b) limited to the SM with ulceration and the diameter is ≤ 2 cm. If curative treatments such as EMR were possible, those treatments had to be given priority. 4)
Figure 1:
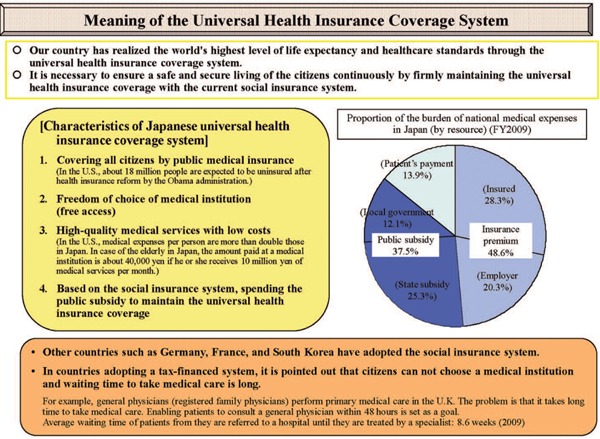
Meaning of Japanese Universal Health Insurance Coverage System (JUHICS) 3).
Later, a more curative endoscopic procedure compared to EMR, endoscopic submucosal dissection (ESD) was developed and became widespread. Since endoscopic resection (EMR or ESD) had the benefit of pathological examination of the resected tumor tissue, the Japanese gastric cancer treatment guidelines by the Japanese Gastric Cancer Association delineated that EMR or ESD was indicated as a standard treatment for the following tumor. A differentiated-type adenocarcinoma without ulcerative findings, of which the depth of invasion is clinically limited to the mucosal layer (M) and the diameter is ≤ 2 cm 5). As for PDT, the Guidelines considered the treatment in the same category ‘Coagulation’ as endoscopic coagulation using LASER or argon-plasma coagulator after non-curative resection by ESD. The nomenclature ‘PDT’ was removed from the guidelines 6) and up to this date PDT for gastric cancer has not gained popularity.
In Japan, PDT for gastric cancer is only approved with the combined use of Photofrin® and EDL or with the later approved YAG-optical parameter oscillator (OPO) laser (YAG-OPO: Ishikawajima-Harima Heavy Industries Co., Ltd., Tokyo, Japan: IHI). However both EDL and YAG-OPO are presently out of production and IHI has withdrawn itself completely from the field of the medical laser industry. Maintenance and repair of EDL will end at the end of March 2017. This means that in the near future, PDT for gastric cancer may be terminated.
2. Purpose
Based on the above background, the authors conducted a national survey of PDT for gastric cancer in order to grasp the present situation and recognize any issue surrounding PDT for gastric cancer as the main purpose. Additional investigation was performed at 3 medical institutions (hospitals) to ascertain the efficacy of Photofrin PDT following its approval for coverage by JUHICS.
3. Materials and Methods.
3.1. National survey of PDT for gastric cancer
A questionnaire (Table 1) was sent to all hospitals performing PDT and known to own or have previously owned an EDL and/or YAG-OPO. The answers were collected and analyzed.
Table 1: Questionnaire.
Japan Society for Laser Surgery and Medicine (JSLSM) National Survey concerning PDT for gastric cancer.
| Japan Society for Laser Surgery and Medicine (JSLSM) National Survey concerning PDT for gastric cancer. | |
| This survey is endorsed as a project of JSLSM. The purpose of this survey is to investigate the present situation of PDT for gastric cancer in Japan. This survey is being sent to hospitals who own (or have previously owned) an excimer-dye laser and/or a YAG-OPO laser. | |
| 1. | Name of your hospital: ____________________ Can we publish the name: Yes / No Name of the responsible doctor: __________ Can we publish the name: Yes / No |
| 2. | Does your hospital perform PDT (or has it done so in the past)? a. Yes, we presently perform PDT. b. We have performed PDT only in the past. (please answer the following question to the best of your ability) |
| 3. | Which laser(s) do you use (or have used) for PDT? Please indicate using ◯ for the laser presently in use, and ● for the laser(s) not presently in use. a. excimer-dye laser b. YAG-OPO laser c. argon dye laser d. diode laser (PD laser: Panasonic) e. others ( ) |
| 4. | Please mark the cancers presently treated by PDT with ◯ and mark cancers that were treated in the past but not in the present with ●. a. early lung cancer b. advanced lung cancer c. superficial esophageal cancer d. advanced esophageal cancer e. superficial early gastric cancer f. advanced gastric cancer g. uterine cervix cancer (including dysplasia) h. other cancers ( ) |
| 5. | Which photosensitizer do you use (or have used) for PDT? a. Photofrin® b. Laserphyrin® c. Hematoporphyrin derivatives d. others ( ) When the cost of PDT is not covered by the Japanese Health Insurance Coverage System (JUHICS), the payment is made by a. the patient b. research funds c. other |
| For the following questions, please answer to the best of your ability, even if you do not perform PDT for gastric cancer. | |
| 6. | Please write the total number of cases and procedures of PDT performed for the treatment of gastric cancer prior to the end of September 2013. a. superficial early gastric cancer ( cases , procedures) b. advanced gastric cancer ( cases, procedures) |
| 7. | Choose from conditions listed below that you think additional indication of PDT for gastric cancer. Multiple choices accepted. (The premise is that there is no metastasis found through diagnostic imaging and that other curative treatments are difficult.) a. after EMR or ESD, remnant or recurrence cancer. b. 0-IIc with ulcerative findings (UL(+)) except differentiated adenocarcinoma. c. superficial early gastric cancer invading SM. d. superficial early gastric cancer in patient receiving hemodialysis. e. advanced gastric cancer simulating early cancer. f. protuberant (Type 1) advanced gastric cancer. g. others ( ) |
