This study is the first to report carotid baroreflex responsiveness to simulated hypotension and hypertension in individuals with MS. The novel findings of our study are twofold. First, carotid baroreflex-mediated increases in blood pressure in response to hypotensive stimuli (NP) were attenuated in individuals with MS. Second, the impaired responsiveness to hypotension appeared to be due to a diminished ability to decrease vascular conductance.
Keywords: baroreceptors, heart rate, sympathetic nervous system, carotid hypotension, carotid hypertension
Abstract
Multiple sclerosis (MS), a progressive neurological disease, can lead to impairments in the autonomic control of cardiovascular function. We tested the hypothesis that individuals with relapsing-remitting MS (n = 10; 7 females, 3 males; 13 ± 4 yr from diagnosis) exhibit impaired carotid baroreflex control of blood pressure and heart rate compared with sex, age, and body weight-matched healthy individuals (CON: n = 10; 7 females, 3 males). At rest, 5-s trials of neck pressure (NP; +40 Torr) and neck suction (NS; −60 Torr) were applied to simulate carotid hypotension and hypertension, respectively, while mean arterial pressure (MAP; finger photoplethysmography), heart rate (HR), cardiac output (CO; Modelflow), and total vascular conductance (TVC) were continuously measured. In response to NP, there was a blunted increase in peak MAP responses (MS: 5 ± 2 mmHg) in individuals with MS compared with healthy controls (CON: 9 ± 3 mmHg; P = 0.005), whereas peak HR responses were not different between groups. At the peak MAP response to NP, individuals with MS demonstrated an attenuated decrease in TVC (MS, −10 ± 4% baseline vs. CON, −15 ± 4% baseline, P = 0.012), whereas changes in CO were similar between groups. Following NS, all cardiovascular responses (i.e., nadir MAP and HR and percent changes in CO and TVC) were not different between MS and CON groups. These data suggest that individuals with MS have impaired carotid baroreflex control of blood pressure via a blunted vascular conductance response resulting in a diminished ability to increase MAP in response to a hypotensive challenge.
NEW & NOTEWORTHY
This study is the first to report carotid baroreflex responsiveness to simulated hypotension and hypertension in individuals with MS. The novel findings of our study are twofold. First, carotid baroreflex-mediated increases in blood pressure in response to hypotensive stimuli (NP) were attenuated in individuals with MS. Second, the impaired responsiveness to hypotension appeared to be due to a diminished ability to decrease vascular conductance.
multiple sclerosis (MS), the most common disabling neurological disorder of young adults, is a progressive autoimmune disease affecting the central nervous system (Edmonds et al. 2010). This disease results in the demyelination of axons leading to slowed or blocked nerve conduction and ultimately axonal loss within the brain and spinal cord. Abnormal nerve conduction resulting from demyelination disrupts communication to, from, and within the central nervous system causing a constellation of neurological clinical signs and symptoms (Lubin 2005; Oger 2007).
Because the underlying pathophysiology of MS becomes more severe as areas of disease progression get larger and involve more of the central nervous system, it is likely the autonomic nervous system is affected (Lubin 2005). Case in point, impaired autonomic control of cardiovascular function has been reported in up to two-thirds of individuals with MS (Acevedo et al. 2000; Nasseri et al. 1998). The most perilous health-related concern related to this autonomic cardiovascular dysfunction is the prevalence of orthostasis-related symptomology (i.e., orthostatic dizziness and orthostatic intolerance) in greater than 50% of people in this clinical population (Adamec and Habek 2013; Kanjwal et al. 2010).
Orthostasis-related symptomology is avoided in healthy individuals by the homeostatic regulation of blood pressure. Arterial baroreflex control of blood pressure is essential for short-term (i.e., beat-to-beat) regulation of blood pressure (Smit et al. 2002; Timmers et al. 2003). The arterial baroreceptor reflex prevents large fluctuations of arterial blood pressure by providing the central nervous system with continuous information from stretch-sensitive baroreceptors originating at the carotid sinus and aortic arch. This classic negative-feedback reflex system is completed by dynamically modulating changes in blood pressure through efferent autonomic neural activity. In its most simplistic form, a decrease in arterial blood pressure elicits decreases in afferent firing of the baroreceptors. This diminished afferent nerve activity, through integration in the nucleus tractus solitareus of the central nervous system, reflexively increases efferent sympathetic nerve activity to the heart and blood vessels and decreases parasympathetic nerve activity to the heart. This results in an increase in cardiac output (CO; i.e., due to increased heart rate), peripheral vasoconstriction, and, ultimately, a corrective increase in blood pressure. In contrast, increases in arterial blood pressure elicit increases in afferent firing of the baroreceptors reflexively decreasing sympathetic nerve activity to the heart and blood vessels and increasing parasympathetic nerve activity to the heart. This results in a decrease in heart rate and CO, peripheral vasodilation, and ultimately a corrective decrease in blood pressure.
