This study demonstrates the patterns of primary afferent activity that can be evoked by microstimulation of the lumbar dorsal root ganglia. We implemented an enhanced method for quantifying selectivity by recording signals from many branches of peripheral nerves simultaneously. Microstimulation at the L5–L7 DRG generated selective activation of primary afferents from nerves throughout the hindlimb, with consistent patterns across animals. These results have important implications for the design and testing of somatosensory neuroprostheses.
Keywords: dorsal root ganglia, sensory feedback, neuroprostheses, selective stimulation
Abstract
Patterned microstimulation of the dorsal root ganglion (DRG) has been proposed as a method for delivering tactile and proprioceptive feedback to amputees. Previous studies demonstrated that large- and medium-diameter afferent neurons could be recruited separately, even several months after implantation. However, those studies did not examine the anatomical localization of sensory fibers recruited by microstimulation in the DRG. Achieving precise recruitment with respect to both modality and receptive field locations will likely be crucial to create a viable sensory neuroprosthesis. In this study, penetrating microelectrode arrays were implanted in the L5, L6, and L7 DRG of four isoflurane-anesthetized cats instrumented with nerve cuff electrodes around the proximal and distal branches of the sciatic and femoral nerves. A binary search was used to find the recruitment threshold for evoking a response in each nerve cuff. The selectivity of DRG stimulation was characterized by the ability to recruit individual distal branches to the exclusion of all others at threshold; 84.7% (n = 201) of the stimulation electrodes recruited a single nerve branch, with 9 of the 15 instrumented nerves recruited selectively. The median stimulation threshold was 0.68 nC/phase, and the median dynamic range (increase in charge while stimulation remained selective) was 0.36 nC/phase. These results demonstrate the ability of DRG microstimulation to achieve selective recruitment of the major nerve branches of the hindlimb, suggesting that this approach could be used to drive sensory input from localized regions of the limb. This sensory input might be useful for restoring tactile and proprioceptive feedback to a lower-limb amputee.
NEW & NOTEWORTHY
This study demonstrates the patterns of primary afferent activity that can be evoked by microstimulation of the lumbar dorsal root ganglia. We implemented an enhanced method for quantifying selectivity by recording signals from many branches of peripheral nerves simultaneously. Microstimulation at the L5–L7 DRG generated selective activation of primary afferents from nerves throughout the hindlimb, with consistent patterns across animals. These results have important implications for the design and testing of somatosensory neuroprostheses.
in the u.s., an estimated 1.6 million people were living with amputation in 2005, a number that is expected to more than double by 2050 (Ziegler-Graham et al. 2008). Of these individuals, 65% had a lower-limb amputation (Ziegler-Graham et al. 2008). Furthermore, Miller et al. (2001) found that individuals with lower-limb loss often struggle with mobility issues and are likely to fear falling. Adding to these numbers are the more than 700 veterans of Operations Iraqi Freedom and Enduring Freedom that have lost limbs, many who have lost multiple limbs (Downs 2008). While lower-limb prostheses typically have higher adoption rates than upper-limb prostheses (Etter et al. 2015), there are still a number of significant problems with current devices that limit their adoption. One major limitation is the lack of sensory feedback provided by these devices. Without direct sensory feedback from the prosthetic limb, the user must infer information about limb state from the pressure exerted on the residual limb by the prosthetic socket. Reduced sensory feedback can make many activities such as stair climbing and walking on uneven terrain difficult and dangerous with a prosthetic limb (Quai et al. 2005). These problems could be reduced by haptic interfaces (Fan et al. 2008) that deliver cutaneous feedback to the residual limb as a substitute for sensory inputs lost in the missing limb.
Electrical stimulation of afferent fibers in residual nerves has been proposed as a method to provide sensory feedback to amputees. An early approach to creating a somatosensory neuroprosthesis was suggested by Clippinger et al. (1982), in which the bending moment of the sole of the foot during early and late stance drove frequency-modulated stimulation of the sciatic nerve. Although the authors reported that patients had used their devices for up to six years, the technology appears to have been abandoned, since there have been no subsequent reports since the initial publication in 1982. Contemporary studies using both epineural and penetrating electrodes have demonstrated successful recruitment of sensory neurons in distal peripheral nerves in humans with arm amputation (Dhillon and Horch 2005; Raspopovic et al. 2014; Tan et al. 2014). These studies have demonstrated that, even decades after amputation, it is possible to generate naturalistic sensory percepts by electrically stimulating peripheral nerves in the residual limb. Additionally, these studies, which have all focused on sensory restoration after upper-limb amputation, have demonstrated improvements in control of prosthetic limbs and the ability to manipulate and detect objects with a prosthetic limb without any visual feedback. Epineural electrodes have also been shown to provide selective recruitment of lower-limb muscles during functional neuromuscular stimulation (FNS) of knee extensors and hip flexors (Fisher et al. 2009; Schiefer et al. 2010) as well as ankle plantar- and dorsiflexors (Schiefer et al. 2013). Despite these successes, electrode placements on peripheral nerves require a compromise between the competing interests of recruitment selectivity, limb coverage, and robustness to mechanical stresses. In particular, the more proximal nerve trunks used to achieve the greatest limb coverage contain a mixture of motor and sensory pathways for large portions of the limb, making selective recruitment of afferents to exclusion of efferents difficult.
Targeted sensory reinnervation (TSR) is another emerging technique for restoring haptic feedback by surgically relocating sensory nerves from the amputated limb to reinnervate the skin of the residual limb and chest. Psychophysical studies with TSR subjects have demonstrated that both electrical and mechanical stimulation of the reinnervated skin evokes percepts that are referred to the amputated hand (Kuiken et al. 2007). However, this technique involves extensive surgical procedures, and it is difficult to control the pattern of reinnervation, which may limit the generalizability of the approach.
The degree of selectivity required to produce useful sensory feedback is currently unknown, although it is expected that more spatially focal stimulation will be more useful as a feedback signal. Intraneural microstimulation has been used to demonstrate that recruitment of even individual cutaneous afferents can produce conscious percepts (Macefield et al. 1990). The same subjects could not detect the recruitment of single muscle spindles, suggesting that mass activation of spindles may be required for salience. Thus, proprioceptive feedback may require selectivity at the population level, i.e., recruitment of many spindles from the same muscle. However, it is also possible that even paresthesias resulting from unnatural recruitment can be modulated to convey discriminable sensations that may offer functional benefits (Anani et al. 1977).
The dorsal root ganglion (DRG) is an attractive target for sensory neuroprostheses, owing to its anatomical segregation of sensory afferents from motor efferents, its mechanical stability, and its inclusion of both cutaneous and proprioceptive afferents. Three to four ganglia account for the innervation of an entire limb (Brown and Koerber 1978), while a DRG at a single spinal level may provide access to the entire sensory representation of the foot. It is anticipated that existing minimally invasive surgical techniques (Francisco et al. 2008) can be adapted for implanting epineural electrodes on the DRG. We have previously shown that microstimulation of the DRG can recruit primary afferent neurons at low intensities both acutely (Gaunt et al. 2009) and over several months after implantation (Fisher et al. 2014). While these studies suggest that DRG microstimulation may be an appropriate technique for sensory feedback, they provide limited information on the specific sensory modality of recruited neurons or their distal innervation patterns. Single unit recordings (Aoyagi et al. 2003) and tracer studies (Kausz and Réthelyi 1985) within the DRG have failed to identify a strong somatotopic organization of sensory fibers in the DRG, although some studies have found evidence of organization along rostrocaudal (Wessels et al. 1994) and mediolateral (Burton and McFarlane 1973) axes of the spinal nerves and DRG. Further investigation is required to determine whether the organization of fibers within the DRG will affect its utility as a substrate for clinical devices.
