Abstract
This work investigated the effects of cellular encapsulation density and differentiation stage on the osteogenic capacity of injectable, dual physically and chemically gelling hydrogels comprised of thermogelling macromers and polyamidoamine crosslinkers. Undifferentiated and osteogenically predifferentiated mesenchymal stem cells (MSCs) were encapsulated within 20 wt% composite hydrogels with gelatin microparticles at densities of 6 or 15 million cells/mL. We hypothesized that a high encapsulation density and predifferentiation would promote increased cellular interaction and accelerate osteogenesis, leading to enhanced osteogenic potential in vitro. Hydrogels were able to maintain the viability of the encapsulated cells over a period of 28 days, with the high encapsulation density and predifferentiation group possessing the highest DNA content at all timepoints. Early alkaline phosphatase activity and mineralization were promoted by encapsulation density, whereas this effect by predifferentiation was only observed in the low seeding density groups. Both parameters only demonstrated short-lived effects when examined independently, but jointly led to greater levels of alkaline phosphatase activity and mineralization. The combined effects suggest that there may be optimal encapsulation densities and differentiation periods that need to be investigated to improve MSCs for biomaterials-based therapeutics in bone tissue engineering.
Keywords: mesenchymal stem cell, predifferentiation, N-isopropylacrylamide, bone tissue engineering
1. Introduction
Tissue engineering strategies may involve the combination of biomaterial scaffolds, cells, and growth factors to repair and replace injured or lost tissue. These strategies are particularly relevant in the aesthetic and functional reconstruction of bone, where the gold standard currently is the use of autologous bone taken from the patient. Autografts, while effective, are scarce, and difficult to contour to irregular-shaped defects, and require invasive surgeries to acquire and implant. Thus, synthetic bone graft substitutes – especially those involving injectable materials that allow for injectable administration, in situ formation and contouring, and localized delivery of cells – are attractive candidates for craniofacial tissue engineering strategies. [1, 2]
Previous work developed and characterized injectable, in situ forming hydrogels comprising copolymers of N-isopropylacrylamide (NiPAAm), dimethyl-γ-butyrolactone acrylate (DBA), glycidyl methacrylate (GMA), and acrylic acid (AA) that can both thermally gel and chemically crosslink with biodegradable, diamine-functionalized polyamidoamine (PAMAM) crosslinkers. These hydrogels have shown promise for bone tissue engineering applications, as they possess tunable physicochemical properties and hydrophobicity-dependent mineralization. Encapsulation of mesenchymal stem cells (MSCs), which undergo established differentiation down the osteogenic lineage [3], in conjunction with gelatin microparticles, which act as sites for cellular attachment and mineral nucleation [4, 5], demonstrate the hydrogel’s ability to support cell viability and differentiation in vitro and bone formation in vivo.[6, 7]
However, despite the performances of these hydrogels for bone regeneration, the resulting bone is not yet robust. Numerous investigations have shown that successful cellular delivery leading to tissue growth not only depends on morphological, adhesive, or mechanical cues from the biomaterial environment, but also biochemical signals from other cells.[8, 9] Cell seeding density has been shown to affect the viability and differentiation of encapsulated stem cells by promoting increased cellular crosstalk.[10] The differentiation stage of MSCs is also an important factor, as shown by increased success of predifferentiated MSCs for hyaline cartilage regeneration in large animal osteochondral defects [11, 12] and bone healing in nonunions.[13] The inclusion of predifferentiated MSCs within hydrogels and altering the seeding density may thus affect the formation of a mineralized matrix and provide the necessary osteoconductive and osteoinductive signals to stimulate cellular activity and bone regeneration.
The objective of this study was to evaluate the effects of cellular parameters such as encapsulation density and predifferentiation on the osteogenic potential of the cell-laden hydrogel composites in vitro. We hypothesized that a high cell seeding density and cellular predifferentiation would promote cellular crosstalk to enhance encapsulated MSC viability and hydrogel mineralization, leading to enhanced osteogenic potential of the hydrogel system for bone tissue engineering applications.
2. Materials and Methods
2.1 Materials
NiPAAm, dimethyl-γ-butyrolactone acrylate (DBA), glycidyl methacrylate (GMA), acrylic acid (AA), 2,2’-azobis(2-methylpropionitrile) (azobisisobutyronitrile, AIBN), N,N’-methylenebisacrylamide (MBA), piperazine (PiP), glycine, and glutaraldehyde were purchased from Sigma Aldrich (Sigma, St. Louis, MO) and used as received. Anhydrous 1,4-dioxane, diethyl ether, and acetone in analytical grade; and water, acetonitrile, chloroform, and methanol in HPLC-grade were purchased from VWR (Radnor, PA) and used as received. Phosphate buffered saline (PBS) (powder, pH 7.4) was obtained from Gibco Life, Grand Island, NY. Ultrapure water was obtained from a Millipore Super-Q water system (Millipore, Billerica, MA). Acidic gelatin (isoelectric point = 5.0) was obtained from Nitta Gelatin (Osaka, Japan). Complete osteogenic medium (COM) was made from minimal essential medium α modification (αMEM) (Gibco Life, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) (Cambrex BioScience, Walkersville, MD), 10−8 M dexamethasone, 10 mM β-glycerol 2-phosphate, 50 mg/L ascorbic acid, and 10 mL/L antibiotic-antimycotic solution (Gibco, Life, Grand Island, NY). Live/Dead viability/cytotoxicity kit was purchased from Molecular Probes (Eugene, OR). The calcium assay was purchased from Genzyme Diagnostics (Cambridge, MA).