Recently, Keller et al. (2014) have demonstrated reduced spontaneous muscle sympathetic nerve activity (MSNA) in individuals with MS compared with healthy controls. MSNA is an index to assess the sympathetic outflow of the central nervous system, which is in part orchestrated by arterial baroreceptors (Charkoudian and Wallin 2014). It is therefore reasonable to suspect that the autonomic neural regulation of beat-to-beat fluctuations in blood pressure by the arterial baroreflex is altered in individuals with MS. Prior investigations on cardiovascular autonomic dysfunction in MS have mostly been descriptive in nature, failing to provide mechanistic insight (Acevedo et al. 2000; Anema et al. 1991; Flachenecker and Reiners 2005; Flachenecker et al. 1999; Frontoni et al. 1997; Nasseri et al. 1998; Pentland and Ewing 1987; Racosta et al. 2015; Senaratne et al. 1984; Thomaides et al. 1993). To our knowledge, there has been only one attempt to assess baroreflex control in individuals with MS. Utilizing dynamic sinusoidal neck suction to induce carotid hypertension, Sanya et al. (2005) demonstrated baroreflex impairments in persons with MS. Although these findings suggest an altered neural regulation of blood pressure in MS, no consideration was given to carotid baroreflex responsiveness to decreases in blood pressure.
With greater than 50% of this clinical population reporting orthostasis-related symptomology, it is therefore critical to more completely understand how MS alters baroreflex control of heart rate and blood pressure, particularly to a hypotensive stress. By using a variable neck pressure collar to manipulate the carotid baroreceptors, we tested the hypothesis that individuals with MS exhibit blunted responses following carotid baroreceptor hypotensive and hypertensive perturbations compared with sex-, age-, and body weight-matched healthy controls. The end goal was to gain a more comprehensive understanding of how MS independently alters carotid baroreflex control of heart rate and blood pressure at rest.
METHODS
Subjects
Participants from the following two groups were investigated: 1) individuals with clinically definite relapsing-remitting MS [MS; n = 10 (7 females, 3 males)]; and 2) healthy body mass index-, age-, race-, and sex-matched controls [CON; n = 10 (7 females, 3 males)]. We focused on relapsing-remitting MS because it is the most common disease course with ∼85% of individuals with MS initially diagnosed with this form of the disease (National Multiple Sclerosis Society 2016a). All individuals with MS reported experiencing dizziness to some degree with standing (orthostatic dizziness). However, dizziness was not reported as one of their top three MS-related symptomatic concerns. No subject had a history or symptoms of cardiovascular or pulmonary disease. There is compounding evidence to show that sex, age, and race influence carotid baroreceptor function (Credeur et al. 2014; Fisher et al. 2007, 2009, 2012; Holwerda et al. 2001, 2013; Kim et al. 2011). Thus, in an effort to minimize these variables, each individual with MS was matched to a healthy control of the same sex, age, race, height, and weight. Two participants with MS were currently on antidepressive medication at the time of the study. Although no studies have directly examined the effects of antidepressive medication on responses to acute carotid baroreceptor perturbation, there is evidence to suggest that antidepressive medication alters sympathetic nerve firing (Esler et al. 1991). To address this potential cofounding variable, the aforementioned two participants with MS were matched to healthy controls taking the exact same antidepressive medication. Female participants were not tested in a specific phase of the menstrual cycle due to recent evidence suggesting minimal influences on carotid baroreflex control (Kim et al. 2012). All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the Institutional Review Board of Southern Methodist University. Participants provided informed written consent before testing.
Experimental Procedures
Neck suction and neck pressure.
For this study, we focused on the baroreceptors located in the carotid sinus due to their accessibility for noninvasive manipulation. The responsiveness of the carotid baroreflex in the short-term regulation of heart rate and blood pressure can be examined quantitatively by utilizing a variable pressure neck chamber system to selectively load (simulated hypertension via neck suction) and unload (simulated hypotension via neck pressure) the carotid baroreceptors (Credeur et al. 2014; Fadel et al. 2003; Ludbrook et al. 1977). In all familiarization and actual experimental trials, subjects were positioned semirecumbent (∼45°) on a patient table. Five-second pulses of −60 Torr neck suction (NS) and +40 Torr neck pressure (NP) were applied to load and unload the carotid baroreceptors, respectively (Credeur et al. 2014; Fadel et al. 2003; Fisher et al. 2010; Kim et al. 2011; Ogoh et al. 2002). The neck collar (Physiology Research Instruments, Austin, TX) was fitted around the anterior two-thirds of the neck with each NS and NP stimulus being delivered 50 ms after the second consecutive R-R interval that did not vary by >50 ms using customized computer-controlled software as previously described (Credeur et al. 2014; Fisher et al. 2007; Potts and Raven 1995). A variable pressure source using a 3-stage vacuum/blower motor (Ametek Lamb, Berwyn, PA) in combination with a computer controlled throttle valve was used to generate the changes in neck collar pressure. To accurately quantify the stimulus applied, a silicon piezoresistive pressure sensor (Freescale Semiconductor, Austin, TX) was connected to a port on the collar. To minimize respiratory-related modulation of HR, the 5-s pulses of NS and NP were delivered to the carotid sinus during a brief 12- to 15-s breath hold at end expiration phase (Eckber et al. 1980; Fisher et al. 2009).
Familiarization sessions.