| 8. | Please write the approximate number of gastric cancer that could be considered cases for PDT (if possible please write the present state and indications of your hospital concerning PDT and ESD). a. superficial early gastric cancer ( cases / year) b. advanced gastric cancer ( cases / year) |
| 9. | The next question is for only those who have given up performing PDT. What were the reasons for giving up performing PDT? (Multiple answers accepted) a. unable to use laser equipment. b. PDT specialist became unavailable due to staff transfer etc. c. patient management and nursing such as light shielding was time consuming. d. the number of patient for PDT were too few. e. PDT was not worthwhile financially. f. others ( ) |
| 10. | Concerning PDT with Laserphyrin® Recently, PDT with Laserphyrin® was only approved for lung cancers. However, as a result of an investigator-initiated clinical trial, insurance coverage by JUHICS for PDT for malignant brain tumors has recently been approved. Presently investigator-initiated clinical trials are taking place for PDT of remnant or recurrence after chemoradiation esophageal cancers and uterine cervix cancers. If, in the near future, a clinical trial or an investigator-initiated clinical study were to be planned to PDT for gastric cancer, how would your hospital respond? a. we would participate in the trial or study. b. we would be interested in the results but would not participate in the study. c. we would have no interest in the study. d. others ( ) |
| 11. | If PDT with Laserphyrin® for gastric cancer were to be approved for insurance coverage by JUHICS what kind of patients would your hospital like to treat by PDT and how (e.g., Perform PDT with Laserphyrin on an outpatient care.)? |
| 12. | If you have anything to add to the above, please feel free to add any comments below. |
| That concludes the questionnaire. Thank you for your cooperation. | |
3.2. Investigation of the efficacy of PDT for gastric cancer
An additional investigation was conducted at the University of Tsukuba Hospital, Dokkyo Medical University Hospital and Gunma University Hospital, all known to have performed PDT in relatively high patient numbers, since the approval for coverage by JUHICS. The efficacy of PDT for gastric cancer and any complications other than photosensitivity were investigated. The criteria for the evaluation of treatment effect were as follows.
Evaluation by endoscopic examination and biopsy specimens performed 1–3 months after PDT procedure.
-
•
Complete response (CR): Fulfills both 1) and 2)
-
1)
No residual tumor at the original lesion examined endoscopically.
-
2)
Biopsy specimen shows no carcinoma cells.
-
1)
-
•
Partial response (PR): Obvious reduction of more than 50 % of the original lesion.
-
•
Stable disease (SD): No obvious change or reduction less than 50% of the original lesion.
-
•
Progressive disease (PD): aggravation or obvious increase of the lesion.
The investigation was conducted separately for superficial early gastric cancer cases (lesions) meeting and outside the range covered by JUHICS and for advanced gastric cancer cases (lesions).
This survey was endorsed as a project of the scientific committee in the Japan Society for Laser Surgery and Medicine (JSLSM). It was approved by the Executive Committee of JSLSM in October 2013.
4. Results
4.1. National survey of PDT for gastric cancer
The survey listed up 39 hospitals at first, but 17 were excluded because of unknown addresses. The survey form (Table 1) was sent to the remaining 22 hospitals with 19 responding (Table 2). Of the 19 responses, one hospital's response was unable to be analyzed and the remaining 18 responses were analyzed. Sixteen out of 18 hospitals were still performing PDT at the present time.
Table 2: Responding hospitals and responsible doctors.
| Institution (Hospital) Responsible Doctors (titles omitted) | |
|---|---|
| Asahikawa Medical University Hospital | Yoshinobu Ohsaki |
| University of Tsukuba Hospital | Tsuyoshi Kaneko |
| Dokkyo Medical University Hospital | Tetsuya Nakamura |
| Gunma University Hospital | Yasuyuki Shimoyama |
| National Cancer Center Hospital East | Tomonori Yano |
| Tokyo Medical University Ibaraki Medical Center | Kinya Furukawa |
| Tokyo Medical University Hospital | Keishi Ohtani |
| Sanno Hospital | Tetsuya Okunaka |
| Shizuoka Cancer Center | Hiroyuki Ono |
| Hamamatsu University Hospital | Satoshi Osawa |
| Hamamatsu Medical Center | Yoshirou Nishiwaki |
| Nagoya City University Hospital | Hiromi Kataoka |
| Fujita Health University Hospital | Tomoyuki Shibata |
| Kyoto University Hospital | Manabu Muto |
| Osaka Medical Center for Cancer and Cardiovascular Diseases | — |
| Hyogo Cancer Center | Yoshinobu Yamamoto |
| Joyo Ejiri Hospital | Yoji Ishii |
| Kurume University Hospital | Satoru Matono |
| Nagasaki University Hospital | Hajime Isomoto |
4.1.1. Laser equipment for PDT
The laser equipment presently or formerly used for PDT was as follows. Twelve hospitals used an EDL, 3 used a YAG-OPO, and 2 used an argon-dye laser (ADL). Twelve hospitals also used a diode laser with wavelength of 664 nm developed for PDT (PD laser: Panasonic Health Care Co., Ltd., Tokyo, Japan denoted as “Diode” in Figure 2). Other lasers included a copper vapor laser, a gold vapor laser and an integrated two-wavelength diode laser were used, from one hospital respectively.
