Abstract
Understanding how the brain works requires understanding how different types of neurons contribute to circuit function and organism behavior. Progress on this front has been accelerated by optogenetics and chemogenetics, which provide an unprecedented level of control over distinct neuronal types in small animals. In primates, however, targeting specific types of neurons with these tools remains challenging. In this review, we discuss existing and emerging strategies for directing genetic manipulations to targeted neurons in the adult primate central nervous system. We review the literature on viral vectors for gene delivery to neurons, focusing on adeno-associated viral vectors and lentiviral vectors, their tropism for different cell types, and prospects for new variants with improved efficacy and selectivity. We discuss two projection targeting approaches for probing neural circuits: anterograde projection targeting and retrograde transport of viral vectors. We conclude with an analysis of cell type-specific promoters and other nucleotide sequences that can be used in viral vectors to target neuronal types at the transcriptional level.
Keywords: gene therapy, optogenetics, primate, targeting, viral vector
decades ago, molecular biology revolutionized cellular neurophysiology. Today, systems neurophysiology is undergoing a similar revolution. Electrodes and amplifiers remain essential tools for understanding neural circuits, but now so too are methods for manipulating genes and delivering them to neurons in vivo. The availability and genetic tractability of small-animal models made them the perfect entry point for these new techniques, but nowadays primate studies are using the tools of optogenetics and chemogenetics with increasing success. Excellent reviews of this small but growing literature already exist (Gerits and Vanduffel 2013; Han 2012; Kinoshita and Isa 2015). The contribution of the present review is the focus on methods for targeting genetic manipulations to specific neuronal types in primates. The review is intended primarily for neurophysiologists who use nonhuman primate models, have minimal background in molecular biology, and are interested in assessing the added value of genetic techniques to their research program. Our focus is on gene delivery to adult animals; we do not cover transgenic models engineered via in vitro gene delivery.
A fundamental technical problem in neurophysiology is that the nervous system comprises many intermixed, distinct neuronal types that cannot be individually manipulated with classical techniques. Transgenic technology has gone much of the way toward solving this problem in small-animal models (e.g., mice and flies), and there is increasing interest and success in extending this technology to primates (Chan 2013; Chan and Yang 2009; Chen et al. 2012; Izpisua Belmonte et al. 2015; Liu et al. 2016; Sasaki 2015). Nevertheless, transgenic primates are unlikely to be economically realistic for most applications in the near term. A more practical means to deliver genetic material to the primate central nervous system (CNS) is by direct injection of a replication-deficient viral vector. Viral vectors are viruses that are devoid of most of their natural genetic material and loaded with engineered DNA or RNA constructs to achieve an experimental or therapeutic goal. Such engineered constructs cannot be packaged into a viral vector if they are much larger than the natural genome of the virus, thus limiting how much genetic material a viral vector can deliver. For example, the two viral vectors most commonly used in the primate CNS, based on adeno-associated virus (AAV) and lentivirus (LV), carry only ∼5,000 and ∼10,000 nucleotides, respectively, which is ∼0.0003% of the human genome. Fortunately, the genes for many useful proteins are quite small; for example, the gene for channelrhodopsin-2 (ChR2), the light-gated ion channel most commonly used in neurophysiology, is ∼1,000 nucleotides. Viral vectors are not the only means to deliver genetic material to the primate CNS, but they are efficient and economical, and their biology is relatively well understood. Alternative techniques for gene delivery are reviewed elsewhere (Maguire et al. 2014; Ramamoorth and Narvekar 2015; Wang et al. 2015b).
The genes delivered by a viral vector, called transgenes, can code for useful proteins. Transgenes that are particularly useful for neurophysiological experiments include those that code for effectors of neural activity (e.g., light- or ligand-gated ion channels), indicators of neural activity (e.g., genetically encoded calcium indicators), DNA recombination enzymes (e.g., Cre-recombinase), and reporter proteins (e.g., fluorescent proteins).
Viral vectors used in neuroscience research are based on a variety of naturally occurring viruses. In this review, we focus on AAV vectors and LV vectors, as these promote stable expression in neurons, are minimally cytotoxic, and have been used successfully in primates. We first describe properties of these vectors that influence which cells they transduce, as these have important implications for all subsequent targeting strategies. We then describe targeting strategies that exploit neuroanatomical connectivity and are starting to enter the mainstream of primate neurophysiology. Finally, we describe targeting techniques based on gene regulatory mechanisms whose broad applicability has yet to be established in primates.
Viral Vector Tropism
Viruses have a natural tendency to infect certain cell types and not others. Understanding how viral vectors differ in this preference, or tropism, is therefore critical for controlling gene expression in specific cell types. In this section, we summarize what is known about AAV and LV vector tropism in the primate CNS.
Viral vectors are typically injected into the brain as liquid suspensions. The liquid diffuses roughly uniformly through extracellular spaces but can also travel preferentially along paths of least resistance: the injection cannula track, white matter tracts, or perivascular spaces (Abbott 2004; Lonser et al. 2015; Wolak and Thorne 2013). To enter a cell, viral particles, called virions, must attach to receptors on the cell membrane (for a review see Dimitrov 2004). Thus which receptors a cell expresses, as well as cell size, geometry, and accessibility to the extracellular milieu, can be strong determinants of viral vector tropism. Once inside a cell, a virion travels toward the nucleus and sheds its protein coat, revealing genes to be transcribed by host cell machinery. Any chemical or environmental factors that affect this complex series of events (e.g., the intracellular concentration of transcription factors or the pH levels of various intracellular compartments) can alter viral vector tropism.
Tropism governed by viral vector outer structure.
Virions bind receptors on the cell membrane via proteins in their outer structure. The outer structure of AAV is a protein coat called a capsid. Different varieties of AAV, called serotypes,1 have different capsids, and small differences in the proteins that make up these capsids can greatly affect cell surface receptor binding (Lisowski et al. 2015; Michelfelder and Trepel 2009). To date, hundreds of functional AAV capsids have been identified and engineered, and while we know some of the receptors to which these capsids bind, most of what we know about which viral vectors transduce which cell types is empirical (but see Controlling tropism by manipulating viral vector outer structure).
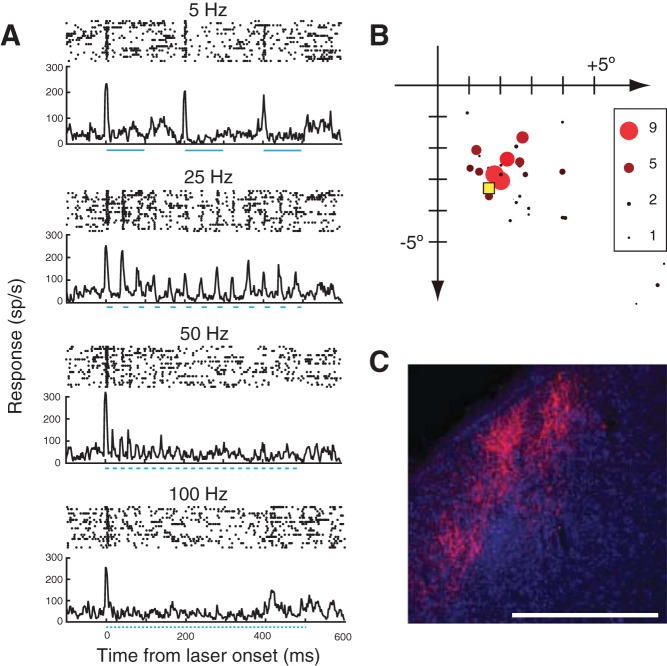
Injections of AAV vectors into the primate CNS do not transduce all neurons uniformly; instead, transduction patterns can be complex. However, across a battery of studies that compared the tropism of different AAV serotypes in the primate CNS, two consistent patterns emerge. First, there is a bias for stronger expression in large cell bodies, possibly owing to the larger surface area and greater number of receptors (Han et al. 2009; Watakabe et al. 2015). Second, transgene expression in neocortex is typically stronger in the deep or superficial cortical layers and weaker in the middle, input layer (Fig. 1), possibly because of the smaller neurons there (Diester et al. 2011; Gerits et al. 2015; Jazayeri et al. 2012; Watakabe et al. 2015).
Fig. 1.
Viral vector tropism in primate neocortex. Coronal sections of visual area V1 of a rhesus macaque. Tissue was stained for SMI-32 (red) and with DAPI (blue), and transduced neurons are green. A: injection of LV-CaMKIIa-ArchT-GFP titered at 1.8 × 107 infectious units/ml. B: injection of AAV9-hSyn-ChR2-eYFP titered at 2.75 × 1013 genomic copies/ml. A total of 5 μl of each viral vector was injected (1 μl injected at each of 5 sites, spaced 500 μm apart). Scale bars, 500 μm. The sparse labeling is consistent with some studies (Gerits et al. 2015) but not others (Diester et al. 2011).
AAV serotypes differ in their ability to transduce different kinds of cells. Neurotropic serotypes include AAV1, 2, 5, 8, and 9. AAV1 and AAV5 may be particularly well suited for transducing neurons in the basal ganglia (Dodiya et al. 2010; Markakis et al. 2010; Sanchez et al. 2011), and these two serotypes, plus AAV9, transduce neocortical neurons efficiently (AAV2 receives conflicting reviews) (Gerits et al. 2015; Lerchner et al. 2014; Watakabe et al. 2015). Overall, AAV vector serotypes differ more in their transduction efficiency (e.g., spread) than in their selectivity for particular neuronal types or brain areas.
LV vectors also have capsids, but these capsids are surrounded by a lipid envelope that is studded with glycoproteins that mediate binding to cell surface receptors. Typical LV vectors are produced with the capsid from one type of LV and the glycoprotein from another. The capsid of many LV vectors is derived from HIV-1, and the glycoprotein is often taken from the vesicular stomatitis virus (VSV-G) because of its ability to bind receptors on a broad range of cells (Cronin et al. 2005; Finkelshtein et al. 2013).
A few studies have surveyed LV tropism in the primate CNS, revealing that LV vectors typically transduce a smaller region of injected tissue than AAV vectors: ∼1 mm for LV vs. ∼2–4 mm for AAV for injections of a few microliters over several minutes (Diester et al. 2011; Lerchner et al. 2014; Watakabe et al. 2015). This difference is likely due to the fact that LV virions are approximately five times larger in diameter than AAV virions, resulting in weaker penetrance of LV into tissue around the injection site (Cetin et al. 2007; Waehler et al. 2007). Transgene expression with LV is more uniform across cortical layers than with AAV in some primate studies but not others, possibly because of differences in injection protocols (Lerchner et al. 2014; Nassi et al. 2015; but see Diester et al. 2011 and Fig. 1).