In this study, we report on the selectivity and dynamic range of microstimulation and the distribution of projected fields in the L5, L6, and L7 DRG via electroneurographic recordings of evoked responses in many of the proximal and distal branches of the femoral and sciatic nerves. Instrumentation of multiple distal nerve branches has been used previously to directly assess the selectivity of peripheral nerve stimulation (Yoo et al. 2004) in the same way that EMG has been used to measure the selectivity of FNS (Branner et al. 2001). This technique can be applied to the study of DRG microstimulation by recording from major nerve trunks of the hindlimb and their distal branches during stimulation. Recording the response to microstimulation in a large number of distal nerve branches allows for approximate determination of the receptive field and fiber type.
METHODS
Acute experiments were performed in four anesthetized male cats (cats E-H). All experimental procedures were performed under the approval of the University of Pittsburgh Institutional Animal Care and Use Committee.
Electrode implantation procedures.
Isoflurane (1–2%) was used to maintain the animals at a surgical anesthetic plane throughout the experiment, and vital signs were monitored continuously. Distal nerve branches (Fig. 1) were instrumented with two-contact nerve cuffs, which were either custom made or purchased (Microprobes, Gaithersburg, MD). Both types of electrodes were made from split silicone tubing with circumferential fine-wire stainless steel electrodes with an interelectrode spacing of 3 or 4 mm. The nerve cuff inner diameters ranged from 1 to 3 mm depending on the size of the targeted nerve. The sciatic and femoral nerves were instrumented with five-contact nerve cuffs (Ardiem Medical, Indiana, PA), which had an interelectrode spacing of 4 mm. Proximal, center, and distal contacts were shorted together and were used as a reference in a virtual tripole configuration when recording from the second and fourth contacts within the cuff (Gaunt et al. 2009).
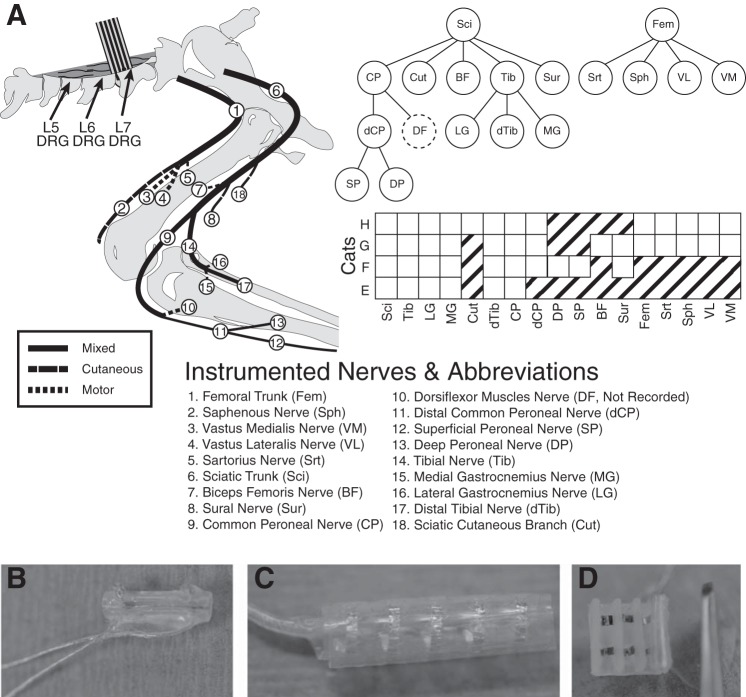
Fig. 1.
A: schematic of nerve cuff location in the left hindlimb. B: most nerves were instrumented with bipolar split-cuff electrodes. C: the femoral and sciatic trunks were each implanted with 5-contact cuffs, while all other nerves received bipolar cuffs. D: in experiment H a custom book electrode was implanted on three femoral nerve branches. The common peroneal and tibial nerves were both implanted with a proximal cuff, close to the initial branch point, and a distal cuff. The ankle dorsiflexor nerves tended to be deep and could not be implanted without significant dissection of the limb, but activity could be inferred by differential activation of the proximal and distal portions of the common peroneal nerve.
Where possible, nerves projecting to members of each major muscle group innervated by the sciatic and femoral trunks were instrumented. The sciatic branches innervating the hamstrings were often too proximal to instrument safely, although a cuff was implanted around the nerve innervating biceps femoris in cat G. It was not possible to instrument the branch of the common peroneal nerve innervating ankle dorsiflexors without reflecting the biceps femoris tendon; however, the common peroneal nerve was always instrumented proximal and distal to this important branch point. Nerve identities were determined using known anatomical landmarks and verified by stimulation using a voltage-controlled stimulator (Grass, Warwick, RI) and finding coarse motor thresholds. Sensory nerves, such as the sural and the sciatic cutaneous branch, were tested to the maximum stimulation intensity (20-V, 200-μs pulse width) to verify that there were no evoked movements. After nerve cuff implantation, the left L5, L6, and L7 DRG were exposed via laminectomy, and the cat was placed in a spinal frame for the duration of the experiment. Motor thresholds were measured again after transfer to the frame to verify that the cuffs still made adequate contact and that the instrumented nerves were still intact. Penetrating floating microelectrode arrays (32-channel FMA Microprobes, Gaithersburg, MD) were inserted in the L6 and L7 DRG of cats E and F. Utah electrode arrays (32-channel UEA; Blackrock Microsystems, Salt Lake City, UT) were inserted in the L5, L6, and L7 DRG of cats G and H. A stainless steel screw in the iliac crest was used as the return for stimulation. In cats E and F, a paralytic (pancuronium bromide, 0.2 mg/kg) was administered after array insertion to minimize reflexive muscle contractions that could contaminate the compound action potential signals recorded in peripheral nerves. This drug blocks transmission at the neuromuscular junction and should not affect the patterns of evoked activity in the peripheral nerve. Cats G and H demonstrated minimal reflexive muscle activation, and, therefore, no paralytic was administered during those experiments.
Experiment design.
The objective of these experiments was to identify the minimum stimulus charge in the DRG that elicited activity in any of the instrumented nerves (threshold), and whether or not activity occurred in one or more nerves (selectivity) at threshold. In the event of nonselective recruitment, any synergies between recruited motor nerves (e.g., nerves innervating multiple heads of the quadriceps) were identified. Finally, we examined the range of intensities over which stimulation achieved selective recruitment, referred to here as dynamic range.