2.2 Thermogelling Macromer (TGM) Synthesis and Characterization
The P(NiPAAm-co-GMA-co-DBA-co-AA) TGM, as shown in Figure 1b, was synthesized according to published protocols.[6] Briefly, 10 g of NiPAAm, GMA, DBA and AA were dissolved in 100 mL of anhydrous 1,4-dioxane under nitrogen at 65°C. AIBN pre-dissolved in the solvent was added at 0.7% of total mol content to thermally initiate free radical polymerization, and the reaction mixture was stirred for 16 h. After solvent removal by rotary evaporation, the material was re-dissolved in pure acetone and purified twice via dropwise precipitation in at least 10× excess diethyl ether. The recovered polymer was air-dried overnight and transferred to a vacuum oven for several days prior to elemental analysis. The chemical composition of the TGMs was determined by proton nuclear magnetic resonance spectroscopy (1H NMR, Bruker, Switzerland) in D2O at a concentration of 20 mg/mL that contained 0.75 wt% 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid, sodium salt as an internal shift reference (Sigma-Aldrich, St. Louis, MO). Acid titration was performed in conjunction with 1H NMR to determine the AA content of the TGMs before hydrolysis. Aqueous gel permeation chromatography using a Waters Alliance HPLC system (Milford, MA) and differential refractometer (Waters, model 410) equipped with a series of analytical columns (Waters Styragel guard column 20 mm, 4.6 × 30 mm; Waters Ultrahydrogel column 1000, 7.8 × 300 mm) was used to determine the molecular weight distributions of the synthesized TGMs. The weight average molecular weight (Mw), number average molecular weight (Mn), and polydispersity index (PDI = Mw/Mn) of the hydrolyzed polymers were determined by comparison to commercially available narrowly dispersed molecular weight poly(ethylene glycol) standards (Waters, Mississauga, ON). For this study, P(NiPAAm85.6-co-GMA6.0-co-DBA5.5-co-AA2.9) with a Mn = 36.4 kDa and PDI of 3.8 was used to form 20 wt% (w/v) hydrogels.
Figure 1.
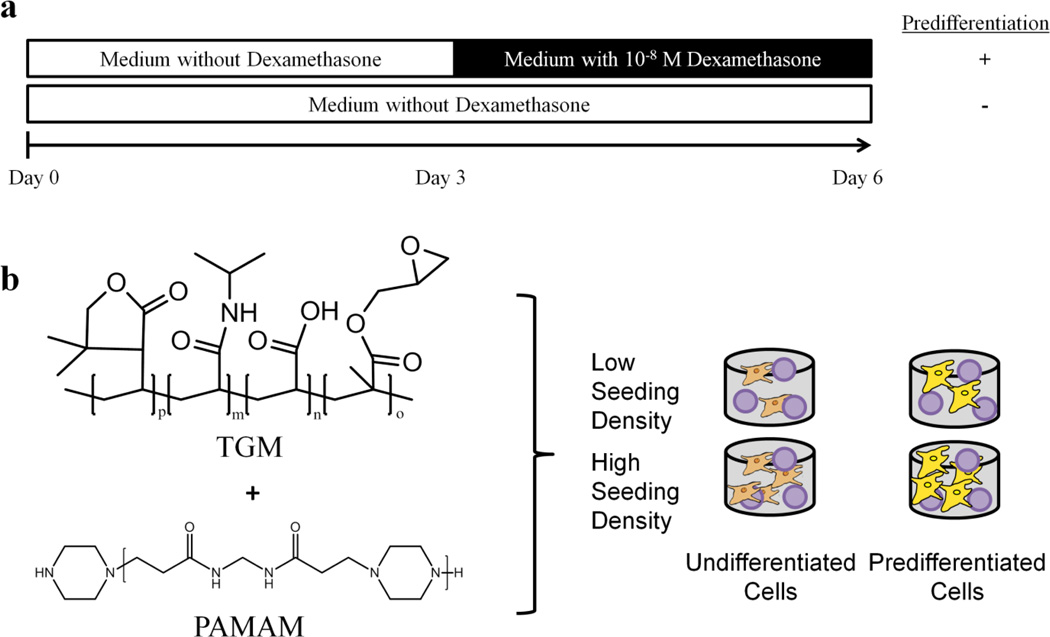
a) Scheme for osteogenic predifferentiation of MSCs in vitro. MSCs were cultured in complete osteogenic medium containing 10−8 M dexamethansone following 3 days of expansion. Cells were encapsulated within hydrogels after 6 days of in vitro culture. b) Chemical structure of the thermogelling macromers (TGM) and polyamidoamine (PAMAM) crosslinker comprising the injectable hydrogel system for encapsulation of gelatin microparticles (purple spheres) with undifferentiated or predifferentiated MSCs.
2.3 PAMAM Synthesis and Characterization
The PAMAM crosslinker, as shown in Figure 1b, was synthesized by the polyaddition of PiP and MBA at a stoichiometric molar ratio of [MBA]/[PiP] = 0.75 following previously reported protocols.[14, 15] Molecular weight distributions of the synthesized PAMAM crosslinkers were analyzed using time-of-flight mass spectroscopy with positive-mode electrospray ionization on a Bruker microTOF ESI spectrometer (Bruker Daltonics, Billerica, MA) equipped with a 1200 series HPLC (Agilent Technologies, Santa Clara, CA) to deliver the mobile phase (50:50 HPLC-grade water and methanol). After data acquisition, all peaks (including degradation and secondary reaction products) were identified using microTOF control software (Bruker). The peaks were corrected for charge state (generally with H+ or Na+ and rarely K+ ions), and quantified for calculation of Mn, Mw, and PDI. PAMAM with Mn = 1440 and PDI = 1.38 was used for the MSC encapsulation studies.
2.4 GMP Synthesis
GMPs with 50–100 µm diameter were synthesized through a water-in-oil emulsion followed by crosslinking in 10 mM glutaraldehyde solution and quenching with glycine as previously described.[16] Following fabrication, the GMPs were vacuum filtered, flash frozen in liquid nitrogen, lyophilized, and sieved. GMPs were incorporated into hydrogels at 20 mg dry GMP mass/100 mg dry hydrogel mass to achieve 20 wt% (w/w) loading as previously established.[17]
2.5 MSC Harvest and Culture
MSCs were harvested from rat femora and tibiae of 6–8 week old Fisher 344 rats (Charles River Laboratories, Wilmington, MA) in accordance to approved protocols by the Rice Institutional Animal Care and Use Committee as previously described.[18] The MSCs were plated in 75 cm2 tissue culture flasks at 37°C under humidified, 5% CO2 atmosphere and cultured in COM without dexamethasone, which was replaced every 2–3 days.[19] The scheme for differentiation is shown in Figure 1a. To induce osteogenic pre-differentiation, MSCs were cultured as above for 3 days and then cultured in COM with dexamethasone for the same duration as previously described.[13, 20] Undifferentiated MSCs were cultured in COM without dexamethasone for 6 days prior to encapsulation.