Controlling tropism by manipulating viral vector outer structure.
The outer structure of viral vectors can be modified to restrict or expand the range of cells that they can transduce. For example, AAV capsids can be engineered to include ligand motifs that bind receptors enriched in a cell type of interest, thereby increasing transduction of that cell type (Buning et al. 2015; Munch et al. 2015). However, producing high-titer AAV vector stocks with these engineered capsids has been challenging (Kwon and Schaffer 2008). An alternative approach involves mutating AAV capsids randomly and then screening them for improved tropism for cell types or tissues of interest (Lisowski et al. 2015; Maheshri et al. 2006). This strategy has been used to find AAVs that transduce retinal neurons after injection into the vitreous humor—a challenge given the barrier posed by the inner limiting membrane (Dalkara et al. 2013). In this case, capsid screening was performed in mice, but one particularly effective AAV vector thus obtained was verified to transduce monkey retinal ganglion cells in vivo (Dalkara et al. 2013; Yin et al. 2014).
The tropism of LV vectors can be changed by replacing their natural envelope glycoproteins with those derived from other enveloped viruses (e.g., rabies virus or Mokola virus) (Cronin et al. 2005; Kato et al. 2013). The specificity of LV vectors can also be modified by ligand bridge proteins—molecular adapters that bind to the viral vector glycoprotein with one domain and to a specific receptor on a cell type of interest with another, thereby promoting vector entry into targeted cells (Choi et al. 2010).
Tropism governed by gene transcription.
Viral vectors typically contain at least two functionally distinct genetic sequences. The first is a transgene sequence that codes for a protein, and the second is a promoter sequence that precedes the transgene and is critical for transgene expression. Transcription of the vector genome occurs in the nucleus of a transduced cell and depends critically on the availability of transcription factors—proteins that promote gene expression by interacting with the promoter sequence. These interactions, in conjunction with the binding of RNA polymerase, trigger the synthesis of messenger RNA from vector-derived DNA. The transcribed messenger RNA then leaves the nucleus and instructs the synthesis of the protein encoded by the transgene sequence. Thus the particular promoter used in a given viral vector can influence tropism profoundly. Subject to size constraints, viral vectors can contain multiple transgenes driven by one or more promoters (de Felipe 2002).
The first generation of viral vectors used in the primate CNS contained promoters from wild-type viruses (e.g., the CMV promoter) or chimeras of viral and cellular promoters (e.g., the CAG promoter) that drive strong expression in a variety of cell types, including neurons and glia. These promoters are commonly referred to as “ubiquitous” despite the fact that they do not drive expression uniformly across all cell types (Gerits et al. 2015; Lerchner et al. 2014; Watakabe et al. 2015; Yaguchi et al. 2013). Ubiquitous promoters are frequently used in the CNS although they can kill neurons through transgene overexpression (Watakabe et al. 2015; but see Sanchez et al. 2011).
More recently, promoters have been found that drive transgene expression in neurons specifically. The two promoters most commonly used to target neurons in primates are the human Synapsin-1 (hSyn) promoter and the mouse calcium-calmodulin kinase-2a (CaMKIIa) promoter. These promoters are relatively small, occupying only 10% and 25% of the AAV vector genome, respectively. Both restrict expression to neurons in the primate CNS (Diester et al. 2011; Gerits et al. 2015; Watakabe et al. 2015). In AAV vectors, the CaMKIIa promoter is more strongly biased to excitatory neurons than the hSyn promoter but still drives expression in inhibitory neurons (Gerits et al. 2015; Watakabe et al. 2015). A truncated version of the CaMKIIa promoter may be more selective for excitatory neurons than longer versions (Gerits et al. 2015). LV vectors containing the CaMKIIa promoter target excitatory neurons (Han et al. 2009; Nassi et al. 2015; Yaguchi et al. 2013). We return to promoter sequences that drive expression in distinct neuronal types in Targeting Neurons at the Transcriptional Level.
Other factors that influence tropism.
The outer structure and promoter sequence of a viral vector are major factors governing tropism, but other factors also contribute. First, vector titer can bias tropism: Low-titer vector stocks transduce their intended targets relatively selectively, whereas higher-titer stocks transduce a greater number of off-target cells (Nathanson et al. 2009b). Second, vector purity can also bias tropism: Nonviral proteins and DNA contaminants of vector stocks can lead to pseudotransduction (Alexander et al. 1997), reduced transduction efficiency (Ayuso et al. 2010), and a specific increase in the number of transduced glia, presumably due to astrogliosis (Klein et al. 2008). Third, transient reductions in pH during vector production have been reported to bias LV vectors toward glia (Torashima et al. 2006). Fourth, the volume and speed of injection into the brain can contribute to the strength and extent of transgene expression: Some groups report broad expression with small and slow injections (Lerchner et al. 2014), whereas others have had success with larger or faster injections (Sanchez et al. 2011; Yazdan-Shahmorad et al. 2016). Fifth, experimental animals can have preexisting immune responses against AAV that prevent these vectors from transducing cells efficiently (reviewed by Calcedo and Wilson 2013). Even in the absence of preexisting immunity, viral vector injections can produce immune responses, decreasing the efficacy of subsequent injections of the same vectors (Hadaczek et al. 2009; Kotterman et al. 2015; Wang et al. 2011). Finally, while most studies of viral vector tropism have varied the outer structure or the promoter sequence individually, it is possible that these factors mediate tropism jointly rather than independently.
Projection Targeting
Projection targeting is the manipulation of specific neuronal populations based on where their axons terminate. It is an established method in mice and rats (Deisseroth 2014; Fenno et al. 2011), and almost all of the studies cited in this section were performed using these animals. However, a few primate studies have used this technique successfully, and we note these studies explicitly. This technique is well suited for use in primates because it achieves cell type specificity on the basis of neuroanatomical connectivity, which is well understood, as opposed to gene regulation, which is not.
There are two main variants of this technique: anterograde and retrograde (Fig. 2). Anterograde projection targeting is based on the trafficking of proteins encoded by viral vectors from cell bodies down to axon terminals. Transduced neurons can then be manipulated with light or ligand at their axon terminals. Retrograde projection targeting is based on the retrograde transport of virions from axon terminals to cell bodies. “Anterograde” and “retrograde” here describe the direction of trafficking along an axon; in neither case do virions cross a synapse. Viruses that do cross synapses are valuable for neuroanatomical studies but not for long-term neurophysiological or behavioral studies in primates because they kill neurons (Card and Enquist 2014; Dum and Strick 2013; Nassi et al. 2015).
Fig. 2.
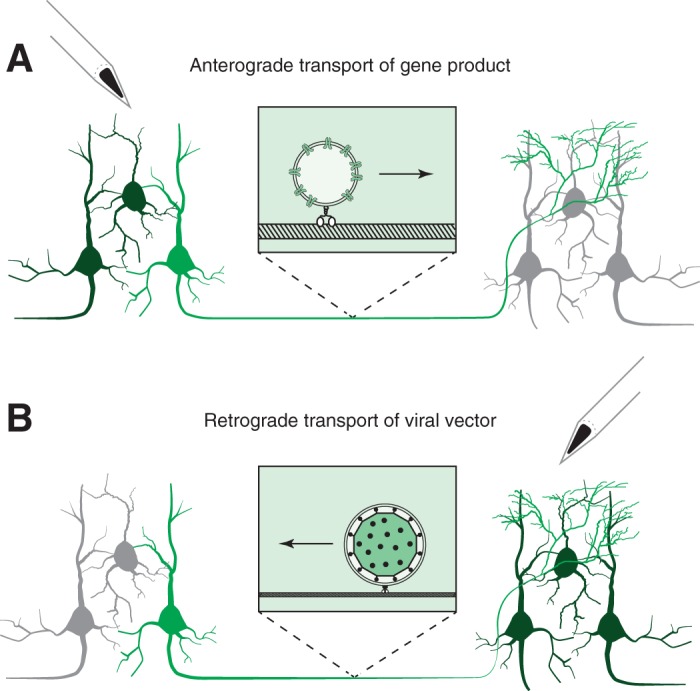
Illustration of projection targeting approaches. A: anterograde projection targeting. A viral vector is injected into a source area (left) where neuronal cell bodies are transduced. Gene products are trafficked along axons (center) to their terminations in a recipient area (right). B: retrograde projection targeting. A viral vector is injected into a recipient area (right). Virions are retrogradely transported along axons to cell bodies (left) where genes are expressed. In both scenarios, some neurons are transduced (light and dark green) whereas others are not transduced (gray). The subset of transduced neurons available for selective manipulation are highlighted (light green).
Anterograde projection targeting.
The fundamental logic of anterograde projection targeting is quite general and can be applied to activating a neural pathway (Deisseroth 2014; Gradinaru et al. 2009; Inoue et al. 2015), shutting down a pathway (Felix-Ortiz and Tye 2014; Kaneda et al. 2011; Stachniak et al. 2014), or interrogating activity in a pathway with optical imaging (Glickfeld et al. 2013; Petreanu et al. 2012; Sadakane et al. 2015). It is particularly easy to explain in the context of optogenetic activation, where it has been most widely used. A viral vector coding for an excitatory opsin (e.g., ChR2) is injected into a brain area. The opsin is expressed in neuronal cell bodies at the injection site, trafficked down axons, and distributed throughout axon terminals. Light delivered to the opsin-containing axon terminals in the recipient area depolarizes them, causing the release of neurotransmitter. Provided the axon terminals are sufficiently far from their cell bodies, the illumination of axon terminals will not activate opsins expressed in the cell bodies. In this way, only the neurons that project from the injection site to the recipient area are activated directly by the light.
This method was recently used for the first time in primates. Two rhesus monkeys received injections of an AAV vector into the frontal eye fields (FEF), a cortical area involved in oculomotor behavior and attention (Inoue et al. 2015). The AAV vector, which contained the ChR2 gene under the control of the CMV promoter, transduced many neurons at the injection site including those that projected to the superior colliculus (SC), several centimeters away in the midbrain. A key result of this study was that illumination of the SC evoked spiking responses in the SC and also evoked saccadic eye movements toward the response fields of the stimulated sites. In addition to representing a technical milestone for primate optogenetics, this result is noteworthy because direct optogenetic manipulation of cell bodies in the FEF and SC, while sufficient to bias saccade probability, latency, and other metrics, was unable to evoke saccades reliably without concurrent electrical stimulation (Cavanaugh et al. 2012; Gerits et al. 2012; Han et al. 2009; Ohayon et al. 2013; Soetedjo et al. 2013).