Electroneurogram (ENG) signals from nerve cuffs were collected using a Grapevine Neural Interface Processor (Ripple, Salt Lake City, Utah), using differential headstages with an input range of 5 mV and a resolution of 0.2 μV. Digitization of signals was performed directly on the headstage at 30 kHz. Stimulation was performed using two IZ2 16-channel stimulus isolators (TDT, Alachua, FL) and custom written LabVIEW software. Stimulation artifacts were generally small and could be blanked out in software using a 1-ms window, which did not exceed the minimum conduction latencies of the most proximal nerves. Following blanking, ENG data were high-pass filtered at 300 Hz. Custom software was written in C++ and Matlab (Mathworks, Natick, MA) to capture and display stimulus-triggered ENG recordings from all cuff electrodes, to detect responses, and to coordinate a binary search for threshold as a function of the injected charge.
Experiment duration was a significant concern due to the time required to collect recruitment data for the many individual nerves and stimulation electrodes. Furthermore, the low signal-to-noise ratio of nerve cuff recordings dictated that a large number of stimulus repetitions be used (n = 600) to resolve responses near threshold. Therefore, significant effort was spent minimizing experiment duration. An initial survey across all electrodes was performed with high charge pulses (2.46–4.38 nC/phase) at a stimulation rate of 58 pulses/s. Individual stimulation pulses were either a cathodic-leading 200-μs pulse with a 400-μs half-amplitude anodic phase (cats E and F) or a cathodic-leading 82 μs/phase symmetric biphasic pulse (cats G and H). Pulse width was shortened in the latter two experiments to prevent artifact from obscuring short-latency ENG responses in the most proximal nerve cuffs. The intent of this initial survey was to identify DRG stimulation electrodes that evoked compound action potentials in any nerve branch. Stimulation electrodes that did not recruit any nerves during the survey trial were not tested further. For electrodes that did evoke responses, only the nerves recruited during that survey trial were considered for further evaluation. The longest latency response recruited by each stimulation electrode was used to set the stimulation pulse repetition rate for the remainder of testing to minimize the duration of testing. Multichannel headstages allowed for recording from multiple nerve cuffs simultaneously, which allowed threshold searching to be performed for multiple nerves in parallel (see below) rather than sequentially.
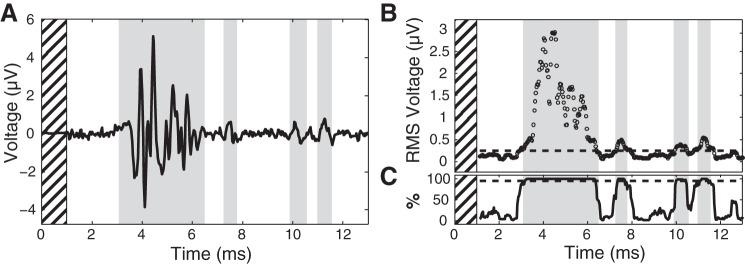
A binary search over stimulation charge was performed on each stimulation electrode to determine the recruitment threshold of each instrumented nerve to a user-specified minimum resolution, which was adjusted between 0.08 and 0.41 nC/phase as time allowed. During the online threshold search, responses were detected (Fig. 2) by comparing the windowed RMS (250-μs duration, 25-μs step size) of stimulus-triggered averages between baseline and stimulation epochs. Baseline data were divided into intervals of the same duration as the stimulation interpulse interval for statistical comparison. RMS values exceeding both 0.5 μV and 3–4 SDs of the mean windowed baseline RMS values for two consecutive windows were accepted as responses. These values were selected empirically to improve accuracy of response detection. In cats E and F, the 600 possible stimulus repetitions were divided into three subsets of 150, 175, and 275 repetitions. The detection algorithm was run after each subset of stimulus repetitions was collected, and if a response was detected the remaining subsets were not collected. The intent was to eliminate unnecessary repetitions when testing at higher stimulation intensities where the ENG signal-to-noise ratio was high, reducing testing time.
Fig. 2.
A: example survey trial stimulus-triggered average (n = 600) showing compound action potentials from the common peroneal nerve. Initial hashed section of time denotes 1-ms blanking period. Detected responses are highlighted in gray. B: average windowed RMS (250-μs width, 25-μs step) values. Broken line denotes 99% confidence interval on baseline mean that was used as the detection threshold. C: to reduce the effect of outliers, 200 individual averages were generated using 80% of the stimulus repetitions (480) drawn with replacement. For an RMS time window to be considered significant, 95% (190) of averages drawn in this manner had to exceed the 99% confidence interval upper bound.
The threshold search was performed on each recruited nerve sequentially, but the multichannel headstage allowed simultaneous measurements from all nerves to be captured and analyzed at each stimulation charge that was tested. For each iteration of the binary search (Fig. 3), the response of a single nerve to stimulation was used to select the subsequent charge, but the upper and lower bound on the threshold estimate for all other nerves was updated in parallel. Intensities chosen to find the recruitment threshold of a single nerve informed subsequent testing on the remaining nerves and in many instances obviated the need for additional iterations for one or more nerve branches. This approach reduced the overall number of search iterations across all experiments by an estimated 77% and significantly shortened experiment duration.
Fig. 3.
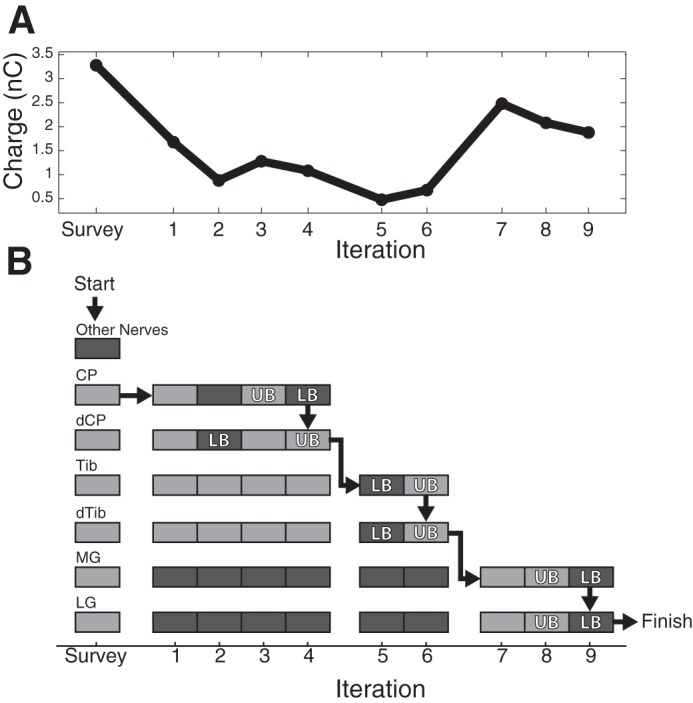
A binary search over stimulation charge was used to find the threshold of each nerve recruited by each dorsal root ganglion (DRG) stimulation electrode. A: the charge injected at each iteration of the binary search. B: whether a response was detected (light gray) or not (dark gray) in each nerve at each stimulus charge. The survey amplitude was used to determine which nerves were recruited by this electrode to limit the scope of the search. Following the survey, the search started with the common peroneal (CP) cuff and proceeded until the upper (UB) and lower (LB) bounds on the recruitment threshold for this cuff were within 0.25 nC/phase. Although the search started with CP, the detection algorithm was run on all the other cuffs such that, by iteration, four thresholds had been found for both CP and distal common peroneal (dCP). Execution continued in this manner until the threshold for all cuffs was determined.