Figure 2:
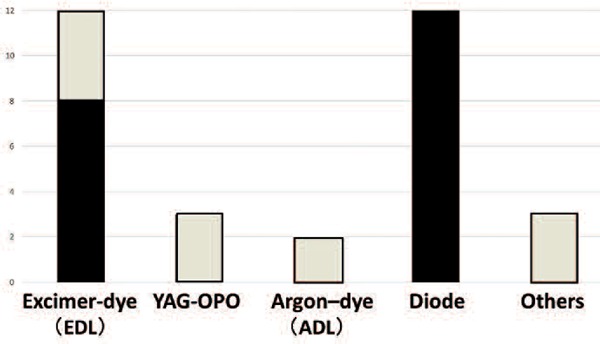
Laser equipment for PDT
(White box: not used now)
Lasers presently unused were EDL and YAG-OPO in 3 hospitals respectively. ADL was out of use in 2 hospitals and the copper vapor, gold vapor and integrated two-wavelength diode laser was not used at each hospital (white boxed area in Figure 2).
4.1.2. Cancers treated by PDT
This survey included cancers other than gastric cancer. Of the 18 hospitals, PDT was being performed on early lung cancer (11 hospitals), advanced lung cancer (4 hospitals), superficial esophageal cancer (14 hospitals), advanced esophageal cancer (5 hospitals), superficial early gastric cancer (13 hospitals), advanced gastric cancer (4 hospitals), and uterine cervical cancer including dysplasia (4 hospitals) (Figure 3). Rectal cancer was treated at 2 hospitals and gastric adenoma, brain tumor, oral cancer, recurrent breast cancer, duodenal cancer and gall bladder cancer was treated respectively at single hospital.
Figure 3:
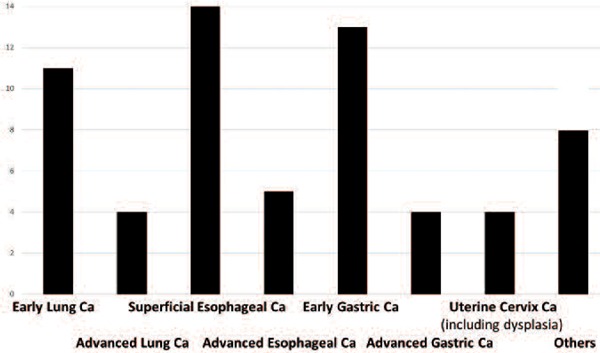
Indication of PDT
Cancers that are not presently being treated by PDT are early lung cancer and superficial early gastric cancer in 4 hospitals, respectively; superficial esophageal cancer (3 hospitals), advanced lung cancer, advanced esophageal cancer, early uterine cervical cancer including dysplasia (2 hospitals), advanced gastric cancer and rectal cancer in one hospital, respectively.
4.1.3. Photosensitizers used for PDT
Of the 18 hospitals, 14 used Photofrin® while 12 used talaporfin sodium (Laserphyrin®: Meiji Seika Pharma Co., Ltd., Tokyo, Japan). Two hospitals used hematoporphyrin derivative (HpD) and 5-aminolevulinic acid (5-ALA) respectively (Figure 4). Discontinued photosensitizers were Photofrin® in 4 hospitals, HpD in 2 hospitals, Laserphyrin® and 5-ALA in one hospital each (white boxed area Figure 4).
Figure 4:
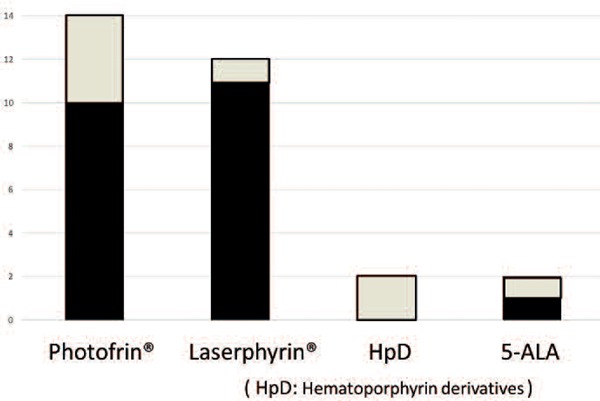
Photosensitizer for PDT
(White box: not used now)
For the treatment of cancer not covered by JUHICS, 6 out of 7 hospitals with valid responses answered that the payment for the treatment was funded from research grants. Two hospitals responded that the patient paid for the treatment.
4.1.4. Number of cases and indications of PDT for gastric cancer
Of the 18 hospitals, data from 2 hospitals; Joyo Ejiri Hospital and Osaka Medical Center for Cancer and Cardiovascular Diseases, were excluded because most of their data was accumulated before coverage by JUHICS. From the remaining 16 hospitals, the number of PDT for gastric cancer performed until the end of September 2013 was 180 cases for superficial early gastric cancer in total of 245 procedures, and 15 cases for advanced gastric cancer in total of 23 procedures. As for Joyo Ejiri Hospital and Osaka Medical Center for Cancer and Cardiovascular Disease, the total number of PDT procedures was not specified but the number of PDT cases was 54 and 152 for superficial early gastric cancer, and 9 and 3 for advanced gastric cancer, respectively. The total number of PDT cases in all 18 hospitals was 386 superficial early gastric cancer and 27 advanced gastric cancer.
