Abstract
Presynaptic inhibition is a very powerful inhibitory mechanism and, despite many detailed studies, its purpose is still only partially understood. One accepted function is that, by reducing afferent inflow to the spinal cord and brainstem, the tonic level of presynaptic inhibition prevents sensory systems from being overloaded. A corollary of this function is that much of the incoming sensory data from peripheral receptors must be redundant, and this conclusion is reinforced by observations on patients with sensory neuropathies or congenital obstetric palsy in whom normal sensation may be preserved despite loss of sensory fibers. The modulation of incoming signals by presynaptic inhibition has a further function in operating a “gate” in the dorsal horn, thereby determining whether peripheral stimuli are likely to be perceived as painful. On the motor side, the finding that even minimal voluntary movement of a single toe is associated with widespread inhibition in the lumbosacral cord points to another function for presynaptic inhibition: to prevent reflex perturbations from interfering with motor commands. This last function, together with the normal suppression of muscle and cutaneous reflex activity at rest, is consistent with Hughlings Jackson's concept of evolving neural hierarchies, with each level inhibiting the one below it.
Keywords: presynaptic inhibition, reflexes, Hughlings Jackson
“Even today we may not have entirely grasped the magnitude of Jackson' ideas, for his intellectual life was a century ahead of his contemporaries” (Macdonald Critchley 1960).
“. . . his conclusion that positive symptoms from destructive lesions must arise from the uncontrolled activity of the healthy parts remaining, simple as it now appears, was the starting point of a revolution in thought which has ever since inspired neurophysiology” (Sir Charles Symonds and W. J. Bishop 1960).
Given the present understanding of the nervous system, it is difficult to appreciate the very limited information that was available a century and a half ago. The latter was a time when the only means of enquiry were detailed clinical descriptions of patients supplemented by anatomical and histological examinations of postmortem material. Few neurologists employed faradic electrical stimulation of peripheral and cranial nerves and animal experiments were rarely performed. Yet, despite these limitations, there was extraordinary interest in the normal functioning of the nervous system and in its disturbance by disease. In the English-speaking world, no one gave more thought to these matters than John Hughlings Jackson, preeminent among his colleagues and subsequently dubbed “the father of British neurology.” Today Jackson is best remembered for his studies in epilepsy and especially for his deduction that the spread of involuntary jerking in a limb, at the start of a seizure, was a consequence of the pattern of representation of movements in the motor cortex. Indeed, despite repeated attempts to define and classify the different types of epilepsy, many neurologists still prefer to talk of “Jacksonian seizures.” However, it is not Jackson's studies of epilepsy that are the stimulus for the present review but rather his concept of a hierarchy of control in the nervous system, with each level inhibiting the one below it (see below). In the light of all that is presently known about descending control of sensory and motor pathways, this concept may appear vague; nevertheless, it was entirely original and of fundamental importance in the understanding of the nervous system. It is significant that Jackson's proposal, given in a Croonian Lecture in 1884, preceded the first clear laboratory demonstration of inhibition in the nervous system (Sherrington 1893), although the nature of inhibition (“resistance”) was a prominent source of speculation around this time.
The discovery of a special form of inhibition, presynaptic, was only made in 1957 (see below) and, as its powerful nature and widespread distribution became evident, presented a puzzle to at least one of its foremost investigators. Thus Patrick Wall was led to write:
“One might assume that, after 60 years of intense study, the existence of a presynaptic control mechanism would now be so fully established that it would be time to move on while simply accepting it as one of the essential components which permits control of the flow of excitation over neural circuits. I do not agree with that because there remain some deep puzzles and paradoxes about the biological function of presynaptic mechanisms which require further consideration” (Wall 1998).
Unlike other reviews of presynaptic inhibition (Rudomin et al. 1998b; Rudomin and Schmidt 1999; Willis 2006), the present one is not exhaustive. It does, however, provide a possible answer to the functional dilemma by suggesting a linkage with Jackson's concept of neural hierarchies. This approach, which includes clinical observations, accepts that presynaptic inhibition may also have local, specific, actions and it does not exclude a role for postsynaptic inhibition. However, the central proposition is that possibly the most important action of presynaptic inhibition lies in its suppression of reflex activity, particularly during voluntary movements. First, however, a note on Hughlings Jackson (see also Swash 1986).
Hughlings Jackson
John Hughlings Jackson was born on April 4, 1835 in Yorkshire, England, the son of a farmer (Fig. 1). After completing his schooling he became an apprentice to a medical practitioner in York and then a medical student in that city. For the completion of his medical education, Jackson enrolled at St. Bartholomew's Hospital in London, qualifying in 1856. After spending a further 2 yr in York, Jackson returned to London where he was to spend the remainder of his medical career, eventually joining the staff of the London Hospital and that of the National Hospital for the Paralyzed and Epileptic in Queen Square. Not the least of his achievements during this period was the founding editorship of Brain. A shy and retiring man, but possessed of a genial nature, Jackson did no laboratory research himself but kept himself abreast of the work of others. More importantly, he was a keen clinical observer and thought deeply about what he saw, ever attempting to provide physiological explanations for the neurological symptoms and signs of his patients. Although Jackson is best known for his work on epilepsy (see above), it is not the latter that is relevant for a discussion of presynaptic inhibition but rather his concept of the different levels of function in the central nervous system. Thus in Jackson's scheme for the motor system, the lowest level of organization consisted of the motor nerve cells in the anterior horns of the spinal cord and in the brain stem. The middle level was the motor cortex (then under investigation by David Ferrier) and the highest level comprised more anterior regions of frontal cortex and the prefrontal areas. Each level had evolved to control the one below it. In the second of his three Croonian Lectures, delivered to the Royal College of Physicians in March 1884, Jackson drew an analogy with government:
Fig. 1.
John Hughlings Jackson (1835–1911). Photogravure by L. Caulkin; courtesy of the Wellcome Library, London.
‘The doctrine of evolution implies the passage from the most organized to the least organized, or, in other terms, from the most general to the most special. Roughly, we say that there is a gradual “adding on” of the more and more special, a continual adding on of new organizations. However, this “adding on” is at the same time a “keeping down.” The higher nervous arrangements evolved out of the lower keep down those lower, just as a government evolved out of a nation controls as well as directs that nation. If this be the process of evolution, then the reverse process of dissolution is not only “a taking off” of the higher, but is at the very same time a “letting go” of the lower. If the governing body of this country were destroyed suddenly, we should have two causes for lamentation: (1) the loss of services of eminent men: and (2) the anarchy of the new uncontrolled people. . . .’