All subjects participated in at least two familiarization sessions before the actual experimental visit. The initial familiarization session included screening subjects to identify the location of the carotid sinus bifurcation using Doppler ultrasound to ensure that the neck collar fully enclosed the carotid sinuses. Although transmission of NS and NP to the carotid sinus has been shown to be near complete, there is variability in the location of the carotid sinuses that requires consideration (Querry et al. 2001). Subsequently, all subjects were familiarized with the study procedures and fitted with a collar based on carotid sinus location and observed neck size. Practice trials of NS and NP were then performed to determine directionally appropriate HR and BP responses. The second familiarization session included additional practice trials of NS and NP to assure subjects were comfortable with the experimental protocol.
Experimental protocol.
Subjects refrained from caffeine, alcohol, and intensive exercise 24 h before the study day. Upon arrival, subjects were positioned semirecumbent (∼45°) on a patient table in a constant ambient room temperature (23–24°C) and instrumented for continuous measures of HR and BP. After instrumentation, subjects were fitted with the neck collar for the application of NS and NP and 5 min of baseline data were collected. Carotid baroreflex-mediated changes in HR and BP were then determined by applying random-ordered single 5-s pulses of NS and NP as described above. Ten trials for each NS and NP were performed with a minimum of 45 s of recovery allotted between trials to allow all physiological variable to return to prestimulus values. The rationale for performing 10 trials of NS and NP was to better characterize individual carotid baroreceptor responses for all cardiovascular variables as previous utilized by Credeur et al. (2014).
Experimental measurements.
HR and respiratory rhythm (electrical impedance) were continuously monitored using a standard lead II surface ECG (Solar 8000i; General Electric) interfaced with a cardiotachometer (CWE; Ardmore). Beat-to-beat blood pressure was measured by continuous finger cuff photoplethysmography (Finometer; FMS, Amsterdam, The Netherlands) with resting values verified by brachial artery auscultation (SunTech; Medical Instruments, Raleigh, NC).
Data analysis.
Data including the ECG, arterial BP waveform, neck chamber pressure, and respiratory signals were sampled at 100 Hz through a commercial data-acquisition system (Biopac System, Santa Barbara, CA). CO was estimated from the arterial BP waveform using the Modelflow method (TNO-TPD; Biomedical Instrumentation, Amsterdam, Netherlands), which incorporates age, sex, weight, and height (Jansen et al. 1990, 2001; Kim et al. 2011). Recently, Fadel et al. (2003) validated the use of Modelflow to measure CO during carotid baroreflex perturbations demonstrating no significant differences between the beat-to-beat CO responses to NS and NP recorded simultaneously by Doppler echocardiography compared with Modelflow. Total vascular conductance (TVC) was calculated as TVC = CO/MAP.
Characterization of carotid baroreflex response variables.
For all carotid baroreflex-mediated changes, the peak changes in HR and MAP were determined in the cardiac cycle (R-R interval) at which the largest change from prestimulus values occurred and compared with the prestimulus (3 cardiac cycle average) for each trial of NS and NP. CO and TVC were calculated from the cardiac cycle at the peak and nadir blood pressure responses and were analyzed as percent changes from the prestimulus value (%baseline). Changes in HR and MAP and percent changes in CO and TVC for each subject were determined for each trial and averaged across all trials of NS and NP, respectively, to provide individual mean responses. These individual mean responses were then averaged to provide group means.
Statistical Analysis
All values are presented as means ± SD. Statistical analyses were conducted using Prism 6 (GraphPad Software, La Jolla, CA). Unpaired t-tests were used to compare group differences in baseline characteristics and cardiovascular responses to NS and NP. Statistical significance was set at P < 0.05.
RESULTS
Baseline Subject Characteristics
General baseline characteristics for individuals with MS and matched healthy controls are summarized in Table 1. All individuals with MS were diagnosed with relapsing-remitting MS with an average diagnosis duration of 12.6 ± 4.2 yr at the time of participating in the study. Disease modifying medications used by individuals with MS included the following: Avonex (interferon-β1a), n = 4; Gilenya (fingolimod), n = 2; Copaxone (glatiramer acetate), n = 2; and Tecifedera (dimethyl fumarate), n = 2. Individuals with MS and their matched healthy controls were similar in age, height, weight, and body mass index. Furthermore, resting heart rate, mean arterial pressure, CO, and TVC were not different between groups.
Table 1.
Baseline subject characteristics
| MS | CON | P Value | |
|---|---|---|---|
| Sex | Male: n = 3; female: n = 7 | Male: n = 3; female: n = 7 | |
| Age, yr | 40 ± 9 | 37 ± 8 | 0.41 |
| Weight, kg | 62 ± 16 | 68 ± 15 | 0.33 |
| Height, cm | 170 ± 12 | 174 ± 12 | 0.57 |
| BMI, kg/m2 | 21 ± 3 | 23 ± 2 | 0.16 |
| Mean arterial pressure, mmHg | 92 ± 10 | 90 ± 9 | 0.53 |
| Systolic blood pressure, mmHg | 117 ± 10 | 118 ± 8 | 0.81 |
| Diastolic blood pressure, mmHg | 80 ± 11 | 75 ± 8 | 0.23 |
| Heart rate, beats/min | 69 ± 13 | 60 ± 12 | 0.11 |
| Cardiac output, l/min | 6.0 ± 1.5 | 5.9 ± 1.0 | 0.90 |
| Total vascular conductance, l·min−1·mmHg−1 | 0.074 ± 0.020 | 0.071 ± 0.016 | 0.69 |
Values are mean ± SD.