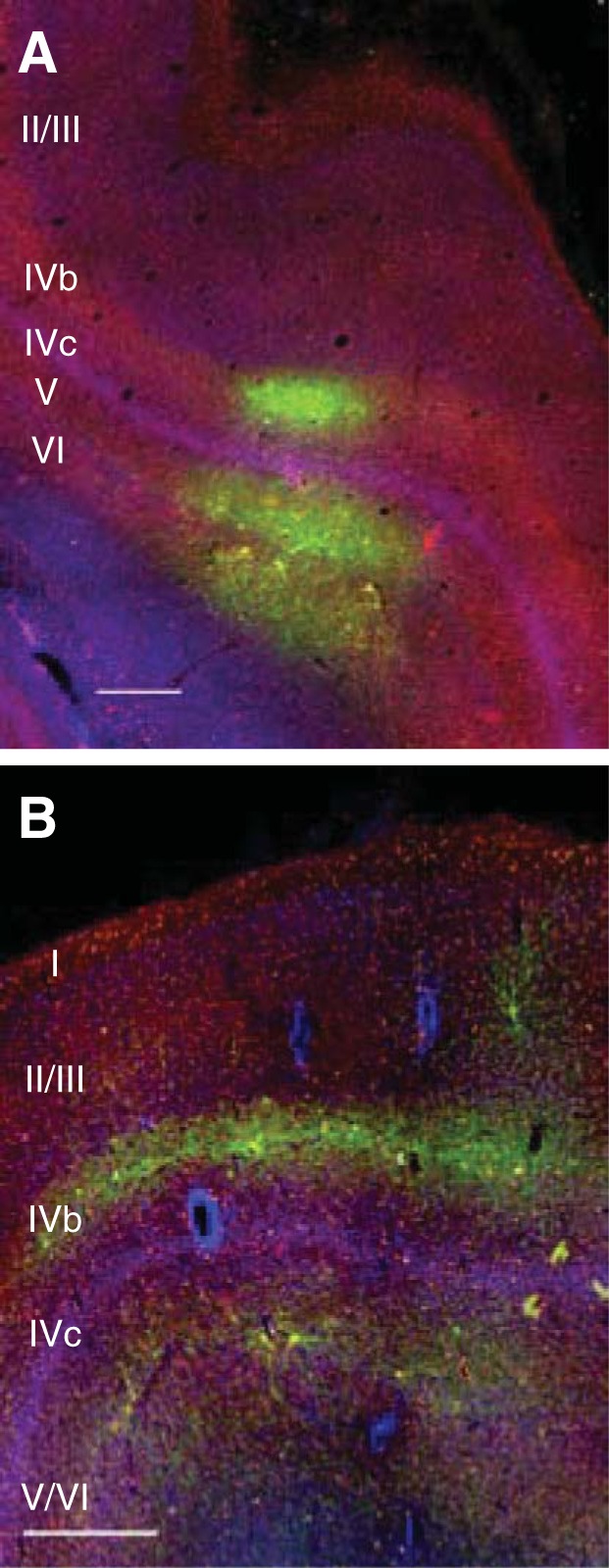
We have recently extended these results by targeting another pathway in the primate oculomotor system: the projection from visual cortical area V1 to the SC. We injected an AAV vector into V1 of one rhesus monkey to express ChR2 under the control of the hSyn promoter. Low-frequency light flashes in the SC resulted in spiking synchronized to the flashes (Fig. 3). This activation was observed only in the region of the SC that was retinotopically matched to the V1 injection site, consistent with the topography of the projections from V1 to the SC. Stimulation of V1 axons in the SC did not evoke saccades, although optogenetic stimulation of V1 neuronal cell bodies in the same animal did evoke saccades in a previous study (Jazayeri et al. 2012).
Fig. 3.
Anterograde projection targeting of V1 axons projecting to the SC (for details of the AAV1-hSyn-ChR2-mCherry vector injection, see Jazayeri et al. 2012). In each experiment, an optical fiber and a tungsten electrode were independently lowered through a common guide tube to the surface of the SC. The tip of the electrode was positioned in the superficial layers, and the tip of the optical fiber was positioned just outside of the SC. Electrical responses to 473-nm light flashes were recorded. A: rasters and PSTHs showing entrainment of multiunit SC responses to periodic illumination (blue dashed lines). B: the position of each dot represents the receptive field location of a tested SC site. The size and color of each dot show the change in spike rate following a pulse of blue light; they are proportional to the number of spikes during the 10 ms after each light pulse divided by the number of spikes during the 10 ms preceding each light pulse. Optogenetic activation was greatest at SC sites with receptive fields that overlapped those at the V1 injection site (yellow square). C: coronal section through the left SC of the same animal showing ChR2-mCherry+ axons (red) and DAPI (blue). Scale bar, 500 μm.
The pathways between the FEF and SC and between V1 and SC differ in many ways, as do the experiments that probed the behavioral consequences of activating them. Conclusions about how each pathway contributes to behavior on the basis of these studies is therefore premature, but we can speculate on why optogenetic stimulation of cell bodies can have effects different from stimulation of axon terminals. Cell bodies are larger and contain more opsin molecules. On the other hand, axon terminals have higher input resistance, so their membrane voltage is modulated more by small numbers of activated opsins. Direct stimulation of cell bodies can activate both excitatory and inhibitory neurons, which may attenuate the net output to downstream structures, whereas stimulation of axon terminals does not affect inhibitory interneurons directly. Direct illumination of widely dispersed cell bodies is expected to be less effective than illumination of their axon terminals if the terminals are tightly clustered in the recipient area. Conversely, divergent axons are more difficult to illuminate than highly clustered cell bodies. Finally, stimulation of cell bodies may activate multiple pathways leaving the injection site simultaneously. Some of these pathways may not participate in a particular behavior, and others may mediate mutually exclusive behaviors resulting in cancellation of evoked behavioral responses (Wang et al. 2015a; Warden et al. 2012; Znamenskiy and Zador 2013).
Caveats of anterograde projection targeting.
Anterograde projection targeting requires efficient trafficking of expressed proteins to axon terminals, and not all proteins are equally well trafficked. The early versions of halorhodopsin trafficked quite poorly, for example, but the addition of targeting sequences to the transgene ameliorated this problem (Gradinaru et al. 2010). In addition, viral vector tropism and the density of axons in the recipient area also affect the quantity of transgene-encoded proteins at axon terminals. It is therefore prudent to confirm the presence of transgene-encoded proteins at axon terminals before embarking on a lengthy neurophysiological study. As a case in point, AAV injections that we have made into V1 of rhesus monkeys have labeled axons densely in the SC and visual area V2 but sparsely in visual area MT and the lateral geniculate nucleus, despite the fact that V1 projects to all four of these areas.
We were able to cause SC neurons to fire synchronously with the illumination of axon terminals, but only up to 25 Hz (Fig. 3), consistent with previous work using ChR2 in rodent slices (Cruikshank et al. 2010; Petreanu et al. 2007). This inability to follow high-frequency optical stimulation may be unique to the axon terminal, as spikes produced by the illumination of ChR2-expressing cell bodies can entrain to higher frequencies (>100 Hz). We also found that constant illumination of axon terminals evoked a transient burst of spikes at light onset whereas constant illumination of cell bodies evoked sustained spike trains. We do not yet know whether these results generalize to other neural pathways or whether they are related to the size of behavioral effects.
The temporal dynamics of optogenetic activation of cell bodies and axon terminals are likely due to the time course of ChR2 inactivation and recovery, interactions between ChR2-mediated currents and those mediated by endogenous channels, and the dynamics of synaptic transmission (in the case of axon terminal illumination measured with postsynaptic recordings). Postsynaptic responses following repetitive optogenetic stimulation of axons are more attenuated than expected from electrical stimulation experiments (Olsen et al. 2012; Zhang and Oertner 2007). The magnitude of this attenuation depends on the particular pathway stimulated and the vector used to deliver the ChR2 gene (Jackman et al. 2014). For experiments in which neural activity in axon terminals must be manipulated at high frequencies, opsins with faster kinetics (e.g., ChIEF or Chronos) might be a better choice than ChR2 (Jurgens et al. 2012; Klapoetke et al. 2014; Lin et al. 2009).
On purely theoretical grounds, optogenetic activation of a pathway should be easier than suppressing it. Even modest ChR2 activation can (indirectly) mediate large calcium currents, increasing the probability of neurotransmitter release (Schoenenberger et al. 2011). In contrast, blocking an action potential requires a greater change in membrane conductance. Nevertheless, optogenetic projection targeting can be used to suppress the transmission of neural signals by illuminating axon terminals expressing halorhodopsin (Kaneda et al. 2011; Stuber et al. 2011; Warden et al. 2012) or archaerhodopsin (Spellman et al. 2015). Under some conditions, activation of archaerhodopsin in axon terminals can paradoxically increase postsynaptic responses, and both halorhodopsin and archaerhodopsin can evoke reboundlike postsynaptic responses following abrupt light termination (Mahn et al. 2016).
Optogenetic stimulation of axon terminals can evoke action potentials that propagate backward to cell bodies. Backward-propagating action potentials do not always occur (Azim et al. 2014; Tye et al. 2011), but when they do, they can be used as an electrophysiological tag to determine whether a recorded neuron projects to a certain target area (Lima et al. 2009). They also complicate the interpretation of behavioral effects, which may be due to nonphysiological routes of signal propagation. In these cases, projection targeting via retrogradely transported viral vectors may provide a solution.
Projection targeting via retrograde transport of viral vectors.
Projection targeting via retrogradely transported viral vectors relies on the ability of virions to enter neurons at their axon terminals, a feat that only some vectors can achieve. Internalized virions, when transported to the nucleus, provide an experimenter with genetic control of neurons projecting to an injection site.
Retrograde projection targeting is particularly useful in several cases. First, it can aid in dissecting the contribution of several distinct afferent pathways that converge onto a single area. For example, a retrogradely transported viral vector injected into the striatum can drive transgene expression in cortical and subcortical areas that project to this structure, priming multiple inputs for neurophysiological investigation. Second, manipulating neurons with light or ligand at their cell bodies may be easier than at their axon terminals, providing more robust control of neural circuits of interest. Finally, retrograde projection targeting avoids signal cancellation that may occur when excitatory and inhibitory neurons are coactivated because most long-range, interareal projections are excitatory.