2.6 MSC Encapsulation and In Vitro Culture
TGM and PAMAM polymers were UV sterilized for 3 h and GMPs were ethylene oxide sterilized for 12 h. 20 wt% (w/v) composite hydrogels (a TGM composition that we previously established for osteogenesis in vitro and in vivo) [6, 7] with 20 wt% (w/w) GMP loading were fabricated by combining the TGM and PAMAM crosslinker that were prepared at twice the desired concentrations in sterile PBS pH 7.4 at 4°C until dissolved. GMPs were partially swollen in PBS pH 7.4 for 15 h at 4°C prior to hydrogel encapsulation as performed previously [16] and transferred to the polymer mixture. After 6 days of culture, the MSCs were passaged and added to the polymer solutions at a final concentration of 6 or 15 million cells/mL hydrogel. The seeding density was chosen based on the sustained viability and successful osteogenic differentiation of rat MSCs in injectable oligo(poly(ethylene glycol) fumarate) hydrogels.[21–24] The solutions were manually mixed, pipetted into 8 mm × 2 mm autoclaved Teflon molds on a heat block, and allowed to crosslink at 37°C in an incubator for 2.5 h before in vitro culture. The formulations selected for in vitro investigation are listed in Table 1. The hydrogels and their acellular controls were placed in 2.5 mL medium in 12-well tissue culture plates and cultured for 0, 7, 14, 21, and 28 days in COM containing 10−8 M dexamethasone. At each timepoint, the hydrogels were soaked in PBS for 30 min, sliced in half, weighed, and processed for Live/Dead confocal imaging (n = 2 halves); DNA Picogreen assay, alkaline phosphatase (ALP) activity, and calcium biochemical assay (n = 4 halves each); and histological staining (n = 2 halves).
Table 1.
Groups for In Vitro Investigation
| Group | TGM wt% (W/v) |
GMP Loading (w/w) |
Encapsulation Density (cells/mL hydrogel) |
Predifferentiation |
|---|---|---|---|---|
| 1 | 20 | 20 | 6 × 106 | − |
| 2 | 20 | 20 | 6 × 106 | + |
| 3 | 20 | 20 | 15 × 106 | − |
| 4 | 20 | 20 | 15 × 106 | + |
2.7 Live/Dead Confocal Microscopy
The samples designated for Live/Dead confocal microscopy were cut into ~0.5 mm cross sectional slices with a hand-held razor blade as previously described [18] and incubated for 30 min with calcein AM (2 µM) and ethidium homodimer-1 (4 µM) in accordance with the Live/Dead viability/cytotoxicity kit instructions. The slices were then analyzed using a confocal microscope (LSM 510 META, Carl Zeiss, Germany) using a 10× objective. Argon and helium–neon lasers were used for excitation at 488 and 543 nm, respectively, and emission filters at 505–526 and 612–644 nm, respectively, were employed.
2.8 Biochemical Assays
Hydrogel halves for biochemical assays were stored in 500 µL of ultrapure water and stored at −20°C. Prior to analysis, samples were manually homogenized, passed through three freeze-thaw cycles, and probe sonicated for 8 s. Detailed protocols for biochemical assays are described elsewhere.[25] Double-stranded DNA correlating with cellularity was evaluated using DNA Picogreen assay (Invitrogen, Eugene, OR) in accordance to the manufacturer’s protocol, adjusted for background with acellular hydrogel controls, and normalized to hydrogel wet mass, which was shown to reach equilibrium swelling after 24 hours.[6] ALP activity was assessed with colorimetric assay using phosphatase substrate capsules in alkaline buffer solution against p-nitrophenol standards. For calcium assay, sample aliquots were digested overnight in equal parts 1N acetic acid to form a final 0.5N acetic acid solution and then evaluated according to the manufacturer’s instructions.
2.9 Histology
Hydrogels (n = 2 halves) were processed at each timepoint for hematoxylin & eosin (H&E) and von Kossa histological staining as previously described.[18, 26] Hydrogels were fixed at 37 °C for 24 h in 10% buffered formalin, dehydrated for 24h in 70% ethanol, and stored in Histo-prep for another 24 h prior to freezing at −20 °C and cryosectioning into 10 µm slices. H&E staining was performed using Mayer hematoxylin counterstained with eosin-Y (Sigma). Von Kossa staining was performed using a 2% silver nitrate solution incubated under UV for 20 min and unreacted silver nitrate was removed using 5% sodium thiosulfate. Sections were imaged with a light microscope (Eclipse E600, Nikon) and the background subtraction to correct for background lighting and dark current effects was performed.
2.10 Statistics
The data are presented as means ± standard deviation in triplicate, unless otherwise stated. Biochemical assays were analyzed using a Tukey’s post-hoc test with JMP v.11 statistics software (SAS Institute, Cary, NC).