MS, multiple sclerosis; CON, control; BMI, body mass index.
Baroreflex Responses to Simulated Carotid Hypotension (Neck Pressure)
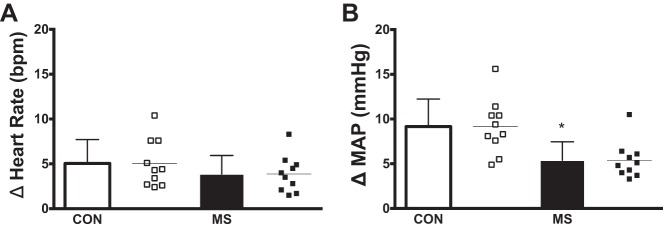
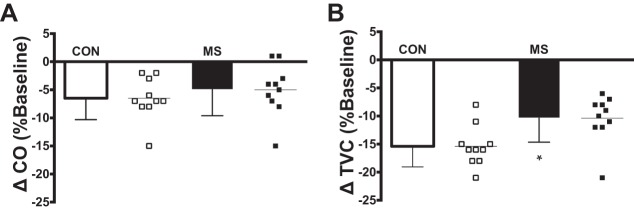
Representative beat-to-beat HR and MAP tracings during NP for an individual with MS and a matched control are presented in Fig. 1. Peak HR and MAP responses for both groups are presented in Fig. 2. In response to selective baroreceptor unloading (NP; +40 Torr), increases in MAP were blunted in subjects with MS compared with healthy controls (P = 0.005; Fig. 2B). However, increases in HR in subjects with MS were not statistically different compared with healthy controls (P = 0.28; Fig. 2A). In response to NP, percent changes in CO and TVC from baseline at the time of the peak MAP response in individuals with MS and matched healthy controls are presented in Fig. 3. Percent changes in CO from baseline were similar between groups (P = 0.44; Fig. 3A). However, individuals with MS demonstrated a significantly reduced percent decrease in TVC from baseline (P = 0.012; Fig. 3B) compared with healthy controls.
Fig. 1.
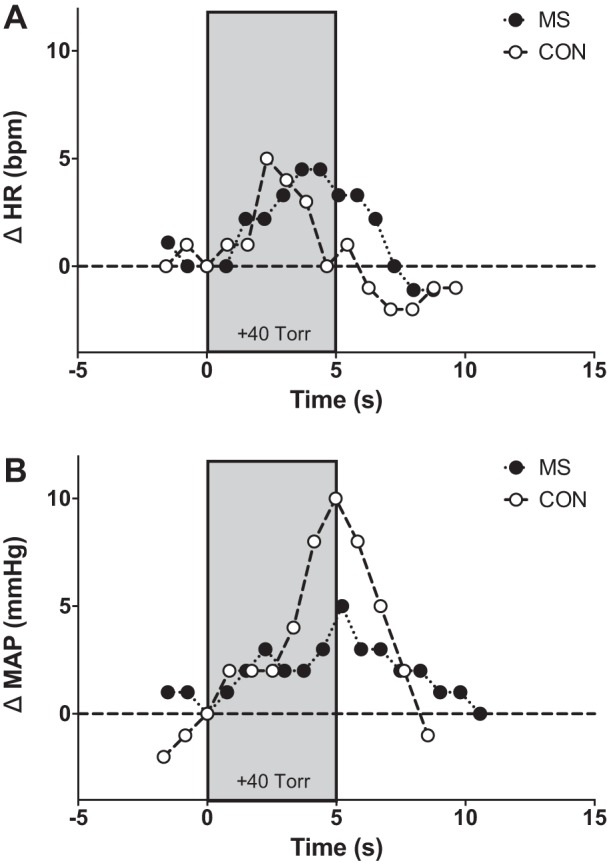
Individual beat-to-beat tracings in heart rate (HR; A) and mean arterial pressure (MAP; B) in response to selective carotid baroreceptor unloading (+40 Torr, neck pressure) for a representative person with multiple sclerosis (MS) and a matched healthy control (CON).
Fig. 2.
Group and individual summary data showing mean peak heart rate responses (A) and mean peak blood pressure responses (B) elicited by selective baroreceptor unloading [neck pressure (NP); +40 Torr] in individuals with multiple sclerosis (MS) and matched healthy controls (CON). *Significantly different from healthy controls (P < 0.05).
Fig. 3.
Group and individual summary data showing percent changes from baseline in cardiac output (CO; A) and total vascular conductance (TVC; B) at the time of the peak MAP response elicited by selective baroreceptor unloading (NP; +40 Torr) in individuals with multiple sclerosis (MS) and matched healthy controls (CON). *Significantly different from healthy controls (P < 0.05).