Although evoked responses were detected online during the experiment, the raw data were reanalyzed offline to perform more rigorous statistical testing. All analyses presented in this paper are based on responses detected using a nonparametric subsampling approach, because runtime efficiency and experiment duration were not limitations. First, a 99% confidence interval about the baseline mean was calculated by subsampling averages using 80% of the windowed baseline RMS repetitions (n = 480) into 200 separate draws. These numbers were chosen to balance computation time and detection sensitivity. The upper bound of the resulting 99% confidence interval was used as the detection threshold for the poststimulation interval. For a time window to be considered significant, 95% of the resulting averages (n = 190) had to be suprathreshold during that time window. This second step was designed to reduce the effect any outliers had on detection. To generate one of the stimulus-trigged averages, a number of repetitions equal to 80% of the total were selected from the entire set. Individual repetitions could be used more than one time to create one of the 200 stimulus-triggered averages. Finally, all responses for each nerve had to fall within a 250-μs window of the responses detected during the survey trial for that same nerve to be considered valid. The 250-μs buffer was added to the survey response time windows to accommodate the preferential recruitment of slower medium-diameter fibers at perithreshold intensities that has been observed in our previous experimental and modeling studies (Bourbeau et al. 2011; Fisher et al. 2014; Gaunt et al. 2009).
RESULTS
Recruitment tests for each electrode began with a survey trial in which a large-amplitude stimulus was used to check for evoked responses in any nerve branch. Figure 2 shows an example of the compound ENG produced during one of these survey trials, which were intended to produce nonselective recruitment of many DRG neurons. The ENG in Fig. 2 shows that multiple responses were evoked in the common peroneal nerve at different latencies, with the largest starting at 3 ms and smaller responses appearing at 7.5, 10, and 11 ms after the stimulus pulse. These different responses likely represent recruitment of fibers having different conduction velocities, with A-α fibers comprising the fastest responses and A-β and smaller fibers generating the responses evoked at longer latencies.
An example threshold search is shown in Fig. 3. An initial high-amplitude survey trial determined which nerves could be recruited by this particular electrode (cat H, L7 DRG). In this example only the common peroneal, distal common peroneal, tibial, distal tibial, and medial and lateral gastrocnemius nerves were recruited. The algorithm then performed a binary search on the common peroneal nerve by testing an amplitude midway between the survey and minimum possible (0.16 nC/phase) amplitudes. A response was detected on the first iteration; therefore, the amplitude was reduced on the second iteration. No response was detected at the second amplitude so the amplitude was increased on the third trial, and the search continued until the upper and lower bounds on the common peroneal nerve threshold were determined to within 0.25 nC/phase. This condition was satisfied on iteration 4. After determining threshold for the common peroneal nerve, the search algorithm switched to find threshold for the distal common peroneal nerve; however, by this point the threshold bounds for this cuff were already within 0.08 nC/phase. The search then continued with proximal and distal portions of the tibial nerve, and gastrocnemius nerves. Upon completion of the threshold search on the medial gastrocnemius nerve, thresholds had been found for all cuffs, including the lateral gastrocnemius nerve, and the algorithm started again with a new stimulation electrode. This continued until the threshold for recruiting all cuffs had been determined for all stimulation electrodes. The search algorithm parameters and number of selective electrodes for each of the four cats is summarized in Table 1.
Table 1.
Binary search summary
| Threshold Resolution |
||||||
|---|---|---|---|---|---|---|
| Cat | L5 | L6 | L7 | Maximum Charge, nC/phase | Total No. | Selective, % |
| E | N/A | 0.08 | 0.08 | 1.23 | 30 | 76.7 |
| F | N/A | 0.08 | 0.08 | 3.28 | 28 | 85.7 |
| G | 0.08 | 0.12 | 0.41 | 2.46 | 95 | 92.6 |
| H | 0.25 | 0.25 | 0.25 | 3.28 | 83 | 79.5 |
Threshold resolution denotes the maximum allowable difference in the upper and lower estimates of threshold, separated here into the values used for each ganglion. Total electrode number is the no. of electrodes that were capable of recruiting any nerve at any charge tested. Selective electrodes were those that recruited only a single nerve, or multiple nerves within a common parent/child innervation pathway, at threshold. N/A, not applicable.
Over the charge range used in this example search, a large number of nerves were recruited, which account for much of the innervation of the ankle and foot. This particular stimulation electrode recruited many distal branches of the sciatic nerve (Fig. 4) to the exclusion of the femoral nerve branches (data not shown). At the survey amplitude, the likely projected fields of this stimulation electrode were the medial and lateral gastrocnemius, the plantar surface of the foot via the distal portion of the tibial nerve, and the proximal and distal portions of the common peroneal nerve, which innervates the ankle dorsiflexors and the foot dorsum, respectively. At threshold, however, this electrode recruited only the proximal and distal portions of the tibial nerve without coactivation of the common peroneal nerve and its branches. It was expected that proximal and distal portions of the same nerve would be coactivated; therefore, stimulation at this charge was considered selective.
Fig. 4.
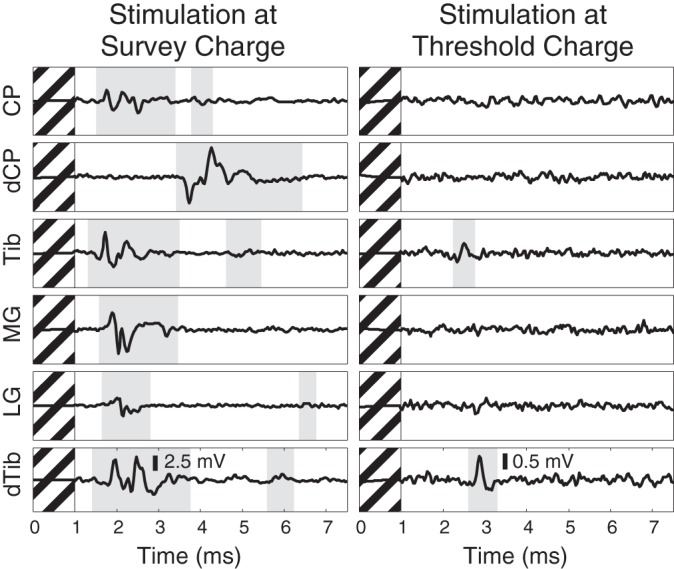
Compound action potentials recorded on distal branches of the sciatic nerve at the survey amplitude (left) and at the lowest amplitude at which any response was detected (right). Detected responses are highlighted in gray. This example highlights a DRG electrode (same as Fig. 3) that, at high amplitude, recruited many of the nerves innervating the ankle and foot but at low amplitude was selective for only the nerves projecting to the plantar surface of the paw. Hashed regions denote 1-ms blanking period.