3. Results
3.1 Live/Dead Confocal Microscopy
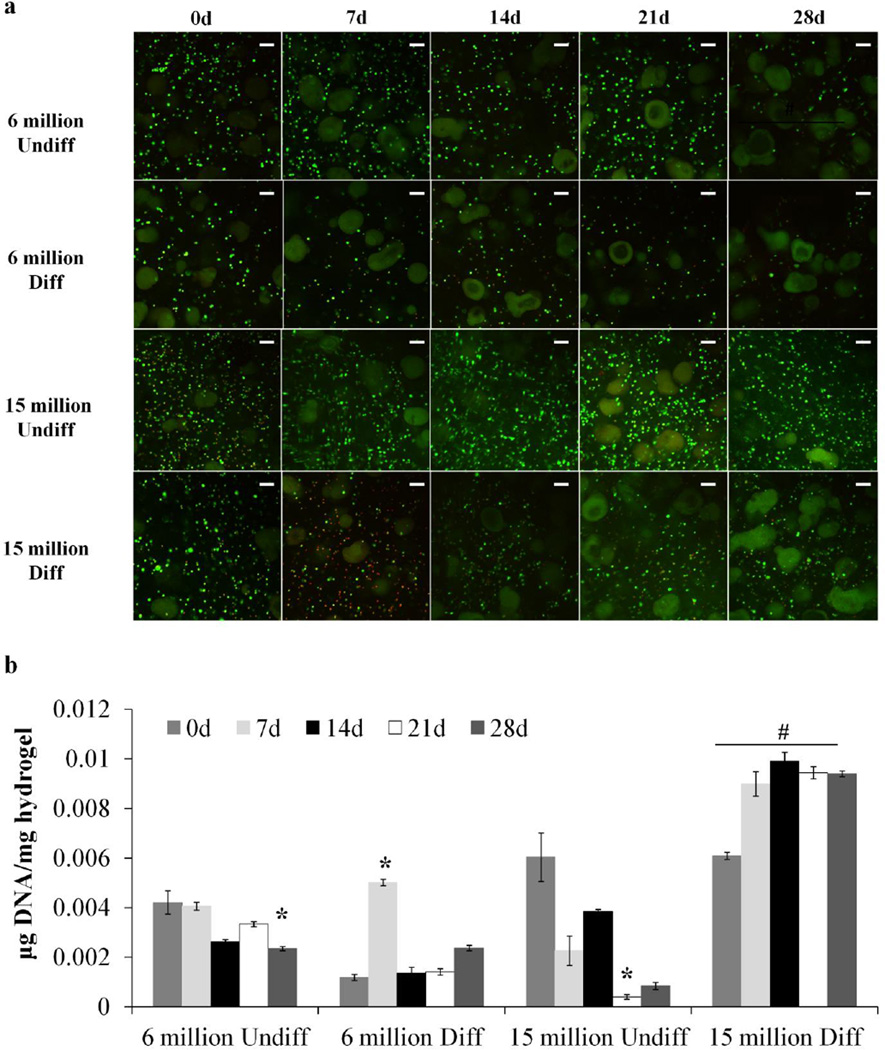
Undifferentiated and predifferentiated MSCs were successfully encapsulated at high and low seeding densities within injectable, dual-gelling composite hydrogels. Live/Dead confocal microscopy images of the samples are shown in Figure 2a. The cells were shown to be rounded, viable at encapsulation, and homogenously distributed within the hydrogels. The composite hydrogels sustained cell survival over the period of 28 days, as seen with the green fluorescent staining. Little red staining was observed due to interfering autofluorescence from the degrading composite gel, a phenomenon which has been observed in hydrogels of similar composition.[18, 27] More viable cells were qualitatively observed at the last timepoint with a high seeding density.
Figure 2.
a) Live/Dead confocal microscopy images of hydrogel composites with high and low seeding density (6 or 15 million cells/mL hydrogel) of undifferentiated (Undiff) or predifferentiated (Diff) MSCs for 28 days. Green stain indicates live cells and red stain indicates dead cells. Large irregular-shaped green particles are autofluorescing GMPs. Scale bar indicates 100 µm. b) DNA content of hydrogel composites with high or low seeding density (6 or 15 million cells/mL hydrogel) of undifferentiated (Undiff) or predifferentiated (Diff) MSCs compared to an acellular control. DNA content is normalized to hydrogel wet mass. (*) indicates significance from 0 day timepoint in the same group and (#) indicates significance from other groups at the same timepoint (p < 0.05).
3.2 Biochemical Assays
The DNA content of the hydrogels corresponding to cellularity is shown in Figure 2b. For the low seeding density groups, DNA content was maintained over time, with a significant decrease observed only at the 28d timepoint for the 6 million undifferentiated group. The discrepancy in the initial DNA content of the low seeding density groups is mostly likely due to variability in encapsulation and sampling following harvest. This trend was observed with the 15 million undifferentiated group, despite significant Live/Dead staining via confocal. However, the group with the high cell density and predifferentiation demonstrated the highest DNA content compared to all the other groups at all timepoints.
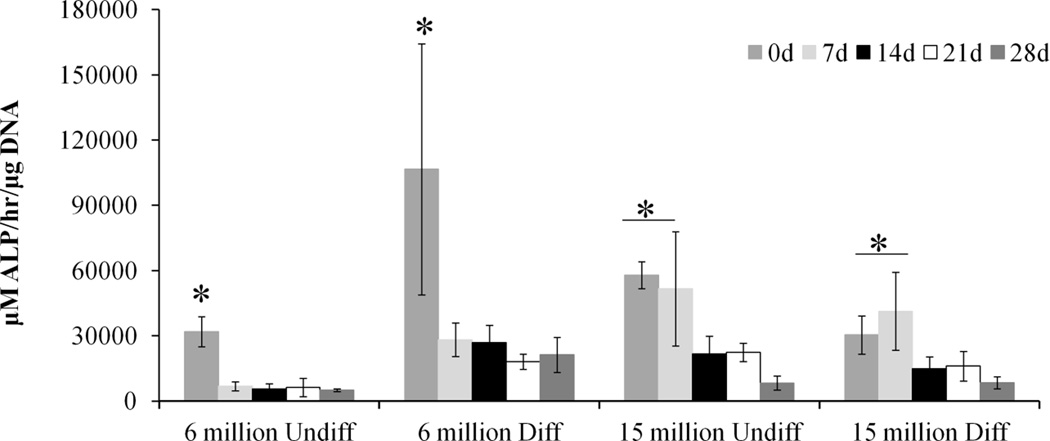
The production of ALP, an early osteogenic marker, was assessed using a p-nitrophenol assay, the results of which are shown in Figure 3. For all groups, ALP activity significantly declines from the 0 day timepoint and remains sustained for the remaining timepoints. A high cell seeding density helps maintain an elevated ALP activity initially, as seen in the 7 day timepoint. Also, predifferentiation improves the level of ALP activity at the 0 day timepoint in the group with low seeding density significantly over the corresponding undifferentiated group. However, the ALP levels at the later timepoints are not significantly different from the other groups.
Figure 3.
Normalized ALP activity of hydrogel composites with high or low seeding density (6 or 15 million cells/mL hydrogel) of undifferentiated (Undiff) or predifferentiated (Diff) MSCs. (*) indicates significance across group (p<0.05).