LV vectors mediate retrograde transduction more efficiently than AAV vectors in macaques (Kato et al. 2013; Oguchi et al. 2015). The retrograde transport of LV vectors is largely due to optimization of their envelope glycoproteins. This optimization is necessary: The VSV glycoprotein, which is commonly used in LV vectors, does not mediate retrograde transduction, and the glycoproteins that do can lose this ability if mutated even slightly (Cronin et al. 2005; Wong et al. 2004). Retrograde transduction can be achieved using LV vectors containing glycoprotein from rabies virus or fusions of the extracellular domain of the rabies glycoprotein to the intracellular domain of the VSV glycoprotein (Kato et al. 2013). These fusion glycoproteins have been used to target several pathways of the primate CNS: the projection from the substantia nigra to the striatum (Kato et al. 2011a, 2011b), from the supplementary motor area to the subthalamic nucleus (Inoue et al. 2012), and from propriospinal neurons to motorneurons (Kinoshita et al. 2012). In rodents, some serotypes of AAV mediate retrograde transduction efficiently (Castle et al. 2014; Kaspar et al. 2002), but in primates this is rare. A few cases of relatively weak retrograde transport of AAV have been documented in the projections from the lateral geniculate nucleus to V1 (Gerits et al. 2015) and from the substantia nigra to the striatum (Masamizu et al. 2011). In general, expression levels via retrograde transport with AAV vectors are much lower than expression levels at the injection site.
Retrogradely transported vectors that are used widely in rodents include those based on rabies virus, herpes simplex virus, and canine adenovirus (Nassi et al. 2015). These vectors mediate efficient retrograde transduction, but none is currently well suited for long-term studies in primates. Rabies viral vectors, even those that are replication-deficient and restricted in their spread, kill neurons in ∼2 wk (Callaway and Luo 2015). Many herpes simplex viral vectors are cytotoxic, and the least cytotoxic variants drive transgene expression transiently (Marconi et al. 2008). We know of no published studies that used canine adenoviral vectors in the primate CNS, and our attempts to use it in the macaque cerebellar and cerebral cortex were unsuccessful (but see Junyent and Kremer 2015).
Caveats of retrograde projection targeting.
The distinction between anterograde and retrograde projection targeting is not always clear-cut. A retrogradely transported viral vector can enter cell bodies at the injection site, which can result in the synthesis of transgene-encoded proteins and their subsequent transport in the anterograde direction, down axons. If these axons terminate near the cell bodies of the targeted neuronal population (those that project to the injection site), then light or ligand may act on both, complicating the interpretation of ensuing effects.
Advances in retrograde projection targeting.
Retrograde vector transport in primates has generally been inefficient, but recent studies have improved its efficacy by using viral vectors that code for a transgene of interest along with a drug-inducible transcription factor that amplifies transgene expression (Boulaire et al. 2009; Cetin and Callaway 2014; Chtarto et al. 2003; Delzor et al. 2012; Kuhn et al. 2012). This system allows transgene expression to be up- or downregulated by oral administration of an antibiotic drug, with effects occurring within a few days. While recent studies have provided evidence that this technique works in primates (Kinoshita et al. 2012; Sadakane et al. 2015), there is some indication that the transcription factor may induce an immune response and cause neuronal death (Chtarto et al. 2013; Latta-Mahieu et al. 2002). Efforts are underway to engineer new transcription factors that are less toxic and to identify new ligands that cross the blood-brain barrier more efficiently (Chtarto et al. 2013).
Transgene expression in this system occurs only in cells that contain the transgene and the transcription factor. This dual requirement is the basis for intersectional strategies for targeting neurons based on combinations of constraints (Fenno et al. 2014). Double virus injections can be used to target neurons whose cell bodies are located at one injection site and whose axon terminals are located at another injection site. For example, one viral vector can be used to deliver the gene for the transcription factor, and another can deliver a transgene that is only transcribed in the presence of that transcription factor. Alternatively, one vector can be used to express Cre recombinase (or other recombinases) in a subset of neurons, and another can deliver a transgene whose expression requires the recombinase. In both cases, only neurons that have been successfully transduced by both viral vectors will express the transgene.
This approach was used to identify a neural pathway from the rat auditory cortex that mediates tone sequence discrimination (Znamenskiy and Zador 2013). This study is particularly noteworthy because it used nontransgenic animals, a well-controlled behavioral task, and an impressive array of optogenetic targeting strategies to reveal the behavioral significance of a particular neural pathway. In one set of experiments, an AAV vector carrying a Cre-dependent ChR2 was injected into the auditory cortex and a retrogradely transported herpes simplex viral vector carrying the Cre recombinase gene was injected at the axon terminals of these neurons in the striatum. The net result was that only the cortical neurons that projected to the striatum could be activated by light.
Exploring a similar technique in primates, Kinoshita and colleagues (2012) injected an AAV vector and a retrogradely transported LV vector into separate locations of the macaque spinal cord. In combination, these vectors drove the expression of a drug-inducible toxin exclusively in neurons whose cell bodies were at the first injection site and whose axons terminated at the second. The net result was the reversible suppression of synaptic transmission in hundreds of the neurons that comprise this pathway. Blockade of this neural pathway impaired the ability to grip with precision. A similar attempt to target projections from the prefrontal cortex to the caudate nucleus and to the FEF of a macaque was less successful (Oguchi et al. 2015). In this case, only ∼30 neurons per pathway were affected, which is presumably too few to mediate robust behavioral effects.
Targeting Neurons at the Transcriptional Level
Every cell in the brain contains the same genetic information. Cells have different properties largely because they express different combinations of genes. The mechanisms by which a set of genes is expressed in one cell and a different set is expressed in another are still incompletely understood, but significant progress has been made on this front over the past few years. Below, we discuss the prospect of harnessing endogenous gene regulation mechanisms to engineer viral vectors that direct gene expression to specific neuronal types.
Promoter-mediated specificity with viral vectors.
Cell type targeting in primates would be greatly facilitated by promoters2 that are packageable into viral vectors and, when delivered this way, drive gene expression exclusively in a targeted cell type (for a review see Kugler 2016). This technology does not exist yet, at least not in a form that can be used to target most neuronal types of interest. However, a growing set of promoters can bias, and in some cases restrict, transgene expression to some neuronal types, and the answers to many neurophysiological questions may be obtainable with a modest degree of specificity.
Characterizing the specificity of a putative cell type-specific promoter is critical for evaluating its utility for neurophysiology but is complicated for several reasons. First, separating the effect of the promoter from other factors that influence transgene expression is challenging. Second, quantifying the rate of off-target cell transduction is difficult, especially when it is low. Third, some gene products are more easily detected than others, and whether a cell expresses the transgene is not always clear. For example, tissues that test positively for viral vector transduction by immunohistochemistry or qPCR may not express the transgene at physiologically relevant levels. Fourth, the region of the brain over which transduced cells are counted influences how specific a promoter appears: At the injection site, where viral vector concentration is high and tissue is injured, off-target expression may be frequent. In tissues where on-target labeling is intense, off-target labeling may be obscured. Finally, studies of promoter selectivity in the primate CNS necessarily use fewer animals than rodent studies, amplifying the effects of individual differences on selectivity estimates.
These caveats notwithstanding, several cell type-specific promoters have been used in viral vectors with varying degrees of success. Recently, Lerchner and colleagues (2014) targeted noradrenergic neurons of the locus coeruleus and substantia nigra in macaques with a tyrosine hydroxylase promoter in an LV vector. In these areas, 96% of transduced cells were tyrosine hydroxylase-expressing neurons, which is presumably higher than would have been obtained with a less restrictive promoter. In rodents, similar attempts to target noradrenergic neurons with selective promoters also achieved some specificity, although methodological differences between these studies preclude a meaningful comparison with the results in primates (Hwang et al. 2001, 2005; Kim et al. 2010; Oh et al. 2009).
Most studies of cell type-specific promoter sequences have used rodent models, and we review this literature below. The particular sequences that drive transgene expression in particular cell types in rodents are unlikely to function identically in the homologous cell types of primates, but we expect the rewards and challenges of this approach to be similar across species. We focus on viral vector-mediated gene delivery and do not review the literature on cell type-specific promoters in transgenic animals, which is less relevant to viral vector-mediated gene delivery in primates.
Cell type-specific promoters have been obtained for rodent rods and cones (Boye et al. 2012; Khani et al. 2007), ON-bipolar cells (Cronin et al. 2014; Doroudchi et al. 2011), Müller glia (Aartsen et al. 2010; Geller et al. 2007; Greenberg et al. 2007), astrocytes (Drinkut et al. 2012; Kugler 2016; Lawlor et al. 2009; Meng et al. 2015; Merienne et al. 2015; von Jonquieres et al. 2013), and oligodendrocytes (Chen et al. 1999; Kagiava et al. 2014; Lawlor et al. 2009; McIver et al. 2005; von Jonquieres et al. 2013). Of these, the promoters for photoreceptors and astrocytes appear the most selective, and the promoters for oligodendrocytes and Müller glia appear the least selective. In mouse and cat V1, a glutamate decarboxylase promoter (GAD1) can restrict expression to GABAergic neurons (Liu et al. 2013). In the mouse brain stem, somatostatin-expressing neurons have been targeted with an off-target transduction rate of ∼20% (Tan et al. 2008). In the rat striatum, cell type-specific promoters have been used to target two endogenous opioid-producing neuronal populations with minor (<10%) off-target transduction for each (Ferguson et al. 2011). In the latter two studies, viral vectors with cell type-specific promoters were used to express chemogenetic receptors that, when activated, hyperpolarized transduced neurons and produced informative behavioral phenotypes (Ferguson et al. 2011; Tan et al. 2008).
Studies of the rat hypothalamus showcase the incisive neurophysiological manipulations that can be made using viral vectors with cell type-specific promoters. In the rat lateral hypothalamus, intermingled populations of neurons express melanin-concentrating hormone and hypocretin. Both populations have been individually targeted (Adamantidis et al. 2007; Carter et al. 2009; van den Pol et al. 2004), and optogenetic stimulation of hypocretin neurons produces wakefulness (Adamantidis et al. 2007; Carter et al. 2009). Elsewhere in the hypothalamus, intermixed oxytocin- and vasopressin-expressing neurons have also been separately targeted (Fields et al. 2012; Knobloch et al. 2014; Ponzio et al. 2012). Optogenetic stimulation of oxytocin-expressing neurons that project from the paraventricular nucleus to the amygdala suppressed fear behaviors (Knobloch et al. 2012). Collectively, these results show that cell type-specific promoters can be used in viral vectors for targeted manipulations of neural activity and behavior in nontransgenic animals. All of these studies were performed in rats, but the technologies used are theoretically feasible in primates.
