Abstract
Background
Peritoneal dialysis (PD) is an effective method of renal replacement therapy for end-stage renal disease patients. The PD catheter could be inserted by surgical (open surgery/laparoscopic-assisted) or percutaneous techniques. However, the efficacy of the techniques, including catheter survival and catheter related complications, is still controversial.
Method
The dataset was defined by searching PubMed, EMBASE, Google Scholar and the Cochrane database that had been published until July 2014. The meta-analysis was performed using Review Manager Software version 5.2.6.
Result
The final analysis was conducted on 10 studies (2 randomized controlled studies (RCTs) and 8 retrospective studies), including 1626 patients. The pooled data demonstrate no significant difference in 1-year catheter survival (OR = 1.04, 95% CI = 0.52–2.10, P = 0.90) between surgical and percutaneous groups. However, the sensitivity analysis of the RCTs demonstrated that the incidence of overall infectious (OR = 0.26, 95% CI = 0.11–0.64, P = 0.003) and overall mechanical complications (OR = 0.32, 95% CI = 0.15–0.68, P = 0.003) were significantly lower in the percutaneous groups than the surgical groups. Furthermore, the subgroup analyses revealed no significant difference in the rates of peritonitis, tunnel and exit site infection, leakage, inflow-outflow obstruction, bleeding and hernia by comparing the methods.
Conclusion
The results showed that the placement modality did not affect 1-year catheter survival. Percutaneous catheter placement is as safe and effective as surgical technique.
Keywords: Peritoneal dialysis catheters, Technical survival, Surgical insertion, Percutaneous insertion, Meta-analysis
Highlights
-
•
Peritoneal dialysis (PD) is an effective and less costly method of renal replacement therapy for end-stage renal disease patients (ESRD). Peritoneal dialysis is more effective in preserves renal function while awaiting renal transplantation, faster restoration of diuresis and better quality of life as a home treatment than hemodialysis.
-
•
Currently, there is no consensus for preferring type of catheter and the catheter placement method because of each modality has its pros, cons, and post-operative complication. Thus, the authors performed a meta-analysis an attempt to clarify the comparison of the outcomes of both techniques (such as a 1-year catheter survival, infectious complication, and mechanical complication).
1. Introduction
Peritoneal dialysis (PD) is an effective and less costly method of renal replacement therapy for end-stage renal disease patients (ESRD). Compared to hemodialysis, PD is more effective in preserving renal function in patients awaiting renal transplant, restoring diuresis, and offering a better quality of life as a home treatment [1], [2], [3]. The peritoneal dialysis catheter is usually placed into the peritoneal cavity either by surgical technique (open surgery or laparoscopic-assisted) or by percutaneous technique (Seldinger or modified Seldinger technique), with or without fluoroscopic guidance [4], [5], [6].
Currently, there is no consensus on the preferred type of catheter and the catheter placement method as each technique has its advantages, disadvantages, and complications [7], [8]. Surgical technique has the advantage of direct visualization, allowing precise catheter placement in the peritoneal cavity. However, this technique is more invasive and requires general anesthesia. In contrast, the percutaneous catheter placement technique could be performed as a bedside procedure using local anesthesia. Failure to advance the guide wire into the peritoneum, development of pain or cramp during the procedure, and limitations of use in patients with previous abdominal surgery were found to be the main drawbacks of this technique [9], [10], [11], [12].
Catheter-related complications were categorized as infectious complications and mechanical complications. Mechanical complications, usually associated with PD technical failure, consequently affect the long-term catheter survival and ultimately patient survival [13], [14], [15], [16], [17].
Although several studies have attempted to compare the outcomes of PD catheter placement techniques, between surgical and percutaneous methods, there has been a significant inconsistency in the findings of these studies. We conducted a meta-analysis based on the published literature in an attempt to clarify and evaluate the comparison of outcomes between the two techniques (such as 1 – year catheter survival, infectious complication, and mechanical complication).
2. Material and methods
2.1. Data sources and search strategies
An electronic literature search was performed on July 2014 by using the PubMed, Embase, Google Scholar, and the Cochrane database. In order to evaluate the postoperative outcomes between catheters placed by percutaneous technique and directed visualized by surgical technique the search terms “Peritoneal dialysis catheter insertion,” “Laparoscopic-assisted peritoneal dialysis catheter insertion,” “Percutaneous peritoneal dialysis catheter insertion” and “Fluoroscopic guide peritoneal dialysis catheter insertion” were used as keywords to identify all relevant studies. This meta-analysis was performed according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 [18].
2.2. Study selection and eligibility criteria
The inclusion criteria were as follows: (1) performed peritoneal catheter insertion for peritoneal dialysis in End-stage renal disease patients, (2) compared the percutaneous group with the surgical group (open/laparoscopic-assisted), (3) the outcomes must evaluate infectious complications, mechanical complication, and 1-year catheter survival.
The percutaneous group was defined as the peritoneal dialysis catheters placed by the percutaneous technique with or without fluoroscopic guidance. The surgery group was defined as the catheters placed under direct visualized by open surgery or laparoscopy-assisted technique. Infectious complications were defined as postoperative peritonitis, tunnel and exit site infection. Peritoneal dialysis fluid leakage, inflow-outflow obstruction, catheter malfunction, bleeding and incisional hernia, were the definitions of the mechanical complications [19].