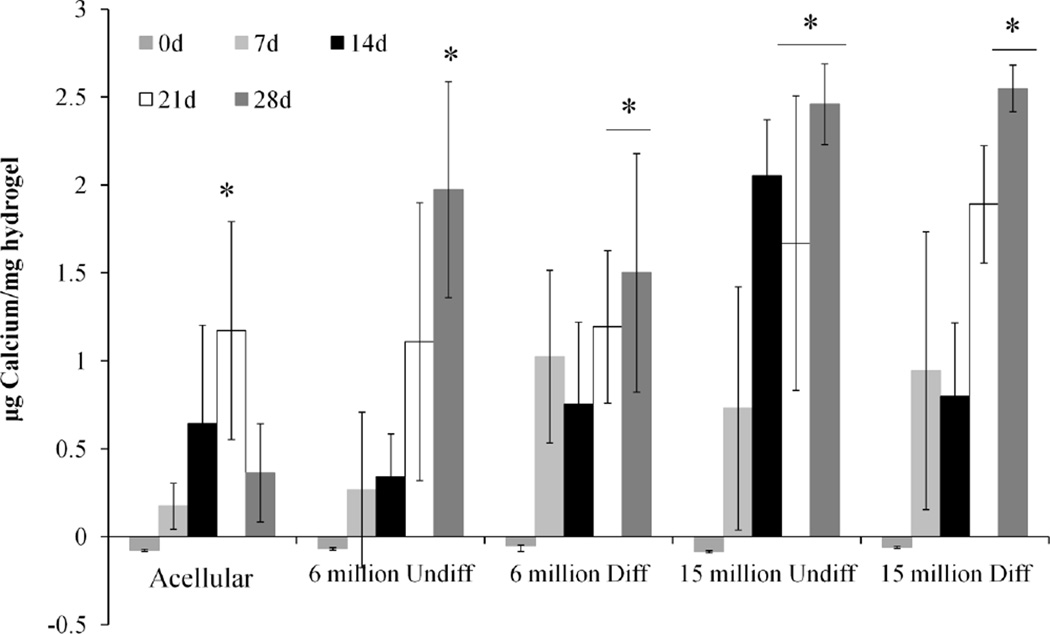
Figure 4 shows the calcium content of the hydrogels corresponding to mineralization. All groups, including the acellular control, demonstrate increases in the calcium content over time, with the cell-laden hydrogels showing significantly higher calcium content at the 28 day timepoint compared to the control. While there were no significant differences in the calcium content of the cell-laden hydrogels with the same seeding density at the 28 day timepoint, groups with high cell seeding density with or without predifferentiation saw enhanced mineralization at earlier timepoints. Predifferentiation at the low seeding density also enhanced mineralization at the earlier timepoint.
Figure 4.
Calcium content of hydrogel composites with high or low seeding density (6 or 15 million cells/mL hydrogel) of undifferentiated (Undiff) or predifferentiated (Diff) MSCs compared to an acellular control. Calcium content is normalized to hydrogel wet mass. (*) indicates significance from 0 day timepoint (p<0.05).
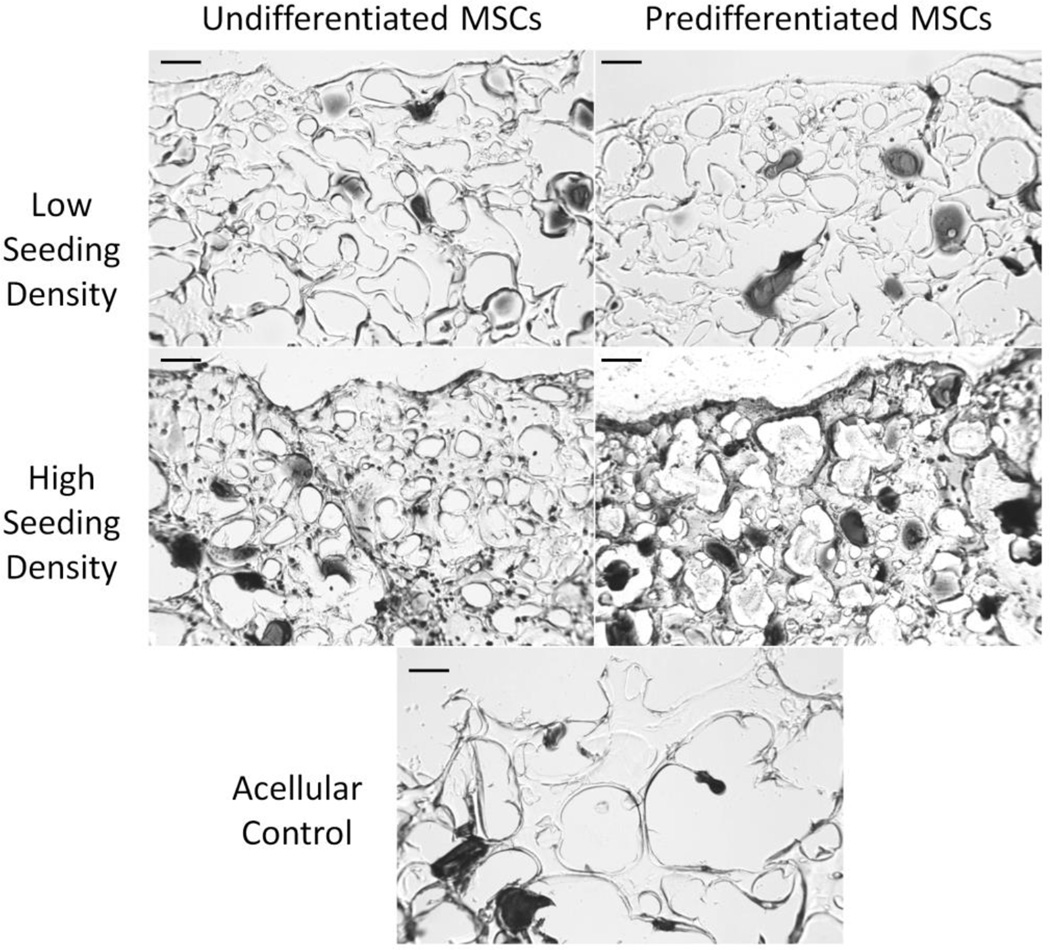
3.3 Histology
The hydrogels were stained for mineralization using a von Kossa reagent, as shown in Figure 5. Histological staining at 28 days shows mineralization primarily around the GMPs and the pericellular matrix around the cells, as seen in the black-brown stain. Hydrogels with predifferentiated cells at a high seeding density demonstrated staining in the hydrogel matrix as well.