Presynaptic Inhibition
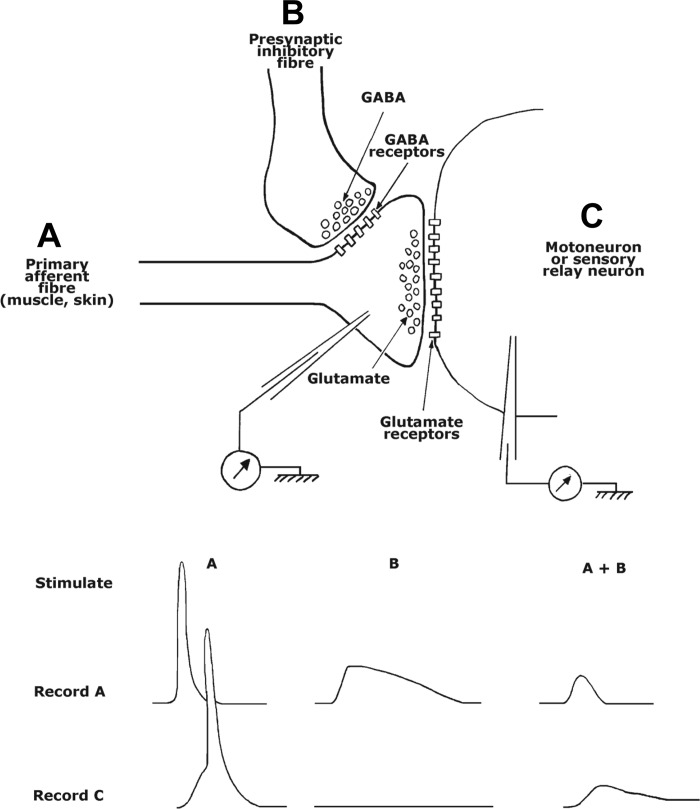
In 1957 Karl Frank and Michelangelo Fuortes, working at the National Institutes of Health in Bethesda, MD, reported some unexpected results while making intracellular recordings from gastrocnemius motoneurons in the cat (Fig. 2). When a group I flexor nerve was stimulated, there was a reduction in the size of the excitatory postsynaptic potential (EPSP) evoked by stimulation of sensory fibers in the gastrocnemius nerve. Since stimulation of the flexor nerve by itself did not evoke a recordable inhibitory postsynaptic potential (IPSP), and since both antidromic invasion and direct stimulation of the motoneurons were unaffected, the inhibitory effect was likely to have been exerted on the sensory fibers in the flexor nerve instead, that is, the inhibition was presynaptic. Subsequently, Frank (1959) considered an alternative explanation, that the flexor nerve endings were situated too remotely on the motoneuron dendrites for the local conductance changes to produce detectable IPSPs in the soma. However, this explanation would not account for the absence of any effect of group I flexor nerve stimulation on EPSPs evoked by descending excitatory pathways to motoneurons (Eccles et al. 1961).
Fig. 2.
Top: schematic of synaptic arrangements between incoming primary afferent fiber (A), a second-order neuron (motoneuron or sensory relay neuron, C), and a regulatory presynaptic fiber (B). Bottom: hypothetical recordings of responses evoked in the primary afferent fiber (A) and second-order neuron (C) by stimulation of the afferent fiber alone (A) and with the presynaptic inhibitory fiber (A + B).
In the years that followed Frank and Fuortes' discovery, the issue of PreI (presynaptic inhibition) was taken up by others, particularly by Eccles in Canberra (Eccles et al. 1961, 1962a,b, 1963) and by Wall in Boston (Wall 1958). It was the Eccles group that provided the definitive proof of PreI, by intracellular recordings of depolarizations in presynaptic fibers (Eccles et al. 1962a,b). They also provided an explanation of the molecular mechanisms likely to be involved, proposing that the inhibitory interneurons involved in the circuit released GABA at synapses with excitatory fibers (c.f. Willis 2006); such axoaxonic synapses were subsequently observed on muscle afferents by Conradi (1969). The GABAA receptors would open chloride channels and partially depolarize the excitatory fibers, effectively reducing their release of transmitter by any approaching impulses: the depolarization was possible because, as was later shown, a membrane Na+-K+-Cl− cotransporter raised the concentration of chloride inside the nerve terminals (Alvarez-Leefmans et al. 1998). The GABA-induced depolarization of the nerve terminals was referred to as “primary afferent depolarization” (PAD; Eccles et al. 1962a,b; Eccles 1964) and shown to have a long duration (Eccles et al. 1962a,b). Figure 2, bottom, shows, in schematic form, the results, in a target neuron, of stimulating an excitatory nerve and a presynaptic inhibitory nerve separately and together. For the sake of completeness, it should be added that, although a GABAA-mediated mechanism is the principal mode of PreI, there are situations in which it may not be the only factor; in the newborn rat; for example, the rise in extracellular K+ accompanying afferent impulse activity might depolarize the axon terminals sufficiently to reduce transmitter release (Kremer and Lev-Tov 1998; for discussion, see Rudomin and Schmidt 1999). Moreover, in the hippocampus, GABAB receptors enable GABA to effect presynaptic inhibition by a second mechanism, in which G proteins act as second messengers to reduce Ca2+ influx in the terminals (see below).
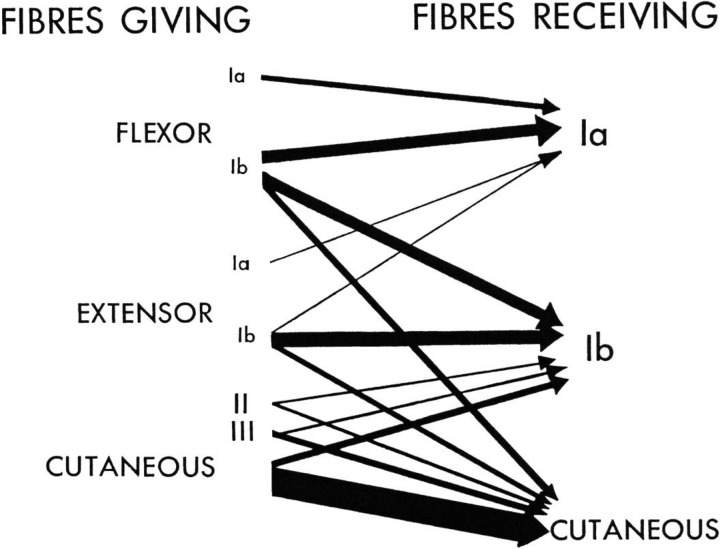
Through intensive studies employing intra- and extracellular recordings of PADs, the Eccles group worked out the patterns of PreI in afferent fibers from skin and muscle receptors (although the PADs would have been partly due to rises in extracellular K+; see above); in addition, interneurons were identified in the dorsal horn which might serve as intermediaries in producing the effects. The authors summarized their work in Fig. 3, the thickness of the arrows being proportional to the strength of the PAD (i.e. the PreI). It can be seen, for example, that only Ia (spindle) afferent terminals are depolarized by Ia fibers from flexor muscles, and that the largest PADs, and therefore the strongest PreIs, are those resulting from interactions between cutaneous nerve fibers. Some of the interactions are surprising, for example, the inhibition of Ib fibers (from Golgi tendon organs) by Ib afferents from all the muscles of the same limb, regardless of whether the muscles normally act as synergists or antagonists. Another surprise is the wide distribution of PAD in the spinal cord following a localized stimulus; for example, in the rat it was later shown that PADs can be detected as many as 17 segments distant from the entry zone of the stimulated fibers and even a force as small as 1 g (<0.01 N) can evoke measurable PAD over several cord segments (Lidierth 2006).
Fig. 3.
Classic diagram of Eccles et al (1963) showing the relative amounts of depolarization evoked in different types of primary afferent fiber by other afferent fibers; the thicker the arrow, the greater the depolarization and hence the degree of presynaptic inhibition. Note that, in the case of jaw muscles (Goldberg and Nakamura 1977), as well as in limb muscles (Rudomin and Schmidt, 1999), there is now evidence that cutaneous fibers can produce PAD in group I afferents. Reproduced from Eccles et al (1963) with permission of the American Physiological Society (see also Rudomin et al. 1986).
At approximately the same time that PreI was being studied in Canberra, Patrick Wall (1958) was employing focal stimulation of afferent endings as a means of identifying PAD in the spinal cord; if the latter was present, the threshold for firing the fibers was lowered. As a corollary, the PAD produced by a peripheral stimulus might, if sufficiently large, initiate impulses that travelled antidromically in the same fibers. These discharges, involving only a small fraction of the sensory fibers at any one time, had been previously observed by Barron and Matthews (1938b) and termed by them “dorsal root reflexes.” It is noteworthy that, although Barron and Matthews (1938a) had employed electrical stimulation of sensory nerves to elicit the dorsal root potentials, upon which the dorsal root reflexes were superimposed, they also showed that similar potentials followed such natural stimuli as touching the skin or stretching a muscle (see also, Lidierth 2006). The dorsal root potentials were later shown to be the depolarizations of the afferent nerve endings, that is, the PADs. Summaries of the known actions of PreI in the vertebrate spinal cord appeared in 1998, as the published proceedings of an international symposium, and also in the following years (Rudomin et al. 1998b; Rudomin and Schmitt 1999; Willis 2006).