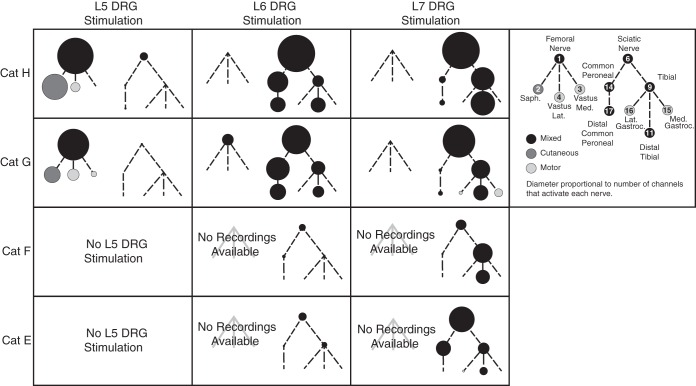
Threshold responses for each DRG electrode were used to map the corresponding innervation target, providing an overview of the limb coverage achieved at each DRG (Fig. 5). These maps agree with the known innervation of the hindlimb (Brown and Koerber 1978; Crouch 1969). The diameter of the circle for each distal nerve was set using the number of electrodes able to recruit that nerve at threshold. The local cross-correlation algorithm, detailed elsewhere (Fisher et al. 2014), was used to detect responses on the femoral and sciatic trunks. From these plots, and in accordance with the physiology, it is clear that L5 stimulation predominantly activates neurons innervating the femoral nerve and its distal branches, L6 stimulation predominantly activates neurons from the plantar and dorsal regions of the foot and the ankle dorsiflexors, and L7 stimulation predominantly activates neurons from the ankle plantarflexors and plantar surface of the foot. As can be seen in the common peroneal nerve branch for the L7 DRG in cat G, there were rare occurrences when a response was detected in a distal branch, but not the corresponding proximal branch of a nerve. This occurrence manifests as a slightly larger diameter in the distal branch than the proximal branch, and is likely a result of the difficulty in detecting threshold-level ENG signals in large-diameter nerves, where the signal may be smaller if the electrode is further from the source. In the vast majority of maps shown in Fig. 5, the diameters of proximal trunks are larger than distal branches, suggesting that this type of error occurs infrequently.
Fig. 5.
Innervation trees in which each circle represents a nerve recruited at threshold. Each of the four cats (row) is shown separately, and the trees have been split by ganglion (column). The relative size of each circle represents the number of DRG electrodes that recruited that nerve. Lines denote innervation of each nerve. Nerves that were never recruited at threshold have been omitted for clarity.
In addition to selectivity at threshold, we also quantified the tendency of multiple nerves to be coactivated at threshold. Given the branching structure of the nerves and our use of multiple cuffs on proximal and distal portions of the same nerve, it was expected that some nerves would be frequently coactivated. For example, the distal ends of the common peroneal and tibial nerve were expected to be coactivated with their respective proximal trunks. A matrix of coactivation was produced to visualize these relationships (Fig. 6) and to help illustrate whether stimulation was selective or not. The matrix was produced by counting the number of times for each nerve (rows) that another nerve was also recruited at threshold (columns). The counts in each row were then normalized by dividing by the total number of times that nerve was recruited. The division between sciatic (Fig. 6, top left) and femoral (Fig. 6, bottom right) innervation is shown with the dashed white line. Six nerves were never recruited at threshold and have been omitted from the matrix: sural, sartorius, biceps femoris, the superficial and deep peroneal nerves, and the cutaneous branch of the sciatic nerve.
Fig. 6.
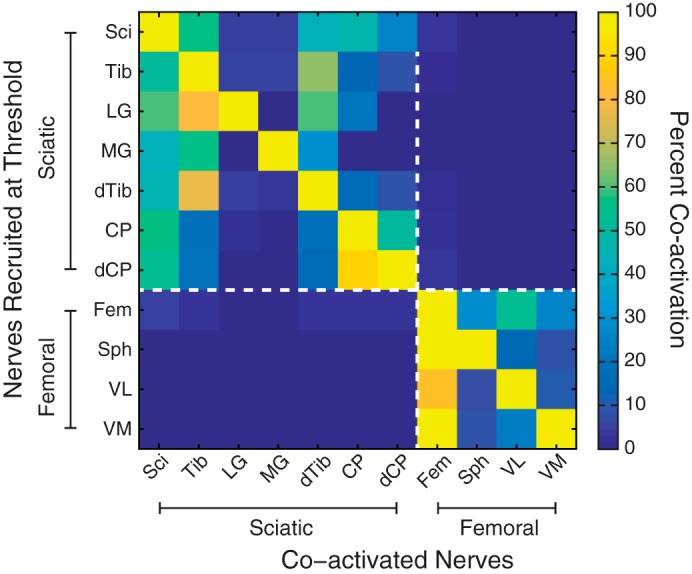
Normalized counts of instances in which a nerve was recruited at threshold and identities of the coactivated nerves. Nerves that were never activated at threshold have been omitted. The broken lines indicate the division between the sciatic and femoral nerves and their branches. Some nerves, such as the tibial, were expected to be recruited along with other nerves in their innervation pathway, such as the distal tibial and branches to the gastrocnemius muscles. Responses on the sciatic and femoral trunks were detected using the local cross correlation (LCC) algorithm (Gaunt et al. 2009; Fisher et al. 2014).
The data from the coactivation matrix were used to summarize the likelihood of selectively recruiting a particular nerve. The instances of selective recruitment of each nerve were tallied using each row of the coactivation matrix (without normalization) and subtracting the number of instances of coactivation with other nerves. In the event of coactivation of multiple nerves within the same innervation pathway, only recruitment of the most distal nerves was counted. Proximal nerves that were recruited to the exclusion of anything else indicated an evoked response that was likely projecting to uninstrumented distal nerve branches. For example, in the case of the common peroneal nerve, any activation of the proximal portion not concomitant with the distal portion suggested possible recruitment of the uninstrumented ankle dorsiflexor nerves (Fig. 1). For functional groups of agonist muscles, namely the two gasctrocnemius muscles and the vastus lateralis and medialis, coactivation was counted for each nerve branch as well as for each functional group. For the purposes of comparison, the raw recruitment numbers at the survey charge were also summarized. Overall, 9 of the 15 instrumented nerves were recruited selectively, and in only 36 cases were multiple nerves recruited at threshold (Fig. 7). Across all animals there were 237 instances of nerve recruitment, of which 201 were selective. There was an overall bias for recruiting branches of the sciatic nerve because these populations are highly represented in the L6 and L7 DRG, although there was significant selective recruitment of the femoral nerve via the L5 DRG. Across all experiments, 84.7% of electrodes achieved selective activation.
Fig. 7.