Figure 5.
von Kossa staining of hydrogel composites with high or low seeding density (6 or 15 million cells/mL hydrogel, respectively) of undifferentiated or predifferentiated MSCs compared to an acellular control at 28 days. Black-brown stains correspond to phosphate deposits. Scale bar indicates 100 µm.
4. Discussion
Cell encapsulation density and predifferentiation have both been shown in the literature to affect the chondrogenesis and osteogenesis of cells, and thus, the therapeutic efficacy, of tissue engineered constructs for bone and cartilage regeneration. Using an injectable, dual-gelling hydrogel system shown to previously support osteogenic differentiation in vitro and promote bone formation and tissue infiltration in vivo [7], we examined the role of the aforementioned cell parameters on encapsulated cell viability and osteogenic differentiation, and hydrogel mineralization. Encapsulation density and predifferentiation independently did not show significant sustained effects as determined by confocal microscopy, histological staining, and biochemical assays. Encapsulation density was found to promote early ALP activity as well as mineralization, but those effects were short-lived if the cells were undifferentiated and did not affect DNA content, despite the greater Live/Dead staining via confocal in the later timepoints. Studies have suggested that successful extraction and potential degradation of the DNA, as well as cell number, may have limited the accurate quantitative assessment of cell viability within 3D scaffolds.[28] Cellular predifferentiation was also found to influence early ALP activity and mineralization for the low seeding density hydrogels, but could not compensate for the lower amount of cells. The decline of ALP activity over time can possibly be explained by the need for cellular attachment to substrates during differentiation, which is disrupted during the encapsulation process.[29] However, hydrogels with high seeding density of predifferentiated cells, showed combined effects that led to higher levels of sustained cell viability and initial ALP activity compared to the other groups. Overall, the encapsulation of cells within the hydrogel system was found to benefit mineralization at the later timepoints compared to the acellular controls – which showed reduced levels at 28 days – correlating with previous studies.[5] It is possible that the swelling experienced by acellular hydrogels with higher polymer content leads to increased diffusion that enables GMP degradation and loss of the accumulated mineral that is not experienced in a cellular system.[4] However, the amount of calcium per cell was not promoted by either predifferentiation or higher seeding density, suggesting that similar levels of mineralization can be achieved with less cellular manipulation.
The effect of cell seeding density has been widely investigated for both bone and cartilage tissue engineering, but the results have been mixed. Previous works report that MSC gene expression increases significantly with cell seeding density from 1 to 10 million cells/mL [21, 23, 24, 30] and plateaus around 25 million cells/mL.[31] However, some studies have shown that cell seeding densities up to 60 million cells/mL provide superior differentiation, mechanical strength, and ECM production in vitro, and tissue formation in vivo [24, 32, 33], while others determined that seeding density provided little functional benefit.[34, 35] In this study, cell encapsulation density did not demonstrate significant long term effects on osteogenic differentiation, which can be explained through several reasons. Although the published results vary, there is consensus that MSC paracrine signaling and direct crosstalk are important, thus controlling mass transport and scaffold architecture are vital to facilitating these interactions. Additionally, the literature suggests that there is an optimal cell encapsulation density to achieve the best tissue engineered construct.[36] It is possible that the seeding densities used in this study and degradation of the GMPs, which were designed to degrade within 2–4 weeks within collagenase-containing PBS [37], were not sufficient to encourage cellular interaction and generate a porous hydrogel structure, respectively. By examining a larger range of seeding densities, accelerating GMP degradation, or modifying hydrogel itself for more directed enzymatic degradation [32, 38] or cellular attachment [39, 40], the osteogenic potential of the system could be improved.
In addition to cellular interaction, the physical properties of a scaffold should be equally considered in combination with cell density to affect specific cellular behavior. Enhanced osteogenic behavior of MSCs has been observed with a previous generation of dual thermogelling and chemically crosslinking hydrogels with high hydrophobicity. Despite a low seeding density of 6×106 cells/mL hydrogel and altered loading ratios and sizes of GMPs, hydrogel mineralization significantly increased over time in vitro [4], peaking at much higher levels than in the present study and correlating with the higher levels bone formation for the acellular hydrophobic hydrogels in vivo.[7] However, when examining the present hydrogel system in a rat cranial defect, incorporation of GMPs with or without undifferentiated MSCs at high seeding density was sufficient to promote bony bridging and implant integration over the previous generation hydrogel.[5] Thus, rational design of the scaffold and cellular components of a tissue engineered construct must consider the differences in performances between in vitro and in vivo.
Cellular preconditioning down a specific lineage has had more consistent results in promoting tissue regeneration, especially for cartilage. Numerous studies have shown that chondrogenically differentiated MSCs and adipose-derived stem cells can promote cartilage gene expression and matrix production in vitro [41], and regenerate osteochondral defects in rabbit and ovine models.[10, 42] When examined for bone, predifferentiated MSCs have shown to significantly improve osteogenic gene expression in vitro and bone formation in vivo.[11, 12] However, in this study, predifferentiation only presented a strong effect with the high seeding density, which again suggests that there may be some optimization required for both cellular parameters to enhance regeneration. The MSCs were precultured in COM with dexamethasone, a potent synthetic glucocorticoid that has been shown to strongly influence osteogenesis of MSCs in vitro.[13, 20] The differentiation period chosen was 3 days, which was determined from studies showing the peak of stimulatory factors in inflammation, chondrogenesis, and osteogenesis.[19, 43] However, this peak of activity was not observed with differences in the ALP results between undifferentiated and predifferentiated MSCs at the 0d timepoint, which suggests that more investigation is needed to understand the differentiation kinetics of MSCs towards osteogenesis. Lam et al. reported on the optimized preconditioning period of rabbit MSCs for cartilage and showed that shorter or longer durations than 7 days led to worse repair.[41] Additionally, as cellular interaction with biomaterials is dependent on the state of differentiation, the incorporation of predifferentiated cells may require additional modifications in the hydrogel design.[29]
5. Conclusion
This work evaluated the effect of cell encapsulation density and predifferentiation on the in vitro osteogenic potential of injectable, dual-gelling cell-laden hydrogels. Rat MSCs were successfully encapsulated and homogenously distributed within hydrogel composites without detriment to cell viability. All groups demonstrated sustained cell viability over 28 days, with the predifferentiated cells at the highest seeding density group maintaining the highest cellularity at all timepoints. ALP activity was shown to decline over time, culminating at levels that were not significantly different from the other groups. Hydrogel mineralization increased significantly over time with only seeding density affecting the final calcium content. Although there were indications that both encapsulation density and predifferentiation state were influential to in vitro osteogenesis of MSCs and osteoconductivity of biomaterials, further investigation is needed to examine these cellular parameters at longer timepoints and with additional levels for bone tissue engineering. These injectable, dual-gelling hydrogels provide a promising platform by which to deliver and evaluate stem cell therapies for bone regeneration.