First described in the spinal cord and dorsal column nuclei, PreI has since been demonstrated throughout the central nervous system, including the hippocampus and cerebellum. Indeed, hippocampal slice experiments have shown that, in addition to the release of glutamate being controlled by presynaptic GABAA receptors, as was first demonstrated in the spinal cord, probably all transmitters have presynaptic control mechanisms (Thompson et al. 1993). In some cases the latter involve G proteins as second messengers and result in reductions in the uptake of Ca2+ by the nerve terminals and hence in the release of transmitter (Ladera et al. 2008). However, anatomical studies of the cortex have only shown axoaxonic synapses on the axon hillocks of pyramidal cells, where they directly influence spike initiation (Somogyi et al. 1982). Finally, not only is PreI present in all the mammalian species that have been examined to date, but it is also present in insects and invertebrates (Clarac and Cattaert 1996; Hedwig and Burrows 1996).
Descending Control Pathways
Evidence that inhibition in the spinal cord could be controlled by descending pathways from the brain came from investigations in animals. In an early study, Hagbarth and Kerr (1954) showed that, when stimulated electrically, not only the primary somatosensory receiving area but the secondary somatosensory area, motor cortex, and cingulate gyrus could all reduce postsynaptic impulse volleys in ascending sensory pathways in the spinal cord and brain stem. Similar effects could be demonstrated when the anterior vermis of the cerebellum or the midbrain and bulbar reticular formation were stimulated. In a more restricted investigation, a single shock to the rat somatosensory cortex was found to attenuate the amplitude of the response evoked in the dorsal column nuclei by stimulation of a peripheral nerve (Dawson 1958). Rather later it was shown that PreI was likely to be the main mechanism involved since electrical stimulation of either the primary or the secondary somatosensory cortical areas was capable of inducing PAD in afferent fibers projecting to the spinal cord and dorsal column nuclei (Carpenter et al. 1963; Andersen et al. 1964a,b).
The properties of some of the cortical neurons projecting to the sensory systems are known; in the rat somatosensory cortex, for example, the cortical neurons with axons running in the pyramidal tract to the dorsal column nuclei have receptive fields and response latencies similar to those of other cells in the same cortical area (McComas and Wilson 1968). Other studies, however, leave doubt as to whether the pyramidal tract is the main pathway involved. Thus when the motor cortex or the pyramidal tract are stimulated electrically, the PAD in group Ia afferents is inhibited rather than increased (Rudomin et al. 1986; Eguibar et al. 1994). Of the other descending pathways, those from the vestibular nuclei have been found to increase PAD in Ia fibers while stimulation of the red nucleus and reticular formation inhibited PAD; opposite effects were observed for Ib fibers (from tendon organs) while cutaneous fibers exhibited mixed effects (for review, see Rudomin and Schmidt 1999) However, these experiments, and indeed most of those employed in studies of PreI, made use of nonphysiological stimuli, massive simultaneous activation of fibers, and only analyzed events immediately after the shock. Clearly there is a need for more studies employing natural stimuli, such as touching the skin or stretching a muscle, and for longer periods of observation. The latter need is well brought out by voluntary contractions, in which an initial short-lived decrease in PreI in Ia fibers is followed by an increase (see below).
The Problem
Despite the profusion of scientific studies on PreI and descending control pathways, there is one overriding question that has still to be answered convincingly: what is the place of such a powerful and widely distributed inhibitory system in the coordinated functioning of the nervous system? Indeed this is the quandary at the heart of Patrick Wall's “deep puzzles and paradoxes” (see Introduction).
The uncertainty over PreI stands in contrast to the known functions of postsynaptic inhibition. This, the other type of inhibition found in the brain and spinal cord, is used to improve spatial contrast in a sensory pathway and to refine muscle contractions in a motor task. In the visual pathway, for example, postsynaptic inhibition enables retinal ganglion cells to be excited while their neighbors are silenced, giving the ganglion cells “center on” or “center off” properties (Kuffler 1953), and the same type of inhibition is also present in the lateral geniculate nucleus and primary visual cortex (Hubel 1960; Hubel and Wiesel 1959). Similarly, in the dorsal column pathway, postsynaptic inhibition sharpens the receptive fields of second order neurons in the gracile and cuneate nuclei (Gordon and Paine 1960). In motor pathways, the Ia fibers of a flexor muscle will, through postsynaptic inhibitory interneurons, reflexly inhibit the motoneurons supplying an extensor muscle operating at the same joint, and vice versa (Eccles et al. 1956). In addition, postsynaptic inhibition, imposed by Renshaw cells in the ventral horn of the spinal cord, is employed to modulate motoneuron firing rates both by a feedback mechanism (Eccles et al. 1954) and by inputs from central pattern generators (Nishimaru et al. 2006).
Presynaptic Inhibition in Different States
Whenever sensory receptors are stimulated, the pattern of PreI neural connections in the brain stem and spinal cord necessarily limits the excitatory influences of the same fibers, whether on motoneurons, sensory pathways, or internuncial neurons of one kind or another. The effect of this suppression will depend on the state of the body, whether it is at rest or undertaking reflex, automatic, or voluntary movements. Furthermore, by means of their extensive divergence, the PreI neurons have the possibility to correlate discharges in higher order neurons of sensory pathways and also to form an essential step in centrally generated movement patterns.
Resting State
If PreI is present during the resting state, then there should be effects following the application of a GABAA antagonist such as picrotoxin, although the latter compound would inhibit GABAA-mediated postsynaptic inhibition also. Wall (1994) found that, following injection of picrotoxin into rats, the incidence of responsive caudally directed axons increased by a factor of 8.7, and similar results could be obtained by cutting dorsal roots above and below the entry zone of the parent fibers. Rather similar results, also indicative of tonically inactive caudal branches of sensory afferents, were obtained in the cat by Eguibar et al. (1994). Taken together, these findings suggested that, even in the absence of signals from the periphery, PreI was operating in the spinal cord.
Reflex and Automatic Movements
Because of its relatively simple nature, the reflex that has been most studied in relation to PreI is the monosynaptic connection between Ia fibers and homonymous motoneurons. In human subjects this reflex can be most readily elicited by stimulating the tibial nerve electrically, while recording from the soleus muscle (the “H” reflex; Hoffmann 1910; Magladery and McDougal 1950). It was noted early that the H reflex was diminished and sometimes completely abolished during passive dorsiflexion of the ankle joint (Hoffmann 1918). Subsequently, it was found that joint afferents were not necessary for this effect (Robinson et al. 1982) and that even passive movement of the whole leg (Staines et al. 1997) could diminish the soleus H reflex. However, while the amount of PreI would certainly affect the amplitude of the H reflex, the latter also depends on the degree of motoneuron excitability, and special strategies are needed to distinguish between the two following any perturbation (Hultborn et al. 1987a,b; see below). In a study of human locomotion it was observed that the H reflex was increased during the stance phase in both walking and running. However the increase was smaller during running, despite motoneurone excitation, as judged by the concurrent EMG activity, being higher than in walking (Capaday and Stein 1987a). Computer modeling of the input-output properties of motoneurons led the same authors to suggest that increased presynaptic inhibition of Ia afferents was responsible for the relatively lower gain of the H reflex during running (Capaday and Stein 1987b). In animal experiments, the situation is clearer, since the amount of PreI can be measured directly by intracellular recording from afferents (Gossard et al. 1991) or inferred from motoneuron EPSPs, PADs, or the amount of antidromic activity in afferents (dorsal root “reflexes”). With the use of a combination of techniques, it has been shown that any change in PreI depends on the nature of the movement, since in the cat PreI is increased during locomotion (Gossard et al. 1991; Gosgnach et al. 2000) but decreased during fictive scratching (Côté and Gossard 2003).