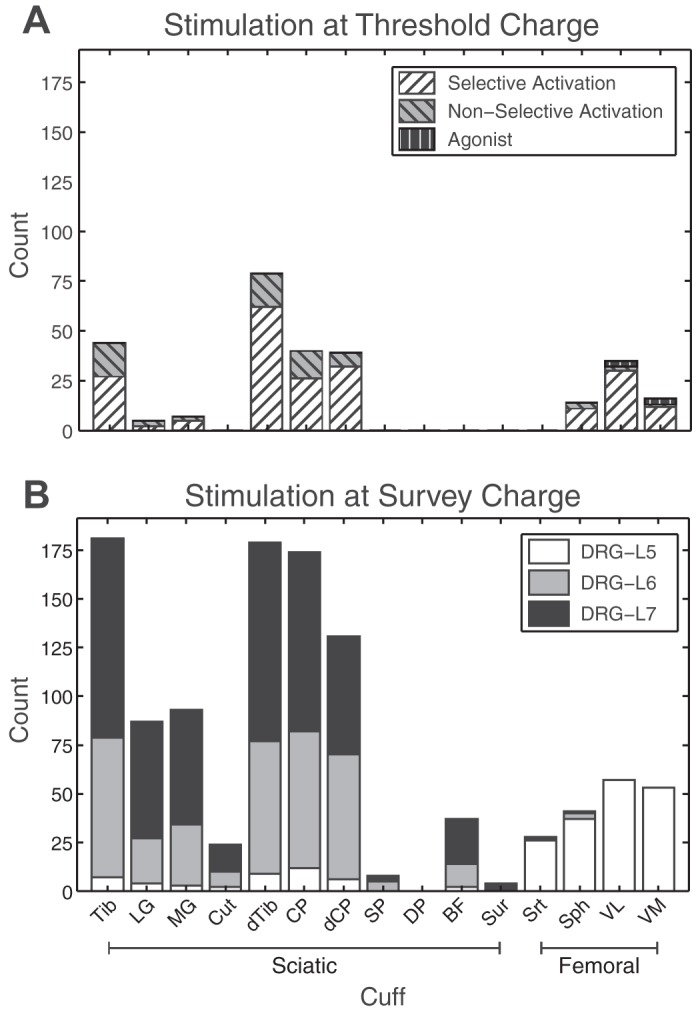
A: counts of selective (white hatched) and nonselective (gray hatched) instances of nerves recruited at threshold. Nerves in the same innervation path (e.g., tibial and distal tibial) were allowed to be coactivated while still being considered selective; however, only activation of the distalmost nerve was counted to highlight differential recruitment of proximal branches. Cases where agonists, such as vastus lateralis and medialis, were coactivated are identified separately (black hatched) from selective and nonselective counts. In total, there were 237 instances of nerve recruitment, 201 of which were selective. B: nerves activated during the high-amplitude survey, split by stimulation location.
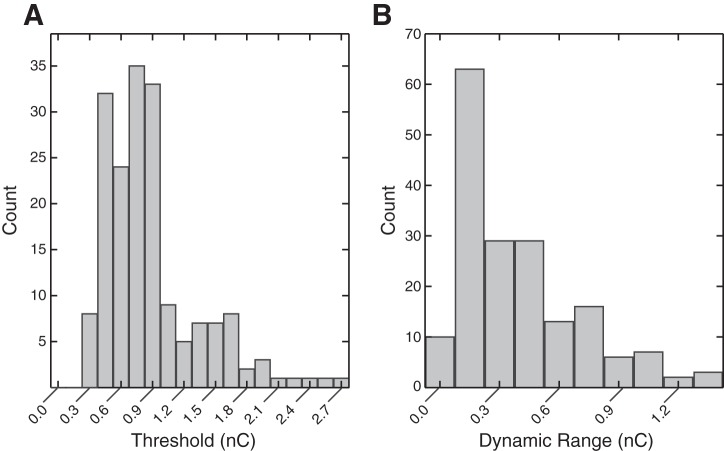
The selective range of stimulation charge was summarized by the recruitment threshold and the dynamic range of stimulation (Fig. 8). The dynamic range was defined as the difference between the threshold charge and the charge that recruited one or more additional nerves nonselectively. As in the other selectivity metrics, recruitment was considered to be selective until a nerve was recruited outside the innervation pathway of the first selectively recruited nerve. Electrodes that did not achieve selective recruitment at threshold and outliers (>95th quantile) were not included in Fig. 8. The median threshold was 0.68 nC/phase, and the median dynamic range was 0.36 nC/phase.
Fig. 8.
A: distribution of recruitment thresholds, median = 0.68 nC/phase (n = 178). Threshold responses outside the 95% percentile were excluded from the plot for clarity. B: distribution of dynamic range, the amount that charge can be increased above threshold before recruitment becomes nonselective, median = 0.36 nC/phase (n = 178).
We used a nonparametric Kruskal-Wallis test (as data were nonnormal) to test for differences in thresholds and dynamic range across cats. There were significant differences in threshold between cats E-F and G–H (P < 0.01), and there was a significant difference in dynamic range for cat F from all other cats (P < 0.01). Because of variations in the experimental setup between cats F and G, there were multiple covarying factors that may contribute to these differences, such as electrode type (FMA vs. UEA) and inclusion of stimulation at the L5 DRG. The overall goal of the study was to characterize the recruitment of somatosensory afferents by lumbar DRG stimulation, and, despite the observed differences in thresholds, these results consistently demonstrate patterns of selective activation of many nerves in the hindlimb of the cat via microstimulation at the L5, L6, and L7 DRG.
DISCUSSION
The goal of this study was to further characterize the recruitment properties of threshold-level microstimulation in the lumbar DRG. First, we wanted to identify which of the major nerve branches in the leg could be accessed with stimulation applied to the L5–L7 DRG. Second, we wanted to know if these nerve branches could be recruited selectively to achieve activation of a localized group of sensory nerve fibers. Finally, we wished to characterize the dynamic range of stimulation within which selective recruitment could be achieved.
Overall, 84.7% of electrodes achieved selective recruitment, and those that did not tended to recruit nerves from similar parts of the hindlimb as shown in the coactivation matrix (Fig. 6). For example, the common peroneal nerve was frequently coactivated with the tibial nerve and not any of the femoral branches. There were also three cases in which the agonists, vastus lateralis and vastus medialis, were coactivated (Fig. 7). Recruitment of these two nerves is not strictly selective, but because these muscles are agonists it may not affect sensory feedback. The division of hindlimb innervation between multiple DRG is such that, even without somatotopy within an individual DRG, the likelihood of recruiting two nerves projecting to wholly different parts of the limb was low. Therefore, the functional consequences of nonselective recruitment may be minimal. This is important because the dynamic range of stimulation was small; increasing stimulus charge 0.36 nC/phase above threshold recruited at least one other nerve.
Through extensive instrumentation of the nerves of the hindlimb, this study provides insight into the distribution of muscular and nonmuscular targets of DRG neurons. If DRG microstimulation is to restore proprioceptive feedback, it must selectively recruit a diverse population of muscular afferents. In these four cats, DRG stimulation selectively recruited 60% of all instrumented nerves. Of the remaining unrecruited nerves, the sciatic cutaneous, superficial and deep peroneal, biceps femoris, and sural nerves were each instrumented in only one animal, so it is difficult to make general conclusions about the relative ease or difficulty of activating those nerves. Additionally, recruitment of the proximal portion of the common peroneal nerve to the exclusion of the distal may indicate recruitment of ankle dorsiflexor nerves. This pattern of activation supports our hypothesis, based on dermatome mapping (Brown and Koerber 1978) and anatomy, that stimulation of three ganglia is sufficient to achieve coverage of at least some portion of the skin and muscles in the foot, ankle, shank, knee, and thigh. There was, however, some animal-to-animal variability in the specific nerves that were recruited by DRG stimulation, making it challenging to determine whether 32 electrodes/root would be an appropriate or sufficient density for a neuroprosthesis. To make this determination, future studies should focus on the use of higher densities of electrodes and current steering approaches to determine the optimal arrangement of electrodes to maximize coverage of the limb and selective recruitment within each DRG.