Indications of PDT for gastric cancer was as follows: 15 out of 18 hospitals identified remnant or recurrence cancer after EMR or ESD. Twelve hospitals identified early superficial gastric cancer with SM invasion. Eleven hospitals identified 0-IIc with ulcerative findings (UL(+)) other than differentiated-type carcinoma. Nine hospitals identified superficial early gastric cancer patients receiving anticoagulant therapy. Seven hospitals identified superficial gastric cancer patients receiving hemodialysis. Six hospitals identified advanced gastric cancer simulating early cancer. Five hospitals identified protuberant (Type 1) advanced gastric cancer. Three hospitals identified gastric tube cancer after esophagectomy for esophageal cancer. One hospital identified a patient complicated with liver cirrhosis, and improvement of the quality of life (QOL) of an inoperable patient, respectively.
The maximum number of PDT cases per year at a single hospital was 6 for superficial early gastric cancer and 5 for advanced gastric cancer.
Some doctors commented that the expanded indication of ESD for gastric cancer has caused a decrease in the number of patient for PDT. Conversely, another doctor commented that the Japanese super ageing society will increase in the number of patient contraindicated for ESD; therefore the number of patient indicated for PDT will increase because PDT is noninvasive and decrease doctor's stress.
The reasons why hospitals that originally performed PDT but discontinued the procedure (multiple answers allowed) were as follows. Three hospitals replied that they could no longer use their laser equipment. Two hospitals answered that management for patients such as light shielding was time consuming, and there were not enough patients indicated for PDT, respectively. One hospital answered that the transfer of PDT specialist from the hospital, and insufficiency of refund money from JUHICS, respectively. There was also a comment that the extension of indication of laparoscopic surgery for gastric cancer has caused a decrease in the patient indicated for PDT.
4.1.5. PDT with Laserphyrin® for gastric cancer
Concerning the question of PDT with Laserphyrin® for gastric cancer, 10 of 18 hospitals replied that they would like to participate in investigator-initiated and/or clinical trials.
If PDT with Laserphyrin® for gastric cancer were covered by JUHICS in the future, desirable indications were as follows. Four hospitals replied a patient not indicated for ESD. An inoperable patient, an elderly patient who cannot endure long time endoscopic treatment, for the recanalization of stenosis caused by gastric cancer, for the improvement of patient's QOL, for combined treatment with EMR or for combination with photodynamic diagnosis (PDD) was replied from one hospital, respectively. There were 5 hospitals answered that they would like to shorten the hospitalization from the present to about 7 or 10 days, and in the future to perform PDT as an outpatient care.
4.2. Efficacy of PDT with Photofrin® for gastric cancer
The efficacy of PDT with Photofrin® for gastric cancer in 3 hospitals (University of Tsukuba Hospital, Dokkyo Medical University Hospital and Gunma University Hospital) that started PDT after the approval by JUHICS, between April 1997 and the end of March 2015 is shown in Table 3. Fifty-seven cases (70 lesions, average age 75.2 years) were meeting the criteria of JUHICS coverage. The criteria mean those patients who are not indicated for other curative treatment such as ESD, had superficial early gastric cancers limited to SM without ulcerative findings (UL(−)) and measuring 1–3 cm in diameter, or UL(+) and the diameter is ≤ 2 cm. Remnant or recurrence cases after EMR or ESD are included. Twenty-five cases (26 lesions, average age 77.5 years) were outside the criteria of JUHICS coverage. All patients showed no sign of lymph node metastasis by diagnostic imaging and were not indicated for other curative treatment including surgery.
Table 3: Efficacy of PDT with for gastric cancer in the 3 selected hospitals.
| Meeting the criteria of JUHICS coverage: 57 cases, 70 lesions (Male 40, Female 17; Average 75.2 years) | ||
| Efficacy of PDT (by cases) | Efficacy of PDT (by lesions) | |
| CR (complete response) | 42/57 (73.7 %) | 54/70 (77.1 %) |
| PR (partial response) | 15/57 (26.3 %) | 16/70 (22.9 %) |
| Outside the criteria of JUHICS coverage: 25 cases, 26 lesions (Male 22, Female 3; Average 77.5 years) | ||
| Efficacy of PDT (by cases) | Efficacy of PDT (by lesions) | |
| CR (complete response) | 6/25 (24.0 %) | 7/26 (26.9 %) |
| PR (partial response) | 19/25 (76.0 %) | 19/26 (73.1 %) |
There was a difference between the CR rate of PDT for superficial early gastric cancer for those meeting the criteria (737%) and those outside the criteria of JUHICS coverage (24%). However, the efficacy rate (the sum of the CR and PR rates) was 100% for both groups.
As for complication of PDT except for photosensitivity, bleeding after treatment was occurred in 2 out of 82 cases; however, which were treated endoscopically. No serious complication such as gastric perforation that would require surgery, were occurred.
In case of PDT for advanced gastric cancer, every case was approved by the relevant ethics committee. The purpose of PDT for advanced gastric cancer was the alleviation of anemia caused by bleeding or the prevention of passage obstruction by stenosis. Three patients with 5 cancerous lesions, (average age 78.3 years old) were treated by PDT. There was no CR, 3 PR for the patient; however, 2 CR (for accompanied 2 superficial early gastric cancer) and 3 PR for the 5 lesions. Thus, the efficacy rate was 100%. No complication except for photosensitivity was observed.