Activity-dependent promoters.
Gene expression is not a static property of cells but rather depends on each cell's activity and environment. For example, the level of c-fos expression in neurons is upregulated after periods of spiking activity (Kovacs 1998). The c-fos promoter, the promoters of other activity-dependent genes, and engineered variants have been used to drive transgene expression in active neurons (Kawashima et al. 2014). This technique has not yet been used in primates but has been used in mice to tag neurons that respond strongly to whisker stimulation or to vertically oriented visual stimuli (Kawashima et al. 2013). Activity-dependent promoters drive expression only briefly, but techniques based on DNA recombination or transcription factor activation, such as those described in Advances in retrograde projection targeting, can prolong transgene expression for several days and theoretically much longer.
microRNA-mediated targeting.
How strongly and in what cell types a viral vector drives expression depends on the sequence of the promoter and also the transgene (Powell et al. 2015). Some transgenes, while successfully transcribed into mRNA, are degraded prior to translation via microRNA-mediated mechanisms. MicroRNAs are short RNA molecules that are naturally expressed in cells and bind to complementary sequences in longer messenger RNAs, suppressing their translation into protein. Different cells express different collections of microRNAs, which causes them to express different genes. To harness this mechanism for a neurophysiological experiment, transgenes delivered by viral vector can be engineered to include sequences that are recognized by the microRNAs in off-target cells, effectively detargeting transgene expression from those cells. Individual microRNA species have only modest effects on gene expression (Gentner and Naldini 2012), but multiple target sites can be concatenated, reducing transgene expression further (Merienne et al. 2015; but see Sayeg et al. 2015). MicroRNA types differ across neuronal types, suggesting that this technique will be useful for targeting subsets of neurons (He et al. 2012; Sayeg et al. 2015). The small size of microRNAs makes this approach particularly amenable for use in viral vectors.
New prospects for cell type-specific promoters.
How will new, more selective cell type-specific promoters be obtained? One approach is to synthesize promoters de novo, based on concatenated copies of transcription factor binding sites or random sequences (Nathanson et al. 2009a; Schlabach et al. 2010). This approach has not yet produced tightly restrictive promoters, but in some cases judicious modification of naturally occurring promoters has boosted expression levels in targeted cell types (Chuah et al. 2014; Hwang et al. 2001). Another approach is to identify a gene that is expressed strongly in the cell type of interest, find the transcription start site of that gene from a bioinformatics database, and use the sequence immediately upstream of it in a viral vector. For example, the widely used CaMKIIa promoter is a 1,300-base pair sequence located just upstream of the mouse CaMKIIa transcription start site. Systematic use of this approach using viral vectors has achieved modest success in rodents (de Leeuw et al. 2014; Delzor et al. 2012; Nathanson et al. 2009a).
There are at least three reasons that this approach has not been more fruitful. First, most neuronal types are distinguished by the graded expression of many genes, not by the binary expression of any single one (Tasic et al. 2016; Toledo-Rodriguez et al. 2005). Second, some indispensable components of viral vector genomes can act as promoters, driving expression even in off-target cell types (Flotte et al. 1993; Haberman et al. 2000; Logan et al. 2004). This issue is more an inconvenience than a profound problem. It has been largely solved in LV vectors (Logan et al. 2004), is weak in neurons, and can be reduced further by virus dilution (Cronin et al. 2014) or the inclusion of transcription blocking sequences (Fitzsimons et al. 2001; Kugler 2016). Third, gene expression is regulated in part by noncoding DNA sequences, called enhancers, that are distinct from promoters and can be quite far from them, either upstream or downstream in the genome.
A key to finding new, highly restrictive cell type-specific promoters may lie in new techniques for identifying active enhancers in the genomic DNA of cells of interest, because these are thought to account for many of the differences among cell types (ENCODE Project Consortium 2012; Furey 2012; Hardison and Taylor 2012; Sanyal et al. 2012; Simonis et al. 2007; Thurman et al. 2012). Recent technical advances in DNA sequencing have greatly facilitated the discovery of new enhancers, and most enhancers that have been discovered are small enough to fit inside viral vectors (Murtha et al. 2014; Narlikar and Ovcharenko 2009).
Two general classes of techniques have proven fruitful for identifying enhancers. The first takes advantage of the fact that enhancers are frequently in regions of genomic DNA that are available to modification by enzymes (Buenrostro et al. 2013; Thurman et al. 2012) and are tagged with particular epigenetic marks (Heintzman et al. 2007). The second measures the ability of a putative enhancer to increase transgene expression in a cell type of interest. This can be done for a small number of candidate enhancers in vivo (Visel et al. 2009) or for a large number in vitro (Arnold et al. 2013; Dailey 2015; Muerdter et al. 2015; Murtha et al. 2014). High-throughput screening in an in vivo system would be a powerful tool (Patwardhan et al. 2012).
Current techniques for finding enhancers require a purified population of the cell type of interest. Collecting such a population from the primate CNS remains challenging, but recent advances are making this easier. For example, laser capture microdissection can be used to collect samples from thin sections of primate CNS (Bernard et al. 2012; Datta et al. 2015). Alternatively, neurons of a common type can be collected by fluorescence-activated cell sorting on the basis of the proteins they express (Bonn et al. 2012; Iglesias-Ussel et al. 2013). Finally, each cell in a heterogeneous tissue sample can be profiled in parallel, and cell types can be categorized through bioinformatic analyses (Schwartzman and Tanay 2015; Shapiro et al. 2013). We anticipate that these tools, combined with viral vector-mediated gene delivery, will transform our ability to direct gene expression in specific cell types in primates.
Conclusion
Transgenes can be expressed in the primate CNS using viral vectors, but targeting manipulations to neuronal types of interest with this technology remains challenging. Methods required to achieve selective targeting generally require tailoring for individual applications. Magic bullets, in the form of highly active, tightly restrictive cell type-specific promoters, are rare but do exist for a handful of neuronal populations. Projection targeting, in contrast, does not rely on cell type restriction at the transcriptional level and takes advantage of the well-studied anatomy of the primate brain. We expect that anterograde projection targeting in primates will lead to new, important discoveries over the next several years. Improvements in retrograde viral vectors would increase the utility of retrograde targeting in the primate CNS. Achieving selectivity based on cell type-specific promoters is expected to be a longer road but a fruitful one for those willing to travel it.
GRANTS
This work was supported by National Institutes of Health Grants R21 EY-024362, P51 OD-010425, and P30 EY-01730.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.E.-S., A.M.N., and G.D.H. conception and design of research; Y.E.-S., A.M.N., and G.D.H. performed experiments; Y.E.-S., A.M.N., and G.D.H. analyzed data; Y.E.-S., A.M.N., and G.D.H. interpreted results of experiments; Y.E.-S., A.M.N., and G.D.H. prepared figures; Y.E.-S., A.M.N., and G.D.H. drafted manuscript; Y.E.-S., A.M.N., and G.D.H. edited and revised manuscript; Y.E.-S., A.M.N., and G.D.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Michael Shadlen (R01 EY-11378) for assistance acquiring the data in Fig. 1 and Jing Huang for expert immunohistochemistry. They also thank Jonathan Nassi, Gabe Murphy, Neeraj Gandhi, and Mehrdad Jazayeri for helpful comments on the manuscript.
Footnotes
A point on nomenclature: Every AAV vector contains a small amount of DNA from a naturally occurring AAV, usually from AAV serotype 2. When this DNA is packaged in the capsid from AAV serotype 5 the resulting vector is called “AAV5,” or sometimes “AAV2/5.”
Promoters used in viral vectors may consist of an assortment of gene-regulatory sequences, only some of which are promoters in a strict sense.