Previous studies have demonstrated the ability of penetrating microelectrodes to recruit medium- and large-diameter fibers both acutely (Gaunt et al. 2009) and chronically (Fisher et al. 2014), but, because only the sciatic trunk was instrumented, the specific peripheral targets were unknown. We have also recorded from area 3a, in primary somatosensory cortex, to characterize the recruitment of DRG microstimulation (Weber et al. 2011), but using this method it was not possible to infer the distal targets of recruited neurons. Conscious reports from human subjects have been crucial in developing an understanding of sensory stimulation, but not all afferents produce conscious percepts when stimulated (Macefield et al. 1990). Likewise, researchers have found the threshold for evoking behavioral responses in cats using muscular afferent stimulation to be higher than that of cutaneous afferent stimulation (Swett et al. 1964).
For this study, we instrumented many nerves throughout the hindlimb to enable us to make definitive statements about specific peripheral targets, recruitment selectivity, and dynamic range of many electrodes that would not have been possible otherwise. It is important to note, however, that we cannot make claims about the perceptual qualities of stimulation from these results. While it is clear that the spatial extent of DRG stimulation is constrained to individual muscle nerves, cutaneous, or mixed nerves, which should lead to localized percepts, we cannot know from the present results if those percepts would feel natural. Furthermore, in other studies of peripheral nerve stimulation in amputees, it has been far more challenging to evoke proprioceptive percepts than to evoke cutaneous sensations (Raspopovic et al. 2014; Tan et al. 2014). Even though we have clearly demonstrated the ability to selectively activate primary afferents that project from muscle nerves, we cannot show definitively that these responses would evoke meaningful proprioceptive percepts.
That neurons can be recruited selectively via microstimulation is a naïve result; however, the diversity of afferents and lack of a clear somatotopic organization in the DRG suggested selective recruitment would be challenging. Modeling of perithreshold recruitment of peripheral nerves using penetrating microelectrodes (Bourbeau et al. 2011) predicted that the reverse recruitment principle would no longer hold. In this model, recruitment favored medium-diameter fibers over large ones because of their relative abundance near the stimulating electrode due to shorter intermodal distances. These results have been confirmed experimentally in multiple studies in our lab (Fisher et al. 2014; Gaunt et al. 2009). Our strategy for achieving a diverse recruitment profile and selective activation of large fibers is to use a large number of electrodes spread across multiple ganglia. In this study, this strategy appears to have been successful, since representatives from all major hindlimb muscle groups were recruited selectively (Fig. 5), excluding the hamstrings and ankle dorsiflexors, which were underinstrumented.
Although many of the distal branches of the sciatic and femoral nerves were instrumented, it was still a relatively coarse sampling due to our desire to limit muscle dissection. The largest groups of missing muscles were the hamstrings, aside from biceps femoris, which was only tested in cat G. The sciatic nerve branches innervating the hamstrings were generally too proximal to access. The sciatic cuff was always placed distal to this branch, so it was not possible to infer semitendinosus or semimembranosus activity from the sciatic recordings. On the other hand, the coactivation matrix suggests the cuff on the proximal portion of the tibial nerve could be removed from future experiments, since it is frequently recruited with the distal portion of the nerve. The same was not true of the proximal common peroneal cuff, which was used to infer activation of ankle dorsiflexor nerves when differentially recruited to the exclusion of the distal portion of the nerve. It should also be noted that extraneural recordings via nerve cuff electrodes are noisy, and, especially near threshold, our detection algorithms may have failed to identify small compound action potentials in some nerves. Failure to identify those responses may result in underestimation of selectivity and dynamic range and an overestimation of stimulation threshold charge. It is currently technically impossible to monitor the response of every neuron in a peripheral nerve, and we believe that the methods described in this study provide the most thorough accounting of stimulation selectivity in the peripheral nerve that has been achieved to date.
In this experiment a very strict definition of selectivity was applied: only nerves within a given innervation pathway could be recruited to the exclusion of all others. In practice, coactivation of synergistic nerves may be acceptable for cases in which broad activation is desired (e.g., recruiting both the common peroneal and tibial nerves to signal ground contact by recruiting afferents from the entire foot). Coactivation may also be acceptable if there is a large difference in the perceived intensities produced by the recruitment of each neural population, such that feedback is still largely unimodal. We chose not to compare relative ENG response magnitudes because there is not necessarily a functional relationship between ENG magnitude and intensity of sensory feedback. In contrast, studies of selective muscle recruitment can rely on EMG (Schiefer et al. 2013), tendon forces (Branner et al. 2001), or the induced torque about a joint (Fisher et al. 2009; Tyler and Durand 2002), all of which provide unambiguous interpretations of the functional consequences of nonselective recruitment.
An important potential application of the experimental model developed here is that it can be used to directly compare the recruitment properties of electrode technologies in targeting the DRG or other sensory and motor targets in the peripheral nerve. Future work may focus on characterizing the selectivity of epineural stimulation at the DRG. It is anticipated that epineural electrodes will provide an easier path to clinical translation, since minimally invasive techniques exist to access cervical (Lynch et al. 2011) and lumbar (Francisco et al. 2008; Liem et al. 2013) DRG.
In conclusion, current prosthesis technology provides limited sensory feedback, a shortcoming that could potentially be addressed by DRG microstimulation. Through extensive instrumentation of peripheral nerves throughout the hindlimb, we have demonstrated in four animals that this technology is capable of selective recruitment of many distal sensory targets. Using a binary search algorithm, selective recruitment of distal branches of the sciatic and femoral nerves was achieved by nearly 85% of tested electrodes. Median threshold across all selective electrodes was 0.68 nC/phase, and the median dynamic range was 0.36 nC/phase. Implanting multiple lumbar ganglia enabled selective recruitment of sensory fibers in most major nerves of the hindlimb, and nonselective recruitment tended to recruit nerves innervating similar regions of the hindlimb. These results suggest that DRG microstimulation may be an effective method for restoring sensation to lower-limb amputees.
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke Grant 5-R01-NS-072342-02.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.A., L.E.F., and R.A.G. conception and design of research; C.A.A., L.E.F., and R.A.G. performed experiments; C.A.A. analyzed data; C.A.A., L.E.F., R.A.G., and D.J.W. interpreted results of experiments; C.A.A. and L.E.F. prepared figures; C.A.A. drafted manuscript; C.A.A., L.E.F., R.A.G., and D.J.W. edited and revised manuscript; C.A.A., L.E.F., R.A.G., and D.J.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank William Cusack, Ameya Nanivadekar, Tyler Simpson, Erin Garia, and Kelly Meyers for help in preparing and performing experiments.