In both instances the cyclic oscillations in dorsal root potential amplitude suggest that PreI is under the control of the respective central pattern generator (Bayev and Kostyuk 1981, 1982). The prolonged duration of the presumptive PreI during fictive locomotion may possibly result from activation of metabotropic GABAB receptors, complementing phasic changes in PreI brought about by the ionotropic GABAA type (Gosgnach et al. 2000).
“Voluntary” Movements
The H reflex has also been employed to examine the effects of a voluntary contraction on PreI. In the case of plantarflexion in human subjects, it was found that there was a rise in reflex excitability even before the soleus motoneurons began firing and that, as the contraction continued, the reflex excitability fell below the resting level (Requin 1969). These early results were consistent with subsequent studies that reported an initial reduction, followed by an increase, of preI in the Ia fibers of the contracting muscle (Hultborn et al. 1987b; Meunier and Pierrot-Deseilligny 1989). However, as with studies of the same reflex during rest (see above), there is a potential problem in human experiments in distinguishing effects due to altered motoneuron excitability from those resulting from PreI. Although ingenious strategies have been devised to deal with this difficulty, they remain indirect, in that there are no recordings of inhibitory potentials, nor are they otherwise free from criticism. For example, in the series of experiments by the Deseilligny group the size of Ia test volley had to be adjusted in the presence of conditioning facilitation or inhibition so that the amplitude of the reference H reflex was kept constant (“compensation”; Hultborn et al. 1987b). For the experiments to work, however, the matching has to be exact, and this is almost impossible to achieve because of the moment-to-moment variations in H-reflex amplitude, even in the resting condition. Also, altering the stimulus for the Ia test volley will affect the potential contributions of other afferents in the same nerve, although the experimenters sought to exclude any polysynaptic effects by examining only the earliest interactions between conditioning and test inputs. An equally important concern is a consequence of the possibility that the respective motoneuron populations examined during rest and voluntary contraction may differ in their excitabilities (Meunier and Pierrot-Deseilligny 1989). With these provisos, one important finding was that, even if only a single muscle or muscle group was made to contract, changes in presynaptic inhibition included Ia afferents to other muscles in the same leg (Hultborn et al. 1987b).
Because of the interpretive difficulties in the human experiments, the results in animal studies are especially noteworthy. Genetic engineering techniques have now made it possible to identify the GABA interneurons responsible for PreI in the mouse, so that the same neurons can then be selectively destroyed by diphtheria toxin (Fink et al. 2014). Animals treated in this way were found to exhibit marked oscillations of their forelimbs as they reached forwards, although the grabbing of food pellets was performed normally. Such oscillations of muscles would be predicted on theoretical grounds if there was insufficient damping of the sensory input in a neuromuscular feedback system (Orguztoreli and Stein, 1975). In this case the group Ia fibers monosynaptically connected to homonymous forearm motoneurons would presumably be operating at high gain, having been deprived of presynaptic inhibitory control. Also relevant is an earlier study, this time in monkeys, in which it was found that prior to, and during, active flexion of the wrist, the responses evoked in spinal interneurons by peripheral nerve stimulation became smaller (Seki et al. 2003). Furthermore, the diminution of the responses was associated with enhancement of the antidromic impulse volleys evoked by focal electrical stimulation of the cord dorsum-Wall's test for PreI.
To summarize, there is good evidence that preI is present in the resting state and that it is increased during reflex, automatic, and voluntary movements. We can now consider the implications of these findings for the function(s) of preI.
Functions of Presynaptic Inhibition
Resting state: sensory redundancy.
Even in the resting state there will be a steady flow of impulses from receptors throughout the body, and this will increase with active or passive movements. On the basis of extensive studies, Rudomin and Schmidt (1999) envisaged PreI as a means of preventing afferent impulses continuously arriving from the periphery from exceeding the information processing capacity of the central nervous system.
Implicit in this proposal is the concept of sensory redundancy, that, through its genetically determined arrays of receptors, the nervous system is bombarded with more information than is required for its normal functioning. Such a concept would be consistent with observations in human subjects with peripheral nerve disorders. Thus patients with Charcot-Marie-Tooth disease (hereditary sensorimotor neuropathy), having presented with difficulty in walking, typically deny having any problems with their arms and may be shown to have normal sensation in their hands. Yet, on the basis of nerve conduction studies such patients may have function in only a few percentage of the normal complement of large-diameter sensory nerve fibers. Although the observation was incidental to the main study, we have seen a similar phenomenon in some child and adult patients with persisting obstetric brachial palsy, in whom the classical sensory tests, light touch assessment with von Frey hairs, two point discrimination, and joint position, were also normal (Brown et al. 2000).
Resting state: pain control.
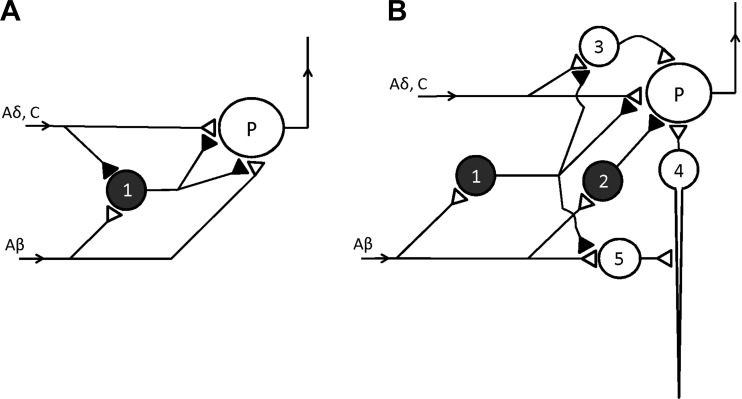
In 1965 Melzack and Wall published a wide-ranging review of pain sensation, drawing on Wall's own neurophysiological studies on the dorsal horn of the spinal cord as well as on descending control mechanisms and psychological factors (Fig. 4). The authors then put forward a “gate” theory, an important feature of which was the ability of neurons in the substantia gelatinosa (SG) to suppress the inputs to those cells, deeper in the dorsal horn, that projected rostrally (P cells; Fig. 4A). The SG cells (1 in Fig. 4A) were thus acting as a gate and were themselves subjected to opposing influences, being excited by large-diameter afferent fibers and inhibited by unmyelinated and small-diameter afferents. When the impulse traffic increased in the small fibers, as the result of a noxious stimulus, the SG cells were inhibited, allowing the small afferents to excite the P cells more strongly. The discharges of the latter to “higher” neurons made it likely that the stimulus would be perceived as painful. A key part of the gate theory was that the SG cells employed presynaptic inhibition of the sensory fibers to achieve their effects, although the possibility of additional postsynaptic mechanisms was not excluded. In the years that followed Melzack and Wall's proposal, the essential features of the gate hypothesis of pain have become generally accepted, although not without some important modifications (Mendell 2014). Importantly, the dorsal horn circuitry responsible for the gate has been shown to be more complex than originally envisaged and it is now known that postsynaptic inhibition is also involved.(Fig. 4B; see Guo and Hu 2014 for review)
Fig. 4.