5. Discussion
5.1. Laser equipment for PDT (Figure 2)
ADL is a continuous wave laser (wavelength 630nm) in which a red color dye is pumping by an argon ion laser, because HpD or Photofrin® is used as photosensitizer in case of PDT. ADL is presently not used in Japan.
EDL is a pulsed laser where a dye (rhodamine 640) is pumping by a XeCl excimer laser (wavelength 308 nm) and the emitted wavelength adjusted to 630 nm. This laser is special because it was designed, manufactured and used only in Japan 7). A study to investigate the anti-tumor effect of PDT with HpD on cancerbearing mice revealed that pulsed laser energy from EDL penetrates significantly deeper into the tumor compared to the continuous wave ADL 8).
The copper vapor laser 9) and the gold vapor laser 10) were introduced to PDT for their advantageous higher anti-tumor effect associated with their pulsed laser emission than continuous wave lasers. However the cost of them was extremely expensive (about 50 million Japanese yen). Moreover, maintenance of them was difficult and only a limited number of hospitals installed these lasers and they were used clinically for a small number of patients. No hospital uses these lasers presently.
YAG-OPO is a pulsed laser emitted from an optical parametric oscillator (OPO) excited by Q-switched Nd:YAG laser energy. It was developed for PDT as a consignment from the Research Development Corporation of Japan (presently Japan Science and Technology Agency) 11). The advantage of YAG-OPO was that it was a tunable laser within the wavelength range of 620 to 670 nm. This allows the laser to use with multiple photosensitizers including Laserphyrin®. This laser hardware can also emit the ordinary 1064 nm high power Nd:YAG laser and hence can also be used for photosurgical or photocoagulative treatment. However, the regulatory body at that time authorized this laser equipment only for a non-tunable output preset at 630 nm, specific for PDT with Photofrin® alone. The cost of the laser was extremely expensive similar to EDL, near 40 million Japanese yen; therefore, a very small number of the systems was sold. Thus, the provider-manufacturer, Ishikawajima-Harima Heavy Industries, withdrew completely from the medical laser hardware business. No hospital presently uses this laser.
PD Laser is a semiconductor laser developed for PDT with Laserphyrin®. This laser equipment emits continuous wave light at the wavelength 664±2 nm, with a maximum output of 500 mW 12). Based on the results of phase II clinical trial in Japan 13), PDT with Laserphyrin® using PD Laser was approved and covered by JUHICS in the treatment of early stage lung cancers in 2004. PD Laser is a small, maintenance free equipment costing only 8 million Japanese yen. However, since coverage by JUHIC was limited to PDT for early lung cancer, PD Laser did not spread. Then, the original manufacturer, Panasonic withdrew from the medical laser business because PD Laser seemed to be unprofitable. Now Panasonic Healthcare succeeded to PD Laser enterprise.
The two-wavelength semiconductor laser is unique laser equipment developed to improve PDT with 5-ALA efficacy. This laser emits two wavelengths of 635 nm and 670 nm. After approval by the local ethics committee in Dokkyo Medical University Hospital, a clinical investigation of PDT with 5-ALA on a single patient suffering from superficial early gastric cancer was performed prior to his surgery. Efficacy of PDT with 5-ALA for this patient was not satisfactory compared to that of PDT with Photofrin® (unpublished data).
5.2. Cancers treated by PDT
‘Superficial esophageal cancer’ was the most popular indication for PDT because 14 hospitals answered that. However, this may be due to the fact that an investigator-initiated clinical trial of PDT with Laserphyrin® for remnant and/or recurrent esophageal cancers after chemoradiation 14) was on going at the same time of this national survey.
Thirteen hospitals were performing PDT for superficial early gastric cancer, 11 hospitals were performing PDT for early lung cancer and 4 hospitals were performing PDT with Photofrin® for early uterine cervix cancer (and dysplasia) which was covered by JUHICS (Figure 3). On the other hand, there were several hospitals that have given up performing PDT due to breakdown of the laser equipment and/or for other reasons.
Recently, PDT with Laserphyrin® for advanced lung cancer and malignant brain tumor was approved for coverage by JUHICS; however, only 4 and 1 hospitals performed PDT, respectively.
And other cancers which does not covered by JUHICS, 5 hospitals stated that PDT should be indicated for advanced esophageal cancer, 4 hospitals for advanced gastric cancer, 2 hospitals for rectal cancer and one hospital for gastric adenoma and duodenal cancer, respectively. It seemed to be the high needs for PDT in the treatment cancer of the digestive tract including gastric cancer.
5.3. Photosensitizers used for PDT
Photofrin® is a product name of porfimer sodium, and porfimer is a mixture of oligomers formed by ether and ester linkages of up to eight porphyrin units. PDT with Photofrin® is performed as follows. First, intravenously injecting 2 mg/kg of Photofrin; and 48 to 72 hours later, the cancerous lesion is irradiated with red laser light (wavelength 630 nm) 4). Presently 14 out of 18 hospitals use Photofrin® (Figure 4) for PDT, but only PDT using EDL or YAG-OPO is covered by JUHICS. As mentioned, there is no hospital presently using YAG-OPO; moreover, when maintenance and repair of EDL will be terminated at the end of March 2017, PDT with Photofrin® for superficial early gastric cancer and early uterine cervix cancer will be impossible to perform. Some doctor mention that importing laser equipment with the same specifications from abroad could solve the problem, but pulsed lasers with such high specifications like EDL or YAG-OPO are thought to be only achievable in Japan and there is no available substitute.