Studies will exclude (1) review articles, (2) non-comparative studies, (3) and studies in pediatric patients. The quality of the studies that were included in the meta-analysis was further evaluated using Newcastle-Ottawa scale. The maximum score possible was 9 points, which represents the highest methodological quality [20].
2.3. Statistical analysis
The meta-analysis was performed using the Review Manager Software (Revman version 5.2.6) provided by the Cochrane Collaboration (Nordic Cochrane Center, Cochrane Collaboration, Copenhagen, Denmark). Cochran's Q-statistic test was applied to access between-study heterogeneity and I2 were used to test for heterogeneity between the studies included. (p < 0.05 is considered for significant heterogeneity).
The postoperative complications and 1-year catheter survival rate outcomes of the patients were analyzed using the Mantel-Haenszel method to generate a pooled odds ratio (OR) with 95% confidence intervals and odds ratio (OR), in order to compare the 1-year catheter survival and postoperative complications between the percutaneous and surgical group. The OR was considered statistically significant at the P < 0.05 level if the 95% CI did not include the value 1.
The authors adopted random-effect models, which is a more conservative way of calculating OR, assuming a high level of variation between studies and using a weighted average of the effect reported in different studies to calculate levels of association. Publication bias was assessed by visual examination of a funnel plot; asymmetry was formally assessed using both Egger's linear regression test and the rank correlation test (Begg's test).
3. Results
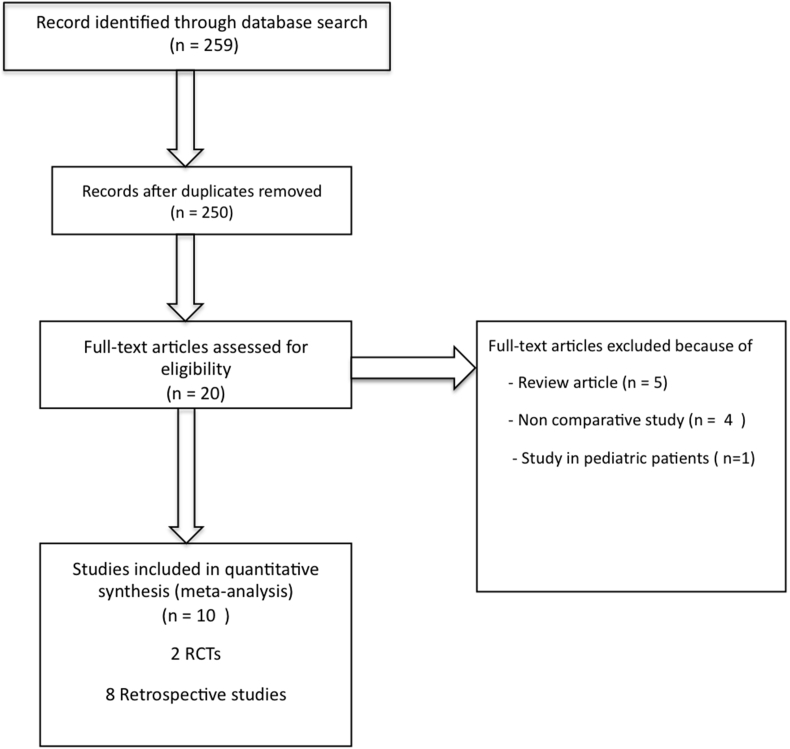
The initial search identified a total of 259 potential articles. After screening, ten articles (2 randomized controlled studies (RCTs) and 8 retrospective studies) matched our criteria and were deemed suitable for inclusion in the meta-analysis. The PRISMA diagram of the search process is shown in Fig. 1. Reviews of data extraction showed 100% agreement between two reviewers. The pooled studies included 1755 peritoneal dialysis catheter placement procedures in 1626 patients. The catheters were placed into the peritoneal cavity by the percutaneous technique, with or without fluoroscopic guidance (percutaneous group) in 841 procedures (47.9%). There were 914 catheters (52.1%) placed into the peritoneal cavity under direct visualization by open surgery or laparoscopic-assisted technique (surgery group). The characteristics of the ten included studies are shown in Table 1.
Fig. 1.
Selection process of studies for inclusion in the meta-analysis.
Table 1.
Characteristics of the 10 studies included in the meta-analysis regarding the PD catheter implantation methods.