Pain pathways in the dorsal horn. A: basic scheme of Melzack ad Wall (1965) in which both the small (Aδ, C) and the large (Aβ) fibers are capable of exciting the projection neuron (P), but their nerve terminals are subject to tonic (presynaptic) inhibition from neuron 1 in the substantia gelatinosa. If the small nociceptor fibers are excited neuron 1 is itself inhibited (Wall and Melzack did not show the inhibitory neuron involved), so that P can now be excited more powerfully. In contrast, large fiber inputs stimulate neuron I and thereby reduce excitation of P by terminals of small (and large) fibers. B: current scheme of the pain pathway and is based on Guo and Hu (2014). Once again neuron 1 exerts presynaptic inhibition on the terminals of the large and small fibers, not only those ending directly on the projection cell (P), but also those innervating excitatory neurons (3, 4, 5) in polysynaptic pathways to P. In addition P receives postsynaptic inhibition via neuron 2.
Reflex and voluntary movements: selectivity or not?
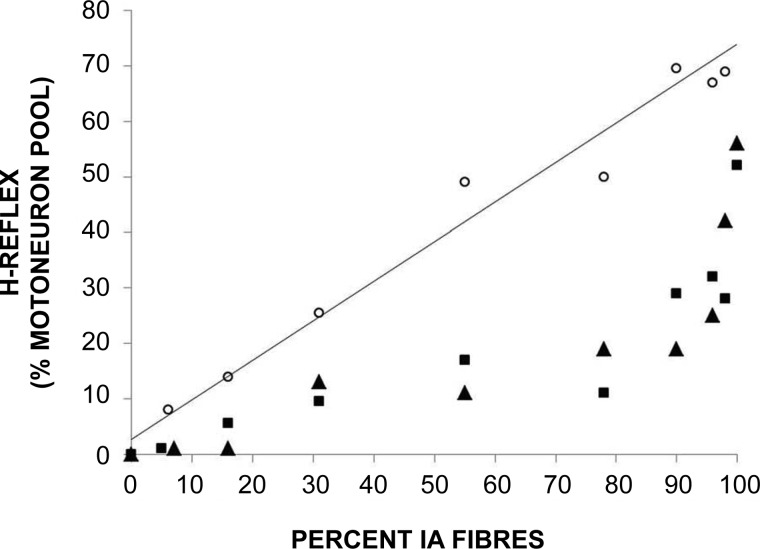
During movements, the barrage of sensory impulses will increase and it has been suggested that PreI will then act selectively, allowing more information to be carried in some neural pathways and less in others. Wall, for example, viewed preI as “a tonically active filter which selects those aspects of the afferent barrage which are permitted to influence deeper nerve cells” (Wall 1998). Initial evidence for the selective application of PreI came from animal studies in which it was possible to record simultaneously from two branches of the same afferent fiber in the spinal cord. It was observed that PAD could be removed in the ascending branch but not in the segmental one by various types of concurrent stimulation (Eguibar et al. 1994). However, in later studies the opposite result was sometimes seen in other pairs of branches (Rudomin et al. 1998a); while the discrepancy could have reflected the ongoing operation of alternative neural programs, even in the resting state, there was also the possibility that the different distributions of PAD arose by chance. In human studies the work that is most frequently cited in favor of selectivity is that by the Pierrot-Deseilligny group in Paris. From experiments on ankle muscles, PreI was thought to be diminished in Ia afferents projecting to motoneurons innervating a contracting muscle and simultaneously increased in Ia fibers projecting to other motoneurons (Hultborn et al. 1987a; Meunier and Pierrot-Deseilligny 1989). However, it appeared that PreI was only reduced at the start of a voluntary ankle movement, being swiftly reimposed as the movement proceeded. In the arm the situation was different, a decrease in PreI being thought to occur in Ia fibers projecting to both wrist flexor and wrist extensor motoneurons (Aymard et al. 2001). As already noted, however, there are serious technical problems with human experiments. Furthermore, experiments in animals show that, following a peripheral stimulus, PAD-and, hence, most likely, PreI-can be detected many spinal cord segments beyond the entry zone of the excited fibers (Lidierth 2006). Other evidence of diffuse, nonselective, inhibition, presumably presynaptic, is our own finding that, following a trivial movement of the great toe, there is usually profound suppression of the soleus muscle H reflex (deBruin et al. 2006; Fig. 5).
Fig. 5.
Relationship between numbers of Ia fibers stimulated and of motoneurons discharging reflexly, with subject relaxed (circles) or performing weak toe dorsiflexion (squares) or ankle plantarflexion (triangles) (McComas AJ, deBruin H, Fu W, Galea A, unpublished observations).
Damping down of reflexes.
Rather than conferring selectivity, a more plausible explanation for the increased PreI in reflex circuits is that it prevents the reflex movements from becoming excessive, or indeed, from appearing at all. That reflexes in the brain stem and spinal cord are normally suppressed by descending pathways is shown by the consequences of an upper motoneuron lesion in the motor cortex, internal capsule, brain stem, or spinal cord. Depending on the location of the lesion, the tendon reflexes may become pathologically brisk in association with an increase in muscle tone; in addition, patients may display a variety of abnormal cutaneous and other reflexes. For example, lightly stroking the lateral sole is sufficient to cause extension of the great toe with fanning out of the other toes (Babinski reflex), while, in some paraplegic patients, pricking the skin over the legs may induce strong reflex withdrawal of the legs and even evacuation of the bowel and bladder (Walshe 1914). Indirect evidence that a loss of PreI is the cause of spasticity after upper motoneurone lesions is that, in such patients, H reflexes can no longer be suppressed by vibration (Burke and Ashby 1972; but see also Pierrot-Deseilligny, 1997). A more direct finding, although involving a single case, is that dorsal root reflexes were absent in a spastic leg but present in the unaffected contralateral limb (Shefner et al. 1992).
Voluntary movements: prevention of reflex contamination.
What is the function of the increased PreI that has been shown to occur in voluntary movements? The most likely explanation is that PreI prevents proprioceptive reflexes from interfering with the centrally planned contractions and the appearance of a coarse tremor during reaching movements in mice without PreI (Fink et al. 2014; see above) provides strong evidence for this view. That the proprioceptive reflexes are not required for increasing the smoothness or the accuracy of the movement is also suggested by clinical observations. First, there are individuals who, although having perfectly normal movements, have either no elicitable tendon reflexes or ones that are barely perceptible even with reinforcement; the H reflexes (see above) are also reduced. The same disparity applies to individuals who lose their tendon reflexes in later life, as in the Holmes-Adie syndrome; in this condition, possibly as the result of an otherwise subclinical viral infection, the tendon reflexes are lost, the H reflexes almost disappear, and the pupil becomes tonically dilated, again, without any impairment of voluntary movement (McComas and Payan 1967; Martinelli et al. 1999). Finally, although long-loop reflexes can be elicited in most subjects by rapidly stretching muscles (see Matthews 1991, for review), the reflex discharges, and hence the numbers of motoneurons participating, are small and require signal averaging to be adequately visualized; moreover, they require a background of voluntary contraction to be seen at all.
Loss of sensory information in voluntary contractions.
The question arises as to whether, in employing PreI to suppress reflex activity during voluntary contractions, the cortex is, at the same time, depriving itself of sensory information. Evidence that this is likely to be the case has come from experiments in which somatosensory potentials have been recorded from the cortex in human subjects during voluntary movements. The results in all cases have been reductions in the amplitudes of the responses (for example, Lee and White 1974; Rushton et al. 1981; Staines et al. 1997). In a recent comprehensive study of instructed movements in the monkey, Seki and Fetz (2012) showed that the evoked responses were diminished not only in the primary somatosensory area but in the motor and premotor cortexes as well (Fig. 6). At least part of this distributed cortical suppression was secondary to gating at the level of the spinal cord. By inserting a delay before the command to move, the authors showed that there was also a suppressive component that was generated in the cortex itself and independent of the subsequent incoming sensory volley. In an earlier study the same authors employed Wall's stimulation of afferent terminals to demonstrate that the inhibition at the level of the spinal cord was mediated, at least in part, by PreI (Seki et al. 2003). That movement also impairs sensory discrimination was shown by Angel and Malenka (1982). These authors determined the thresholds for perceiving electric shocks delivered to the index finger, both at rest and while the subjects were required to move the finger in a tracking task. The authors found that the detection threshold rose in proportion to the speed of movement. Essentially similar results were noted in a subsequent study in which a matching technique between the two hands was employed (Milne et al. 1988).