Acknowledgments
We acknowledge support from the National Institutes of Health (R01 DE017441 and R01 AR068073). T.N.V. acknowledges support from a Ruth L. Kirschstein fellowship from the National Institute of Dental and Craniofacial Research (F31 DE023999). We would also like to acknowledge Esther J. Lee for her assistance in MSC harvest.
Abbreviations
- 3D
Three dimensional
- αMEM
Minimal essential medium α modification
- AA
Acrylic acid
- AIBN
Azobisisobutyronitrile
- ALP
Alkaline phosphatase
- COM
Complete osteogenic medium
- DBA
Dimethyl-γ-butyrolactone acrylate
- ECM
Extracellular matrix
- FBS
Fetal bovine serum
- GMA
Glycidyl methacrylate
- GMP
Gelatin microparticle
- H&E
Hematoxylin & eosin
- 1H NMR
Proton Nuclear Magnetic Spectroscopy
- HPLC
High Performance Liquid Chromatography
- MBA
Methylene bisacrylamide
- Mn
Number average molecular weight
- Mw
Weight average molecular weight
- MSC
Mesenchymal stem cell
- NiPAAm
N-isopropylacrylamide
- PiP
Piperazine
- PAMAM
Polyamidoamine
- PBS
Phosphate buffered saline
- PDI
Polydispersity index
- TGM
Thermogelling macromer
References
- 1.Kretlow JD, Young S, Klouda L, Wong M, Mikos AG. Injectable biomaterials for regenerating complex craniofacial tissues. Adv Mater. 2009;21:3368–3393. doi: 10.1002/adma.200802009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev. 2012;64:1292–1309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caplan AI, Correa D. The msc: An injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzouanas SN, Ekenseair AK, Kasper FK, Mikos AG. Mesenchymal stem cell and gelatin microparticle encapsulation in thermally and chemically gelling injectable hydrogels for tissue engineering. J Biomed Mater Res A. 2014;102:1222–1230. doi: 10.1002/jbm.a.35093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vo TN, Shah SR, Lu S, Tatara AM, Lee EJ, Roh TT, Tabata Y, Mikos AG. Injectable dual-gelling cell-laden composite hydrogels for bone tissue engineering. Biomaterials. 2016;83:1–11. doi: 10.1016/j.biomaterials.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vo TN, Ekenseair AK, Kasper FK, Mikos AG. Synthesis, physicochemical characterization, and cytocompatibility of bioresorbable, dual-gelling injectable hydrogels. Biomacromolecules. 2014;15:132–142. doi: 10.1021/bm401413c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vo TN, Ekenseair AK, Spicer PP, Watson BM, Tzouanas SN, Roh TT, Mikos AG. In vitro and in vivo evaluation of self-mineralization and biocompatibility of injectable, dual-gelling hydrogels for bone tissue engineering. J Control Release. 2015;205:25–34. doi: 10.1016/j.jconrel.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrere F, Mahmood TA, de Groot K, Van Blitterswijk CA. Advanced biomaterials for skeletal tissue regeneration: Instructive and smart functions. Mater Sci Eng R. 2008;59:38–71. [Google Scholar]
- 9.Bensaid W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497–2502. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- 10.Zscharnack M, Hepp P, Richter R, Aigner T, Schulz R, Somerson J, Josten C, Bader A, Marquass B. Repair of chronic osteochondral defects using predifferentiated mesenchymal stem cells in an ovine model. Am J Sports Med. 2010;38:1857–1869. doi: 10.1177/0363546510365296. [DOI] [PubMed] [Google Scholar]
- 11.Grayson WL, Bhumiratana S, Grace Chao PH, Hung CT, Vunjak-Novakovic G. Spatial regulation of human mesenchymal stem cell differentiation in engineered osteochondral constructs: Effects of pre-differentiation, soluble factors and medium perfusion. Osteoarthr Cartil. 2010;18:714–723. doi: 10.1016/j.joca.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters A, Toben D, Lienau J, Schell H, Bail HJ, Matziolis G, Duda GN, Kaspar K. Locally applied osteogenic predifferentiated progenitor cells are more effective than undifferentiated mesenchymal stem cells in the treatment of delayed bone healing. Tissue Eng Part A. 2009;15:2947–2954. doi: 10.1089/ten.TEA.2009.0058. [DOI] [PubMed] [Google Scholar]
- 13.Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254:317–330. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- 14.Ekenseair AK, Boere KW, Tzouanas SN, Vo TN, Kasper FK, Mikos AG. Synthesis and characterization of thermally and chemically gelling injectable hydrogels for tissue engineering. Biomacromolecules. 2012;13:1908–1915. doi: 10.1021/bm300429e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekenseair AK, Peppas NA. Tuning the transport dynamics of small penetrant molecules in glassy polymers. Polymer. 2012;53:4010–4017. [Google Scholar]
- 16.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101:111–125. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klouda L, Perkins KR, Watson BM, Hacker MC, Bryant SJ, Raphael RM, Kasper FK, Mikos AG. Thermoresponsive, in situ cross-linkable hydrogels based on N-isopropylacrylamide: Fabrication, characterization and mesenchymal stem cell encapsulation. Acta Biomater. 2011;7:1460–1467. doi: 10.1016/j.actbio.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothenberg AR, Ouyang L, Elisseeff JH. Mesenchymal stem cell stimulation of tissue growth depends on differentiation state. Stem Cell Dev. 2011;20:405–414. doi: 10.1089/scd.2010.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peter SJ, Liang CR, Kim DJ, Widmer MS, Mikos AG. Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta-glycerolphosphate, and l-ascorbic acid. J Cell Biochem. 1998;71:55–62. doi: 10.1002/(sici)1097-4644(19981001)71:1<55::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Hoemann CD, Sun J, Legare A, McKee MD, Buschmann MD. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthr Cartil. 2005;13:318–329. doi: 10.1016/j.joca.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Temenoff JS, Park H, Jabbari E, Sheffield TL, LeBaron RG, Ambrose CG, Mikos AG. In vitro osteogenic differentiation of marrow stromal cells encapsulated in biodegradable hydrogels. J Biomed Mater Res A. 2004;70:235–244. doi: 10.1002/jbm.a.30064. [DOI] [PubMed] [Google Scholar]