REFERENCES
- Aartsen WM, van Cleef KW, Pellissier LP, Hoek RM, Vos RM, Blits B, Ehlert EM, Balaggan KS, Ali RR, Verhaagen J, Wijnholds J. GFAP-driven GFP expression in activated mouse Müller glial cells aligning retinal blood vessels following intravitreal injection of AAV2/6 vectors. PLoS One 5: e12387, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45: 545–552, 2004. [DOI] [PubMed] [Google Scholar]
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450: 420–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander IE, Russell DW, Miller AD. Transfer of contaminants in adeno-associated virus vector stocks can mimic transduction and lead to artifactual results. Hum Gene Ther 8: 1911–1920, 1997. [DOI] [PubMed] [Google Scholar]
- Arnold CD, Gerlach D, Stelzer C, Boryn LM, Rath M, Stark A. Genome-wide quantitative enhancer activity maps identified by STARR-seq. Science 339: 1074–1077, 2013. [DOI] [PubMed] [Google Scholar]
- Ayuso E, Mingozzi F, Montane J, Leon X, Anguela XM, Haurigot V, Edmonson SA, Africa L, Zhou S, High KA, Bosch F, Wright JF. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther 17: 503–510, 2010. [DOI] [PubMed] [Google Scholar]
- Azim E, Fink AJ, Jessell TM. Internal and external feedback circuits for skilled forelimb movement. Cold Spring Harb Symp Quant Biol 79: 81–92, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A, Lubbers LS, Tanis KQ, Luo R, Podtelezhnikov AA, Finney EM, McWhorter MM, Serikawa K, Lemon T, Morgan R, Copeland C, Smith K, Cullen V, Davis-Turak J, Lee CK, Sunkin SM, Loboda AP, Levine DM, Stone DJ, Hawrylycz MJ, Roberts CJ, Jones AR, Geschwind DH, Lein ES. Transcriptional architecture of the primate neocortex. Neuron 73: 1083–1099, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn S, Zinzen RP, Perez-Gonzalez A, Riddell A, Gavin AC, Furlong EE. Cell type-specific chromatin immunoprecipitation from multicellular complex samples using BiTS-ChIP. Nat Protoc 7: 978–994, 2012. [DOI] [PubMed] [Google Scholar]
- Boulaire J, Balani P, Wang S. Transcriptional targeting to brain cells: engineering cell type-specific promoter containing cassettes for enhanced transgene expression. Adv Drug Deliv Rev 61: 589–602, 2009. [DOI] [PubMed] [Google Scholar]
- Boye SE, Alexander JJ, Boye SL, Witherspoon CD, Sandefer KJ, Conlon TJ, Erger K, Sun J, Ryals R, Chiodo VA, Clark ME, Girkin CA, Hauswirth WW, Gamlin PD. The human rhodopsin kinase promoter in an AAV5 vector confers rod- and cone-specific expression in the primate retina. Hum Gene Ther 23: 1101–1115, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods 10: 1213–1218, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buning H, Huber A, Zhang L, Meumann N, Hacker U. Engineering the AAV capsid to optimize vector-host-interactions. Curr Opin Pharmacol 24: 94–104, 2015. [DOI] [PubMed] [Google Scholar]
- Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol 4: 341, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Luo L. Monosynaptic circuit tracing with glycoprotein-deleted rabies viruses. J Neurosci 35: 8979–8985, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Enquist LW. Transneuronal circuit analysis with pseudorabies viruses. Curr Protoc Neurosci 68: 1–1.5, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci 29: 10939–10949, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle MJ, Gershenson ZT, Giles AR, Holzbaur EL, Wolfe JH. Adeno-associated virus serotypes 1, 8, and 9 share conserved mechanisms for anterograde and retrograde axonal transport. Hum Gene Ther 25: 705–720, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh J, Monosov IE, McAlonan K, Berman R, Smith MK, Cao V, Wang KH, Boyden ES, Wurtz RH. Optogenetic inactivation modifies monkey visuomotor behavior. Neuron 76: 901–907, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin A, Callaway EM. Optical control of retrogradely infected neurons using drug-regulated “TLoop” lentiviral vectors. J Neurophysiol 111: 2150–2159, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin A, Komai S, Eliava M, Seeburg PH, Osten P. Stereotaxic gene delivery in the rodent brain. Nat Protoc 1: 3166–3173, 2007. [DOI] [PubMed] [Google Scholar]
- Chan AW. Progress and prospects for genetic modification of nonhuman primate models in biomedical research. ILAR J 54: 211–223, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AW, Yang SH. Generation of transgenic monkeys with human inherited genetic disease. Methods 49: 78–84, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, McCarty DM, Bruce AT, Suzuki K. Oligodendrocyte-specific gene expression in mouse brain: use of a myelin-forming cell type-specific promoter in an adeno-associated virus. J Neurosci Res 55: 504–513, 1999. [DOI] [PubMed] [Google Scholar]
- Chen Y, Niu Y, Ji W. Transgenic nonhuman primate models for human diseases: approaches and contributing factors. J Genet Genomics 39: 247–251, 2012. [DOI] [PubMed] [Google Scholar]
- Choi J, Young JA, Callaway EM. Selective viral vector transduction of ErbB4 expressing cortical interneurons in vivo with a viral receptor-ligand bridge protein. Proc Natl Acad Sci USA 107: 16703–16708, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtarto A, Bender HU, Hanemann CO, Kemp T, Lehtonen E, Levivier M, Brotchi J, Velu T, Tenenbaum L. Tetracycline-inducible transgene expression mediated by a single AAV vector. Gene Ther 10: 84–94, 2003. [DOI] [PubMed] [Google Scholar]
- Chtarto A, Bockstael O, Tshibangu T, Dewitte O, Levivier M, Tenenbaum L. A next step in adeno-associated virus-mediated gene therapy for neurological diseases: regulation and targeting. Br J Clin Pharmacol 76: 217–232, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah MK, Petrus I, De Bleser P, Le Guiner C, Gernoux G, Adjali O, Nair N, Willems J, Evens H, Rincon MY, Matrai J, Di Matteo M, Samara-Kuko E, Yan B, Acosta-Sanchez A, Meliani A, Cherel G, Blouin V, Christophe O, Moullier P, Mingozzi F, VandenDriessche T. Liver-specific transcriptional modules identified by genome-wide in silico analysis enable efficient gene therapy in mice and non-human primates. Mol Ther 22: 1605–1613, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther 5: 387–398, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin T, Vandenberghe LH, Hantz P, Juttner J, Reimann A, Kacso AE, Huckfeldt RM, Busskamp V, Kohler H, Lagali PS, Roska B, Bennett J. Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol Med 6: 1175–1190, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65: 230–245, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey L. High throughput technologies for the functional discovery of mammalian enhancers: new approaches for understanding transcriptional regulatory network dynamics. Genomics 106: 151–158, 2015. [DOI] [PubMed] [Google Scholar]
- Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med 5: 189ra176, 2013. [DOI] [PubMed] [Google Scholar]
- Datta S, Malhotra L, Dickerson R, Chaffee S, Sen CK, Roy S. Laser capture microdissection: big data from small samples. Histol Histopathol 30: 1255–1269, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felipe P. Polycistronic viral vectors. Curr Gene Ther 2: 355–378, 2002. [DOI] [PubMed] [Google Scholar]
- de Leeuw CN, Dyka FM, Boye SL, Laprise S, Zhou M, Chou AY, Borretta L, McInerny SC, Banks KG, Portales-Casamar E, Swanson MI, D'Souza CA, Boye SE, Jones SJ, Holt RA, Goldowitz D, Hauswirth WW, Wasserman WW, Simpson EM. Targeted CNS delivery using human minipromoters and demonstrated compatibility with adeno-associated viral vectors. Mol Ther Methods Clin Dev 1: 5, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. Circuit dynamics of adaptive and maladaptive behaviour. Nature 505: 309–317, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzor A, Dufour N, Petit F, Guillermier M, Houitte D, Auregan G, Brouillet E, Hantraye P, Deglon N. Restricted transgene expression in the brain with cell-type specific neuronal promoters. Hum Gene Ther Methods 23: 242–254, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K, Shenoy KV. An optogenetic toolbox designed for primates. Nat Neurosci 14: 387–397, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov DS. Virus entry: molecular mechanisms and biomedical applications. Nat Rev Microbiol 2: 109–122, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodiya HB, Bjorklund T, Stansell J 3rd, Mandel RJ, Kirik D, Kordower JH. Differential transduction following basal ganglia administration of distinct pseudotyped AAV capsid serotypes in nonhuman primates. Mol Ther 18: 579–587, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroudchi MM, Greenberg KP, Liu J, Silka KA, Boyden ES, Lockridge JA, Arman AC, Janani R, Boye SE, Boye SL, Gordon GM, Matteo BC, Sampath AP, Hauswirth WW, Horsager A. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol Ther 19: 1220–1229, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinkut A, Tereshchenko Y, Schulz JB, Bahr M, Kugler S. Efficient gene therapy for Parkinson's disease using astrocytes as hosts for localized neurotrophic factor delivery. Mol Ther 20: 534–543, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Transneuronal tracing with neurotropic viruses reveals network macroarchitecture. Curr Opin Neurobiol 23: 245–249, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489: 57–74, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci 34: 586–595, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci 34: 389–412, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J, Selimbeyoglu A, Berndt A, Grosenick L, Zalocusky KA, Bernstein H, Swanson H, Perry C, Diester I, Boyce FM, Bass CE, Neve R, Huang ZJ, Deisseroth K. Targeting cells with single vectors using multiple-feature Boolean logic. Nat Methods 11: 763–772, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci 14: 22–24, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RL, Ponzio TA, Kawasaki M, Gainer H. Cell-type specific oxytocin gene expression from AAV delivered promoter deletion constructs into the rat supraoptic nucleus in vivo. PLoS One 7: e32085, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci USA 110: 7306–7311, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons HL, McKenzie JM, During MJ. Insulators coupled to a minimal bidirectional tet cassette for tight regulation of rAAV-mediated gene transfer in the mammalian brain. Gene Ther 8: 1675–1681, 2001. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Afione SA, Solow R, Drumm ML, Markakis D, Guggino WB, Zeitlin PL, Carter BJ. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem 268: 3781–3790, 1993. [PubMed] [Google Scholar]
- Furey TS. ChIP-seq and beyond: new and improved methodologies to detect and characterize protein-DNA interactions. Nat Rev Genet 13: 840–852, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller SF, Ge PS, Visel M, Greenberg KP, Flannery JG. Functional promoter testing using a modified lentiviral transfer vector. Mol Vis 13: 730–739, 2007. [PMC free article] [PubMed] [Google Scholar]
- Gentner B, Naldini L. Exploiting microRNA regulation for genetic engineering. Tissue Antigens 80: 393–403, 2012. [DOI] [PubMed] [Google Scholar]