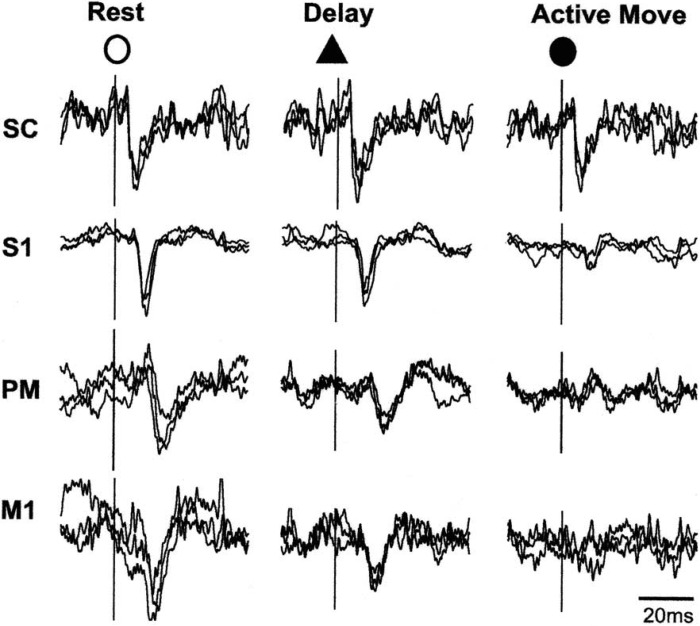
Fig. 6.
Superimposed responses recorded from cervical spinal cord (SC), primary somatosensory cortex (S1), premotor cortex (PM), and primary motor cortex (M1) following stimulation of the superficial radial nerve in a monkey. During active flexion of the wrist the cortical responses are markedly diminished from the resting values, and there is also some reduction in the motor and premotor areas when the animal is waiting for the signal to contract. Reproduced from Seki and Fetz (2012), with permission of K. Seki.
Postnatal development of presynaptic inhibition.
In the mouse it has been shown that PreI of the Ia proprioceptive fibers in the spinal cord is weak or absent at birth, but appears within the next few days (Somner and Ladle 2013). In humans also, it is likely that the neural substrates of PreI are not fully functional immediately after birth. A delay is suggested by the presence in neonates of certain reflexes, which would be abnormal in adults and older children, as well as two types of automatic movements. The reflexes include the tonic neck and palmar grasp reflexes as well as the plantar response and the Moro reflex. The automatic movements include those associated with walking and swimming. (A newborn infant, when supported, will step when the feet are placed on a flat surface, particularly if the surface is a moving one. Similarly, an infant will kick and paddle if placed face-down in water.) All of these reflexes and automatic movements disappear during the first year, usually by 2–6 mo.
Conclusions
Despite its powerful nature and widespread presence, PreI has been largely neglected in neuroscience textbooks because of doubts about its function (Rose and Scott 2003). Starting with the speculations of Hughlings Jackson, however, it is now possible to provide a solution to “the deep puzzles and paradoxes” about this form of inhibition (Wall 1998). By reducing sensory inflow to the nervous system, it serves two main functions: 1) preventing the various sensory pathways in the brain and spinal cord from being overloaded with redundant information, some of which might otherwise be perceived as painful, and 2) damping down spinal reflexes that could have interfered both with the resting state and with reflex and voluntary contractions. It is in undertaking the latter function that PreI best fulfils Jackson's need for the “higher nervous arrangements” to keep down the lower. One of the surprising consequences of this action is restriction of sensory information during voluntary movement, with an attendant impairment of sensory discrimination in the moving part. The impairment, however, is a price that the sensorimotor systems in the brain are evidently prepared to pay, in return for the ability to generate patterns of muscle contraction free of reflexly-induced perturbations (Rose and Scott 2003). In carrying out its suppression of reflex and ascending sensory pathways, PreI does not need to target small groups of fibers but, instead, has evolved to be applied widely (Fig. 7).
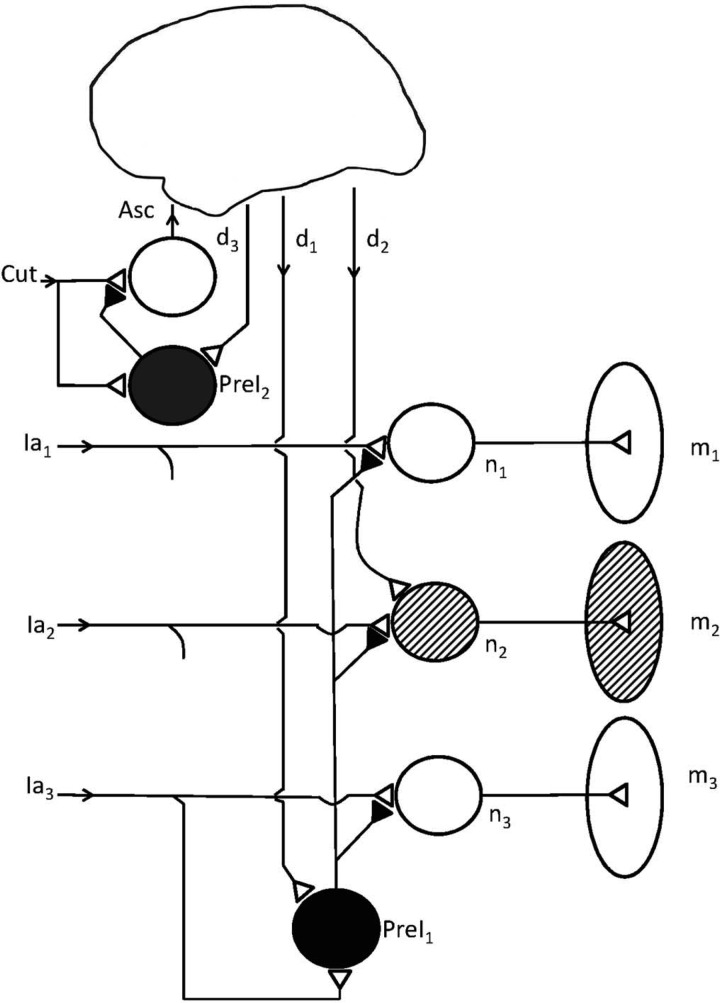
Fig. 7.
Some of the most important actions of PreI. A presynaptic inhibitory neuron (PreI1) inhibits terminals of a Ia fiber (Ia2) synapsing with a homonymous motoneuron (n2); the latter is excited by a descending voluntary input (d2) so as to activate a motor unit in a muscle (m2). However, the PreI interneuron also inhibits Ia fibers (Ia1, Ia3) belonging to other muscles (m1, m3) that are not involved in the desired movement. At the left another PreI interneuron (PreI2) receives inputs from cutaneous peripheral nerve fibers (Cut) and inhibits the terminals of these and other cutaneous afferents. Both PreI neurons are under descending control (d1, d3).