Baroreflex Responses to Simulated Carotid Hypertension (Neck Suction)
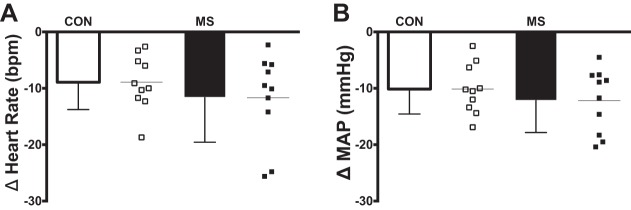
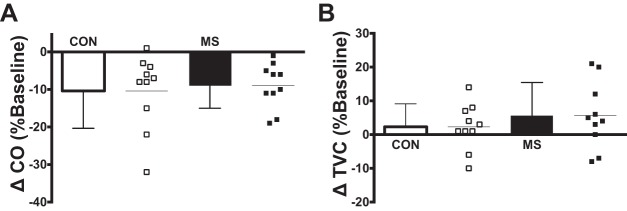
Individual beat-to-beat MAP and HR tracings during NS from a representative individual with MS and a matched healthy control are presented in Fig. 4. Nadir HR and MAP responses for both groups are presented in Fig. 5. In response to selective baroreceptor loading (NS; −60 Torr), nadir decreases in MAP and HR were not statistically different between groups (MAP: P = 0.38; HR: P = 0.36; Fig. 5). In response to NS, changes in CO and TVC at the time of the nadir MAP response in individuals with MS and matched healthy controls are presented in Fig. 6. Similar to the HR and MAP data, there was no significant difference between groups in percent change from baseline of CO (P = 0.71; Fig. 6A) or TVC (P = 0.40; Fig. 6B).
Fig. 4.
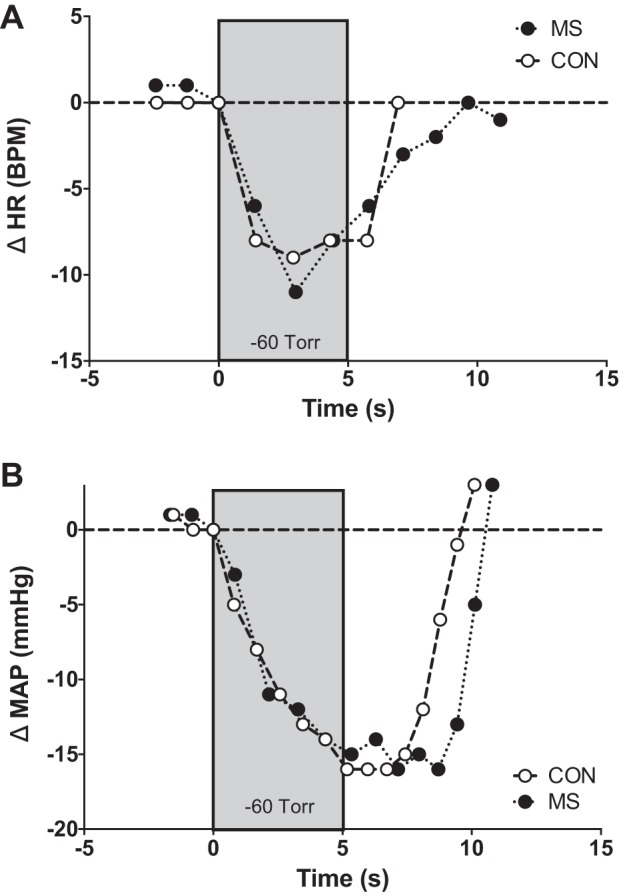
Individual beat-to-beat tracings in heart rate (A) and mean arterial responses (B) in response to selective carotid baroreceptor loading [−60 Torr, neck suction (NS)] for a representative person with multiple sclerosis (MS) and a matched healthy control (CON).
Fig. 5.
Group and individual summary data showing mean nadir heart rate responses (A) and mean nadir blood pressure responses (B) elicited by selective baroreceptor loading (NS; −60 Torr) in individuals with multiple sclerosis (MS) and matched healthy controls (CON).
Fig. 6.
Group and individual summary data showing percent changes from baseline in cardiac output (A) and total vascular conductance (B) at the time of the nadir MAP response elicited by selective baroreceptor loading (NS; −60 Torr) in individuals with multiple sclerosis (MS) and matched healthy controls (CON).
DISCUSSION
To the authors' knowledge, the present study is the first to demonstrate the independent influence of MS on carotid baroreflex-mediated responses to simulated hypertension and hypotension (i.e., carotid baroreceptor loading and unloading); as well as determine the influence of the heart (CO) and peripheral vasculature (TVC) at the time of the peak BP responses. The novel findings of our study are twofold. First, carotid baroreflex-mediated increases in MAP in response to hypotensive stimuli (neck pressure) were reduced in individuals with relapsing-remitting MS. Second, causes of this reduced corrective increase in MAP in response to a hypotensive stimulus appeared to be due to a diminished decrease in TVC. In contrast to NP, carotid baroreflex responses to simulated hypertension (neck suction) were similar in MS and matched healthy controls.
Recently, the interplay between autonomic dysfunction and the pathogenesis and progression of MS has gained traction (Cosentino and Marino 2013). There is accumulating evidence that MS affects cardiovascular function. The incidence of orthostatic dizziness is as high as 50% in individuals with MS (Acevedo et al. 2000; Flachenecker 2007; Flachenecker et al. 1999; Racosta et al. 2015). The findings of this study not only provides evidence that MS abnormally influences carotid baroreflex control of blood pressure to a hypotensive stimulus, but more importantly gives implications on how this impairment may factor into orthostasis-related symptomology.