- 23.Huang CY, Reuben PM, D'Ippolito G, Schiller PC, Cheung HS. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat Rec. 2004;278:428–436. doi: 10.1002/ar.a.20010. [DOI] [PubMed] [Google Scholar]
- 24.Na K, Kim SW, Sun BK, Woo DG, Yang HN, Chung HM, Park KH. Osteogenic differentiation of rabbit mesenchymal stem cells in thermo-reversible hydrogel constructs containing hydroxyapatite and bone morphogenic protein-2 (BMP-2) Biomaterials. 2007;28:2631–2637. doi: 10.1016/j.biomaterials.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Thibault RA, Scott Baggett L, Mikos AG, Kasper FK. Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue Eng Part A. 2010;16:431–440. doi: 10.1089/ten.tea.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson BM, Vo TN, Tatara AM, Shah SR, Scott DW, Engel PS, Mikos AG. Biodegradable, phosphate-containing, dual-gelling macromers for cellular delivery in bone tissue engineering. Biomaterials. 2015;67:286–296. doi: 10.1016/j.biomaterials.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson BM, Vo TN, Engel PS, Mikos AG. Biodegradable, in situ-forming cell-laden hydrogel composites of hydroxyapatite nanoparticles for bone regeneration. Ind Eng Chem Res. 2015;54:10206–10211. [Google Scholar]
- 28.Forsey RW, Chaudhuri JB. Validity of DNA analysis to determine cell numbers in tissue engineering scaffolds. Biotechnol Lett. 2009;31:819–823. doi: 10.1007/s10529-009-9940-5. [DOI] [PubMed] [Google Scholar]
- 29.Hsiong SX, Carampin P, Kong HJ, Lee KY, Mooney DJ. Differentiation stage alters matrix control of stem cells. J Biomed Mater Res A. 2008;85:145–156. doi: 10.1002/jbm.a.31521. [DOI] [PubMed] [Google Scholar]
- 30.Kim K, Dean D, Lu A, Mikos AG, Fisher JP. Early osteogenic signal expression of rat bone marrow stromal cells is influenced by both hydroxyapatite nanoparticle content and initial cell seeding density in biodegradable nanocomposite scaffolds. Acta Biomater. 2011;7:1249–1264. doi: 10.1016/j.actbio.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kavalkovich KW, Boynton RE, Murphy JM, Barry F. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell Dev Biol. 2002;38:457–466. doi: 10.1290/1071-2690(2002)038<0457:cdohms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Erickson IE, Kestle SR, Zellars KH, Farrell MJ, Kim M, Burdick JA, Mauck RL. High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater. 2012;8:3027–3034. doi: 10.1016/j.actbio.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang SC, Rowley JA, Tobias G, Genes NG, Roy AK, Mooney DJ, Vacanti CA, Bonassar LJ. Injection molding of chondrocyte/alginate constructs in the shape of facial implants. J Biomed Mater Res. 2001;55:503–511. doi: 10.1002/1097-4636(20010615)55:4<503::aid-jbm1043>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 34.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable rgd-modified peg hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 35.Grayson WL, Bhumiratana S, Cannizzaro C, Chao PH, Lennon DP, Caplan AI, Vunjak-Novakovic G. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng Part A. 2008;14:1809–1820. doi: 10.1089/ten.tea.2007.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Seshareddy K, Weiss ML, Detamore MS. Effect of initial seeding density on human umbilical cord mesenchymal stromal cells for fibrocartilage tissue engineering. Tissue Eng Part A. 2009;15:1009–1017. doi: 10.1089/ten.tea.2008.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holland TA, Tabata Y, Mikos AG. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J Control Release. 2003;91:299–313. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 38.Anderson SB, Lin CC, Kuntzler DV, Anseth KS. The performance of human mesenchymal stem cells encapsulated in cell-degradable polymer-peptide hydrogels. Biomaterials. 2011;32:3564–3574. doi: 10.1016/j.biomaterials.2011.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benoit DS, Durney AR, Anseth KS. The effect of heparin-functionalized peg hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007;28:66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 40.Shin H, Temenoff JS, Bowden GC, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Osteogenic differentiation of rat bone marrow stromal cells cultured on arg-gly-asp modified hydrogels without dexamethasone and beta-glycerol phosphate. Biomaterials. 2005;26:3645–3654. doi: 10.1016/j.biomaterials.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 41.Lam J, Lu S, Meretoja VV, Tabata Y, Mikos AG, Kasper FK. Generation of osteochondral tissue constructs with chondrogenically and osteogenically predifferentiated mesenchymal stem cells encapsulated in bilayered hydrogels. Acta Biomater. 2014;10:1112–1123. doi: 10.1016/j.actbio.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliveira JT, Gardel LS, Rada T, Martins L, Gomes ME, Reis RL. Injectable gellan gum hydrogels with autologous cells for the treatment of rabbit articular cartilage defects. J Orthop Res. 2010;28:1193–1199. doi: 10.1002/jor.21114. [DOI] [PubMed] [Google Scholar]
- 43.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]