REFERENCES
- Anani AB, Ikeda K, Körner LM. Human ability to discriminate various parameters in afferent electrical nerve stimulation with particular reference to prostheses sensory feedback. Med Biol Eng Comput 15: 363–373, 1977. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y, Stein R, Branner A, Pearson K, Normann RA. Capabilities of a penetrating microelectrode array for recording single units in dorsal root ganglia of the cat. J Neurosci Methods 128: 9–20, 2003. [DOI] [PubMed] [Google Scholar]
- Bourbeau DJ, Hokanson JA, Rubin JE, Weber DJ. A computational model for estimating recruitment of primary afferent fibers by intraneural stimulation in the dorsal root ganglia. J Neural Eng 8: 056009, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branner A, Stein R, Normann RA. Selective stimulation of cat sciatic nerve using an array of varying-length microelectrodes. J Neurophysiol 85: 1585–1594, 2001. [DOI] [PubMed] [Google Scholar]
- Brown PBB, Koerber HR. Cat hindlimb tactile dermatomes determined with single-unit recordings. J Neurophysiol 41: 260–267, 1978. [DOI] [PubMed] [Google Scholar]
- Burton H, McFarlane JJ. The organization of the seventh lumbar spinal ganglion of the cat. J Comp Neurol 149: 215–232, 1973. [DOI] [PubMed] [Google Scholar]
- Clippinger FW, Seaber AV, McElhaney JH, Harrelson JM, Maxwell GM. Afferent sensory feedback for lower extremity prosthesis. Clin Orthop Relat Res 169: 202–206, 1982. [PubMed] [Google Scholar]
- Crouch JE. Text-Atlas of Cat Anatomy. Philadelphia, PA: Lea & Febiger, 1969. [Google Scholar]
- Dhillon GS, Horch KW. Direct neural sensory feedback and control of a prosthetic arm. IEEE Trans neural Syst Rehabil Eng 13: 468–472, 2005. [DOI] [PubMed] [Google Scholar]
- Downs F. Prosthetics in the VA: past, present and future. In: U.S. Naval Institute Proceedings 134, no. 2 (February 2008), p. 56–61. [Google Scholar]
- Etter K, Borgia M, Resnik L. Prescription and repair rates of prosthetic limbs in the VA healthcare system: implications for national prosthetic parity. Disabil Rehabil Assist Technol 10: 493–500, 2015. [DOI] [PubMed] [Google Scholar]
- Fan RE, Culjat MO, King CH, Franco ML, Boryk R, Bisley JW, Dutson E, Grundfest WS. A haptic feedback system for lower-limb prostheses. IEEE Trans Neural Syst Rehabil Eng 16: 270–277, 2008. [DOI] [PubMed] [Google Scholar]
- Fisher LE, Ayers CA, Ciollaro M, Ventura V, Weber DJ, Gaunt RA. Chronic recruitment of primary afferent neurons by microstimulation in the feline dorsal root ganglia. J Neural Eng 11: 036007, 2014. [DOI] [PubMed] [Google Scholar]
- Fisher LE, Tyler DJ, Anderson JS, Triolo RJ. Chronic stability and selectivity of four-contact spiral nerve-cuff electrodes in stimulating the human femoral nerve. J Neural Eng 6: 046010, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco S, Pirris SM, Dhall S, Mummaneni PV, Kanter AS. Minimally invasive approach to extraforaminal disc herniations at the lumbosacral junction using an operating microscope: case series and review of the literature. Neurosurg Focus 25: E10, 2008. [DOI] [PubMed] [Google Scholar]
- Gaunt R, Hokanson J, Weber D. Microstimulation of primary afferent neurons in the L7 dorsal root ganglia using multielectrode arrays in anesthetized cats: thresholds and recruitment properties. J Neural Eng 6: 055009, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausz M, Réthelyi M. Lamellar arrangement of neuronal somata in the dorsal root ganglion of the cat. Somatosens Mot Res 2: 193–204, 1985. [DOI] [PubMed] [Google Scholar]
- Kuiken TA, Marasco PD, Lock BA, Harden RN, Dewald JPA. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. Proc Natl Acad Sci USA 104: 20061–20066, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem L, Russo M, Huygen FJPM, Van Buyten JP, Smet I, Verrills P, Cousins M, Brooker C, Levy R, Deer T, Kramer J. A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic pain. Neuromodulation 16: 471–482; discussion 482, 2013. [DOI] [PubMed] [Google Scholar]
- Lynch PJ, McJunkin T, Eross E, Gooch S, Maloney J. Case report: successful epiradicular peripheral nerve stimulation of the C2 dorsal root ganglion for postherpetic neuralgia. Neuromodulation 14: 58–61, 2011. [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC, Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J Physiol 429: 113–129, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WC, Speechley M, Deathe B. The prevalence and risk factors of falling and fear of falling among lower extremity amputees. Arch Phys Med Rehabil 82: 1031–1037, 2001. [DOI] [PubMed] [Google Scholar]
- Quai TM, Brauer SG, Nitz JC. Somatosensation, circulation and stance balance in elderly dysvascular transtibial amputees. Clin Rehabil 19: 668–676, 2005. [DOI] [PubMed] [Google Scholar]
- Raspopovic S, Capogrosso M, Petrini FMM, Bonizzato M, Rigosa J, Di Pino G, Carpaneto J, Controzzi M, Boretius T, Fernandez E, Granata G, Oddo CMM, Citi L, Ciancio ALL, Cipriani C, Carrozza MCC, Jensen W, Guglielmelli E, Stieglitz T, Rossini PMM, Micera S, Pino Di GD, Carpaneto J, Controzzi M, Boretius T, Fernandez E, Granata G, Oddo CMM, Citi L, Ciancio ALL, Cipriani C, Carrozza MCC, Jensen W, Guglielmelli E, Stieglitz T, Rossini PMM, Micera S, Di Pino G, Carpaneto J, Controzzi M, Boretius T, Fernandez E, Granata G, Oddo CMM, Citi L, Ciancio ALL, Cipriani C, Carrozza MCC, Jensen W, Guglielmelli E, Stieglitz T, Rossini PMM, Micera S. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci Transl Med 6: 222ra19, 2014. [DOI] [PubMed] [Google Scholar]
- Schiefer MA, Freeberg M, Pinault GJC, Anderson J, Hoyen H, Tyler DJ, Triolo RJ. Selective activation of the human tibial and common peroneal nerves with a flat interface nerve electrode. J Neural Eng 10: 056006, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer MA, Polasek KH, Triolo RJ, Pinault GCJ, Tyler DJ. Selective stimulation of the human femoral nerve with a flat interface nerve electrode. J Neural Eng 7: 26006, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swett JE, Bourassa CM, Inoue S. Effects of cutaneous and muscle sensory nerve volleys in awake cats: a study in perception. Science 145: 1071–1073, 1964. [PubMed] [Google Scholar]
- Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler J, Tyler DJ. A neural interface provides long-term stable natural touch perception. Sci Transl Med 6: 257ra138, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D, Durand D. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng 10: 294–303, 2002. [DOI] [PubMed] [Google Scholar]
- Weber DJ, London BM, Hokanson JA, Ayers CA, Gaunt RA, Torres RR, Zaaimi B, Miller LE. Limb-state information encoded by peripheral and central somatosensory neurons: implications for an afferent interface. IEEE Trans Neural Syst Rehabil Eng 19: 501–513, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels WJT, Feirabend HKP, Marani E. The rostrocaudal organization in the dorsal root ganglia of the rat: a consequence of plexus formation? Anat Embryol (Berl) 190: 1–11, 1994. [DOI] [PubMed] [Google Scholar]
- Yoo PB, Sahin M, Durand D. Selective stimulation of the canine hypoglossal nerve using a multi-contact cuff electrode. Ann Biomed Eng 32: 511–519, 2004. [DOI] [PubMed] [Google Scholar]
- Ziegler-Graham K, MacKenzie E, Ephraim P, Travison T, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil 89: 422–429, 2008. [DOI] [PubMed] [Google Scholar]