One of the advantages to utilizing the application of brief, sustained periods of neck pressure/suction is the ability to quantify the influence of CO and TVC to carotid baroreflex-mediated changes in arterial pressure. With this quantification, further insight into the influence of MS on short-term blood pressure control was gained. Prior studies have demonstrated that compensatory blood pressure responses to carotid baroreceptor perturbation were predominantly attributable to changes in peripheral vascular tone (i.e., TVC) (Kim et al. 2012; Ogoh et al. 2002, 2003). In this regard, the primary finding of a significant reduced peak blood pressure response after hypotensive perturbations suggests impairments in vasomotor adjustments in MS are potentially due to abnormal sympathetic modulation of blood vessels. This notion is supported by the current findings that the percent decrease from baseline in TVC to NP is significantly lower in the MS group compared with healthy controls.
Corroborating this conjecture of altered sympathetic blood pressure regulation, our laboratory has recently observed low MSNA at rest in persons with MS (Keller et al. 2014). Given the involvement of MSNA in blood pressure regulation, reductions in MSNA in individuals with MS, taken together with the current findings, indicate an impaired sympathetic modulation of the vasculature in MS in response to hypotensive stimuli. Given that women are two to three times more likely to be diagnosed with MS along with strong evidence that suggest vasoconstrictor responsiveness is blunted in young women, the impact of reduced baroreflex-mediated vasoconstriction may be compounded (Hogarth et al. 2007; Joyner et al. 2015; Kneale et al. 2000; National Multiple Sclerosis Society 2016b). Furthermore, men living with MS progress more rapidly, which may lead to greater carotid baroreflex dysfunction (20). Therefore, more studies are needed to further investigate mechanism(s) impairing the baroreflex control of BP to hypotension in MS with attention on potential sex differences.
In the current study, nadir heart rate and blood pressure responses to simulated baroreceptor loading (i.e., neck suction) were not different between the two experimental groups. In 2005, Sanya et al. reported baroreflex impairments in persons with MS subjected to only neck suction compared with healthy controls. While the exact reason for the differences with the findings from the present study are not clear, contrasting methodologies may be involved. Sanya et al. utilized dynamic sinusoidal neck suction stimulation (0 to −30 mmHg at 6 cycles/min and 12 cycles/min) compared with the 5-s static stimulation utilized in our study. Furthermore, they utilized spectral analysis to separately evaluate the cardiac and blood pressure responses to the baroreceptor stimulations. Nevertheless, despite these differences, the interpretive conclusions of their study are complementary to ours in that there appears to be an impairment in baroreflex responsiveness in MS individuals. Future studies quantifying full carotid baroreflex stimulus response curves are warranted to gain further insight into baroreflex function in MS.
In regards to clinical implications, presently there is a lack of consensus regarding a clear definition of cardiovascular autonomic dysfunction in persons with MS, making diagnosis and treatment more difficult (Racosta et al. 2015). The current results provide experimental evidence demonstrating the inability of individuals with MS to reflexively increase their blood pressure in response to a hypotensive stimulus. This leads to a possible mechanistic explanation of why this clinical population often experiences orthostasis-related symptomology. Indeed, our findings emphasize a potentially deleterious aspect of the disease that likely impacts these individuals on a daily basis. As such, the carotid baroreflex abnormalities observed in this study highlight the importance for recognition and continued research of this dysfunction as it relates to daily activities in this relatively understudied patient population.
A potential limitation of the study that requires consideration is that all individuals with MS that participated in our study were on disease-modifying therapies. According to the 2015 consensus paper by the Multiple Sclerosis Coalition on the use of disease modifying therapies, FDA-approved disease-modifying treatment is recommended and should be continued indefinitely (Costello et al. 2015). Taking persons with MS off of their disease-modifying medication would be impractical and not suggested by treating clinicians. In light of this circumstance, it is difficult to distinguish with certainty that the abnormal carotid baroreflex responses were due to the disease process itself, the sequela of using disease-modifying therapies, or a combination of both. Regardless, physicians and health care providers need to be aware of this clinical feature that affects the majority of individuals with MS.
Conclusion
In conclusion, individuals living with MS demonstrate a diminished ability to increase blood pressure in response to a hypotensive stimuli compared with healthy controls. Furthermore, this diminished ability to increase blood pressure can be attributed to a blunted vascular response. Our findings suggest that carotid baroreceptor control of blood pressure is compromised in this clinical population that may be contributing towards orthostasis-related symptomology associated with MS.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R15-HL-117224 (to S. L. Davis) and National Multiple Sclerosis Society Grant RG4696A3/2 (to S. L. Davis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.H., D.R.A., and S.L.D. performed experiments; M.H., D.R.A., D.M.K., P.J.F., and S.L.D. analyzed data; M.H., D.R.A., D.M.K., P.J.F., E.M.F., and S.L.D. interpreted results of experiments; M.H. and S.L.D. prepared figures; M.H. and S.L.D. drafted manuscript; M.H., D.R.A., D.M.K., P.J.F., E.M.F., and S.L.D. edited and revised manuscript; M.H., D.R.A., D.M.K., P.J.F., E.M.F., and S.L.D. approved final version of manuscript; D.M.K., P.J.F., E.M.F., and S.L.D. conception and design of research.
ACKNOWLEDGMENTS
The considerable time and effort of the participants are greatly appreciated.