Figure 5:
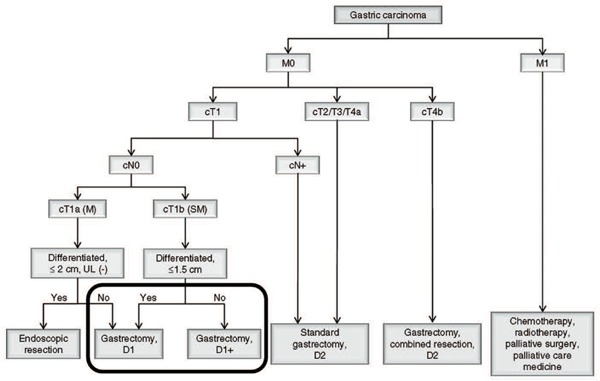
Algorithm of standard treatments for gastric cancer to be recommended in clinical practice, partially edited by authors.
(Available at http://www.jgca.jp/pdf/JGCA_Guidelines_ver3.pdf)
Laserphyrin® is a product name of talaporfin (also known as aspartyl chlorin, mono-L-aspartyl chlorin e6, NPe6); which is a photosensitizer derived from chlorophyll of plant origin created by Nippon Petrochemicals Co. Ltd. It is a single compound where the chlorin skeleton is linked to aspartic acid through an amide linkage and has an absorption peak at 664 nm 15). In this survey, 12 out of 18 hospitals performed PDT with Laserphyrin® for early lung cancer and superficial esophageal cancers, and only one hospital performed it for gastric cancer. Based on the results of the first investigator-initiated clinical trial in Japan 16), PDT with Laserphyrin® using PD Laser for primary malignant brain tumors was approved coverage by JUHIC (but only when combined with tumor resection) in 2014. Laserphyrin® presently cites ‘malignant brain tumors’ as one of its effective targets and is designated as medicine for rare diseases 15). Recently, an investigator-initiated clinical trial of PDT with Laserphyrin® for remnant or recurrent esophageal cancer after chemoradiation has ended. Laserphyrin® was approved again as medicine for rare diseases by JUHICS. An investigator-initiated clinical trial of PDT with Laserphyrin® using PD Laser for gastric cancer and uterine cervix cancer is currently being planned. The authors strongly hope that these trials would start as soon as possible, and that PDT with Laserphyrin® for gastric cancer and uterine cervix cancer would be approved by JUHIC to the same indication as PDT with Photofrin®.
HpD is no longer in use in Japan, since the approval of Photofrin®.
5-ALA is a substance commonly found in vivo among many species including human. It is biosynthesized in cellular mitochondrias from glycine and succinyl-CoA. The photosensitizing compound protoporphyrin IX (PpIX) is synthesized through the metabolic pathway of 5-ALA to heme 17). PpIX preferentially accumulates in tumor cells and when such tumors are irradiated with purple-blue light (wavelength 400∼410 nm), excitation of PpIX causes visible red fluorescence, which is used for PDD. In 2007, European Union approved the use of 5-ALA as a PDD agent for the tumor resection of neuroglioblastoma, while in Japan it was first designated as a medicine for rare diseases and then approved in 2013. Presently it is marketed under the trade name Alaglio® oral 17).
PDT with 5-ALA is possible by irradiating tumor cells, in which PpIX has preferentially accumulated, with red light (wavelength 600∼700 nm). However, the targets of PDT with 5-ALA are believed to be limited to superficial lesions such as skin surface and mucosa 18). Therefore, clinical applications have been conducted mostly in the field of dermatology in Japan 19).
5.4. Number of cases and indication of PDT for gastric cancer
The total number of PDT cases from all 18 hospitals was 386 for superficial early gastric cancer, and 27 for advanced gastric cancer. This number exceeded our expectations. However this number contains many cases of PDT performed prior to coverage by JUHIC, the recent number of cases is estimated to have decreased.
The most popular indication of PDT for gastric cancer was remnant and/or recurrent case after EMR or ESD (15 of 18 hospitals). This is probably due to the fact that PDT is regarded as ‘Coagulation (using LASER or argon-plasma coagulator)’ under the Japanese gastric cancer treatment guidelines 6).
Other indications of PDT were superficial early gastric cancer invading SM (12 hospitals), UL(+) 0-IIc except differentiated adenocarcinoma (11 hospitals), superficial early gastric cancer case receiving anti-coagulant therapy (9 hospitals), superficial early gastric cancer case receiving hemodialysis (7 hospitals) and gastric tube cancer (3 hospitals). Most of these indications meet the criteria of JUHICS coverage (above mentioned). Algorithm of standard treatments to be recommended in clinical practice by Japanese Gastric Cancer Association is shown Figure 4 5). Patients surrounded by bold lines Figure 4 and who are inoperable are indication for PDT and meet the criteria of JUHICS coverage. If the misunderstanding for PDT in the Japanese gastric cancer treatment guidelines were resolved and the criteria of JUHICS coverage were well understood by doctors, much more patients suffering from gastric cancer could be treated by PDT.
According to this survey, the approximate number of patients for PDT at single hospital per year is 6 cases for superficial early, and 5 cases for advanced gastric cancer. This is a relatively little less than our anticipation. However, as Japan is already entering super ageing society, the number of patients suffering from gastric cancer who are not indicated for surgery or ESD will increase. In this situation, there are high expectations for PDT which is minimally invasive and safe endoscopic treatment.