| Study | Country | Year of publication | Study design | Number of patients | Number of procedures | Age | Sex (male) | Comparison | Follow up peroid | Catheter type | Number of previous abdominal operations | Matching | Newcastle Ottawa quality score | 1-year catheter survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rosenthal MA. |
USA | 2008 | Retrospective study | 101 | 107 | 56.1 ± 15.4 | 47 | Open and laparoscopic surgery Versus percutaneous (fluoroscopic guide) |
1 year | Swan neck tenckhoff, Double cuff catheter (Kendoll Healthcare,M assachusetts, USA) | None (patients with previous abdominal operation were excluded) | a,b,c,d,e,f,g,h | 7 | None |
| Park SY. | South Korea | 2014 | Retrospective study | 167 | 167 | – | 100 | Open surgery versus percutaneous | 16 ± 10 months | Swan neck tenckhoff, Double cuff catheter | Surgery group = 17 Percutaneous group = 4 | b,,c,d,e,f,g,i,j | 8 | -Surgery group = 93.3% -Percutaneous group = 89.9% |
| Voss D | New Zealand | 2012 | Randomized controlled study | 113 | 102 6 | 0.8 (51–69.7) | 58 | Laparoscopic surgery versus percutaneous (fluoroscopic guide) | 1 years | Double cuff peritoneal dialysis catheter | None (patients with previous abdominal operation were excluded) | a,b,c,d,e,h,i,j | 7 | -Surgery group = 73.7% -Percutaneous group = 84.0% |
| Atapour A. | Iran | 2011 | Randomized controlled study | 64 | 61 | 55.10 ± 17.20 | 33 | Open surgery versus percutaneous | 2 months | Swan neck tenckhoff, Double cuff catheter | None (patients with previous abdominal operation were excluded) | a,b,d,e,f,g | 6 | None |
| Perakis EK. | Greece | 2009 | Retrospective study | 152 | 170 | 62.8 ± 15.7 | 88 | Open surgery versus percutaneous | 33 ± 29.5 months | -Tenkchoff straight or coiled double cuff catheter - Toronto Western Hospital-II catheter | Surgery group = 14 Percutaneous group = 11 | a,b,i,j | 7 | -Surgery group = 89.5% -Percutaneous group = 91.1% |
| Medani S. | Ireland | 2011 | Retrospective study | 313 | 313 | 50.4 ± 15.3 | 193 | Open surgery versus percutaneous | 12-15 months | Swan neck tenckhoff, Double cuff catheter | Surgery group = 78 Percutaneous group = 14 | a,b,e,f,h,i,j | 7 | -Surgery group = 68.7% -Percutaneous group = 77.7% |
| Roueff S. | France | 2002 | Retrospective study | 104 | 104 | – | – | Open surgery versus percutaneous | – | Single deep cuff tenckhoff catheter | None | b,c,d,e,f,i,j | 6 | -Surgery group = 71.0% -Percutaneous group = 75.0% |
| Ozener C. | Turkey | 2001 | Retrospective study | 191 | 215 | – | 117 | Open surgery versus percutaneous | 21 ± 18 months in surgerygroup, 17 ± 12 months in percutaneous group | Straight or coiled tip double cuff tenckhoff catheter | None | b,e,f,h,i,j | 8 | -Surgery group = 73.0% -Percutaneous group = 90.0% |
| Melotte JG. | UK | 1993 | Retrospective study | 172 | 230 | 66 ± 10.5 | – | Open surgery versus percutaneous | 2583 patients months | Silastic curve- catheter with double cuff | None | a,c,d,e,f,g,i,j | 6 | -Surgery group = 60.0% -Percutaneous group = 33.0% |
| Maher E. | New Zealand | 2014 | Retrospective study | 249 | 286 | 57.4 | 160 | Open surgery versus percutaneous (fluoroscopic guide) | 1 year | Curl peritoneal catheter,Baxter, Deerfield,Illinois | None | a,b,c,d,e,f,h | 8 | None |
Abbreviations: a = age, b = sex, c = peritonitis, d = tunnel and exit site infection, e = leakage, f = inflow and outflow obstruction, g = bleeding, h = hernia, I = early complication, j = 1-year catheter survival.
3.1. 1-Year catheter survival
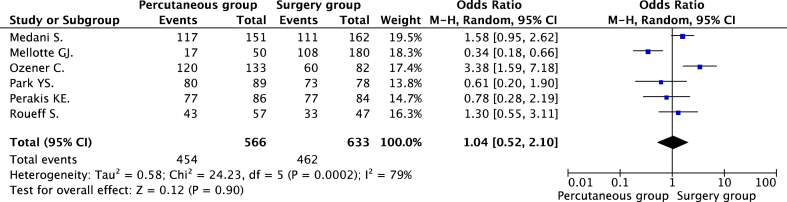
The main purpose of our study was to compare the 1- year catheter survival rate between the percutaneous group and surgery group. Six observational studies [21], [23], [24], [25], [26], [27] including 1199 procedures reported 1-year catheter survival as an outcome. The rate of 1-year catheter survival in the percutaneous group and surgery group were 80.2% (455/566) and 73.0% (462/633), respectively. The authors truncated 1 RCTs from the meta-analysis of the association between 1-year catheter survival and the catheter placement techniques to minimize the risk of heterogeneity. However, the evidence of the significance of heterogeneity between studies was still observed (P = 0.0002, I2 = 79%). The forest plots displaying the results of the meta-analysis of the 1-year catheter survival between the percutaneous group and surgery group is illustrated in Fig. 2.
Fig. 2.
Forest plots of the association between 1-year catheter survival and catheter implantation methods.
The pooled analysis did not demonstrate a significant difference in 1-year catheter survival between the percutaneous and surgery groups. (OR = 1.04, 95% CI = 0.52–2.10, P = 0.90). No evidence of publication bias was observed by either Egger's test (P = 0.740) or rank correlation test (P = 0.348).