- Gerits A, Farivar R, Rosen BR, Wald LL, Boyden ES, Vanduffel W. Optogenetically induced behavioral and functional network changes in primates. Curr Biol 22: 1722–1726, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerits A, Vancraeyenest P, Vreysen S, Laramée ME, Michiels A, Gijsbers R, Van den Haute C, Moons L, Debyser Z, Baekelandt V, Arckens L. Serotype-dependent transduction efficiencies of recombinant adeno-associated viral vectors in monkey neocortex. Neurophotonics 2: 031209, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerits A, Vanduffel W. Optogenetics in primates: a shining future? Trends Genet 29: 403–411, 2013. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Andermann ML, Bonin V, Reid RC. Cortico-cortical projections in mouse visual cortex are functionally target specific. Nat Neurosci 16: 219–226, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science 324: 354–359, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141: 154–165, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg KP, Geller SF, Schaffer DV, Flannery JG. Targeted transgene expression in Müller glia of normal and diseased retinas using lentiviral vectors. Invest Ophthalmol Vis Sci 48: 1844–1852, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman RP, McCown TJ, Samulski RJ. Novel transcriptional regulatory signals in the adeno-associated virus terminal repeat A/D junction element. J Virol 74: 8732–8739, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadaczek P, Forsayeth J, Mirek H, Munson K, Bringas J, Pivirotto P, McBride JL, Davidson BL, Bankiewicz KS. Transduction of nonhuman primate brain with adeno-associated virus serotype 1: vector trafficking and immune response. Hum Gene Ther 20: 225–237, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X. Optogenetics in the nonhuman primate. Prog Brain Res 196: 215–233, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron 62: 191–198, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison RC, Taylor J. Genomic approaches towards finding cis-regulatory modules in animals. Nat Rev Genet 13: 469–483, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Liu Y, Wang X, Zhang MQ, Hannon GJ, Huang ZJ. Cell-type-based analysis of microRNA profiles in the mouse brain. Neuron 73: 35–48, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, Wang W, Weng Z, Green RD, Crawford GE, Ren B. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39: 311–318, 2007. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Carlezon WA Jr, Isacson O, Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Hum Gene Ther 12: 1731–1740, 2001. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Hwang MM, Kim HS, Kim KS. Genetically engineered dopamine beta-hydroxylase gene promoters with better PHOX2-binding sites drive significantly enhanced transgene expression in a noradrenergic cell-specific manner. Mol Ther 11: 132–141, 2005. [DOI] [PubMed] [Google Scholar]
- Iglesias-Ussel M, Marchionni L, Romerio F. Isolation of microarray-quality RNA from primary human cells after intracellular immunostaining and fluorescence-activated cell sorting. J Immunol Methods 391: 22–30, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Koketsu D, Kato S, Kobayashi K, Nambu A, Takada M. Immunotoxin-mediated tract targeting in the primate brain: selective elimination of the cortico-subthalamic “hyperdirect” pathway. PLoS One 7: e39149, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Takada M, Matsumoto M. Neuronal and behavioural modulations by pathway-selective optogenetic stimulation of the primate oculomotor system. Nat Commun 6: 8378, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izpisua Belmonte JC, Callaway EM, Caddick SJ, Churchland P, Feng G, Homanics GE, Lee KF, Leopold DA, Miller CT, Mitchell JF, Mitalipov S, Moutri AR, Movshon JA, Okano H, Reynolds JH, Ringach D, Sejnowski TJ, Silva AC, Strick PL, Wu J, Zhang F. Brains, genes, primates. Neuron 86: 617–631, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Beneduce BM, Drew IR, Regehr WG. Achieving high-frequency optical control of synaptic transmission. J Neurosci 34: 7704–7714, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri M, Lindbloom-Brown Z, Horwitz GD. Saccadic eye movements evoked by optogenetic activation of primate V1. Nat Neurosci 15: 1368–1370, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junyent F, Kremer EJ. CAV-2—why a canine virus is a neurobiologist's best friend. Curr Opin Pharmacol 24: 86–93, 2015. [DOI] [PubMed] [Google Scholar]
- Jurgens CW, Bell KA, McQuiston AR, Guido W. Optogenetic stimulation of the corticothalamic pathway affects relay cells and GABAergic neurons differently in the mouse visual thalamus. PLoS One 7: e45717, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagiava A, Sargiannidou I, Bashiardes S, Richter J, Schiza N, Christodoulou C, Gritti A, Kleopa KA. Gene delivery targeted to oligodendrocytes using a lentiviral vector. J Gene Med 16: 364–373, 2014. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Kasahara H, Matsui R, Katoh T, Mizukami H, Ozawa K, Watanabe D, Isa T. Selective optical control of synaptic transmission in the subcortical visual pathway by activation of viral vector-expressed halorhodopsin. PLoS One 6: e18452, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar BK, Erickson D, Schaffer D, Hinh L, Gage FH, Peterson DA. Targeted retrograde gene delivery for neuronal protection. Mol Ther 5: 50–56, 2002. [DOI] [PubMed] [Google Scholar]
- Kato S, Kobayashi K, Inoue K, Kuramochi M, Okada T, Yaginuma H, Morimoto K, Shimada T, Takada M, Kobayashi K. A lentiviral strategy for highly efficient retrograde gene transfer by pseudotyping with fusion envelope glycoprotein. Hum Gene Ther 22: 197–206, 2011a. [DOI] [PubMed] [Google Scholar]
- Kato S, Kobayashi K, Inoue K, Takada M, Kobayashi K. Vectors for highly efficient and neuron-specific retrograde gene transfer or gene therapy of neurological diseases. In: Gene Therapy—Tools and Potential Applications, edited by Martin DF. Haverhill, MA: InTech, 2013. [Google Scholar]
- Kato S, Kuramochi M, Takasumi K, Kobayashi K, Inoue K, Takahara D, Hitoshi S, Ikenaka K, Shimada T, Takada M, Kobayashi K. Neuron-specific gene transfer through retrograde transport of lentiviral vector pseudotyped with a novel type of fusion envelope glycoprotein. Hum Gene Ther 22: 1511–1523, 2011b. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Kitamura K, Suzuki K, Nonaka M, Kamijo S, Takemoto-Kimura S, Kano M, Okuno H, Ohki K, Bito H. Functional labeling of neurons and their projections using the synthetic activity-dependent promoter E-SARE. Nat Methods 10: 889–895, 2013. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Okuno H, Bito H. A new era for functional labeling of neurons: activity-dependent promoters have come of age. Front Neural Circuits 8: 37, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khani SC, Pawlyk BS, Bulgakov OV, Kasperek E, Young JE, Adamian M, Sun X, Smith AJ, Ali RR, Li T. AAV-mediated expression targeting of rod and cone photoreceptors with a human rhodopsin kinase promoter. Invest Ophthalmol Vis Sci 48: 3954–3961, 2007. [DOI] [PubMed] [Google Scholar]
- Kim ML, Han S, Lee SB, Kim JH, Ahn HK, Huh Y. Evaluation of recombinant adenovirus-mediated gene delivery for expression of tracer genes in catecholaminergic neurons. Anat Cell Biol 43: 157–164, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Isa T. Potential of optogenetics for the behavior manipulation of non-human primates. In: Optogenetics, edited by Yawo H. Tokyo: Springer Japan, 2015, p. 279–290. [Google Scholar]
- Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, Watakabe A, Yamamori T, Nishimura Y, Alstermark B, Watanabe D, Kobayashi K, Isa T. Genetic dissection of the circuit for hand dexterity in primates. Nature 487: 235–238, 2012. [DOI] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, Wang J, Xie Y, Yan Z, Zhang Y, Chow BY, Surek B, Melkonian M, Jayaraman V, Constantine-Paton M, Wong GK, Boyden ES. Independent optical excitation of distinct neural populations. Nat Methods 11: 338–346, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Tatom JB, Henderson KM, Henning PP. AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol Ther 16: 89–96, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73: 553–566, 2012. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Stoop R, Grinevich V. Viral vectors for optogenetics of hypothalamic neuropeptides. In: Viral Vector Approaches in Neurobiology and Brain Diseases, edited by Brambilla R. New York: Humana, 2014, p. 311–329. [Google Scholar]
- Kotterman MA, Yin L, Strazzeri JM, Flannery JG, Merigan WH, Schaffer DV. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther 22: 116–126, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int 33: 287–297, 1998. [DOI] [PubMed] [Google Scholar]
- Kugler S. Tissue-specific promoters in the CNS. Methods Mol Biol 1382: 81–91, 2016. [DOI] [PubMed] [Google Scholar]
- Kuhn B, Ozden I, Lampi Y, Hasan MT, Wang SS. An amplified promoter system for targeted expression of calcium indicator proteins in the cerebellar cortex. Front Neural Circuits 6: 49, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon I, Schaffer DV. Designer gene delivery vectors: molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer. Pharm Res 25: 489–499, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latta-Mahieu M, Rolland M, Caillet C, Wang M, Kennel P, Mahfouz I, Loquet I, Dedieu JF, Mahfoudi A, Trannoy E, Thuillier V. Gene transfer of a chimeric trans-activator is immunogenic and results in short-lived transgene expression. Hum Gene Ther 13: 1611–1620, 2002. [DOI] [PubMed] [Google Scholar]
- Lawlor PA, Bland RJ, Mouravlev A, Young D, During MJ. Efficient gene delivery and selective transduction of glial cells in the mammalian brain by AAV serotypes isolated from nonhuman primates. Mol Ther 17: 1692–1702, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerchner W, Corgiat B, Der Minassian V, Saunders RC, Richmond BJ. Injection parameters and virus dependent choice of promoters to improve neuron targeting in the nonhuman primate brain. Gene Ther 21: 233–241, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Hromadka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One 4: e6099, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J 96: 1803–1814, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowski L, Tay SS, Alexander IE. Adeno-associated virus serotypes for gene therapeutics. Curr Opin Pharmacol 24: 59–67, 2015. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Ehrengruber MU, Negwer M, Shao HJ, Cetin AH, Lyon DC. Tracing inputs to inhibitory or excitatory neurons of mouse and cat visual cortex with a targeted rabies virus. Curr Biol 23: 1746–1755, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li X, Zhang JT, Cai YJ, Cheng TL, Cheng C, Wang Y, Zhang CC, Nie YH, Chen ZF, Bian WJ, Zhang L, Xiao J, Lu B, Zhang YF, Zhang XD, Sang X, Wu JJ, Xu X, Xiong ZQ, Zhang F, Yu X, Gong N, Zhou WH, Sun Q, Qiu Z. Autism-like behaviours and germline transmission in transgenic monkeys overexpressing MeCP2. Nature 530: 98–102, 2016. [DOI] [PubMed] [Google Scholar]
- Logan AC, Haas DL, Kafri T, Kohn DB. Integrated self-inactivating lentiviral vectors produce full-length genomic transcripts competent for encapsidation and integration. J Virol 78: 8421–8436, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonser RR, Sarntinoranont M, Morrison PF, Oldfield EH. Convection-enhanced delivery to the central nervous system. J Neurosurg 122: 697–706, 2015. [DOI] [PubMed] [Google Scholar]
- Maguire CA, Ramirez SH, Merkel SF, Sena-Esteves M, Breakefield XO. Gene therapy for the nervous system: challenges and new strategies. Neurotherapeutics 11: 817–839, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat Biotechnol 24: 198–204, 2006. [DOI] [PubMed] [Google Scholar]
- Mahn M, Prigge M, Ron S, Levy R, Yizhar O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat Neurosci 19: 554–556, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi P, Argnani R, Epstein AL, Manservigi R. HSV as a vector in vaccine development and gene therapy. Hum Vaccin 4: 91–105, 2008. [DOI] [PubMed] [Google Scholar]