5.5. PDT with Laserphyrin® for gastric cancer
Expectations for PDT with Laserphyrin® for gastric cancer are thought to be very high. There were opinions that PDT should be approved for not only superficial gastric cancer meeting the criteriona of JUHICS coverage, but also for advanced gastric cancer. In case of advanced gastric cancer, PDT is expected to prevent of stenosis, and/or to increase QOL of patients, or to combine with EMR for the purpose of tumor reduction.
PDT with Laserphyrin® is much better compared to that with Photofrin® in term of extremely elderly patient's QOL, because the period required for hospitalization and light shielding is much shorter 20). Moreover, when Laserphyrin® is used for PDD, the red florescence is much bright and clear than that of 5-ALA 20). Recently, clinical application of PDD and PDT by Laserphyrin® using a newly developed high-resolution electronic endoscope was reported 21).
5.6. Efficacy of PDT with Photofrin® for gastric cancer
In the first clinical trial of PDT with Photofrin® using EDL for early gastric cancer between 1990 and 1992, CR was achieved in 21 of 24 cases (87.5 %) 2). The details were as follows: 14 CR of 16 cases (87.5%) in M, and 7 CR of 8 cases (87.5%) in SM cancer. However, 3 cases of SM cancer judged as CR initially, recurred later on. The size of these recurred cancerous lesions was greater than 2 cm.
In the clinical trial of PDT with Photofrin® using YAG-OPO for early gastric cancer between 1995 and 1996, CR was achieved in 9 of 12 cases (75%) 22). The details were as follows: CR in all 4 cases of M (100 %), and 5 CR of 8 cases (62.5%) in SM cancer 22). No recurrences were seen in the CR patients during the 20 months follow up period 22).
In a clinical investigation of PDT with Photofrin® using EDL for early gastric cancer at Dokkyo Medical University Hospital between 2003 and 2008, CR was achieved in 17 of 20 cases (85 %) 23). The details were as follows: 4 CR of 5 cases (80%) in M, and 13 CR of 15 cases (86.7%) in SM cancer. Of the 3 cases judged as PR initially, 2 cases became CR following additional PDT 23).
In the 1990s, PDT using EDL or YAG-OPO was performed by single laser irradiation to the cancerous lesion during 48 to 72 hours after injection of Photofrin®. This procedure of PDT is suspected to be the reason for the limitation of efficacy for SM cancer. In the 21st century, a novel ‘two days method of PDT, was designed where the cancerous lesion was irradiated by EDL at not only 48 hours but also 72 hours after Photofrin® injection 23). As a result, the efficacy of PDT for SM cancer increased.
The novel ‘two days method of PDT’ is applied for early gastric cancer in the selected 3 hospitals (University of Tsukuba Hospital, Dokkyo Medical University Hospital and Gunma University Hospital). As a result, a very good efficacy of PDT for early gastric cancer (CR rate of 73.7% (42 of 57 cases), Table 3) meeting the criteria of JUHICS coverage (patients surrounded by bold lines Figure 4 and who are inoperable) must be achieved in these hospitals.
PDT is regarded as ‘Coagulation (using LASER or argon-plasma coagulator)’ under the Japanese gastric cancer treatment guidelines 5); however, it is a minimally invasive treatment involving tumor-selective destruction through the photochemical reaction with laser light and photosensitizers 1). It is clearly different form direct physical destructive treatments using high power laser or argon plasma coagulator which might be occurred gastric perforation. The fact that not a single complication which would require surgery such as gastric perforation has been reported, strongly supports for the safety of PDT.
6. Conclusions
PDT which has a characteristic of tumor-selective destruction is a safe and effective treatment for a patient suffering from gastric cancer that are not indicated surgery or endoscopic treatments such as EMR or ESD. PDT is safe and minimally invasive treatment; therefore, it will be one of important endoscopic treatments for gastric cancer especially in a super ageing society like Japan.
[Acknowledgements]
The authors would like to deeply thank the Chairman of the Academic Committee of the Japan Society for Laser Surgery and Medicine, President of Yotsuya Medical Cube (and former Professor of Clinical Research, Innovation and Education Center, Tohoku University Hospital) Dr. Yoshimochi Kurokawa, for his pronounced assistance concerning this national survey of PDT for gastric cancer in Japan.
Editor's Note: This paper was originally published in Japanese in The Journal of Japan Society for Laser Surgery and Medicine, Vol. 36.2:124–132, 2015, and has been specially translated for inclusion in Laser Therapy as an English Original Article.
Conflict of interests
No conflict of interest exists.