3.2. Overall infectious complication
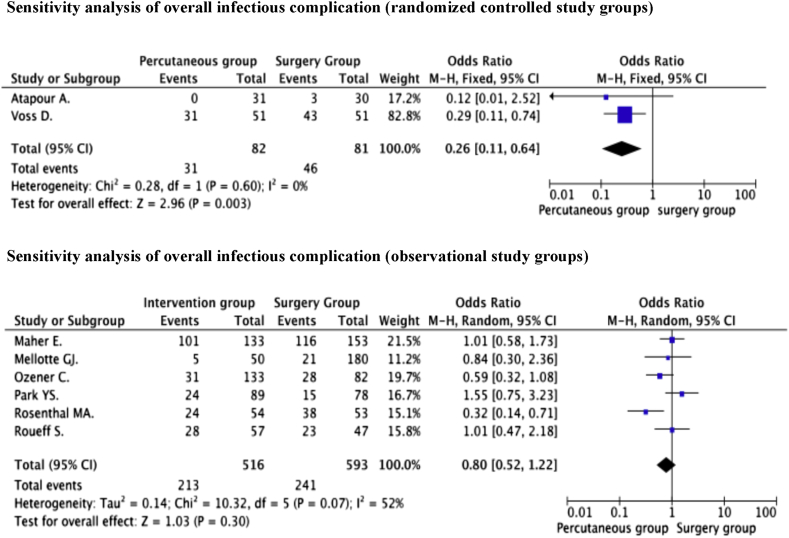
Eight studies [21], [22], [23], [26], [27], [28], [29], [30] reported the incidence of overall infectious complication in the percutaneous group and surgery group as 40.8% (244/598) and 42.6% (287/674), respectively. The authors conducted a meta-analysis to compare the infectious complication rates between the percutaneous group and surgery group Since the evidence of significant heterogeneity was observed between the grouped RCTs and the grouped observational studies, separation of sensitivity analysis was preformed on the respective groups.
The pooled data of the sensitivity analysis from 2 RCTs [22], [28] reported that incidence of overall infectious complications was 37.8% (31/82) and 56.8% (46/81) in the percutaneous and surgery groups respectively. The pooled analysis of the odds ratio indicated that the incidence of overall infectious complications was significantly lower in the percutaneous group compared to the surgery group (OR = 0.26, 95% CI = 0.11–0.64, P = 0.003) without evidence of significant heterogeneity (P = 0.60, I2 = 0%).
In contrast, the sensitivity analysis from 6 observational studies [21], [25], [26], [27], [29], [30], demonstrates no significant difference in the incidence of overall infectious complication between both catheter placement techniques [41.3% and 40.6% (percutaneous vs. surgery)] (OR = 0.80, 95% CI = 0.52–1.22, P = 0.30). The evidence of moderate heterogeneity was observed between the included studies (P = 0.07, I2 = 52%). No evidence of publication bias was identified through either Egger's test (P = 0.774) or by the rank correlation test (P = 0.348). The forest plots displayed the results of the sensitivity-analysis of the overall infectious complications between the percutaneous group and surgery group is illustrated in Fig. 3.
Fig. 3.
The forest plots displayed the results of the sensitivity-analysis of the overall infectious complications.
3.3. Overall mechanical complication
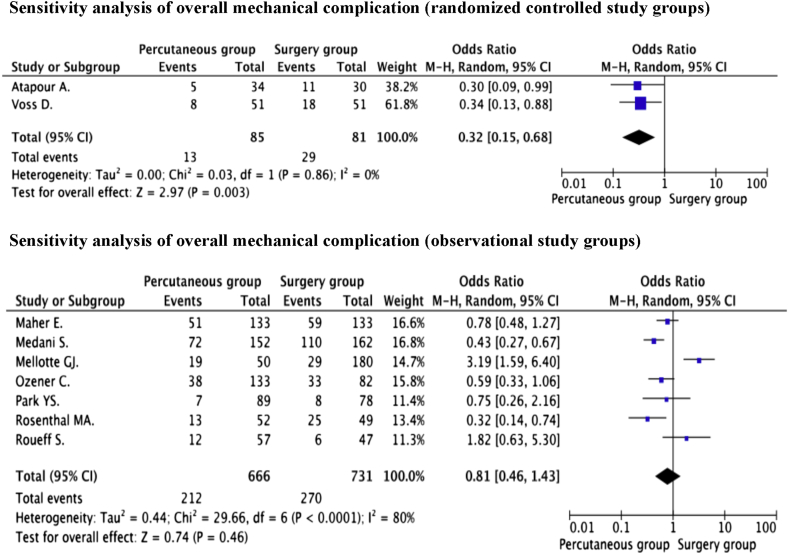
From nine studies [21], [22], [23], [24], [26], [27], [28], [29], [30], 1582 procedures were enrolled to assess the association between overall mechanical complication and peritoneal catheter placement technique. The incidence of overall mechanical complication in the percutaneous group and surgery group was 30.0% (225/750) and 35.9% (299/832), respectively. Given that significant heterogeneity was observed between studies concerning both infectious and mechanical complications, the authors thus conducted a separate sensitivity analysis of RCTs and observational studies in both study groups.