REFERENCES
- Acevedo AR, Nava C, Arriada N, Violante A, Corona T. Cardiovascular dysfunction in multiple sclerosis. Acta Neurol Scand 101: 85–88, 2000. [DOI] [PubMed] [Google Scholar]
- Adamec I, Habek M. Autonomic dysfunction in multiple sclerosis. Clin Neurol Neurosurg 115, Suppl 1: S73–S78, 2013. [DOI] [PubMed] [Google Scholar]
- Anema JR, Heijenbrok MW, Faes TJ, Heimans JJ, Lanting P, Polman CH. Cardiovascular autonomic function in multiple sclerosis. J Neurol Sci 104: 129–134, 1991. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Wallin BG. Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Compr Physiol 4: 825–850, 2014. [DOI] [PubMed] [Google Scholar]
- Cosentino M, Marino F. Adrenergic and dopaminergic modulation of immunity in multiple sclerosis: teaching old drugs new tricks? J Neuroimmune Pharmacol 8: 163–179, 2013. [DOI] [PubMed] [Google Scholar]
- Costello K, Halper J, Kalb R, Skutnik L, Rapp R. The Use Of Disease-Modifying Therapies in Multiple Sclerosis: Principles and Current Evidence–a Consensus Paper by the Multiple Sclerosis Coalition. Cherry Hill, NJ: MS Coalition, 2015. [Google Scholar]
- Credeur DP, Holwerda SW, Boyle LJ, Vianna LC, Jensen AK, Fadel PJ. Effect of aging on carotid baroreflex control of blood pressure and leg vascular conductance in women. Am J Physiol Heart Circ Physiol 306: H1417–H1425, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL, Kifle YT, Roberts VL. Phase relationship between normal human respiration and baroreflex responsiveness. J Physiol 304: 489–502, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds P, Hart S, Wei GN, Vivat B, Burman R, Silber E, Higginson IJ. Palliative care for people severely affected by multiple sclerosis: evaluation of a novel palliative care service. Mult Scler 16: 627–636, 2010. [DOI] [PubMed] [Google Scholar]
- Esler MD, Wallin G, Dorward PK, Eisenhofer G, Westerman R, Meredith I, Lambert G, Cox HS, Jennings G. Effects of desipramine on sympathetic nerve firing and norepinephrine spillover to plasma in humans. Am J Physiol Regul Integr Comp Physiol 260: R817–R823, 1991. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Keller DM, Raven PB. Recent insights into carotid baroreflex function in humans using the variable pressure neck chamber. Exp Physiol 88: 671–680, 2003. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Kim A, Hartwich D, Fadel PJ. New insights into the effects of age and sex on arterial baroreflex function at rest and during dynamic exercise in humans. Auton Neurosci Basic Clin 172: 13–22, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Kim A, Young CN, Fadel PJ. Carotid baroreflex control of arterial blood pressure at rest and during dynamic exercise in aging humans. Am J Physiol Regul Integr Comp Physiol 299: R1241–R1247, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Kim A, Young CN, Ogoh S, Raven PB, Secher NH, Fadel PJ. Influence of ageing on carotid baroreflex peak response latency in humans. J Physiol 587: 5427–5439, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Ogoh S, Ahmed A, Aro MR, Gute D, Fadel PJ. Influence of age on cardiac baroreflex function during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 293: H777–H783, 2007. [DOI] [PubMed] [Google Scholar]
- Flachenecker P. Autonomic dysfunction in Guillain-Barré syndrome and multiple sclerosis. J Neurol 254, Suppl 2: II96–II101, 2007. [DOI] [PubMed] [Google Scholar]
- Flachenecker P, Reiners K. Abnormal baroreflex responses in multiple sclerosis. Clin Auton Res 15: 419 author reply 420, 2005. [DOI] [PubMed] [Google Scholar]
- Flachenecker P, Wolf A, Krauser M, Hartung HP, Reiners K. Cardiovascular autonomic dysfunction in multiple sclerosis: correlation with orthostatic intolerance. J Neurol 246: 578–586, 1999. [DOI] [PubMed] [Google Scholar]
- Frontoni M, Fiorini M, Strano S, Cerutti S, Giubilei F, Urani C, Bastianello S, Pozzilli C. Power spectrum analysis contribution to the detection of cardiovascular dysautonomia in multiple sclerosis. Acta Neurol Scand 93: 241–245, 1996. [DOI] [PubMed] [Google Scholar]
- Harbo HF, Gold R, Tintoré M. Sex and gender issues in multiple sclerosis. Ther Adv Neurol Disord 6: 237–248, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth AJ, Mackintosh AF, Mary DA. Gender-related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin Sci (Lond) 112: 353–361, 2007. [DOI] [PubMed] [Google Scholar]
- Holwerda SW, Fulton D, Eubank WL, Keller DM. Carotid baroreflex responsiveness is impaired in normotensive African American men. Am J Physiol Heart Circ Physiol 301: H1639–H1645, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwerda SW, Samels MR, Keller DM. Carotid baroreflex responsiveness in normotensive African Americans is attenuated at rest and during dynamic leg exercise. Front Physiol 4: 29, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JR, Schreuder JJ, Mulier JP, Smith NT, Settels JJ, Wesseling KH. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br J Anaesth 87: 212–222, 2001. [DOI] [PubMed] [Google Scholar]