In retrospect, the overriding function of PreI in humans and primates seems obvious; rather than shaping neural activity in discrete circuits, it serves to suppress all unwanted activity in lower levels of the nervous system. That this has not been recognized or at least stated explicitly is perhaps a consequence of the present tendency not to view the system as a whole. One suspects it is a trap that Hughlings Jackson, had he been alive today, would not have fallen into, although the eminent neurologist would certainly have been pleased by the continuing and careful elaboration of his basic idea.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.J.M. interpreted results of experiments; A.J.M. prepared figures; A.J.M. drafted manuscript; A.J.M. edited and revised manuscript; A.J.M. approved final version of manuscript.
REFERENCES
- Alvarez-Leefmans FJ, Nani A, Marquez S. Chloride transport, osmotic balance, and presynaptic inhibition. In: Presynaptic Inbinition and Neural Control, edited by Rudomin P, Romo R, Mendell LM. New York: Oxford Univ. Press, 1998, p. 50–79. [Google Scholar]
- Andersen P, Eccles JC, Sears TA. Cortically evoked depolarization of primary afferent fibers in the spinal cord. J Neurophysiol 27: 63–77, 1964a. [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Schmidt RF, Yokota T. Slow potential waves produced in the cuneate nucleus by cutaneous volleys and by cortical stimulation. J Neurophysiol 27: 78–91, 1964b. [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Schmidt RF, Yokota T. Depolarization of presynaptic fibres in the cuneate nucleus. J Neurophysiol 27: 92–106, 1964c. [DOI] [PubMed] [Google Scholar]
- Angel RW, Malenka RC. Velocity-dependent suppression of cutaneous sensitivity during movement. Exp Neurol 77: 266–274, 1982. [DOI] [PubMed] [Google Scholar]
- Aymard C, Baret M, Katz R, Lafitte C, Pénicaud A, Raoul S. Modulation of presynaptic inhibition of Ia afferents during voluntary wrist flexion and extension in man. Exp Brain Res 137: 127–131, 2001. [DOI] [PubMed] [Google Scholar]
- Barron DH, Matthews BH. The interpretation of potential changes in the spinal cord. J Physiol 92: 276–321, 1938a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron DH, Matthews BH. Dorsal root reflexes. J Physiol 94: 26P–27P, 1938b.16995033 [Google Scholar]
- Bayev KV, Kostyuk PG. Primary afferent depolarization evoked by the activity of spinal scratching generator. Neuroscience 6: 205–215, 1981. [DOI] [PubMed] [Google Scholar]
- Bayev KV, Kostyuk PG. Polarization of primary afferent terminals of lumbosacral cord elicited by the activity of spinal locomotor generator. Neuroscience 7: 1401–1409, 1982. [DOI] [PubMed] [Google Scholar]
- Brown T, Cupido C, Scarfone H, Pape K, Galea V, McComas A. Developmental apraxia following neonatal brachial plexus palsy. Neurology 55: 24–30, 2000. [DOI] [PubMed] [Google Scholar]
- Burke D, Ashby P. Are spinal “presynaptic” inhibitory mechanisms suppressed in spasticity? J Neurol Sci 15: 321–326, 1972. [DOI] [PubMed] [Google Scholar]
- Caccia MR, McComas AJ, Upton AR, Blogg T. Cutaneous reflexes in small muscles of the hand. J Neurol Neurosurg Psychiatry 36: 960–977, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Difference in the amplitude of the human soleus H reflex during walking and running. J Physiol 392: 513–522, 1987a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Stein RB. A method for simulating the reflex output of a motoneuron pool. J Neurosci Methods 21: 91–104. 1987b. [DOI] [PubMed] [Google Scholar]
- Carpenter D, Lundberg A, Norrsell U. Primary afferent depolarization evoked from sensorimotor cotex. Acta Physiol Scand 59: 126–142, 1963. [DOI] [PubMed] [Google Scholar]
- Clarac F, Cattaert D. Invertebrate presynaptic inhibition and motor control. Exp Brain Res 112: 163–180, 1996. [DOI] [PubMed] [Google Scholar]
- Conradi S. On motoneuron synaptology in adult cats. Acta Physiol Scand Suppl 332: 1–115, 1969. [PubMed] [Google Scholar]
- Côté MP, Gossard JP. Task-dependent presynaptic inhibition. J Neurosci 23: 1886–1893, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley M. The contribution of Hughlings Jackson to neurology. Dev Med Child Neurol 2: 7–9, 1960. [Google Scholar]
- Dawson GD. The effect of cortical stimulation on transmission through the cuneate nucleus in the anaesthetized rat. J Physiol 142: 2P–3P, 1958. [Google Scholar]
- deBruin H, Fu W, Galea V, McComas A. Speculations surrounding a spinal reflex. J Neurol Sci 242: 75–82, 2006. [DOI] [PubMed] [Google Scholar]
- Eccles JC. The Physiology of Synapses. Berlin, Germany: Springer, 1964. [Google Scholar]
- Eccles JC, Eccles RM, Magni F. Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys. J Physiol 159: 147–166, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol 126: 524–562, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Fatt P, Landgren S. The central pathway for the direct inhibitory action of impulses in the largest afferent nerve fibres to muscle. J Neurophysiol 19: 75–98, 1956. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Magni F, Willis WD. Depolarization of central terminals of group I afferent fibres from muscle. J Physiol 160: 62–93, 1962a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Schmidt RF, Willis WD. Presynaptic inhibition of the spinal monosynaptic reflex pathway. J Physiol 161: 282–297, 1962b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Schmidt RF, Willis WD. Depolarization of the central terminals of cutaneous afferent fibers. J Neurophysiol 26: 646–661, 1963. [DOI] [PubMed] [Google Scholar]
- Eguibar JR, Quevedo J, Jimenez I, Rudomin P. Selective cortical control of information flow through different intraspinal collaterals of the same muscle afferent fiber. Brain Res 643: 328–333, 1994. [DOI] [PubMed] [Google Scholar]
- Fink AJ, Croce KR, Huang ZJ, Abbott LF, Jessell TM, Azim E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 509: 43–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K. Basic mechanisms of synaptic transmission in the central nervous system. IEEE Trans Med Electr 6: 85–88, 1959. [Google Scholar]
- Frank K, Fuortes MG. Presynaptic and postsynaptic inhibition of monosynaptic reflexes. Fed Proc 16: 39–40, 1957. [Google Scholar]
- Goldberg LJ, Nakamura Y. Production of primary afferent depolarization in group Ia fibers from the masseter muscle by stimulation of trigeminal cutaneous afferents. Brain Res 134: 561–567, 1977. [DOI] [PubMed] [Google Scholar]
- Gordon G, Paine CH. Functional organization of nucleus gracilis of cat. J Physiol 153: 311–349, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosgnach S, Quevedo J, Fedirchuk B, McCrea DA. Depression of group Ia monosynaptic EPSPs in cat hindlimb motoneurones during fictive locomotion. J Physiol 526: 639–652, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard JP, Cabelguen JM, Rossingnol S. Intra-axonal recordings of cutaneous primary afferents during fictive locomotion in the cat. J Neurophysiol 65: 914–926, 1991. [DOI] [PubMed] [Google Scholar]
- Guo D, Hu J. Spinal presynaptic inhibition in pain control. Neuroscience 283: 95–216, 2014. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Kerr DI. Central influences on spinal afferent conduction. J Neurophysiol 17: 295–307, 1954. [DOI] [PubMed] [Google Scholar]
- Hedwig B, Burrows M. Presynaptic inhibition of sensory neurons during kicking movements in the locust. J Neurophysiol 75: 1221–1232, 1996. [DOI] [PubMed] [Google Scholar]
- Hoffmann P. Beitrag zur Kenntnis der menschlichen Reflexe emit besonderer Berucksichtigung der elektrischen Erscheinungen. Arch Anat Physiol 1: 223–246, 1910. [Google Scholar]
- Hoffmann P. Über die Beziehungen der Sehnenreflexe sur willkürlichen Bewegung und zum Tonus. Z Biol 68: 351–370, 1918. [Google Scholar]