The pooled ORs of the sensitivity analysis from 2 RCTs [22], [28] indicated that the incidence of overall mechanical complication was significantly lower in the percutaneous group than in the surgery group [15.3% and 35.8% (percutaneous vs. surgery)] (OR = 0.32, 95% CI = 0.15–0.68, P = 0.003) without evidence of significant heterogeneity (P = 0.86, I2 = 0%).
In contrast, the pooled data of the sensitivity analysis from 7 observational studies [21], [24], [25], [26], [27], [29], [30] showed no significant difference in the incidence of overall mechanical complications in either catheter placement techniques [31.8% and 36.9% (percutaneous vs. surgery)] (OR = 0.81, 95% CI = 0.46–1.43, P = 0.46). Significant heterogeneity between the studies was found (P < 0.0001, I2 = 80%). No evidence of publication bias was identified by either Egger's test (P = 0.453) or the rank correlation test (0.293). The forest plots displayed the results of the sensitivity-analysis of the overall infectious complications between the percutaneous group, and surgery group is illustrated in Fig. 4.
Fig. 4.
The forest plots displayed the results of the sensitivity-analysis of the overall mechanical complications.
3.4. Subgroup analysis
The authors conducted the subgroup analysis to assess the association between catheter placement technique and catheter –related complications in the purpose to identify the source of heterogeneity in the study.
Sixes studies [21], [22], [25], [27], [29], [30] reported the rate of peritonitis as 25.9% (110/424) and 23.1% (132/572) in the percutaneous and surgery groups respectively. The pooled analysis of the odds ratio indicated no significant difference in the peritonitis rate (OR = 0.93, 95% CI = 0.54–1.60, P = 0.79) between both techniques. The evidence of significant heterogeneity was also observed (P = 0.02, I2 = 63%).
The pooled ORs from 7 studies [21], [22], [25], [27], [28], [29], [30] indicated that the rate of the tunnel and exit site infection was not significantly different between either catheter placement techniques [22.2% and 21.5% (percutaneous vs. surgery)] (OR = 0.85, 95% CI = 0.62–1.17, P = 0.33.
Nine studies [21], [22], [24], [25], [26], [27], [28], [29], [30] reported the leakage rate as 11.0% (82/749) and 12.0% (100/836) in the percutaneous and surgery group respectively. The pooled data demonstrated no significant differences in the leakage between both techniques (OR = 1.04, 95% CI = 0.58–1.86, P = 0.91) with evidence of moderate heterogeneity (P = 0.02, I2 = 57%).
Eight studies [21], [24], [25], [26], [27], [28], [29], [30] reported the inflow-outflow obstruction and catheter malfunction rate of 12.8% (90/701) and 15.5% (122/785). [Percutaneous vs. surgery group]. The result demonstrated that the inflow-outflow obstruction and catheter malfunction rate were not significantly different between both techniques (OR = 0.73, 95% CI = 0.50–1.08, P = 0.11) Furthermore, the rate of postoperative bleeding [2.9% and 4.4% (percutaneous vs. surgery)] (OR = 0.76, 95% CI = 0.31–1.87, P = 0.55) and incisional hernia [5.4% and 8.0% (percutaneous vs. surgery)] (OR = 0.75, 95% CI = 0.41–1.39, P = 0.37) were not found to be significantly different between percutaneous and surgery groups. The result of the meta-analyses of the association between postoperative complications with catheter placement techniques is summarized in Table 2.
Table 2.
The result of the meta-analyses of the association between postoperative complications with catheter placement techniques.
| Postoperative complications | No. of studies | n | Or | 95% CI | P value | Heterogeneity I2P | Egger's test | Rank-correlation test | |
|---|---|---|---|---|---|---|---|---|---|
| Infectious compilation | |||||||||
| Peritonitis | 7 | 996 | 0.93 | 0.54–1.60 | 0.79 | 63% | 0.02a | 0.879 | 0.851 |
| Tunnel and Exit site infection | 7 | 1057 | 0.85 | 0.62–1.17 | 0.33 | 0% | 0.88 | 0.067 | 0.652 |
| Mechanical complication | |||||||||
| Leakage | 9 | 1585 | 1.04 | 0.58–1.86 | 0.91 | 57% | 0.02a | 0.632 | 0.677 |
| Inflow-outflow obstruction and catheter malfunction | 8 | 1486 | 0.73 | 0.50–1.08 | 0.11 | 30% | 0.19 | 0.589 | 0.805 |
| Bleeding | 4 | 565 | 0.76 | 0.31–1.87 | 0.55 | 35% | 0.20 | 0.737 | 1.000 |
| Hernia | 4 | 710 | 0.75 | 0.41–1.39 | 0.11 | 0% | 0.71 | 0.863 | 0.497 |
OR = Odds ratio, Cl = confidence interval.
Statistical significant.
4. Discussion
The crucial role for the success of long-term peritoneal dialysis is to mimic the risk of catheter-related complication. Catheter-related complications may lead to technical failure, which directly affect the long-term catheter survival and can ultimately lead to permanent conversion to hemodialysis in up to 20% of patients [31]. Several techniques are established for the PD catheter implantation including conventional open surgical and percutaneous placement methods. The open surgery technique is associated with high incidence of catheter malfunction and the percutaneous technique may cause visceral organ perforation and catheter malposition [32].