References
- 1: Nakamura T, Matsui H, Narahara H: Endoscopic laser treatment guidelines. In gastroenterological endoscopy guidelines (3rd Edit.). Edited by postgraduate educational committee of Japan Gastroenterological Endoscopy Society. Igaku-Shoin Ltd., Tokyo, 299-306, 2006. [Google Scholar]
- 2: Mimura S, Ito Y, Nagayo T, Ichii M, Kato H, Sakai H, Goto K, Noguchi Y, Tanimura Y, Nagai Y, Suzuki S, Hiki Y, Hayata Y: Cooperative clinical trial of photodynamic therapy with photofrinlland excimer dye laser for early gastric cancer. Laser in Surgery and Medicine. 19: 168-172, 1996. [DOI] [PubMed] [Google Scholar]
- 3: Meaning of universal health insurance coverage system. (Available at http://www.mhlw.go.jp/bunya/iryouhoken/iryouhoken01/dl/01_eng.pdf)
- 4: Photofrin injection 75mg, package insert, 9th edition (authorization code: 22000AMX00978), 2010. [Google Scholar]
- 5: The types and indications of treatment methods. Edited by Japan Gastric Cancer Assoiciation, in Guidelines for treatment of gastric cancer: for doctors, revised May 2014, 4th edit., 6-8, 2014, Kanehara & Co., Ltd. [Google Scholar]
- 6: Enodoscopic resection. Edited by Japan Gastric Cancer Assoiciation, in Guidelines for treatment of gastric cancer: for doctors, revised May 2014, 4th edit., 20-23, 2014, Kanehara & Co., Ltd. [Google Scholar]
- 7: Hirano T, Suzuki K. (1995): Excimer dye laser for PDT application. Jour. Japan Soc. laser Surg. and Med.. 16: 29-35. [Google Scholar]
- 8: Okunaka T, Kato H, Konaka C, Sakai H, Kawabe H, Aizawa: A comparison between argon-dye and excimer-dye laser for photodynamic effect in transplanted mouse tumor. Jpn J Cancer Res, 83: 226-231, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9: Ichi M, Mimura S, Tatsuta M, Okuda S. (1988): Photodynamic therapy for early gastric cancer with a copper vapor laser. Jour. Japan Soc. Laser Surg. and Med.. 9: 125-126. [Google Scholar]
- 10: Nakamura T, Ejiri M, Fujisawa T, Akiyama H, Ejiri K, Ishida M, Fujimori T, Maeda S, Saeki S, Baba S: Photodynamic therapy for early gastric cancer using a pulsed gold vapor laser. J Clin Laser Med Surg, 8: 63-67, 1990. [DOI] [PubMed] [Google Scholar]
- 11: Udagawa T, Inoue K, Fukutomi S. (1995): YAG-OPO laser for PDT application. Jour. Japan Soc. Laser Surg. and Med.. 16: 25-30. [Google Scholar]
- 12: Machinery and hardware (31). Medical ablative semiconductor laser for PDT. PD Laser attached instructions 8th edit. (authorization number 21600BZZ26000) revised April 1, 2012. [Google Scholar]
- 13: Kato H, Furukawa K, Sato M, Okunaka T, Kusunoki Y, Kawahara M, Fukuoka M, Miyazawa T, Yana T, Matsui K, Shiraishi T, Horinouchi H: Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer, 42: 103-111, 2003. [DOI] [PubMed] [Google Scholar]
- 14: Yano T, Muto M, Yoshimura K, Niimi M, Ezoe Y, Yoda Y, Yamamoto Y, Nishisaki H, Higashino K, Iishi H: Phase I study of photodynamic therapy using talaporfin sodiumand diode laser for local failure after chemoradiotherapy for esophageal cancer. Radiat Oncol, 7: 113, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15: Agent for photodynamic therapy separandum, prescription medicine, Laserphyrin injection 100mg. Interview form for medicine, revised September, 2014. (7th edition). [Google Scholar]
- 16: Muragaki Y, Akimoto J, Maruyama T, Iseki H, Ikuta I, Nitta M, Maebayashi K, Saito T, Okada Y, Kaneko S, Matsumura A, Kuroiwa T, Karasawa K, Nakazato Y, Kayama T: Phase II clinical study on intraoperative photodynamic therapy with tala porfin sodium and semiconductor laser in patients with malignant brain tumors. J Neurosurg, 119: 845-852, 2013. [DOI] [PubMed] [Google Scholar]
- 17: Agent for photodynamic diagnosis, Alaglio oral 1.5 g (aminolevulin dihydrochloride). Interview form for medicine, September, 2013. (2nd edition). [Google Scholar]
- 18: Petersen BT, Chuttani R, Croffie J, DiSario J, Liu J, Mishkin D, Shah R, Somogyi L, Tierney W, Wong Kee Song LM : Photodynamic therapy for gastrointestinal disease. Gastrointest Endosc, 63: 927-932, 2006. [DOI] [PubMed] [Google Scholar]
- 19: Matsumoto Y, Akita Y, Kawamura C, Nakaseko H, Watanebe D, Tamada Y. (2008): Photodynamic therapy for the treatment of skin tumors. Jour. Japan Soc. Laser Surg. and Med.. 29: 160-163. [Google Scholar]
- 20: Nakamura T, Oinuma T. (2014). Usefulness of photodynamic diagnosis and therapy using talaporfin sodium for an advanced-aged patient with inoperable gastric cancer (a secondary publication). Laser Therapy 23: 201-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21: Nakamura T, Oinuma T, Yamagishi H, Masuyama H, Terano A: Evaluation of a novel high-resolution magnifying videoendoscope that is capable of photodynamic diagnosis and therapy for gastric cancer. PDPDT, 12: 115-122, 2015. [DOI] [PubMed] [Google Scholar]
- 22: Mimura S, Narahara H, Hirashima T, Fukutomi H, Nakahara A, Kashimura H., Matsui H., Tanimura H., Nagai Y., Suzuki S., Murata Y., Yoshida K., Isono K., Kozu T., Ide H., Kato H.: Cooperative clinical trial of photodynamic therapy for early gastric cancer with photofrin injection and YAG-OPO laser. Diagn Ther Endosc, 4: 165-171, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23: Oinuma T, Masuyama H, Nakamura T. (2013): Efficacy of two days method PDT using porfimer sodium for early gastric cancer unsuitable for endoscopic submucosal dissection Jour. Japan Soc. Laser Surg. and Med.. 34: 118-123. [Google Scholar]