- Hubel DH. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol 150: 91–104, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat's striate cortex. J Physiol 148: 574–591, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn J, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol 389: 757–772, 1987a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia fibres: a study in man and the cat. J Physiol 389: 729–756, 1987b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JH. Croonian Lecture II. Evolution and dissolution of the nervous system. BMJ 1: 660–663, 1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer E, Lev-Tov A. GABA-receptor-independent dorsal root afferents depolarization in the neonatal rat spinal cord. J Neurophysiol 79: 2581–2592, 1998. [DOI] [PubMed] [Google Scholar]
- Kuffler SW. Discharge patterns and functional organization of mammalian retina. J Neurophysiol 16: 37–68, 1953. [DOI] [PubMed] [Google Scholar]
- Ladera C, Godino MD, Canañero MJ, Torres M, Watanabe M, Luján R, Sánchez-Pieto J. Pre-synaptic receptors inhibit glutamate release through GIRK channels in rat cerebral cortex. J Neurochem 107: 1506–1517, 2008. [DOI] [PubMed] [Google Scholar]
- Lee RG, White DG. Modification of the human somatosensory evoked response during voluntary movement. Electroencephalogr Clin Neurophysiol 36: 53–62, 1974. [DOI] [PubMed] [Google Scholar]
- Lidierth M. Local and diffuse mechanisms of primary afferent depolarization and presynaptic inhibition in the rat spinal cord. J Physiol 576: 309–327, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magladery JW, McDougal DB Jr. Electrophysiogical studies of nerve and reflex activity in normal man. I. Identification of certain reflexes in the electromyogram and the conduction velocity of peripheral nerve fibres. Bull Johns Hopkins Hosp 86: 265–290, 1950. [PubMed] [Google Scholar]
- Martinelli P, Minardi C, Ciucci G, Dalpozzo F, Giuliani S, Scaglione C. Neurophysiological evaluation of areflexia in Holmes-Adie syndrome. Neurophysiol Clin 29: 255–262, 1999. [DOI] [PubMed] [Google Scholar]
- Matthews PB. The human stretch reflex and the motor cortex. Trends Neurosci 14: 87–91, 1991. [DOI] [PubMed] [Google Scholar]
- McComas AJ, Payan JA. Motoneurone excitability in the Holmes-Adie syndrome. In: Control and Innervation of Skeletal Muscle, edited by Andrew BL. Edinburgh: Livingstone: 1966. [Google Scholar]
- McComas AJ, Wilson P. An investigaton of pyramidal tract cells in the somatosensory cortex of the rat. J Physiol 194: 271–288, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 250: 971–979, 1965. [DOI] [PubMed] [Google Scholar]
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain 155: 210–216, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Gating of the afferent volley of the monosynaptic stretch reflex during movement in man. J Physiol 419: 753–763, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne RJ, Aniss AM, Kay NE, Gandevia SC. Reduction in perceived intensity of cutaneous stimuli during movement: a quantitative study. Exp Brain Res 70: 569–576, 1988. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Restrepo CE, Kiehn O. Activity of Renshaw cells during locomotor-like rhythmic activity in the isolated spinal cord of neonatal mice. J Neurosci 26: 5320–5328, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orguztöreli MN, Stein RB. An analysis of oscillations in neuro-muscular systems. J Math Biol 2: 87–105, 1975. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia afferents during movement in humans. J Neurosci Methods 74: 189–199, 1997. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Mazavet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiol Clin 30: 67–80, 2000. [DOI] [PubMed] [Google Scholar]
- Requin J. Some data on neurophysiological processes involved in the preparatory motor activity to reaction time performance. Acta Psychol 30: 358–367, 1969. [DOI] [PubMed] [Google Scholar]
- Robinson KL, McComas AJ, Belanger AY. Control of soleus motoneurone excitability during muscle stretch in man. J Neurol Neurosurg Psychiatry 45: 699–704, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose PK, Scott SH. Sensory-motor control: a long-awaited behavioral correlate of presynaptic inhibition. Nat Neurosci 6: 1243–1245, 2003. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmitt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129: 1–137, 1999. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Jiménez I, Quevedo J. Selectivity of the presynaptic control of synaptic effectiveness of group I afferents in the mammalian spinal cord. In: Presynaptic Inhibition and Neural Control, edited by Rudomin P, Romo R, Mendell LM. New York: Oxford Univ. Press, 1998a, p. 282–302. [Google Scholar]
- Rudomin P, Romo R, Mendell LM. Presynaptic Inhibition, and Neural Control. New York: Oxford Univ. Press, 1998b. [Google Scholar]
- Rudomin P, Solodkin M, Jiménez I. PAD and PAH response patterns of group Ia- and Ib-fibers to cutaneous and descending inputs in the cat spinal cord. J Neurophysiol 56: 987–1006, 1986. [DOI] [PubMed] [Google Scholar]
- Rushton DN, Rothwell JC, Craggs MD. Gating of somatosensory evoked potentials during different kinds of movement in man. Brain 104: 465–491, 1981. [DOI] [PubMed] [Google Scholar]
- Seki K, Fetz E. Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J Neuroscience 32: 890–902, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki K, Perlmutter SI, Fetz E. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci 6: 1309–1316, 2003. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Buchthal F, Krarup C. Recurrent potentials in human peripheral sensory nerve. Muscle Nerve 15: 1354–1363, 1992. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Note on the knee-jerk and the correlation of action of antagonistic muscles. Proc R Soc London 52: 556–564, 1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somner PM, Ladle DR. Early postnatal development of GABAergic presynaptic inhibition of Ia proprioceptive afferent connections in the mouse spinal cord. J Neurophysiol 109: 2118–2128, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Freund TF, Cowey A. The axo-axonic interneuron in the cerebral cortex of the rat, cat and monkey. Neuroscience 7: 2577–2607, 1982. [DOI] [PubMed] [Google Scholar]
- Staines WR, Brooke JD, Cheng J, Misiaszek JE, MacKay WA. Movement-induced gain modulation of somatosensory potentials and soleus H-refexes evoked from the leg. I. Kinaesthetic task demands. Exp Brain Res 115: 147–155, 1997. [DOI] [PubMed] [Google Scholar]
- Swash M. John Hughlings Jackson: a sesquicentennial tribute. J Neurol Neurosurg Psychiatry 49: 981–985, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds C, Bishop WJ. Hughlings Jackson. Dev Med Child Neurol 2: 3–4, 1960. [Google Scholar]
- Thompson SM, Capogna M, Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci 16: 222–227, 1993. [DOI] [PubMed] [Google Scholar]
- Wall PD. Excitability changes in afferent fibre terminations and their relation to slow potential. J Physiol 142: 1–21, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD. Control of impulse conduction in long range afferents. Eur J Neurosci 6: 1136–1142, 1994. [DOI] [PubMed] [Google Scholar]
- Wall PD. Some unanswered questions about the mechanisms and function of presynaptic inhibition. In: Presynaptic Inhibition and Neural Control, edited by Rudomin P, Romo R, Mendell LM. New York: Oxford Univ. Press, 1998, p. 228–241. [Google Scholar]
- Walshe FM. The physiological significance of the reflex phenomena in spastic paralysis of the lower limbs. Brain 87: 269–336, 1914. [DOI] [PubMed] [Google Scholar]
- Willis WD. John Eccles' studies of spinal cord presynaptic inhibition. Progress Neurobiol 78: 189–214, 2006. [DOI] [PubMed] [Google Scholar]