Currently, laparoscopic-assisted technique is an alternative method for PD catheter placement; it offers less pain, earlier recovery and the proper catheter placement. Laparoscopic-assisted technique provides a higher one-year catheter survival than that of open surgery. However, there are no significant differences in length of hospital stay, early and late complications, including infection, migration, leakage, and hernia [33], [34].
Jacobs et al. reported a fluoroscopic guidance technique for PD catheters placement in 1992 [6]. This technique can be perform under local anesthesia and demonstrates a safe and cost-effective for alternative for PD placement with a low complication rate. The benefit of radiologic guidance technique is ease of correct catheter placement. However, a major disadvantage of this method is the inability to perform other surgical interventions simultaneously [12], [22].
In the present study, a meta-analysis was conducted to evaluate the post-operative outcomes, comparing the catheters inserted by surgical versus percutaneous method. This meta-analysis aims to evaluate the 1-year catheter survival. The pooled result demonstrated that the 1- year survival rate in both techniques is nearly 80%, which was not significantly different and is considered as a satisfactory outcome [20], [26].
Our results were somewhat complicated by the fact that significant heterogeneity was identified in almost all of the results. We found the evidences of heterogeneity in the association between catheter implantation methods and 1-year catheter survival, overall infectious/mechanical complication. Therefore, the pooled data were separated into randomized controlled study groups and observational study groups in the sensitivity analysis of the post-operative outcomes to mimic the risk of heterogeneity between the included studies.
The percutaneous group reported lower overall unfavorable outcomes. The sensitivity analysis on the association between overall infectious and mechanical complications in the observational study groups did not demonstrate a significant difference between surgical and percutaneous groups. In contrast, the sensitivity analysis of the RCTs revealed that the incidence of overall infectious and mechanical complications were significantly lower in the percutaneous group compared to the surgical group.
This finding could be explained by the varied patient characteristics and selection criteria in the studies which may affect the incidence of post-operative complications: for instance the presence of diabetes mellitus, severe co-morbidities, history of previous abdominal surgery, the use of prophylactic antibiotic, and obesity (BMI > 28–35 kg/m2) [21], [25], [30]. 2) There was a limited number of randomized controlled studies in this meta-analysis. Thus, the positive results in a small sample size may contribute to a significant difference in post-operative outcomes.
Infectious and mechanical complications are associated with catheter failure and usually leading to conditions requiring catheter removal, affecting the 1-year catheter survival [30], [35], [36]. The subgroup analysis revealed no significant differences in incidence of complications between both groups; this includes mechanical complications such as leakage, bleeding, hernia, inflow-outflow obstruction and catheter malfunctions, and infectious complications such as peritonitis, tunnel and exit site infection. Consequently, this may result in similar 1-year catheter survival rates in both study groups.
As described above, the heterogeneity observed in the meta-analysis can be explained by; 1) the methodology that combined the two patient groups—conventional open surgery and laparoscopic-assisted technique for PD catheter insertion—into the surgery group as there was a small number of studies utilizing laparoscopic assisted techniques, 2) the percutaneous group included both fluoroscopic guided and non fluoroscopic guided techniques, and 3) the variety of types of PD catheters used in different studies, which may affect catheter infection rates [37].
In order to compensate for this effect, we adopted a random-effects model to calculate the OR, which renders more conservative results in situations where significant heterogeneity occurred. Furthermore, seven of ten studies included have excluded patients with previous abdominal surgery and body mass index (BMI) > 28–35 kg/m2. This may have led to a potential selection bias in the studies. However, evidence of publication bias was not observed in the Egger's linear regression test and the rank correlation test.
The present report also strongly suggests that percutaneous technique is safe, effective, and offers equivalent and satisfactory outcomes as a surgical technique for peritoneal dialysis catheter placement, especially in the selected patients (such as no previous abdominal surgery, BMI < 28 kg/m2.). Established on the result of 1-year catheter survival, a power calculation with 0.05% significance level and 80% power was used. Future RCTs will need to enroll 620 peritoneal dialysis catheter insertion procedures in each arm to rule out a 12.0% relative risk reduction (7.2% absolute risk reduction) in the 1-year catheter survival rates.
5. Conclusion
The present study shows the incidence of overall infectious and mechanical complications were more frequent in the surgery group compared to the percutaneous group. However, there was no difference in the 1-year catheter survival rate associated with the PD catheter placement techniques. Thus, percutaneous placement of PD catheter offers an effective and safe alternative surgical technique and should be considered in selected patients (such as no previous abdominal operation, BMI < 28 kg/m2.).
Disclosure statement
This study receives a grant support from Srinakharinwirot University.
Acknowledgements
The authors thank the surgical staff at the Department of Surgery, Faculty of Medicine, Srinakharinwirot University, for the acquisition of data.