- Markakis EA, Vives KP, Bober J, Leichtle S, Leranth C, Beecham J, Elsworth JD, Roth RH, Samulski RJ, Redmond DE Jr. Comparative transduction efficiency of AAV vector serotypes 1–6 in the substantia nigra and striatum of the primate brain. Mol Ther 18: 588–593, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamizu Y, Okada T, Kawasaki K, Ishibashi H, Yuasa S, Takeda S, Hasegawa I, Nakahara K. Local and retrograde gene transfer into primate neuronal pathways via adeno-associated virus serotype 8 and 9. Neuroscience 193: 249–258, 2011. [DOI] [PubMed] [Google Scholar]
- McIver SR, Lee CS, Lee JM, Green SH, Sands MS, Snider BJ, Goldberg MP. Lentiviral transduction of murine oligodendrocytes in vivo. J Neurosci Res 82: 397–403, 2005. [DOI] [PubMed] [Google Scholar]
- Meng X, Yang F, Ouyang T, Liu B, Wu C, Jiang W. Specific gene expression in mouse cortical astrocytes is mediated by a 1740bp-GFAP promoter-driven combined adeno-associated virus 2/5/7/8/9. Neurosci Lett 593: 45–50, 2015. [DOI] [PubMed] [Google Scholar]
- Merienne N, Delzor A, Viret A, Dufour N, Rey M, Hantraye P, Deglon N. Gene transfer engineering for astrocyte-specific silencing in the CNS. Gene Ther 22: 830–839, 2015. [DOI] [PubMed] [Google Scholar]
- Michelfelder S, Trepel M. Adeno-associated viral vectors and their redirection to cell-type specific receptors. Adv Genet 67: 29–60, 2009. [DOI] [PubMed] [Google Scholar]
- Muerdter F, Boryn LM, Arnold CD. STARR-seq—principles and applications. Genomics 106: 145–150, 2015. [DOI] [PubMed] [Google Scholar]
- Munch RC, Muth A, Muik A, Friedel T, Schmatz J, Dreier B, Trkola A, Pluckthun A, Buning H, Buchholz CJ. Off-target-free gene delivery by affinity-purified receptor-targeted viral vectors. Nat Commun 6: 6246, 2015. [DOI] [PubMed] [Google Scholar]
- Murtha M, Tokcaer-Keskin Z, Tang Z, Strino F, Chen X, Wang Y, Xi X, Basilico C, Brown S, Bonneau R, Kluger Y, Dailey L. FIREWACh: high-throughput functional detection of transcriptional regulatory modules in mammalian cells. Nat Methods 11: 559–565, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narlikar L, Ovcharenko I. Identifying regulatory elements in eukaryotic genomes. Brief Funct Genomic Proteomic 8: 215–230, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassi JJ, Cepko CL, Born RT, Beier KT. Neuroanatomy goes viral! Front Neuroanat 9: 80, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson JL, Jappelli R, Scheeff ED, Manning G, Obata K, Brenner S, Callaway EM. Short promoters in viral vectors drive selective expression in mammalian inhibitory neurons, but do not restrict activity to specific inhibitory cell-types. Front Neural Circuits 3: 19, 2009a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson JL, Yanagawa Y, Obata K, Callaway EM. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience 161: 441–450, 2009b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguchi M, Okajima M, Tanaka S, Koizumi M, Kikusui T, Ichihara N, Kato S, Kobayashi K, Sakagami M. Double virus vector infection to the prefrontal network of the macaque brain. PLoS One 10: e0132825, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MS, Hong SJ, Huh Y, Kim KS. Expression of transgenes in midbrain dopamine neurons using the tyrosine hydroxylase promoter. Gene Ther 16: 437–440, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon S, Grimaldi P, Schweers N, Tsao DY. Saccade modulation by optical and electrical stimulation in the macaque frontal eye field. J Neurosci 33: 16684–16697, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H, Scanziani M. Gain control by layer six in cortical circuits of vision. Nature 483: 47–52, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan RP, Hiatt JB, Witten DM, Kim MJ, Smith RP, May D, Lee C, Andrie JM, Lee SI, Cooper GM, Ahituv N, Pennacchio LA, Shendure J. Massively parallel functional dissection of mammalian enhancers in vivo. Nat Biotechnol 30: 265–270, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Gutnisky DA, Huber D, Xu NL, O'Connor DH, Tian L, Looger L, Svoboda K. Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature 489: 299–303, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 10: 663–668, 2007. [DOI] [PubMed] [Google Scholar]
- Ponzio TA, Fields RL, Rashid OM, Salinas YD, Lubelski D, Gainer H. Cell-type specific expression of the vasopressin gene analyzed by AAV mediated gene delivery of promoter deletion constructs into the rat SON in vivo. PLoS One 7: e48860, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SK, Rivera-Soto R, Gray SJ. Viral expression cassette elements to enhance transgene target specificity and expression in gene therapy. Discov Med 19: 49–57, 2015. [PMC free article] [PubMed] [Google Scholar]
- Ramamoorth M, Narvekar A. Non viral vectors in gene therapy—an overview. J Clin Diagn Res 9: GE01–GE06, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadakane O, Masamizu Y, Watakabe A, Terada S, Ohtsuka M, Takaji M, Mizukami H, Ozawa K, Kawasaki H, Matsuzaki M, Yamamori T. Long-term two-photon calcium imaging of neuronal populations with subcellular resolution in adult non-human primates. Cell Rep 13: 1989–1999, 2015. [DOI] [PubMed] [Google Scholar]
- Sanchez CE, Tierney TS, Gale JT, Alavian KN, Sahin A, Lee JS, Mulligan RC, Carter BS. Recombinant adeno-associated virus type 2 pseudotypes: comparing safety, specificity, and transduction efficiency in the primate striatum. Laboratory investigation. J Neurosurg 114: 672–680, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature 489: 109–113, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki E. Prospects for genetically modified non-human primate models, including the common marmoset. Neurosci Res 93: 110–115, 2015. [DOI] [PubMed] [Google Scholar]
- Sayeg MK, Weinberg BH, Cha SS, Goodloe M, Wong WW, Han X. Rationally designed microRNA-based genetic classifiers target specific neurons in the brain. ACS Synth Biol 4: 788–795, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlabach MR, Hu JK, Li M, Elledge SJ. Synthetic design of strong promoters. Proc Natl Acad Sci USA 107: 2538–2543, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger P, Scharer YP, Oertner TG. Channelrhodopsin as a tool to investigate synaptic transmission and plasticity. Exp Physiol 96: 34–39, 2011. [DOI] [PubMed] [Google Scholar]
- Schwartzman O, Tanay A. Single-cell epigenomics: techniques and emerging applications. Nat Rev Genet 16: 716–726, 2015. [DOI] [PubMed] [Google Scholar]
- Shapiro E, Biezuner T, Linnarsson S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat Rev Genet 14: 618–630, 2013. [DOI] [PubMed] [Google Scholar]
- Simonis M, Kooren J, de Laat W. An evaluation of 3C-based methods to capture DNA interactions. Nat Methods 4: 895–901, 2007. [DOI] [PubMed] [Google Scholar]
- Soetedjo R, Kojima Y, Horwitz GD. Optogenetic activation of primate superior colliculus, a substitute for electrical stimulation? (Abstract). Neuroscience Meeting Planner 2013: 365.01, 2013. [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature 522: 309–314, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron 82: 797–808, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475: 377–380, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci 11: 538–540, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, Levi B, Gray LT, Sorensen SA, Dolbeare T, Bertagnolli D, Goldy J, Shapovalova N, Parry S, Lee C, Smith K, Bernard A, Madisen L, Sunkin SM, Hawrylycz M, Koch C, Zeng H. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci 19: 335–346, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, Sheffield NC, Stergachis AB, Wang H, Vernot B, Garg K, John S, Sandstrom R, Bates D, Boatman L, Canfield TK, Diegel M, Dunn D, Ebersol AK, Frum T, Giste E, Johnson AK, Johnson EM, Kutyavin T, Lajoie B, Lee BK, Lee K, London D, Lotakis D, Neph S, Neri F, Nguyen ED, Qu H, Reynolds AP, Roach V, Safi A, Sanchez ME, Sanyal A, Shafer A, Simon JM, Song L, Vong S, Weaver M, Yan Y, Zhang Z, Zhang Z, Lenhard B, Tewari M, Dorschner MO, Hansen RS, Navas PA, Stamatoyannopoulos G, Iyer VR, Lieb JD, Sunyaev SR, Akey JM, Sabo PJ, Kaul R, Furey TS, Dekker J, Crawford GE, Stamatoyannopoulos JA. The accessible chromatin landscape of the human genome. Nature 489: 75–82, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Goodman P, Illic M, Wu C, Markram H. Neuropeptide and calcium-binding protein gene expression profiles predict neuronal anatomical type in the juvenile rat. J Physiol 567: 401–413, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torashima T, Yamada N, Itoh M, Yamamoto A, Hirai H. Exposure of lentiviral vectors to subneutral pH shifts the tropism from Purkinje cell to Bergmann glia. Eur J Neurosci 24: 371–380, 2006. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471: 358–362, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron 42: 635–652, 2004. [DOI] [PubMed] [Google Scholar]
- Visel A, Blow MJ, Li Z, Zhang T, Akiyama JA, Holt A, Plajzer-Frick I, Shoukry M, Wright C, Chen F, Afzal V, Ren B, Rubin EM, Pennacchio LA. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature 457: 854–858, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Jonquieres G, Mersmann N, Klugmann CB, Harasta AE, Lutz B, Teahan O, Housley GD, Frohlich D, Kramer-Albers EM, Klugmann M. Glial promoter selectivity following AAV-delivery to the immature brain. PLoS One 8: e65646, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet 8: 573–587, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Bell P, Lin J, Grant RL, Siegel DL, Wilson JM. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther 22: 1389–1401, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen IZ, Lin D. Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron 85: 1344–1358, 2015a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Miao L, Satterlee A, Huang L. Delivery of oligonucleotides with lipid nanoparticles. Adv Drug Deliv Rev 87: 68–80, 2015b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 492: 428–432, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watakabe A, Ohtsuka M, Kinoshita M, Takaji M, Isa K, Mizukami H, Ozawa K, Isa T, Yamamori T. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci Res 93: 144–157, 2015. [DOI] [PubMed] [Google Scholar]
- Wolak DJ, Thorne RG. Diffusion of macromolecules in the brain: implications for drug delivery. Mol Pharm 10: 1492–1504, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LF, Azzouz M, Walmsley LE, Askham Z, Wilkes FJ, Mitrophanous KA, Kingsman SM, Mazarakis ND. Transduction patterns of pseudotyped lentiviral vectors in the nervous system. Mol Ther 9: 101–111, 2004. [DOI] [PubMed] [Google Scholar]
- Yaguchi M, Ohashi Y, Tsubota T, Sato A, Koyano KW, Wang N, Miyashita Y. Characterization of the properties of seven promoters in the motor cortex of rats and monkeys after lentiviral vector-mediated gene transfer. Hum Gene Ther Methods 24: 333–344, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdan-Shahmorad A, Diaz-Botia C, Hanson TL, Kharazia V, Ledochowitsch P, Maharbiz MM, Sabes PN. A large-scale interface for optogenetic stimulation and recording in nonhuman primates. Neuron 89: 927–939, 2016. [DOI] [PubMed] [Google Scholar]
- Yin L, Masella B, Dalkara D, Zhang J, Flannery JG, Schaffer DV, Williams DR, Merigan WH. Imaging light responses of foveal ganglion cells in the living macaque eye. J Neurosci 34: 6596–6605, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Oertner TG. Optical induction of synaptic plasticity using a light-sensitive channel. Nat Methods 4: 139–141, 2007. [DOI] [PubMed] [Google Scholar]
- Znamenskiy P, Zador AM. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature 497: 482–485, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]