- Jansen JR, Wesseling KH, Settels JJ, Schreuder JJ. Continuous cardiac output monitoring by pulse contour during cardiac surgery. Eur Heart J 11, Suppl I: 26–32, 1990. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Barnes JN, Hart EC, Wallin BG, Charkoudian N. Neural control of the circulation: how sex and age differences interact in humans. Compr Physiol 5: 193–215, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjwal K, Karabin B, Kanjwal Y, Grubb BP. Autonomic dysfunction presenting as postural orthostatic tachycardia syndrome in patients with multiple sclerosis. Int J Med Sci 7: 62–67, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Fadel PJ, Harnsberger MA, Remington GM, Frohman EM, Davis SL. Reduced spontaneous sympathetic nerve activity in multiple sclerosis patients. J Neurol Sci 344: 210–214, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Deo SH, Fisher JP, Fadel PJ. Effect of sex and ovarian hormones on carotid baroreflex resetting and function during dynamic exercise in humans. J Appl Physiol 112: 1361–1371, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Deo SH, Vianna LC, Balanos GM, Hartwich D, Fisher JP, Fadel PJ. Sex differences in carotid baroreflex control of arterial blood pressure in humans: relative contribution of cardiac output and total vascular conductance. Am J Physiol Heart Circ Physiol 301: H2454–H2465, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000. [DOI] [PubMed] [Google Scholar]
- Lublin FD. Clinical features and diagnosis of multiple sclerosis. Neurol Clin 23: 1–15, 2005. [DOI] [PubMed] [Google Scholar]
- Ludbrook J, Mancia G, Ferrari A, Zanchetti A. The variable-pressure neck-chamber method for studying the carotid baroreflex in man. Clin Sci Mol Med 53: 165–171, 1977. [DOI] [PubMed] [Google Scholar]
- Nasseri K, TenVoorde BJ, Adèr HJ, Uitdehaag BM, Polman CH. Longitudinal follow-up of cardiovascular reflex tests in multiple sclerosis. J Neurol Sci 155: 50–54, 1998. [DOI] [PubMed] [Google Scholar]
- National Multiple Sclerosis Society. Types of MS (Online). http://www.nationalmssociety.org/What-is-MS/Types-of-MS [8 March 2016a].
- National Multiple Sclerosis Society. Who Gets MS? (Online). http://www.nationalmssociety.org/What-is-MS/Who-Gets-MS [10 March 2016b].
- Oger J. Multiple Sclerosis for the Practicing Neurologist. New York: Demos Medical Publishing, 2007. [Google Scholar]
- Ogoh S, Fadel PJ, Monteiro F, Wasmund WL, Raven PB. Haemodynamic changes during neck pressure and suction in seated and supine positions. J Physiol 540: 707–716, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Nissen P, Jans Ø, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentland B, Ewing DJ. Cardiovascular reflexes in multiple sclerosis. Eur Neurol 26: 46–50, 1987. [DOI] [PubMed] [Google Scholar]
- Potts JT, Raven PB. Effect of dynamic exercise on human carotid-cardiac baroreflex latency. Am J Physiol Heart Circ Physiol 268: H1208–H1214, 1995. [DOI] [PubMed] [Google Scholar]
- Querry RG, Smith SA, Strømstad M, Ide K, Secher NH, Raven PB. Anatomical and functional characteristics of carotid sinus stimulation in humans. Am J Physiol Heart Circ Physiol 280: H2390–H2398, 2001. [DOI] [PubMed] [Google Scholar]
- Racosta JM, Sposato LA, Morrow SA, Cipriano L, Kimpiski K, Kremenchutzky M. Cardiovascular autonomic dysfunction in multiple sclerosis: a meta-analysis. Mult Scler Relat Disord 4: 104–111, 2015. [DOI] [PubMed] [Google Scholar]
- Sanya EO, Tutaj M, Brown CM, Goel N, Neundörfer B, Hilz MJ. Abnormal heart rate and blood pressure responses to baroreflex stimulation in multiple sclerosis patients. Clin Auton Res 15: 213–218, 2005. [DOI] [PubMed] [Google Scholar]
- Senaratne MP, Carroll D, Warren KG, Kappagoda T. Evidence for cardiovascular autonomic nerve dysfunction in multiple sclerosis. J Neurol Neurosurg Psychiatry 47: 947–952, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AA, Timmers HJ, Wieling W, Wagenaar M, Marres HA, Lenders JW, van Montfrans GA, Karemaker JM. Long-term effects of carotid sinus denervation on arterial blood pressure in humans. Circulation 105: 1329–1335, 2002. [DOI] [PubMed] [Google Scholar]
- Thomaides TN, Zoukos Y, Chaudhuri KR, Mathias CJ. Physiological assessment of aspects of autonomic function in patients with secondary progressive multiple sclerosis. J Neurol 240: 139–143, 1993. [DOI] [PubMed] [Google Scholar]
- Timmers HJ, Karemaker JM, Wieling W, Marres HA, Lenders JW. Baroreflex control of muscle sympathetic nerve activity after carotid body tumor resection. Hypertension 42: 143–149, 2003. [DOI] [PubMed] [Google Scholar]