References
- 1.Fenton S.S., Schaubel D.E., Desmeules M., Morrison H.I., Mao Y., Copleston P. Hemodialysis versus peritoneal dialysis: a comparison of adjusted mortality rates. Am. J. Kidney Dis. 1997;30(3):334–342. doi: 10.1016/s0272-6386(97)90276-6. [DOI] [PubMed] [Google Scholar]
- 2.Popovich R.P., Moncrief J.W., Nolph K.D., Ghods A.J., Twardowski Z.J. Pyle WK.Continuous ambulatory peritoneal dialysis. Ann. Intern Med. 1978;88(4):449–456. doi: 10.7326/0003-4819-88-4-449. [DOI] [PubMed] [Google Scholar]
- 3.Alloatti S., Manes M., Paternoster G., Gaiter A.M., Molino A., Rosati C. Peritoneal dialysis compared with hemodialysis in the treatment of end-stage renal disease. J. Nephrol. 2000;13(5):331–342. [PubMed] [Google Scholar]
- 4.Crabtree J.H. Fluoroscopic placement of peritoneal dialysis catheters: a harvest of the low-hanging fruits. Perit. Dial. Int. 2008;28(2):134–137. [PubMed] [Google Scholar]
- 5.Zaman F. Peritoneal dialysis catheter placement by nephrologist. Perit. Dial. Int. 2008;28(2):138–141. [PubMed] [Google Scholar]
- 6.Jacobs I.G., Gray R.R., Elliott D.S., Grosman H. Radiologic placement of peritoneal dialysis catheters: preliminary experience. Radiology. 1992;182(1):251–255. doi: 10.1148/radiology.182.1.1727292. [DOI] [PubMed] [Google Scholar]
- 7.Veys N., Biesen W.V., Vanholder R., Lameire N. Peritoneal dialysis catheters: the beauty of simplicity or the glamour of technicality? Percutaneous vs surgical placement. Nephrol. Dial. Transpl. 2002;17(2):210–212. doi: 10.1093/ndt/17.2.210. [DOI] [PubMed] [Google Scholar]
- 8.Eklund B.H., Honkanen E.O., Kala A.R., Kyllönen L.E. Peritoneal dialysis access: prospective randomized comparison of the Swan neck and Tenckhoff catheters. Perit. Dial. Int. 1995;15(8):353–356. [PubMed] [Google Scholar]
- 9.Goh B.L., Ganeshadeva Y.M., Chew S.E., Dalimi M.S. Does peritoneal dialysis catheter insertion by interventional nephrologists enhance peritoneal dialysis penetration? Semin. Dial. 2008;21(6):561–566. doi: 10.1111/j.1525-139X.2008.00478.x. [DOI] [PubMed] [Google Scholar]
- 10.Crabtree J.H., Burchette R.J. Effective use of laparoscopy for long-term peritoneal dialysis access. Am. J. Surg. 2009;198(1):135–141. doi: 10.1016/j.amjsurg.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Henderson S., Brown E., Levy J. Safety and efficacy of percutaneous insertion of peritoneal dialysis catheters under sedation and local anaesthetic. Nephrol. Dial. Transpl. 2009;24(11):3499–3504. doi: 10.1093/ndt/gfp312. [DOI] [PubMed] [Google Scholar]
- 12.Vaux E.C., Torrie P.H., Barker L.C., Naik R.B., Gibson M.R. Percutaneous fluoroscopically guided placement of peritoneal dialysis catheters–a 10-year experience. Semin. Dial. 2008;21(5):459–465. doi: 10.1111/j.1525-139X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 13.McCormick B.B., Bargman J.M. Noninfectious complications of peritoneal dialysis: implications for patient and technique survival. J. Am. Soc. Nephrol. 2007;18(12):3023–3025. doi: 10.1681/ASN.2007070796. [DOI] [PubMed] [Google Scholar]
- 14.Stuart S., Booth T.C., Cash C.J., Hameeduddin A., Goode J.A., Harvey C. Complications of continuous ambulatory peritoneal dialysis. Radiographics. 2009;29(2):441–460. doi: 10.1148/rg.292085136. [DOI] [PubMed] [Google Scholar]
- 15.Li P.K., Szeto C.C., Piraino B., Bernardini J., Figueiredo A.E., Gupta A. International Society for Peritoneal Dialysis. Peritoneal dialysis-related infections recommendations: 2010 update. Perit. Dial. Int. 2010;30(4):393–423. doi: 10.3747/pdi.2010.00049. [DOI] [PubMed] [Google Scholar]
- 16.Singh N., Davidson I., Minhajuddin A., Gieser S., Nurenberg M., Saxena R. Risk factors associated with peritoneal dialysis catheter survival: a 9-year single-center study in 315 patients. J. Vasc. Access. 2010;11(4):316–322. doi: 10.5301/jva.2010.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo A., Mujais S. Patient and technique survival on peritoneal dialysis in the United States: evaluation in large incident cohorts. Kidney Int. Suppl. 2003;88:S3–S12. doi: 10.1046/j.1523-1755.2003.08801.x. [DOI] [PubMed] [Google Scholar]
- 18.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Gokal R., Alexander S., Ash S., Chen T.W., Danielson A., Holmes C. Peritoneal catheters and exit-site practices toward optimum peritoneal access: 1998 update. (Official report from the International Society for Peritoneal Dialysis) Perit. Dial. Int. 1998;18(1):11–33. [PubMed] [Google Scholar]
- 20.Wells G., Shea B., O'Connell D. Ottawa Health Research Institute; Ottawa (ON): 2010. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. [Google Scholar]
- 21.Park Y.S., Min S.I., Kim D.K., Oh K.H., Min S.K., Kim S.M. The outcomes of percutaneous versus open placement of peritoneal dialysis catheters. World J. Surg. 2014;38(5):1058–1064. doi: 10.1007/s00268-013-2346-5. [DOI] [PubMed] [Google Scholar]
- 22.Voss D., Hawkins S., Poole G., Marshall M. Radiological versus surgical implantation of first catheter for peritoneal dialysis: a randomized non-inferiority trial. Nephrol. Dial. Transpl. 2012;27(11):4196–4204. doi: 10.1093/ndt/gfs305. [DOI] [PubMed] [Google Scholar]
- 23.Perakis K.E., Stylianou K.G., Kyriazis J.P., Mavroeidi V.N., Katsipi I.G., Vardaki E.A. Long-term complication rates and survival of peritoneal dialysis catheters: the role of percutaneous versus surgical placement. Semin. Dial. 2009;22(5):569–575. doi: 10.1111/j.1525-139X.2009.00621.x. [DOI] [PubMed] [Google Scholar]
- 24.Medani S., Shantier M., Hussein W., Wall C., Mellotte G. A comparative analysis of percutaneous and open surgical techniques for peritoneal catheter placement. Perit. Dial. Int. 2012;32(6):628–635. doi: 10.3747/pdi.2011.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roueff S., Pagniez D., Moranne O., Roumilhac D., Talaszka A., Le Monies De Sagazan H. Simplified percutaneous placement of peritoneal dialysis catheters: comparison with surgical placement. Perit. Dial. Int. 2002;22(2):267–269. [PubMed] [Google Scholar]
- 26.Ozener C., Bihorac A., Akoglu E. Technical survival of CAPD catheters:comparison between percutaneous and conventional surgical placement techniques. Nephrol. Dial. Transpl. 2001;16(9):1893–1899. doi: 10.1093/ndt/16.9.1893. [DOI] [PubMed] [Google Scholar]
- 27.Mellotte G.J., Ho C.A., Morgan S.H., Bending M.R., Eisinger A.J. Peritoneal dialysis catheters: a comparison between percutaneous and conventional surgical placement techniques. Nephrol. Dial. Transpl. 1993;8(7):626–630. [PubMed] [Google Scholar]
- 28.Atapour A., Asadabadi H.R., Karimi S., Eslami A., Beigi A.A. Comparing the outcomes of open surgical procedure and percutaneously peritoneal dialysis catheter (PDC) insertion using laparoscopic needle: a two month follow-up study. J. Res. Med. Sci. 2011;16(4):463–468. [PMC free article] [PubMed] [Google Scholar]
- 29.Maher E., Wolley M.J., Abbas S.A., Hawkins S.P., Marshall M.R. Fluoroscopic versus laparoscopic implantation of peritoneal dialysis catheters: a retrospective cohort study. J. Vasc. Interv. Radiol. 2014;25(6):895–903. doi: 10.1016/j.jvir.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal M.A., Yang P.S., Liu I.L., Sim J.J., Kujubu D.A., Rasgon S.A. Comparison of outcomes of peritoneal dialysis catheters placed by the fluoroscopically guided percutaneous method versus directly visualized surgical method. J. Vasc. Interv. Radiol. 2008;19(8):1202–1207. doi: 10.1016/j.jvir.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Maio R., Figueiredo N., Costa P. Laparoscopic placement of Tenckhoff catheters for peritoneal dialysis: a safe, effective, and reproducible procedure. Perit. Dial. Int. 2008;28:170–173. [PubMed] [Google Scholar]
- 32.Lund L. Peritoneal dialysis catheter placement: is laparoscopy an option? Int. Urol. Nephrol. 2007;39:625–628. doi: 10.1007/s11255-007-9193-y. [DOI] [PubMed] [Google Scholar]
- 33.Xie H., Zhang W., Cheng J., He Q. Laparoscopic versus open catheter placement in peritoneal dialysis patients: a systematic review and meta-analysis. BMC Nephrol. 2012;27(13):69. doi: 10.1186/1471-2369-13-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagen S.M., Lafranca J.A., Steyerberg E.W., IJzermans J.N., Dor F.J. Laparoscopic versus open peritoneal dialysis catheter insertion: a meta-analysis. PLoS One. 2013;8(2):e56351. doi: 10.1371/journal.pone.0056351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy C., Dybbro P.E., Guest S. Fluoroscopically guided percutaneous peritoneal dialysis catheter placement: single center experience and review of the literature. Ren. Fail. 2010;32(3):294–299. doi: 10.3109/08860220903548932. [DOI] [PubMed] [Google Scholar]
- 36.Peppelenbosch A., van Kuijk W.H.M., Bouvy N.D., van der Sande F.M., Tordoirl J.H.M. Peritoneal dialysis catheter placement technique and complications. NDT Plus. 2008;1(Suppl. 4):iv23–iv28. doi: 10.1093/ndtplus/sfn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eklund B.H., Honkanen E.O., Kala A.R., Kyllönen L.E. Peritoneal dialysis access: prospective randomized comparison of the Swan neck and Tenckhoff catheters. Perit. Dial. Int. 1995;15(8):353–356. [PubMed] [Google Scholar]